Abstract
The clinical and pathological records of 44 domestic, female rabbits with an age ranging from 6–124 months (median age: 63.5 month) were assessed retrospectively for ovarian lesions. Included were all rabbits that underwent an ovariohysterectomy with a subsequent pathological examination of the genital tract between March 1997 and June 2016. Pathological examination revealed ovarian lesions in 12 of the 44 rabbits including follicular cysts (n=7), cystic rete ovarii (n=3), widespread ovarian necrosis with dystrophic calcification (n=2), ovarian adenoma (n=1). Clinical examination including radiographs only suggested ovarian disorders in two cases of ovarian necrosis with dystrophic calcification and in two cases of cystic rete ovarii. Clinical significance was only conclusive in a case of cystic rete ovarii.
Keywords: cystic rete ovarii, follicular cyst, ovarian cyst
Many studies investigated genital tract disorders in rabbits. However, most investigations focus on uterine disorders because they are one of the most common findings in intact female rabbits [8, 18, 19, 24, 27, 28]. In contrast, only a few ovarian disorders are scientifically described in rabbits, including ovarian cysts [6], ovarian neoplasia [28], and ovarian abscesses [12].
Intraovarian cysts can be found in many species including dogs [1, 2], cats [9], mice [17, 20], and particularly in guinea pigs [13, 21]. Those cysts can derive from different structures within the ovary and the origin can only be determined by histology or by immunohistochemistry [7]. Recently, cystic rete ovarii have been scientifically described for the first time in rabbits [6, 7]. These cysts arised from the intraovarian rete ovarii, which is a tubular structure that extends from the ovarian hilus into the medulla. In contrary to cystic rete ovarii, follicular cysts are often considered to be hormonally active in different species including cattle [22], dogs [16] and guinea pigs [3]. Single or multiple follicular cysts develop from unovulated and dilated Graafian follicles. Lode et al. described one follicular cyst in one of 8 rabbits [19]. In contrast to intraovarian cysts, parovarian cysts (also paraovarian or periovarian cysts) are located in the mesosalpinx or mesovarium and arise from the remnants of the mesonephric respectively paramesonephric duct. They are not connected to the ovary [13]. They are considered to be rare and usually do not cause symptoms in dogs and guinea pigs [15, 25].
Based on their origin, primary ovarian tumors are assigned into groups: Sex cord stromal tumor (granulosa cell tumor, theca cell tumor and interstitial cell tumor), germ cell tumor (dysgerminoma and teratoma), epithelial tumor (ovarian adenoma and adenocarcinoma) and other tumors originating from connective tissue [2, 14]. The incidence of ovarian neoplasms in rabbits is considered to be low [28]. In most reported cases, ovarian neoplasia were incidental findings in rabbits with uterine adenocarcinoma [10, 26, 28]. Mainly ovarian granulosa cell tumors were mentioned in the literature [11, 28], but also adenocarcinoma [28], hemangioma [10] and one unclassified soft tissue tumor of the ovaries [26] were described.
The aim of the present study was to investigate the occurrence, clinical and pathological findings of ovarian disorders in pet rabbits.
In this retrospective study, the pathological and clinical records of all intact female rabbits that were transferred to the Institute of Veterinary Pathology at the Freie University of Berlin by the Small Animals Clinic at the Freie University of Berlin between March 1997 and June 2016 due to owner’s interest were assessed. During that period, a total of 44 genital tract biopsy examinations (including the uterus, the uterine tube and the ovaries) and 181 post-mortem examinations were conducted. Rabbits that underwent postmortem examination were excluded from the study, because the ovaries were not routinely examined by histopathology. Ethical approval was not required as specimens were part of routine diagnostic service.
Forty-one of the 44 rabbits, that were included in the study, were presented to the veterinarian because of sickness (bloody vaginal discharge and hematuria (n=24); inappetence (n=13); pseudopregnancy (n=5); diarrhea (n=4), pain during micturition (n=3); apathy (n=2)) and the remaining three for elective ovariohysterectomy. For diagnostic work-up, a complete clinical examination always included palpation of the abdomen, in 39 cases radiographs of the abdomen in two projections and in 23 cases sonography of the abdomen were performed. Genital tract disorder, especially uterine disorders, were suspected and all rabbits underwent an ovariohysterectomy under general anesthesia and the genital tract was forwarded for gross and histopathological examination due to the owner’s interest. The surgically removed genital tract was routinely processed for histopathological examination. Representative sections of the three organs were stained with hematoxylin and eosin (HE). In selected cases (two cystic rete ovarii, two follicular cysts and a parovarian cysts) immunohistochemistry was performed according to Table 1.
