SUMMARY
Falling between the classical characteristics of innate immune cells and adaptive T and B cells are a group of lymphocytes termed “unconventional.” These cells express antigen-specific T or B cell receptors, but behave with innate characteristics. Well-known members of this group include the gamma-delta T cell and the Natural Killer T cell. Recent literature has greatly expanded scientific knowledge of unconventional lymphocytes, but key questions remain unresolved in the field, including why these cells have been maintained concurrently with conventional innate and adaptive immune cells. Here we summarize current literature that suggests what their unique purposes may be, including specialized functions with the microbiota and in early development. From the consensus literature, we discuss where we see unconventional lymphocytes fit into the logical organization of the complete immune system.
Keywords: Unconventional, lymphocytes, MAIT, NKT, γδ, microbiota
INTRODUCTION: IMMUNE CELLS AS A SPECTRUM
Immunology textbooks teach that there are two separate immune systems, the innate and adaptive, that work in a stepwise fashion to protect against foreign assault on the body(1). But are they really so distinct? In the past decade, considerable research has revealed a spectrum of ‘in between’ cells of various types (Figure 1).
Figure 1. A functional spectrum of immunity.
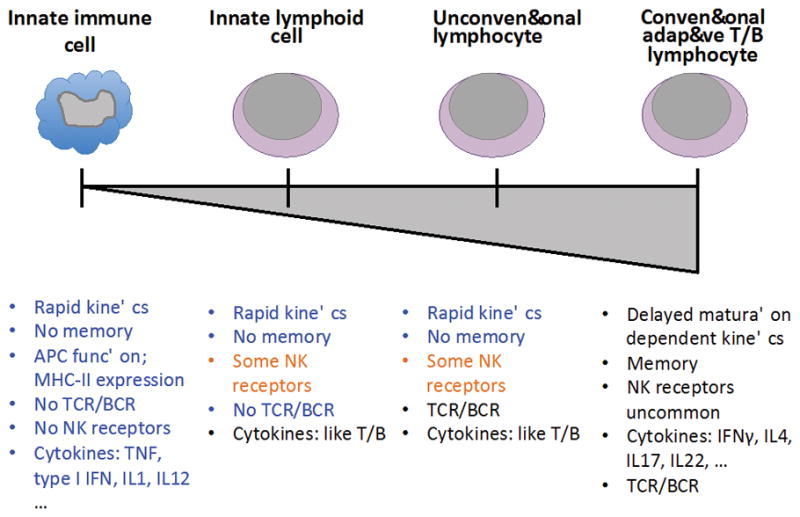
Innate immunity (left) is the germline encoded, immediate response that is unchanging with repeated exposure. This response is a basic characteristic of all cell types when they are infected and is orchestrated by a specialized subset of innate immune cells including macrophages, dendritic cells, neutrophils, basophils, and mast cells. Adaptive immunity (right) is initially delayed, educated upon first stimulation, and increases in kinetic speed and response amplitude upon re-exposure. The two mammalian adaptive immune cells are the conventional T and B cell, each of which has its own subsets delineated by functionality. Between these two ends of the spectrum lie multiple cell types that are neither entirely innate nor adaptive. This review focuses on unconventional lymphocytes, T and B cells that express antigen-specific receptors but do not behave with full adaptive functionality. We also touch on innate lymphoid cells, cells that do not express antigen-specific receptors but carry out similar adaptive functional cytokine responses with innate kinetics.
There have been many studies characterizing and demonstrating their function, not all of which will be reviewed here. However, key questions remain unresolved: How do these cells fit into the logical organization of immune responses? Do they represent evolutionary steps in the formation of the modern adaptive immune response? Are they retained for unique purposes, or are they the ‘appendices’ of the mammalian immune system—a vestigial organ left over from previous iterations in evolution? (Indeed, whether the appendix itself is vestigial in humans or is still required for optimal health continues to be debated(2).) These questions coalesce into two general areas that will be the focus of this review:
What is the current role of “in-between” cells in host immunity?
Why are these cells been maintained during evolution?
These two questions are superficially similar, but their answers may differ depending on the host and its specialized development and environment. We think that, as the field moves beyond an initial phase of observational characterization, it will head towards answering these questions and establishing a new framework of interlocking layers or spectra of immune response organization.
1. IMMUNE CELLS AND THEIR HISTORY
Innate Immune Cells
Innate immunity relies on rapid responses to the recognition of non-self signatures via signaling of germline-encoded receptors. Thus innate cells can be classified into two groups: non-immune-specialized cells with innate immune signaling capacity, and immune-specialized cells with prioritized roles for higher-level immune response coordination.
Innate immunity can be defined as germline encoded, non-adaptive defenses that form the earliest barriers to infection(1). Originally discovered more than a century ago(1), innate immune cells demonstrate immediate activation with rapid kinetics post stimulation. They received renewed attention in the 1990s upon the discovery of pattern recognition receptors (PRRs)(3–5). This family of receptors recognizes conserved structures of bacteria, viruses, and fungi. The PRR system allows for recognition of a broad range of potential infectious agents with minimal investment in different receptors and signaling pathways: a handful of receptors can recognize almost all non-self. PRRs, however, are found on an array of different cell types, not just specialized innate immune cells. For non-immune cells such as epithelial cells(6), neurons(7), and even conventional adaptive immune cells(8), PPR expression establishes intracellular innate immune protective pathways that allow these cells to inform specialized immune cells of a potential infection.
In addition to carrying PRRs, specialized innate immune cells can internalize foreign antigen and translate this antigen into identifying signatures for the coordination of further immune responses. These antigen-presenting cells (APCs) can initiate various pathways to breakdown the objects that stimulate their PRRs and generate small peptides for presentation on major histocompatibility complex class II (MHC-II) molecules—specialized presentation receptors expressed on the cell surface (Figure 2). Indeed, non-immune-specialized cells can similarly process and present antigen from infectious agents on MHC class I (MHC-I) molecules, but only specialized innate immune cells can internalize external antigen for presentation without being infected themselves. These specialized APCs, notably macrophages and dendritic cells (DCs), initiate signaling cascades to immediately recruit effector functions that are non-discriminate, while also initiating the activation and maturation process of antigen-specific, discriminate adaptive immune cells. Specialized effector immune cells include mast cells, basophils, and eosinophils. These cells act as the immediate, but less discriminate, effector arm of innate immunity through release of cytolytic molecules, pro-inflammatory cytokines, and initiation of expulsion responses(1).
Figure 2. T cells are restricted by MHC.
