Abstract
Necrotizing enterocolitis (NEC) remains one of the highest causes of mortality and of acute and long-term morbidity in premature infants. Multiple factors are involved in the pathophysiology of NEC including the immaturity of the immune system and the complex changing composition of the intestinal microbiome. This is compounded by the fact that the premature infant should ideally still be a developing fetus and has an immature intestinal tract. Because these complexities are beyond the scope of studies in single cell cultures, animal models are absolutely essential to understand the mechanisms involved in the pathophysiology of NEC and the effects of inflammation on the immature intestinal tract. To this end, investigators have utilized many different species (e.g. rats, mice, rabbits, quails, piglets, and non-human primates) and conditions to develop models of NEC. Each animal has distinct advantages and drawbacks related to its preterm viability, body size, genetic variability, and cost. The choice of animal model is strongly influenced by the scientific question being addressed. While no model perfectly mimics human NEC, each has greatly improved our understanding of disease. Examples of recent discoveries in NEC pathogenesis and prevention underscore the importance of continued animal research in NEC.
Keywords: Necrotizing enterocolitis, animal models, formula, hypoxia, rodent, piglet
Introduction
Necrotizing enterocolitis (NEC) is the leading cause of gastrointestinal morbidity and mortality in preterm infants. NEC currently affects 5–10% of very low birth weight (VLBW) infants, carries a mortality rate as high as 40%(1, 2), and many of these patients will progress to severe NEC requiring surgical intervention (Figure 1). The pathophysiology of NEC remains to be fully elucidated, but several risk factors have been identified, including prematurity, bacterial colonization, enteral feeds, and an immature immune system(3). While observational studies in humans have led to the identification of these risk factors, manipulation of these variables is not ethical or plausible in humans. Likewise, the complex interactions between microbiota, immune cells, enterocytes, and premature physiology are too broad to be studied in single-cell cultures. As a result, scientific studies utilizing animal models have been foundational in discovering much of what we know about NEC pathogenesis. These models allow for additional studies that are otherwise not feasible in humans. These include manipulation of biochemical pathways, examinations of specific cellular receptors or cell types, targeted examinations of the microbiome, and direct measurements of bowel perfusion and intestinal permeability. Additionally, animal models permit investigation of promising preventive or treatment strategies, providing an invaluable proof-of-concept prior to human trials (4, 5).
Figure 1.
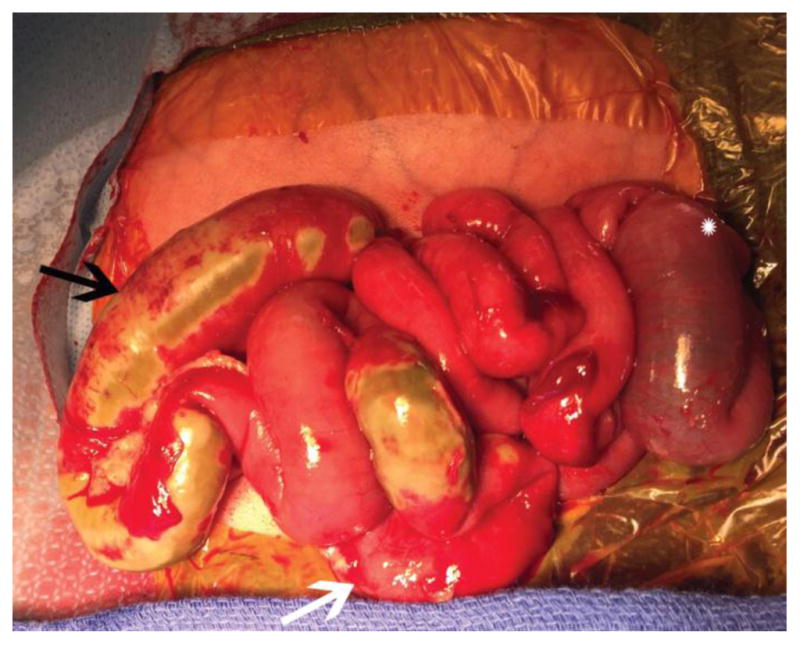
Exploratory laparotomy of premature neonate with severe NEC requiring surgical intervention. Note the patchy areas of necrosis (black arrow), distended bowel (star), and inflammation (white arrow).
