Abstract
Background and Objectives
Opioid use disorder (OUD) is a chronic disorder with relapse based on both desire for reinforcement (craving) and avoidance of withdrawal. The aversive aspect of dependence and relapse has been associated with a small brain structure called the habenula, which expresses large numbers of both opioid and nicotinic receptors. Additionally, opioid withdrawal symptoms can be induced in opioid-treated rodents by blocking not only opioid, but also nicotinic receptors. This receptor co-localization and cross-induction of withdrawal therefore might lead to genetic variation in the nicotinic receptor influencing development of human opioid dependence through its impact on the aversive components of opioid dependence.
Methods
We studied habenular resting state functional connectivity with related brain structures, specifically the striatum. We compared abstinent psychiatric patients who use opioids (N=51) to psychiatric patients who don’t (N=254) to identify an endophenotype of opioid use that focused on withdrawal avoidance and aversion rather than the more commonly examined craving aspects of relapse.
Results
We found that habenula – striatal connectivity was stronger in opioid-using patients. Increased habenula-striatum connectivity was observed in opioid-using patients with the low risk rs16969968 GG genotype, but not in patients carrying the high risk AG or AA genotypes.
Conclusions
We propose that increased habenula – striatum functional connectivity may be modulated by the nicotinic receptor variant rs16969968 and may lead to increased opioid use.
Scientific Significance
Our data uncovered a promising brain target for development of novel anti-addiction therapies and may help the development of personalized therapies against opioid abuse.
Introduction
Abuse of both prescription opioids and heroin has increased over the last several years, leading to more reported cases of Opioid Use Disorder (OUD). Between 2000 and 2014, annual deaths from abuse of prescription opioids increased from 4,400 to 18,900, while the number of deaths due to heroin overdose increased from 1,800 to 10,600 in the USA1. Motivational drivers for continued opioid use include first, seeking the rewards and positive reinforcement of acute opioid use and second, avoiding withdrawal and its negative reinforcement after dependence has developed. The second component, withdrawal avoidance, can include both acute opioid withdrawal syndrome (1–2 weeks) and protracted withdrawal that can persist for months after discontinuation2.
While there are no good animal models of protracted withdrawal and negative reinforcement, precipitated opioid withdrawal in rodents serves as an animal model of acute withdrawal. In this model, a mouse or rat is semi-chronically treated with morphine, followed by precipitated withdrawal using an opioid receptor antagonist, such as naloxone. A rodent model of withdrawal also has been successfully used for nicotine, precipitating withdrawal following semi-chronic nicotine treatment with the nicotinic receptor blocker mecamylamine3. Interestingly, in rodents nicotine withdrawal can be precipitated with naloxone4, while morphine withdrawal can be modified by mecamylamine5. This opioid/nicotine cross-talk is likely to occur within a brain region expressing high numbers of receptors responsive to both drugs, such as the habenula.
The habenula is a small brain region activated by negative reinforcers, such as lack of expected reward. The habenula has substantial concentrations of both µ-opioid and α5 and β4 subunits of the nicotinic receptors (Allen Brain Atlas, www.brain-map.org6). We previously showed that blocking nicotinic receptors with mecamylamine in the habenula precipitates nicotine withdrawal, and that the α5 and β4 nicotinic receptor subunits are both expressed in the habenula and necessary to observe nicotine withdrawal. One of the habenula’s main downstream targets, the interpeduncular nucleus, also expresses the α2 nicotinic receptor subunit, which may also be important for nicotine withdrawal7,8. Thus, we hypothesize that cross-talk between the nicotinic and the opioid systems may occur within the habenula. Three of the nicotinic receptor subunits necessary for mouse nicotine withdrawal and other nicotine-induced behaviors (α3, α5, β4)7–11 were later shown to be important for tobacco abuse and related diseases in humans. The genes coding for the α3, α5, and β4 nicotinic receptors are clustered on the human chromosome 15q25.1, where a large number of single nucleotide polymorphisms (SNPs) in high linkage disequilibrium have been found to be associated with tobacco abuse, terminal lung cancer, and other tobacco-related phenotypes12,13. The rs16969968 SNP is located in the α5 subunit of the nicotinic acetylcholine receptor gene and has been linked not only to tobacco, but also to opioid, alcohol, and cocaine use disorders. Presence of the rs16969968 A allele increases risk of use and disease related to tobacco12,13 and alcohol14 while also increasing risk of high severity dependence for opioid users15. A allele carriers, however, have a decreased risk of abuse of cocaine16.
The habenula indirectly decreases dopamine availability in downstream striatal areas. Activation of the habenula following negative events results in inhibition of midbrain dopaminergic cells, ultimately affecting dopamine levels in the striatum17. Given the role dopamine plays in reward, habenula-striatum connectivity is likely to be a major player in OUD. The habenula also affects the noradrenergic, serotonergic, and cholinergic systems via its anatomical connections to the locus coeruleus, raphe nucleus, and interpeduncular nucleus, respectively18. In addition to modulating the brain’s response to drugs of abuse8,19–21, the habenula regulates affect and has been implicated in mood disorders22 and anxiety-like behaviors23,24. In addition, the habenula is an important locus for pain signaling and sensitivity25, likely due to high µ-opioid receptor expression.
To determine the role of the habenula in opioid use and whether the α5 nicotinic acetylcholine receptor SNP rs16969968 is associated with the possible habenular mechanisms of opioid use, we studied the resting state functional connectivity (RSFC) between the habenula and the striatum using functional MRI (fMRI) in psychiatric inpatients with and without opioid use at The Menninger Clinic, a psychiatric clinic in Houston, TX.
