Abstract
A 16-year-old castrated male mongrel cat presented with swelling under the left pinna and a 3-month history of voice change. Laryngeal endoscopy revealed circumferential oedema around the arytenoid cartilages and hypersecretion of saliva. Histopathological examination of the mass around the left ear canal was considered the primary lesion that originated from cutaneous apocrine adenocarcinoma or parotid gland adenocarcinoma, and it metastasized to the larynx, lung and medial retropharyngeal lymph nodes. This report provides new insights into feline laryngeal diseases which could result in laryngeal metastasis with slight mucosal irregularity alone and without obvious radiographic abnormalities. Therefore, histopathological examination should be performed when a cat presents clinical signs such as stridor, dysphonia or voice change without any mass-forming laryngeal lesion.
Keywords: adenocarcinoma, cat, endoscopy, laryngeal disease
Feline laryngeal diseases include laryngeal paralysis, neoplasia and inflammation. The clinical signs of these laryngeal disease are similar [5, 7, 11, 12]. Moreover, when the endoscopic appearance of the laryngeal lesion is mass forming, it could be similar between inflammation and neoplasia and histopathological diagnosis is needed to distinguish them [11, 12]. This report describes the development of an adenocarcinoma in the head and a laryngeal lesion, both of which were histopathologically identical. The laryngeal disease with slight mucosal irregularity had resulted from the metastasis of the primary lesion without any mass-forming lesion.
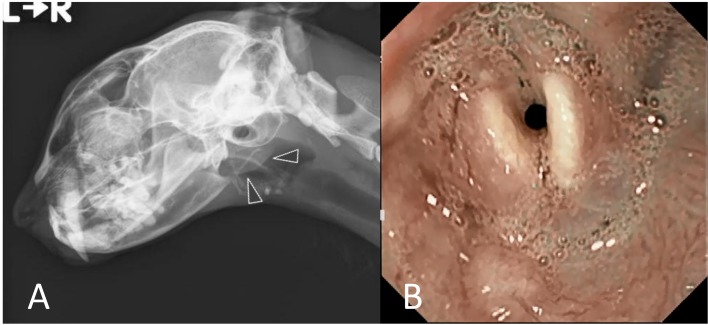
A 16-year-old castrated male mongrel cat presented to the referring veterinarian with swelling under the left pinna. Although the cat’s activity and appetite were normal, it had a 3-month history of voice change. Furthermore, the cat ate slower than before and antibiotic treatment using amoxicillin hydrate (Pasetocin; Kyowa Hakko Kirin, Tokyo, Japan) was performed for 2 weeks, but his condition remained unchanged. Therefore, the cat was referred to our veterinary hospital for further evaluation 1 month after the initial visit. At first examination, a 2.5-cm-diameter mass was easily palpated under the left pinna; it was hard and firmly fixed to the muscle. Although his voice got hoarse, respiratory condition and sounds were normal. Radiographs of the head to larynx and chest were acquired. The head-to-larynx radiographs revealed a soft tissue mass around the base of the left pinna without obvious bony destruction and no obvious laryngeal abnormalities (Fig. 1A). The chest radiographs revealed an interstitial pattern with miliary lesions, suggesting pulmonary metastasis. Complete blood cell counts and serum biochemistry showed no abnormal findings. Fine-needle aspiration from the mass yielded epithelial cells which had anisokaryosis and obvious nucleolus; therefore, epithelial tumor was suspected.
Fig. 1.
Radiographs of the head (1A) and laryngeal endoscopic findings (1B). 1A, a soft tissue mass was seen around the left pinna (arrowheads) without obvious bony destruction, and laryngeal abnormalities were absent. 1B, circumferential mucosal edema and redness around the arytenoid cartilages and hypersecretion of saliva were observed.