The use of animal models has been pivotal to improving our current understanding of NEC and identifying potential therapeutic strategies. For example, animal models have advanced our understanding of the protective effects of individual elements of maternal milk on the intestinal epithelium, modulators of biochemical pathways with therapeutic potential such as Toll-like Receptors, the role of the microbiome in the protection and propagation of NEC, and even the role of individual cell types in NEC pathogenesis(5–9). To better understand the pathophysiology of NEC and the effects of inflammation on the immature intestinal tract, investigators have utilized many different species (e.g. rats, mice, rabbits, quails, piglets, and non-human primates)(5, 10–13) and conditions to develop models which simulate the pathophysiology seen in NEC. Each animal has distinct advantages and drawbacks related to their preterm viability, body size, genetic variability, and cost. The choice of animal model should be strongly influenced by the scientific question being addressed.
While no model perfectly mimics human NEC, there are distinct advantages and disadvantages of each, and each has greatly improved our understanding of disease. A thorough understanding of each model will better allow the investigator to choose the appropriate one (based on the question being addressed), and will allow reviewers and funders to determine the value of future studies. The purpose of this review is to 1) Justify the value of animal models in the study of NEC, 2) Discuss the three most commonly used animal species for the study of NEC, and 3) Identify the advantages and disadvantages of each model and highlight recent discoveries from each.
Modeling NEC in the Rat
The earliest animal models of NEC were done in adult animals and mainly examined the effects of different feeding compositions on closed loops of bowel. In these models, ischemia/reperfusion injury was induced by temporary occlusion of the superior mesenteric artery (SMA) or surgical creation of a small bowel closed loop. By the mid-1970’s, clinicians and scientists realized that a new model needed to be developed that took into account the immaturity of the intestine, formula feedings, and bacteria. The first model to successfully achieve this was described by Barlow et al. in 1974(10). In this study, she demonstrated that gut flora and lack of breast milk were important contributors to the development of NEC-like injury; facts that are still applicable in our understanding of NEC today. In 1975, using rats, she included hypoxia/ hypothermia/ formula-feeding (HHF) to enhance the model(14). These experiments laid the foundation for animal models of NEC, establishing that these models must rely on mechanistic, histopathological, and physiologic changes similar to human NEC to be considered relevant(15). The initial histologic grading system described by Barlow remains pertinent for disease confirmation in all current rodent models.
NEC models utilizing rats have several benefits including their low cost per litter size, a higher resilience to stressors than other species such as mice, and relative large size compared to mice. The major disadvantages to using rats include a lack of biomolecular reagents such as commercially available antibodies, and a lack of the ability to utilize genetic techniques to understand mechanisms. Additionally, the resilience to stress is also disadvantageous when inducing NEC, as rats have a superior tolerance to bacterial contamination and endotoxins compared to humans.
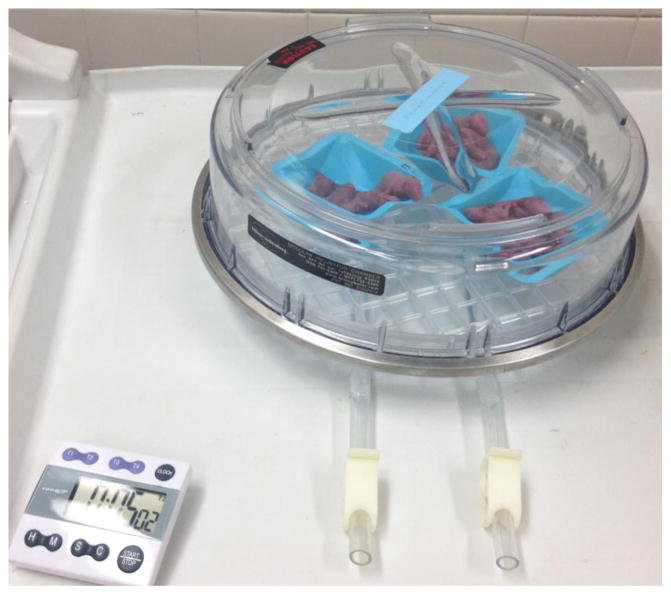
One important point about NEC models that utilize rats is that there is great variation reported in the literature as to how injury is induced. This includes how rat pups are fed, what composition of formula they are fed, and how often the formula is given (most often three, four, or six times per day). Both term and pre-term delivery is utilized. The pups are delivered via cesarean section to avoid breast feeding or oxytocin injection to induce labor(16). Following delivery, rat pups may be exposed to varying durations of hypoxia, (Figure 2), hypothermia, and/or hyperoxia(17). To boost the disease severity of the model, an additional “bonus” factor is often added by investigators, which may be lipopolysaccharide (LPS)(18), enteric bacteria(19), Cronobacter sakazakii(20), or casein(21), among others. These may be administered enterally (Figure 3), intravenously, or even intraperitoneally(22). One of the most striking features of the rat HHF NEC model is that exposure to hypoxia and hypothermia in pups that are dam fed essentially never leads to NEC.