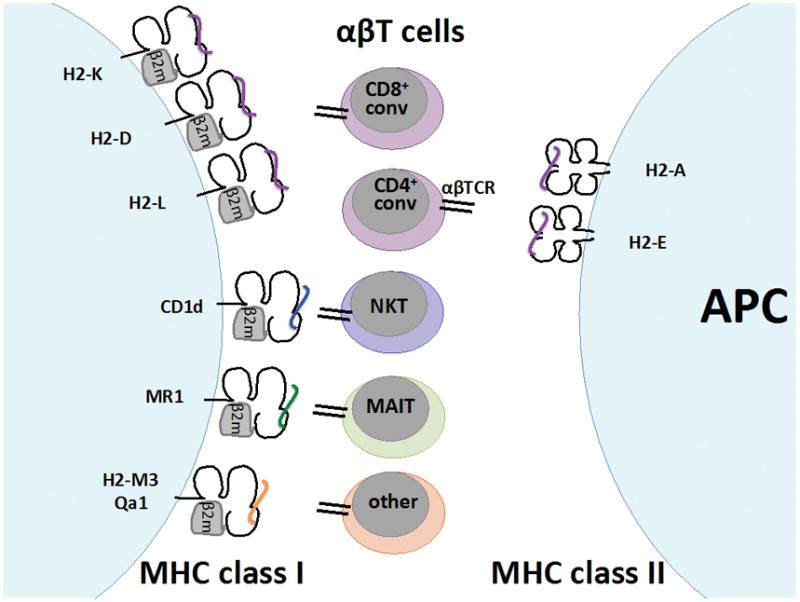
T cells have antigen-specific receptors that interact with specialized antigen-presenting molecules called major histocompatibility complexes (MHCs). There are two general classes of MHC—class I and class II—are found in the mouse clustered on chromosome 17. Class II MHC molecules are formed by heterodimerization of an alpha (α) chain and a beta (β) chain. Class I MHC molecules are formed by one α chain that combines with β2-microglobulin. The outward-facing surfaces of the α:β chains for Class II and the α chain of class I have a cleft that holds the antigen being presented. The class I genes H2-K, H2-D, and H2-L exclusively interact with (“restrict”) the TCRs of CD8+ conventional T cells of the mouse. The class II genes H2-A and H2-E restrict the TCRs of CD4+ conventional lymphocytes of the mouse. MHC restriction is educated during thymic development in two steps of positive and negative selection. Developing immature T cells that are double-positive (DP) for CD4/CD8 expression interact with thymic stromal cells expressing MHC loaded with peptide. This stimulus provides a survival signal that selects positively for T cells that are able to interact well with MHC:peptide complexes, those T cells that cannot interact well with MHC:peptide die off. Surviving T cells progress in maturation to single-positive (SP) CD4+ or CD8+ thymocytes, which then interact with a second population of thymic cells expressing MHC:peptide enriched for peptides from peripheral self-antigen. In this case, if the TCR binds strongly to the MHC:peptide complex, the cell induces apoptosis. This negative pressure selects against TCRs that may be autoreactive to the host’s own healthy tissue. What remains is a population of adaptive CD4+ and CD8+ T cells that can recognize and respond to MHC:peptide complexes, but only when those peptides are non-self. This system is not perfect, and some autoreactive T cells can be found in the periphery after thymic T cell development.
There are also a number of non-classical MHC genes or MHC-like genes which resemble the classical MHC genes but do not restrict conventional CD4+ or CD8+ T cells; these are referred to class Ib genes. Some, but not all, are linked within the MHC complex on chromosome 17. Of these class Ib molecules, MR1 restricts MAIT cells and CD1d restricts NKT cells. Additional subsets of TCR-bearing lymphocytes have been found that are restricted to non-classical class I molecule H2-M3 and Qa1. Additional non-classical class Ib genes exist that have either been shown to not interact with TCR or whose interaction status remains undetermined. References: Janeway’s Immunobiology(1); Godfrey et al 2015(36); Rodgers et al 2005(77).
Conventional Adaptive Immune Cells
There are two types of adaptive immune cells: the conventional αβ-TCR T cell and the B cell. A defining characteristic of these cells is their expression of antigen-specific receptors—the T cell receptor (TCR) and the B cell receptor (BCR), respectively. T cells mature in the thymus via positive selection of MHC interaction (ensuring the removal of cells that are not responsive to MHC) and negative selection of MHC expressing self-antigen (removing cells that are too responsive to antigen expressed by the host under non-inflammatory conditions). In a complex mechanism involving recombination-activating genes 1 and 2 (Rag1 and Rag2), TCRs are constructed via post-somatic recombination from four possible loci—alpha α, beta β, gamma γ, or delta δ, leading to either heterodimeric αβ TCRs or γδ TCRs(9). T cells can be restricted to either MHC-I or MHC-II (Figure 2). There are a number of MHC types, but conventional adaptive αβT cells are restricted to the classical H2-K, H2-D, and H2-L (class I) or H2-A and H2-E (class II) in mice, or to human leukocyte antigen (HLA)-A, HLA-B and HLA-C (class I) and HLA-DR, HLA-DP, and HLA–DQ (class II) in humans(1). These MHC molecules present short peptide antigens and activate the TCR via co-stimulation with the receptors CD8 or CD4. Thus all conventional T cells are also positive for either CD8 or CD4. After developmental maturation in the thymus, these cells will circulate throughout the blood and lymphatics, and certain subsets will also seed peripheral tissues, where they can be retained upon local stimulation.
Beyond this CD4+ and CD8+ dichotomy, CD4+ αβT cells can also be separated into different functional effector groups based on their cytokine production. T helper cells, named because of their function in aiding the activation of CD8+ cytolytic function and B cell effector functions, are divided into four main groups: TH1 (producers of interferon [IFN]γ), TH2 (producers of effector cytokines interleukin [IL]4 and IL13), TH17 (producers of IL17A), and regulatory T cells (TREG; producers of IL10 with suppressive function). These arms of adaptive immunity are well characterized and have been described elsewhere(1). In addition to the four best-characterized groups, there are other less studied arms, including TH9 (producers of IL-9 and IL-21)(10), TH22 (producers of IL-22)(11), and follicular helper T cells (TFH; cells specialized for B cell interaction in germinal centers)(12), among others.
B cells express a surface BCR, but they can also secrete their BCR extracellularly in the form of antibodies. BCRs undergo Rag-dependent recombination in a similar manner to TCRs, with two additional steps during post-BCR-stimulated maturation called somatic hypermutation and class switch recombination that create even greater repertoire diversity. Somatic hypermutation is dependent on a cytidine deaminase, activation-induced deaminase (AID), and creates mutations along the structure of the BCR especially within the antigen-binding zones(13). Class-switch recombination allows B cells to switch their antibody type among several options (isotypes IgM, IgD, IgE, IgA, and IgG, including subtypes of IgG and IgA) that confer different characteristics, such as higher affinity for bacterial opsonization, immune complex formation, complement activation, and other functions(14). Rag-dependent recombination and AID-dependent somatic hypermutation and class-switch recombination are complex processes that are important to the understanding of conventional adaptive immune cells and have been well described elsewhere(9, 13, 14).
“In-betweeners”: Innate lymphoid cells and unconventional lymphocytes
Between these two ends of the innate-adaptive immune spectrum fall a handful of cell types that do not fit neatly into either category (Figure 1). The “leftward” group on the spectrum entails the recently discovered innate lymphoid cells (ILCs)(15). The “rightward” group contains a mix of cells that express TCR or BCR but behave with innate-like functionality, referred to collectively as either unconventional, or innate-like lymphocytes. For simplicity these will be referred to with the broader term unconventional lymphocytes for the remainder of this review.
