Abstract
BACKGROUND
A cohort of heart failure (HF) patients receiving left ventricular assist devices (LVADs) has decoupling of their diastolic pulmonary artery pressure and pulmonary capillary wedge pressure. However, the clinical implications of this decoupling remain unclear.
METHODS AND RESULTS
In this prospective study, patients with LVADs underwent routine invasive hemodynamic ramp testing with right heart catheterization, during which LVAD speeds were adjusted. Inappropriate decoupling was defined as a >5 mm Hg difference between diastolic pulmonary artery pressure and pulmonary capillary wedge pressure. The primary outcomes of survival and heart failure readmission rates after ramp testing were assessed. Among 63 LVAD patients (60±12 years old and 25 female [40%]), 27 patients (43%) had inappropriate decoupling at their baseline speed. After adjustment of their rotation speed during ramp testing, 30 patients (48%) had inappropriate decoupling. Uni/multivariable Cox analyses demonstrated that decoupling was the only significant predictor for the composite end point of death and heart failure readmission during the 1 year following the ramp study (total of 18 events; hazards ratio, 1.09; 95% confidence interval, 1.04–1.24; P<0.05). Furthermore, normalization of decoupling (n=8) during ramp testing was significantly associated with higher 1-year heart failure readmission–free survival rate compared with the non-normalized group (n=19, 100% versus 53%; P=0.035).
CONCLUSIONS
The presence of inappropriate decoupling was associated with worse outcomes in patients with LVADs. Prospective, large-scale multicenter studies to validate the result are warranted.
Keywords: heart failure, hemodynamics, pulmonary artery, survival rate
Left ventricular assist device (LVAD) therapy significantly improves long-term survival rates in patients with stage D heart failure (HF), particularly in the era of modern devices such as HVAD (HeartWare International, Framingham, MA) and HeartMate II (Abbott, Pleasanton, CA).1,2 Improved optimization of patient selection and sophisticated management strategies for complications have further improved postimplantation quality of life and survival rates.3,4 However, recurrent decompensated HF remains a common cause of hospitalization in these patients.
Recently, several institutions have implemented LVAD speed optimization using right heart catheterization to adjust LVAD speed and patient medical therapy.5 A portion of these patients exhibit an excessive difference between diastolic pulmonary artery pressure (dPAP) and pulmonary capillary wedge pressure (PCWP). Such decoupling, defined as an excessive increase of dPAP gradient, provides an index of precapillary pulmonary hypertension (PH) which may be superimposed on post-capillary PH in patients with left heart disease.6
There is a paucity of data on the mechanism and prognostic implications of decoupling in LVAD patients, particularly on the recurrence of HF. Furthermore, the clinical impact of changes in the degree of decoupling during rotation speed change is unknown. In this study, we investigated the clinical significance of decoupling and its reversibility in patients who undergo ramp testing while on LVAD support.
METHODS
Patient Selection
We prospectively collected data on clinically stable outpatients with LVADs (HVAD or HeartMate II) followed at our institution who were evaluated with a hemodynamic ramp testing. All patients received guideline-directed medical therapy before testing.7 Patients with suspicion for LVAD thrombosis or device malfunction were excluded. Written informed consent was obtained from all participants before ramp testing. The study protocol was approved by the Ethics Committee at our institution.
Ramp Testing Protocol
Ramp tests evaluated hemodynamics by right heart catheterization in combination with transthoracic echocardiography. Serial measurements were performed starting with the patient’s baseline speed and then again after the devices were turned down to a minimal acceptable speed; subsequently, measurements were taken at progressively increasing speeds to a maximal value.5 Cardiac output and cardiac index were calculated by the indirect Fick method. At the conclusion of each test, an attending cardiologist reviewed the data, and LVAD speed was adjusted to the final speed to achieve hemodynamic optimization. Primary goals were central venous pressure <12 mm Hg and PCWP <18 mm Hg, with secondary goals of intermittent aortic valve opening and minimal mitral regurgitation.
Variables Evaluated
Patients’ preoperative background characteristics, including demographic, echocardiographic, and hemodynamic data, were obtained within the 1-month period preceding LVAD implantation. During ramp testing, hemodynamic and echocardiographic data were obtained per protocol. All hemodynamic data were reviewed by the attending cardiologist, who was blinded to the study results to ensure accuracy of measurements. Inappropriate decoupling was defined as dPAP–PCWP, difference of >5 mm Hg. Pulmonary artery pulsatility index was calculated according to (systolic PAP–dPAP)/central venous pressure. Pulmonary artery compliance was calculated according to stroke volume/(systolic PAP–dPAP). All patients were followed at our institution at the final speed determined according to the results of the ramp test.
Statistical Analyses
Baseline data were expressed as mean±SD, unless otherwise indicated. Continuous variables were compared between groups using the unpaired t test or Mann–Whitney U test as appropriate, and categorical variables were compared between groups using the χ2 test or Fisher exact test as appropriate. Correlation between decoupling and other hemodynamic variables was assessed by Pearson correlation coefficient.