Figure 2.
Rat pups are exposed to hypoxia in a chamber created using 100% nitrogen to washout room air, then clamped shut. Hypoxia duration may vary between investigators from 1 to 10 minutes. Note the cyanotic discoloration of the pups.
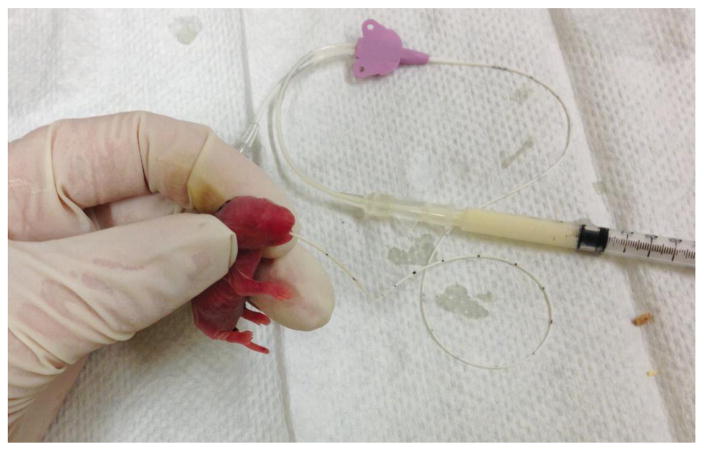
Figure 3.
Gavage feeding of 1 day old rat pup using silicone orogastric tubing and syringe. A variety of small-sized catheters may be used, such as angio-catheters or as in this case, a 1.9F central venous catheter.
Rat NEC models have been useful in demonstrating prevention of NEC with administration of probiotics, growth factors, stem cells, human milk oligosaccharides, TNF blockers, and various anti-oxidants. Rat NEC models have also been useful in temporal biomarker studies (e.g. correlation on intestinal fatty acid binding protein with timing of ischemia and severity of tissue injury(23)). Currently, the administration of a protein kinase A inhibitor(24) and milk-derived exosomes(25) are being studied in a rat model with promising results as potential treatments for NEC.
