Abstract
The aim of this study was to investigate osteoclastogenic properties of inflammatory cytokines at different time-points of osteoclastogenesis. Bone marrow-derived macrophages from five healthy dogs were stimulated with the macrophage colony-stimulating factor, receptor activator of nuclear factor-κB ligand and inflammatory cytokines such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α and IL-17. Osteoclasts (OC) formation and function were enhanced with TNF-α regardless of temporal differences. But in contrast, IL-1β suppressed the osteoclastogenesis at early phase of the process while upregulating at the late phase. Furthermore, differentiation of OC precursors into OC was suppressed at high concentrations of IL-17. Collectively, the results revealed that suppressing TNF-α would be a promising strategy to inhibit inflammation-associated bone destruction in dogs.
Keywords: cytokine, dog, osteoclast, osteoclastogenesis, rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic autoimmune disease, ultimately presents with extensive joint destruction as a consequence of severe inflammatory process. Chronic inflammation is the key mediator for local and systemic bone loss in arthritic joints where cytokines abundantly present in the synovium [2, 13]. Composed interaction of pro-inflammatory cytokines with T and B cells plays a key role in the pathophysiology of RA [18, 19]. Immune and bone cells are functionally interconnected and derived from same progenitors in the bone marrow sharing common microenvironment [27]. Well-balanced activity of osteoclasts (OC) at the healthy joint is deregulated with excessive activation of the immune mediators under the inflammatory conditions [3]. Based on the current evidence, pro-inflammatory cytokines are categorized into three groups, called osteoclastogenic (IL-1, 6, 8, 11 and 17, TNF-α), anti-osteoclastogenic (IL-4, 10, 13 and 18, IFN-γ, IFN-β) and both osteoclastogenic and anti-osteoclastogenic (IL-7, 12, 23, and 6, TGF-β) [9, 20]. However, the theories on activity of pro-inflammatory cytokines in different stages of osteoclastogenesis are still controversial. Recently, Moon et al., (2013) observed the enhancement of osteoclastogenesis with the treatment of IL-1β, regardless of the maturation status of mouse-derived precursor cells. However, previous human study shows that early treatment of IL-1β prior to or together with receptor activator of nuclear factor-κB ligand (RANKL) strongly inhibits osteoclastogenesis of human-derived precursor cells. The explanation for strong inhibition of human osteoclastogenesis induced by IL-1β is due to suppression of RANK expression by IL-1β-induced proteolytic shedding of the macrophage colony-stimulating factor (M-CSF) receptor, c-Fms which is essential for receptor activator of nuclear factor-κB (RANK) expression [10]. Further, TNF-α could stimulate the proliferation and differentiation of OC precursors [15, 23] in addition to maturation of OC [7, 12, 22]. The disputes regarding the cytokine-induced osteoclastogenesis which stems from the fact that previous experiments were performed under different conditions among different species were considered in this study. However, the direct effect of IL-1β, TNF-α and IL-17 on the differentiation stages and function of OC have not been fully understood in dogs. To our knowledge, this study was the first attempt to examine the role(s) of IL-1β, TNF-α and IL-17 on OC differentiation, function and expression of OC marker genes, cathepsin K (CTK) and matrix metallopeptidase 9 (MMP9) using canine bone marrow-derived OC precursors.