Innate lymphoid cells
Our understanding of ILCs has greatly expanded in recent years, with several simultaneous reports defining new cell types and a seminal reorganization of ILC nomenclature in 2013(15). Discovered as the source of T helper cytokines in Rag-deficient mice, ILCs are now generally grouped into three types: ILC1, ILC2, and ILC3s.
ILC1s are “TH1-like” and produce predominantly IFNγ upon stimulation(16). Crucially, this group includes Natural Killer (NK) cells, a well-established population discovered decades before the recent expansion of the literature on ILCs. In the mouse model, ILC1s are defined by their surface molecule signature of ‘lineage negative’ (combined panel of antigens: [T cell] CD3, CD4, CD8, [B cell] CD19, B220, [DC/macrophage] CD11b, CD11c, [neutrophil] GR1, [mast cell] FcεRI, [epithelial cell]TER119 aka ‘Lin-’(16)), Rorγ−, Thy1+ and Sca1+. NK cells are similarly defined with additional NK receptors such as NKp46 and NK1.1 (the expressions of which is dependent on the mouse strain)(16). These cells also produce cytolytic molecules such as perforin and granzymes, similar to effector CD8+ T effector cytotoxic lymphocytes (CTLs).
ILC2s are “TH2-like,” producing IL5, IL9, IL13, and other tissue-protective factors such as amphiregulin(16). ILC2s are defined by their surface marker expression as Lin−, ICOS+, Sca1+, and IL7Rα+. Like TH2 cells, ILC2s are dependent on the transcription factors RORα and GATA3(16). These ILCs are predominantly involved in expulsion responses of both parasitic and allergic settings, and are found several peripheral sites including the lungs, gut, spleen and liver(16).
ILC3s are “TH17/22-like,” producing predominantly IL-22, as well as some IL-17(16). ILC3s are found in mucosal tissues; they were originally discovered and have been especially well studied in the gut(17–19). This group also includes (somewhat controversially) the lymphoid tissue inducer (LTi) cell. Like NK cells, LTis were discovered before the recent literature expansion on and reorganization of ILCs. LTis are precursor cells that help initiate the development of lymphoid structures during embryogenesis(20, 21) and potentially later in life as well(22).
The recent literature expansion on ILCs has greatly helped clarify the logical organization of the different ILC populations, including for previously known cell types such as NK cells and LTi cells. This organization clearly mirrors that of conventional adaptive T cells.
Unconventional lymphocytes
Unconventional lymphocytes encompass all T and B cells that carry TCR or BCR but that do not display other characteristics canonically attributed to conventional adaptive lymphocytes. These characteristics include highly polyclonal expression of their antigen receptors, delayed initiation in naïve settings, and unambiguous memory in secondary responses. Cells reported thus far that fall into this category include the following:
Gamma-delta (γδ) T cells
γδT cells were discovered during studies investigating the mechanism of VDJ (variable, diversity, joining) recombination of conventional T cells(23). They are defined by their carriage of a TCR with the gamma (γ) and delta (δ) components (as apposed to the alpha (α) and beta (β) of conventional αβT cells). These two TCRs are mutually exclusive because the δ locus resides within the α locus, thus upon the expression of one, the other is germline excised(23). γδT cells have a limited repertoire of TCRs, unlike the polyclonal TCRs of αβT cells. In early development, individual populations of specific invariant TCR-γδT cells radiate to different peripheral sites including the lung, skin, intestine, and liver, among others(23). γδT cells can express cytokines similar to those expressed by αβT cells, but they respond with innate kinetics. In murine infection models, γδT cells are the predominant source of early-response IFNγ and IL-4, prior to the expansion of adaptive αβT cells(24). Some γδT cells can be directly stimulated by antigen via the TCR similar to αβT cells ( “induced” γδT cells), but they can also be directly stimulated by PRRs and cytokines without TCR signaling (“natural” γδT cells)(23).
Natural Killer T cells
Natural killer T (NKT) cells develop from the CD4+/CD8+ double-positive (DP) stage of conventional T cell development in the thymus(25). They express αβTCRs and diverge after the γδ/αβ T cell split, which occurs during the CD4−/CD8− double-negative (DN) stage of thymic development. They can express adaptive T cell cytokines like IFNγ, IL-4, and IL-17, but respond with innate kinetics, and can be stimulated by antigen via the TCR as well as by PRRs and cytokines(25). NKT cells were originally defined as NK cell marker NK1.1+ αβT cells with invariant TCRs (thus “NK like” T cells). However, over time their definition has become both broader and more specific to be MHC-I non-classical molecule CD1d-responsive αβT cells that are also positive for some NK-cell markers, though not necessarily NK1.1 (as some mouse strains do not express NK1.1)(8). CD1d is the molecule of its type expressed in mice, though humans have several CD1 types including CD1a, b, c, and d(25). Invariant NKT cells (iNKTs or Type I NKTs) commonly express the TCR Vα14-Jα18 and either Vβ8, Vβ7 or Vβ2 in mice, or Vα24-Jα18 and Vβ11 in humans(25). In addition to iNKTs, there are also NKT cells that respond to CD1d but are less restricted in their TCR repertoire and do not share the canonical TCR of iNKTs. These are known as variant or type II NKT cells.
iNKTs respond to lipid antigens of similar structure to glycosphingolipid alpha-galactosylceramide (αGalCer). The first ligand discovered to stimulate iNKT cells was an αGalCer from the marine sea sponge Agelas mauritianus(25). Work by our lab and others have demonstrated that members of the Bacteroides phylum, one of the most abundant bacterial phyla in the mammalian intestinal microbiota, express related glycosphingolipids and interact with iNKT cells(26, 27). Flow cytometric staining reagents have been developed made of four CD1d molecules loaded with appropriate antigen ligand, such as synthetic KRN7000 or other αGalCer molecules, which can label iNKT cells in vivo. The ligand(s) for variant NKT cells is more disputed and probably variable, thus these cells currently cannot be stained with tetramer.
Mucosal Associated Invariant T Cells
Mucosal associated invariant T cells (MAIT) cells were definitively identified in 1999(28). As their name implies, they are found in higher frequencies in mucosal tissues than in non-mucosal, non-lymphoid tissues. They can express T helper cytokines and cytolytic molecules but respond as effectors with innate kinetics. MAIT cells are defined by restriction to the highly-conserved MHC-I non-classical molecule MR1(29). Invariant MAIT cells commonly express the Vα19/TRAV1-2-Jα33 in mice and humans, paired with Vβ8 or Vβ6 in mice(30) and BV6 and BV20 in humans(31). Like other lymphocytes, MAIT cells develop in the thymus. However, MAIT cell retention in the periphery is dependent on presence of the microbiota, and in the gut, on a local B cell population(29, 32). Recent development of an MR1 tetramer now allows for improved identification of these cells in both mice(30) and humans(31).
