Abstract
Dampness affects a substantial percentage of homes and is associated with increased risk of respiratory ailments; yet the effects of dampness on indoor chemistry are largely unknown. We hypothesize that the presence of water-soluble gases and their aqueous processing alters the chemical composition of indoor air and thereby affects inhalation and dermal exposures in damp homes.
Herein, we use the existing literature and new measurements to examine the plausibility of this hypothesis, summarize existing evidence, and identify key knowledge gaps. While measurements of indoor volatile organic compounds (VOCs) are abundant, measurements of water-soluble organic gases (WSOGs) are not. We found that concentrations of total WSOGs were, on average, 15 times higher inside homes than immediately outside (N=13). We provide insights into WSOG compounds likely to be present indoors using peer-reviewed literature and insights from atmospheric chemistry. Finally, we discuss types of aqueous chemistry that may occur on indoor surfaces and speculate how this chemistry could affect indoor exposures. Liquid water quantities, identities of water-soluble compounds, the dominant chemistry, and fate of aqueous products are poorly understood. These limitations hamper our ability to determine the effects of aqueous indoor chemistry on dermal and inhalation exposures in damp homes.
Keywords: Damp homes, Interfacial chemistry, Aqueous chemistry, Water-soluble organic compounds, OVOC, Human exposure
Introduction
Dampness in buildings is common in the United States, with estimates ranging from 18 to 50% percent of buildings affected, where buildings were defined as “damp” based on observation of standing water, water damaged materials, presence of mold and/or high measured relative humidity (RH)1,2. Dampness and high RH indoors can be caused by high humidity outdoors, water leakage into buildings, leakage of pipes, flooding, water infiltration into building materials, and moisture resulting from human activities such as cooking and bathing 3. Dampness can also occur as a result of water vapor condensation in the building structure or on surfaces indoors. Building dampness is associated with increased risk of respiratory symptoms such as cough, wheeze, asthma, and respiratory infections 4. Dampness is also associated with mold exposures. Two scientific reviews report that dampness or mold exposures result in increased respiratory symptoms with a range of odds ratios from 1.30–1.75 and 1.4–2.2, respectively 3,4. Mold and mildew indoors and its health effects have been studied extensively. However, to date, mold and mildew have only been able to partially explain the associations between damp buildings and adverse health outcomes, and causal linkages remain weak 4. Certainly, there could be additional chemical, physical, or biological factors that contribute to adverse health in damp spaces.
We hypothesize that aqueous uptake and processing of water-soluble gases in damp homes alters the chemical composition of indoor air and can affect dermal and inhalation exposure. If true, aqueous chemistry indoors could plausibly affect health in damp homes. Aqueous chemistry outdoors (in clouds, fogs, and wet particles) has been demonstrated to alter the concentrations and composition of outdoor gases and particles;5–8 aqueous chemistry may also occur indoors in damp buildings where the surface area for water condensation is large. In fact, the observation of HONO production in residences 9,10 provides definitive evidence that indoor liquid water concentrations are sufficient, at least in certain homes/times, to drive aqueous chemistry because HONO is produced on surfaces (from NO2 hydrolysis) only in the presence of liquid water11.
For aqueous processing indoors to be an important factor for indoor air chemical composition, liquid water and water-soluble gases must be present indoors. When dampness occurs in homes, liquid water can be present in and on indoor surfaces as well as in hygroscopic airborne particles. Knowledge about water-soluble gases indoors is limited, although nonpolar volatile organic compounds (VOCs) have been extensively measured. VOCs are frequently measured at much higher concentrations indoors than outdoors. A systematic review of indoor and outdoor VOC measurements found indoor/outdoor residential VOC ratios range from 1 to 150 12. One study that measured 30 VOCs using GC-FID inside and outside homes in Helsinki, Finland, found indoor/outdoor VOC ratios of measured compounds ranged from 0.25 to 55 with an average ratio of 6 13 (compounds with higher concentrations outside homes tended to be associated with vehicle emissions). We speculate that, similar to non-polar VOCs, polar and water-soluble VOCs are present at higher concentrations indoors than outdoors. While measurements of formaldehyde are relatively common and other targeted polar VOCs are sometimes measured indoors 14–16, measurement of polar and water-soluble VOCs (also called oxygenated VOCs, OVOCs; or water-soluble organic gases, WSOGs) is, in general, more challenging.
WSOGs are emitted from indoor sources and formed through oxidation. They will be taken up into liquid water, when present, and subsequently react. Thus, aqueous chemistry could affect exposure by acting as a sink for certain water-soluble gases in indoor air and a source of other volatile products (altering inhalation exposure) and condensed phase products (altering particle inhalation and dermal exposure).
This paper provides insights into WSOGs likely to be in residential indoor air and examines the potential for aqueous chemistry indoors to alter the chemical composition of this air, with the motivation of further understanding dermal and inhalation exposure to gaseous and particulate species in homes. We demonstrate that water-soluble organic gases are elevated in residential indoor environments. We make use of the literature to document water-soluble compounds measured or likely to be present in homes. Finally, we discuss knowledge and knowledge gaps concerning indoor aqueous chemistry and its implications, and we make recommendations for future research.
Approach
First, we address the potential importance of indoor water-soluble organic gases (WSOGs). Because, to our knowledge, measurements of total WSOGs have not previously been made indoors, we conducted these measurements inside 13 homes and directly outside for comparison. We then provide a list of water-soluble gas-phase compounds that have been measured in residential indoor air. Next, we use knowledge about emissions, non-polar VOC concentrations, and oxidation chemistry to identify additional water-soluble organic compounds that are likely to be present indoors. This is followed by presentation of the case for indoor aqueous chemistry. We familiarize the reader with outdoor atmospheric aqueous chemistry and speculate about wet chemistry on indoor surfaces. Finally, we discuss the potential for aqueous chemistry to alter indoor exposures and summarize major knowledge gaps.
Indoor and outdoor concentrations of total WSOG were determined by collecting the ambient mix of water-soluble gases into water using Cofer scrubbers 17, also called mist chambers, and measuring the total carbon collected. While it is possible to target specific water-soluble compounds by optimizing analytical methods and through the use of authentic standards, such an approach would likely only provide a small fraction of total. The goal of these measurements is to determine whether or not total WSOG is enriched indoors compared to outdoors.
For this purpose, field samples were collected from a convenience sample of 3 homes in central New Jersey and 10 homes in the Triangle region of North Carolina between June and October 2015; each home was sampled once. WSOGs were collected from particle-filtered air (particles filtered with pre-baked quartz fiber filters (Pall, 47 mm)) using 2 mist chambers 17 in parallel sampling from a common area of the home and 2 simultaneously sampling directly outside the home. Each mist chamber scrubbed the air with an air flow rate of 25 L/min and water collection volume of 25 mL (ultra-pure deionized water, conductivity = 17.8 ±0.5 MΩ) for a duration of two hours, twice consecutively. Indoor and outdoor samples were composited separately for each home, analyzed for total organic carbon using a Shimadzu 5000a TOC analyzer as described previously (18,19), and the remaining volume was frozen for future research.
In order to gain additional insights into WSOGs in indoor air, a literature search was performed on September 14–15, 2015 using the Scopus database. The search terms using the Boolean operators “and” and “or” were:
-
One of the following: Volatile, semi-volatile, gas phase, gas-phase, organic, organic compounds, organic gases, aldehydes, aromatic hydrocarbons, terpenes, alcohols, alkanes, alkenes, epoxides, phenols, ethers, carboxylic acids, amines, pesticides, termiticides, insecticides, flame retardants, cooking emissions, cleaning, repellent, deodorant, deodorizer, PBDE, or PAH;
And: indoor or indoors
And: air, gas phase or gas-phase;
And: concentration or concentrations;
And: measured or calculated.
The search yielded 517 results. From among these, 37 articles provided concentration data on gas-phase species measured in homes in the United States. Compounds with Henry’s law constants greater than 1 M/atm were considered water-soluble, as recommended by Sander (2015)20. Potential aqueous phase concentrations were calculated from typical indoor air concentrations reported in the literature and Henry’s law constants. This provides a “first assessment” of compounds that could be important to indoor aqueous chemistry. (Note that the magnitude of the aqueous reservoir depends also on the liquid water volume.)
Although the literature search yielded an extensive list of WSOGs in indoor air, we expect that there are many more water-soluble gases present in indoor air that have not been measured, because advances in measurement methods for oxidized compounds are a recent phenomenon. Thus, we made use of chemical insights from the outdoor (atmospheric) chemistry literature (e.g., smog chamber studies) to propose additional types of oxidized compounds that we expect to be present indoors. Knowledge of indoor source emissions, including cooking, microbial activity, and occupant skin off-gassing provided a few additional WSOGs.
Finally, we used knowledge about outdoor aqueous chemistry and indoor spaces to speculate on how indoor aqueous chemistry may affect indoor air composition and therefore affect exposure.
Total water-soluble organic gases measured during this study
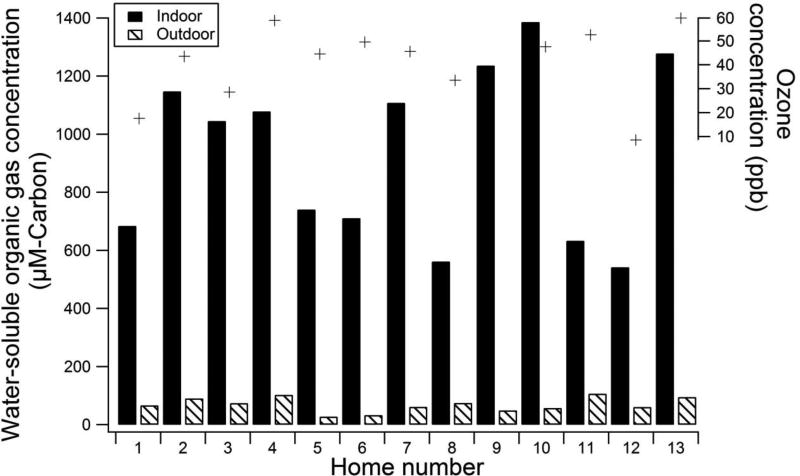
Figure 1 compares the concentrations of organic carbon collected concurrently in the mist chamber samples inside and immediately outside the 13 homes. Indoor samples contained 540–1,400 µM-C, with mean indoor WSOG concentration of 940 µM-C, standard deviation of 300 µM-C and the 95% confidence interval of 770 – 1,100 µM-C. In contrast, concurrently-collected samples from residential outdoor air contained only 28 – 110 µM-C, with a mean of 69 µM-C, standard deviation of 25 µM-C, and 95% confidence interval of 56 – 83 µM-C.
