Abstract
Stem cell therapy is a promising treatment modality for necrotizing enterocolitis. Among the many promising stem cells identified to date, it is likely that mesenchymal stem cells will be the most useful and practical cell-based therapies for this condition. Using acellular components such as exosomes or other paracrine mediators are promising as well. Multiple mechanisms are likely at play in the positive effects provided by these cells, and further research is underway to further elucidate these effects.
Keywords: necrotizing enterocolitis, stem cell therapy, premature, neonatal, intestine, ischemia
Introduction
Necrotizing enterocolitis (NEC) is a leading cause of death in premature infants, with mortality remaining as high as 30% [1]. Despite extensive efforts to elucidate the pathophysiology of the disease, and to identify potential treatments, mortality and morbidity remain unacceptably high. Survivors are faced with lifelong complications including short gut syndrome and neurological sequelae. Treatment options for infants affected by NEC are limited to supportive care at diagnosis, with many babies progressing to surgical intervention for resection of necrotic bowel. It is important to note that although breast milk may reduce the incidence of NEC, it does not eliminate it [2]. Despite decades of research, a cure for the disease has still not been found.
The identification of potential treatments for NEC is still hampered by an incomplete understanding of the precise pathogenesis of the disease. NEC is a disease that predominantly affects premature infants, occurring most commonly after the introduction of enteral feeding. Differences exist in microbial colonization of pre-term infants and term infants, as well as those delivered vaginally and those delivered by Caesarean-section [3]. The microbiome of infants who go on to develop NEC differs from that of babies that do not develop the disease [4, 5]. In addition, the inflammatory response is heightened in infants affected with NEC [6]. This combination of pro-inflammatory and infectious components certainly contributes to the development of disease.
Animal models of experimental NEC have been utilized over the decades to examine different potential therapies for the disease, including stem cell (SC) therapy. Stem cells have been shown in several disease models to have anti-inflammatory properties and to lead to improvements in tissue health and function [7–9]. The ability of SC to self-replicate, differentiate, prevent apoptosis and reduce inflammation has raised interest in the potential therapeutic value of these cells in NEC.
Types of Stem Cells (Figure 1)
Figure 1.
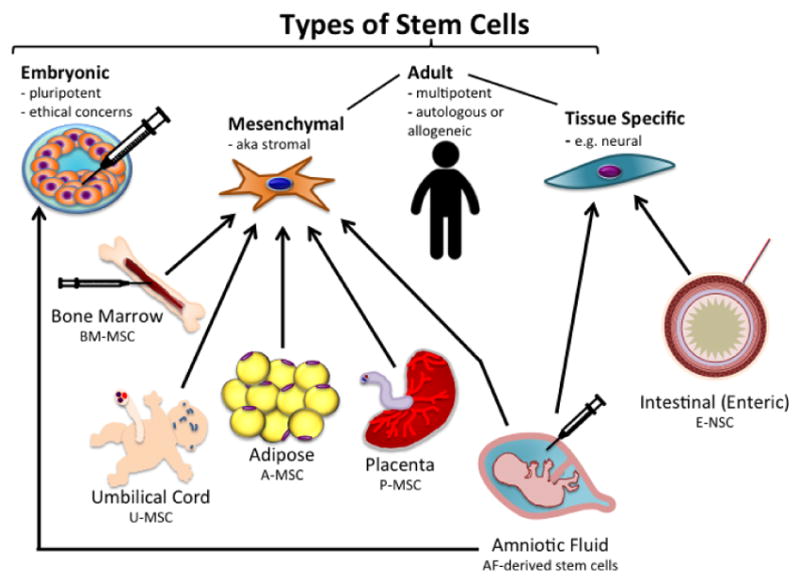
Different types of stem cells available for NEC treatment. Embryonic, while able to differentiate into any tissue, are not used due to ethical concerns with their procurement from the inner mass of the blastocyst of human embryos. Amniotic fluid can give rise to embryonic stem cells as well. Both mesenchymal stem cells and neural stem cells have been used with success in animal models of NEC.
Early stem cell research focused on embryonic stem cells (ESC), totipotent cells derived from embryos in mice [10]. Subsequent work showed that these same cells could be derived from human embryos. These human ESC (hESC) were also totipotent, however ethical concerns limited the research that could be done with hESC and researchers continued to search for alternative sources of stem cells.
Bone Marrow-derived Mesenchymal Stem Cells (BM-MSC)
Bone marrow-derived cells have been studied extensively in both animals and humans. They can be readily derived from human donors without being encumbered by the ethical challenges faced by ESC. The culture process is straight-forward: marrow is harvested from long bones of donors and placed into culture to select for MSC [11]. As the cells are cultured, they must be passaged several times in order to minimize contamination with hematopoietic precursors that are present in the initial marrow sample. Confirmation of the presence of CD44 and CD90 on cells, and the absence of cells expressing CD45, helps to confirm that the cell population of interest is composed of MSC. BM-MSC derived from mice, rats and humans have been shown to be effective in reducing the incidence and severity of NEC in mouse and rat models [11–14].
Amniotic Fluid-derived Stem Cells
The first report of successful culture and expansion of mesenchymal stem cells from amniotic fluid (AF) was published in 2003 [15]. These cells not only express surface markers typical of mesenchymal stem cells (MSC) such as CD29, CD44, and CD90 among others, but they also express stage-specific embryonic antigen (SSEA)-4 and the transcription factor Oct4, both of which are associated with ESCs and not MSCs, and help to ensure that the cells remain undifferentiated. It appears that AF-MSCs may be closer to embryonic in nature than other types of MSCs, which may contribute to their enhanced pluripotency and growth. De Coppi et al isolated human and rodent amniotic fluid-derived stem (AFS) cells that express both embryonic and adult stem cell markers [16]. AFS cells can be induced to form cells from all three germ layers, but have not been demonstrated to be tumorigenic [17, 18].
In addition to their promising properties, collection and culture of AF-MSC is straight-forward and easily translated to humans. These cells can be cultured from AF samples collected during routine amniocentesis or at planned caesarean section [17, 18]. After collection, these cells can be cultured in media with few supplements and grow rapidly and reliably [11]. In comparison to BM-MSC, AF-MSC grow significantly faster and can be more easily and readily cultured and expanded into clinically useful cell numbers.