MAIT cells were recently reported to respond to microbially derived riboflavin metabolites(33). Riboflavin, also known as vitamin B2, is a water-soluble vitamin found in many foods with highest levels found in dairy, offal meats, fatty fish and some vegetables(34). It can also be synthetized by gram-positive and gram-negative bacteria in the gut, especially lactic acid bacteria(35). It is a precursor component for flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD), important electron carrier co-enzymes in the metabolism of vitamins B3, B6, and folate, as well as other redox activities(34). Deficiency of riboflavin intake has been associated with a number of health hazards, including fetal developmental abnormalities, iron absorption defects and anemia, and neurodegeneration(34). Its diffuse and pleiotropic effects are likely due to its intermediate role in many metabolic pathways as an electron carrier. While MAIT cells have been shown to respond in an MR1-dependent fashion to riboflavin derivatives, and the bacteria that have been shown to induce MAIT cells can synthesize riboflavin, the exact ligand(s) of MAIT cells remains to be determined(33).
Several other smaller or less explored groups of unconventional lymphocytes have been identified. These include unconventional T cells restricted to other non-classical MHC-I molecules, including H2-M3, Qa1 and HFE(36), as well as unconventional B cells such as B-1 cells(8) and marginal zone (MZ) B cells(37). There is also a heterogeneously complex population of cells called intraepithelial lymphocytes (IELs) that have unconventional lymphocyte behavior and include members of unconventional lymphocyte populations described above as well as other cell types. IELs will be discussed in more detail later in this review.
2. UNCONVENTIONAL MIDLIFE CRISIS: WHAT IS MY PURPOSE?
In the dichotomous setting of innate and adaptive immune cells, why do we have and maintain these “in-betweeners”? Adaptive cells are set apart due to their memory response, as well as their antigen-specific receptors. ILCs may thus be viewed as ‘all but -” cells that have an organization and functional cytokine capacity similar to those of conventional T cells but without the antigen specificity and memory response. But what is (and/or was) the role of the ‘in between’ unconventional T cells?
These in-between cells are often classified as ‘innate-like’ because they display some of the key aspects of innate immune cells: (1) they exhibit quick kinetics with immediate effector functions that, unlike adaptive cells, do not require a maturation phase, and (2) they carry PRRs, respond to PRR stimulation, and, according to some reports in the literature, can play the role of APCs(8, 38). However, these are classical functions of innate immune cells found in every tissue that unconventional lymphocytes can also be found. Why are these cells maintained if they are redundant with innate immune cells?
One argument for the contemporary role of unconventional lymphocytes is that they bridge the kinetics gap between innate and adaptive responses in a naïve setting, behaving with innate speed to produce cytokines otherwise usually produced by conventional lymphocytes, such as IL-17A. Although this may indeed be an important and productive function, it is not the sole reason for the maintenance of unconventional lymphocytes, as there is evidence of innate cells producing IL-17 as well, such as paneth cells in the gut(39) and neutrophils in the lungs(40, 41). However, this bridging role does suggest that the functional and kinetic gap between innate and adaptive cells could, in theory, have been filled by migration of either cell type into this “niche.” We suggest three reasons why this migration likely came from adaptive cells, and why these cells have been maintained despite presence of conventional adaptive lymphocytes:
(1) Unconventional cells evolved concurrently with conventional lymphocytes
A glimpse of the current uses of unconventional lymphocytes suggests that these cells might be an evolutionary “stepping stone” that preceded the development of conventional adaptive immune responses reliant on highly variable TCRs and BCRs. Perhaps these cells can compensate in a situation where a species does not have adaptive immune cells. If this were the case, there should be evidence of the existence of these cell types preceding the existence of conventional adaptive cells in distant relatives of mammals. Instead, the data thus far seem to indicate coincident evolution of unconventional and conventional lymphocytes. αβT cells restricted to non-classical MHC molecules have recently been reported in a number of jawed vertebrates, human’s furthest relatives to share conventional T and B cells(42). These animals do not share CD1 or MR1, but nonetheless have broad non-classical MHC molecules.
Edholm et al. identified putative innate-like T cells in Xenopus, a common laboratory amphibian model that represents an important evolutionary bridge between mammals and more evolutionarily ancient vertebrate lineages(43). These are αβT cells but display limited repertoire usage similar to that of unconventional T cells in mammals. Non-classical and MHC-like molecules have been reported in vertebrates as distance from humans as cartilaginous fish(42), but this new example of unconventional lymphocytes in amphibians suggests that this relationship of non-classical MHC-restricted unconventional lymphocytes extends far beyond mammals. This widespread use of unconventional lymphocytes suggests that these cells serve important, non-redundant functions, though those functions may have adapted for different purposes in different species.
Jawless vertebrates like the lamprey and its relative the hagfish, even more distant from humans than the jawed vertebrates, have been shown to have their own adaptive immune system with many parallels to our own T/B cell system. Based on variable lymphocyte receptors (VLRs), this system involves three cell types: VLR-A, VLR-B, and VLR-C(44). VLR-A is a surface protein expressed on T cell-like lymphocytes, while VLR-B is surface-expressed and secreted similar to BCR and antibody by B cell-like lymphocytes. Both VLR-A and VLR-B have a repertoire diversified via cytidine deaminase-dependent gene conversion mechanisms(44), with similarities to the underlying principles of AID-dependent class-switch and somatic hypermutation in our adaptive immune system (AID itself being a cytidine deaminase)(44, 45). VLR-C, however, is less variant, and VLR-C+ cells are responsive to PRRs and react innately without a required maturation time. Like mammalian IELs, VLR-C+ cells tend to be found in epithelial tissues(46). VLR-C+ cells are thus very similar conceptually to mammalian unconventional lymphocytes, and indeed are considered the lamprey-equivalent “γδ T-like cell.” Therefore, even in this parallel system of adaptive immunity, the unconventional lymphocyte is present and maintained but does not seem to have preceded the conventional adaptive VLR-A+ and VLR-B+ cells. The lamprey VLR immune system and our own T/B immune system were initially hypothesized to represent convergent evolution. This concept is now up for debate as similarities continue to be documented, and it is now suggested these systems may actually have a common pre-RAG-expressing ancestor(45, 47).
If unconventional lymphocytes are not evolutionarily older than conventional lymphocytes but have been maintained across a wide array of species despite the presence of conventional adaptive cells, they likely serve some current unique role that cannot be fulfilled by those conventional adaptive cells. Their widespread co-occurrence with conventional adaptive cells also suggests strong evolutionary pressure to create and maintain some type of antigen receptor-responsive innate-like cell within a cellular adaptive immune response. Comparing the development and functions of unconventional versus conventional lymphocytes suggests two potential unique roles for unconventional lymphocytes: a specialized relationship with the commensal microbiota, and a bridging role in early host development prior to fully functional adaptive immunity.
(2) Unconventional lymphocytes have specialized function for microbiota
In infection, all immune cells have functions in various responses against “bad” non-self, be it viral, bacterial, fungal or parasitic. Defense against infection is a primary responsibility of the collective immune system. However, many of our tissues are also colonized with “good” non-self: the microbiota of our intestines, skin, reproductive systems, and upper respiratory tracts. These “good” microbial interactions differ from infection in part because they are constant and ongoing, but unlike a chronic infection, they do not cause pathology and indeed provide many benefits to their host. This constant interaction between microbiota and host during mammalian development and evolution has greatly impacted the shape of the microbiota(48) and of the immune system at those interactive tissues(49). It is in these interactive sites that unconventional lymphocytes seem to shine.