Figure 1.
Total organic carbon (µM-C) in aqueous mist chamber samples collected concurrently from particle-filtered ambient air inside (black bars) and directly outside (striped bars) 13 New Jersey and North Carolina homes. Indoor concentrations were significantly higher (paired t-test, α=0.05, p=0.00000018) than outdoor concentrations. (Source blanks were below the detection limit and field blanks = 7±2.5 µM-C. Neither were not subtracted from the sample totals) Nearby outdoor ozone concentrations for each home are plotted directly above each pair of bars. For homes in New Jersey and North Carolina, outdoor ozone concentrations were reported from the New Jersey Department of Environmental Protection Rider University Campus and the North Carolina Department of Environmental Quality Durham Armory ambient air quality sites respectively (ozone was not available for home 9). Indoor relative humidity ranged from 53 to 67%, indoor temperatures ranged from 20 to 26 °C, and the absolute difference between indoor and outdoor temperatures ranged from 0.5 to 8 °C.
Indoor concentrations of WSOG were, on average, 15 times higher than outdoor concentrations (Figure 1), suggesting that the vast majority of indoor WSOG is emitted or formed indoors. A lower-bound estimate of the percentage of indoor WSOG that is of indoor origin can be calculated by assuming that all water-soluble organic gases that originated outdoors penetrate and persist indoors with 100% efficiency (100% penetration, no indoor losses). Using this lower-bound assumption, the mean, median and range of indoor-origin WSOG are 86%, 92%, and 83% to 98%. Thus, the indoor residential environment is substantially enriched in water-soluble organic gases and this WSOG is predominantly emitted or formed indoors.
Comparison of indoor and outdoor organic carbon concurrently collected under identical conditions enables a quantitative comparison of WSOG indoors relative to outdoors. However, to calculate gas phase WSOG concentrations (µg-C/m3 air) rather than the concentration of WSOG in the collected aqueous samples (µg-C/L water) would require the average Henry’s Law constant for the mixture. In this work, sample collection times were long to increase organic compound concentrations in aqueous samples for more detailed chemical characterization and laboratory experiments. At long collection times, mist chamber water approaches Henry’s Law equilibrium with the sampled air. Thus, for long collection times, calculation of WSOG concentrations in µg-C/m3 requires an estimate of the average Henry’s Law constant of the collected WSOG mixture. To provide an example, if the average Henry’s Law concentration of the mixture were 3000 M/atm (the same as formaldehyde), the average indoor WSOG concentration would be 350 ppbv (470 µg/m3). However, Henry’s Law constants vary tremendously. For example, the Henry’s Law constant is 4×106 M/atm for glyoxal and 20 M/atm for 2-butanone20, both water-soluble gases found in air. Thus since the composition of the WSOG mixture is unknown, the error in such a calculation could potentially be large. Despite this limitation, the knowledge that WSOG concentrations indoors are substantially greater than outdoors, and thus indoor WSOG sources/production is substantial, constitutes a major step forward in understanding indoor WSOG.
Below we use knowledge from the existing literature to infer what water-soluble organic gases are likely to be available to participate in indoor aqueous chemistry.
Water-soluble organic gases in indoor air
Compounds previously measured
Table 1 shows water-soluble organic compounds identified in the literature search that have a potential aqueous concentration equal to or greater than 10−3 µM, organized by potential aqueous-phase concentrations. Concentrations are the product of the Henry’s law constant20 and typical gas-phase concentration.
Table 1.
Water-soluble organic gases measured inside homes in the United States
| Compound | Structures | Henry’s law constant (M/ atm) |
Mean (and range) gas phase concentrations (ppb) |
Mean (and range) potential aqueous concentration (µM) |
References |
|---|---|---|---|---|---|
| Glyoxal |
|
4,000,000 | 1 (5 – 1.8) | 4,300 (1,900 – 7,500) | 21 |
| Formaldehyde |
|
3,000 | 22 (4 – 100) | 66 (10 – 300) | 14,15,22–25 |
| Methylglyoxal |
|
32,000 | 0.9 (0.4 – 1.6) | 30 (10 – 50) | 14 |
| Trichloroacetic acid (TCA) |
|
74,000 | 0.07 (NA) | 5.2 | 26 |
| Bisphenol A (BPA) |
|
25,000,000 | 0.00015 (BDL – 0.02) | 3.8 (≤ 500) | 27,28 |
| Dibutyl phthalate (DBP) |
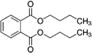
|
1,000 | (0.018 – 0.11) | (1.3 – 8.1) | 27 |
| Acetone |
|
31 | 16 (0.4 – 280) | 0.5(0.01 – 9) | 14–16,22–24,29 |
| Acetaldehyde |
|
15 | 7.4 (0.7 – 23) | 0.11 (0.01 – 0.3) | 14–16,21,23,24 |
| 2-Butanone (methyl-ethyl-ketone) |
|
18 | 2 (BDL – 8.4) | 0.035 (≤ 0.15) | 15,23,26,30 |
| Propionaldehyde (propanal) |
|
13 | 1 (BDL – 7.3) | 0.012 (≤ 0.1) | 14–16,23 |
| Crotonaldehyde |
|
60 | 0.15 (BDL – 1.1) | 0.012 (≤ 0.07) | 14 |
| Benzaldehyde |
|
20 | 0.5 (BDL – 1.4) | 0.010 (≤ 0.03) | 14–16,23,24 |
| Valeraldehyde (pentanal, pentanaldehyde) |
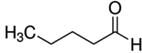
|
6.1 | 1 (BDL – 23) | 0.007 (≤ 0.14) | 15,16,23,24 |
| Hexaldehyde (hexanal) |
|
5.1 | 1.2 (BDL – 8.5) | 0.006 (≤ 0.0078) | 14–16,23,24 |
| Chlordane |
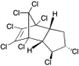
|
18 | 0.26 (BDL – 5.7) | 0.0047 (≤ 0.1) | 25,27 |
| Tetrahydrofuran |
|
10 | 0.44 (NA – 83) | 0.0044 (≤ 0.84) | 26 |
| Butyraldehyde (butanal) |
|
6.5 | 0.66 (BDL – 4.7) | 0.0044 (≤ 0.31) | 15,16,23,24 |
| Nonylaldehyde (nonanal) |
|
2 | 2 (NA – 4.9) | 0.0044 (≤ 0.01) | 23 |
| Acrolein (propenal) |
|
10 | 0.25 (BDL – 2.4) | 0.0026 (≤ 0.024) | 14 |
| Methyl isobutyl ketone, 3-methyl 2-pentanone (MIBK) |
|
7.4 | 0.3 (BDL – 81) | 0.0024 (≤ 0.6) | 26,30 |
| Octylaldehyde (octanal) |
|
2 | 0.9 (NA – 2.4) | 0.0018 (≤ 0.0044) | 23 |
| Heptaldehdye (heptanal) |
|
4 | 0.4 (NA – 1.1) | 0.0017 (≤ 0.01) | 23 |
| 2-Hexanone |
|
11 | 0.13 (NA – 0.8) | 0.0014 (≤ 0.009) | 23 |
NA = not available, values not reported. BDL = below detection limit
Compounds predicted to be present
In addition to the water-soluble gases measured in air, many more are expected to be present. Some of these compounds may be directly emitted while others will be formed through gas phase reactions. Table 2 provides examples of water-soluble gases measured in emissions from sources typical of indoor environments, specifically from building occupants, cooking, wood burning, and microbial metabolism. We also included volatile disinfection byproducts (DBPs) from drinking water because, if present in the household water supply, they can partition to indoor air as a result of activities such as showering and water boiling. Microbial VOCs (MVOCs) are released from metabolism of bacteria and fungi; the majority of MVOCs listed are emitted from other indoor sources as well 31. Although not included here, off gassing from building materials, furniture, tobacco smoke, and consumer products can also produce a wide variety of VOCs including WSOGs 32–35. In most cases listed compounds were measured in controlled chamber studies using analytical techniques optimized to particular compound classes (e.g. phenols and syringols using GC-FID 36). We expect that as sophisticated methods for measurement of polar gases are applied to indoor air measurement, these and other WSOGs will be increasingly found in indoor spaces.
Table 2.
Water-soluble organic compounds measured in emissions from indoor sources types. These are expected to be released into indoor air and may then partition into any liquid water present.
| Emission source | WSOG emitted | Source |
|---|---|---|
| Building occupants | Acids such as:
|
37,38 |
| Cooking | Aldehydes, such as:
|
39 |
| Fireplace | Phenols and substituted phenols, such as:
|
36 |
| Disinfection byproducts in drinking water | Volatile trihalomethanes such as:
|
40 |
Volatile DBPs:
|
41 | |
| Microbial VOCs | Aldehydes, such as:
|
31 |
NA = not available
In addition to WSOG directly released into indoor air, WSOG will also be formed indoors from gas-phase VOC oxidation and oxidation of organic surfaces. Gas-phase oxidation has been observed to form WSOGs on time scales that are competitive with the air exchange rate, for example from d-limonene42. Oxidation of surface materials is considered the major formation pathway for WSOGs because surface oxidation can produce indoor WSOGs even when reaction times are long relative to the time-scale for air exchange. Three oxidants are of particular importance to the formation of WSOG indoors: ozone, hydroxyl radical (OH·) and nitrate radical (NO3·). Ozone infiltrates from outdoors while OH· and NO3· are produced indoors 43–45. Nitrogen pentoxide (N2O5) and chlorine radical may also play an important role occasionally, such as in homes with natural gas combustion and ozone (NO2 + O3) or cleaning and photolysis, respectively.
Ozone oxidation of unsaturated VOCs has long been recognized as an indoor source of polar organics (e.g., gas phase aldehydes);46 the importance of ozonolysis of surface-bound organics to WSOG formation has more recently been recognized47,48. The predominant source of indoor residential ozone is infiltration from outdoors, yielding indoor concentrations are typically 20–70% of those outside 45. It is not uncommon for indoor ozone concentrations to range from negligible to 40 ppb, depending on outdoor levels, air exchange rate and indoor sinks 43. Higher concentrations usually occur midday, during summertime, although high concentrations can also occur in wintertime under certain circumstances 49. In addition to formation of oxidized organic gases, ozone – VOC reactions can produce indoor OH·.