The primary end point of this study was the composite of all-cause mortality and HF readmission from the time of the ramp test (time zero) through 1-year follow-up. Patients were censored at the termination of LVAD therapy because of explantation or heart transplantation. The prognostic impact of hemodynamic variables at the final speed setting was analyzed by Cox hazards ratio analysis. Variables with P <0.05 of significance in univariable analyses were entered into multivariable analyses after the confirmation that there was no significant multicollinearity among them (variance inflation factor <5 was considered nonsignificant). Cutoff values of variables predicting prognosis were calculated by using receiver operating characteristic analysis. Patients’ prognosis stratified by the existence of inappropriate decoupling was assessed by Kaplan–Meier analyses and compared by log-rank test. All statistical analyses were performed using SPSS Statistics 22 (SPSS Inc, Chicago, IL).
RESULTS
Baseline Characteristics
Sixty-three LVAD patients (19 HVAD and 44 HeartMate II) were enrolled (Table 1). Patients were 59.9±11.5 years old, and 25 (40%) were female. The majority of patients were implanted as destination therapy (79%), and 33 (52%) had an ischemic cause of their cardiomyopathy. The median duration between time of LVAD implantation and ramp testing was 280 days (13–1954 days).
Table 1.
Comparison of Background Characteristics Stratified by the Existence of Decoupling at the Final Speed
| Total (n=63) | Inappropriate Decoupling (n=30) |
Noninappropriate Decoupling (n=33) |
P Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 59.9±11.5 | 57.9±10.4 | 61.7±12.3 | 0.20 |
| Sex (female) | 25 (40) | 15 (50) | 10 (30) | 0.09 |
| Race (white) | 32 (51) | 12 (40) | 20 (61) | 0.31 |
| Nonischemic cause | 33 (52) | 17(57) | 16 (48) | 0.35 |
| Body height, cm | 170±19 | 173±10 | 173±11 | 0.84 |
| Body weight, kg | 89.0±23.2 | 85.6±21.8 | 92.1±24.4 | 0.27 |
| HeartMate II | 44 (70) | 20 (66) | 24 (73) | 0.60 |
| HVAD | 19 (30) | 10 (33) | 19 (58) | 0.60 |
| Destination therapy | 50 (79) | 27 (90) | 23 (70) | 0.045* |
| Diabetes mellitus | 23 (37) | 9 (30) | 14 (42) | 0.22 |
| Hypertension | 35 (56) | 14 (47) | 21 (64) | 0.14 |
| Peripheral artery disease | 2 (3) | 0 (0) | 2 (6) | 0.17 |
| Atrial fibrillation | 24 (38) | 13 (43) | 11 (33) | 0.29 |
| History of ventricular tachyarrhythmia | 14 (22) | 7 (23) | 7 (21) | 0.54 |
| Chronic obstructive pulmonary disease | 11 (17) | 8 (27) | 3 (9) | 0.066 |
| LVAD duration before ramp testing, d | 507±534 | 443±447 | 565±604 | 0.39 |
| Echocardiography | ||||
| LVDd, cm | 7.50±1.09 | 7.54±0.76 | 7.47±1.33 | 0.77 |
| Hemodynamics | ||||
| PCWP, mm Hg | 24.0±9.6 | 25.5±10.1 | 22.7±9.1 | 0.34 |
| CI, L min−1 m−2 | 1.86±0.58 | 1.88±0.50 | 1.84±0.65 | 0.81 |
| mPAP, mm Hg | 36.4±12.0 | 36.7±11.9 | 36.1±12.3 | 0.87 |
| dPAP, mm Hg | 27.7±11.2 | 29.9±12.2 | 25.8±10.0 | 0.21 |
| CVP, mm Hg | 11.9±7.5 | 11.9±8.7 | 11.8±6.5 | 0.96 |
| PVR, WU | 3.65±2.79 | 3.28±2.65 | 4.02±2.95 | 0.39 |
CI indicates cardiac index; CVP, central venous pressure; dPAP, diastolic pulmonary artery pressure; LVAD, left ventricular assist device; LVDd, left ventricular diastolic diameter; mPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; and PVR, pulmonary vascular resistance.
P<0.05 by χ2 test.
Decoupling and Other Clinical Variables
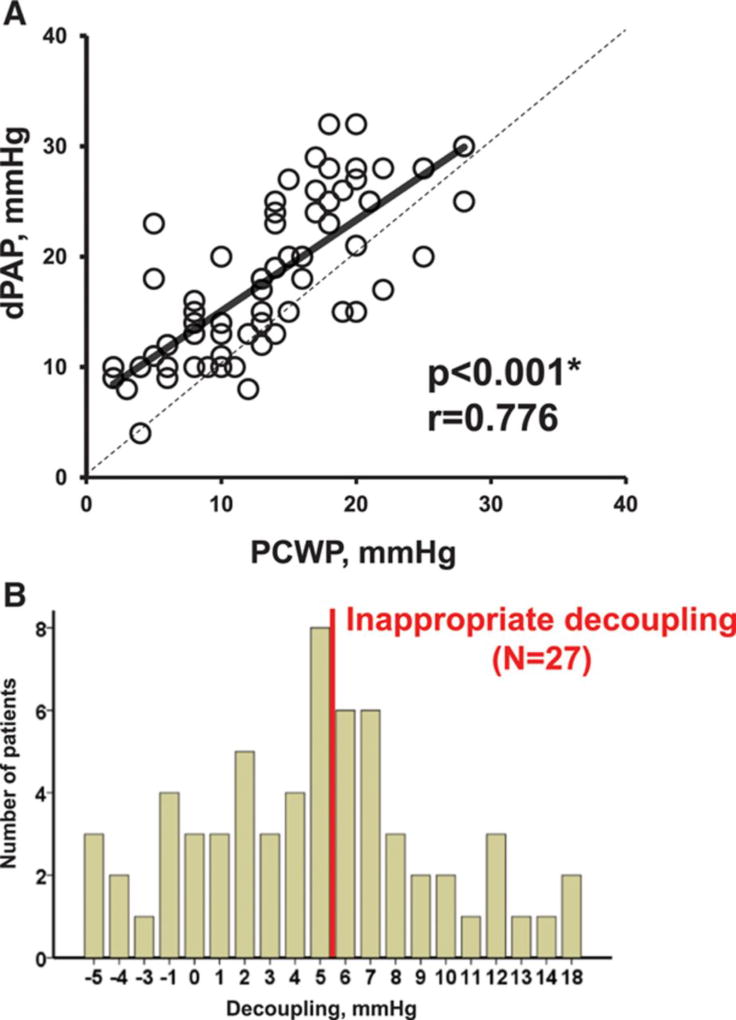
At the baseline speed, there was a significant correlation between dPAP and PCWP (Figure 1A). Decoupling was normally distributed (P=0.290 by Shapiro–Wilk test) and averaged 4.7±5.2 mm Hg, ranging between −5 and 18 mm Hg. Twenty-seven patients (43%) had inappropriate decoupling at their baseline speed (Figure 1B).