Methods
Participants
Participants were psychiatric inpatients from a larger study at the Menninger Clinic in Houston, TX, USA (McNair Initiative for Neuroscience Discovery – Menninger/Baylor, or MIND-MB project26–28. All participation was voluntary with approximately 72% of eligible patients consenting to participate. Psychiatric disorder diagnoses were assessed using the research versions of the Structured Clinical Interview for DSM-IV Disorders (SCID-I/II). The SCID-I29 and SCID II30 were administered by master’s level researchers after reviewing pertinent psychiatric and psychosocial evaluations as well as consulting with the attending psychiatrist. Most inpatients showed co-occurring psychiatric illnesses, with the most common diagnoses being major depressive disorder (MDD), anxiety disorders (ANX), personality disorders (PD), and substance use disorders (SUD). We used the World Health Organization ASSIST scale, which contains 10 subscales for specific drugs31 to study opioid use. We used the ASSIST scale to divide patients into two groups: low versus moderate/high opioid use (called non-users versus users from now on). From an original group of 316 patients, two were removed for missing ASSIST scale data. Of the remaining 314 patients, nine patients were removed for missing or low quality MRI data, leaving a final sample of 305 patients (Table 1). Procedures were approved by the Baylor College of Medicine Institutional Review Board and the Research and Development Committee of the MEDVAMC. All patients had been drug-free at least since time of admission and were imaged an average of 4.3 days following clinic admission.
Table 1.
Demographic characteristics of the sample. No differences were statistically significant between the two groups (Chi square test).
| Opioid Non-Users |
Opioid Users |
|
|---|---|---|
| N | 254 | 51 |
| Gender (% male) | 53 | 75 |
| Age (years; SD) | 31.4; 12.4 | 28.1; 11.4 |
| MDD Spectrum (%) | 64 | 57 |
| Anxiety Spectrum (%) | 56 | 63 |
| Any PD (%) | 34 | 43 |
| rs16969968 GG (%) | 50 | 51 |
PD: personality disorders.
Imaging
Informed consent was obtained prior to scanning in a 3T Siemens Trio MR scanner in the Core for Advanced MR Imaging at Baylor College of Medicine in Houston, TX, USA. A 4.5 min structural MPRAGE sequence (TE = 2.66 ms, TR = 1200 ms, flip angle = 12°, 256 × 256 matrix, 160 one mm axial slices at 1×1×1 mm voxels) was collected, followed by a 5 min resting state scan (RSFC: TE = 40 ms, TR = 2 s, flip angle = 90, 3.4 × 3.4 × 4 mm voxels). During the resting state scan, subjects were instructed to relax. A large “X” was displayed on the screen during the resting state scan.
RSFC data were pre-processed using the CONN Functional Connectivity Toolbox32. The preprocessing pipeline consisted of realignment to the first time series image, slice-timing correction, structural segmentation and normalization to the MNI EPI template, functional normalization, ART-based outlier detection using default settings, and smoothing with an 8 mm full width at high maximum (FWHM) Gaussian smoothing kernel (although importantly, the habenula ROI was not smoothed).
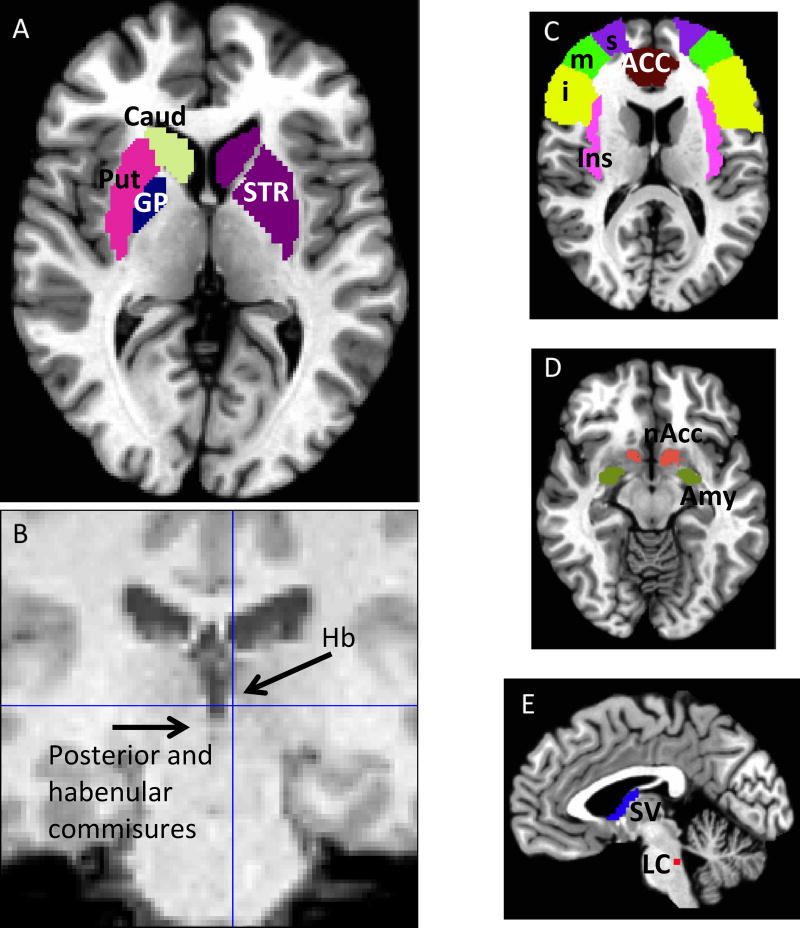
The main region of interest (ROI) was the striatum, defined as the sum of caudate, putamen, and globus pallidus. Additional ROIs for exploratory analyses included the inferior, medial, and superior frontal gyri (iFG, mFG, sFG), insula, nucleus accumbens, septum verum, locus coeruleus, amygdala, and anterior cingulate cortex (ACC) and were created in AFNI33 using the MNI atlas Figure 1A and C–E). Those regions were chosen because they are known to be important for habenular circuits18. Left and right habenula ROIs were generated for each subject using SPM 8 (http://imaging.mrc-cbu.cam.ac.uk/imaging). Functional images were downsampled to 3×3×3 to try to mitigate partial volume issues derived from the habenula size being comparable to voxel size. Each habenula was identified using brain landmarks and a 3×3×3 mm cube was manually placed for each individual subject (Figure 1B). RSFC data were then analyzed using the CONN functional connectivity toolbox. White matter, cerebrospinal fluid, realignment, and scrubbing were considered variables of no interest confounders.