Indoor hydroxyl and nitrate radical concentrations are not well constrained, but are expected to be substantial at times. Ozone - NO2 reactions provide a source of nitrate radicals 43, particularly when natural gas combustion provides an indoor source of NO2 50,51. Because there is less photolysis indoors, NO3· can persist longer in indoor spaces than outdoors. NO3· concentrations on the order of 10−3 ppb 52,54 can occur indoors. When ozone concentrations are elevated (e.g., through outdoor-to-indoor transport), or NO2 is elevated indoors, OH can be produced through VOC oxidation reactions 44 or with adequate infiltrated sunlight, through HONO photolysis 10. New evidence suggests that indoor OH can reach concentrations comparable to daytime outdoor concentrations through HONO photolysis 10 (10−5 to 10−6 ppb). However, indoor OH concentrations are probably frequently lower than peak daytime outdoor levels. Given that indoor oxidants co-exist with elevated indoor VOCs and organic-rich surface materials, formation of water-soluble organic compounds in indoors spaces is inevitable.
To provide insights into secondary oxidized compounds expected from gas phase or surface oxidation chemistry in indoor air, Table 3 shows compounds identified indoors and their oxidation products gleaned from the atmospheric and indoor chemistry literature. Smog chamber studies designed to better understand atmospheric chemistry and indoor literature examining oxidation products of cleaning agents and human skin lipids provide the bulk of the insights provided in Table 3. Several examples of non-polar VOC precursors and soluble oxidation products are given. However, this list is not exhaustive.
Table 3.
VOCs identified indoors and water-soluble gas-phase oxidation products expected to be found indoors based on knowledge from atmospheric (smog chamber) and indoor chemistry studies.
| VOC | Oxidant | Water-soluble product | Source |
|---|---|---|---|
| C5-C8 n-alkanes (from attached garages) | OH· | Hydroxycarbonyls, such as:
|
55 |
| Monoterpenes (from cleaning products, wood floors, e.g. α-pinene, β-pinene, limonene) | Ozone | Aldehydes and acids, such as:
|
56,57 |
| OH· | Aldehydes and ketones, such as:
|
57–59 | |
| Alkylbenzenes (building materials, furniture, attached garages) | OH· | Quinones, such as:
|
60 |
| Squalene (human skin lipid) | Ozone |
|
48 |
| isoprene (indoor plants, people) | OH· | Carbonyls, such as:
|
61–63 |
| NO3· |
|
64 |
NA = not available
As shown in Table 3, oxidation of organics leads to the formation of water-soluble gases, such as ketones, acids, alcohols, organic nitrates, peroxides, and epoxides. Some of these have been measured indoors and more are likely to be present. These water-soluble organic gases, have the potential to play a key role in aqueous chemistry indoors when liquid water is present.
Aqueous chemistry in outdoor air
Since water-soluble organic gases are plentiful indoors and liquid water is expected to be present in a substantial fraction of homes, it is plausible that aqueous chemistry could alter the concentrations of gas and condensed phase compounds indoors, like it does outdoors. In outdoor air, aqueous chemistry occurs because WSOGs partition into clouds, fog and wet aerosols and react through radical and non-radical reactions 65. This reactive uptake is a substantial sink for some atmospheric WSOGs 7. Low-volatility products of atmospheric chemistry in clouds, fog and wet aerosol remain in the particle phase even after water evaporation; thus aqueous organic chemistry is a source of secondary atmospheric organic particulate matter (secondary organic aerosol; SOA; 5,66,68). Aqueous chemistry also alters the gas phase composition via volatile products 65.
There is substantial atmospheric evidence for the importance of aqueous atmospheric chemistry. Perhaps the most well-established and best-known example is aqueous oxidation of SO2 by H2O2. Dramatic co-depletion of SO2 and H2O2 has been documented with cloud on-set, for example at Whiteface Mountain, NY 69. Aqueous SO2 oxidation is much more rapid than gas phase oxidation, and forms the majority of particulate sulfate and acid rain69,71. Additionally, aqueous processing (heterogeneous hydrolysis of NO2) is a major source of HONO 72. Global modeling suggests that SOA formation via OH oxidation of aldehydes and acetic acid in clouds is comparable in magnitude to SOA formation via gas phase chemistry and vapor pressure driven partitioning 73, and this chemistry largely explains the global atmospheric loading of oxalate, the most abundant dicarboxylic acid in the atmosphere 74. Isoprene-derived epoxides and peroxides are known to react in wet acidic sulfate aerosol; the products of these reactions are substantial contributors to organic aerosol in the southeastern U.S. 75. Likewise, a variety of compelling atmospheric evidence suggests that aqueous uptake can be a major sink for WSOGs, such as formic and acetic acids 76, formaldehyde and acetaldehyde 77, and glyoxal 7,78.
In the atmosphere, liquid water is found in the form of clouds, fog, and wet aerosols. Interestingly, liquid water is the largest component of fine particulate matter in the atmosphere (PM2.5), globally 79 and the largest summertime component of PM2.5 in the eastern United States 80. Additionally, water-soluble gases are ubiquitous and abundant outdoors, 80,81 mostly due to atmospheric oxidation of VOCs. For example, glyoxal and other aldehydes are formed from the hydroxyl radical oxidation of isoprene and 2-methyl-3-buten-2-ol (MBO) emitted from trees 82 and from the oxidation of aromatics emitted from fossil fuel combustion 83. There are also primary sources of water-soluble gases such as acetone, formaldehyde, acetic acid, and phenols from biomass burning 84,85.
Once in the atmosphere, water-soluble gases can partition into liquid water 5 and hydrate, dissociate, undergo hydrolysis, nucleophilic or other acid-base and catalytic reactions, or be oxidized by ozone, H2O2, OH or NO3 radicals 66,67,86 dissolved from the gas phase or formed in the aqueous phase (e.g. aqueous OH formation though Fenton reaction, or HONO, nitrate, or peroxide photolysis). Lifetimes with respect to OH are frequently shorter in the aqueous phase than in the gas phase. For example, for OH in equilibrium between the gas phase and aqueous phase (i.e., for [OH] = 106 molecules/cm3 in the gas phase and [OH] = 10−12 M in the aqueous phase), the lifetimes of glyoxal, formaldehyde and phenol are 1.2 days, 1.4 days and 10 hours, respectively, in the gas phase 87 and 15 min, 40 s, and 3 min., respectively, in the aqueous phase 88. OH radicals are so reactive, that their uptake or aqueous formation rate controls product formation rates 89, and concentrations will be lower than equilibrium values unless aqueous OH formation is substantial. In clouds and fogs, where solute concentrations are low, OH radicals dominate daytime aqueous organic chemistry due to their abundance, high reactivity and water solubility 90,91. NO3 radical and Fenton reactions are more important contributors at night 92,93. Formaldehyde, for example, partitions into the aqueous-phase (H = 3000 M/atm 94), hydrates and reacts with OH radical to make formic acid, HO2 radicals and water 95,96. Nucleophilic addition to formaldehyde is also possible; for example, addition of bisulfite leads to the formation of hydroxymethanesulfonate 97.
Aqueous-phase reaction can form different products than gas-phase reaction. For example, gas-phase glyoxal oxidation produces formaldehyde 98,99, whereas aqueous-phase glyoxal oxidation makes glyoxylic and oxalic acids 100,101; subsequent neutralization (e.g., by ammonium) can produce low volatility salts (e.g., ammonium oxalate) that remain in the condensed phase (in particles or on surfaces) even after water evaporation 102,103. This difference in chemistry occurs because glyoxal is doubly hydrated in the aqueous phase (exists as a tetrol) 100.
Chemistry in wet aerosols differs from that in clouds and fogs because aerosol liquid water is highly concentrated with solutes. Non-radical reactions can compete with radical reactions in wet aerosols because high solute concentrations make up for their lower rate constants 104. For example, acid-catalyzed ring opening and nucleophilic ammonium reaction with isoprene epoxydiols lead to the formation of oligomers and organosulfates in wet atmospheric particles 105,106. The process of water evaporation (i.e. in evaporating fogs and clouds) speeds otherwise slow reactions, e.g., between aldehydes and amines,107 facilitating chemical transformation. Further, photosensitized radical reactions involving humic-like substances that are found in wet aerosols and at air-sea interfaces have been shown to produce oxygenated organic gases 108. Thus, aqueous chemistry can be both a sink for water-soluble gases and a source of both gases and condensed phase species in airborne particles and on surfaces5,67,73.
The now substantial body of research being conducted to understand atmospheric aqueous chemistry provides insights into the potential for aqueous chemistry to alter concentrations and exposures indoors.
The case for aqueous chemistry in indoor air
Surfaces play an important role in indoor chemistry. Indoor surface area-to-volume (S/V) ratios are greater than 3 m2/m3, and much greater when internal surface areas are considered for materials such as carpets and upholstery 109,110. This is orders of magnitude greater than the <0.01 m2/m3 typical of ambient outdoor air. Even a 1 nm water film on indoor surfaces, a film consistent with simple water adsorption, will provide more than 1000 times the volume of liquid water as is found in aerosols in outdoor air (assuming 3 μg/m3 of aerosol water). Combined with the knowledge that WSOGs are present at much higher concentrations indoors than outdoors, the potential for aqueous chemistry to play an important role indoors, as it does outdoors, is substantial.
The measurement of HONO indoors provides clear evidence that there is sufficient liquid water to drive aqueous chemistry in residences, and that aqueous chemistry can alter the composition of indoor air. HONO is produced from the heterogeneous hydrolysis of NO2. HONO has been measured in many homes over the last few decades, including the early study of 10 homes by Spengler and Brauer, who found HONO concentrations ranging from 2 to 8 ppb.9 Recently, measured indoor OH radical concentrations of 1.8 × 106 molecules/m3 were linked to HONO photolysis on windows10. Interestingly, this suggests that OH radicals can be produced through photolysis in indoor liquid water films on windows. Those OH radicals can then go on to oxidize aldehydes (e.g., glyoxal), organic acids (e.g., acetic, lactic) and phenols (e.g., guaiacol, phenol) in the aqueous phase to produce oxalic acid, oligomers and other products111–113. Aqueous OH oxidation of formaldehyde will be a source of gas phase formic acid65. We also expect that ammonia/amines, which have varying water-solubilities and could also be elevated indoors114, will react with aldehydes as water evaporates from indoor surfaces. These reactions will form brown surface films115. Other likely chemistry is described below.