Figure 1. Relationship between diastolic pulmonary artery pressure (dPAP) and pulmonary capillary wedge pressure (PCWP; A) and distribution of the decoupling (B).
*P<0.05 by Pearson correlation coefficiency.
At the final speed setting, 30 patients (48%) had inappropriate decoupling. Comparison of preoperative background characteristics demonstrated that more patients with inappropriate decoupling received LVAD as destination therapy than those without. Otherwise, there were no significant differences in baseline characteristics between these 2 groups (Table 1). At the baseline speed setting, the decoupling group had higher PAP and pulmonary vascular resistance (PVR) compared with the nondecoupling group (Table 2; P<0.05 for all). Pulmonary artery pulsatility index and pulmonary artery compliance were comparable irrespective of inappropriate decoupling.
Table 2.
Postoperative Echocardiographic and Hemodynamic Data Stratified by the Existence of Inappropriate Decoupling at Final Speed
| Inappropriate Decoupling Group (n=30) |
Noninappropriate Decoupling Group (n=33) |
P Value | |
|---|---|---|---|
| Echocardiography | |||
| LVDd, cm | 6.08±1.00 | 6.10±1.22 | 0.96 |
| Hemodynamics | |||
| PCWP, mm Hg | 14.1±5.9 | 13.8±6.8 | 0.84 |
| CI, L min−1 m−2 | 2.52±0.38 | 2.89±0.84 | 0.062 |
| mPAP, mm Hg | 27.6±7.3 | 21.8±6.7 | 0.003* |
| dPAP, mm Hg | 20.9±6.4 | 16.2±6.3 | 0.007* |
| Heart rate, beats per minute | 84.5±11.7 | 82.4±11.0 | 0.49 |
| RVSWI, g/m2 | 7.38±2.65 | 6.43±3.31 | 0.25 |
| CVP, mm Hg | 10.3±4.6 | 8.2±5.9 | 0.15 |
| PVR, WU | 2.84±1.35 | 1.51±0.84 | <0.001* |
| PAPi | 2.74±2.21 | 3.09±2.01 | 0.51 |
| PAC, mL/mm Hg | 3.64±1.37 | 4.17±1.36 | 0.38 |
CI indicates cardiac index; CVP, central venous pressure; dPAP, diastolic pulmonary artery pressure; LVDd, left ventricular diastolic diameter; mPAP, mean pulmonary artery pressure; PAC, pulmonary artery compliance; PAPi, pulmonary artery pulsatility index; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; and RVSWI, right ventricular stroke work index.
P<0.05 by unpaired t test.
Decoupling and Patient Prognosis
The group of patients with inappropriate decoupling experienced 6 deaths and 8 HF readmissions. In comparison, the group of patients without inappropriate decoupling experienced 1 death and 3 HF readmissions. In univariable Cox regression analysis of hemodynamic characteristics at the final speed setting, mean PAP, dPAP, and decoupling were significant predictors of death or HF readmission during the 1-year study period (Table 3; P<0.05 for all analyses). PVR was not a significant predictor of the outcome (P=0.094). Multivariable analysis revealed that decoupling was the only significant predictor of death or HF readmission (Table 3; P=0.046; hazards ratio, 1.09; 95% confidential interval, 1.04–1.24). Variance inflation factors of decoupling, dPAP, and mean PAP were 1.482, 13.52, and 13.35, respectively, and dPAP was not included in the multivariable analysis because of its high collinearity with mean PAP. By receiver operating characteristic analysis, the cutoff level for decoupling was found to be values >5 mm Hg (Appendix Figure I in the Data Supplement).
Table 3.
Univariable/Multivariable Cox Hazard Ratio Analysis for the Prediction of Death or Heart Failure Readmission Among Hemodynamics at Final Speed
| Univariable | Multivariable | |||
|---|---|---|---|---|
| P Value | Hazards Ratio (95% CI) |
P Value | Hazards Ratio (95% CI) |
|
| mPAP, mm Hg | 0.005* | 1.10 (1.03–1.17) | 0.22 | 1.05 (0.97–1.15) |
| dPAP, mm Hg | 0.002* | 1.13 (1.05–1.22) | ||
| PVR, WU | 0.094 | 1.33 (0.95–1.86) | ||
| Decoupling, mm Hg | 0.003* | 1.16 (1.05–1.27) | 0.046* | 1.09 (1.04–1.24) |
CI indicates confidence interval; dPAP, diastolic pulmonary artery pressure; mPAP, mean pulmonary artery pressure; and PVR, pulmonary vascular resistance.