Figure 1.
Regions of interest. A) Striatum (Str) (subdivided into caudate (Caud), putamen (Put) and globus pallidus (GP) on different colors on the left side). B) Habenula region of interest was one 3×3×3 cube on each side, manually located using the T1 and functional images as guide. C), D), E) Exploratory ROIs: C) superior, medial, inferior frontal gyri (s, m, i), insula (Ins), anterior cingulate cortex (ACC); D) nucleus accumbens (nAcc), amygdala (Amy); E) Septum verum (SV), Locus coeruleus (LC).
Statistics
Fisher’s z-transformed correlation coefficients between the different seeds for each subject were used in statistical analyses. ANOVA was used to test the primary outcome: RSFC between the habenula (separated in right and left) and the striatum. Next we subdivided the striatum into its constituent parts: right and left caudate, globus pallidus, and putamen. Because gender was found to be different between groups, we then used ANCOVA to control for the effect of gender after finding initial differences in habenula-whole striatum. ANCOVA was also used to further explore differences in RSFC activation in striatal sub-regions.
Since we were interested in the possible relationship between opioid use and habenular connectivity, the high co-use of different drugs of abuse was dealt with by stepwise regression. First, we separated patients into users and non-users of each drug. The WHO-ASSIST questionnaire31 was used to assess drug use. The ASSIST is divided into ten subcategories (tobacco, alcohol, cannabis, cocaine, amphetamine, inhalants, sedatives, hallucinogens, opioids, and other). With the exception of alcohol, each sub-category of drug was divided into groups of low (scores of 0–3) and pooled moderate to high (4+) use. Alcohol use was scored as low (0–10) and moderate to high (11+), as defined in the ASSIST questionnaire. Individual patients could be a “user” of more than one drug. We tested whether opioid use would predict the habenula-striatum RSFC when the effect of additional drugs was considered by entering each drug in a stepwise multiple regression analysis.
We then investigated possible genotype differences within the opioid users and non-users on the Right habenula/Left caudate RSFC. We used ANCOVA to test Right habenula/Left caudate RSFC after separating participants according to their rs16969968 genotype. We pooled those with an AG or AA genotype, since the phenotype seems to be dominant for the A allele12,13. In this case, we used population structure, despite the patients being ethnically homogenous (97% Caucasian), and sex as covariates. Ten ancestry informative markers (AIMs) were genotyped and used to calculate population structure. Data from the current participant sample were compared to the Centre d'Etude du Polymorphisme Humain–Human Genome Diversity Panel (CEPH-HGDP) samples (1,035 subjects of 51 populations) as previously described34. It has been shown before that 94.6% of the maximum informativity value is obtained using these ten AIMs35.
Finally, in a series of exploratory experiments we studied the connectivity between the habenula and several brain regions known or hypothesized to be related to habenular activity (iFG, mFG, sFG, insula, striatum, nucleus accumbens, septum verum, locus coeruleus, amygdala, and ACC). These data are presented as exploratory since we did not perform any corrections for multiple comparisons.