Table 1. Antibodies used for immunohistochemistry.
| Antibody | Dilution | Pretreatmenta | Antigen source | Manufacturer |
|---|---|---|---|---|
| Anti-vimentin (clone V9) | 1:100 | Heatingb | Monoclonal mouse | Dako, Carpinteria, CA, U.S.A. |
| Anti-cytokeratin (clones AE1/AE3) | 1:500 | Heatingb | Monoclonal mouse | Dako, Carpinteria, CA, U.S.A. |
| Anti-alpha smooth muscle actin (clone ASM-1) | 1:700 | Heatingb | Monoclonal mouse | Progen, Heidelberg, Germany |
a) Pretreatment for antigen retrieval: b) heating: microwave heating with citrate buffer for 12 min at 600 w.
Ovarian disorders were diagnosed by histopathology in 12 of the 44 rabbits (27.3%; Table 2). The median age of the rabbits was 62 months (range 6–124 month). One type of ovarian disorder was found in 11 cases and two types in 1 case.
Table 2. Neoplastic and non-neoplastic ovarian disorders in 12 of 44 rabbits.
| Ovarian disorder | n | |
|---|---|---|
| Neoplastic | ||
| Ovarian adenoma | 1 | |
| Non-neoplastic | ||
| Follicular cysts | 7 | |
| Cystic rete ovarii | 3 | |
| Necrosis with dystrophic Calcification | 2 | |
| None | 32 | |
Intraovarian cysts were discovered in 10 cases (22.7%). Follicular cysts were identified in 7 cases (15.9%) and cystic rete ovarii in three cases (6.8%).
The age of the rabbits with confirmed follicular cysts ranged between 11 to 61 months (median 26 months). Two rabbits without symptoms were presented for elective ovariohysterectomy. The other rabbits were referred for examination with bloody vaginal discharge in three cases as well as hematuria, pseudopregnancy and inappetence in one case each. On pathological examination, follicular cysts were found to be single and unilaterally multiple in four and three cases, respectively. The spherical follicular cysts were located in the cortex ovarii bulging the surface. Histopathological examination revealed that the wall of the cysts was comparatively thin and usually consisted of a single layer of cuboidal to flattened granulosa cells. In immunohistochemical staining the granulosa cells of the cyst as well of normal Graafian follicles were positive for anti-vimentin (clone V9) and negative for anti-cytokeratin (clones AE1/AE3) (Table 3). Both, follicular cyst and normal Graafian follicle, were surrounded by a layer of spindle-shaped cells resembling fibrocytes, which stained slightly positive for anti-alpha smooth muscle actin (clone ASM-1) in immunohistochemistry.
Table 3. Results of immunohistochemical of Graafian follicles, two follicular cysts, two cystic rete ovarii and a parovarian cyst.
| Immunhistochemistry | Graafian follicle | Follicular cyst | Cystic rete ovarii | Parovarian cyst |
|---|---|---|---|---|
| Anti-vimentin (clone V9) | +a | +a | −b | −b |
| Anti-cytokeratin (clone AE1/AE3) | −a | −a | +b | +b |
| Anti-alpha smooth muscle actin (clone ASM-1) | +/−c fibrocytes | +/−c fibrocytes | +/−c fibrocytes | + c smooth muscle layer |
a) granulosa cells; b) epithelial cells; c) surrounding tissue.