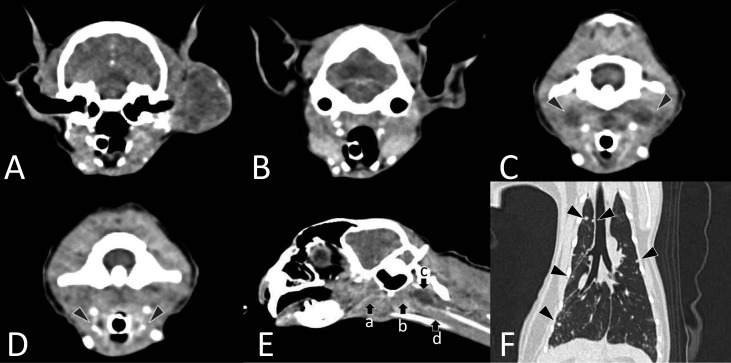
Although malignant tumors under the left pinna and pulmonary metastasis were suspected, a detailed examination under anesthesia was performed for definitive diagnosis. Prior to surgical resection, endoscopy was performed for observing the larynx and investigating the cause of the hoarseness of voice, and computed tomography (CT) was performed to investigate local invasion and/or metastasis from the lesion. Circumferential oedema around the arytenoid cartilages, slight mucosal irregularity and hypersecretion of saliva were observed (Fig. 1B), and a biopsy was performed. Head CT revealed a soft tissue mass extending around the left ear canal and temporal bone, but no destruction of the ear canal was observed. Additionally, both sides of the medial retropharyngeal lymph nodes were enlarged and their surrounding areas were heterogeneously enhanced by contrast injection. The laryngeal region was markedly enhanced by contrast injection (Fig. 2A–C). No continuity between the periauricular mass and laryngeal lesion was observed. Thoracic CT revealed multiple pulmonary nodules throughout the lung lobes (Fig. 2D). Abnormalities were not detected on abdominal CT. Because CT findings suggested that extensive resection was difficult and because the nodules were considered metastatic lesions, a punch biopsy of the mass extending around the left ear canal and temporal bone was performed to confirm the histopathological diagnosis.
Fig. 2.
Computed Tomography images of the head (2A–E) and thorax (2F). 2A, a soft tissue mass was seen extending around the left ear canal and temporal bone, and it was heterogeneously enhanced by contrast injection. Invasion of the bone tissue was not observed. 2B, image at the level of the mandibular gland and the fore level of the retropharyngeal lymph nodes. Continuity to the periauricular mass is not observed. 2C, medial retropharyngeal lymph nodes (arrowheads) were enlarged and surroundings areas were heterogeneously enhanced by contrast injection. 2D, the laryngeal region (arrowheads) was markedly enhanced by contrast injection. 2E, each position of the mass (a, b), retropharyngeal lymph nodes (c), and laryngeal lesion (d). There are no continuity from periauricular mass to laryngeal lesion. 2F, multiple nodules (arrowheads) were detected throughout the lung lobes.
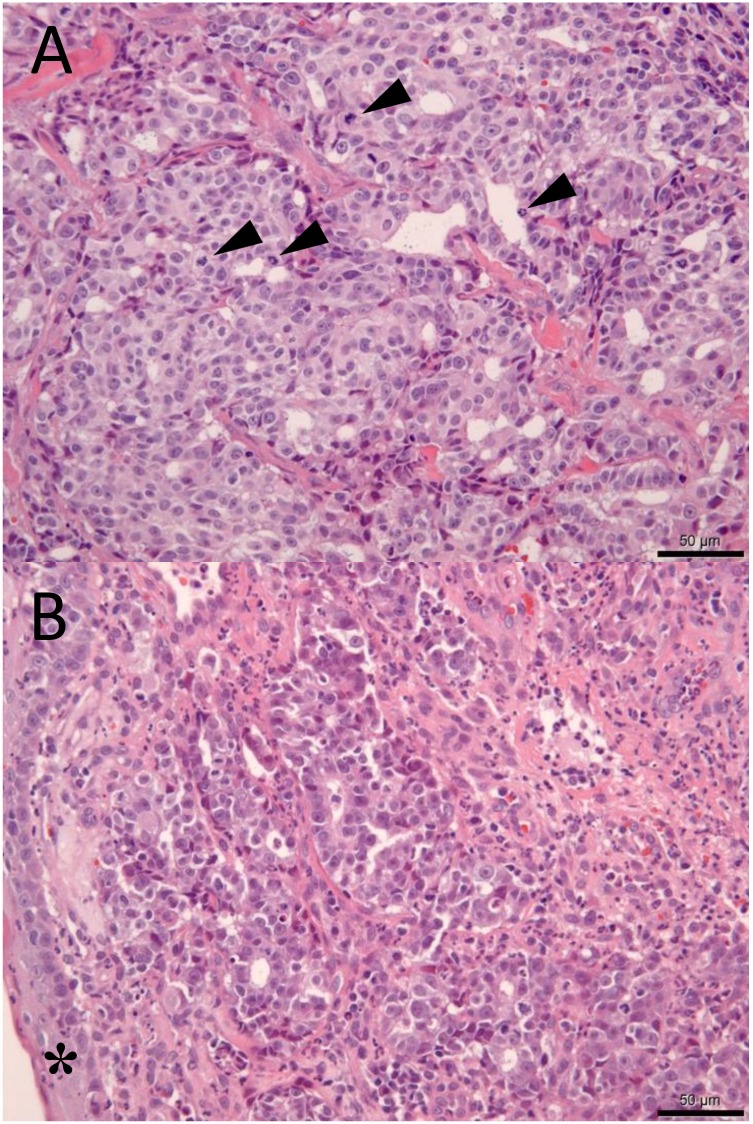
Histopathological examination of the mass around the left ear canal revealed adenocarcinoma (Fig. 3A). Tumor cells derived from the glandular epithelium were highly atypical and proliferated at the dermis. Tubular proliferation of tumor cells was observed, and a proportion of the cells showed solid growth. Normal glandular tissue was absent around the tumor proliferation, and the cells invaded the skeletal muscles. However, punch biopsy analysis revealed no intravascular tissue invasion. The laryngeal lesion was also diagnosed to be an adenocarcinoma composing cells identical to those in the mass around the ear