P<0.05 by Cox hazard ratio analysis.
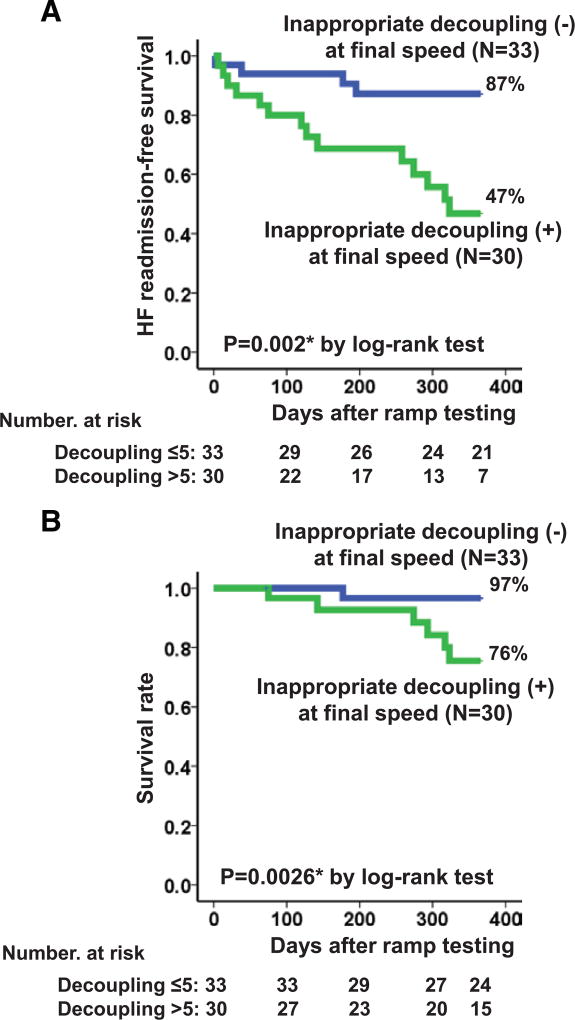
Kaplan–Meier analysis showed that patients with inappropriate decoupling had significantly lower HF readmission–free survival compared with those without inappropriate decoupling during the 1-year study period (Figure 2A; 47% versus 87%; P=0.002). Overall mortality was also significantly higher in the group with inappropriate decoupling (Figure 2B; P=0.026).
Figure 2. Comparison of heart failure (HF) readmission–free survival rate (A) and survival rate (B) stratified by the existence of inappropriate decoupling at the final speed setting.
*P<0.05 by the log-rank test.
Among 19 HVAD patients, 10 patients with inappropriate decoupling had a trend toward lower HF readmission–free survival compared with 9 without inappropriate decoupling (47% versus 80%; P=0.16). Among 44 HeartMate II patients, 20 patients with inappropriate decoupling had significantly lower HF readmission–free survival compared with 24 without inappropriate decoupling (48% versus 91%; P=0.005).
Changes in Decoupling During Ramp Testing and Patients’ Prognosis
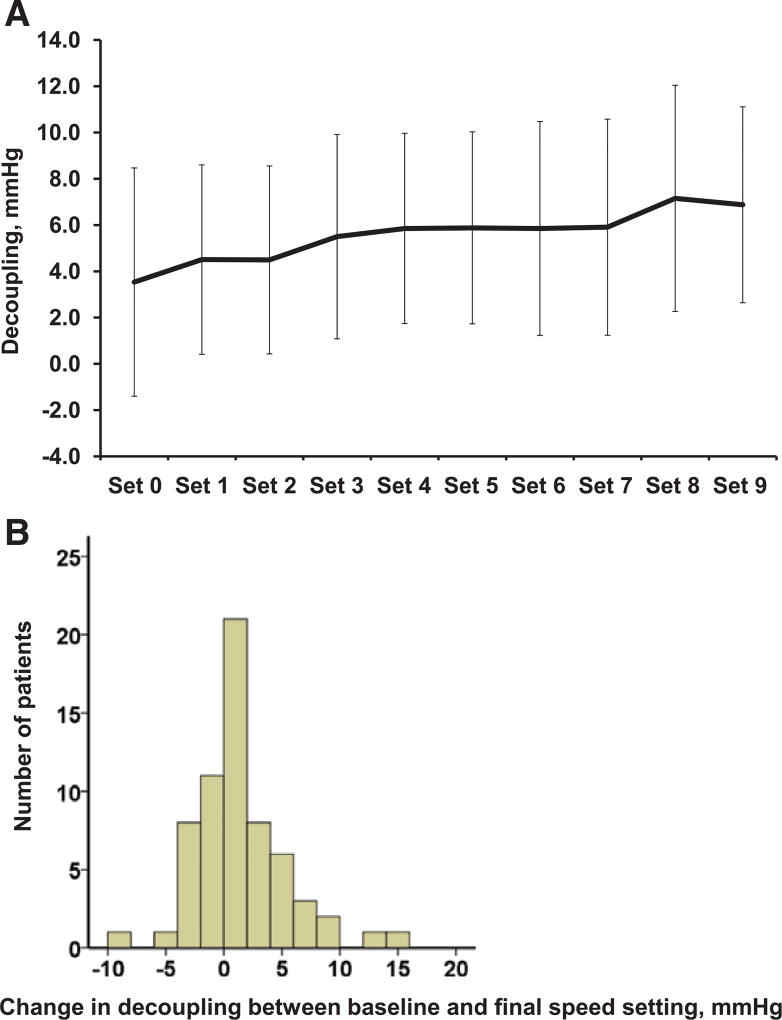
During ramp testing, 49 patients (78%) had increasing degrees of decoupling at incremental rotation speed. The relationship between decoupling and rotation speed (expressed as speed steps) is shown in Figure 3A. Eventually, the change in decoupling between baseline and final speed averaged 0.95±3.97 mm Hg (range, −9 to 15 mm Hg), and 26 patients (41%) experienced an increase in decoupling between baseline and final speed settings (Figure 3B).