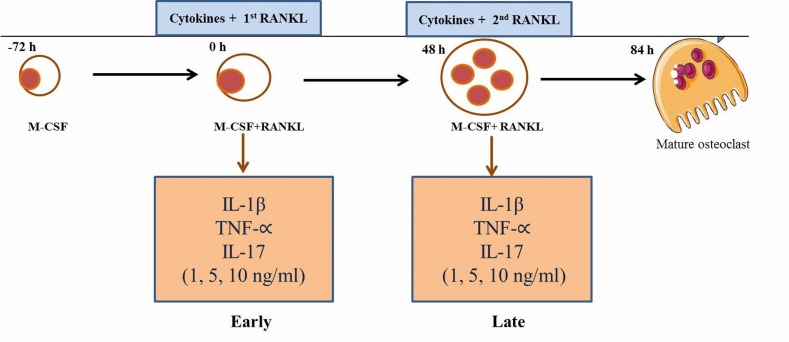
Proximal femurs of 1-year-old, healthy beagle dogs (n=5) were used to collect the 5 ml of bone marrow samples into 10 ml syringe containing 1 ml Dulbecco’s modified eagle’s medium (DMEM, Life technologies, Grand Island, NY, U.S.A.) and 1,000 U/ml of heparin (Nipro, Osaka, Japan). The use of clinical samples and all samples from experimental dogs were in accordance with Hokkaido University Institutional Animal Care and Use Committee guidelines (approval number: 12-0059). The bone marrow was preceded as described previously [25, 26]. Briefly, the bone-marrow derived monocyte-macrophages (BMMs) fraction was obtained by density gradients centrifugation over lymphoprep (Axis-sheild PoC AS, Oslo, Norway) to remove red blood cells. Isolated BMMs fraction (5 × 106 cells/ml) was incubated with DMEM containing penicillin/streptomycin (100 units/ml, Wako Pure Chemical, Tokyo, Japan) and 10% heat-inactivated fetal bovine serum (FBS, Nichirei Bioscience Inc., Tokyo, Japan) for 24 hr to separate the non-adherent and adherent cells. Non-adherent cells were collected as a source of immature OC precursors, suspended in DMEM and counted. The isolated cells were then cultured in DMEM with the presence of 20 ng/ml recombinant human M-CSF (Invitrogen, Frederick, MD, U.S.A.) on 48-wells plates (Corning Inc., Corning, NY, U.S.A.) at 2 × 105 cells/well for 3 days. After 3 days, adherent cells were used as OC precursors after washing out the non-adherent cells, including lymphocytes and further cultured in the presence of 25 ng/ml M-CSF, 50 ng/ml recombinant human RANKL (Sigma-Aldrich, St. Louis, MO, U.S.A.) to generate OC. Various concentrations of canine recombinant IL-1β, TNF-α and IL17 (1, 5, 10 ng/ml) (Kingfisher Biotech, Inc., St. Paul, MN, U.S.A.) and RANKL were added into the osteoclastogenesis culture system at two different time-points; early cytokine treatment was performed with the first RANKL administration and late cytokine treatment was performed on day 2, when the second RANKL treatment was administered to the osteoclast culture media (Fig. 1). On day 2, the media was replaced with fresh medium containing M-CSF, RANKL and cytokines. Selected concentrations of cytokines were within the previous proved non-cytotoxic range for bone marrow derived cells [14]. Precursor cells began to fuse between 36 and 48 hr and mature osteoclasts were observed at 60 hr after RANKL stimulation.
Fig. 1.
Schematic description of the osteoclastogenesis process and cytokine treatments. Early and late challenges with cytokines were performed concurrently with the first RANKL stimulation (at 0 hr) and the second RANKL stimulation (after 48 hr).
Cultured BMMs with M-CSF and RANKL in the presence or absence of cytokines were stained for tartrate-resistant acid phosphatase (TRAP) (Cosmo Bio Co., Ltd., Tokyo, Japan). Cells were washed with 1% phosphate buffered saline (PBS) and fixed with 10% formalin neutral buffer solution for 5 min at room temperature. After washing with 500 µl deionized water 3 times, cells were stained for TRAP as per the manufacturer’s instructions. Cells containing ≥3 nuclei were considered as OC. Four wells were measured in total per one condition and these results were expressed as mean ± SEM.
Bone marrow cells were cultured on bone resorption assay plate 48 (PG Research, Tokyo, Japan) coated with calcium phosphate (CaP-coated, Sigma-Aldrich) and followed the same cytokines treatments. The resorption pit area was analyzed with image-J software (Image J software version 1.43, National Institute of Health).