One important knowledge gap pertains to indoor liquid water. The hygroscopicity of indoor surfaces, indoor liquid water quantities and chemical characteristics of aqueous solutions are not well understood. Evidence for the presence of water has been documented in a substantial percentage of buildings 1,2, and water can be present indoors for a variety of reasons. Some indoor locations are constantly wet, like toilet bowl and sink traps, but have limited interfacial surface areas for chemical mass transport. Other locations are seasonally wet, such as air conditioning coils (summer) and windows/exterior walls (winter) that are colder than the indoor air and thus condense moisture from that air. Still other locations are subject to regular condensation/ evaporation cycles such as sinks, showers and bathroom mirrors. Liquid water can also be found indoors due to snow melt, rain water, leaks and faulty plumbing3. Building occupants themselves have liquid water in their respiratory tract and hygroscopic salts on human skin also take up water when the RH exceeds the deliquescence point 116. Finally, a wide variety of hygroscopic materials (e.g., clothing, furniture, building materials, aerosols and surface grime) absorb water vapor,117–122 creating highly concentrated aqueous solutions where reactions can take place. For example, a room with walls consisting of lime–gypsum interior plaster can reduce the RH by 10% 121. This accounts for a water uptake of 3 mL per square meter of plaster (equivalent to a water layer 3 µm thick, were it all to exist as a surface film). Airborne particles are also hygroscopic, increasing their water content with increasing RH. While the hygroscopicity of indoor-generated particles is not well known, roughly half of indoor fine particle mass is of outdoor origin. Globally, ambient fine particles are 70% water, on average79. At particularly high RH, ambient particle mass can increase by a factor of five due to water uptake123. Indoor surfaces are known to accumulate surface films 1–14 nm thick 124–128. Although polar compounds have been measured in surface films124, the hygroscopicity of these films is not known. Based on aerosol hygroscopicity, it is not unreasonable to think that the film thickness could double or triple at high RH, due to hygroscopic growth, leading to highly concentrated aqueous mixtures.
The extent of WSOG uptake to wet surfaces depends on the volume of liquid water indoors, which is poorly understood, the Henry’s law constant, and on subsequent reactions in the aqueous phase. For example, assuming aqueous uptake to reach Henry’s Law equilibrium but no subsequent aqueous reaction, 20 ppbv of formaldehyde (a typical indoor concentration) would decrease by 1% in a 40 m3 room that has a liquid water film 1 nm thick (surface water adsorption only) and 15% if the liquid water film were 30 nm thick. Once taken up, formaldehyde will hydrate and reactions in liquid water will enhance uptake to reestablish Henry’s law equilibrium.
Unfortunately, little work has been done to characterize the quantity and the physical and chemical properties of liquid water on indoor surfaces. Many factors may affect the aqueous chemistry that could occur indoors including, but not limited to:
The hygroscopicity of surface materials. If the hygroscopicity is high (e.g. fabric furniture, unpainted walls, salty skin, infiltrated outdoor particles), then water vapor can be taken up into the material, sometimes even at modest humidities 118, forming highly concentrated aqueous solutions 129
The surface area and volume of liquid water films. The capacity to remove water-soluble gases depends on the water volume but also on compound removal through aqueous reaction; the (reactive) uptake rate can depend on the water surface area or on the rate of reaction after uptake 89.
The features of the aqueous solution such as pH, water activity, presence of salts (which can change Henry’s law constants and affect hygroscopicity) 130, production of oxidants 131, photosensitizers 132, nucleophiles 105, transition metals 133, and other reactants.
The chemical composition of the surface. Indoor surfaces can become soiled with semi-volatile organic compounds that partition and continuously re-partition between the gas and adsorbed phases, particles released by occupant activities, and occupant skin flakes 134. Soiled surfaces can also become oxidized, e.g., by ozone transported from outdoors 47,48. The properties of the soiled surfaces will affect hygroscopicity 135 and aqueous reactivity.
Chemical composition affects surface water uptake. While some atmospheric aerosol constituents (i.e., sulfuric acid) take up water even at 10% RH, others do not deliquesce until high RH (e.g., 85% RH, inorganic salts). Non-polar hydrocarbons are not hygroscopic, but some aerosol organics do retain water even as low as 10% RH 136,137 (e.g., malonic, citric, tartaric acids). We expect that indoor surface films will be comprised of complex mixtures. Aerosol particles comprised of complex mixtures take up water at lower RH values than single component particles138. For example, a particle mixture of ammonium sulfate, ammonium nitrate, levoglucosan, fulvic acid and succinic acid experiences deliquescence behavior at relative humidities from 55 to 70%. Independently, the inorganic salts would not be in solution until an RH of 85% 86,138. Also, because of the presence of ammonia and amines indoors and atmospheric aerosol studies of their effect on hygroscopicity, we anticipate that reaction of indoor organic surface films with ammonia and amines will increase water uptake by indoor surfaces 114,139. Additionally, as surfaces age, they become oxidized leading to carbonyl, alcohol, and carboxylic acid functional groups.47 Methods to predict atmospheric aerosol water uptake as a function of RH and surface composition (oxygen-to-carbon ratio or functional group contributions)135 have the potential to predict the hygroscopicity of indoor surfaces, if they are chemically-characterized.
Once partitioned in the aqueous phase, water-soluble compounds could undergo several types of reactions including radical reactions, acid-base, nucleophilic and hydrolysis reactions. We expect that aqueous surface films will contain high concentrations of solutes, and thus the chemistry will be more similar to the chemistry in wet aerosols rather than clouds. Compounds taken up from the gas phase may react with carbonyl, alcohol, and carboxylic acid functional groups present in surface films 47 or with compounds taken up from the gas phase. Since ammonia and amines are water soluble, they are likely to be taken up from the gas phase and to participate in aqueous chemistry. TiO2, present in most paints, is a photosensitizer140 and thus can initiate radical reactions in aqueous solutions in the presence of light, as can humic-like organics108,141. Because solar radiation penetrates through windows, photosensitized reactions could yield volatile products, as has been observed previously for photosensitizers at air-ocean interfaces 108 and could be a major pathway for OH formation in water films. This is especially true when condensation forms on windows. When window condensate evaporates, it will release volatile products to the gas phase 107,115. Aqueous photolysis 90 of HONO 10, HOCl142, organic peroxides 131 and nitrate are also likely sources of OH in indoor water films. While OH radicals can also be taken up from the gas phase, they are so reactive that diffusion would be the rate limiting step to product formation in the absence of aqueous phase sources. Even though reaction rate constants are much smaller, non-radical reactions are competitive with OH radical reactions in wet aerosols because solute concentrations are so high (e.g., molar). We expect the same to be true in indoor water films. Note also that, unlike gas phase reactions, surface reactions do not have to be faster than air exchange in order to be important, because molecules sorbed to surfaces have much longer residence times indoors.
pH is likely to affect the types of chemical reactions that occur in liquid water. Wet airborne particles are frequently acidic, while thin aqueous films on indoor surfaces may be basic or acidic depending on the composition of the surface and adsorbed gases. For example, indoor carbon dioxide concentrations are quite high due to the presence of occupants; dissolved carbon dioxide will lead to the formation of carbonic acid making the aqueous film acidic. Or ammonia may dominate pH since it can be present in high quantities indoors143 leading to more basic conditions. The presence of salts, for example on skin, can also affect uptake and reactions. Compounds that may undergo hydrolysis in liquid water partitioned onto surfaces include phthalates from plasticizers and organophosphates from pesticides 144 as well other compounds that can be easily protonated or deprotonated, such as alcohols and amines. OH radicals (H=30 M/atm), ozone (H=10−2 M/atm) and peroxides (H=varies, high) will also be taken up into liquid water, and can react with WSOG in the aqueous phase to form volatile and low volatility products.
Some reactions will produce products that remain in the condensed phase (e.g. on surfaces) even after water evaporation. For example, OH radical oxidation of acetic acid and glyoxal may occur in indoor water films. In dilute solution these reactions produce oxalic acid, which remains in the condensed phase as an ammonium salt 112,145. In concentrated aqueous solution low volatility oligomers form 112,145. Evaporation of water films containing aldehydes and amines or amino acids, also found indoors, enables the formation of low-volatility imidazoles and other nitrogen containing oligomers 107,115. Oligomers are also expected from nucleophilic reaction of organic epoxides, e.g. in the presence of ammonium 105. Organic hydroperoxides are very water soluble and very reactive in the condensed phase. If present, we expect that they will react with aldehydes or carbonyl functionalities in indoor surface films to form peroxyhemiacetals111.
Chemistry in liquid water indoors may also be a source of volatile compounds that will be released into the gas phase upon formation or undergo additional chemistry before they are released. For example, aqueous OH radical oxidation of phenols emitted from wood combustion forms formic and acetic acids that will evaporate when the surface dries (and oxidized aromatic oligomers that remain in the condensed phase) 146. Formaldehyde oxidation by OH radicals also produces formic acid 65. The fate of oxalic acid, an aqueous oxidation product of phenol, glyoxal, and acetic acid 100,112,146, depends on whether it remains a volatile acid or is neutralized and present as a low volatility salt 102.
Human skin contains hygroscopic salts that take up water as relative humidity increases, therefore building occupants themselves are expected to be a medium for aqueous chemistry. In addition to the types of reactions discussed above, indoors, these salts may participate in displacement reactions such as those observed in wet marine aerosols. Chlorine present in marine aerosol, is displaced by organic acids and is released into the atmosphere as HCl 147. This could happen on wet skin with organic acids such as acetic acid (which has been measured indoors) 147. Damp skin may be a source of reactive halogen gases such as Cl2 and Br2 as well as nitrate especially if there is sufficient sunlight indoors 148. These halogens can then drive further gas phase chemistry. Also, there is evidence that NO2 and HCl forms ClNO and ClNO2 in the presence of water molecules at air-surface interfaces. These products are highly volatile and reactive and can further drive indoor chemistry149.
Lung fluid, a saline aqueous solution, is also a probable location for aqueous chemistry of indoor water-soluble gases. Formaldehyde, is known to dissolve in lung fluid and produce reactive oxygenated species 150. Water-soluble organic peroxides and quinones found in atmospheric particles have been shown to produce strong oxidants in synthetic lung fluid 151,152. Chemically similar WSOGs may also be expected to do so. However, little is known about the potential health effects of inhalation exposures to most WSOG and how their aqueous products that are released from lung fluid may affect indoor chemistry.