Results
Menninger inpatients show strong comorbidity
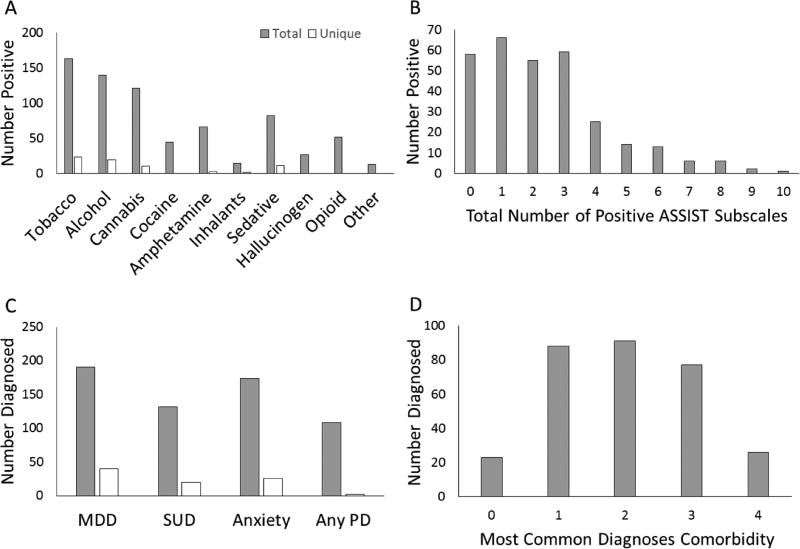
Figure 2A shows the number of patients who were positive (moderate or high) for use of each drug (solid bars) and the number of patients who were positive for only one individual drug (open bars). Eight percent of the patients were exclusively positive for tobacco. Interestingly, all opioid users also were positive for other drug use. Figure 2B shows the number of patients who were positive (according to ASSIST) for none, one, or more drugs of abuse. Figure 2C shows the most common diagnoses (MDD, SUD, ANX, and PD). Solid bars show the total numbers of patients with each diagnosis, while open bars show the number of patients with only one diagnosis. Figure 2D shows the number of patients with none, one, or more of the most common diagnoses (MDD, SUD, ANX, PD). Comorbidities of two or more diagnoses were present in more than half of patients. Additional diagnoses included psychotic and bipolar patients who were not diagnosed with MDD, SUD, ANX, or PD.
Figure 2.
Sample description. A) Number of patients that were positive for moderate/high use for each drug of abuse according to the ASSIST questionnaire. Both total positives (gray bars) and unique positives (white bars, patients who were positive for only one drug) are shown. B) Number of patient positive use of for none, one, or more drugs (according to ASSIST questionnaire). C) Number of patients diagnosed for each of the four most common DSM-IV diagnoses in the sample: Major depressive disorder (MDD), substance use disorder (SUD), anxiety disorders (ANX), and personality disorders (PD). Both total diagnosed (gray bars) and unique positives (white bars, patients who had only one diagnosis) are shown. D) Number of patient with none, one, or more diagnoses (counting only the four most common diagnoses, therefore zero are patients other than MDD, SUD, ANX or PD).
The right habenula shows increased striatal connectivity in opioid-dependent patients
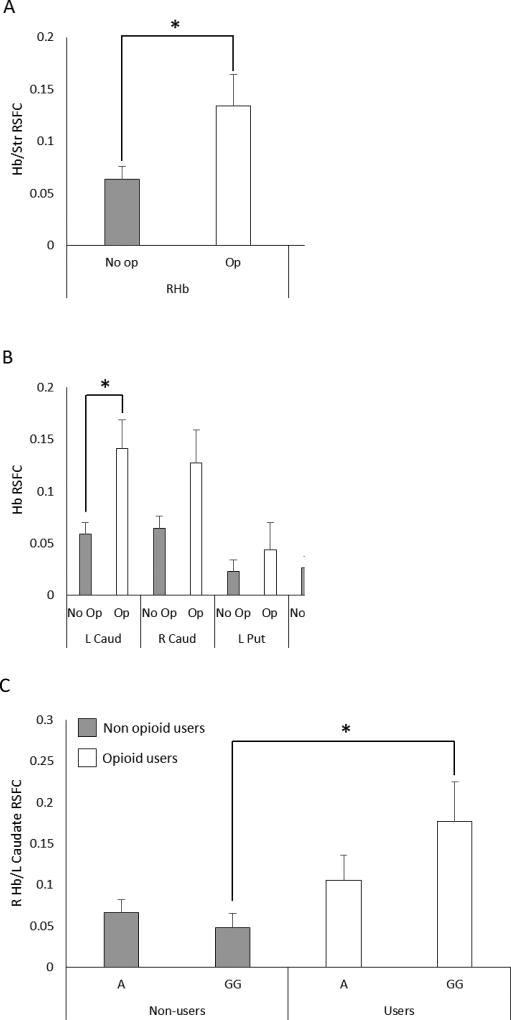
In our initial analysis, we found a significant RSFC difference between the Right habenula/striatumopioid (0.13 ± 0.03) and Right habenula/striatumnon-opioid (0.06 ± 0.01) (F(304,1) = 5.152, p<0.03) (Nopioid = 51, Nnon-opioid = 254) (primary outcome, Figure 3A). After controlling for gender, a nearly significant difference between the Right habenula/striatum remained (F(304),1) = 3.694, p = 0.056). No difference was found for the left habenula. No other drug showed a difference for the right habenula. For Right habenula/striatum, the Cohen’s d effect size for users vs. non-users was 0.17.
Figure 3.
Habenula/striatum resting state functional connectivity (Hb/Str RSFC) in opioid users and psychiatric patient controls. A) RSFC between the right and the left habenula (RHb, LHb) and the whole striatum (composed of caudate, putamen and globus pallidus). B) RSFC between the right and the left habenula and each sub-region of the striatum. No op: patients with no opioid use, Op: patients with opioid use. * p<0.05. C) Right habenula/left caudate connectivity and alpha 5 nicotinic acetylcholine receptor subunit genetic variant rs16969968 genotype. The low risk GG genotype shows increased connectivity in opioid-dependent patients compared to those carrying an A-allele and non-opioid users with GG genotype. No opi: patients with no opioid use, opi, patients with opioid use. * p<0.05 (LSD).