Total RNA from chondrocytes was extracted using RNeasy Mini Kit® (QIAGEN, Germantown, MD, U.S.A.) as per the manufacture’s protocol. Total RNA was quantified by spectrophotometry at 260 nm. Total RNA was reverse transcribed into cDNA with M-MLV RT kit (Takara Bio, Tokyo, Japan) according to manufacturer’s recommended procedures. Quantitative real-time PCR analysis was performed with KAPA SYBR® FAST qPCR kit (KAPA Biosystems, Woburn, MA, U.S.A.). The amount of 2 µl of cDNA template was added to each 10 µl of premixture with specific primers. The following primer sets were used: CTK 5′- ACCCATATGTGGGACAGGAT-3′ (forward) and 5′-TGGAAAGAGGTCAGGCTTGC-3′ (reverse); MMP9, 5′-GGCAAATTCCAGACCTTTGA-3′ (forward) and 5′-TACACGCGAGTGAAGGTGAG-3′ (reverse); glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-CTGAACGGGAAGCTCACTGG-3′ (forward) and 5′-CGATGCCTGCTTCACTACCT-3′ (reverse). All reactions were normalized to the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase GAPDH. Relative gene expression of level of MMP9 and CTK were analyzed with the t test using SPSS statistical software (ver. 16.0; SPSS, Armonk, NY, U.S.A.) and expressed as the mean ± standard error of mean (SEM). P values <0.05 were considered statistically significant and determined using the Bonferroni modification of ANOVA. Each data point represents the mean ± SEM of 5 samples unless otherwise indicated.
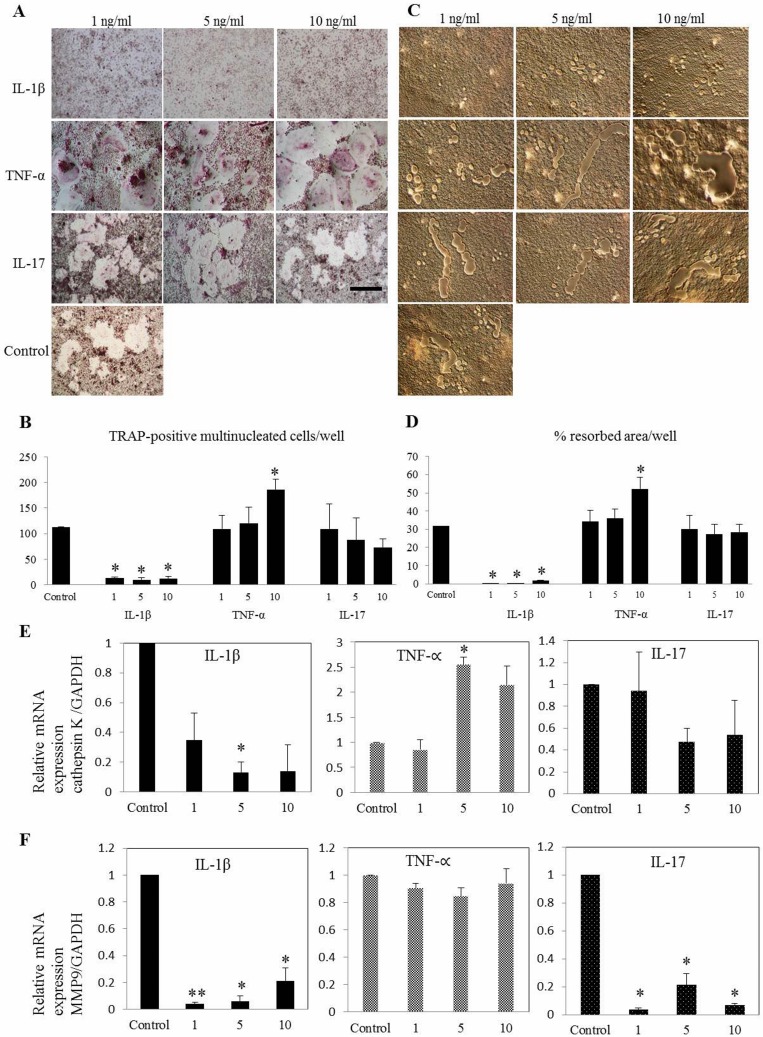
This study demonstrated that cytokines have specific characteristic osteoclastogenic properties throughout the maturation stages. Consistent with the results of previous human study, our results confirmed that the rate of IL-1β driven dog osteoclastogenesis which restrained by its inhibitory action on early osteoclasts precursors, could limit the extent of inflammation in arthritis [11]. Challenging many previous reports, this study found temporal differential effect of IL-1β, determined by varying degree of osteoclastogenesis, bone resorption and mRNA expression levels of OC-related specific genes [4, 5, 8, 17]. At all given concentration of IL-1β, number of TRAP-positive multinucleated cells and resorbed area were decreased compared with that in the control group at early phase (Fig. 2A–F).