Conclusions and future work
Aqueous chemistry has been found to be an important factor in the atmospheric processing of organic compounds. Because the indoor surface area to volume ratios are so much higher than the outdoor values, even a thin water surface coating would provide more water per unit volume than found outdoors. Since the concentrations of WSOGs and several other potential reactants are also much higher indoors than out, we argue that aqueous chemistry is likely to affect indoor air composition and affect inhalation and dermal exposures in damp homes.
Oxidized, polar, water-soluble organic compounds are poorly characterized because methods for their gas-phase measurement are less developed than measurement methods for non-polar VOCs. Thus, herein we report measurements of total water-soluble organic gases in 13 real homes, and find for the first time that WSOG concentrations are substantially higher indoors than outdoors where photochemistry is known to make WSOG ubiquitous and abundant 80. Literature-based evidence suggests that indoor WSOG includes carbonyl compounds, carboxylic acids, epoxides, organic peroxides, organic nitrates, amines, and phenols. When liquid water is present as indoor surface films, on skin and in wet particles (i.e., at elevated RH), we expect that WSOG will partition into that water and react further. This chemistry is likely to remove some compounds from the gas phase, while introducing others to the gas phase, thereby altering indoor air composition and inhalation exposure. When reactions occur on damp skin, dermal exposures could also be altered.
While we argue that aqueous chemistry will alter exposures in damp indoor spaces, the following are critically needed to assess of the magnitude of this effect and are poorly understood:
The composition of indoor WSOG
The hygroscopicity of indoor surfaces
The chemical and physical properties of indoor aqueous surface films
The relative importance of radical and no-radical chemistry and thermodynamic properties of the products
Ultimately we wish to know the degree to which “dampness” alters indoor air composition, inhalation and dermal exposures through indoor aqueous chemistry, and whether this chemistry helps to explain adverse health effects in damp homes.
Practical Implications.
Associations between “dampness” in homes and adverse health effects are only partially explained by mold and mildew. We provide evidence that aqueous chemistry could plausibly alter inhalation and dermal exposures in damp homes. However, key knowledge gaps hamper efforts to understand the magnitude of these effects and whether changes in exposure due to aqueous chemistry could help to explain associations observed between “dampness” and adverse health effects.
Acknowledgments
The authors gratefully acknowledge the support of the Alfred P. Sloan Foundation (grant #G-2015-13886), the National Institute of Environmental Health Sciences Exposure Training Grant (grant #T32ES019854), and the assistance of Jason Surratt, Glenn Morrison, Ronald Lauck, Neha Sareen, and Jeffrey Kirkland.
Literature cited
- 1.Gunnbjörnsdóttir MI, Franklin KA, Norbäck D, et al. Prevalence and incidence of respiratory symptoms in relation to indoor dampness: the RHINE study. Thorax. 2006;61:221–225. doi: 10.1136/thx.2005.057430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mudarri D, Fisk WJ. Public health and economic impact of dampness and mold. Indoor Air. 2007;17:226–235. doi: 10.1111/j.1600-0668.2007.00474.x. [DOI] [PubMed] [Google Scholar]
- 3.Bornehag C-G, Blomquist G, Gyntelberg F, et al. Dampness in Buildings Health. Nordic Interdisciplinary Review of the Scientific Evidence on Associations between Exposure to ‘Dampness’ in Buildings and Health Effects (NORDDAMP) Indoor Air. 2001;11:72–86. doi: 10.1034/j.1600-0668.2001.110202.x. [DOI] [PubMed] [Google Scholar]
- 4.Mendell MJ, Mirer AG, Cheung K, Tong M, Douwes J. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: a review of the epidemiologic evidence. Environ. Health Perspect. 2011;119:748–756. doi: 10.1289/ehp.1002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blando JD, Turpin BJ. Secondary organic aerosol formation in cloud and fog droplets: A literature evaluation of plausibility. Atmos. Environ. 2000;34:1623–1632. [Google Scholar]
- 6.Crahan KK, Hegg D, Covert DS, Jonsson H. An exploration of aqueous oxalic acid production in the coastal marine atmosphere. Atmos. Environ. 2004;38:3757–3764. [Google Scholar]
- 7.Volkamer R, San Martini F, Molina LT, et al. A missing sink for gas-phase glyoxal in Mexico City: Formation of secondary organic aerosol. Geophys. Res. Lett. 2007;34 doi: 10.1029/2007GL030752. [DOI] [Google Scholar]
- 8.Ervens B, Wang Y, Eagar J, et al. Dissolved organic carbon (DOC) and select aldehydes in cloud and fog water: The role of the aqueous phase in impacting trace gas budgets. Atmos. Chem. Phys. 2013;13:5117–5135. [Google Scholar]
- 9.Spengler JD, Brauer M, Samet JM, Lambert WE. Nitrous acid in Albuquerque, New Mexico, homes. Environ. Sci. Technol. 1993;27:841–845. [Google Scholar]
- 10.Gómez Alvarez E, Amedro D, Afif C, et al. Unexpectedly high indoor hydroxyl radical concentrations associated with nitrous acid. Proc. Natl. Acad. Sci. U. S. A. 2013;110:13294–13299. doi: 10.1073/pnas.1308310110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finlayson-Pitts BJ, Wingen LM, Sumner AL, et al. The heterogeneous hydrolysis of NO2 in laboratory systems and in outdoor and indoor atmospheres: An integrated mechanism. Phys. Chem. Chem. Phys. 2003;5:223–242. [Google Scholar]
- 12.Paciência I, Madureira J, Rufo J, Moreira A, de Oliveira Fernandes E. A Systematic Review of Evidence and Implications of Spatial and Seasonal Variations of Volatile Organic Compounds (VOC) in Indoor Human Environments. J. Toxicol. Environ. Health. B. Crit. Rev. 2016;19:47–64. doi: 10.1080/10937404.2015.1134371. [DOI] [PubMed] [Google Scholar]
- 13.Edwards RD, Jurvelin J, Saarela K, Jantunen M. VOC concentrations measured in personal samples and residential indoor, outdoor and workplace microenvironments in EXPOLIS-Helsinki, Finland. Atmos. Environ. 2001;35:4531–4543. [Google Scholar]
- 14.Liu W, Zhang J, Zhang L, et al. Estimating contributions of indoor and outdoor sources to indoor carbonyl concentrations in three urban areas of the United States. Atmos. Environ. 2006;40:2202–2214. [Google Scholar]
- 15.Reiss R, Ryan PB, Tibbetts SJ, Koutrakis P. Measurement of organic acids, aldehydes, and ketones in residential environments and their relation to ozone. J. Air Waste Manag. Assoc. 1995;45:811–822. doi: 10.1080/10473289.1995.10467411. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, He Q, Lioy PJ. Characteristics of aldehydes: Concentrations, sources, and exposures for indoor and outdoor residential microenvironments. Environ. Sci. Technol. 1994;28:146–152. doi: 10.1021/es00050a020. [DOI] [PubMed] [Google Scholar]
- 17.Cofer WR, Collins VG, Talbot RW. Improved aqueous scrubber for collection of soluble atmospheric trace gases. Environ. Sci. Technol. 1985;19:557–560. doi: 10.1021/es00136a012. [DOI] [PubMed] [Google Scholar]
- 18.Perri MJ, Seitzinger S, Turpin BJ. Secondary organic aerosol production from aqueous photooxidation of glycolaldehyde: Laboratory experiments. Atmos. Environ. 2009;43:1487–1497. [Google Scholar]
- 19.Tan Y, Perri MJ, Seitzinger SP, Turpin BJ. Effects of Precursor Concentration and Acidic Sulfate in Aqueous Glyoxal−OH Radical Oxidation and Implications for Secondary Organic Aerosol. Environ. Sci. Technol. 2009;43:8105–8112. doi: 10.1021/es901742f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sander R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015;15:4399–4981. [Google Scholar]
- 21.Dodson RE, Levy JI, Spengler JD, Shine JP, Bennett DH. Influence of basements, garages, and common hallways on indoor residential volatile organic compound concentrations. Atmos. Environ. 2008;42:1569–1581. [Google Scholar]
- 22.Ross Highsmith V, Zweidinger RB, Merrill RG. Characterization of indoor and outdoor air associated with residences using woodstoves: A pilot study. Environ. Int. 1988;14:213–219. [Google Scholar]
- 23.Jurvelin JA, Edwards RD, Vartiainen M, Pasanen P, Jantunen MJ. Residential indoor, outdoor, and workplace concentrations of carbonyl compounds: Relationships with personal exposure concentrations and correlation with sources. J. Air Waste Manag. Assoc. 2003;53:560–573. doi: 10.1080/10473289.2003.10466190. [DOI] [PubMed] [Google Scholar]
- 24.Sax SN, Bennett DH, Chillrud SN, Kinney PL, Spengler JD. Differences in source emission rates of volatile organic compounds in inner-city residences of New York City and Los Angeles. J. Expo. Anal. Environ. Epidemiol. 2004;14(Suppl 1):S95–109. doi: 10.1038/sj.jea.7500364. [DOI] [PubMed] [Google Scholar]
- 25.Zhu X, Jia C. Apportioning variability of polycyclic aromatic hydrocarbons (PAHs) and chlordanes in indoor and outdoor environments. J. Environ. Monit. 2012;14:1926–1934. doi: 10.1039/c2em30127j. [DOI] [PubMed] [Google Scholar]
