Key Points
The red blood cell metabolic signature of Down syndrome is identified
Trisomy 21 impacts red blood cell redox, amino acid, purine, and bile acid metabolism in an age- and sex-dependent fashion.
Abstract
Red blood cells (RBCs) are the most abundant cell in the human body. During their ∼120-day life span in the circulatory system, RBCs release oxygen to all human tissues while being exposed to tissue metabolic activity. Owing to the relative simplicity of their intrinsic metabolism and the abundance of metabolite transporters in RBC membranes, the metabolism of mature erythrocytes indirectly mirrors systemic metabolic homeostasis and its alterations as a function of physiological factors, such as aging. Trisomy 21 (T21), the etiological factor of Down syndrome (DS), has been shown to cause chronic autoinflammation, promoting alterations in RBC life span, size (macrocytosis), and redox homeostasis. Here, we provide the first mass spectrometry–based relative and absolute quantitative metabolomic description of human RBCs from volunteer disomic and trisomic donors (n = 97). The results indicate a widespread deregulation of T21 RBC metabolism, including significant intracellular accumulation of lactate, amino acids (except methionine), purine catabolites, glutathione metabolites, carboxylic acids, bile acids (especially conjugated ones), and acyl-conjugated carnitines. These changes may underlie some of the well-established comorbidities in DS. Finally, we identify sex- and/or T21-specific metabolic signatures of aging.
Visual Abstract
Introduction
Red blood cells (RBCs) are the most abundant host cell in the human body, accounting for ∼83% of the 30 trillion total host cells in an adult individual.1 Despite the lack of nuclei and organelles, the RBC proteome is complex enough (∼2800 proteins)2 to include a variety of receptors and transporters, which allow RBCs to uptake exogenous (eg, drugs)3 or endogenous metabolites4 as they circulate for ∼120 days in the bloodstream. RBC metabolism thus mirrors systemic metabolic homeostasis and its pathological derangements beyond traditional RBC-specific pathologies, such as sickle cell disease5 (eg, HbA1c and diabetes).1 More recently, it has been suggested that RBC metabolism might mirror acute (eg, hemorrhagic shock6 or physical activity7) and/or chronic metabolic aberrations, such as in aging4 and inflammation.7
Trisomy 21 (T21) is the etiological factor of Down syndrome (DS), the most common chromosomal abnormality in the human population, occurring in 1 in ∼700 live births in the United States.8 Individuals with DS display an altered disease spectrum, whereby they are protected from some medical conditions, but are highly predisposed to others. For example, rates of most solid malignancies are lower among people with DS, yet they are highly predisposed to develop Alzheimer’s disease (AD), several autoimmune disorders, leukemia, pulmonary hypertension, and various hearing and vision problems.8-13 Some of the more prevalent comorbidities could be explained by the altered dosage of expression of genes encoded on chromosome 21, such as amyloid protein or interferon receptors, factors likely contributing to the early onset of AD and autoimmune disorders.14 Of note, T21 has been associated with RBC alterations, such as increased cell size (macrocytosis),15 increased micronutrient levels (eg, copper and zinc),16 altered adenine17 and fatty acid/phospholipid levels,18,19 as well as impaired redox homeostasis, especially with respect to superoxide dismutase (coded by a gene on chromosome 21, 21q22.1), glutathione peroxidase, and lipid peroxidation activity.18,20 Of note, children with DS presented higher levels of plasma and RBC monounsaturated fatty acids and altered proportions of n-6 choline phosphoacylglycerol species in comparison with disomic siblings.18 Despite the common presence of the extra copy of chromosome 21 among people with DS, the clinical manifestations of comorbidities in DS (eg, cognitive impairment21) vary widely among individuals, which complicates cooccurring diagnoses and intervention. Factors such as sex would, for example, contribute at least to redox homeostasis by influencing dosage and activity of key antioxidant enzymes coded by genes on chromosome X, such as glucose 6-phosphate dehydrogenase.22 Within this framework, we investigated the RBC metabolome in disomic (D21) and T21 subjects as a function of sex and age.
Materials and methods
Extensive details are provided in supplemental Methods.
Sample collection and hematology
Blood was collected from D21 (n = 67; 23 male and 44 female; age range, 12-76.5 years) and T21 (n = 30; 13 male and 17 female; 0.5- 53.6 years old) study participants in accordance with the Declaration of Helsinki and stored at the Linda Crnic Institute for Down Syndrome within the framework of the Human Trisome Project Biobank (Colorado Multiple Institutional Review Board protocol #15-2170). Hematological parameters were measured through standard clinical hemochromocytometric assays at the University of Colorado Hospital (Aurora, CO).