Fig. 2.
Differential effect of IL-1β, TNF-α and IL-17 on osteoclast differentiation of canine bone marrow-derived macrophages (A) at early phase is shown. Early challenges with cytokines were performed concurrently with the first RANKL stimulation (at 0 hr). TNF-α increased the (B) number of osteoclast and (C, D) area of resorption while IL-1β inhibits osteoclast formation in all concentrations. (E) Relative mRNA expression of cathepsin K was significantly upregulated with the presence of 5 ng/ml of TNF-α. (F) Expression of MMP9 was significantly lower in all the treatments in early phase of maturation. Values are the means ± SEM of 5 independent experiments. *P<0.05 and **P<0.001 compared with control group. Scale bar=200 µm.
However, previous work has also found that IL-1β could suppress bone resorption in selective in vivo models [1, 10, 24]. In this context, our findings suggest that suppressive functions of IL-1β on osteoclastogenesis may become apparent and biologically important in limiting the extent of bone resorption under conditions where exposure to IL-1β precedes initial steps of differentiation in response to RANKL. But the inhibitory action of IL-1β at early phase was not enough to overcome the stimulatory effects on bone erosion which is given by other factors. Lee et al. explained that the inhibitor action of IL-1β in human due to its down regulatory activity over receptor of M-CSF, ultimately ended up with concurrent inhibition of M-CSF-induced RANK expression. Additionally, the effect of IL-1β on osteoclastogenesis is strikingly time dependent and species dependent when considering that in human [10], murine [14] and current canine-related study.
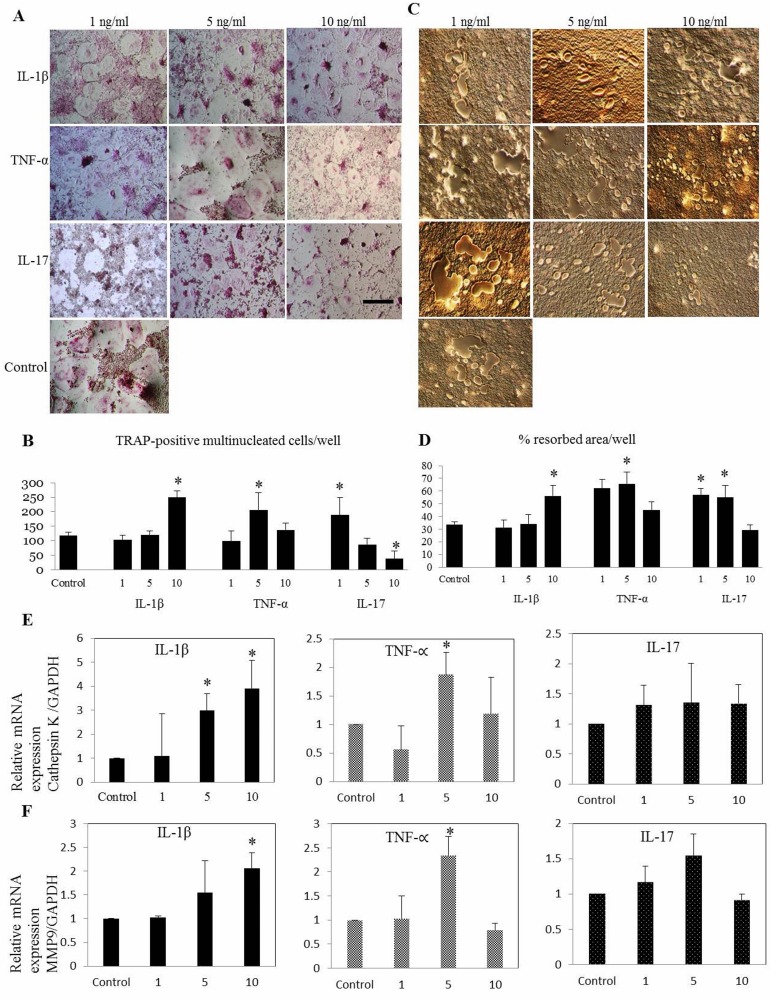
Osteoclast differentiation, bone resorption and CTK expression were amplified with TNF-α in dose dependent manner, while keeping 10 ng/ml as significant concentration (P<0.05) (Fig. 2E). Remarkably, IL-17 suppressed OC functional genes, CTK (Fig. 2E) and MMP9 (P<0.05) (Fig. 2F) with all the concentrations at early phase where those genes starting to express [21]. Bone resorption-related genes CTK and MMP9 are highly expressed in OC and play an important role in skeletal remodeling [16]. Various patterns of osteoclastogenesis were observed with supplementation of TNF-α and IL-17. At the concentration of 5 ng/ml, TNF-α markedly upregulate all functional genes (P<0.05) while increasing OC differentiation and bone resorption (Fig. 