Modeling NEC in the Mouse
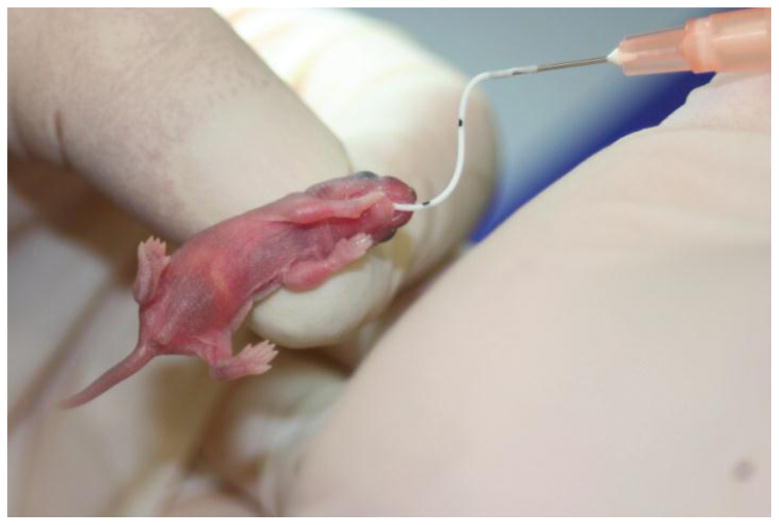
Mice have been widely used in biomedical research. The similarities between mice and humans in anatomy, physiology, and genetics have led to numerous advances in human biology, and the advanced knowledge of mouse genetics has greatly facilitated mechanistic studies. In addition, their comparatively low maintenance cost, high reproductive rates, and short life cycle offer great advantages. As such, mice are an attractive species to use in modeling NEC. The earliest attempt to use mice in NEC research was an adult isolated ileal loop model in 1986 by former U.S. Surgeon General CE Koop(26). However, the first true advance in utilizing mice was in 2006 when the rat model of NEC was adapted to mice(11). Similar to the rat model described above, mice in this model were delivered by cesarean section prior to term, and then gavage fed every 2 hours with 200kcal/kg/day with Esbilac puppy formula using a small orogastric feeding catheter (Figure 4). Mouse pups are exposed to hypoxia and cold stress using 100% nitrogen exposure for 1 minute and 4°C exposure for 10 minutes, twice daily for a total of 72 hours. Since the first description of this model, several notable variations have been developed including: the use of hypothermia, ranging from no hypothermia to 10 minutes at 4°C; the type, amount, and frequency of formula feedings; the type of delivery; the age of initiation of injury; and the use of formula adjuncts such as live bacteria, LPS, or other inflammatory agents.
Figure 4.
A 4 day old mouse pup undergoing gavage feeding, note the milk bubble (white area) on the pup’s abdomen indicating that milk is filling the stomach.
The primary advantages of mice include the ability to use genetically altered animals and animals that were raised in germ free conditions. Transgenic mice have been used to study a host of receptors and mechanistic pathways that are thought to be of relevance to the pathophysiology of NEC, including Toll-like receptor 4, tumor necrosis factor, and interleukin 18(11, 27, 28). In addition, alterations to the intestinal microbiome are now thought to be fundamental to the development of NEC. Utilizing mice that are bred and raised in the absence of bacteria, investigators have been able to begin to understand the effects of varying bacterial compositions on the immature intestinal tract(29).
Recent concerns have arisen regarding the HHF models including a lack of advances in treatment options, the developmental stage of disease, and the severity of the conditions utilized. In response to this, several new mouse models have been developed that attempt to leverage inflammatory pathways rather than hypoxia as a driver of injury. Trinitrobenzenesulfonic acid (TNBS) has been utilized in 10-day old mice. TNBS acts as a hapten, binding to host proteins and generating an immune response that results in a mucosal injury similar to that of NEC(30). A second model utilizes disruption of Paneth cells followed by enteral gavage of bacteria to induce disease in 14-day old mice that also induces injury similar to NEC(9). These new models offer additional insight into mechanistic pathways of how the immature intestinal tract can be injured and strongly support the new theory that NEC may a final common pathway of multiple initiating events(31). The Paneth cell disruption model has the distinct advantage of timing that most closely mimics the human intestinal tract, i.e. NEC-like injury is only seen in a narrow developmental window that correlates with the timing of highest risk in premature infants.
The mouse model has been useful in demonstrating mechanisms of prevention of NEC by probiotics and anti-oxidants, NEC prevention by fecal microbial transplantation(32), and the lack of impact of osmolality of enteral feeding on NEC severity(33). The mouse model has also been useful in confirming mechanisms underlying platelet consumption in NEC(34). The TLR4 knockout mouse has been essential in discovering the roles of this receptor in NEC (35) and in demonstrating that NEC can occur with Paneth cell insult in the absence of TLR4 (9). Recent studies using transgenic mouse models of NEC have also discovered important immune modulating milk proteins, such as the LPS recognition receptor sCD14, stressing the need for improvement of infant formulas(25).
While the use of mice to model NEC has greatly advanced our understanding of the immature intestine and its injury, the small size of mouse pups makes studies with them technically challenging, including limiting the ability to obtain serial samples, obtaining maternal milk, and gavage feeding animals.