Figure 3. Trend of decoupling during speed ramp testing (A) and distribution of change in decoupling between baseline and the final speed setting (B).
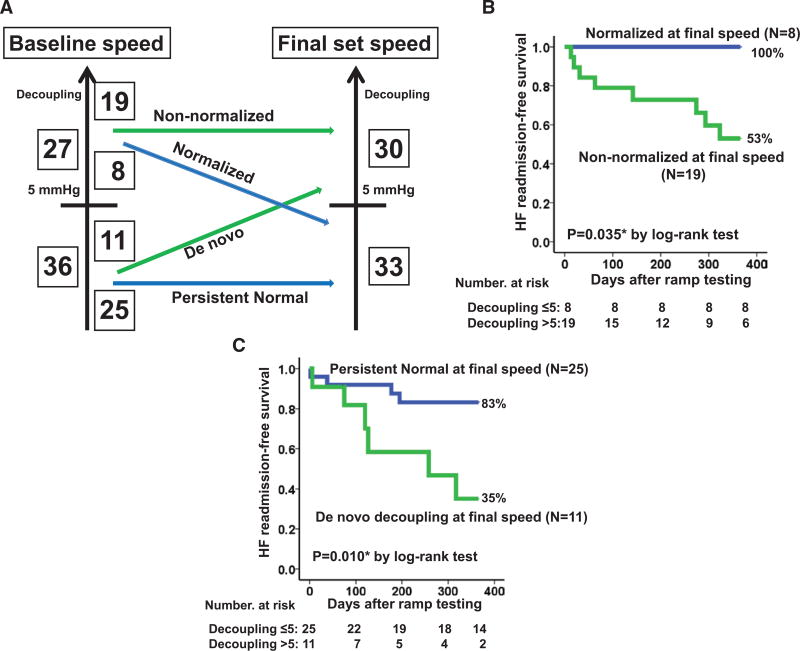
Among 27 patients with inappropriate decoupling at baseline speed, 8 (30%) achieved normalization of decoupling at the final speed (Figure 4A). This cohort had higher HF readmission–free survival rates than the non-normalized group (Figure 4B; 100% versus 53%; P=0.035). There were no significant differences in preoperative background characteristics between the normalized and non-normalized groups (data not shown). Patients in the normalized group had comparable LVAD duration to those in the non-normalized group (530.1±757.1 versus 347.2±341.3 days; P=0.87).
Figure 4. Changes in the proportion of inappropriate decoupling during ramp test and its prognostic impact.
Changes in the patients number with/without inappropriate decoupling between baseline and the final speed setting (A) and comparison of heart failure (HF) readmission–free survival rate stratified by the changes in decoupling during the ramp testing (B, decoupling >5 mm Hg at baseline speed setting; C, decoupling ≤5 mm Hg at baseline speed setting). *P<0.05 by the log-rank test.
Among 36 patients without inappropriate decoupling at their baseline speed, 11 (31%) experienced de novo inappropriate decoupling at their final speed (Figure 4A). This cohort had significantly lower HF readmission–free survival compared with the persistent normal group (Figure 4C; 35% versus 83%; P=0.010). There were no significant differences in preoperative background variables between these groups (data are not shown).
These trends were similar for HVAD and HeartMate II subgroups. Among 19 HVAD patients, normalization of decoupling was associated with a trend toward higher HF readmission–free survival compared with the persistent decoupling group (100% versus 54%; P=0.14). Among 44 HeartMate II patients, normalization of decoupling was associated with a trend toward higher HF readmission–free survival compared with the persistent decoupling group (100% versus 53%; P=0.16). De novo decoupling was associated with lower HF readmission–free survival compared with the persistent normal group (38% versus 89%; P=0.010).
Several differences were noted in hemodynamic characteristics between the baseline and final speed settings (Table 4). Among patients with inappropriate decoupling at baseline speed, PCWP tended to increase in the normalized group (LVAD speed was increased in 3 patients, decreased in 4 patients, and unchanged in 1 patient), whereas PCWP tended to decrease at incremental rotation speed in the persistent decoupling group. Among patients without inappropriate decoupling at baseline speed, dPAP and PVR increased significantly accompanied by a trend toward decrease in PCWP in the de novo decoupling group (LVAD speed was increased in 4 patients, decreased in 4 patients, and unchanged in 3 patients). Rotation speed could be increased without the development of decoupling in the persistent normal group.