2A–E). But challenging the previous study [6], our findings of IL-17 indicated remarkable suppression of osteoclastogenesis (P<0.05) at highest concentration (10 ng/ml) in later phase by implementing the variation on differentiation capability of cytokines in temporal manner (Fig. 3A–F). Interestingly this study was able to postulate the differentiation patterns of inflammatory cytokines at different time-points of osteoclastogenesis.
Fig. 3.
Differential effect of IL-1β, TNF-α and IL-17 on osteoclast differentiation of canine bone marrow-derived macrophages (A) at late phase is shown. Late challenges with cytokines were performed concurrently with second RANKL stimulation (after 48 hr). IL-1β at 10 ng/ml and TNF-α at 5 ng/ml significantly increased the (B) number of osteoclast and (C, D) area of bone resorption. At the late phase, 10 ng/ml of IL-17 attenuate the osteoclastogenesis. Relative mRNA expression of (E) cathepsin K and (F) MMP9 was significantly upregulated with IL-1β and TNF-α. Values are the means ± SEM of 5 independent experiments. *P<0.05 compared with control group. Scale bar=200 µm.
The present study validated that cytokines have specific characteristics throughout the osteoclastogenesis process. Regardless temporal differentiation, TNF-α and IL-17 enhanced OC formation and their function at particular concentrations. But IL-1β suppressed the osteoclastogenesis at early phase of the process while stimulating at the late stage (Fig. 3A–F). Therefore, additional studies are needed to test differential effect of other cytokines involved in canine osteoclastogenesis to identify their roles and implement the therapeutics strategies targeting bone resorption. Moreover, this study might be a manifesto for future investigation of finding other factors involved in the IL-1β–induced inhibition of canine osteoclastogenesis and to unveil the rationale of MMP9 downregulation by IL-17 in addition to determine the mechanisms of action of IL-17 in bone erosion. Collectively, understanding the precise mechanisms of immune-mediated bone destruction would increase opportunities for target-specific inhibition of bone erosion or osteoporosis. The identification of the OC and its role in joint destruction has enabled the development of therapies aimed at reducing its resorptive capacity. Thereby, therapeutic interventions specifically targeting osteoclastogenesis might enable veterinarians to spare bone mass in canine patients with arthritis.