canal (Fig. 3B). Tubular proliferation of tumor cells was observed but intravascular tissue invasion was not observed in the tumor tissue obtained using biopsy forceps. The mass around the left ear canal was considered the primary lesion owing to its site and size, and it metastasized to the larynx. Furthermore, the primary lesion was considered a cutaneous apocrine adenocarcinoma or a salivary adenocarcinoma that originated from the parotid gland. Although the histopathological diagnosis was not determined, the nodules throughout the lungs and medial retropharyngeal lymph nodes were clinically considered metastatic lesions. The owner did not desire aggressive treatment such as chemotherapy and hence, palliative therapy including fluids and antibiotics was performed. After diagnosis, his appetite and activity began to decrease and the cat died at home 34 days after the first visit to our veterinary hospital. Although a necropsy was not performed, the cause of death was considered to be multiple organ dysfunction associated with tumor progression.
Fig. 3.
Histopathological examination of the mass around the left ear canal (3A) and laryngeal lesion (3B). 3A, higher magnification image of the mass around the left ear canal (hematoxylin and eosin [H&E] stain, ×400). Mitotic counts (arrowheads) are in the range of 3–9/high-power field (bar=50 µm). 3B, higher magnification image of the laryngeal lesion (H&E stain, ×400). Tubular proliferation of tumor cells is observed beneath the mucosa (*), and these cells are identical to the cells obtained from the mass around the ear canal (bar=50 µm).
Concurrent laryngeal disease was also suspected in this cat because of the 3-month history of voice change. Endoscopy revealed circumferential oedema around the larynx, but a mass forming lesion indicating a malignant tumor was not detected. Although no continuity between the periauricular mass and laryngeal lesion was observed, extension from the primary lesion at a microscopic level could not be ruled out. However, our examinations revealed no obvious CT findings suggestive of the invasion of a periauricular mass; therefore, a laryngeal lesion resulting from metastasis was considered the final diagnosis. A study on 35 cats with laryngeal disease revealed laryngeal paralysis to be the most common manifestation (40%), followed by neoplasia (28.6%) and inflammation (17.1%) [12]. Another study on 69 cats with laryngeal disease revealed laryngeal paralysis (42%), neoplasia (34.8%) and inflammation (20.3%) [7]. However, the clinical signs were similar among these laryngeal diseases [5, 7, 11, 12]. Dyspnea, stridor, dysphonia or voice change and coughing or gagging were frequently observed. Furthermore, the endoscopic appearance of the laryngeal lesion with mass forming can be similar between inflammation and neoplasia, and histopathological diagnosis is needed to distinguish them [11, 12], especially because of the difficulty in diagnosis using clinical signs and appearance of laryngeal lesions.