Several γδT cell populations with specific TCR repertoires seed different peripheral tissues. Two of the best-studied populations are the fetal Vγ3(50) (also known as Vγ5(51)) dendritic epidermal γδT cells (DETCs), and the Vγ7 intestinal IELs (see IMGT database for clarification of repertoire nomenclature(52)). The skin and the intestine are also the main two organs harboring a commensal microbiota. Intestinal IELs are a heterogeneous population but are dominated by γδT cells. They promote anti-inflammatory and tolerance responses at the epithelial barrier. Intestinal IELs heavily express CD8αα, a homodimer of the CD8α molecule that is also a component in the conventional CD8 (CD8αβ) co-receptor. However, CD8αα is thought to repress TCR responsiveness by recruiting and sequestering TCR signaling components away from the TCR/CD3 complex, rather than serve as a co-receptor like CD8αβ or CD4(53). This repressive and tolerizing function is critical for their role as first-line lymphocytes at the epithelial layer bordering non-self microbiota signals.
Skin DETCs are seeded within skin epithelial layers, thus making them an intraepithelial lymphocyte similar to the intestinal IEL population. DETCs interact closely with neighboring keratinocytes and epithelial cells, allowing for quick immune and reparative responses in loss of barrier function(23, 54). Almost all DETCs express the same Vγ usage, suggesting strong evolutionary selection pressure for a unique function with the skin exogenous environment. However, the exact ligand(s) recognized by DETCs remains unknown.
Our laboratory and colleagues in Richard Blumberg’s laboratory recently demonstrated that the microbiota interacts in significant ways with the intestinal and lung iNKT cell population(55). Germ-free (GF) Swiss-Webster mice have significantly higher iNKT cell frequency and counts as compared to colonized specific pathogen free (SPF) Swiss-Webster mice at examined mucosal sites, but not in other peripheral tissues where there is less microbial contact such as the liver and thymus. Colonization of neonatal GF animals with SPF stool reduced the intestinal iNKT population in a CD1d-dependent manner, confirmed through administration of monoclonal CD1d-blocking antibodies. iNKT cells increased in the lung due to increased local expression of the chemokine CXCL16 in GF animals as compared to SPF animals. This CXCL16 expression was reduced upon microbial exposure of young GF animals(55).
But how do these cells and the microbiota interact? This remains a difficult question to answer in many of the phenomena reported of the microbiotal effect on health and disease. The Kasper lab showed that the human commensal Bacteroides fragilis has unique membrane lipids called glycosphingolipids that serve beneficial purposes both to the microbe and to the host in the context of the intestinal microbiota. Compared to a mutant strain deficient in the capacity to produce sphingolipids (ΔSPT), wild-type (WT) B. fragilis survived better in stress environments that mimic those of the mammalian intestine. In addition, WT B. fragilis monocolonization of GF animals was able to limit the early life iNKT population similar to SPF levels, below the levels seen in littermates that remained GF(27). The failure of monocolonization with the glycosphingolipid deficient ΔSPT mutant to reproduce this effect suggested that B. fragilis glycosphingolipids directly influenced the iNKT population(27). Indeed, direct delivery of isolated B. fragilis glycosphingolipids to GF animals was sufficient to replicate the active suppression of iNKT cell levels to levels similar as in microbial conditions (both SPF and B. fragilis monocolonization)(27).
Glycosphingolipids are ligands for CD1d, the non-classical MHC molecule that restricts NKT cells. Though sphingolipids are rare in bacteria in general, they are highly expressed by Bacteroides species (including B. fragilis)(26, 56), members of the Bacteroidetes phylum that is heavily enriched in mammalian microbiota(48). This bacterial glycosphingolipid was one of the first commensal microbial components demonstrated to directly modulate mammalian host immune response, influencing an unconventional lymphocyte. The Kasper lab has also demonstrated that another commensal microbial surface molecule, the zwitterionic capsular Polysaccharide A (PSA) of B. fragilis, can be presented on MHC-II(57) and modulate intestinal DC and TREG cells to protect against disease(58, 59). Indeed, B. fragilis sphingolipids and PSA, along with a few other metabolite candidates(60–63), remain the very few commensal microbial molecules shown to play direct roles in the mechanisms of the microbial/host immune balance(64).
Most strikingly of all unconventional lymphocytes, MAIT cells have been shown to be entirely dependent on the presence of the microbiota(29). These unconventional cells are predominantly found in mucosal sites, which are also the body’s primary sites of microbial and other xenobiotic/environmental exposures. Their ligand is likely a bacterial metabolic derivative of riboflavin biosynthesis or dietary riboflavin(33). Thus the relevance of the microbiota to MAIT cells is obvious, but the purposes and mechanisms of this relationship remain unclear. Following on the examples of the logical organization of γδT cells and ILCs, it is likely with time that we will develop an understanding of logical organization for MAIT cells and their interaction with the microbiota in each mucosal location.
Lastly, conventional lymphocytes are, of course, influenced by commensal microbes also, as we have recently catalogued with our colleagues in the laboratory of Diane Mathis and Christophe Benoist(65). However, the mechanisms underlying most of these effects remain unknown. In the interactions with known mechanisms, however, unconventional lymphocytes or other “in-betweeners” are often instrumental.
(3) Unconventional lymphocytes have specialized function for early host development
In many respects, γδT cells overlap in function and cytokine production with αβT cells, but they have a strikingly unique development in the early establishment of peripheral immunity. γδT cells are the first T cells to appear during embryonic thymic development(23). As the fetus develops, specific Vγ/Vδ subsets radiate to different peripheral tissues, including major entry sites of xenobiotic and infectious agents like the lung, skin, intestine, and liver(23). Conventional adaptive B cells and αβT cells do not begin to circulate in the periphery until later in post-natal development. This timing positions γδT cells as the first (and sometimes only) effector lymphocytes in these locations during a developmentally vulnerable time frame encompassing not only first exposure to and immune maturation “balancing” with commensal microbiota, but also often first encounter with many pathogens. Unsurprisingly, γδT cells have been shown to have strong population expansions and protective roles in several early-childhood diseases, including infections with Epstein-Barr virus (EBV)(66, 67), herpes viruses(67), and food-borne microbes like Listeria(68).
We found a similar important influence of age in our studies of microbial interaction with intestinal iNKT cells. Animals GF from birth displayed larger populations of colonic iNKT cells as compared to SPF animals from birth, but exposure of GF animals to microbes as adults (>5weeks of age) did not influence this dynamic. The fact that only GF neonates showed a reduction of iNKT cell levels upon microbial exposure suggests the existence of a temporal window for modulation of the intestinal immune system during early postnatal development. This early “education” of the intestinal iNKT cell population suggests that these cells may be involved in the initial balancing act between newly colonizing commensal microbes and the “uneducated” local immune system of the gut, as tolerance is developed to preferred “good” commensal microbes. Perhaps it is beneficial for this initial tolerance to be developed by lymphocytes that have adaptive cytokine functionality, but do not develop a memory of their stimulants or future amplifications of their effector responses.