Table 4.
Changes in Hemodynamic Variables Between Baseline and the Final Speed Setting Stratified by Changes in Decoupling
| Baseline Speed | Final Speed | P Value | |
|---|---|---|---|
| Normalized group (n=8) | |||
| Rotation speed | |||
| HeartMate II (n=4), rpm | 9533±416 | 9600±600 | 0.67 |
| HVAD (n=4), rpm | 2725±143 | 2705±175 | 0.35 |
| PCWP, mm Hg | 9.6±8.0 | 12.6±8.4 | 0.078 |
| CI, L min−1 m−2 | 2.79±0.68 | 2.50±0.49 | 0.16 |
| dPAP, mm Hg | 17.0±7.7 | 16.6±8.1 | 0.56 |
| CVP, mm Hg | 6.9±6.1 | 7.1±4.9 | 0.90 |
| PVR, WU | 2.03±0.53 | 1.79±0.66 | 0.27 |
| Non-normalized group (n=19) | |||
| Rotation speed | |||
| HeartMate II (n=14), rpm | 9015±377 | 9267±421 | 0.013* |
| HVAD (n=5), rpm | 2704±182 | 2792±168 | 0.28 |
| PCWP, mm Hg | 13.2±5.7 | 12.2±5.6 | 0.20 |
| CI, L min−1 m−2 | 2.57±0.38 | 2.70±0.67 | 0.29 |
| dPAP, mm Hg | 22.8±6.5 | 21.8±7.0 | 0.18 |
| CVP, mm Hg | 10.4±4.8 | 9.3±4.1 | 0.021* |
| PVR, WU | 3.43±1.29 | 3.40±1.39 | 0.87 |
| Persistent normal group (n=25) | |||
| Rotation speed | |||
| HeartMate II (n=20), rpm | 9219±375 | 9486±415 | 0.013* |
| HVAD (n=5), rpm | 2580±86 | 2644±124 | 0.040* |
| PCWP, mm Hg | 15.0±6.0 | 14.3±5.8 | 0.33 |
| CI, L min−1 m−2 | 2.93±0.89 | 2.92±0.87 | 0.99 |
| dPAP, mm Hg | 15.9±6.0 | 16.1±6.6 | 0.84 |
| CVP, mm Hg | 8.6±5.9 | 8.9±6.9 | 0.56 |
| PVR, WU | 1.34±0.86 | 1.54±0.70 | 0.15 |
| De novo decoupling group (n=11) | |||
| Rotation speed | |||
| HeartMate II (n=8), rpm | 9429±214 | 9486±279 | 0.36 |
| HVAD (n=3), rpm | 2733±181 | 2780±72 | 0.75 |
| PCWP, mm Hg | 15.6±6.2 | 12.7±4.2 | 0.10 |
| CI, L min−1 m−2 | 2.61±0.28 | 2.68±0.35 | 0.61 |
| dPAP, mm Hg | 17.7±5.0 | 20.8±3.8 | 0.009* |
| CVP, mm Hg | 10.0±4.6 | 9.1±5.4 | 0.33 |
| PVR, WU | 1.80±0.43 | 2.34±0.41 | 0.018* |
CI indicates cardiac index; CVP, central venous pressure; dPAP, diastolic pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; and PVR, pulmonary vascular resistance.
P<0.05 by paired t test.
DISCUSSION
In this prospective study, we analyzed the prognostic implications of decoupling during LVAD support. The main finding is that inappropriate decoupling is common in clinically stable outpatients with LVAD and a strong predictor of the composite end point of death or HF readmission. More than 40% of stable outpatients with LVAD had inappropriate decoupling without a trend toward PH in preoperative hemodynamics. In addition, the degree of decoupling often changed during ramp testing, and this change also had prognostic implications: those whose decoupling normalized as a result of ramp test–guided speed adjustment had a better prognosis compared with those that did not.
Inappropriate Decoupling During LVAD Therapy
We quantified the degree of decoupling in clinically stable LVAD patients irrespective of the existence of PH; as a result, the decoupling group included many patients without PH. Considering that patients with inappropriate decoupling had higher PVR, inappropriate decoupling may indicate damage to the pulmonary vasculature. Pathological studies may provide further insight into the mechanism underlying the current results.
Prognostic Impact of Decoupling in LVAD Population
Decoupling was the strongest predictor of the composite of HF readmission and mortality among all considered hemodynamic parameters. This easily calculable variable is unique because inappropriate decoupling can exist irrespective of PH. PVR was not a significant predictor in univariable analysis, probably because of the dependency of mean PAP and cardiac output on volume and flow.8
There are no prior studies evaluating the prognostic implications of decoupling in LVAD patients. However, prior research has been conducted on decoupling in other clinical settings. Tedford et al9 showed that preoperative decoupling had no effect on postheart transplant survival in patients with reactive PH. However, in this study, preoperative decoupling may be diminished after drastic improvement in hemodynamics by heart transplantation. In contrast, among HF patients with reactive PH, Gerges et al10 demonstrated that decoupling identified those with significant pulmonary vascular disease and increased mortality. They enrolled only patients with reactive PH, whereas we expanded the research population to include all LVAD patients, including those without PH.