- 26.Jia C, Batterman S, Godwin C. VOCs in industrial, urban and suburban neighborhoods, Part 1: Indoor and outdoor concentrations, variation, and risk drivers. Atmos. Environ. 2008;42:2083–2100. [Google Scholar]
- 27.Weschler CJ, Nazaroff WW. Semivolatile organic compounds in indoor environments. Atmos. Environ. 2008;42:9018–9040. [Google Scholar]
- 28.Wilson NK, Chuang JC, Morgan MK, Lordo RA, Sheldon LS. An observational study of the potential exposures of preschool children to pentachlorophenol, bisphenol-A, and nonylphenol at home and daycare. Environ. Res. 2007;103:9–20. doi: 10.1016/j.envres.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Quackenboss JJ, Lebowitz MD, Michaud JP, Bronnimann D. Formaldehyde exposure and acute health effects study. Environ. Int. 1989;15:169–176. [Google Scholar]
- 30.Weisel CP, Alimokhtari S, Sanders PF. Indoor Air VOC Concentrations in Suburban and Rural New Jersey. Environ. Sci. Technol. 2008;42:8231–8238. doi: 10.1021/es8005223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korpi A, Järnberg J, Pasanen A-L. Microbial volatile organic compounds. Crit. Rev. Toxicol. 2009;39:139–193. doi: 10.1080/10408440802291497. [DOI] [PubMed] [Google Scholar]
- 32.Hodgson AT, Rudd A, Beal D, Chandra S. Volatile organic compound concentrations and emission rates in new manufactured and site-built houses. Indoor Air. 2000;10:178–192. doi: 10.1034/j.1600-0668.2000.010003178.x. [DOI] [PubMed] [Google Scholar]
- 33.Singer BC, Coleman BK, Destaillats H, et al. Indoor secondary pollutants from cleaning product and air freshener use in the presence of ozone. Atmos. Environ. 2006;40:6696–6710. doi: 10.1021/es052198z. [DOI] [PubMed] [Google Scholar]
- 34.Salthammer T. Emission of Volatile Organic Compounds from Furniture Coatings. Indoor Air. 1997;7:189–197. [Google Scholar]
- 35.Charles SM, Batterman SA, Jia C. Composition and emissions of VOCs in main- and side-stream smoke of research cigarettes. Atmos. Environ. 2007;41:5371–5384. [Google Scholar]
- 36.Schauer JJ, Kleeman MJ, Cass GR, Simoneit BRT. Measurement of Emissions from Air Pollution Sources 3. C1 −C29 Organic Compounds from Fireplace Combustion of Wood. Environ. Sci. Technol. 2001;35:1716–1728. doi: 10.1021/es001331e. [DOI] [PubMed] [Google Scholar]
- 37.Liu S, Li R, Wild RJ, et al. Contribution of human-related sources to indoor volatile organic compounds in a university classroom. Indoor Air. 2016;26:925–938. doi: 10.1111/ina.12272. [DOI] [PubMed] [Google Scholar]
- 38.Liu S, Thompson SL, Stark H, Ziemann PJ, Jimenez JL. Gas-Phase Carboxylic Acids in a University Classroom: Abundance, Variability, and Sources. Environ. Sci. Technol. 2017;51:5454–5463. doi: 10.1021/acs.est.7b01358. [DOI] [PubMed] [Google Scholar]
- 39.Katragadda HR, Fullana A, Sidhu S, Carbonell-Barrachina Á. A Emissions of volatile aldehydes from heated cooking oils. Food Chem. 2010;120:59–65. [Google Scholar]
- 40.Nieuwenhuijsen MJ, Toledano MB, Eaton NE, Fawell J, Elliott P. Chlorination disinfection byproducts in water and their association with adverse reproductive outcomes: a review. Occup. Environ. Med. 2000;57:73–85. doi: 10.1136/oem.57.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richardson S. Disinfection by-products and other emerging contaminants in drinking water. TrAC Trends Anal. Chem. 2003;22:666–684. [Google Scholar]
- 42.Carslaw N. A mechanistic study of limonene oxidation products and pathways following cleaning activities. Atmos. Environ. 2013;80:507–513. [Google Scholar]
- 43.Weschler CJ, Brauer M, Koutrakis P. Indoor ozone and nitrogen dioxide: A potential pathway to the generation of nitrate radicals, dinitrogen pentaoxide, and nitric acid indoors. Environ. Sci. Technol. 1992;26:179–184. [Google Scholar]
- 44.Weschler CJ, Shields HC. Production of the Hydroxyl Radical in Indoor Air. Environ. Sci. Technol. 1996;30:3250–3258. [Google Scholar]
- 45.Weschler CJ. Ozone in indoor environments: concentration and chemistry. Indoor Air. 2000;10:269–288. doi: 10.1034/j.1600-0668.2000.010004269.x. [DOI] [PubMed] [Google Scholar]
- 46.Weschler CJ, Hodgson AT, Wooley JD. Indoor chemistry: ozone, volatile organic compounds, and carpets. Environ. Sci. Technol. 1992;26:2371–2377. [Google Scholar]
- 47.Wang H, Morrison G. Ozone-surface reactions in five homes: surface reaction probabilities, aldehyde yields, and trends. Indoor Air. 2010;20:224–234. doi: 10.1111/j.1600-0668.2010.00648.x. [DOI] [PubMed] [Google Scholar]
- 48.Wisthaler A, Weschler CJ. Reactions of ozone with human skin lipids: sources of carbonyls, dicarbonyls, and hydroxycarbonyls in indoor air. Proc. Natl. Acad. Sci. U. S. A. 2010;107:6568–6575. doi: 10.1073/pnas.0904498106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schnell RC, Oltmans SJ, Neely RR, et al. Rapid photochemical production of ozone at high concentrations in a rural site during winter. Nat. Geosci. 2009;2:120–122. [Google Scholar]
- 50.Yamanaka S, Hirose H, Takada S. Nitrogen oxides emissions from domestic kerosene-fired and gas-fired appliances. Atmos. Environ. 1979;13:407–412. doi: 10.1016/0004-6981(79)90297-x. [DOI] [PubMed] [Google Scholar]
- 51.Zhang J, Smith KR, Ma Y, et al. Greenhouse gases and other airborne pollutants from household stoves in China: a database for emission factors. Atmos. Environ. 2000;34:4537–4549. [Google Scholar]
- 52.Weschler CJ. Chemical reactions among indoor pollutants: what we’ve learned in the new millennium. Indoor Air. 2004;14:184–194. doi: 10.1111/j.1600-0668.2004.00287.x. [DOI] [PubMed] [Google Scholar]
- 53.Carslaw N. A new detailed chemical model for indoor air pollution. Atmos. Environ. 2007;41:1164–1179. [Google Scholar]
- 54.Nøjgaard JK. Indoor measurements of the sum of the nitrate radical, NO3, and nitrogen pentoxide, N2O5 in Denmark. Chemosphere. 2010;79:898–904. doi: 10.1016/j.chemosphere.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 55.Reisen F, Aschmann SM, Atkinson R, Arey J. 1,4-Hydroxycarbonyl products of the OH radical initiated reactions of C5-C8 n-alkanes in the presence of NO. Environ. Sci. Technol. 2005;39:4447–4453. doi: 10.1021/es0483589. [DOI] [PubMed] [Google Scholar]
- 56.Yu J, Flagan RC, Seinfeld JH. Identification of Products Containing −COOH, −OH, and −CO in Atmospheric Oxidation of Hydrocarbons. Environ. Sci. Technol. 1998;32:2357–2370. [Google Scholar]
- 57.Atkinson R, Arey J. Gas-phase tropospheric chemistry of biogenic volatile organic compounds: A review,in. Atmospheric Environment. 2003;37:197–219. [Google Scholar]
- 58.Capouet M, Peeters J, Nozi`ere B, Müller J-F. Alpha-pinene oxidation by OH: simulations of laboratory experiments. Atmos. Chem. Phys. 2004;4:2285–2311. [Google Scholar]
- 59.Larsen BR, Bella DDi, Glasius M, et al. Gas-Phase OH Oxidation of Monoterpenes: Gaseous and Particulate Products. J. Atmos. Chem. 2001;38:231–276. [Google Scholar]
- 60.Yu J, Jeffries HE, Sexton KG. Atmospheric photooxidation of alkylbenzenes—I. Carbonyl product analyses. Atmos. Environ. 1997;31:2261–2280. [Google Scholar]
- 61.Paulot F, Crounse JD, Kjaergaard HG, et al. Unexpected epoxide formation in the gas-phase photooxidation of isoprene. Science. 2009;325:730–733. doi: 10.1126/science.1172910. [DOI] [PubMed] [Google Scholar]
- 62.Crutzen PJ, Williams J, Pöschl U, et al. High spatial and temporal resolution measurements of primary organics and their oxidation products over the tropical forests of Surinam. Atmos. Environ. 2000;34:1161–1165. [Google Scholar]
- 63.Reeves CE, Penkett SA. Measurements of peroxides and what they tell us. Chem. Rev. 2003;103:5199–5218. doi: 10.1021/cr0205053. [DOI] [PubMed] [Google Scholar]
- 64.Rollins AW, Kiendler-Scharr A, Fry JL, et al. Isoprene oxidation by nitrate radical: alkyl nitrate and secondary organic aerosol yields. Atmos. Chem. Phys. 2009;9:6685–6703. [Google Scholar]
- 65.Jacob DJ. Chemistry of OH in remote clouds and its role in the production of formic acid and peroxymonosulfate. J. Geophys. Res. 1986;91:9807–9826. [Google Scholar]
- 66.McNeill VF. Aqueous organic chemistry in the atmosphere: Sources and chemical processing of organic aerosols. Environ. Sci. Technol. 2015;49:1237–1244. doi: 10.1021/es5043707. [DOI] [PubMed] [Google Scholar]
- 67.Ervens B, Turpin BJ, Weber RJ. Secondary organic aerosol formation in cloud droplets and aqueous particles (aqSOA): A review of laboratory, field and model studies. Atmospheric Chemistry and Physics. 2011;11:11069–11102. [Google Scholar]
- 68.Nozière B, Kalberer M, Claeys M, et al. The Molecular Identification of Organic Compounds in the Atmosphere: State of the Art and Challenges. Chem. Rev. 2015;115:3919–3983. doi: 10.1021/cr5003485. [DOI] [PubMed] [Google Scholar]
- 69.Mohnen V, Kadlecek J. Cloud chemistry research at Whiteface Mountain. Tellus B. 1989;41B:79–91. [Google Scholar]