Modeling NEC in the Pig
A relatively recent but exciting model used to study NEC is the piglet model, which utilizes the larger body size of the piglet as a distinct advantage. Piglets can be used for repeated tissue sampling, analysis of tissue perfusion and blood flow, and differential analysis of feeding route, nutritional components, and volumes. In this model, piglets are delivered by C-section at 90% term. Following birth, preterm piglets go through a period of natural hypoxia/hypothermia, and then are formula fed to induce injury(5). A variation uses total parental nutrition at birth followed by a transition to enteral formula feeds on the 2nd day of life(4, 36). The advantages of piglets as a model for NEC include their size, their physiology and anatomy which closely resembles that of a human infant, and the ability to utilize premature offspring which allows for the study of an immature intestine in an immature host(37). This is advantageous over rat and mouse models, where immature intestines can be studied but the hosts themselves are more mature(38). However, piglets are extremely costly to maintain, and there are limited analytical tools available such as antibodies. Additionally, there is little to no current availability to utilize genetic techniques to study disease mechanisms. Lastly, the piglet models of NEC cause intestinal injury that is global (stomach through rectum) which differs from rodent models which more specifically affect the distal small intestine. However, it is also important to note that while most human NEC occurs at the distal small intestine, NEC totalis is a significant NEC variant that like the pig model, affects the entire bowel.
The piglet model has been useful in demonstrating prevention of NEC with enteral but not parenteral antibiotics, in identification of NEC prior to the onset of symptoms by NIRS monitoring and plasma intestinal fatty-acid binding protein (4), and in prevention of NEC with bovine colostrum. A recent study has successfully identified elevated plasma levels of circulating cell-free DNA and neutrophil proteins as early markers associated with NEC(39). Ongoing research with piglet models continues to highlight the anti-inflammatory effects of human milk oligosaccharides and elucidate their role in maturation of intestinal function(40).
Novel Models and Future Directions
Rabbits and hamsters have been also used in the study of NEC (12, 41). These models are variations of the hypoxia/ hypothermia/ formula feed method, and some include addition of LPS. A rabbit model uses formula feedings with Enterobacter cloacae to produce a NEC-like mucosal injury, in addition to creating a distal obstruction by anal blockage to simulate the intestinal dysmotility of premature infants. This model has allowed for the study of management of non-surgical NEC(42). A gnotobiotic quail model has also been used successfully to delineate the contribution of individual bacterial species in the pathophysiology of NEC(12). Innoculation of germ-free quails with bacteria associated with NEC have improved our understanding of the iNOS pathway activation prior to the development of macroscopic lesions in NEC(43). Another noteworthy model is the premature baboon, delivered via C-section at 125 days gestation, which is equivalent to a 27-week gestation human(13). The baboons were treated with mechanical ventilation, antibiotics, enteral feeds, and other necessary treatments similar to those provided to a neonate with sepsis. Outcomes were compared showing a difference in the Transforming growth factor-beta expression in premature intestines during NEC. These less commonly used models have a role in NEC research; however, they are relatively understudied and their associated costs are significant, making them less attractive than more conventional models.
In addition, several novel systems show promise in the study of intestinal injury and development of NEC. Enteroids, while not strictly “animal” models, are intestinal stem cells harvested from human or mouse crypt cells and grown in culture to produce self-renewing intestinal tissue(44). These 3-dimensional structures can be manipulated to study biochemical signaling pathways in NEC and pharmacologically and genetically altered in the search for therapeutic targets. A novel study using mice to implant human intestine subcutaneously, while not physiologic, allows for the manipulation of human tissue to study NEC(45). Another new application is gene editing technology, which may lead to further transgenic studies in rats and large animals, although it has not yet been used in published models of NEC(46).