Since their discovery, interest in these cells has remained high. Intraperitoneal (IP) injection of AF derived stem cells has been shown to significantly reduce the incidence and severity of NEC in a murine model [19]. Subsequent studies confirmed that IP injection of these cells significantly decreases histologic injury and leads to improved gut barrier function during experimental NEC [11, 20].
Beneficial effects of AF have also been identified when experimental models utilize whole AF without purification of AF-derived cells. Both mice and pigs that received whole AF via enteral administration have significant protection from experimental NEC [21, 22] The concentration of stem cells in whole AF is generally low, on the order of 1–2% of cellular content, however this appears to be enough to have a positive effect, with the fluid functioning as a modulator of inflammation in the gut [19, 22].
Other Birth-Associated Mesenchymal Stem Cells (U-MSC, P-MSC)
Both the umbilical cord and the placenta have been recently appreciated as bountiful sources of mesenchymal stem cells. These cells are typically thought to be a more primitive progenitor population compared to adult tissue-derived MSC [23]. Umbilical cord blood-derived MSC (U-MSC) can be isolated with relatively high efficiency through cell culture, with best results noted from blood that is less than 15 hours old and with a total volume of over 33 mL [24]. Other groups have demonstrated success with direct culture of pieces of the umbilical cord [25, 26]. U-MSC have been shown to be as beneficial as other types of MSC in acute intestinal ischemia [27]. Placental MSC (P-MSC) are also relatively easy to obtain through culture of the products of placenta tissue digestion. Like AF-MSC, these cells are positive for MSC markers as well as typical ESC-associated cell markers, indicating their high potential for differentiation [28]. Both U-MSC and P-MSC have been successful in preventing injury in animal models of ischemia [29, 30]
All birth-associated stem cell products represent a unique opportunity for autologous cell use in at-risk infants. Potentially, these tissues could be harvested during delivery of any premature infant or infant delivered via C-section for isolation and preservation in preparation for future administration.
Amniotic Fluid-derived Neural Stem Cells (AF-NSC)
Although much research has focused on the use of MSC in NEC therapy, there are significant changes to the enteric nervous system (ENS) that occur during the development of NEC [31]. This has led to the investigation of neural stem cells (NSC) as a therapy for NEC. NSC can be cultured and isolated from AF and selectively grown. The culture process is somewhat more complicated than that of AF-MSC, and the cells do not grow as quickly. In brief, AF is collected in a similar fashion as for AF-MSC. The culture medium into which the cells are placed is significantly different for AF-NSC – the presence of epidermal growth factor (EGF) and fibroblast growth factor (FGF) helps to ensure that NSCs remain in an undifferentiated state [11]. This process also helps to minimize the likelihood of survival of other SC that are present in AF. As the cells develop and grow, they begin to aggregate into neurospheres, which contain predominantly NSC. These neurospheres can be mechanically separated to isolate individual NSC. These cells tend to grow more slowly than MSC, and it can take several weeks before usable quantities of cells are available. After culturing, AF-NSC identity can be confirmed by the expression of nestin, an intermediate filament protein present in developing NSC but downregulated when the cells become mature neural cells [32]. AF-NSC have been shown to have beneficial effects in animal models of ENS-based conditions such as Hirschsprung disease, and are also effective at reducing the incidence and severity of experimental NEC when injected IP [11].
Enteric Neural Stem Cells (E-NSC)
The complexity of the ENS and its interactions with the central nervous system (CNS) have become better understood in recent years, with a very high concentration of nerve endings and NSC located within the gut [33]. ENS abnormalities have been identified in infants diagnosed with NEC [31]. Interestingly, these ENS abnormalities last long after recovery from the acute NEC event.
E-NSC derived from the gut have similar properties to AF-NSC, and the culture medium used to support the growth of the cells is of the same composition. E-NSC are somewhat more challenging to obtain, with current experimental protocols purifying these cells through a combination of mechanical and enzymatic digestion of the gut to obtain E-NSC from the muscular layer of the small intestine [11]. After digestion, the cells are placed into culture to allow NSC to replicate and form neurospheres. These neurospheres can then be mechanically separated to yield individual NSC, which can be confirmed positive for nestin expression. E-NSC administered IP have been shown in several studies to be beneficial in treating experimental NEC and improving gut barrier function in these animals [11, 20, 31, 34].
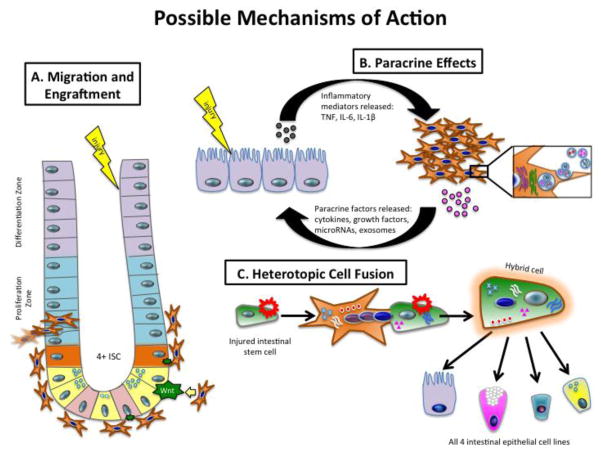
Stem Cell Mechanisms of Action (Figure 2)
Figure 2.
Mechanisms by which mesenchymal stem cells work to protect the intestine against NEC. Beneficial effects provided by stem cells are likely achieved through one or more of these pathways. A) MSC homing, engraftment and differentiation. B) Paracrine release of anti-inflammatory mediators, exosomes, microRNA, and/or cytokines. C) Heterotopic cell fusion with direct transfer of cellular biomaterials.
Migration and Engraftment
Stem cells have the unique ability to migrate and home to injured tissues and organs. Additionally, because stem cells are multipotent, they are able to differentiate into multiple cell types, including intestinal epithelia, endothelia, and connective tissue cells [16, 35, 36]. Therefore, stem cell transplantation may contribute to repair directly by integrating into the damaged tissue and replacing the injured cells.
Stem cells can travel to a wide variety of tissues with a high level of engraftment, particularly in the presence of injury or inflammation; however, the mechanism of this phenomenon is not yet fully understood [37]. It is possible that injured tissues express specific receptors or ligands that facilitate trafficking, adhesion, and infiltration of stem cells to the site of injury [38]. The intestine of premature neonates with NEC responds to injury by activating molecular pathways that increase the release of inflammatory mediators, tissue chemokines, and chemokine receptors, which may attract stem cells to the damaged intestine, where they may home and differentiate [39].