- 70.Munger JW, Jacob DJ, Waldman JM, Hoffmann MR. Fogwater chemistry in an urban atmosphere. J. Geophys. Res. Ocean. 1983;88:5109–5121. [Google Scholar]
- 71.Aleksic N, Roy K, Sistla G, et al. Analysis of cloud and precipitation chemistry at Whiteface Mountain, NY. Atmos. Environ. 2009;43:2709–2716. [Google Scholar]
- 72.Heikes BG, Thompson AM. Effects of heterogeneous processes on NO3 , HONO, and HNO3 chemistry in the troposphere. J. Geophys. Res. 1983;88:10883–10895. [Google Scholar]
- 73.Liu J, Horowitz LW, Fan S, Carlton AG, Levy H. Global in-cloud production of secondary organic aerosols: Implementation of a detailed chemical mechanism in the GFDL atmospheric model AM3. J. Geophys. Res. Atmos. 2012;117 doi: 10.1029/2012JD017838. [DOI] [Google Scholar]
- 74.Myriokefalitakis S, Tsigaridis K, Mihalopoulos N, et al. In-cloud oxalate formation in the global troposphere: a 3-D modeling study. Atmos. Chem. Phys. 2011;11:5761–5782. [Google Scholar]
- 75.Budisulistiorini SH, Baumann K, Edgerton ES, et al. Seasonal characterization of submicron aerosol chemical composition and organic aerosol sources in the southeastern United States: Atlanta, Georgia, and Look Rock, Tennessee. Atmos. Chem. Phys. 2016;16:5171–5189. [Google Scholar]
- 76.Chebbi A, Carlier P. Carboxylic acids in the troposphere, occurrence, sources, and sinks: A review. Atmos. Environ. 1996;30:4233–4249. [Google Scholar]
- 77.Grosjean D, Wright B. Carbonyls in urban fog, ice fog, cloudwater and rainwater. Atmos. Environ. 1983;17:2093–2096. [Google Scholar]
- 78.Hodas N, Sullivan AP, Skog K, et al. Aerosol liquid water driven by anthropogenic nitrate: Implications for lifetimes of water-soluble organic gases and potential for secondary organic aerosol formation. Environ. Sci. Technol. 2014;48:11127–11136. doi: 10.1021/es5025096. [DOI] [PubMed] [Google Scholar]
- 79.Liao H, Seinfeld JH. Global impacts of gas-phase chemistry-aerosol interactions on direct radiative forcing by anthropogenic aerosols and ozone. J. Geophys. Res. 2005;110 doi: 10.1029/2005JD005907. [DOI] [Google Scholar]
- 80.Carlton AG, Turpin BJ. Particle partitioning potential of organic compounds is highest in the Eastern US and driven by anthropogenic water. Atmos. Chem. Phys. 2013;13:10203–10214. [Google Scholar]
- 81.Goldstein AH, Galbally IE. Known and unexplored organic consistuents in the Earth’s atmosphere. Environ. Sci. Technol. 2007;41:1514. doi: 10.1021/es072476p. [DOI] [PubMed] [Google Scholar]
- 82.Spaulding RS. Characterization of secondary atmospheric photooxidation products: Evidence for biogenic and anthropogenic sources. J. Geophys. Res. 2003;108 doi: 10.1029/2002JD002478. [DOI] [Google Scholar]
- 83.Volkamer R, Platt U, Wirtz K. Primary and Secondary Glyoxal Formation from Aromatics: Experimental Evidence for the Bicycloalkyl−Radical Pathway from Benzene, Toluene, and p -Xylene. J. Phys. Chem. A. 2001;105:7865–7874. [Google Scholar]
- 84.Christian TJ, Kleiss B, Yokelson RJ, et al. Comprehensive laboratory measurements of biomass-burning emissions: 1. Emissions from Indonesian, African, and other fuels. J. Geophys. Res. 2003;108 doi: 10.1029/2003JD003704. [DOI] [Google Scholar]
- 85.Yokelson RJ, Susott R, Ward DE, Reardon J, Griffith DWT. Emissions from smoldering combustion of biomass measured by open-path Fourier transform infrared spectroscopy. J. Geophys. Res. Atmos. 1997;102:18865–18877. [Google Scholar]
- 86.Seinfeld JH, Pandis SN. Atmospheric chemistry and physics?: from air pollution to climate change. J. Wiley; 2006. [Google Scholar]
- 87.Atkinson R. Gas-Phase tropospheric chemistry of volatile organic compounds: 1 alkanes and alkenes. J. Phys. Chem. Ref. Data. 1997;26:215–290. [Google Scholar]
- 88.Doussin J-F, Monod A. Structure-activity relationship for the estimation of OH-oxidation rate constants of carbonyl compounds in the aqueous phase. Atmos. Chem. Phys. 2013;13:11625–11641. [Google Scholar]
- 89.Ervens B, Sorooshian A, Lim YB, Turpin BJ. Key parameters controlling OH-initiated formation of secondary organic aerosol in the aqueous phase (aqSOA) J. Geophys. Res. Atmos. 2014;119:3997–4016. [Google Scholar]
- 90.Faust BC, Allen JM. Aqueous-phase photochemical formation of hydroxyl radical in authentic cloudwaters and fogwaters. Environ. Sci. Technol. 1993;27:1221–1224. [Google Scholar]
- 91.Ervens B, George C, Williams JE, et al. CAPRAM 2.4 (MODAC mechanism): An extended and condensed tropospheric aqueous phase mechanism and its application. J. Geophys. Res. 2003;108 doi: 10.1029/2002JD002202. [DOI] [Google Scholar]
- 92.Atkinson R, Winer AM, Pitts JN. Estimation of night-time N2O5 concentrations from ambient NO2 and NO3 radical concentrations and the role of N2O5 in night-time chemistry. Atmos. Environ. 1986;20:331–339. [Google Scholar]
- 93.Moonshine M, Rudich Y, Katsman S, Graber ER. Atmospheric HULIS enhance pollutant degradation by promoting the dark Fenton reaction. Geophys. Res. Lett. 2008;35 doi: 10.1029/2008GL035285. [DOI] [Google Scholar]
- 94.Betterton EA, Hoffmann MR. Henry’s law constants of some environmentally important aldehydes. Environ. Sci. Technol. 1988;22:1415–1418. doi: 10.1021/es00177a004. [DOI] [PubMed] [Google Scholar]
- 95.Chameides WL, Davis DD. Aqueous-phase source of formic acid in clouds. Nature. 1983;304:427–429. [Google Scholar]
- 96.Bothe E, Schulte-Frohlinde D. Reaction of dihydroxymethylradical with molecular oxygen in aqueous solutions. Z.Naturforsch. 1980;35b:1035–1039. [Google Scholar]
- 97.Munger JW, Tiller C, Hoffmann MR. Identification of hydroxymethanesulfonate in fog water. Science. 1986;231:247–9. doi: 10.1126/science.231.4735.247. [DOI] [PubMed] [Google Scholar]
- 98.Ervens B, Feingold G, Frost GJ, Kreidenweis SM. A modeling of study of aqueous production of dicarboxylic acids: 1. Chemical pathways and speciated organic mass production. J. Geophys. Res. D Atmos. 2004;109 doi: 10.1029/2003JD004387. [DOI] [Google Scholar]
- 99.Atkinson R. Gas-phase tropospheric chemistry of organic compounds: A review. Atmos. Environ. Part A. Gen. Top. 1990;24:1–41. [Google Scholar]
- 100.Carlton AG, Turpin BJ, Altieri KE, et al. Atmospheric oxalic acid and SOA production from glyoxal: Results of aqueous photooxidation experiments. Atmos. Environ. 2007;41:7588–7602. [Google Scholar]
- 101.Karpel Vel Leitner N, Doré M. Mecanisme d’action des radicaux OH sur les acides glycolique, glyoxylique, acetique et oxalique en solution aqueuse: Incidence sur la consammation de peroxyde d’hydrogene dans les systemes H2O2UV et O3H2O2. Water Res. 1997;31:1383–1397. [Google Scholar]
- 102.Mcneill VF, Riipinen I. Effect of Inorganic Salts on the Volatility of Organic Acids. Environ. Sci. Techn. 2014;48:13718–13726. doi: 10.1021/es5033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ortiz-Montalvo DL, Lim YB, Perri MJ, Seitzinger SP, Turpin BJ. Volatility and Yield of Glycolaldehyde SOA Formed through Aqueous Photochemistry and Droplet Evaporation. Aerosol Sci. Technol. 2012;46:1002–1014. [Google Scholar]
- 104.Dziedzic P, Co A. Products and Kinetics of the Liquid-Phase Reaction of Glyoxal Catalyzed by Ammonium. J. Phys. Chem. A. 2009;113:231–237. doi: 10.1021/jp8078293. [DOI] [PubMed] [Google Scholar]
- 105.Nguyen TB, Coggon MM, Bates KH, et al. Organic aerosol formation from the reactive uptake of isoprene epoxydiols (IEPOX) onto non-acidified inorganic seeds. Atmos. Chem. Phys. 2014;14:3497–3510. [Google Scholar]
- 106.Surratt JD, Chan AWH, Eddingsaas NC, et al. Reactive intermediates revealed in secondary organic aerosol formation from isoprene. Proc. Natl. Acad. Sci. 2010;107:6640–6645. doi: 10.1073/pnas.0911114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.De Haan DO, Hawkins LN, Kononenko JA, et al. Formation of nitrogen-containing oligomers by methylglyoxal and amines in simulated evaporating cloud droplets. Environ. Sci. Technol. 2011;45:984–991. doi: 10.1021/es102933x. [DOI] [PubMed] [Google Scholar]
- 108.Fu H, Ciuraru R, Dupart Y, et al. Photosensitized Production of Atmospherically Reactive Organic Compounds at the Air/Aqueous Interface. J. Am. Chem. Soc. 2015;137:8348–8351. doi: 10.1021/jacs.5b04051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Singer BC, Hodgson AT, Hotchi T, et al. Sorption of organic gases in residential rooms. Atmos. Environ. 2007;41:3251–3265. [Google Scholar]
- 110.Morrison GC, Nazaroff WW. The Rate of Ozone Uptake on Carpets: Experimental Studies. Environ. Sci. Technol. 2000;34:4963–4968. [Google Scholar]
- 111.Docherty KS, Wu W, Lim YBin, Ziemann PJ. Contributions of Organic Peroxides to Secondary Aerosol Formed from Reactions of Monoterpenes with O 3. Environ. Sci. Technol. 2005;39:4049–4059. doi: 10.1021/es050228s. [DOI] [PubMed] [Google Scholar]
- 112.Tan Y, Lim YB, Altieri KE, Seitzinger SP, Turpin BJ. Mechanisms leading to oligomers and SOA through aqueous photooxidation: Insights from OH radical oxidation of acetic acid and methylglyoxal. Atmos. Chem. Phys. 2012;12:801–813. [Google Scholar]