UHPLC-MS metabolomics.
RBCs were processed via ultra high-performance liquid chromatography–mass spectrometry (UHPLC-MS)23 as extensively reported in supplemental Methods.
Statistics
Statistical analysis, including Student t test (disomic vs trisomic) or two-way analysis of variance (D21 vs T21, either male or female) as well as multivariate analysis were performed with GraphPad Prism version 5.0 and MetaboAnalyst version 3.0. Metabolic linkage analyses24 are extensively described in supplemental Methods.
Results
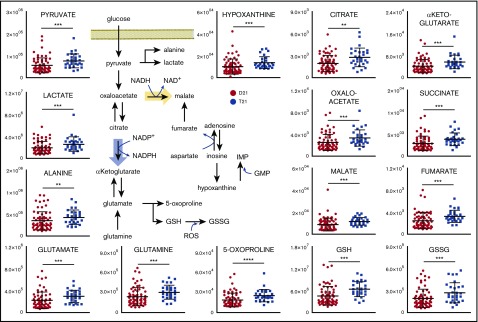
Consistent with the literature,15 RBCs from T21 individuals displayed larger mean cell volumes in comparison with D21 RBCs (mean ± standard deviation: 92.5 ± 5.5 fL and 92.7 ± 5.5 fL for D21 males and females, respectively; 97.1 ± 7.3 fL and 97.3 ± 5.1 fL for T21 males and females, respectively; P < .001, analysis of variance; supplemental Figure 1). The mean corpuscular hemoglobin was significantly higher in female T21 subjects than D21 counterparts, whereas other parameters showed sex and age dependency, but no significant association with T21. A total of 225 named metabolites (>20 000 features monitored) were quantified in RBCs from D21 and T21 samples (Figure 1A; supplemental Table 1). Multivariate analyses of the results were performed to determine significant differences between D21 and T21 subjects (Figure 1B). In addition, metabolic linkage analyses24 (supplemental Methods) revealed a widespread derangement of metabolic homeostasis (Figure 1B-C). Focusing on a subset of the total cohort only including age-matched subjects (n = 72, 43 D21 and 29 T21 from age 12-54 years; supplemental Figure 2), we show that metabolites significantly (P < .05, Student t test) affected by T21 were involved in glycolysis (pyruvate and lactate), purine catabolism (hypoxanthine), glutaminolysis and glutathione homeostasis (glutamine, glutamate, 5-oxoproline, and reduced glutathione [GSH] and oxidized glutathione [GSSG]), transamination products (alanine, α-ketoglutarate, and oxaloacetate) and other carboxylic acids (citrate, succinate, fumarate, and malate; Figure 2). Some of these changes (especially purines and carboxylic acids) were specific to female T21 donors (supplemental Figure 3). Additional significant increases were observed in T21 RBCs in the levels of amino acids and amino acid catabolites (supplemental Figures 4A and 5A), with the notable exception of methionine (supplemental Figure 4A and absolute quantification in supplemental Figure 4B). Some of the amino acid metabolic changes occurred in a sex-specific fashion (supplemental Figures 4B and 5C). Increases in acyl-conjugated fatty acids as well as conjugated and deconjugated bile acids were observed in T21 RBCs compared with D21, especially in women (supplemental Figures 2, 6, and 7).
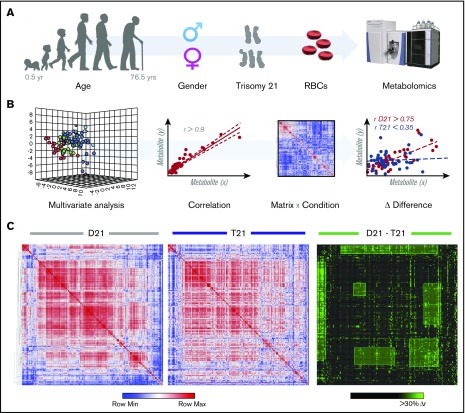
Figure 1.