In normal rat pups, the intra-peritoneal administration of AFS cells leads to the migration, homing and integration of AF-MSC into the intestine, kidneys, liver, and spleen [40]. In the rat pup NEC model, within 48 to 72 hours after intraperitoneal injection, the AFS cells adhered to the mesentery and migrated to the intestinal serosa, smooth muscle, submucosal layers, and to the villi. Colonization with AFS cells occurred almost exclusively in the intestine of the pups with experimental NEC, indicating that AFS cells preferentially localize to damaged tissue [19]. Similarly, a previous study in experimental NEC demonstrated that intra-peritoneal administration of human BM-MSC exhibited high engraftment in the injured intestine resulting in reduced intestinal damage [13]. Once cells are engrafted, they can either directly differentiate to replace damaged cells, or interact with native intestinal stem cells (ISC) to upregulate the Wnt/β-catenin pathway as discussed below, which promotes auto-regeneration of intestinal epithelium [19].
Paracrine Effects
Although stem cells have a systemic distribution and integration into tissues, the beneficial effects seen after administration occur within hours of injection at a time when the amount of stem cells in the intestine is still relatively low. It seems more likely that the SC secrete anti-inflammatory mediators that enhance the host tissue’s ability to repair itself [8]. SC may act indirectly via the release of factors that support the regeneration of damaged cells, suggesting a predominantly paracrine effect [13, 19]. This has been shown in models of NEC and in numerous other disease processes including neurologic injury, ischemia-reperfusion injury [8, 9].
Most studies of SC efficacy have examined individual cell types in their ability to protect the intestines from NEC. However, given the observation that these cells appear to function through reducing inflammation, it is possible that cells harvested from different tissues may ultimately have similar effects. In ischemia/reperfusion injury, BM-MSC and umbilical cord U-MSC were shown to have similar effects [27]. When comparing AF-MSC, BM-MSC, AF-NSC, and E-NSC in a rodent model, all four cell types equivalently reduced the incidence and severity of experimental NEC [11]. These studies support the notion that there may be mediators common to multiple SC types that are responsible for their beneficial effects.
Conditioned media can be generated by placing stem cells into culture for several days, allowing their extracellular products to accumulate in the media, and then centrifuging to remove the cells. In animals that received an IP injection of this conditioned medium, a statistically significant reduction in NEC was observed equivalent to the reduction seen when animals were treated with the same kind of SC [19].
Zani et al have shown that AFS cells may act via a paracrine mechanism related to cyclooxygenase-2 (COX-2) activity [19]. An increased migration of COX-2+ cells into the crypts was observed after AFS cell injection, which was inversely correlated with intestinal damage. Furthermore, COX-2 inhibitors abolished the protective effects of AFS cells, confirming that AFS cells act in a COX-2-dependant manner to attenuate the NEC injury [19].
Similarly, BM-MSC produce many soluble mediators such as cytokines, growth factors, microRNAs, and exosomes [41], which may be responsible for their beneficial effect of attenuating the NEC related injury. It has been shown that MSCs can increase intestinal epithelial cell viability and proliferative capacity via the paracrine release of IL-6, HGF, and VEGF, after hypoxic injury [42].
Stem cell-derived exosomes are nanovesicles 30–100 nm in size that are released by exocytosis into the extracellular space and can be purified from conditioned media through commercially available kits or by differential ultracentrifugation [43, 44]. They contain various types of RNA (including microRNAs important in post-translational gene regulation), DNA, and protein, and are important paracrine factors mediating cell-cell and cell-environment communication [45, 46]. Recently, Rager et al. isolated exosomes from BM-MSCs and tested their effects in a neonatal rat model of NEC. They found a reduction in incidence and severity of NEC as well as preserved gut barrier function [12]. Stem Cell derived exosomes also function as mediators of inflammation [44].
Further work is needed to understand the precise mechanisms by which SC and SC-derived exosomes exert their beneficial effects in NEC. It may be possible to identify specific components in the extracellular milieu or within the nanovesicles that are the driving factors in these effects. These components could subsequently be isolated and purified and used therapeutically without the theoretical risks associated with stem cell based therapies.
Heterotopic Cell Fusion
Heterotopic cell fusion is a third mechanism by which stem cells may provide their beneficial effects. It occurs when two cells from different lineages merge into one cell, transmitting information and mediators in the process [47]. Fusion has been demonstrated with BM-MSC and hepatocytes, Purkinje neurons, and cardiac myocytes [48], but the data in intestinal injury have not been convincing. Some studies have demonstrated fusion events between BM-MSC and intestinal epithelial cells [49], however others have noted that this is so rare that it is likely not the mechanism by which these cells are providing protective effects [50].
Beneficial Effects of Stem Cells
Preservation of Intestinal Barrier Function
While the mechanisms are still in question, stem cells certainly provide significant benefit to the intestine in NEC. The intestinal epithelium is the most important barrier preventing the passage of foreign antigens and toxins from the external environment into the systemic circulation [51]. Preterm infants have diminished intestinal barrier function, leading to increased intestinal permeability. This may allow pathogens to translocate from the intestinal lumen and trigger the exuberant inflammatory response that characterizes NEC [52].
One of the potential beneficial effects of stem cell administration in NEC is the reestablishment of intestinal epithelial integrity. In the rat pup NEC model, all four stem cell types preserve and maintain intestinal epithelial integrity [20]. Previous studies have demonstrated that heparin-binding EGF-like growth factor (HB-EGF) promotes neural stem cell (NSC) proliferation and migration, and that both HB-EGF and NSC reduce intestinal injury, improve gut barrier function, and enhance intestinal motility in experimental NEC [34]. In addition, BM-MSC and BM-MSC-derived exosomes preserve intestinal permeability during NEC induction [12, 13]. MSC administration reduces bacterial translocation in NEC, indicating the protective role MSCs have in barrier function [13]. Similarly, AFS cell administration during experimental NEC improves intestinal barrier function and attenuates intestinal injury [19].