- 113.Yu L, Smith J, Laskin A, et al. Chemical characterization of SOA formed from aqueous-phase reactions of phenols with the triplet excited state of carbonyl and hydroxyl radical. Atmos. Chem. Phys. 2014;14:13801–13816. [Google Scholar]
- 114.Ongwandee M, Bettinger SS, Morrison GC. The influence of ammonia and carbon dioxide on the sorption of a basic organic pollutant to a mineral surface. Indoor Air. 2005;15:408–419. doi: 10.1111/j.1600-0668.2005.00380.x. [DOI] [PubMed] [Google Scholar]
- 115.De Haan DO, Corrigan AL, Smith KW, et al. Secondary organic aerosol-forming reactions of glyoxal with amino acids. Environ. Sci. Technol. 2009;43:2818–2824. doi: 10.1021/es803534f. [DOI] [PubMed] [Google Scholar]
- 116.Finlayson-Pitts BJ, Pitts JN. Chemistry of the Upper and Lower Atmosphere. Elsevier Inc.; 2000. [Google Scholar]
- 117.Hill CAS, Norton A, Newman G. The water vapor sorption behavior of natural fibers. J. Appl. Polym. Sci. 2009;112:1524–1537. [Google Scholar]
- 118.Svennberg K, Hedegaard L, Rode C. Moisture Buffer Performance of a Fully Furnished Room. Proc. Build. IX, Clear. 2004 [Google Scholar]
- 119.Straube J, Onysko D, Schumacher C. Methodology and Design of Field Experiments for Monitoring the Hygrothermal Performance of Wood Frame Enclosures. J. Build. Phys. 2002;26:123–151. [Google Scholar]
- 120.Baergen AM, Styler SA, van Pinxteren D, et al. Chemistry of Urban Grime: Inorganic Ion Composition of Grime vs Particles in Leipzig, Germany. Environ. Sci. Technol. 2015;49:12688–12696. doi: 10.1021/acs.est.5b03054. [DOI] [PubMed] [Google Scholar]
- 121.Künzel HM, Holm AH, Sedlbauer K. Predicting indoor temperature and humidity conditions including hygrothermal interactions with the building envelope. ASHRAE Transactions. 2004;110(PART I):820–826. [Google Scholar]
- 122.Saxena P, Hildemann LM. Water-soluble organics in atmospheric particles: A critical review of the literature and application of thermodynamics to identify candidate compounds. J. Atmos. Chem. 1996;24:57–109. [Google Scholar]
- 123.Nguyen TKV, Zhang Q, Jimenez JL, Pike M, Carlton AMG. Liquid water: ubiquitous contributor to aerosol mass. Environ. Sci. Technol. Lett. 2016;3:257–263. [Google Scholar]
- 124.Liu Q-T, Chen R, McCarry BE, Diamond ML, Bahavar B. Characterization of Polar Organic Compounds in the Organic Film on Indoor and Outdoor Glass Windows. Environ. Sci. Technol. 2003;37:2340–2349. doi: 10.1021/es020848i. [DOI] [PubMed] [Google Scholar]
- 125.Gao W, Wu J, Wang Y, Jiang G. Distribution and congener profiles of short-chain chlorinated paraffins in indoor/outdoor glass window surface films and their film-air partitioning in Beijing, China. Chemosphere. 2016;144:1327–1333. doi: 10.1016/j.chemosphere.2015.09.075. [DOI] [PubMed] [Google Scholar]
- 126.Huo CY, Liu LY, Zhang ZF, et al. Phthalate esters in indoor window films in a northeastern Chinese urban center: Film growth and implications for human exposure. Environ. Sci. Technol. 2016;50:7743–7751. doi: 10.1021/acs.est.5b06371. [DOI] [PubMed] [Google Scholar]
- 127.Li J, Lin T, Pan S-H, et al. Carbonaceous matter and PBDEs on indoor/outdoor glass window surfaces in Guangzhou and Hong Kong, South China. Atmos. Environ. 2010;44:3254–3260. [Google Scholar]
- 128.Weschler CJ, Nazaroff WW. Growth of organic films on indoor surfaces. Indoor Air. 2017 doi: 10.1111/ina.12396. [DOI] [PubMed] [Google Scholar]
- 129.Simonson CJ, Salonvaara M, Ojanen T, Walker I, Levin H. Moderating indoor conditions with hygroscopic building materials and outdoor ventilation. ASHRAE Trans. 2004;110:804–819. [Google Scholar]
- 130.Waxman EM, Elm J, Kurtén T, et al. Glyoxal and Methylglyoxal Setschenow Salting Constants in Sulfate, Nitrate, and Chloride Solutions: Measurements and Gibbs Energies. Environ. Sci. Technol. 2015;49:11500–11508. doi: 10.1021/acs.est.5b02782. [DOI] [PubMed] [Google Scholar]
- 131.Lim YB, Turpin BJ. Laboratory evidence of organic peroxide and peroxyhemiacetal formation in the aqueous phase and implications for aqueous OH. Atmos. Chem. Phys. 2015;15:12867–12877. [Google Scholar]
- 132.Aregahegn KZ, Nozière B, George C, et al. Organic aerosol formation photo-enhanced by the formation of secondary photosensitizers in aerosols. Faraday Discuss. 2013;165:123. doi: 10.1039/c3fd00044c. [DOI] [PubMed] [Google Scholar]
- 133.Vidrio E, Jung H, Anastasio C. Generation of hydroxyl radicals from dissolved transition metals in surrogate lung fluid solutions. Atmos. Environ. 2008;42:4369–4379. doi: 10.1016/j.atmosenv.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Weschler CJ. Roles of the human occupant in indoor chemistry. Indoor Air. 2016;26:6–24. doi: 10.1111/ina.12185. [DOI] [PubMed] [Google Scholar]
- 135.Duplissy J, DeCarlo PF, Dommen J, et al. Relating hygroscopicity and composition of organic aerosol particulate matter. Atmos. Chem. Phys. 2011;11:1155–1165. [Google Scholar]
- 136.Peng C, Chan MN, Chan CK. The hygroscopic properties of dicarboxylic and multifunctional acids: Measurements and UNIFAC predictions. Environ. Sci. Technol. 2001;35:4495–4501. doi: 10.1021/es0107531. [DOI] [PubMed] [Google Scholar]
- 137.Man NC, Kreidenweis SM, Chan CK. Measurements of the hygroscopic and deliquescence properties of organic compounds of different solubilities in water and their relationship with cloud condensation nuclei activities. Environ. Sci. Technol. 2008;42:3602–3608. doi: 10.1021/es7023252. [DOI] [PubMed] [Google Scholar]
- 138.Svenningsson B, Rissler J, Swietlicki E, et al. Hygroscopic growth and critical supersaturations for mixed aerosol particles of inorganic and organic compounds of atmospheric relevance. Atmos. Chem. Phys. Atmos. Chem. Phys. 2006;6:1937–1952. [Google Scholar]
- 139.Smith JN, Barsanti KC, Friedli HR, et al. Observations of aminium salts in atmospheric nanoparticles and possible climatic implications. Proc. Natl. Acad. Sci. U. S. A. 2010;107:6634–9. doi: 10.1073/pnas.0912127107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fujishima A, Rao TN, Tryk DA. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000;1:1–21. [Google Scholar]
- 141.Ciuraru R, Fine L, van Pinxteren M, et al. Photosensitized production of functionalized and unsaturated organic compounds at the air-sea interface. Sci. Rep. 2015;5 doi: 10.1038/srep12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wong JP, Zhao R, Zhou S, Carslawc N, Abbatt JPD. Observations and impacts of bleach washing on indoor air chlorine chemistry. Indoor Air. 2017 doi: 10.1111/ina.12402. [DOI] [PubMed] [Google Scholar]
- 143.Šišović A, Šega K, Kalinić N. Indoor/outdoor relationship of ammonia concentrations in selected office buildings. Sci. Total Environ. 1987;61:73–77. [Google Scholar]
- 144.Weschler CJ. Chemistry in indoor environments: 20 years of research. Indoor Air. 2011;21:205–218. doi: 10.1111/j.1600-0668.2011.00713.x. [DOI] [PubMed] [Google Scholar]
- 145.Lim YB, Tan Y, Perri MJ, Seitzinger SP, Turpin BJ. Aqueous chemistry and its role in secondary organic aerosol (SOA) formation. Atmos. Chem. Phys. 2010;10:10521–10539. [Google Scholar]
- 146.Li YJ, Huang DD, Cheung HY, Lee AKY, Chan CK. Aqueous-phase photochemical oxidation and direct photolysis of vanillin - a model compound of methoxy phenols from biomass burning. Atmos. Chem. Phys. 2014;14:2871–2885. [Google Scholar]
- 147.Laskin A, Moffet RC, Gilles MK, et al. Tropospheric chemistry of internally mixed sea salt and organic particles: Surprising reactivity of NaCl with weak organic acids. J. Geophys. Res. Atmos. 2012;117 doi: 10.1029/2012JD017743. [DOI] [Google Scholar]
- 148.Richards-Henderson NK, Callahan KM, Nissenson P, et al. Production of gas phase NO2 and halogens from the photolysis of thin water films containing nitrate, chloride and bromide ions at room temperature. Phys. Chem. Chem. Phys. 2013;15:17636–17646. doi: 10.1039/c3cp52956h. [DOI] [PubMed] [Google Scholar]
- 149.Raff JD, Njegic B, Chang WL, et al. Chlorine activation indoors and outdoors via surface-mediated reactions of nitrogen oxides with hydrogen chloride. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13647–54. doi: 10.1073/pnas.0904195106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jung W-W, Kim E-M, Lee E-H, et al. Formaldehyde exposure induces airway inflammation by increasing eosinophil infiltrations through the regulation of reactive oxygen species production. Environ. Toxicol. Pharmacol. 2007;24:174–182. doi: 10.1016/j.etap.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 151.Lin Y-H, Arashiro M, Martin E, et al. Isoprene-Derived Secondary Organic Aerosol Induces the Expression of Oxidative Stress Response Genes in Human Lung Cells. Environ. Sci. Technol. Lett. 2016;3:250–254. [Google Scholar]
- 152.Charrier JG, McFall AS, Richards-Henderson NK, Anastasio C. Hydrogen peroxide formation in a surrogate lung fluid by transition metals and quinones present in particulate matter. Environ. Sci. Technol. 2014;48:7010–7017. doi: 10.1021/es501011w. [DOI] [PMC free article] [PubMed] [Google Scholar]