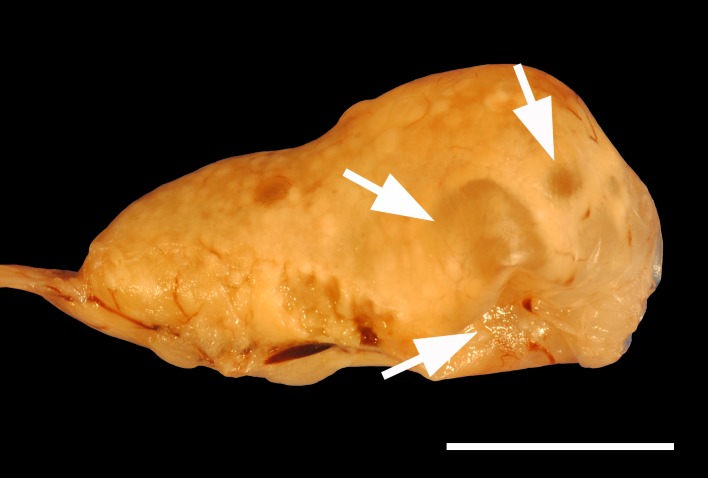
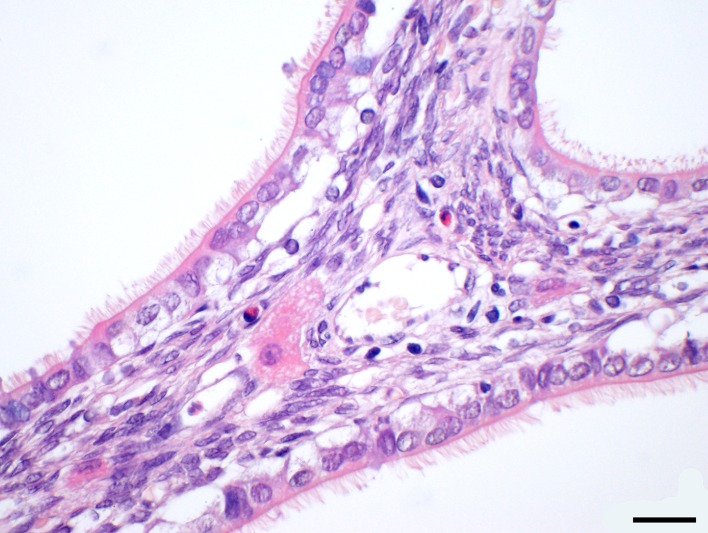
The three rabbits with the cystic rete ovarii had a median age of 87 months (range: 73–88 months). The youngest rabbit was presented with inappetence and the radiography revealed a large mass in the caudoventral abdomen and a smaller mass in the left, caudodorsal abdomen with soft tissue opacity. Pathological examination discovered a uterine adenocarcinoma and the left ovary was approximately double the size of the right ovary due to a 1.2 cm in diameter large, multilobulated cystic rete ovarii (Figs. 1 and 2). In immunohistochemical staining, the epithelial cells of the cystic rete ovarii were positive for anti-cytokeratin and negative for anti-vimentin (Table 3). The second rabbit was 87-month-old and was presented with bloody vaginal discharge. A radiographic and sonographic examination was not performed. The multilobulated cystic rete ovarii was approximately 0.6 × 0.4 × 0.4 cm large. In the uterus, an endometrial hyperplasia and a hemomucometra were diagnosed histopathologically. The third rabbit was presented with inappetence and inability to defecate. Palpation, radiographic as well as sonographic examination revealed a large mass in the caudal to middle abdomen, which sized approximately five cm in diameter. Sonography however did not reveal a connection to the ovary. Histopathology confirmed a single cystic rete ovarii. Additionally, an ovarian adenoma and endometrial hyperplasia was diagnosed. Gross pathology of the three cases of cystic rete ovarii revealed unilateral and single respectively in one case multilobulated cysts. Histopathological examination showed that the ovarian cysts bulged from the ovarian hilus and extended into the medulla of the ovary. The cystic rete ovarii were lined by a simple cuboidal to flattened epithelium that was characterized by tufts of cilia (Fig. 2).
Fig. 1.
Photography of a multilobulated cystic rete ovarii (arrows) bulged from the ovarian hilus. The ovary is formalin-fixed. Bar=1 cm.
Fig. 2.
Histopathological picture of the ovary in Fig. 2: a simple cuboidal to flattened epithelium with tufts of cilia are consistent with cystic rete ovarii. H&E stain, magnification 600×, bar=20 γm.