Given the initial result and strong trend toward significance after controlling for gender, we divided the striatum into its sub-components: right and left caudate, putamen, and globus pallidus. When we analyzed the RSFC between the right habenula and striatal sub-regions, we found that the Right habenula/Left caudate RSFC was significantly higher in those with opioid use (0.14 ± 0.03) than those without (0.06 ± 0.01) (F(304,1) = 6.73, p<0.01, Figure 3B). The right caudate showed a similar, but not statistically significant, difference. Both the putamen and the globus pallidus showed a trend in the same direction as the caudate. Finally, the Right habenula/Left caudate RSFC difference remained significant when we included the additional drugs in the ANCOVA models.
The nicotinic acetylcholine receptor variant rs16969968 moderates habenula/caudate connectivity in opioid users
Given the hypothesis that the CHRNA5 nicotinic acetylcholine receptor α5 subunit variant, rs16969968 may be a risk factor for higher opioid use, the sample was divided according to genotype into pooled AA or AG genotypes (high risk) and GG genotype (low risk). Figure 3C shows that opioid users carrying the GG genotype have higher Right habenula/Left caudate RSFC (0.18 ± 0.05) compared to non-opioid users carrying the GG genotype (non-opioid GG) (0.05 ± 0.02) (F(288,1) = 3.17, p<0.03). Post-hoc comparisons showed significant differences (p<0.05 LSD corrected) between both opioid GG vs. non-opioid GG and opioid GG vs. non-opioid AA/AG. There was no interaction between genotype and opioid use (F(285,1)=2.034, p=0.16). For Right Hb/Left caudate, the Cohen’s d effect size for users vs. non-users was 0.26. The effect sizes for GG vs. AG/AA were 0.49 (users) and 0.08 (non-users).
Exploratory analysis shows habenula/amygdala RSFC is possibly involved in opioid dependence
We studied a number of regions known or hypothesized to be part of the habenular circuit18: superior, medial, and inferior frontal gyrus (s, m, iFG); insula; anterior cingulate cortex (ACC); nucleus accumbens; amygdala (right and left); septum verum; and locus coeruleus. When opioid users were compared to non-opioid users, significant differences in RSFC were observed between the following brain regions and habenula (p<0.05, not corrected for multiple comparisons): Right habenula/Left Insula, Right habenula/Right insula, Right habenula/Left ACC, and Left habenula/Left sFG. Interestingly, the connectivity between the right and left habenula was also higher in opioid users than in non-opioid user patients. Both the habenula/insula and habenula/ACC RSFC data recapitulated the results found in the caudate, in that among those with a GG genotype, opioid-using participants had higher RSFC than non-using participants (not shown).
Discussion
RSFC between the habenula and sub-regions of the striatum, particularly the caudate, was greater in opioid-using participants than in non-users in our inpatient psychiatric sample. This increase was driven by patients with the CHRNA rs16969968 GG genotype, which is known to confer a lower risk of being a tobacco smoker in humans12,13, and less severe opioid dependence15. Less severe dependence and withdrawal symptoms when stopping opioids may make using opioids less aversive and consequently more attractive.
We focused on the habenula due to the role this small brain structure plays in negative reinforcement. Following unexpectedly rewarding events, dopamine release in the striatum is hypothesized to be an important learning signal. Activation of habenula following negative prediction errors, however, decreases dopamine release in the striatum17,36. This same habenular signaling pathway is likely to be modulated by drugs of abuse. We previously showed that habenular activity is, at least in mice, critical for the appearance of nicotine withdrawal7,8. In nicotine treated mice, both systemic mecamylamine (a non-specific nicotinic acetylcholine receptor blocker) and systemic naloxone (a μ-opioid receptor competitive antagonist) precipitate withdrawal symptoms3,4, pointing to cross-talk between the nicotinic and the opioid systems. Given the high habenular expression of both nicotinic receptors and μ-opioid receptors (Allen Brain Atlas, www.brain-map.org6) and the fact that nicotine withdrawal can be precipitated by microinjection of mecamylamine in the habenula8, we hypothesized that the habenula, and specifically nicotinic receptor activity within the habenula, may be associated with the effects of opioid use.
The A allele of rs16969968 in the CHRNA5 gene (nicotinic acetylcholine receptor α5 subunit) confers higher risk of tobacco abuse12,13, greater opioid dependence severity15 and alcohol use14. However, this same allele confers lower risk of cocaine abuse16. We therefore examined if opioid use alters RSFC between the habenula and dopaminergic striatal regions.
Higher habenula/striatum RSFC was observed in opioid-using participants, which could point to a role for the habenula in OUD. Specifically, the effect was observed mainly between the right habenula and the caudate. Habenular asymmetry is important for animal behaviors and has been observed in human healthy controls37. Although most habenular fibers are lateralized, as evidenced by diffusion tensor imaging (DTI) measurements38,39, the habenular commissure, which is very close to the posterior commissure (both are often observed as a single structure in MRI, see Figure 2E), likely transmits information between the hemispheres. Indeed, we observed increased RSFC between the right and the left habenula in opioid users. The RSFC between the habenula and the striatum is not likely to reflect direct anatomical connections, but be dependent on indirect connectivity (e.g. habenula → rostromedial tegmental nucleus → ventral tegmental area/substantia nigra compacta → striatum)38,39.