Metabolomics analyses of T21 vs D21 RBCs. (A) UHPLC-MS metabolomic analyses were performed on RBCs from male or female disomic and trisomic volunteers. (B) Metabolite levels were correlated to each other and to age to identify variations (Δ|r| > 30%) in metabolite levels secondary to metabolic rewiring in DS, sex, or aging. The rationale behind this analysis is that, even though correlation does not necessarily imply causation, metabolites in the same pathway will show significant correlations to each other owing to biochemical constraints governing the kinetics of the rate-limiting enzymes from that pathway, a concept we previously referred to as the metabolic linkage.24 Phenotypic alterations that disrupted this fine tuning of the kinetics of specific metabolic pathways would be highlighted by a differential analysis of correlation of metabolites across conditions (ie, in this case D21 and T21). (C) Shown from left to right is an overview of correlations across metabolites in D21 and T21 subjects (blue to red = −1 < r < +1) as well as a highlight of correlations varying >30% between these 2 conditions (highlighted in green).
Figure 2.
DS promotes a significant metabolic reprogramming of RBCs. Levels of metabolites involved in glycolysis, glutathione homeostasis, carboxylic acids, and purine metabolism are significantly increased in RBCs from individuals with T21 (dark blue) when compared with age-matched disomic individuals (red) (y-axis = arbitrary units). **P < .01; ***P < .001; ****P < .0001, Student t test.
Candidate metabolic markers of aging were identified in the total population and T21 subjects in a sex-specific fashion (supplemental Figure 7).
Discussion
The present study represents the first report of global RBC metabolic alterations in D21 and T21 as a function of sex or age. Age-related signatures were exacerbated in individuals with DS, indicative of accelerated aging. T21 RBCs had increased levels of redox markers (eg, glutathione homeostasis), consistent with previous reports on antioxidant enzyme activity.18,19 Increases in purine oxidation expand on previous reports of deranged adenine nucleoside/energy metabolism in T21 RBCs17 and may relate to the herein observed signature in carboxylates accumulating in T21 RBCs, potentially explained by compensatory hyperactivation of NADPH-generating isoforms of Krebs cycle enzymes (eg, malate and isocitrate dehydrogenase 1, which are both identified and active in mature erythrocytes)23 or purine salvage reactions (eg, fumarate, to the extent that these reactions are still active in mature erythrocytes). Increases in RBC levels of pyruvate and lactate as well as acyl-conjugated fatty acids may relate to diabetes and obesity, which are recurring comorbidities in the T21 population,13 complementing previous lipidomics reports on n-6 fatty acid dysregulation in RBCs from children with T21.18 RBC metabolic markers of T21 included purine catabolites like hypoxanthine as well as immunomodulatory/inflammation-related metabolites like (1) conjugated bile acids (markers of alteration to the microbiome),25 (2) the carboxylic acids fumarate and succinate,7,26 and (3) the tryptophan oxidation product kynurenine,27 linking the present observations with the autoimmune and inflammatory comorbidities of DS.13,14 Increases in plasma purines, specifically adenosine and its deaminated byproducts, have been previously reported in individuals with DS, where increased adenosine deaminase activity and hyperuricemia result from excessive purine synthesis secondary to the overexpression of genes in the purine de novo synthesis pathway GARS-AIRS-GART located on chromosome 21.28,29
Although merely speculative at this stage, it is fascinating to highlight the observed decreases in methionine levels of T21 RBCs and previous reports on the role of this metabolite in cognitive impairment, such as AD.30 Of note, in D21 individuals, excess plasma methionine or methionine supplementation are noted to promote the onset of AD,30,31 an observation that may not hold true in T21 subjects. Because cystathionine-β-synthase is coded by a gene on chromosome 21,32 increased expression of this enzyme impacts homocysteine metabolism by impairing folate-dependent methionine resynthesis and availability of active folate (folate trap),32 an etiological factor of macrocytosis and shorter survival of RBCs,15 the richest cell by iron content in the human body. Notably, lower levels of essential amino acids and, specifically, significantly lower levels of methionine have been previously observed in the plasma of age- and sex-matched individuals with DS,33 suggesting a compromised homocysteine metabolism in this population.33 Faster RBC turnover may impact iron homeostasis and affect iron-dependent nonapoptotic cell death (ferroptosis), an etiological factor of AD,34 suggesting a potential therapeutic window, especially at an early age when the brain is still plastic, through dietary intervention with folinic acid, methyl-B12, thymidine, dimethylglycine, and/or methionine supplementation32 or iron metabolism management in children and adults with DS.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Rani Powers for her support with the logistics of sample delivery from the biobank to the analytical laboratories and acknowledge support from Tom Blumenthal. The authors also thank the volunteers and their families, who made this study possible.