In an 80 months old rabbit with bloody vaginal discharge a cyst was located in the broad ligament close to the ovary but it was neither connected to the ovary nor the uterine tube. This parovarian cyst was approximately 0.9 cm in diameter large and was filled with a transparent, colorless fluid. On histopathological examination, the cyst was lined by a single and cuboidal to flattened epithelium with tufts of cilia that was positive for anti-cytokeratin and negative for anti-vimentin in immunohistochemistry (Table 3). The positive immunostaining for anti-alpha smooth muscle actin revealed a smooth muscular layer with a maximal thickness of 35 µm underneath the epithelium. The wall of the cyst showed multifocal dystrophic calcification. Also a uterine leiomyoma, endometrial hyperplasia and a hemomucometra were confirmed by histopathology.
Two rabbit had ovarian necrosis. The younger rabbit was 90 months old and was submitted to examination with diarrhea and multiple palpable firm masses in the abdomen. On radiographs, a bilateral calcification in the caudal third of the abdomen was present. Histopathological examination additionally revealed a uterine adenocarcinoma and pyometra. The other rabbit was 118 months old and was submitted due to inappetence. On radiographs, a mass with mineral opacity in the location of the right ovary was evident. Fatty tissue necrosis with dystrophic calcification in the abdominal cavity was overlapping the left ovary and therefore limiting its radiographic examination. No uterine disorder was diagnosed in this case. Histopathologic examination of both cases revealed bilateral and extensive necrosis and only remnants of ovarian tissue were detected in histopathology without evidence for neoplastic transformation or inflammation. The underlying cause remained unknown.
On histopathological examination, the ovaries bilaterally consisted of more than 75% of corpora lutei in 33 rabbits. The residual ovarian tissue was largely displaced to the margin of the ovary. The youngest rabbit with this finding was 11 months old (median 57.5 months; range 11–124 month).
Uterine disorders are a common finding in female rabbits [8] whereas only anecdotal reports exist about ovarian disorders [6, 7, 19, 28]. In our study ovarian disorders have been found in high frequency. Ovarian cysts have been found in 10 of 44 cases (22.7%). Additionally, there are only two case reports about cystic rete ovarii [6, 7]. This study identifies 3 cases of cystic rete ovarii in 44 rabbits, one of which has been previously scientifically described in a case report [6]. The present results suggest that parovarian cysts are rare in rabbits. Walter et al. states that ovarian neoplasia occurs rarely in rabbits [28]. This statement might be supported by the results of this study, as only one ovarian adenoma was diagnosed in the current study.
Limitations of the current study include the fact that only a small number of animals were available for retrospective analysis. Furthermore, the authors were not able to assess the total number of rabbits that were presented to the small animal clinic during the observation period. Therefore, it is difficult to determine the true prevalence of ovarian disorders. Also, most rabbits in the current study had concurrent uterine disorders. This is also true for most ovarian disorders described in the literature [6, 12, 19, 28]. Additionally, in the current study further causes of disease cannot be conclusively excluded, because only the genital tract was examined pathologically. Therefore, clinical symptoms and clinical significance of ovarian disorders are difficult to interpret and subsequent studies need to be conducted.
For guinea pigs many different symptoms, like bilateral, nonpruritic alopecia, decreased appetite and increased aggression, are described in association with intraovarian cysts [3, 23]. Most symptoms in the current study might have been associated with the uterine disorders rather than caused by the ovarian alterations. In the current study, one rabbit with a large (5 cm in diameter) cystic rete ovarii was presented with absence of defecation. This was most likely due to the size of the cyst and a subsequent obstruction of the gastrointestinal tract. Also in guinea pigs cystic rete-ovarii with larger diameters are more likely to cause clinical signs [21].
Clinical examination only rarely suggested ovarian disorders in the present study. Radiographic examination revealed dystrophic calcification of the ovaries in two cases as well as two abdominal mass, which was further characterized to be fluid-filled and thin-walled by sonography in one case. It was however not possible to reveal a connection to the ovary. The second case was not examined by sonography. It is well accepted that a sonographic examination in rabbits is not successful in every case to demonstrate the ovaries [6] but further systematic studies are required to determine the accuracy of diagnosing ovarian disorders. At least in guinea pigs [4, 5, 21] and dogs [2] sonography is described to be appropriate to reliably diagnose ovarian cysts. In rabbits, sonography is a well-established method to diagnose uterine disorders [18, 24, 28]. A sonographic examination of the female genital tract in rabbits should therefore always include the ovaries.