Summary
Animal models of NEC have been in use for more than forty years. The current animal models, albeit varied, provide invaluable mechanistic understandings of the physiology of the immature intestine, and the pathogenesis of NEC. Without these animal models, our current understanding of the biochemical pathways leading to the development of NEC, the protective effects of breastmilk, and the physiologic implications of an immature intestine would be severely limited. Furthermore, animal models provide a unique testing venue for potential therapeutic options prior to application in clinical trials. Each animal has distinct advantages and drawbacks related to their preterm viability, body size, genetic variability, and cost. The choice of animal model is strongly influenced by the scientific question being addressed. While no model perfectly mimics human NEC, each has greatly improved our understanding of the immature intestinal tract, and the pathophysiology that leads to NEC. Further development and funding of animal research is essential to reducing the impact of this devastating disease of the most vulnerable humans.
Footnotes
Disclosures: No disclosures from the authors
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holman RC, Stoll BJ, Curns AT, et al. Necrotising enterocolitis hospitalisations among neonates in the United States. Paediatr Perinat Epidemiol. 2006;20(6):498–506. doi: 10.1111/j.1365-3016.2006.00756.x. [DOI] [PubMed] [Google Scholar]
- 2.Hull MA, Fisher JG, Gutierrez IM, et al. Mortality and management of surgical necrotizing enterocolitis in very low birth weight neonates: a prospective cohort study. J Am Coll Surg. 2014;218(6):1148–55. doi: 10.1016/j.jamcollsurg.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Jiang PP, Smith B, Qvist N, et al. Intestinal proteome changes during infant necrotizing enterocolitis. Pediatric Research. 2013;73(3):268–76. doi: 10.1038/pr.2012.182. [DOI] [PubMed] [Google Scholar]
- 4.Zamora IJ, Stoll B, Ethun CG, et al. Low Abdominal NIRS Values and Elevated Plasma Intestinal Fatty Acid-Binding Protein in a Premature Piglet Model of Necrotizing Enterocolitis. Plos One. 2015;10(6) doi: 10.1371/journal.pone.0125437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sangild PT, Siggers RH, Schmidt M, et al. Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology. 2006;130(6):1776–92. doi: 10.1053/j.gastro.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 6.Zhou W, Lv H, Li MX, et al. Protective effects of bifidobacteria on intestines in newborn rats with necrotizing enterocolitis and its regulation on TLR2 and TLR4. Genet Mol Res. 2015;14(3):11505–14. doi: 10.4238/2015.September.28.2. [DOI] [PubMed] [Google Scholar]
- 7.Wu W, Wang Y, Zou J, et al. Bifidobacterium adolescentis protects against necrotizing enterocolitis and upregulates TOLLIP and SIGIRR in premature neonatal rats. BMC Pediatr. 2017;17(1):1. doi: 10.1186/s12887-016-0759-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Good M, Sodhi CP, Egan CE, et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol. 2015;8(5):1166–79. doi: 10.1038/mi.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White JR, Gong H, Pope B, et al. Paneth-cell-disruption-induced necrotizing enterocolitis in mice requires live bacteria and occurs independently of TLR4 signaling. Dis Model Mech. 2017;10(6):727–36. doi: 10.1242/dmm.028589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barlow B, Santulli TV, Heird WC, et al. An experimental study of acute neonatal enterocolitis--the importance of breast milk. J Pediatr Surg. 1974;9(5):587–95. doi: 10.1016/0022-3468(74)90093-1. [DOI] [PubMed] [Google Scholar]
- 11.Jilling T, Simon D, Lu J, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol. 2006;177(5):3273–82. doi: 10.4049/jimmunol.177.5.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waligora-Dupriet AJ, Dugay A, Auzeil N, et al. Evidence for clostridial implication in necrotizing enterocolitis through bacterial fermentation in a gnotobiotic quail model. Pediatr Res. 2005;58(4):629–35. doi: 10.1203/01.PDR.0000180538.13142.84. [DOI] [PubMed] [Google Scholar]