Attenuation of Inflammation
Inflammation is an important contributing factor in the pathogenesis of NEC [53]. TLR4 signalling in the gut is required to maintain intestinal homeostasis and triggers the immune response to Gram-negative bacterial translocation. Activated TLR4 signalling is associated with NEC in mice, rats, and premature infants [54, 55]. AFS cells have been shown to reduce gut inflammation during NEC, however the mechanism of this action is not fully understood [19]. Stem cells secrete a wide variety of cytokines and chemokines that have beneficial actions during tissue repair [56, 57], to offset TLR4-induced intestinal inflammation [58, 59]. MSC also reduce gut inflammation and epithelium damage during NEC induction [13].
Promotion of Intestinal Regeneration
To maintain the integrity and viability of the epithelial layer, intestinal epithelial cells are in constant turnover and are replenished by intestinal stem cells (ISC). These cells, located in the intestinal crypts, are rapidly proliferating and critical for the intestinal epithelial regeneration that follows injury [60–62]. The ISC population is depleted in NEC proportionally to the severity of intestinal damage, indicating the crucial role of ISC in intestinal repair [63, 64]. AFS cells enhance enterocyte proliferation and improve epithelial regeneration [19], indicating cross-talk between exogenous stem cells and endogenous ISC.
NEC is associated with Wnt/β-catenin pathway dysfunction; this pathway is required for ISC function and gut epithelial maintenance [19, 65]. AFS cell administration led to upregulation of gene expression of the Wnt/β-catenin pathway, suggesting that this could be a potential mechanism by which AFS cells contribute to intestinal regeneration [19]. Similarly, MSCs have been shown to upregulate growth factors which protect intestinal stem cells, maintain gut epithelial regeneration and reduce mucosal injury [14, 66].
Clinical Applications
Premature infants have relatively high levels of circulating hematopoietic stem cells at birth compared to term infants[67]. Since stem cells protect and heal injured tissues, those babies with suboptimal cell counts may be at increased risk for developing NEC. Relative stem cell deficiency could be due to an overall paucity of stem cells, dysfunctional stem cells, or an inability to mobilize functional stem cells to areas of injury. Mouse studies have demonstrated an increase in circulating stem cells after major intestinal resection or injury, indicating that stem cell mobilization is a native protective response in this patient population [68]. Therefore, it is reasonable to expect that providing exogenous stem cells to affected patients would promote intestinal healing and decreased injury during NEC [35, 69].
Route of Administration
Based on previous studies in both NEC and intestinal ischemia-reperfusion injury, direct application of cellular therapy to injured or at-risk bowel may be more optimal compared to systemic intravenous or intraarterial administration [70–72]. As previously discussed, a likely mechanism of action of the stem cells is through the paracrine release of vital mediators. Thus, applying the stem cells directly to the injured bowel may be the most effective way to deliver the cell products at the highest local concentrations and improve recovery in the injured intestine [42].
In previous human studies for other conditions, systemic adverse effects such as infections and viral reactivation secondary to immune suppression occurred less frequently with local application of cells as compared to systemic application [73, 74]. Although both IP and intravenous (IV) routes of administration had equivalent efficacy in an animal model, many cells become entrapped in the lungs after IV injection, leading to a reduction in the number of cells available for therapeutic purposes [14, 75, 76].
Patient Selection-Therapy
The significant variation in NEC disease course makes decisions regarding timing of treatment with cellular therapy difficult [77]. Ideally, stem cell therapy would be provided to infants who have definitive NEC but who have not progressed to the point of requiring surgical intervention. Currently this would include Bell’s Stages II A, II B, and IIIA. However, given that many infants with stage IIA NEC recover with observation and bowel rest alone, the use of stem cell therapy in infants with non-surgical NEC beyond stage IIA may be considered optimal to limit progression of disease.
Although infants who present with perforation require surgical resection of the perforated and necrotic segments, they also may be able to be treated with stem cell therapy for other areas of the intestine that appear marginally ischemic at the time of surgery. Optimal patients for initial trials would be those with marginally ischemic bowel who will require reassessment at a second-look operation [35, 77]. Since these infants already require multiple operations, no additional procedure would be needed to directly administer stem cells or to monitor their effects. Goals of therapy in this group would be to mitigate ongoing ischemia and necrosis and avoid the need for further resection at the second look operation. These patients represent an important subset of the population who could most benefit from this therapy, and would be an ideal first patient population to study.
Patient Selection-Prophylaxis
The prophylactic administration of stem cells to extremely premature infants with high risk for development of NEC could also be considered. A recent systematic review of prognostic studies identified many risk factors for NEC. Significant prognostic factors identified in multivariable analysis included low birth weight, early gestational age, sepsis, ethnicity, hypotension, outborn status, need for assisted ventilation, premature rupture of membranes, and small size for gestational age. Low birth weight (≤1500g) was the most commonly reported prognostic factor, which is likely linked to gestational age (studies varied from <36 weeks to <26 weeks) [78]. If prophylactic or early stage therapy were to be considered, these risk factors would be useful in determining the most at-risk population.
Ethically, however, it would be challenging to justify the administration of cellular therapy to patients without definitive disease due to the perceived risks of tumorigenicity and immunogenicity as discussed below. Importantly, as discussed previously, acellular therapy could be administered without risk of these complications associated with cellular formulations. Because it is very likely that stem cells are acting in a paracrine fashion, delivery of exosomes or another secreted mediator could be equally effective, as shown in animal models [12, 44].
Use of Stem cell therapy for treatment of long-term NEC sequelae
Another important use of stem cell therapy would be in preventing or treating short bowel syndrome (SBS), often a long-term complication of NEC secondary to extensive bowel resection [69]. Unfortunately, despite surgical techniques such as the serial transverse enteroplasty and Bianchi procedures, which are aimed at lengthening the intestine and expanding the functional epithelial surface area, SBS remains a difficult disease to manage. Rehabilitation of injured bowel and/or integration of new functional bowel into an SBS patient would be groundbreaking.
Stem cell therapy alone may be able to improve intestinal epithelial absorptive function and bowel adaption, both of which are extremely important in recovery from SBS [35, 79]. For more severe cases, a stem cell-engrafted neo-intestine could be considered to provide length either at the time of initial resection or later in treatment, to allow the SBS patient to achieve freedom from parenteral nutrition [79]. A promising study in rats has demonstrated the feasibility of creating decellularized small bowel, leaving the villus-crypt architecture intact and able to be repopulated with new intestinal stem cells [80]. Intestinal organoids have been cultured in-vitro and subsequently administered back to rats, thereby resulting in renewal and repair of the grafted intestinal epithelium [81].