Like unconventional T cells, unconventional B cells also display roles during interaction with the microbiota and early-life as well. Unconventional B cells function at important interface tissues where mixing between lymphoid cells and environmental signals occur, such as the marginal zone of the spleen (MZ B cells)(37) and peritoneal and pleural cavities (B-1 B cells)(69). Both B cell populations express high levels of PRRs on their surface and respond with innate kinetics upon stimulation. Crucially, MZ B cell and B-1 B cells are the main producers of “natural antibodies”—primarily IgM antibody with low affinity and broad specificity that are produced without stimulation by inflammatory signals(37). This is at least partially influenced by the presence of microbiota, as GF mice have impaired natural antibody responses when stimulated with microbial compounds(37, 70).
Lastly, this relationship with early-age development also holds true for ILCs, or at least for LTi cell. Rather than being immune effectors, LTi cells function predominantly in the early development of lymphoid tissues, as well as in the later formation of tertiary lymphoid structures in adult animals. However, LTi cells still produce many of the same cytokines as other ILC3s(16). One wonders whether LTi cells might be viewed as “unconventional” ILCs.
3. DEFINING WHAT COMES NEXT
Early-development γδT cells have a neat logic of specific subsets of Vγ/Vδ TCRs radiating to different peripheral sites, and ILCs and iNKT cells have been delineated into functional subsets via cytokine expression profiles that parallel those of the helper subsets of conventional αβT cells. Thus we are beginning to decipher the logical organization of some unconventional and “in-between” cells. Nonetheless, many of the roles and functions of these cells remain obscure and will be the focus of continuing research in this field. A few factors have made the field of research on unconventional lymphocytes more unwieldy, especially the heavy overlap of cellular functions and markers among different cell types and the limited information of the ligands and relevance of each TCR type.
Unconventional lymphocytes are often still distinguished by whether they have (or do not have) a set of defined surface “markers” (e.g., B220 or CD8αα for IELs), which may (or may not) be expressed by other closely related cell types as well. While this delineation by expression of a panel of surface proteins offers a logical strategy for distinctions until a lineage-defining or cellular-unique marker is found for each cell type, these surface proteins or transcription factors are not present to serve as flags to facilitate the easy identification of their host cells by scientists. Rather, they are proteins serving specific functions. Most individual functions are not unique to a specific cell, but instead are performed by other cell types as well. For example, CD103 was used as a defining marker of tissue-resident versus circulating memory adaptive T and B cells(71, 72). CD103 functions as the receptor for E-cadherin, commonly expressed on epithelial cells. Thus while CD103 is expressed on some tissue-resident conventional memory adaptive cells (T, BRMs) and not on circulating adaptive cells, the purpose of its expression is to promote that cell’s retention in the local tissue when there are E-cadherin+ epithelial cells. Unsurprisingly, local innate cell types such as macrophages and dendritic cells also express CD103, even though these cells are not memory cells. This leads to the question: are CD103− TRMs no longer TRMs? Are they a different cell type, or a different lineage/arm of TRMs? Or was it perhaps overly limiting to use CD103 as a marker? This example illustrates why research on unconventional lymphocytes must address similar questions, assignments, and reassignments of cell types as we better characterize the unique and redundant functions of these cells.
These questions plague the investigation of unconventional lymphocytes especially because many of the populations have surface proteins and functions overlapping with those of both innate and conventional adaptive cells, as well as different unconventional populations. The case of type II variant NKT cells argues for the inadequacy of using surface markers for identification purposes but also highlights the difficulty of not doing so. Upon their discovery, NKT cells were defined as CD3+ TCRαβ+ cells that were also positive for specific surface receptors, notably NK1.1(36). While this classification remains useful in B6 mice, it was quickly realized that these same markers did not accurately reflect the same cell types in different mouse strains or in humans. Thus the defining characteristic of NKT cells became interaction with the MHC-I non-classical CD1d molecule. In mice, NKT cells have been semantically separated into invariant αGalCer/CD1d-responsive (type I) and variant ligand-unknown/CD1d-responsive (type II) NKT cells. Fluorescently labeled CD1d/αGalCer tetramers can be used to label type I NKT cells, but they do not label type II. Without a distinguishing set of markers, or a lineage-defining characteristic, we can only examine type II NKT cells by comparing CD1d-deficient mice, which lose all NKT cells, and TRAJ18-deficient mice, which lose only invariant Vα14-Jα18+ Type I NKT cells. This comparison would be restrictive and difficult in most experimental settings, and has limited the ability to study type II NKT cells.
Indeed, all of these proposed roles of and issues around unconventional lymphocytes apply to intestinal IELs. These lymphocytes are certainly unconventional and innate: they respond with rapid kinetics and do not display memory. They are non-circulating and maintained at specific epithelial sites. Moreover, they can be defined by a specific set of markers (especially CD8αα+) and functionalities. Thus, by current defining metrics, these cells could be a singular population of unconventional lymphocytes. However, IELs are in fact not one homogeneous population. Intestinal IELs seed the epithelium in two distinct waves. An initial early-life wave consists of truly unconventional populations consisting predominantly of γδT cells—so called “natural IELs”, one of the arms of γδT cells that radiate to different peripheral sites in pre- and postnatal development(23). A second, gradual wave of “induced IELs” accumulate over time. Induced IELs are not a homogeneous population either, but instead can be seeded from adaptive cells that get “caught” at the epithelial border as well as from additional γδT cells from the thymus(73). Recent work by Daniel Mucida’s laboratory suggests a mechanism of how this “catching” could occur by conventional intestinal CD4+ Foxp3+ regulatory αβT cells(74), though IELs are also seeded by conventional effector T cells(75). Upon joining the epithelial layer, these cells seem to drop some of the transcriptional signatures and functions of conventional T cells and adopt new ones similar to those of natural IELs, such as the induction of CD8αα expression and reduction of Foxp3(74). Thus IELs show the characteristics of innate or unconventional lymphocytes, and also highlight the difficulties encountered as we continue to work towards stable definitions of cell types. When grouping cells or identifying new ones, should we focus on TCR repertoire? Functionality? Surface markers?
There are two competing aspects to T cell biology: TCR organization and cellular function. For conventional T cells, separate lineages are divided by function and thus by marker expression, because they are already canonically defined as conventional T cells on the basis of their post-germline capacity for TCR somatic recombination. Thus an early definition of unconventional T cells was at the TCR level and was originally assumed to be the γδTCR. However, we now know that there are unconventional αβTCR-expressing cells, which can be secondarily defined by their TCR oligoclonality or by their function. At this level, there is some confusion because it is no longer obvious which defining characteristic should carry more weight than the other. In the IEL example, IELs are generally oligoclonal, CD8αα+, and respond rapidly. Nevertheless, they are composed of γδT cells, early-seeded αβT cells, as well as conventional αβT cells that are pulled into the tissue and lose their conventional markers upon microbial antigen interaction(74, 75). Do these all remain separate cell populations, or are they cells from different sources that coalesce into a new population? Lineage tracing allows us to track where they come from, but does a natural αβT IEL respond differently from an conventional later-seeded αβT that has acquired natural IEL features? At what point do we maintain a distinction? These semantic decisions matter because (1) it can make it difficult to distinguish the cells described in the older literature (e.g., “innate” IL-17 was originally described to be expressed in “αβTCR+CD4−CD8− thymocytes”(76), a category that we now know includes NKT cells, MAIT cells, and IELs(41)), and (2) arbitrarily applied definitions may obscure the physiological logic of why these cells have been maintained.