Supplementary Material
REFERENCES
- 1.Bajayo A., Goshen I., Feldman S., Csernus V., Iverfeldt K., Shohami E., Yirmiya R., Bab I.2005. Central IL-1 receptor signaling regulates bone growth and mass. Proc. Natl. Acad. Sci. U.S.A. 102: 12956–12961. doi: 10.1073/pnas.0502562102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brennan F. M., McInnes I. B.2008. Evidence that cytokines play a role in rheumatoid arthritis. J. Clin. Invest. 118: 3537–3545. doi: 10.1172/JCI36389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao Y., Grassi F., Ryan M. R., Terauchi M., Page K., Yang X., Weitzmann M. N., Pacifici R.2007. IFN-γ stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J. Clin. Invest. 117: 122–132. doi: 10.1172/JCI30074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jimi E., Shuto T., Koga T.1995. Macrophage colony-stimulating factor and interleukin-1 alpha maintain the survival of osteoclast-like cells. Endocrinology 136: 808–811. doi: 10.1210/endo.136.2.7835314 [DOI] [PubMed] [Google Scholar]
- 5.Jimi E., Nakamura I., Duong L. T., Ikebe T., Takahashi N., Rodan G. A., Suda T.1999. Interleukin 1 induces multinucleation and bone-resorbing activity of osteoclasts in the absence of osteoblasts/stromal cells. Exp. Cell Res. 247: 84–93. doi: 10.1006/excr.1998.4320 [DOI] [PubMed] [Google Scholar]
- 6.Kitami S., Tanaka H., Kawato T., Tanabe N., Katono-Tani T., Zhang F., Suzuki N., Yonehara Y., Maeno M.2010. IL-17A suppresses the expression of bone resorption-related proteinases and osteoclast differentiation via IL-17RA or IL-17RC receptors in RAW264.7 cells. Biochimie 92: 398–404. doi: 10.1016/j.biochi.2009.12.011 [DOI] [PubMed] [Google Scholar]
- 7.Kitazawa R., Kimble R. B., Vannice J. L., Kung V. T., Pacifici R.1994. Interleukin-1 receptor antagonist and tumor necrosis factor binding protein decrease osteoclast formation and bone resorption in ovariectomized mice. J. Clin. Invest. 94: 2397–2406. doi: 10.1172/JCI117606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi K., Takahashi N., Jimi E., Udagawa N., Takami M., Kotake S., Nakagawa N., Kinosaki M., Yamaguchi K., Shima N., Yasuda H., Morinaga T., Higashio K., Martin T. J., Suda T.2000. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J. Exp. Med. 191: 275–286. doi: 10.1084/jem.191.2.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotake S., Nanke Y., Yago T., Kawamoto M., Yamanaka H.2009. Human osteoclastogenic T cells and human osteoclastology. Arthritis Rheum. 60: 3158–3163. doi: 10.1002/art.24886 [DOI] [PubMed] [Google Scholar]
- 10.Lee B., Kim T. H., Jun J. B., Yoo D. H., Woo J. H., Choi S. J., Lee Y. H., Song G. G., Sohn J., Park-Min K. H., Ivashkiv L. B., Ji J. D.2010. Direct inhibition of human RANK+ osteoclast precursors identifies a homeostatic function of IL-1β. J. Immunol. 185: 5926–5934. doi: 10.4049/jimmunol.1001591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S. E., Chung W. J., Kwak H. B., Chung C. H., Kwack K. B., Lee Z. H., Kim H. H.2001. Tumor necrosis factor-alpha supports the survival of osteoclasts through the activation of Akt and ERK. J. Biol. Chem. 276: 49343–49349. doi: 10.1074/jbc.M103642200 [DOI] [PubMed] [Google Scholar]
- 12.Lerner U. H., Ohlin A.1993. Tumor necrosis factors alpha and beta can stimulate bone resorption in cultured mouse calvariae by a prostaglandin-independent mechanism. J. Bone Miner. Res. 8: 147–155. doi: 10.1002/jbmr.5650080205 [DOI] [PubMed] [Google Scholar]