The creation of these decellularized grafts led to development of functional tissue-engineered small intestines which have been anastomosed with native bowel in piglets. Graft therapy resulted in weight gain and improved recovery after bowel resection [82]. More recently, a study in dogs demonstrated that intestinal crypt cultures can grow and differentiate into functional, vascularized bowel on 3D synthetic tubularized villous structures, and this neo-intestine can integrate into existing intestine and support native mucosal regeneration [83].
Human cadaver donor intestine has also been decellularized and reconstituted with BM-MSC. The neo-intestine was found to have epithelial, smooth muscle, and endothelial cells and morphology similar to normal intestine [84]. While these haven’t been re-implanted into humans yet, the results are quite promising.
Safety Considerations
Tumorigenicity is a major concern in stem cell therapy. The same molecular pathways that allow a cell to be pluripotent are involved in tumorigenicity, and therefore more differentiated stem cells have a lower risk of this complication. Stem cells with a capacity for rapid growth but a low incidence of spontaneous differentiation are ideal for avoiding these complications. Neither AF-MSC nor BM-MSC have been shown to have high tumor formation potential in animal models [17, 18, 85, 86]. In fact, a recent systematic review looking at 36 clinical trials found no association between MSC administration and de novo tumor formation or increased susceptibility to infection [87]. This meta-analysis suggests the potential benefit of MSC therapy outweighs the potential risk, and that these theoretical risks may be less significant than previously believed.
Alternatively, recent work has indicated that human pluripotent stem cells have the capacity to develop loss-of-homozygosity mutations at the p53 locus, potentially increasing risk of cancer formation and demonstrating that comprehensive genetic testing may be necessary before cell therapy [88].
Immunogenicity is another potential adverse outcome of stem cell therapy. It can be reduced by autologous cell transfer or use of stem cells that have low expression of major histocompatibility complex (MHC) such as MSC [89]. Nevertheless, these risks cannot be eliminated, and thus the risks and benefits of this therapy need to be carefully considered.
Lastly, intravenous administration of stem cells has been demonstrated to cause trapping of cells within the lung parenchyma with sarcoma like lesions in the lungs in mice [90, 91]. Fortunately, this effect is lessened with intraperitoneal administration, another factor in favor of direct application.
Future Directions
The collective goal of clinicians and translational researchers in the field of stem cell therapy for the treatment of NEC is to progress to human clinical trials for stem cell delivery. There are several limitations to overcome before this can become a reality. First, regulatory hurdles to ensure safety for an extremely vulnerable population will need to be carefully addressed.
Secondly, as discussed earlier, it is challenging to determine which patients will benefit the most from this therapy. We would propose starting first with the surgical NEC population, Bell’s stage III – those patients who require one or multiple trips to the operating room for bowel resection. The stem cell therapy could be delivered directly to injured bowel intraoperatively and assessed at future operations or by clinical improvement in the patients. In this population, there is little risk of providing unnecessary treatment, with great potential for benefit.
A third concern is the long-term implications of cellular therapy as discussed above. The ability of these cells to transform into malignant cells over time has been a long-time concern, despite evidence that the therapeutic benefits likely outweigh these risks [86].
Lastly, we still do not understand the full extent of their mechanisms of action. If stem cells protect by paracrine mechanisms, which factors are most important? How many factors need to be present? And most importantly, if paracrine factors are the key to protection, could an acellular cocktail be made of these factors to avoid the need for a cellular based therapy altogether? These questions need to be answered before widespread clinical use.
Conclusions
Stem cell therapy is promising as a treatment for necrotizing enterocolitis. It is likely that AF derived stem cells or BM-MSC will be the most useful and practical cell-based therapies for this condition. Acellular therapies such as exosomes or other paracrine mediators still to be identified are promising as well. Multiple mechanisms are likely at play in the positive effects provided by these cells, and further research is underway to fully understand these. Moving to human clinical trials is feasible and reasonable, and with good patient selection, success is anticipated.
Footnotes
Disclosures: The authors have no relevant disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neu J, Walker WA. Necrotizing enterocolitis. The New England journal of medicine. 2011;364(3):255–64. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herrmann K, Carroll K. An exclusively human milk diet reduces necrotizing enterocolitis. Breastfeed Med. 2014;9(4):184–90. doi: 10.1089/bfm.2013.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li QY, An Y, Liu L, Wang XQ, Chen S, Wang ZL, et al. Differences in the Clinical Characteristics of Early-and Late-Onset Necrotizing Enterocolitis in Full-Term Infants: A Retrospective Case-Control Study. Scientific reports. 2017;7:43042. doi: 10.1038/srep43042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coggins SA, Wynn JL, Weitkamp JH. Infectious causes of necrotizing enterocolitis. Clinics in perinatology. 2015;42(1):133–54. ix. doi: 10.1016/j.clp.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warner BB, Deych E, Zhou Y, Hall-Moore C, Weinstock GM, Sodergren E, et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet. 2016;387(10031):1928–36. doi: 10.1016/S0140-6736(16)00081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma R, Tepas JJ, 3rd, Hudak ML, Mollitt DL, Wludyka PS, Teng RJ, et al. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. Journal of pediatric surgery. 2007;42(3):454–61. doi: 10.1016/j.jpedsurg.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 7.Diaco N, Diamandis Z, Borlongan C. Amniotic fluid-derived stem cells as an effective cell source for transplantation therapy in stroke. Brain Circ. 2015;1(2):119. [Google Scholar]