The strong role for interaction and discrimination of commensal microbes by unconventional lymphocytes points to another obvious avenue of continuing research in this field. Several unconventional T cells in the skin, lungs, and gut have been demonstrated to be partially or wholly microbiota-dependent. However, the mechanism and functional goal of interaction with the microbiota remains an underexplored area. The Kasper lab has shown the role of the microbiota, and specifically microbial glycosphingolipids, on the early education of iNKT cell populations in the gut, demonstrating that improper expansion of these iNKT cells leads to increased sensitivity to colitis later in life(27, 55). MAIT cells, which are lost under GF conditions(29), clearly depend on interactions with the microbiota but what that dependence represents remains unclear. Is it evolutionary convenience that their local mucosal population happens to be regulated by the microbiota? Do they serve some type of role in cross-communication, tissue barrier maintenance, or sensing? Why have they been selected to recognize riboflavin derivatives? Is the reason nutritional health of the host, or do riboflavin derivatives signify some information that the host can use to differentiate and encourage colonization specific microbes in the microbiota? Similar questions remain to be answered for other unconventional lymphocytes as well, and many of the lesser explored populations such as H2-M3 restricted T cells and B-1 cells are fruitful candidates for exploring relationships of these cells with their local microbiota. In our recent survey of immune responses to individual human commensal microbes, we reported an intestinal TCRαβ+ CD4− CD8α − population that is regulated by, but not entirely dependent on, the microbiota(65). The purpose of these cells and the group of unconventional T cells to which they belong are being explored.
Clearly, unconventional lymphocytes have co-evolved with the conventional adaptive immune system, in both the B/T cell system of jawed vertebrates and the VLR system of jawless vertebrates. This history indicates strong evolutionary pressure to maintain a tripartite system consisting of an adaptive cellular component, an adaptive humoral component, and an adaptive “innate” or rapidly responsive component. CD1 and MR1 both have high levels of conservation across the species in which they are found(42), but there are several other non-classical MHC molecules and MHC-like molecules. These extra molecules suggest two non-exclusive possibilities: that there may yet be even more unconventional lymphocytes or lymphocyte-like cells, and that the functions of these cells may have diverged for different species. There remains a large open field of exploration for research on unconventional lymphocytes.
Acknowledgments
We thank Julie McCoy for excellent editorial support and Christophe Benoist for helpful discussion. D.L.K. has funding by a Sponsored Research Agreement from UCB Pharma and by the International MS Microbiome Study (iMSMS). L.P. is supported by the NIH NRSA postdoctoral fellowship (1F32DK111126).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Murphy KM, Travers P, Walport M. Janeway’s Immunobiology. 7. New York: Garland Science; 2007. [Google Scholar]
- 2.Smith HF, Fisher RE, Everett ML, Thomas AD, Randal Bollinger R, Parker W. Comparative anatomy and phylogenetic distribution of the mammalian cecal appendix. J Evol Biol. 2009;22:1984–1999. doi: 10.1111/j.1420-9101.2009.01809.x. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 4.Medzhitov R, Janeway C. Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- 5.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 6.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 7.Santoni G, Cardinali C, Morelli MB, Santoni M, Nabissi M, Amantini C. Danger- and pathogen-associated molecular patterns recognition by pattern-recognition receptors and ion channels of the transient receptor potential family triggers the inflammasome activation in immune cells and sensory neurons. J Neuroinflammation. 2015;12 doi: 10.1186/s12974-015-0239-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz-Adalia A, Veiga E. Close Encounters of Lymphoid Cells and Bacteria. Front Immunol. 2016;7:405. doi: 10.3389/fimmu.2016.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schatz DG, Ji Y. Recombination centres and the orchestration of V(D)J recombination. Nat Rev Immunol. 2011;11:251–263. doi: 10.1038/nri2941. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan MH. Th9 cells: differentiation and disease. Immunol Rev. 2013;252:104–115. doi: 10.1111/imr.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eyerich S, Eyerich K, Pennino D, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol. 2012;8:337–347. doi: 10.1038/nrrheum.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teng G, Papavasiliou FN. Immunoglobulin Somatic Hypermutation. Annu Rev Genet. 2007;41:107–120. doi: 10.1146/annurev.genet.41.110306.130340. [DOI] [PubMed] [Google Scholar]
- 14.Stavnezer J, Jeroen E, Guikema J, Schrader CE. Mechanism and Regulation of Class Switch Recombination. Annu Rev Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spits H, Artis D, Colonna M, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 16.Walker JA, Barlow JL, McKenzie ANJ. Innate lymphoid cells--how did we miss them? Nat Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 17.Satoh-Takayama N, Vosshenrich CAJ, Lesjean-Pottier S, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Luci C, Reynders A, Ivanov II, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 19.Sanos SL, Bui VL, Mortha A, et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mebius RE, Streeter PR, Michie S, Butcher EC, Weissman IL. A developmental switch in lymphocyte homing receptor and endothelial vascular addressin expression regulates lymphocyte homing and permits CD4+ CD3- cells to colonize lymph nodes. Proc Natl Acad Sci U S A. 1996;93:11019–11024. doi: 10.1073/pnas.93.20.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3- LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 22.Scandella E, Bolinger B, Lattmann E, et al. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat Immunol. 2008;9:667–675. doi: 10.1038/ni.1605. [DOI] [PubMed] [Google Scholar]
- 23.Chien Y, Meyer C, Bonneville M. γδ T Cells: First Line of Defense and Beyond. Annu Rev Immunol. 2014;32:121–155. doi: 10.1146/annurev-immunol-032713-120216. [DOI] [PubMed] [Google Scholar]
- 24.Ferrick DA, Schrenzel MD, Mulvania T, Hsieh B, Ferlin WG, Lepper H. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature. 1995;373:255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 25.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 26.An D, Na C, Bielawski J, Hannun YA, Kasper DL. Membrane sphingolipids as essential molecular signals for Bacteroides survival in the intestine. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4666–4671. doi: 10.1073/pnas.1001501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.An D, Oh SF, Olszak T, et al. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell. 2014;156:123–133. doi: 10.1016/j.cell.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tilloy F, Treiner E, Park SH, et al. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med. 1999;189:1907–1921. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treiner E, Duban L, Bahram S, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 30.Rahimpour A, Koay HF, Enders A, et al. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J Exp Med. 2015;212:1095–1108. doi: 10.1084/jem.20142110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reantragoon R, Corbett AJ, Sakala IG, et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med. 2013;210:2305–2320. doi: 10.1084/jem.20130958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin E, Treiner E, Duban L, et al. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009;7:e54. doi: 10.1371/journal.pbio.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kjer-Nielsen L, Patel O, Corbett AJ, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491:717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 34.Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr. 2003;77:1352–1360. doi: 10.1093/ajcn/77.6.1352. [DOI] [PubMed] [Google Scholar]