This work was supported by the Linda Crnic Institute for Down Syndrome, the Global Down Syndrome Foundation, the Anna and John J. Sie Foundation, the University of Colorado School of Medicine, and a Shared Instrument grant by the National Institutes of Health (S10OD021641) (K.C.H. and A.D.). A.D. is a recipient of the Webb-Waring Biomedical Research Award sponsored by the Boettcher Foundation.
Authorship
Contribution: T.N., K.C.H., and A.D. designed the experiments; R.C.-H., C.Z., and J.A.R. performed sample extraction and UHPLC-MS analyses; R.C.-H. analyzed the data and prepared the figures; R.C.-H. and A.D. finalized the manuscript figures; A.D. wrote the first draft of the manuscript; A.R., E.B., R.G., K.S., and J.M.E. are members of the Crnic Institute’s Human Trisome Project, which recruited all participants, collected blood samples, purified RBCs, and annotated all the participants' data and associated clinical metadata; and all authors critically contributed to the finalization of the manuscript.
Conflict-of-interest disclosure: T.N., K.C.H., and A.D. are part of Omix Technologies, Inc. A.D. is a consultant for New Health Sciences, Inc. The remaining authors declare no competing financial interests.
Correspondence: Angelo D’Alessandro, Department of Biochemistry and Molecular Genetics, University of Colorado Denver–Anschutz Medical Campus, 12801 East 17th Ave, L18-9118, Aurora, CO, 80045; e-mail: angelo.dalessandro@ucdenver.edu.
References
- 1.D’Alessandro A, Zolla L. Proteomic analysis of red blood cells and the potential for the clinic: what have we learned so far? Expert Rev Proteomics. 2017;14(3):243-252. [DOI] [PubMed] [Google Scholar]
- 2.Wilson MC, Trakarnsanga K, Heesom KJ, et al. . Comparison of the proteome of adult and cord erythroid cells, and changes in the proteome following reticulocyte maturation. Mol Cell Proteomics. 2016;15(6):1938-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bordbar A, Jamshidi N, Palsson BO. iAB-RBC-283: A proteomically derived knowledge-base of erythrocyte metabolism that can be used to simulate its physiological and patho-physiological states. BMC Syst Biol. 2011;5(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaleckis R, Murakami I, Takada J, Kondoh H, Yanagida M. Individual variability in human blood metabolites identifies age-related differences. Proc Natl Acad Sci USA. 2016;113(16):4252-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Dai Y, Wen J, et al. . Detrimental effects of adenosine signaling in sickle cell disease. Nat Med. 2011;17(1):79-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reisz JA, Slaughter AL, Culp-Hill R, et al. . Red blood cells in hemorrhagic shock: a critical role for glutaminolysis in fueling alanine transamination in rats. Blood Adv. 2017;1(17):1296-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavalli G, Justice JN, Boyle KE, et al. . Interleukin 37 reverses the metabolic cost of inflammation, increases oxidative respiration, and improves exercise tolerance. Proc Natl Acad Sci USA. 2017;114(9):2313-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander M, Petri H, Ding Y, Wandel C, Khwaja O, Foskett N. Morbidity and medication in a large population of individuals with Down syndrome compared to the general population. Dev Med Child Neurol. 2016;58(3):246-254. [DOI] [PubMed] [Google Scholar]
- 9.Ramia M, Musharrafieh U, Khaddage W, Sabri A. Revisiting Down syndrome from the ENT perspective: review of literature and recommendations. Otorhinolaryngol Head Neck Surg. 2014;271(5):863-869. [DOI] [PubMed] [Google Scholar]
- 10.Sobey CG, Judkins CP, Sundararajan V, Phan TG, Drummond GR, Srikanth VK. Risk of major cardiovascular events in people with Down syndrome. PLoS One. 2015;10(9):e0137093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasle H, Friedman JM, Olsen JH, Rasmussen SA. Low risk of solid tumors in persons with Down syndrome. Genet Med. 2016;18(11):1151-1157. [DOI] [PubMed] [Google Scholar]
- 12.Bull MJ; Committee on Genetics. Health supervision for children with Down syndrome. Pediatrics. 2011;128(2):393-406. [DOI] [PubMed] [Google Scholar]