Immunohistochemical staining may be applied in doubtful cases of histopathological examination to reveal the type of cyst. However, in rabbits, the immunohistochemical characteristics of only one rete-ovarii cysts has been determined so far [7]. Approving the results of Chambers et al., the epithelial cells of a cystic rete ovarii was positive for anti-cytokeratin and negative for anti-vimentin [7]. The reverse was true for the granulosa cells in follicular cysts and graphian follicles, which allows to differentiate cystic rete ovarii and follicular cysts. Similar to cystic rete ovarii, parovarian cysts are lined by a cuboidal to flattened epithelium with tufts of cilia. Parovarian cysts can be identified by a smooth muscle layer underneath the epithelium, which may be identified by immunohistochemistry with primary antibodies for anti-alpha smooth muscle actin [7].
REFERENCES
- 1.Akihara Y., Shimoyama Y., Kawasako K., Komine M., Hirayama K., Kagawa Y., Omachi T., Matsuda K., Okamoto M., Kadosawa T., Taniyama H.2007. Immunohistochemical evaluation of canine ovarian cysts. J. Vet. Med. Sci. 69: 1033–1037. doi: 10.1292/jvms.69.1033 [DOI] [PubMed] [Google Scholar]
- 2.Arlt S. P., Haimerl P.2016. Cystic ovaries and ovarian neoplasia in the female dog - a systematic review. Reprod. Domest. Anim. 51Suppl 1: 3–11. doi: 10.1111/rda.12781 [DOI] [PubMed] [Google Scholar]
- 3.Bean A. D.2013. Ovarian cysts in the guinea pig (Cavia porcellus). Vet. Clin. North Am. Exot. Anim. Pract. 16: 757–776. doi: 10.1016/j.cvex.2013.05.008 [DOI] [PubMed] [Google Scholar]
- 4.Beregi A., Zorn S., Felkai F.1999. Ultrasonic diagnosis of ovarian cysts in ten guinea pigs. Vet. Radiol. Ultrasound 40: 74–76. doi: 10.1111/j.1740-8261.1999.tb01841.x [DOI] [PubMed] [Google Scholar]
- 5.Beregi A., Molnár V., Perge E., Felkai C.2001. Radiography and ultrasonography in the diagnosis and treatment of abdominal enlargements in five guinea pigs. J. Small Anim. Pract. 42: 459–463. doi: 10.1111/j.1748-5827.2001.tb02503.x [DOI] [PubMed] [Google Scholar]
- 6.Bertram C., Klopfleisch R., Pischon H., Traeger B., Müller K.2016. Rete-ovarii-Zyste bei zwei Zwergkaninchen (Oryctolagus cuniculus). Kleintierpraxis 61: 374–380. [Google Scholar]
- 7.Chambers J. K., Uchida K., Ise K., Nakayama H.2014. Cystic rete ovarii and uterine tube adenoma in a rabbit. J. Vet. Med. Sci. 76: 909–912. doi: 10.1292/jvms.14-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuchs-Baumgartinger A., Heckermann H., Gruber A., Künzel F.2009. Ein Beitrag zur Art und Häufigkeit von Uterusveränderungen beim Kaninchen. Wien. Tieraerztl. Monatsschr. 25: 272–278. [Google Scholar]
- 9.Gelberg H. B., McEntee K., Heath E. H.1984. Feline cystic rete ovarii. Vet. Pathol. 21: 304–307. doi: 10.1177/030098588402100307 [DOI] [PubMed] [Google Scholar]
- 10.Greene H. S., Strauss J. S.1949. Multiple primary tumors in the rabbit. Cancer 2: 673–691. doi: [DOI] [PubMed] [Google Scholar]
- 11.Iyer P., Majumdar G.1979. Note on spontaneous granulosa cell tumour of ovary in a rabbit (Oryctolagus cuniculus). Indian J. Anim. Sci. 49: 242–244. [Google Scholar]
- 12.Johnson J. H., Wolf A. M.1993. Ovarian abscesses and pyometra in a domestic rabbit. J. Am. Vet. Med. Assoc. 203: 667–669. [PubMed] [Google Scholar]