Patients with inappropriate decoupling were more likely to have an HF readmission during the study period. Pulmonary vascular damage, as indicated by the presence of decoupling, may present a greater hemodynamic load on the right ventricle, leading to subsequent right ventricular failure and hospitalizations. Although there was no association between decoupling and other hemodynamic variables associated with right ventricular failure, repeated hemodynamic and echocardiographic studies may demonstrate worsening of right ventricular function over time.6
Changes in Decoupling by Ramp Testing and Its Prognostic Impact
Normalization of decoupling was associated with improvement in overall patient prognosis, whereas patients with de novo decoupling had a worse overall prognosis. Considering these results, decoupling may be a novel therapeutic target during LVAD therapy, although this should be validated in a larger-scale study. Anti-PH medications such as phosphodiesterase inhibitors and endothelin receptor antagonists may have potential benefit in decreasing decoupling.11 Very limited data exist on their efficacy in LVAD recipients with residual PH, likely because of their side effects including liver and renal dysfunction.12,13
No background factors were identified in association with changes in decoupling during speed changes. Longer duration of LVAD support before the ramp testing was not associated with changes in decoupling during the ramp test. Duration of preoperative HF to LVAD implant may be associated with reversibility of decoupling; however, these data were not available in this study. Patients experiencing long-term HF may be more likely to develop de novo decoupling during LVAD speed changes because of the presence of irreversible damage to the pulmonary vasculature.
Among those with inappropriate decoupling at baseline speed, irreversible pulmonary vasculature damage may exist. In this situation, unnecessarily aggressive reduction of PCWP leads to worsening of decoupling accompanied by persistently elevated dPAP during ramp test (non-normalized group) and may not be recommended. Instead, leaving PCWP unchanged may be recommended to normalize decoupling (normalized group).
Among those without decoupling at baseline speed, some patients may have advanced pulmonary vasculature pathology, and others may not. In patients with such advanced pulmonary vasculature pathology, reduction in PCWP may lead to the development of decoupling accompanied by persistently elevated dPAP during ramp tests (these are the de novo decoupling group). During hemodynamic ramp testing, decreases in PCWP can occur even when the final speed is reduced compared with the baseline speed, and, therefore, it is more important to give attention to the changes in hemodynamics than the change in speed. In contrast, decreases in PCWP may not necessarily be associated with the development of decoupling in patients without significant pulmonary vascular pathology (these are the persistent normal group).
Repeated Adjustment of Rotation Speed to Reduce Decoupling
On the basis of the results of the present study, the use of ramp testing to adjust rotation speed to reduce the degree of decoupling may lead to improved outcomes in LVAD patients, although prospective large-scale study is required to validate our results before such an approach can be recommended. Recently, pulmonary artery sensors (CardioMEMS, Inc, Atlanta, GA) have become widely used to adjust medical therapies in outpatients with HF or PH.14,15 Noninvasive technologies such as remote dielectric sensing are also being used to quantify lung fluid content and estimate filling pressures.16 Although the clinical implication of these technologies in LVAD patients remains uncertain, our study highlights the importance of understanding both the PCWP and PAP in LVAD patients.
Study Limitations
Several potential limitations of this study should be considered. First, the cohort is a small population from a single center, and the number of events was very low. In particular, the analyses about changes in decoupling during the ramp test included small subgroups, and the strength of statistical precision and power is limited. Furthermore, speed adjustments were performed to optimize central venous pressure and PCWP but not to optimize decoupling. Accordingly, a randomized, multicenter large-scale study would be useful to validate our findings and the implications for clinical practice. Second, we did not specify any medication adjustments during the study period; patient management was dependent on the clinical decisions of multiple attending physicians. The physicians were blinded to the data on decoupling, and it seems unlikely to have changed management. Third, we did not show longitudinal data showing how decoupling and other clinical parameters vary during long-term LVAD support. Fourth, LVAD duration before the ramp testing varied widely. Eight patients had experienced HF readmission between LVAD implantation and ramp testing. This wide variation may set up a time bias because it relates to subsequent outcome events. However, all participants were clinically stable outpatients until the time of ramp test and such bias may therefore be minimized. Fifth, deceased LVAD patients could not receive ramp testing, and this study has a selection bias. Sixth, although our results showed similar trends in the HVAD and HeartMate II subgroups, some of the comparisons did not reach statistical significance because of the small size of the subgroup. A larger multicenter study could provide more detailed information about differences between the 2 devices.
Conclusions
The presence of inappropriate decoupling was associated with worse outcomes in patients with LVAD. A prospective, multicenter, large-scale study to validate the results is warranted.
Supplementary Material
WHAT IS NEW?
Assessment of the pulmonary vascular system is an established practice in durable left ventricular assist device therapy and pulmonary vascular abnormalities can be quantified in a number of ways.
Inappropriate decoupling, defined as difference >5 mmHg between diastolic pulmonary artery pressure and pulmonary capillary wedge pressure, e.g. the diastolic pulmonary pressure gradient (DPG), was a strong prognostic predictor of death and HF readmission rates in left ventricular assist device (LVAD) patients when assessed at the time of ramp testing.
Many asymptomatic outpatients with LVAD had inappropriate decoupling despite no pre-LVAD pulmonary hypertension.
Furthermore, the degree of decoupling often changed during ramp testing, and these changes also had a prognostic impact.
WHAT ARE THE CLINICAL IMPLICATIONS?
Inappropriate decoupling, defined as an increased DPG, in severe heart failure may be an important hemodynamic variable of the pulmonary vascular system in patients undergoing LVAD support, regardless of patient symptoms.