- 35.Thakur K, Tomar SK, De S. Lactic acid bacteria as a cell factory for riboflavin production. Microb Biotechnol. 2015;9:441–451. doi: 10.1111/1751-7915.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nat Immunol. 2015;16:1114–1123. doi: 10.1038/ni.3298. [DOI] [PubMed] [Google Scholar]
- 37.Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol. 2013;13:118–132. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science. 2005;309:264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi N, Vanlaere I, de Rycke R, et al. IL-17 produced by Paneth cells drives TNF-induced shock. J Exp Med. 2008;205:1755–1761. doi: 10.1084/jem.20080588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li L, Huang L, Vergis AL, et al. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120:331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 42.Edholm E-S, Banach M, Robert J. Evolution of innate-like T cells and their selection by MHC class I-like molecules. Immunogenetics. 2016;68:525–536. doi: 10.1007/s00251-016-0929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edholm E-S, Albertorio Saez L-M, Gill AL, et al. Nonclassical MHC class I-dependent invariant T cells are evolutionarily conserved and prominent from early development in amphibians. Proc Natl Acad Sci U S A. 2013;110:14342–14347. doi: 10.1073/pnas.1309840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boehm T, McCurley N, Sutoh Y, Schorpp M, Kasahara M, Cooper MD. VLR-Based Adaptive Immunity. Annu Rev Immunol. 2012;30:203–220. doi: 10.1146/annurev-immunol-020711-075038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carmona LM, Schatz DG. New insights into the evolutionary origins of the recombination-activating gene proteins and V(D)J recombination. FEBS J. 2016 doi: 10.1111/febs.13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirano M, Guo P, McCurley N, et al. Evolutionary implications of a third lymphocyte lineage in lampreys. Nature. 2013;501:435–438. doi: 10.1038/nature12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasahara M, Sutoh Y. Two forms of adaptive immunity in vertebrates: similarities and differences. Adv Immunol. 2014;122:59–90. doi: 10.1016/B978-0-12-800267-4.00002-X. [DOI] [PubMed] [Google Scholar]
- 48.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 49.Chung H, Pamp SJ, Hill JA, et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149:1578–1593. doi: 10.1016/j.cell.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garman RD, Doherty PJ, Raulet DH. Diversity, rearrangement, and expression of murine T cell gamma genes. Cell. 1986;45:733–742. doi: 10.1016/0092-8674(86)90787-7. [DOI] [PubMed] [Google Scholar]
- 51.Heilig JS, Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature. 1986;322:836–840. doi: 10.1038/322836a0. [DOI] [PubMed] [Google Scholar]
- 52.Lefranc M-P, Giudicelli V, Ginestoux C, Bodmer J, Muller W, Bontrop R, Lemaitre M, Malik A, Barbie V, Chaume D. IMGT, the international ImMunoGeneTics database. Correspondence between nomenclatures: Mouse (Mus musculus) TRG. 1999 doi: 10.1093/nar/27.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11:445–456. doi: 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jameson J, Ugarte K, Chen N, et al. A role for skin gammadelta T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 55.Olszak T, An D, Zeissig S, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kato M, Muto Y, Tanaka-Bandoh K, Watanabe K, Ueno K. Sphingolipid composition in Bacteroides species. Anaerobe. 1995;1:135–139. doi: 10.1006/anae.1995.1009. [DOI] [PubMed] [Google Scholar]
- 57.Cobb BA, Wang Q, Tzianabos AO, Kasper DL. Polysaccharide processing and presentation by the MHCII pathway. Cell. 2004;117:677–687. doi: 10.016/j.cell.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 59.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An Immunomodulatory Molecule of Symbiotic Bacteria Directs Maturation of the Host Immune System. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 60.Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 61.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Surana NK, Kasper DL. Deciphering the tête-à-tête between the microbiota and the immune system. J Clin Invest. 2014;124:4197. doi: 10.1172/JCI72332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Geva-Zatorsky N, Sefik E, Kua L, et al. Mining the Human Gut Microbiota for Immunomodulatory Organisms. Cell. 2017;168:928–943. e11. doi: 10.1016/j.cell.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Farnault L, Gertner-Dardenne J, Gondois-Rey F, et al. Clinical evidence implicating gamma-delta T cells in EBV control following cord blood transplantation. Bone Marrow Transplant. 2013;48:1478–1479. doi: 10.1038/bmt.2013.75. [DOI] [PubMed] [Google Scholar]
- 67.Wallace M, Malkovsky M, Carding SR. Gamma/delta T lymphocytes in viral infections. J Leukoc Biol. 1995;58:277–283. doi: 10.1002/jlb.58.3.277. [DOI] [PubMed] [Google Scholar]
- 68.Vantourout P, Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu L, Shao T, Nie L, Zhu L, Xiang L, Shao J. Evolutionary implication of B-1 lineage cells from innate to adaptive immunity. Mol Immunol. 2016;69:123–130. doi: 10.1016/j.molimm.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 70.Bos NA, Kimura H, Meeuwsen CG, et al. Serum immunoglobulin levels and naturally occurring antibodies against carbohydrate antigens in germ-free BALB/c mice fed chemically defined ultrafiltered diet. Eur J Immunol. 1989;19:2335–2339. doi: 10.1002/eji.1830191223. [DOI] [PubMed] [Google Scholar]
- 71.Shin H, Iwasaki A. Tissue-resident memory T cells. Immunol Rev. 2013;255:165–181. doi: 10.1111/imr.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guy-Grand D, Vassalli P, Eberl G, et al. Origin, trafficking, and intraepithelial fate of gut-tropic T cells. J Exp Med. 2013;210:1839–1854. doi: 10.1084/jem.20122588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sujino T, London M, Hoytema van Konijnenburg DP, et al. Tissue adaptation of regulatory and intraepithelial CD4+ T cells controls gut inflammation. Science. 2016;352:1581–1586. doi: 10.1126/science.aaf3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Staton TL, Habtezion A, Winslow MM, Sato T, Love PE, Butcher EC. CD8+ recent thymic emigrants home to and efficiently repopulate the small intestine epithelium. Nat Immunol. 2006;7:482–488. doi: 10.1038/ni1319. [DOI] [PubMed] [Google Scholar]
- 76.Kennedy J, Rossi DL, Zurawski SM, et al. Mouse IL-17: a cytokine preferentially expressed by alpha beta TCR + CD4-CD8-T cells. J Interferon Cytokine Res Off J Int Soc Interferon Cytokine Res. 1996;16:611–617. doi: 10.1089/jir.1996.16.611. [DOI] [PubMed] [Google Scholar]
- 77.Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol. 2005;5:459–471. doi: 10.1038/nri1635. [DOI] [PubMed] [Google Scholar]


