Abstract
A 16-year-old female Indian peafowl (Pavo cristatus) died two days after recognition of conjunctivitis in the right eye, anorexia and depression. Gross necropsy revealed a thick pseudomembrane under the eyelid and hydropericardium. Histopathological examination revealed hepatocellular necrosis, sinusoidal and vascular congestion and infiltrated inflammatory cells. Infiltration by inflammatory cells was noted in the epicardium. The lungs had mild interstitial pneumonia with the extensive congestion within the capillaries of the air sacs. Tubular interstitial congestion and necrosis was noted in the kidneys. Bacterial culture and nucleotide sequencing of the inflammatory specimens identified the causative agent as Serratia marcescens, an uncommon bacterium in birds. In summary, this study describes the sudden death of an Indian peafowl due to S. marcescens infection, which is rarely seen in animals.
Keywords: antimicrobial susceptibility test, conjunctivitis, Indian peafowl, Pavo cristatus, Serratia marcescens
Serratia marcescens is a Gram-negative bacillus of the Enterobacteriaceae family; it is an important cause of nosocomial infection in humans and can cause serious infections in animals [3, 7, 11]. This bacterium is saprophytic with wide distribution, and has been found in food as well as in a variety of environments [3, 12]. This opportunistic pathogen causes a broad range of infections, including wound infections, septicemia, pneumonia and eye infections [6, 7]. Recently, multidrug-resistant strains of S. marcescens have presented a public health concern [6, 13].
The environmental strains of S. marcescens produce a red pigment known as prodigiosin (2-methyl-3-pentyl-6-methoxyprodigiosin), which is similar in color to blood. However, not all isolates of the bacteria contain this red pigment, and most clinical strains are not pigmented [3]. In the present report, we describe the sudden death of an Indian peafowl due to non-pigmented S. marcescens infection.
A 16-year-old female Indian peafowl, which had been kept in an aviary with other birds, got conjunctivitis in the right eye and had a two-day history of anorexia and depression. The bird had been housed in an enclosed facility at the Daejeon O-World Theme Park, which is located in the middle of Korea (36°17ʹ19.00ʺ N, 127°23ʹ52.04ʺ E). It was fed a diet of commercial bird feed and vegetables. The bird was treated using penicillin G (10,000 IU/kg; Tardomyocel, Bayer, Leverkusen, Germany) and dexamethasone (0.25 mg/kg; Dexorone, Handong Pharmacy, Seoul, Korea), which were delivered via intramuscular injection. The bird was then washed with sterile normal saline. During the treatment period, the complete blood count and blood chemistry results were monitored. Nonetheless, it died following a two-day treatment period.
The complete blood count indicated leukocytosis, with increased numbers of neutrophils, monocytes and eosinophils. Increased creatine phosphokinase, glutamate oxaloacetate transaminase (GOT), lactate dehydrogenase (LDH), total bilirubin and uric acid were noted on chemistry profile (Table 1) [4].
Table 1. Abnormalities of blood count and blood chemistry in a 16-year-old female Indian peafowl.
| Variablea) | Value | Reference range [4] |
|---|---|---|
| WBC (×103 cells/µl) | 59.00 | 8.7–44 |
| neutrophil (×103 cells/µl) | 33.67 | 4–26.4 |
| lymphocyte (×103 cells/µl) | 12.98 | 2.44–17.2 |
| monocyte (×103 cells/µl) | 8.85 | 0.087–4.7 |
| eosinophil (×103 cells/µl) | 2.95 | 0.162–2.6 |
| basophil (×103 cells/µl) | 0.59 | 0.087–1.32 |
| CPK (U/l) | >2,000 | 544–1,924 |
| GOT (U/l) | 480 | 88–307 |
| LDH (U/l) | 763 | 83–388 |
| Total bilirubin (mg/dl) | 1 | 0.1–0.8 |
| Uric acid (mg/dl) | 28 | 1.1–10.8 |
a) CPK, creatine phosphokinase; GOT, glutamate oxaloacetate transaminase; LDH, lactate dehydrogenase.