The adjustment of LVAD speed during ramp testing to optimize coupling may be an important strategy to improve patient outcomes following LVAD implantation but will require validation in a prospective randomized study.
Therapies targeting the pulmonary vasculature in patients exhibiting decoupling after VAD implantation warrant further investigation.
Acknowledgments
Dr Imamura receives financial support from Japan Heart Foundation/Bayer Yakuhin Research Grant Abroad and Postdoctoral Fellowship for Research Abroad of Japan Society for the Promotion of Science. Dr Uriel receives consultant fees and grant support from St Jude and Medtronic. Dr Jeevanandam receives consultant fees from St Jude. Dr Burkhoff receives consultant fees from Medtronic, Corvia Medical, Sensible Medical, Impulse Dynamics, Cardiac Implants, and educational grant support from Abiomed.
Footnotes
DISCLOSURES
The other authors report no conflicts.
The Data Supplement is available at http://circheartfailure.ahajournals.org/lookup/suppl/doi:10.1161/CIRCHEARTFAILURE.117.003882/-/DC1.
Circ Heart Fail is available at http://circheartfailure.ahajournals.org.
References
- 1.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34:1495–1504. doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, III, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 3.Estep JD, Starling RC, Horstmanshof DA, Milano CA, Selzman CH, Shah KB, Loebe M, Moazami N, Long JW, Stehlik J, Kasirajan V, Haas DC, O’Connell JB, Boyle AJ, Farrar DJ, Rogers JG ROADMAP Study Investigators. Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients: results from the ROADMAP study. J Am Coll Cardiol. 2015;66:1747–1761. doi: 10.1016/j.jacc.2015.07.075. [DOI] [PubMed] [Google Scholar]
- 4.Ikegami H, Kurlansky P, Takeda K, Naka Y. Challenges faced in long term ventricular assist device support. Expert Rev Med Devices. 2016;13:727–740. doi: 10.1080/17434440.2016.1208557. [DOI] [PubMed] [Google Scholar]
- 5.Uriel N, Sayer G, Addetia K, Fedson S, Kim GH, Rodgers D, Kruse E, Collins K, Adatya S, Sarswat N, Jorde UP, Juricek C, Ota T, Jeevanandam V, Burkhoff D, Lang RM. Hemodynamic Ramp tests in patients with left ventricular assist devices. JACC Heart Fail. 2016;4:208–217. doi: 10.1016/j.jchf.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barberà J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol Ç, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Völler H, Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 7.Hunt SA American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:e1–e82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Mejía Chew CR, Alcolea Batres S, Ríos Blanco JJ. Update in pulmonary arterial hypertension. Rev Clin Esp. 2016;216:436–444. doi: 10.1016/j.rce.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Tedford RJ, Beaty CA, Mathai SC, Kolb TM, Damico R, Hassoun PM, Leary PJ, Kass DA, Shah AS. Prognostic value of the pre-transplant diastolic pulmonary artery pressure-to-pulmonary capillary wedge pressure gradient in cardiac transplant recipients with pulmonary hypertension. J Heart Lung Transplant. 2014;33:289–297. doi: 10.1016/j.healun.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerges C, Gerges M, Lang MB, Zhang Y, Jakowitsch J, Probst P, Maurer G, Lang IM. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest. 2013;143:758–766. doi: 10.1378/chest.12-1653. [DOI] [PubMed] [Google Scholar]
- 11.Jensen CW, Goldstone AB, Woo YJ. Treatment and prognosis of pulmonary hypertension in the left ventricular assist device patient. Curr Heart Fail Rep. 2016;13:140–150. doi: 10.1007/s11897-016-0288-6. [DOI] [PubMed] [Google Scholar]
- 12.Tedford RJ, Hemnes AR, Russell SD, Wittstein IS, Mahmud M, Zaiman AL, Mathai SC, Thiemann DR, Hassoun PM, Girgis RE, Orens JB, Shah AS, Yuh D, Conte JV, Champion HC. PDE5A inhibitor treatment of persistent pulmonary hypertension after mechanical circulatory support. Circ Heart Fail. 2008;1:213–219. doi: 10.1161/CIRCHEARTFAILURE.108.796789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaRue SJ, Garcia-Cortes R, Nassif ME, Vader JM, Ray S, Ravichandran A, Rasalingham R, Silvestry SC, Ewald GA, Wang IW, Schilling JD. Treatment of secondary pulmonary hypertension with bosentan after left ventricular assist device implantation. Cardiovasc Ther. 2015;33:50–55. doi: 10.1111/1755-5922.12111. [DOI] [PubMed] [Google Scholar]
- 14.Benza RL, Raina A, Abraham WT, Adamson PB, Lindenfeld J, Miller AB, Bourge RC, Bauman J, Yadav J. Pulmonary hypertension related to left heart disease: insight from a wireless implantable hemodynamic monitor. J Heart Lung Transplant. 2015;34:329–337. doi: 10.1016/j.healun.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS CHAMPION Trial Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 16.Amir O, Azzam ZS, Gaspar T, Faranesh-Abboud S, Andria N, Burkhoff D, Abbo A, Abraham WT. Validation of remote dielectric sensing (ReDS™) technology for quantification of lung fluid status: comparison to high resolution chest computed tomography in patients with and without acute heart failure. Int J Cardiol. 2016;221:841–846. doi: 10.1016/j.ij-card.2016.06.323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.