The increase in Right habenula/Left caudate RSFC was greater in patients with the GG CHRNA5 genotype (lower tobacco, opioid, and alcohol risk), compared to A-allele carriers. The current study suggests RSFC between habenula and striatal sub-regions is altered in opioid users, possibly the result of (or resulting from) differences in negative reinforcement among those who are dependent versus non-dependent as well as carriers of the GG versus the AA/AG CHRNA5 genotypes. In rodents, habenular expression of α5-containing nicotinic receptors is necessary for the appearance of nicotine’s negative effects, including withdrawal symptoms. For example, α5 subunit null mice self-administer enormous amounts of nicotine because they are sensitive to the positive (reward) but not to the negative (withdrawal) effects of nicotine use19. Since the rs16969968 A-allele may decrease nicotinic receptor activity40, it is tempting to speculate that the high risk for tobacco and opioid use associated with this allele may be due to decreased negative drug reinforcement. We postulate that high-risk opioid users feel less negative reinforcement, which may result in increased use. The functional significance of the ra16969968 variant is not completely clear but simplistically, it may work by decreasing the receptor’s response to nicotine13. In that sense, humans carrying the rs16969968 A variant may be a mild version of α5 nicotinic receptor knock-out mice. These animals self-administer enormous amounts of nicotine19 and show no nicotine withdrawal signs8.
Our data showed a possible significant functional difference between the right and the left habenula in the context of opioid abuse. Habenular asymmetry has been shown to be behaviorally important in vertebrates41. In humans, habenular RSFC has been shown to be asymmetric42,43. However, the functional significance of this asymmetry is not clear. Our data opens questions about the possibility that habenular functional asymmetry may play a role in addiction.
The current sample consisted of opioid-using and non-using participants within an inpatient population at The Menninger Clinic, rather than between opioid-using participants and healthy controls. By using other patients as controls and including comorbidities as covariates in the analysis, we attempted to control for the possible confounding effects of multiple diagnoses. In addition, by studying all consenting patients without excluding patients because of comorbidities when comparing user versus non-user participants, our sample arguably has higher ecological validity than the more traditional imaging approach, where a small sample of narrowly defined patients is compared to healthy controls. Our sample included comorbid diagnoses and abusers of multiple dugs. Despite this heterogeneity, opioid use was the one variable that influenced RSFC between habenula and striatal sub-regions.
Given the small size of the habenula, comparable to the size of the functional voxel we used, it is difficult to be confident that the observed results are indeed differences in habenular connectivity. The thalamus is in close proximity and much larger than the habenula, so thalamic contamination could influence the results. Although this is a possibility, we believe it is unlikely for a number of reasons. First, the habenula is known to be (indirectly) connected to the striatum, and habenula/striatum connectivity is known to affect reward processes17. Second, the habenula expresses nicotinic receptor alpha 5 subunit mRNA, which is absent in the thalamus11, and mu-opioid receptors6. Third, habenular resting state connectivity has been studied using a smaller voxel42, and the results are qualitatively very similar to ours: Although in this manuscript we focused only on the striatum and areas known or hypothesized to be important for the habenular reward-related circuit, high habenular connectivity with thalamus, anterior cingulate, posterior cingulate, superior frontal gyrus, and high "negative connectivity" with lingual gyrus and cuneus were observed both in the Ely manuscript and in our data (not shown). We believe that only a qualitative comparison between both sets of data should be done, since there are many differences between both manuscripts, including scanner, imaging parameters, data processing, and importantly sample characteristics.
A limitation of this work is the fact that we can't separate the medial from the lateral habenula at the resolution achieved in our MRI. The medial and the lateral habenula are functionally and transcriptionally very different44, and both the alpha 5 subunit of the nicotinic receptor and the mu-opioid receptor are expressed predominantly in the medial habenula6. Thus, we hypothesize that our results may be associated to medial habenular connectivity, but major improvements on equipment and/or analysis techniques are necessary to explore that question.
An additional limitation of this work is the use of a particularly short scan time. We collected resting state data over only 5 minutes, which may lead to high within-subject variability in our sample. Previous work has shown that a resting state of at least 7 minutes is preferable to reduce variability and strengthen the statistical power of our analyses45. A longer scan time of 7–10 minutes may significantly reduce within-subject variability.
Our initial finding of increased right habenula/striatum RSFC in opioid users compared to non-users was statistically not very strong. Furthermore, we did not correct for multiple comparisons of laterality and subregions of the striatum. Additional studies or replications of the analysis will be needed to strengthen these findings.
As with most human brain imaging studies, our results must be interpreted with care. The use of a heterogeneous sample of psychiatric inpatients diagnosed with several different disorders, without the use of a control sample limits this work. Although the study population provides insight into the role of the habenula in opioid use in a psychiatric population, it may be difficult to interpret the findings without a healthy sample of controls with which to compare results. Furthermore, despite the relatively large number of patients analyzed, false positives and hard to replicate data are common in these types of studies. In addition, we can only speculate about causality between habenular RSFC and opioid abuse. However, we believe that the inclusion of genotypic data in imaging research moves us closer to being able to infer causality in some studies, particularly for brain regions expressing high levels of particular genes of interest. Finally, all these patients were medicated at the time of MRI. Since we used controls with roughly the same diagnoses as the opioid users (see table 1), any effects of medications on imaging parameters are very likely to cancel each other.
In conclusion, we have shown that RSFC between the habenula and caudate is increased in opioid users and further mediated by a coding variant in the CHRNA α5 nicotinic acetylcholine subunit receptor rs16969968 A allele.