- 13.Namachivayam K, Blanco CL, MohanKumar K, et al. Smad7 inhibits autocrine expression of TGF-beta2 in intestinal epithelial cells in baboon necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2013;304(2):G167–80. doi: 10.1152/ajpgi.00141.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barlow B, Santulli TV. Importance of multiple episodes of hypoxia or cold stress on the development of enterocolitis in an animal model. Surgery. 1975;77(5):687–90. [PubMed] [Google Scholar]
- 15.Sodhi C, Richardson W, Gribar S, et al. The development of animal models for the study of necrotizing enterocolitis. Dis Model Mech. 2008;1(2–3):94–8. doi: 10.1242/dmm.000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadler EP, Dickinson E, Knisely A, et al. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res. 2000;92(1):71–7. doi: 10.1006/jsre.2000.5877. [DOI] [PubMed] [Google Scholar]
- 17.Tayman C, Uckan D, Kilic E, et al. Mesenchymal stem cell therapy in necrotizing enterocolitis: a rat study. Pediatr Res. 2011;70(5):489–94. doi: 10.1203/PDR.0b013e31822d7ef2. [DOI] [PubMed] [Google Scholar]
- 18.Rentea RM, Liedel JL, Fredrich K, et al. Enteral intestinal alkaline phosphatase administration in newborns decreases iNOS expression in a neonatal necrotizing enterocolitis rat model. J Pediatr Surg. 2013;48(1):124–8. doi: 10.1016/j.jpedsurg.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Fatheree NY, Mangalat N, et al. Lactobacillus reuteri strains reduce incidence and severity of experimental necrotizing enterocolitis via modulation of TLR4 and NF-kappaB signaling in the intestine. Am J Physiol Gastrointest Liver Physiol. 2012;302(6):G608–17. doi: 10.1152/ajpgi.00266.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunter CJ, Singamsetty VK, Chokshi NK, et al. Enterobacter sakazakii enhances epithelial cell injury by inducing apoptosis in a rat model of necrotizing enterocolitis. J Infect Dis. 2008;198(4):586–93. doi: 10.1086/590186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatia AM, Feddersen RM, Musemeche CA. The role of luminal nutrients in intestinal injury from mesenteric reperfusion and platelet-activating factor in the developing rat. J Surg Res. 1996;63(1):152–6. doi: 10.1006/jsre.1996.0239. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Watkins D, Chen CL, et al. Heparin-binding epidermal growth factor-like growth factor and mesenchymal stem cells act synergistically to prevent experimental necrotizing enterocolitis. J Am Coll Surg. 2012;215(4):534–45. doi: 10.1016/j.jamcollsurg.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simoes AL, Figueira RL, Goncalves FL, et al. Temporal profile of intestinal tissue expression of intestinal fatty acid-binding protein in a rat model of necrotizing enterocolitis. Clinics (Sao Paulo) 2016;71(7):412–9. doi: 10.6061/clinics/2016(07)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blackwood BP, Wood DR, Yuan C, et al. A Role for cAMP and Protein Kinase A in Experimental Necrotizing Enterocolitis. Am J Pathol. 2017;187(2):401–17. doi: 10.1016/j.ajpath.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hock A, Miyake H, Li B, et al. Breast milk-derived exosomes promote intestinal epithelial cell growth. J Pediatr Surg. 2017;52(5):755–9. doi: 10.1016/j.jpedsurg.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 26.Krasna IH, Howell C, Vega A, et al. A mouse model for the study of necrotizing enterocolitis. J Pediatr Surg. 1986;21(1):26–9. doi: 10.1016/s0022-3468(86)80645-5. [DOI] [PubMed] [Google Scholar]
- 27.Markel TA, Crisostomo PR, Wang M, et al. Activation of individual tumor necrosis factor receptors differentially affects stem cell growth factor and cytokine production. Am J Physiol Gastrointest Liver Physiol. 2007;293(4):G657–62. doi: 10.1152/ajpgi.00230.2007. [DOI] [PubMed] [Google Scholar]