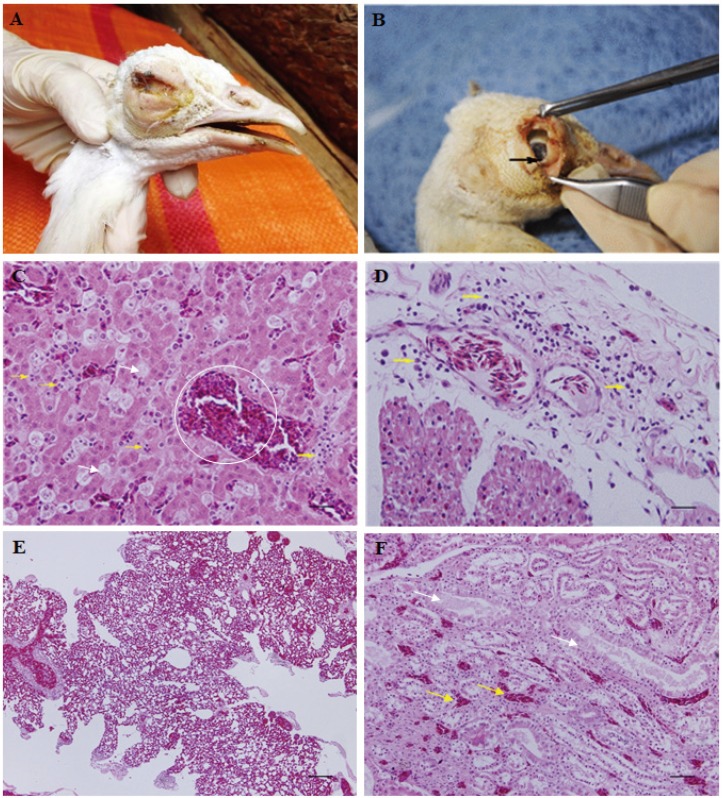
To determine the cause of death, gross and microscopic necropsy was performed. The carcass was emaciated with the conjunctivitis of the right eye (Fig. 1A). There was a thick pseudomembrane under the eyelid (Fig. 1B). The heart revealed yellowish hydropericardium and mildly swollen epicardial lesions (data not shown). Samples taken from the surroundings of the right eye and the liver were submitted to the zoo laboratory for bacterial culture and antimicrobial susceptibility test and the College of Veterinary Medicine, Kyungpook National University, Daegu, Korea, for molecular diagnosis.
Fig. 1.
Serratia marcescens infection in an Indian peafowl (Pavo cristatus) that died in a zoo. (A) Conjunctivitis of the right eye caused by S. marcescens (B) Thick pseudomembrane (black arrow) caused by conjunctivitis under the eyelid (C) Hepatocellular necrosis (white arrows), sinusoidal congestion (white circle) and infiltrated inflammatory cells (yellow arrows) in the liver (×400) (D) Congestion within cardiac muscle and infiltration of inflammatory cells (yellow arrows) within the epicardium (×400) (E) Severe congestion and interstitial pneumonia within the lung parenchyma (×100) (F) Tubular interstitial congestions (yellow arrows) and necrotic changes with exfoliation and nuclear condensation in tubular epithelial cells (white arrows) of the kidney (×200). All tissues were stained with hematoxylin and eosin. Scale bars=20 µm.
For histopathological analysis, the heart, liver, lungs and kidneys were collected and fixed in 10% neutral buffered formalin for two weeks, then embedded in paraffin, sectioned at 4 µm and stained with hematoxylin and eosin for the microscopic examination. Histopathologically, hepatocellular necrosis, sinusoidal congestion and inflammatory infiltration were observed in the liver (Fig. 1C). The portal areas and hepatic perivenules showed mild to marked infiltration with heterophils and mononuclear inflammatory cells. Mild capillary congestion was noted within cardiac muscle, and an inflammatory cell infiltrate was observed in the edematous lesions of the epicardium (Fig. 1D). There was severe congestion in the air sacs of the lung parenchyma and mild interstitial pneumonia (Fig. 1E). Tubular interstitial congestion and necrotic changes, including exfoliation and nuclear condensation of tubular epithelial cells, were observed in the medullary region of the kidney (Fig. 1F).
With regards to the bacteria culture, the inflammatory samples were cultured on blood agar (Asan Pharmacy, Seoul, Korea) at 37°C for 18 hr. A pure culture of hemolytic and dewdrop colonies was isolated following the incubation period. The colonies were composed of short, rod-shaped, Gram-negative bacteria. The bacteria were identified as S. marcescens using a biochemical API 20NE identification kit (bioMérieux, Marcy l’Etoile, France).
The susceptibility of the isolated microorganisms to antibiotics was determined using the disc diffusion method, as previously described [5, 8]. The S. marcescens strain isolated in this study was sensitive to amikacin, chloramphenicol, ciprofloxacin, colistin, gentamicin, kanamycin, enrofloxacin, norfloxacin and trimethoprim-sulfamethoxazole. Conversely, it was resistant to ampicillin, bacitracin, cephalotin, cefazolin, erythromycin, novobiocin, penicillin, streptomycin, oxytetracycline and vancomycin.