Acknowledgments
This work was supported in part by Merit Review Award # VHA5I01CX000994 from the U.S. Department of Veterans Affairs Clinical Sciences Research and Development Program. This work was also supported by the McNair Medical Institute; American Foundation for Suicide Prevention (SRG-2-125-14); NARSAD (19295); and the National Institute of health (NIDA DA026539, DA09167). This research was partially supported by the Menninger Clinic Foundation, The Brown Foundation, Inc. of Houston, Texas, and George and Mary Josephine Hamman Foundation, Vivian L. Smith Foundation; Robert J. Kleberg, Jr. & Helen C. Kleberg Foundation; Huffington Foundation; Ray C. Fish Foundation; the Gordon A. Cain Foundation; and the Toomim Family Fund. Drs. Frueh and Madan are McNair Scholars. This material is partly the result of work supported with resources and the use of facilities at the Michael E. DeBakey VA Medical Center, Houston, TX.
The authors thank the Core for Advanced MRI at Baylor College of Medicine, and Dr. Charles Neblett. The study follows the guidelines on good publication practices. The study sponsors were not involved in any aspect of research activities and did not approve the specific protocol or manuscript. Thus, the authors were independent from study sponsors in the context of research.
The authors alone are responsible for the content and writing of this paper.
Footnotes
Declaration of Interest
The authors report no conflicts of interest.
References
- 1.Prevention CfDCa. Wide-ranging Online Data for Epidemiologic Research (WONDER), Multiple-Cause-of-Death file, 2000–2014. 2015 http://www.cdc.gov/nchs/data/health_policy/AADR_drug_poisoning_involving_OA_Heroin_US_2000-2014.pdf.
- 2.Satel SL, Kosten TR, Schuckit MA, Fischman MW. Should protracted withdrawal from drugs be included in DSM-IV? The American journal of psychiatry. 1993 May;150(5):695–704. doi: 10.1176/ajp.150.5.695. [DOI] [PubMed] [Google Scholar]
- 3.Malin DH, Lake JR, Carter VA, et al. The nicotinic antagonist mecamylamine precipitates nicotine abstinence syndrome in the rat. Psychopharmacology. 1994 Jun;115(1–2):180–184. doi: 10.1007/BF02244770. [DOI] [PubMed] [Google Scholar]
- 4.Malin DH, Lake JR, Carter VA, Cunningham JS, Wilson OB. Naloxone precipitates nicotine abstinence syndrome in the rat. Psychopharmacology. 1993;112(2–3):339–342. doi: 10.1007/BF02244930. [DOI] [PubMed] [Google Scholar]
- 5.Jhamandas K, Sutak M, Bell S. Modification of precipitated morphine withdrawal syndrome by drugs affecting cholinergic mechanisms. European journal of pharmacology. 1973 Dec;24(3):296–305. doi: 10.1016/0014-2999(73)90153-2. [DOI] [PubMed] [Google Scholar]
- 6.Lein ES, Hawrylycz MJ, Ao N, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007 Jan 11;445(7124):168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 7.Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004 Nov 10;24(45):10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009 Mar 11;29(10):3014–3018. doi: 10.1523/JNEUROSCI.4934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salas R. Altered anxiety-related responses in mutant mice lacking the beta4 subunit of the nicotinic receptor. Neuroscience letters. 2003 Jul 16;23(15):6255–6263. doi: 10.1523/JNEUROSCI.23-15-06255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salas R, Cook KD, Bassetto L, De Biasi M. The alpha3 and beta4 nicotinic acetylcholine receptor subunits are necessary for nicotine-induced seizures and hypolocomotion in mice. Neuropharmacology. 2004 Sep;47(3):401–407. doi: 10.1016/j.neuropharm.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Salas R, Orr-Urtreger A, Broide RS, Beaudet A, Paylor R, De Biasi M. The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Molecular pharmacology. 2003 May;63(5):1059–1066. doi: 10.1124/mol.63.5.1059. [DOI] [PubMed] [Google Scholar]
- 12.Amos CI, Wu X, Broderick P, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nature genetics. 2008 May;40(5):616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bierut LJ, Stitzel JA, Wang JC, et al. Variants in nicotinic receptors and risk for nicotine dependence. The American journal of psychiatry. 2008 Sep;165(9):1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang JC, Grucza R, Cruchaga C, et al. Genetic variation in the CHRNA5 gene affects mRNA levels and is associated with risk for alcohol dependence. Molecular psychiatry. 2009 Apr 15;14(5):501–510. doi: 10.1038/mp.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erlich PM, Hoffman SN, Rukstalis M, et al. Nicotinic acetylcholine receptor genes on chromosome 15q25.1 are associated with nicotine and opioid dependence severity. Human genetics. 2010 Nov;128(5):491–499. doi: 10.1007/s00439-010-0876-6. [DOI] [PubMed] [Google Scholar]
- 16.Grucza RA, Wang JC, Stitzel JA, et al. A risk allele for nicotine dependence in CHRNA5 is a protective allele for cocaine dependence. Biological psychiatry. 2008 Dec 1;64(11):922–929. doi: 10.1016/j.biopsych.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007 Jun 28;447(7148):1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 18.Klemm WR. Habenular and interpeduncularis nuclei: shared components in multiple-function networks. Med Sci Monit. 2004 Nov;10(11):RA261–273. [PubMed] [Google Scholar]