- 28.Halpern MD, Khailova L, Molla-Hosseini D, et al. Decreased development of necrotizing enterocolitis in IL-18-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G20–6. doi: 10.1152/ajpgi.00168.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Hoenig JD, Malin KJ, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. The ISME journal. 2009;3(8):944–54. doi: 10.1038/ismej.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MohanKumar K, Kaza N, Jagadeeswaran R, et al. Gut mucosal injury in neonates is marked by macrophage infiltration in contrast to pleomorphic infiltrates in adult: evidence from an animal model. Am J Physiol Gastrointest Liver Physiol. 2012;303(1):G93–102. doi: 10.1152/ajpgi.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon P, Christensen R, Weitkamp JH, et al. Mapping the New World of Necrotizing Enterocolitis (NEC): Review and Opinion. The e-journal of neonatology research. 2012;2(4):145–72. [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Li X, Shang Q, et al. Fecal microbiota transplantation (FMT) could reverse the severity of experimental necrotizing enterocolitis (NEC) via oxidative stress modulation. Free Radic Biol Med. 2017;108:32–43. doi: 10.1016/j.freeradbiomed.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Miyake H, Chen Y, Koike Y, et al. Osmolality of enteral formula and severity of experimental necrotizing enterocolitis. Pediatr Surg Int. 2016;32(12):1153–6. doi: 10.1007/s00383-016-3998-7. [DOI] [PubMed] [Google Scholar]
- 34.Namachivayam K, MohanKumar K, Garg L, et al. Neonatal mice with necrotizing enterocolitis-like injury develop thrombocytopenia despite increased megakaryopoiesis. Pediatr Res. 2017;81(5):817–24. doi: 10.1038/pr.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y, Li Y, Zhou B, et al. Inflammation and Apoptosis: Dual Mediator Role for Toll-like Receptor 4 in the Development of Necrotizing Enterocolitis. Inflammatory bowel diseases. 2017;23(1):44–56. doi: 10.1097/MIB.0000000000000961. [DOI] [PubMed] [Google Scholar]
- 36.Jensen ML, Sangild PT, Lykke M, et al. Similar efficacy of human banked milk and bovine colostrum to decrease incidence of necrotizing enterocolitis in preterm piglets. Am J Physiol-Reg I. 2013;305(1):R4–R12. doi: 10.1152/ajpregu.00094.2013. [DOI] [PubMed] [Google Scholar]
- 37.Sangild PT, Thymann T, Schmidt M, et al. Invited review: the preterm pig as a model in pediatric gastroenterology. J Anim Sci. 2013;91(10):4713–29. doi: 10.2527/jas.2013-6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McElroy SJ, Weitkamp JH. Innate Immunity in the Small Intestine of the Preterm Infant. NeoReviews. 2011;12(9):e517–e26. doi: 10.1542/neo.12-9-e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen DN, Stensballe A, Lai JC, et al. Elevated levels of circulating cell-free DNA and neutrophil proteins are associated with neonatal sepsis and necrotizing enterocolitis in immature mice, pigs and infants. Innate Immun. 2017;23(6):524–36. doi: 10.1177/1753425917719995. [DOI] [PubMed] [Google Scholar]
- 40.Rasmussen SO, Martin L, Ostergaard MV, et al. Human milk oligosaccharide effects on intestinal function and inflammation after preterm birth in pigs. J Nutr Biochem. 2017;40:141–54. doi: 10.1016/j.jnutbio.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Cassutto BH, Misra HP, Pfeiffer CJ. Intestinal post-ischemic reperfusion injury: studies with neonatal necrotizing enterocolitis. Acta Physiol Hung. 1989;73(2–3):363–9. [PubMed] [Google Scholar]
- 42.Bozeman AP, Dassinger MS, Birusingh RJ, et al. An animal model of necrotizing enterocolitis (NEC) in preterm rabbits. Fetal Pediatr Pathol. 2013;32(2):113–22. doi: 10.3109/15513815.2012.681426. [DOI] [PubMed] [Google Scholar]
- 43.Waligora-Dupriet AJ, Dugay A, Auzeil N, et al. Short-chain fatty acids and polyamines in the pathogenesis of necrotizing enterocolitis: Kinetics aspects in gnotobiotic quails. Anaerobe. 2009;15(4):138–44. doi: 10.1016/j.anaerobe.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016;18(3):246–54. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 45.Nanthakumar N, Meng D, Goldstein AM, et al. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: an immature innate immune response. PLoS One. 2011;6(3):e17776. doi: 10.1371/journal.pone.0017776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu P, Sodhi CP, Jia H, et al. Animal models of gastrointestinal and liver diseases. Animal models of necrotizing enterocolitis: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol. 2014;306(11):G917–28. doi: 10.1152/ajpgi.00422.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]