- 13.van Schrojenstein Lantman-de Valk HM, Haveman MJ, Crebolder HF. Comorbidity in people with Down’s syndrome: a criteria-based analysis. J Intellect Disabil Res. 1996;40(Pt 5):385-399. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan KD, Lewis HC, Hill AA, et al. . Trisomy 21 consistently activates the interferon response. eLife. 2016;5:e16220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wachtel TJ, Pueschel SM. Macrocytosis in Down syndrome. Am J Ment Retard. 1991;95(4):417-420. [PubMed] [Google Scholar]
- 16.Saghazadeh A, Mahmoudi M, Dehghani Ashkezari A, Oliaie Rezaie N, Rezaei N. Systematic review and meta-analysis shows a specific micronutrient profile in people with Down Syndrome: Lower blood calcium, selenium and zinc, higher red blood cell copper and zinc, and higher salivary calcium and sodium. PLoS One. 2017;12(4):e0175437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stocchi V, Magnani M, Cucchiarini L, Novelli G, Dallapiccola B. Red blood cell adenine nucleotides abnormalities in Down syndrome. Am J Med Genet. 1985;20(1):131-135. [DOI] [PubMed] [Google Scholar]
- 18.Bueno AA, Brand A, Neville MM, Lehane C, Brierley N, Crawford MA. Erythrocyte phospholipid molecular species and fatty acids of Down syndrome children compared with non-affected siblings. Br J Nutr. 2015;113(1):72-81. [DOI] [PubMed] [Google Scholar]
- 19.Pastor MC, Sierra C, Doladé M, et al. . Antioxidant enzymes and fatty acid status in erythrocytes of Down’s syndrome patients. Clin Chem. 1998;44(5):924-929. [PubMed] [Google Scholar]
- 20.Brooksbank BW, Balazs R. Superoxide dismutase, glutathione peroxidase and lipoperoxidation in Down’s syndrome fetal brain. Brain Res. 1984;318(1):37-44. [DOI] [PubMed] [Google Scholar]
- 21.de Sola S, de la Torre R, Sánchez-Benavides G, et al. ; TESDAD Study Group. A new cognitive evaluation battery for Down syndrome and its relevance for clinical trials. Front Psychol. 2015;6:708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tzounakas VL, Kriebardis AG, Georgatzakou HT, et al. . Glucose 6-phosphate dehydrogenase deficient subjects may be better “storers” than donors of red blood cells. Free Radic Biol Med. 2016;96:152-165. [DOI] [PubMed] [Google Scholar]
- 23.D’Alessandro A, Nemkov T, Yoshida T, Bordbar A, Palsson BO, Hansen KC. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion. 2017;57(2):325-336. [DOI] [PubMed] [Google Scholar]
- 24.D’alessandro A, Nemkov T, Reisz J, Dzieciatkowska M, Wither MJ, Hansen KC. Omics markers of the red cell storage lesion and metabolic linkage. Blood Transfus. 2017;15(2):137-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014;30(3):332-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tannahill GM, Curtis AM, Adamik J, et al. . Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496(7444):238-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mondanelli G, Bianchi R, Pallotta MT, et al. . A relay pathway between arginine and tryptophan metabolism confers immunosuppressive properties on dendritic cells. Immunity. 2017;46(2):233-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puukka R, Puukka M, Perkkilä L, Kouvalainen K. Levels of some purine metabolizing enzymes in lymphocytes from patients with Down’s syndrome. Biochem Med Metab Biol. 1986;36(1):45-50. [DOI] [PubMed] [Google Scholar]
- 29.Peeters MA, Megarbane A, Cattaneo F, Rethore MO, Lejeune J. Differences in purine metabolism in patients with Down’s syndrome. J Intellect Disabil Res. 1993;37(Pt 6):491-505. [DOI] [PubMed] [Google Scholar]
- 30.Tapia-Rojas C, Lindsay CB, Montecinos-Oliva C, Arrazola MS, Retamales RM, Bunout D, Hirsch S, Inestrosa NC. Is L-methionine a trigger factor for Alzheimer's-like neurodegeneration?: Changes in Aβ oligomers, tau phosphorylation, synaptic proteins, Wnt signaling and behavioral impairment in wild-type mice. Mol Neurodegener. 2015;10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffin JW, Bradshaw PC. Amino acid catabolism in Alzheimer's disease brain: friend or foe? Oxid Med Cell Longev. 2017;2017:5472792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pogribna M, Melnyk S, Pogribny I, Chango A, Yi P, James SJ. Homocysteine metabolism in children with Down syndrome: in vitro modulation. Am J Hum Genet. 2001;69(1):88-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coppus AW, Fekkes D, Verhoeven WMA, et al. . Plasma amino acids and neopterin in healthy persons with Down’s syndrome. J. Neural Transm. 2007;114(5):1041-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dixon SJ, Lemberg KM, Lamprecht MR, et al. . Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.