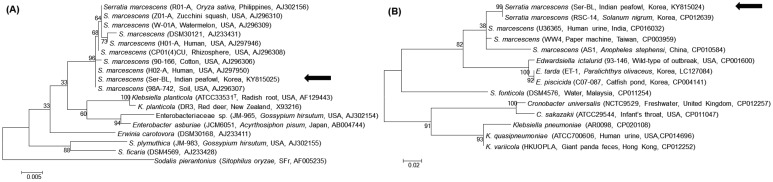
For molecular diagnosis, genomic DNA sample was prepared from the pure cultured colonies using the DNeasy® Blood & Tissue kit (Qiagen, Hilden, Germany). PCR was performed using the AccuPower® HotStart PCR PreMix (Bioneer, Daejeon, Korea) to amplify the 16S rRNA and S-adenosylhomocysteine nucleosidase (pfs) gene fragments, according to a previous study [17]. The PCR products were sent to Solgent (Daejeon, Korea) for bidirectional sequencing. Subsequently, 1,387-bp 16S rRNA was obtained, as was a 193-bp pfs gene fragment. A basic local alignment search tool (NCBI, Bethesda, MD, U.S.A.) revealed that the sequences identified in this study showed 100% identity with the 16S rRNA (KP241785) and pfs gene (CP012639) of S. marcescens, which are deposited in the GenBank database. Phylogenetic trees were then constructed, based on the maximum-likelihood method, using MEGA 6.0 with 100 replicates. These also showed that the isolate constituted S. marcescens (Fig. 2A and 2B) [14]. The sequences obtained from the 16S rRNA and pfs gene were submitted to GenBank database (accession numbers KY815025 and KY815024).
Fig. 2.
Phylogenetic tree of (A) the 16S rRNA and (B) the pfs gene of the Serratia marcescens strain isolated from an Indian peafowl. The trees were constructed using the maximum-likelihood method with 100 replicates. Data regarding isolate, host, country and GenBank accession number are described in parentheses. An arrow indicates the isolate from this study. The scale bar indicates the number of substitutions per nucleotide.
In animals, there have been multiple reports of S. marcescens infections, encompassing septicemia, necrotizing fasciitis, pneumonia, mastitis, endocarditis, osteomyelitis and purulent meningoencephalitis [9, 11]. Generally, Gram-negative bacteria are not considered normal flora in an avian species [11]. However, several previous studies have reported isolation of S. marcescens with or without clinical symptoms in birds, including houbara bustards (Chlamydotis undulata macqueenii), brown-headed cowbirds (Molothrus ater), raptors (Falconiformes), parrots and wedge-tailed shearwater chicks (Puffinus pacificus) [1, 2, 10, 11, 15]. Clinical signs in birds include regurgitation or vomiting, hepatitis, nephritis and septicemia [11]. However, to our knowledge, conjunctivitis caused by S. marcescens has not been reported in birds.
Predisposing factors for S. marcescens infection in humans include the use of antibiotics or steroids; contaminated surgical instruments, catheters or injection sites and the presence of wounds and ulcers [7]. In birds, antibiotic treatment and immunosuppression are predisposing factors [11]. In the present study, the opportunistic infection of S. marcescens in the right eye of the bird attributes to the immunosuppression or liver damage, as indicated by the abnormal GOT and LDH values because the bacterium is not considered normal flora in avian species. These symptoms may in turn have been the result of old age, as well as the stress of being kept in a closed aviary [16].
Probably, the bacteria then replicated within the bird, causing septicemia and ultimately death. Isolation of S. marcescens from the inflammatory liver sample, as well as the leukocytosis of neutrophils, monocytes and eosinophils, provides evidence of S. marcescens septicemia.
The antimicrobial susceptibility test indicated that the S. marcescens isolated in this study was resistant to penicillin, cephalosporin, macrolides, tetracycline and vancomycin. A study reported that all S. marcescens isolates of human origin were resistant to ampicillin, cefotaxime and gentamicin [13]. Because many S. marcescens strains have shown resistance to multiple antibiotics, it represents a growing public health concern [6, 13].
In conclusion, this study described the sudden death of an Indian peafowl (Pavo cristatus) due to septicemia caused by non-pigmented S. marcescens infection, which is rare in animals, especially birds. In addition, this study described the first case of S. marcescens conjunctivitis in a bird. Although S. marcescens is not considered normal flora in avian species, opportunistic infection can result in death [11]. Therefore, zoo veterinarians should be aware of S. marcescens infection in captive birds.