The most frequently reported laryngeal neoplasia in cats is lymphoma, followed by squamous cell carcinoma, adenocarcinoma and poorly differentiated round cell carcinoma [5, 12]. These laryngeal tumors could be primary lesions, but laryngeal metastasis has not been reported. Although tumor metastasis to the laryngeal region is rare, it should be considered as the differential diagnosis when a cat has a primary lesion with clinical signs indicating laryngeal disease.
Adenocarcinomas such as cutaneous apocrine adenocarcinomas in the head [6] and salivary gland adenocarcinomas from the parotid gland [1] are rarer in cats than in dogs. This report described the development of an adenocarcinoma in the larynx resulting from the metastasis of a primary lesion. The primary lesion increased in size, and therefore, whether the primary tumor originated from a cutaneous apocrine adenocarcinoma or from a parotid gland adenocarcinoma could not be determined. The salivary gland is normally located below the hypodermis, and the tumor cells proliferate at the dermis. Therefore, the primary tumor was likely a cutaneous apocrine adenocarcinoma rather than a salivary gland adenocarcinoma.
In a previous study on 340 cats with cutaneous neoplasia, only 11 were diagnosed with apocrine adenocarcinoma [8]. Although these tumors occur commonly on the head, pinna, neck, axilla, limbs, tail and anal sac [3, 6, 9, 14], laryngeal metastasis has rarely been documented. Moreover, the biological behavior of apocrine adenocarcinomas in cats remains unclear. Currently, the treatment of choice for apocrine adenocarcinomas of the anal sac in cats is surgical resection, with adjuvant therapies including carboplatin and radiotherapy [2, 3, 14]. Nevertheless, early diagnosis and treatment can provide long-term control of apocrine adenocarcinomas [3]. The behavior of apocrine adenocarcinomas in the head with laryngeal metastasis has not been reported; according to the current report, their behavior could be similar to that of apocrine adenocarcinomas of the anal sac.
Salivary gland disease in cats is also rare [10], and salivary gland tumors account for only 0.6% of all feline tumors [1]. The majority of feline salivary tumors have been reported to be malignant, and adenocarcinomas account for 77–79% of salivary tumors [1, 4]. Salivary tumors have been reported to originate from the mandibular gland in 59% of cats, from the parotid gland in 19% and from other organs in 22% [4]. Regional lymph nodes were involved in 39% of cats at diagnosis [4], and distant metastasis to the lung, liver, spleen, pancreas, heart, adrenal gland, diaphragm, body wall and bone has been reported [1, 13]. However, to our knowledge, laryngeal metastasis has not yet been documented. As for the prognosis of feline salivary gland tumor, one study reported a median survival time of 516 days in 30 cats treated via surgical resection with or without adjuvant therapy including radiotherapy and chemotherapy [4].
In conclusion, histopathological diagnosis is needed to distinguish laryngeal neoplasia from non-cancerous laryngeal disease. Moreover, early diagnosis and systemic treatment are warranted for apocrine adenocarcinomas because of the high rate of local recurrence and metastasis [2, 3, 14]. In this cat, the voice change was observed 3 months before the first examination; therefore, the primary lesion may have progressed previously. This report provides new insights into feline laryngeal diseases which could result in laryngeal metastasis with slight mucosal irregularity alone and without obvious radiographic abnormalities, as well as into the behavior of feline adenocarcinoma. Histopathological examination of the laryngeal region should be performed even if a cat presents clinical signs such as stridor, dysphonia or voice change without mass-forming laryngeal lesion.
CONFLICT OF INTEREST
None of the authors of this paper have financial or personal relationships with other people or organizations that could inappropriately influence or bias the content of the paper.
Acknowledgments
The authors would like to acknowledge Dr. Masaya Tsuboi, Veterinary Medical Center, The University of Tokyo, for his advice for diagnosis.