- 8.Galindo LT, Filippo TR, Semedo P, Ariza CB, Moreira CM, Camara NO, et al. Mesenchymal stem cell therapy modulates the inflammatory response in experimental traumatic brain injury. Neurol Res Int. 2011;2011:564089. doi: 10.1155/2011/564089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowart P, Erpicum P, Detry O, Weekers L, Gregoire C, Lechanteur C, et al. Mesenchymal Stromal Cell Therapy in Ischemia/Reperfusion Injury. J Immunol Res. 2015;2015:602597. doi: 10.1155/2015/602597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bongso A, Richards M. History and perspective of stem cell research. Best Pract Res Clin Obstet Gynaecol. 2004;18(6):827–42. doi: 10.1016/j.bpobgyn.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 11.McCulloh CJ, Olson JK, Zhou Y, Wang Y, Besner GE. Stem cells and necrotizing enterocolitis: A direct comparison of the efficacy of multiple types of stem cells. Journal of pediatric surgery. 2017;52(6):999–1005. doi: 10.1016/j.jpedsurg.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rager TM, Olson JK, Zhou Y, Wang Y, Besner GE. Exosomes secreted from bone marrow-derived mesenchymal stem cells protect the intestines from experimental necrotizing enterocolitis. Journal of pediatric surgery. 2016;51(6):942–7. doi: 10.1016/j.jpedsurg.2016.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tayman C, Uckan D, Kilic E, Ulus AT, Tonbul A, Murat Hirfanoglu I, et al. Mesenchymal stem cell therapy in necrotizing enterocolitis: a rat study. Pediatr Res. 2011;70(5):489–94. doi: 10.1203/PDR.0b013e31822d7ef2. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Watkins D, Chen CL, Bhushan B, Zhou Y, Besner GE. Heparin-binding epidermal growth factor-like growth factor and mesenchymal stem cells act synergistically to prevent experimental necrotizing enterocolitis. J Am Coll Surg. 2012;215(4):534–45. doi: 10.1016/j.jamcollsurg.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.itA PS. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102(4):1548–9. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- 16.De Coppi P, Bartsch G, Jr, Siddiqui MM, Xu T, Santos CC, Perin L, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25(1):100–6. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 17.Bottai D, Cigognini D, Nicora E, Moro M, Grimoldi MG, Adami R, et al. Third trimester amniotic fluid cells with the capacity to develop neural phenotypes and with heterogeneity among sub-populations. Restor Neurol Neurosci. 2012;30(1):55–68. doi: 10.3233/RNN-2011-0620. [DOI] [PubMed] [Google Scholar]
- 18.Murphy SV, Atala A. Amniotic fluid and placental membranes: unexpected sources of highly multipotent cells. Semin Reprod Med. 2013;31(1):62–8. doi: 10.1055/s-0032-1331799. [DOI] [PubMed] [Google Scholar]
- 19.Zani A, Cananzi M, Fascetti-Leon F, Lauriti G, Smith VV, Bollini S, et al. Amniotic fluid stem cells improve survival and enhance repair of damaged intestine in necrotising enterocolitis via a COX-2 dependent mechanism. Gut. 2014;63(2):300–9. doi: 10.1136/gutjnl-2012-303735. [DOI] [PubMed] [Google Scholar]
- 20.McCulloh CJ, Olson JK, Wang Y, Vu J, Gartner S, Besner GE. Evaluating the efficacy of different types of stem cells in preserving gut barrier function in necrotizing enterocolitis. The Journal of surgical research. 2017;214:278–85. doi: 10.1016/j.jss.2017.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Good M, Siggers RH, Sodhi CP, Afrazi A, Alkhudari F, Egan CE, et al. Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(28):11330–5. doi: 10.1073/pnas.1200856109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siggers J, Ostergaard MV, Siggers RH, Skovgaard K, Molbak L, Thymann T, et al. Postnatal amniotic fluid intake reduces gut inflammatory responses and necrotizing enterocolitis in preterm neonates. Am J Physiol Gastrointest Liver Physiol. 2013;304(10):G864–75. doi: 10.1152/ajpgi.00278.2012. [DOI] [PubMed] [Google Scholar]
- 23.Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(10):3828–32. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bieback K, Kern S, Kluter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem cells. 2004;22(4):625–34. doi: 10.1634/stemcells.22-4-625. [DOI] [PubMed] [Google Scholar]
- 25.Dehkordi MB, Madjd Z, Chaleshtori MH, Meshkani R, Nikfarjam L, Kajbafzadeh AM. A Simple, Rapid, and Efficient Method for Isolating Mesenchymal Stem Cells from the Entire Umbilical Cord. Cell Transplant. 2016;25(7):1287–97. doi: 10.3727/096368915X582769. [DOI] [PubMed] [Google Scholar]
- 26.Hassan G, Kasem I, Soukkarieh C, Aljamali M. A Simple Method to Isolate and Expand Human Umbilical Cord Derived Mesenchymal Stem Cells: Using Explant Method and Umbilical Cord Blood Serum. Int J Stem Cells. 2017 doi: 10.15283/ijsc17028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen AR, Manning MM, Khaneki S, Drucker NA, Markel TA. Harvest tissue source does not alter the protective power of stromal cell therapy after intestinal ischemia and reperfusion injury. The Journal of surgical research. 2016;204(2):361–70. doi: 10.1016/j.jss.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yen BL, Huang HI, Chien CC, Jui HY, Ko BS, Yao M, et al. Isolation of multipotent cells from human term placenta. Stem cells. 2005;23(1):3–9. doi: 10.1634/stemcells.2004-0098. [DOI] [PubMed] [Google Scholar]
- 29.Watkins DJ, Yang J, Matthews MA, Besner GE. Synergistic effects of HB-EGF and mesenchymal stem cells in a murine model of intestinal ischemia/reperfusion injury. Journal of pediatric surgery. 2013;48(6):1323–9. doi: 10.1016/j.jpedsurg.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koponen JK, Kekarainen T, SEH, Laitinen A, Nystedt J, Laine J, et al. Umbilical cord blood-derived progenitor cells enhance muscle regeneration in mouse hindlimb ischemia model. Molecular therapy: the journal of the American Society of Gene Therapy. 2007;15(12):2172–7. doi: 10.1038/sj.mt.6300302. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y, Yang J, Watkins DJ, Boomer LA, Matthews MA, Su Y, et al. Enteric nervous system abnormalities are present in human necrotizing enterocolitis: potential neurotransplantation therapy. Stem Cell Res Ther. 2013;4(6):157. doi: 10.1186/scrt387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60(4):585–95. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 33.Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12(8):453–66. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei J, Zhou Y, Besner GE. Heparin-binding EGF-like growth factor and enteric neural stem cell transplantation in the prevention of experimental necrotizing enterocolitis in mice. Pediatr Res. 2015;78(1):29–37. doi: 10.1038/pr.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markel TA, Crisostomo PR, Lahm T, Novotny NM, Rescorla FJ, Tector J, et al. Stem cells as a potential future treatment of pediatric intestinal disorders. Journal of pediatric surgery. 2008;43(11):1953–63. doi: 10.1016/j.jpedsurg.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsumoto T, Okamoto R, Yajima T, Mori T, Okamoto S, Ikeda Y, et al. Increase of bone marrow-derived secretory lineage epithelial cells during regeneration in the human intestine. Gastroenterology. 2005;128(7):1851–67. doi: 10.1053/j.gastro.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 37.Allers C, Sierralta WD, Neubauer S, Rivera F, Minguell JJ, Conget PA. Dynamic of distribution of human bone marrow-derived mesenchymal stem cells after transplantation into adult unconditioned mice. Transplantation. 2004;78(4):503–8. doi: 10.1097/01.tp.0000128334.93343.b3. [DOI] [PubMed] [Google Scholar]