- 13.Matei I., Matei L.2002. Cytokine patterns and pathogenicity in autoimmune diseases. Rom. J. Intern. Med. 40: 27–41. [PubMed] [Google Scholar]
- 14.Moon S. J., Ahn I. E., Jung H., Yi H., Kim J., Kim Y., Kwok S. K., Park K. S., Min J. K., Park S. H., Kim H. Y., Ju J. H.2013. Temporal differential effects of proinflammatory cytokines on osteoclastogenesis. Int. J. Mol. Med. 31: 769–777. doi: 10.3892/ijmm.2013.1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfeilschifter J., Chenu C., Bird A., Mundy G. R., Roodman G. D.1989. Interleukin-1 and tumor necrosis factor stimulate the formation of human osteoclastlike cells in vitro. J. Bone Miner. Res. 4: 113–118. doi: 10.1002/jbmr.5650040116 [DOI] [PubMed] [Google Scholar]
- 16.Reponen P., Sahlberg C., Munaut C., Thesleff I., Tryggvason K.1994. High expression of 92-kDa type IV collagenase (gelatinase) in the osteoclast lineage during mouse development. Ann. N. Y. Acad. Sci. 732: 472–475. doi: 10.1111/j.1749-6632.1994.tb24789.x [DOI] [PubMed] [Google Scholar]
- 17.Ritchlin C.2000. Fibroblast biology. Effector signals released by the synovial fibroblast in arthritis. Arthritis Res. 2: 356–360. doi: 10.1186/ar112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smolen J. S., Steiner G.2003. Therapeutic strategies for rheumatoid arthritis. Nat. Rev. Drug Discov. 2: 473–488. doi: 10.1038/nrd1109 [DOI] [PubMed] [Google Scholar]
- 19.Smolen J. S., Aletaha D., Koeller M., Weisman M. H., Emery P.2007. New therapies for treatment of rheumatoid arthritis. Lancet 370: 1861–1874. doi: 10.1016/S0140-6736(07)60784-3 [DOI] [PubMed] [Google Scholar]
- 20.Takayanagi H., Kim S., Taniguchi T.2002. Signaling crosstalk between RANKL and interferons in osteoclast differentiation. Arthritis Res. 4Suppl 3: S227–S232. doi: 10.1186/ar581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeshita S., Kaji K., Kudo A.2000. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J. Bone Miner. Res. 15: 1477–1488. doi: 10.1359/jbmr.2000.15.8.1477 [DOI] [PubMed] [Google Scholar]
- 22.Thomson B. M., Mundy G. R., Chambers T. J.1987. Tumor necrosis factors alpha and beta induce osteoblastic cells to stimulate osteoclastic bone resorption. J. Immunol. 138: 775–779. [PubMed] [Google Scholar]
- 23.van der Pluijm G., Most W., van der Wee-Pals L., de Groot H., Papapoulos S., Löwik C.1991. Two distinct effects of recombinant human tumor necrosis factor-alpha on osteoclast development and subsequent resorption of mineralized matrix. Endocrinology 129: 1596–1604. doi: 10.1210/endo-129-3-1596 [DOI] [PubMed] [Google Scholar]
- 24.Vargas S. J., Naprta A., Glaccum M., Lee S. K., Kalinowski J., Lorenzo J. A.1996. Interleukin-6 expression and histomorphometry of bones from mice deficient in receptors for interleukin-1 or tumor necrosis factor. J. Bone Miner. Res. 11: 1736–1744. doi: 10.1002/jbmr.5650111117 [DOI] [PubMed] [Google Scholar]
- 25.Wagner E. F., Eferl R.2005. Fos/AP-1 proteins in bone and the immune system. Immunol. Rev. 208: 126–140. doi: 10.1111/j.0105-2896.2005.00332.x [DOI] [PubMed] [Google Scholar]
- 26.Yagi M., Miyamoto T., Toyama Y., Suda T.2006. Role of DC-STAMP in cellular fusion of osteoclasts and macrophage giant cells. J. Bone Miner. Metab. 24: 355–358. doi: 10.1007/s00774-006-0697-9 [DOI] [PubMed] [Google Scholar]
- 27.Zupan J., Jeras M., Marc J.2013. Osteoimmunology and the influence of pro-inflammatory cytokines on osteoclasts. Biochem Med (Zagreb) 23: 43–63. doi: 10.11613/BM.2013.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.