- 13.Keller L. S., Griffith J. W., Lang C. M.1987. Reproductive failure associated with cystic rete ovarii in guinea pigs. Vet. Pathol. 24: 335–339. doi: 10.1177/030098588702400408 [DOI] [PubMed] [Google Scholar]
- 14.Kennedy P., Cullen J., Edwards J., Goldschmidt M., Larsen S., Munson L., Nielsen S.1998. Tumors of the ovary. pp. 24–29. In: Histological Classification of Tumors of the Genital System of Domestic Animals, 4th ed., Armed Forces Institute of Pathology, Washington, D. C. [Google Scholar]
- 15.Kim H., Choi H., Kim H., Choi J.2012. A giant parovarian cyst in a dog with a granulosa cell tumor. J. Vet. Med. Sci. 74: 385–389. doi: 10.1292/jvms.11-0414 [DOI] [PubMed] [Google Scholar]
- 16.Knauf Y., Bostedt H., Failing K., Knauf S., Wehrend A.2014. Gross pathology and endocrinology of ovarian cysts in bitches. Reprod. Domest. Anim. 49: 463–468. doi: 10.1111/rda.12311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kon Y., Konno A., Hashimoto Y., Endoh D.2007. Ovarian cysts in MRL/MpJ mice originate from rete ovarii. Anat. Histol. Embryol. 36: 172–178. doi: 10.1111/j.1439-0264.2006.00728.x [DOI] [PubMed] [Google Scholar]
- 18.Künzel F., Grinninger P., Shibly S., Hassan J., Tichy A., Berghold P., Fuchs-Baumgartinger A.2015. Uterine disorders in 50 pet rabbits. J. Am. Anim. Hosp. Assoc. 51: 8–14. doi: 10.5326/JAAHA-MS-5812 [DOI] [PubMed] [Google Scholar]
- 19.Lode J., Sassenburg L., Münnich A., Haider W.2003. Endometriale Hyperplasie beim Kaninchen–eine Darstellung von 8 Fällen. Kleintierpraxis 48: 203–206. [Google Scholar]
- 20.Long G. G.2002. Apparent mesonephric duct (rete anlage) origin for cysts and proliferative epithelial lesions in the mouse ovary. Toxicol. Pathol. 30: 592–598. doi: 10.1080/01926230290105785 [DOI] [PubMed] [Google Scholar]
- 21.Nielsen T. D., Holt S., Ruelokke M. L., McEvoy F. J.2003. Ovarian cysts in guinea pigs: influence of age and reproductive status on prevalence and size. J. Small Anim. Pract. 44: 257–260. doi: 10.1111/j.1748-5827.2003.tb00152.x [DOI] [PubMed] [Google Scholar]
- 22.Peter A. T.2004. An update on cystic ovarian degeneration in cattle. Reprod. Domest. Anim. 39: 1–7. doi: 10.1046/j.0936-6768.2003.00466.x [DOI] [PubMed] [Google Scholar]
- 23.Pilny A.2014. Ovarian cystic disease in guinea pigs. Vet. Clin. North Am. Exot. Anim. Pract. 17: 69–75. doi: 10.1016/j.cvex.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 24.Saito K., Nakanishi M., Hasegawa A.2002. Uterine disorders diagnosed by ventrotomy in 47 rabbits. J. Vet. Med. Sci. 64: 495–497. doi: 10.1292/jvms.64.495 [DOI] [PubMed] [Google Scholar]
- 25.Shi F., Petroff B. K., Herath C. B., Ozawa M., Watanabe G., Taya K.2002. Serous cysts are a benign component of the cyclic ovary in the guinea pig with an incidence dependent upon inhibin bioactivity. J. Vet. Med. Sci. 64: 129–135. doi: 10.1292/jvms.64.129 [DOI] [PubMed] [Google Scholar]
- 26.Sommerville L. M.1998. Treatment of a uterine adenocarcinoma in a domestic rabbit by ovariohysterectomy. Vet. Rec. 142: 550–551. doi: 10.1136/vr.142.20.550-b [DOI] [PubMed] [Google Scholar]
- 27.Streicher M., Hach V.2006. Das Uterus-Adenokarzinom des Kaninchens. Kleintierpraxis 51: 309–314. [Google Scholar]
- 28.Walter B., Poth T., Böhmer E., Braun J., Matis U.2010. Uterine disorders in 59 rabbits. Vet. Rec. 166: 230–233. doi: 10.1136/vr.b4749 [DOI] [PubMed] [Google Scholar]