- 38.Sordi V. Mesenchymal stem cell homing capacity. Transplantation. 2009;87(9 Suppl):S42–5. doi: 10.1097/TP.0b013e3181a28533. [DOI] [PubMed] [Google Scholar]
- 39.Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Semin Perinatol. 2008;32(2):70–82. doi: 10.1053/j.semperi.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Ghionzoli M, Cananzi M, Zani A, Rossi CA, Leon FF, Pierro A, et al. Amniotic fluid stem cell migration after intraperitoneal injection in pup rats: implication for therapy. Pediatric surgery international. 2010;26(1):79–84. doi: 10.1007/s00383-009-2504-x. [DOI] [PubMed] [Google Scholar]
- 41.Bruno S, Collino F, Tetta C, Camussi G. Dissecting paracrine effectors for mesenchymal stem cells. Adv Biochem Eng Biotechnol. 2013;129:137–52. doi: 10.1007/10_2012_149. [DOI] [PubMed] [Google Scholar]
- 42.Weil BR, Markel TA, Herrmann JL, Abarbanell AM, Meldrum DR. Mesenchymal stem cells enhance the viability and proliferation of human fetal intestinal epithelial cells following hypoxic injury via paracrine mechanisms. Surgery. 2009;146(2):190–7. doi: 10.1016/j.surg.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 43.Lasser C. Exosomal RNA as biomarkers and the therapeutic potential of exosome vectors. Expert Opin Biol Ther. 2012;12(Suppl 1):S189–97. doi: 10.1517/14712598.2012.680018. [DOI] [PubMed] [Google Scholar]
- 44.Lo Sicco C, Reverberi D, Balbi C, Ulivi V, Principi E, Pascucci L, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization. Stem cells translational medicine. 2017;6(3):1018–28. doi: 10.1002/sctm.16-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, et al. Functional delivery of viral miRNAs via exosomes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(14):6328–33. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thery C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doster DL, Jensen AR, Khaneki S, Markel TA. Mesenchymal stromal cell therapy for the treatment of intestinal ischemia: Defining the optimal cell isolate for maximum therapeutic benefit. Cytotherapy. 2016;18(12):1457–70. doi: 10.1016/j.jcyt.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425(6961):968–73. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 49.Davies PS, Powell AE, Swain JR, Wong MH. Inflammation and proliferation act together to mediate intestinal cell fusion. PLoS One. 2009;4(8):e6530. doi: 10.1371/journal.pone.0006530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Jong JH, Rodermond HM, Zimberlin CD, Lascano V, De Sousa EMF, Richel DJ, et al. Fusion of intestinal epithelial cells with bone marrow derived cells is dispensable for tissue homeostasis. Scientific reports. 2012;2:271. doi: 10.1038/srep00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124(1):3–20. doi: 10.1016/j.jaci.2009.05.038. quiz 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Halpern MD, Denning PW. The role of intestinal epithelial barrier function in the development of NEC. Tissue Barriers. 2015;3(1–2):e1000707. doi: 10.1080/21688370.2014.1000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kandasamy J, Huda S, Ambalavanan N, Jilling T. Inflammatory signals that regulate intestinal epithelial renewal, differentiation, migration and cell death: Implications for necrotizing enterocolitis. Pathophysiology. 2014;21(1):67–80. doi: 10.1016/j.pathophys.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu P, Sodhi CP, Hackam DJ. Toll-like receptor regulation of intestinal development and inflammation in the pathogenesis of necrotizing enterocolitis. Pathophysiology. 2014;21(1):81–93. doi: 10.1016/j.pathophys.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arciero J, Bard Ermentrout G, Siggers R, Afrazi A, Hackam D, Vodovotz Y, et al. Modeling the interactions of bacteria and Toll-like receptor-mediated inflammation in necrotizing enterocolitis. Journal of theoretical biology. 2013;321:83–99. doi: 10.1016/j.jtbi.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gnecchi M, Melo LG. Bone marrow-derived mesenchymal stem cells: isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol Biol. 2009;482:281–94. doi: 10.1007/978-1-59745-060-7_18. [DOI] [PubMed] [Google Scholar]
- 57.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;166(3):585–92. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 58.Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7(1):125. doi: 10.1186/s13287-016-0363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prockop DJ. Concise review: two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem cells. 2013;31(10):2042–6. doi: 10.1002/stem.1400. [DOI] [PubMed] [Google Scholar]
- 60.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 61.Metcalfe C, Kljavin NM, Ybarra R, de Sauvage FJ. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell. 2014;14(2):149–59. doi: 10.1016/j.stem.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 62.Li B, Lee C, Filler T, Hock A, Wu RY, Li Q, et al. Inhibition of corticotropin-releasing hormone receptor 1 and activation of receptor 2 protect against colonic injury and promote epithelium repair. Scientific reports. 2017;7:46616. doi: 10.1038/srep46616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Afrazi A, Branca MF, Sodhi CP, Good M, Yamaguchi Y, Egan CE, et al. Toll-like receptor 4-mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis. J Biol Chem. 2014;289(14):9584–99. doi: 10.1074/jbc.M113.526517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neal MD, Richardson WM, Sodhi CP, Russo A, Hackam DJ. Intestinal stem cells and their roles during mucosal injury and repair. The Journal of surgical research. 2011;167(1):1–8. doi: 10.1016/j.jss.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sodhi CP, Shi XH, Richardson WM, Grant ZS, Shapiro RA, Prindle T, Jr, et al. Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology. 2010;138(1):185–96. doi: 10.1053/j.gastro.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen CL, Yu X, James IO, Zhang HY, Yang J, Radulescu A, et al. Heparin-binding EGF-like growth factor protects intestinal stem cells from injury in a rat model of necrotizing enterocolitis. Lab Invest. 2012;92(3):331–44. doi: 10.1038/labinvest.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bizzarro MJ, Bhandari V, Krause DS, Smith BR, Gross I. Circulating stem cells in extremely preterm neonates. Acta paediatrica. 2007;96(4):521–5. doi: 10.1111/j.1651-2227.2007.00194.x. [DOI] [PubMed] [Google Scholar]