REFERENCES
- 1.Bailey T. A., Silvanose C., Manvell R., Gough R. E., Kinne J., Combreau O., Launay F.2002. Medical dilemmas associated with rehabilitating confiscated houbara bustards (Chlamydotis undulata macqueenii) after avian pox and paramyxovirus type 1 infection. J. Wildl. Dis. 38: 518–532. doi: 10.7589/0090-3558-38.3.518 [DOI] [PubMed] [Google Scholar]
- 2.Gerlach H.1994. Bacteria. pp. 949–983. In: Avian Medicine: Principles and Application, 1st ed. (Ritchie, B. W., Harrison, G. J. and Harrison, L. R. eds.), Wingers Publishing Inc, Lake Worth. [Google Scholar]
- 3.Hejazi A., Falkiner F. R.1997. Serratia marcescens. J. Med. Microbiol. 46: 903–912. doi: 10.1099/00222615-46-11-903 [DOI] [PubMed] [Google Scholar]
- 4.International Species Information System1997. Physiological Data Reference Values. Apple Valley. [Google Scholar]
- 5.Kim K. T., Lee S. H., Kwak D.2015. Prevalence, biochemical characteristics, and antibiotic susceptibility of aeromonads, vibrios, and plesiomonads isolated from different sources at a zoo. J. Zoo Wildl. Med. 46: 298–305. doi: 10.1638/2014-0194R.1 [DOI] [PubMed] [Google Scholar]
- 6.Kim S. B., Jeon Y. D., Kim J. H., Kim J. K., Ann H. W., Choi H., Kim M. H., Song J. E., Ahn J. Y., Jeong S. J., Ku N. S., Han S. H., Choi J. Y., Song Y. G., Kim J. M.2015. Risk factors for mortality in patients with Serratia marcescens bacteremia. Yonsei Med. J. 56: 348–354. doi: 10.3349/ymj.2015.56.2.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lakhani N. A., Narsinghani U., Kumar R.2015. Necrotizing fasciitis of the abdominal wall caused by Serratia marcescens. Infect. Dis. Rep. 7: 5774. doi: 10.4081/idr.2015.5774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards1999. Performance standards for antimicribial susceptibility testing; ninth informational supplement. National Committee for Clinical Laboratory Standards, Wayne. [Google Scholar]
- 9.Plavec T., Zdovc I., Juntes P., Svara T., Suhadolc Scholten S., Nemec A., Domanjko Petric A., Tozon N.2008. Necrotizing fasciitis caused by Serratia marcescens after tooth extraction in a doberman pinscher: a case report. Vet. Med. 53: 629–635. [Google Scholar]
- 10.Radwan A. I., Lampky J. R.1972. Enterobacteriaceae isolated from cowbirds (Molothrus ater) and other species of wild birds in Michigan. Avian Dis. 16: 343–350. doi: 10.2307/1588799 [DOI] [PubMed] [Google Scholar]
- 11.Saidenberg A. B. S., Teixeira R. H. F., Astolfi-Ferreira C. S., Knöbl T., Ferreira A. J.2007. Serratia marcescens infection in a swallow-tailed hummingbird. J. Wildl. Dis. 43: 107–110. doi: 10.7589/0090-3558-43.1.107 [DOI] [PubMed] [Google Scholar]
- 12.Son D., Lee J. S., Cheong M. H., Lee K., Park B. D., Lee M. H., Kim J. J.2008. Deep cutaneous ulcer caused by Serratia marcescens after fresh water exposure. Infect. Chemother. 40: 330–332. doi: 10.3947/ic.2008.40.6.330 [DOI] [Google Scholar]
- 13.Sung M. J., Chang C. H., Yoon Y. K., Park S. E.2006. Clinical aspects of an outbreak of Serratia marcescens infections in neonates. Korean J. Pediatr. 49: 500–506(in Korean with English abstract). doi: 10.3345/kjp.2006.49.5.500 [DOI] [Google Scholar]
- 14.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S.2013. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30: 2725–2729. doi: 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Work T. M., Rameyer R. A.1999. Mass stranding of wedge-tailed shearwater chicks in Hawaii. J. Wildl. Dis. 35: 487–495. doi: 10.7589/0090-3558-35.3.487 [DOI] [PubMed] [Google Scholar]
- 16.Yan J., Li S., Li S.2014. The role of the liver in sepsis. Int. Rev. Immunol. 33: 498–510. doi: 10.3109/08830185.2014.889129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou L., Pan X., Wu Q., Luo Y., Liu S., Lin C., Li B., Wang X., Long M., Guo F.2011. First detection of OKP-A β-lactamase in two Serratia marcescens isolates in China. New Microbiol. 34: 371–378. [PubMed] [Google Scholar]