- 19.Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011 Mar 31;471(7340):597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frahm S, Slimak MA, Ferrarese L, et al. Aversion to nicotine is regulated by the balanced activity of beta4 and alpha5 nicotinic receptor subunits in the medial habenula. Neuron. 2011 May 12;70(3):522–535. doi: 10.1016/j.neuron.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Velasquez KM, Molfese DL, Salas R. The role of the habenula in drug addiction. Frontiers in human neuroscience. 2014;8:174. doi: 10.3389/fnhum.2014.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sartorius A, Kiening KL, Kirsch P, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biological psychiatry. 2010 Jan 15;67(2):e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 23.Okamoto H, Aizawa H. Fear and anxiety regulation by conserved affective circuits. Neuron. 2013 May 8;78(3):411–413. doi: 10.1016/j.neuron.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 24.Pang X, Liu L, Ngolab J, et al. Habenula cholinergic neurons regulate anxiety during nicotine withdrawal via nicotinic acetylcholine receptors. Neuropharmacology. 2016 Mar 26;107:294–304. doi: 10.1016/j.neuropharm.2016.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shelton L, Becerra L, Borsook D. Unmasking the mysteries of the habenula in pain and analgesia. Progress in neurobiology. 2012 Feb;96(2):208–219. doi: 10.1016/j.pneurobio.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madan A, Fowler JC, Patriquin MA, et al. A novel approach to identifying a neuroimaging biomarker for patients with serious mental illness. J Neuropsychiatry Clin Neurosci. 2016 doi: 10.1176/appi.neuropsych.16090174. in press. [DOI] [PubMed] [Google Scholar]
- 27.Ridgewell C, Bray A, Curtis K, et al. Enhanced Olfactory Cortex Connectivity in a Patient With PTSD With Olfactory Hallucinations. J Neuropsychiatry Clin Neurosci. 2015;27(1–2) doi: 10.1176/appi.neuropsych.14070156. [DOI] [PubMed] [Google Scholar]
- 28.Viswanath H, Velasquez KM, Savjani R, et al. Interhemispheric insular and inferior frontal connectivity are associated with substance abuse in a psychiatric population. Neuropharmacology. 2015 Jan 12;92C:63–68. doi: 10.1016/j.neuropharm.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 29.First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured clinical interview for DSM-IV axis II personality disorders, (SCID-II) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 30.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. (SCID-I/P) 2002 [Google Scholar]
- 31.Humeniuk R, Ali R, Babor TF, et al. Validation of the Alcohol, Smoking And Substance Involvement Screening Test (ASSIST) Addiction. 2008 Jun;103(6):1039–1047. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- 32.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain connectivity. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 33.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996 Jun;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 34.Kosten TR, Domingo CB, Hamon SC, Nielsen DA. DBH gene as predictor of response in a cocaine vaccine clinical trial. Neuroscience letters. 2013 Apr 29;541:29–33. doi: 10.1016/j.neulet.2013.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lao O, van Duijn K, Kersbergen P, de Knijff P, Kayser M. Proportioning whole-genome single-nucleotide-polymorphism diversity for the identification of geographic population structure and genetic ancestry. American journal of human genetics. 2006 Apr;78(4):680–690. doi: 10.1086/501531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salas R, Baldwin P, de Biasi M, Montague PR. BOLD Responses to Negative Reward Prediction Errors in Human Habenula. Frontiers in human neuroscience. 2010;4:36. doi: 10.3389/fnhum.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hetu S, Luo Y, Saez I, D'Ardenne K, Lohrenz T, Montague PR. Asymmetry in functional connectivity of the human habenula revealed by high-resolution cardiac-gated resting state imaging. Human brain mapping. 2016 Apr 1; doi: 10.1002/hbm.23194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savjani RR, Velasquez KM, Thompson-Lake DG, et al. Characterizing white matter changes in cigarette smokers via diffusion tensor imaging. Drug and alcohol dependence. 2014 Dec 1;145:134–142. doi: 10.1016/j.drugalcdep.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Vadovicova K. Affective and cognitive prefrontal cortex projections to the lateral habenula in humans. Frontiers in human neuroscience. 2014;8:819. doi: 10.3389/fnhum.2014.00819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang JC, Cruchaga C, Saccone NL, et al. Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Human molecular genetics. 2009 Aug 15;18(16):3125–3135. doi: 10.1093/hmg/ddp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aizawa H. Habenula and the asymmetric development of the vertebrate brain. Anatomical science international. 2013 Jan;88(1):1–9. doi: 10.1007/s12565-012-0158-6. [DOI] [PubMed] [Google Scholar]
- 42.Ely BA, Xu J, Goodman WK, Lapidus KA, Gabbay V, Stern ER. Resting-state functional connectivity of the human habenula in healthy individuals: Associations with subclinical depression. Human brain mapping. 2016 Jul;37(7):2369–2384. doi: 10.1002/hbm.23179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hetu S, Luo Y, Saez I, D'Ardenne K, Lohrenz T, Montague PR. Asymmetry in functional connectivity of the human habenula revealed by high-resolution cardiac-gated resting state imaging. Human brain mapping. 2016 Jul;37(7):2602–2615. doi: 10.1002/hbm.23194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Viswanath H, Carter AQ, Baldwin PR, Molfese DL, Salas R. The medial habenula: still neglected. Frontiers in human neuroscience. 2013;7:931. doi: 10.3389/fnhum.2013.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomasi DG, Shokri-Kojori E, Volkow ND. Temporal Evolution of Brain Functional Connectivity Metrics: Could 7 Min of Rest be Enough? Cerebral cortex. 2016 Aug 12; doi: 10.1093/cercor/bhw227. [DOI] [PMC free article] [PubMed] [Google Scholar]