- 68.Dekaney CM, Fong JJ, Rigby RJ, Lund PK, Henning SJ, Helmrath MA. Expansion of intestinal stem cells associated with long-term adaptation following ileocecal resection in mice. Am J Physiol Gastrointest Liver Physiol. 2007;293(5):G1013–22. doi: 10.1152/ajpgi.00218.2007. [DOI] [PubMed] [Google Scholar]
- 69.Zani A, De Coppi P. Stem cell therapy as an option for pediatric surgical conditions. European journal of pediatric surgery: official journal of Austrian Association of Pediatric Surgery [et al] = Zeitschrift fur Kinderchirurgie. 2014;24(3):219–26. doi: 10.1055/s-0034-1378150. [DOI] [PubMed] [Google Scholar]
- 70.McCulloh CJ, Olson JK, Zhou Y, Wang Y, Besner GE. Stem cells and necrotizing enterocolitis: A direct comparison of the efficacy of multiple types of stem cells. Journal of pediatric surgery. 2017 doi: 10.1016/j.jpedsurg.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jensen AR, Manning MM, Khaneki S, Drucker NA, Markel TA. Harvest tissue source does not alter the protective power of stromal cell therapy after intestinal ischemia and reperfusion injury. The Journal of surgical research. 2016;204(2):361–70. doi: 10.1016/j.jss.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jensen AR, Doster DL, Hunsberger EB, Manning MM, Stokes SM, Barwinska D, et al. Human Adipose Stromal Cells Increase Survival and Mesenteric Perfusion Following Intestinal Ischemia and Reperfusion Injury. Shock. 2016;46(1):75–82. doi: 10.1097/SHK.0000000000000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hawkey CJ. Stem cells as treatment in inflammatory bowel disease. Dig Dis. 2012;30(Suppl 3):134–9. doi: 10.1159/000342740. [DOI] [PubMed] [Google Scholar]
- 74.Ryska O, Serclova Z, Mestak O, Matouskova E, Vesely P, Mrazova I. Local application of adipose-derived mesenchymal stem cells supports the healing of fistula: prospective randomised study on rat model of fistulising Crohn’s disease. Scand J Gastroenterol. 2017;52(5):543–50. doi: 10.1080/00365521.2017.1281434. [DOI] [PubMed] [Google Scholar]
- 75.Furlani D, Ugurlucan M, Ong L, Bieback K, Pittermann E, Westien I, et al. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc Res. 2009;77(3):370–6. doi: 10.1016/j.mvr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 76.Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39(2):573–6. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 77.Eaton S, Zani A, Pierro A, De Coppi P. Stem cells as a potential therapy for necrotizing enterocolitis. Expert Opin Biol Ther. 2013;13(12):1683–9. doi: 10.1517/14712598.2013.849690. [DOI] [PubMed] [Google Scholar]
- 78.Samuels N, van de Graaf RA, de Jonge RCJ, Reiss IKM, Vermeulen MJ. Risk factors for necrotizing enterocolitis in neonates: a systematic review of prognostic studies. BMC pediatrics. 2017;17(1):105. doi: 10.1186/s12887-017-0847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grant CN, Grikscheit TC. Tissue engineering: a promising therapeutic approach to necrotizing enterocolitis. Semin Pediatr Surg. 2013;22(2):112–6. doi: 10.1053/j.sempedsurg.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 80.Totonelli G, Maghsoudlou P, Garriboli M, Riegler J, Orlando G, Burns AJ, et al. A rat decellularized small bowel scaffold that preserves villus-crypt architecture for intestinal regeneration. Biomaterials. 2012;33(12):3401–10. doi: 10.1016/j.biomaterials.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5(+) stem cell. Nat Med. 2012;18(4):618–23. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 82.Sala FG, Kunisaki SM, Ochoa ER, Vacanti J, Grikscheit TC. Tissue-engineered small intestine and stomach form from autologous tissue in a preclinical large animal model. The Journal of surgical research. 2009;156(2):205–12. doi: 10.1016/j.jss.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 83.Shaffiey SA, Jia H, Keane T, Costello C, Wasserman D, Quidgley M, et al. Intestinal stem cell growth and differentiation on a tubular scaffold with evaluation in small and large animals. Regen Med. 2016;11(1):45–61. doi: 10.2217/rme.15.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Patil PB, Chougule PB, Kumar VK, Almstrom S, Backdahl H, Banerjee D, et al. Recellularization of acellular human small intestine using bone marrow stem cells. Stem cells translational medicine. 2013;2(4):307–15. doi: 10.5966/sctm.2012-0108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85.Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7(10):e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prockop DJ, Brenner M, Fibbe WE, Horwitz E, Le Blanc K, Phinney DG, et al. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy. 2010;12(5):576–8. doi: 10.3109/14653249.2010.507330. [DOI] [PubMed] [Google Scholar]
- 87.Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, et al. Safety of Cell Therapy with Mesenchymal Stromal Cells (SafeCell): A Systematic Review and Meta-Analysis of Clinical Trials. PLoS ONE. 2012;7(10):e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Merkle FT, Ghosh S, Kamitaki N, Mitchell J, Avior Y, Mello C, et al. Human pluripotent stem cells recurrently acquire and expand dominant negative P53 mutations. Nature. 2017;545(7653):229–33. doi: 10.1038/nature22312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mousavinejad M, Andrews PW, Shoraki EK. Current Biosafety Considerations in Stem Cell Therapy. Cell J. 2016;18(2):281–7. doi: 10.22074/cellj.2016.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aguilar S, Nye E, Chan J, Loebinger M, Spencer-Dene B, Fisk N, et al. Murine but not human mesenchymal stem cells generate osteosarcoma-like lesions in the lung. Stem cells. 2007;25(6):1586–94. doi: 10.1634/stemcells.2006-0762. [DOI] [PubMed] [Google Scholar]
- 91.Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem cells. 2007;25(2):371–9. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]