REFERENCES
- 1.Carberry C. A., Flanders J. A., Harvey H. J., Ryan A. M.1987. Salivary gland tumors in dogs and cats: A literature and case review. J. Am. Anim. Hosp. Assoc. 24: 561–567. [Google Scholar]
- 2.Chun R., Jakovljevic S., Morrison W. B., DeNicola D. B., Cornell K. K.1997. Apocrine gland adenocarcinoma and pheochromocytoma in a cat. J. Am. Anim. Hosp. Assoc. 33: 33–36. doi: 10.5326/15473317-33-1-33 [DOI] [PubMed] [Google Scholar]
- 3.Elliott J. W., Blackwood L.2011. Treatment and outcome of four cats with apocrine gland carcinoma of the anal sac and review of the literature. J. Feline Med. Surg. 13: 712–717. doi: 10.1016/j.jfms.2011.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammer A., Getzy D., Ogilvie G., Upton M., Klausner J., Kisseberth W. C.2001. Salivary gland neoplasia in the dog and cat: survival times and prognostic factors. J. Am. Anim. Hosp. Assoc. 37: 478–482. doi: 10.5326/15473317-37-5-478 [DOI] [PubMed] [Google Scholar]
- 5.Jakubiak M. J., Siedlecki C. T., Zenger E., Matteucci M. L., Bruskiewicz K. A., Rohn D. A., Bergman P. J.2005. Laryngeal, laryngotracheal, and tracheal masses in cats: 27 cases (1998-2003). J. Am. Anim. Hosp. Assoc. 41: 310–316. doi: 10.5326/0410310 [DOI] [PubMed] [Google Scholar]
- 6.Kalaher K. M., Anderson W. I., Scott D. W.1990. Neoplasms of the apocrine sweat glands in 44 dogs and 10 cats. Vet. Rec. 127: 400–403. [PubMed] [Google Scholar]
- 7.Lam A. L., Beatty J. A., Moore L., Foster D. J., Brain P., Churcher R., Angles J., Lam R. W., Barrs V. R.2012. Laryngeal disease in 69 cats: a retrospective multicentre study. Aust. Vet. Pract. 42: 321–326. [Google Scholar]
- 8.Miller M. A., Nelson S. L., Turk J. R., Pace L. W., Brown T. P., Shaw D. P., Fischer J. R., Gosser H. S.1991. Cutaneous neoplasia in 340 cats. Vet. Pathol. 28: 389–395. doi: 10.1177/030098589102800506 [DOI] [PubMed] [Google Scholar]
- 9.Shoieb A. M., Hanshaw D. M.2009. Anal sac gland carcinoma in 64 cats in the United kingdom (1995–2007). Vet. Pathol. 46: 677–683. doi: 10.1354/vp.08-VP-0257-S-FL [DOI] [PubMed] [Google Scholar]
- 10.Spangler W. L., Culbertson M. R.1991. Salivary gland disease in dogs and cats: 245 cases (1985–1988). J. Am. Vet. Med. Assoc. 198: 465–469. [PubMed] [Google Scholar]
- 11.Tasker S., Foster D. J., Corcoran B. M., Whitbread T. J., Kirby B. M.1999. Obstructive inflammatory laryngeal disease in three cats. J. Feline Med. Surg. 1: 53–59. doi: 10.1016/S1098-612X(99)90010-4 [DOI] [PubMed] [Google Scholar]
- 12.Taylor S. S., Harvey A. M., Barr F. J., Moore A. H., Day M. J.2009. Laryngeal disease in cats: a retrospective study of 35 cases. J. Feline Med. Surg. 11: 954–962. doi: 10.1016/j.jfms.2009.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volmer C., Benal Y., Caplier L., Rakotovao F., Fontaine J. J.2009. Atypical vimentin expression in a feline salivary gland adenocarcinoma with widespread metastases. J. Vet. Med. Sci. 71: 1681–1684. doi: 10.1292/jvms.001681 [DOI] [PubMed] [Google Scholar]
- 14.Wright Z. M., Fryer J. S., Calise D. V., Oliveira F. N.2010. Carboplatin chemotherapy in a cat with a recurrent anal sac apocrine gland adenocarcinoma. J. Am. Anim. Hosp. Assoc. 46: 66–69. doi: 10.5326/0460066 [DOI] [PubMed] [Google Scholar]