Abstract
Recombinant human interferon-β (rhIFN-β), a therapeutic protein, is produced using both prokaryotic and eukaryotic expression systems. However, instability of recombinant plasmid during cultivation of Escherichia coli results in low yield of the recombinant proteins. In addition, use of antibiotics during the cultivation imposes a major concern. In this study, we have compared the expression yield of rhIFN-β in E. coli BL21 (DE3) and E coli SE1 cells. Gene-encoding rhIFN-β was expressed in E. coli BL21 (DE3) and SE1 cells and the cultivation of recombinant E. coli cells was done in a laboratory scale bioreactor. Our results suggest that, compared to BL21(DE3) cells, the SE1 cells expressing rhIFN-β protein can be cultivated in the medium without antibiotic and provide increased stability of recombinant plasmid and higher expression yield of rhIFN-β protein. This system can be used for the production of rhIFN-β proteins for biomedical applications.
Keywords: Recombinant human interferon-beta, Plasmid stability, Antibiotic, ELISA, Expression yield
Introduction
Human interferon-beta (hIFN-β) is a ~ 20 kDa cytokine produced by many cell types in the body and exerts a wide range of effects including antiviral, antibacterial, antitumor, antiproliferative, proapoptotic, and cytotoxic effects, and is thus clinically important (Derynck et al. 1980; Reder and Feng 2014). Recombinantly produced human interferon-beta (rhIFN-β) is used for the treatment of multiple sclerosis (MS) and is also a potential candidate for the development of therapeutics against other diseases (Reder and Feng 2014). The mechanism as to how hIFN-β elicits its therapeutic effect in MS is poorly understood, although it is proposed that the beneficial effects of IFN-β are due to its anti-inflammatory and immune-modulatory properties. It is also proposed that rhIFN-β reduces the leukocyte migration across BBB (blood–brain barrier) and regulates autoimmunity (Reder and Feng 2014).
For commercial use, rhIFN-β proteins are now being produced using both prokaryotic and eukaryotic expression systems (Reder and Feng 2014; Rudick and Goelz 2011). Two different forms of rhIFN-β are approved by US FDA for the treatment of MS: rhIFN-β1a and rhIFN-β1b (Reder and Feng 2014). The rhIFN-β1a is a glycosylated protein produced using eukaryotic expression systems (CHO cells) and possesses identical amino acid sequence to that of naturally occurring hIFN-β, while IFN-β1b is a non-glycosylated form of hIFN-β produced by E. coli (prokaryotic expression system) (Reder and Feng 2014). It is reported that the stability and efficacy of recombinantly produced IFN-β are influenced by the level and pattern of post-translational modifications. However, it has also been shown that recombinantly produced non-glycosylated form (rhIFN-β1b) is biologically active and exhibits activities similar to its glycosylated counterpart.
Due to several advantages, E. coli is one of the most widely used host system for the production of recombinant proteins (Baneyx 1999; Huang et al. 2012). However, there are several limitations associated with the use of E. coli expression system for the production of recombinant proteins for commercial and biomedical applications. Instability of recombinant plasmids (carrying the gene-encoding target recombinant protein) during high-density cultivation of host cells is known to result in low yield of the target recombinant proteins (Peubez et al. 2010; Sodoyer et al. 2012). In addition, the use of antibiotics during the cultivation of host cells (recombinant E. coli cells) expressing target recombinant proteins is a major concern (Peubez et al. 2010; Sodoyer et al. 2012). Thus, there is a need for the development of effective and safe system for the production of rhIFN-β proteins for biomedical applications. E. coli SE1–pStaby expression system offers many advantages over BL21(DE3)–pET expression system. It is reported that using SE1–pStaby expression system, the yield of target recombinant protein can be increased up to three–five times than the other system (Sodoyer et al. 2011).
In this study, we have compared the expression of rhIFN-β protein in E. coli BL21(DE3) and SE1 cells. Our results suggest that, the recombinant E. coli SE1 cells expressing rhIFN-β protein can be cultivated in the medium without antibiotic and provide increased stability of recombinant plasmid and higher expression yield of rhIFN-β protein, as compared to E. coli BL21(DE3) cells.
Materials and methods
Materials
Gene-encoding rhIFN-β was obtained from GenScript, NJ, USA. E. coli SE1 cells and regeneration media were procured from Delphi Genetics SA, Charleroi, Belgium. Mouse anti-human IFN-β1b antibody was purchased from Santa Cruz Biotechnology, Santa Cruz, USA. Protein molecular weight markers and Bradford reagent were obtained from Bio-Rad, Gurgaon, India. VeriKine™ Human IFN Beta ELISA Kit (Catalog No. 41410) was purchased from Essence Life Sciences, Chandigarh, India. All other chemicals used in this study were of reagent (analytical) grade or higher quality. Buffers used were prepared in double distilled water.
Construction of recombinant plasmids containing rhIFN-β gene
Construction of recombinant expression plasmids containing gene for rhIFN-β was done following standard molecular biology protocols as described previously (Sambrook et al. 2001; Satvik Iyengar et al. 2015; Beladiya et al. 2015; Bajaj et al. 2015). Briefly, amino acid sequence of naturally occurring hIFN-β (GenBank # GI: 50593016) was used to design a gene for the expression of rhIFN-β protein in E. coli cells. The designed gene (cloned in pUC57 plasmid) was purchased commercially. The gene was sub-cloned into pET-23a(+) and pStaby plasmids and the recombinant plasmids [i.e., pET-23a(+)–rhIFN-β and pStaby–rhIFN-β] were transformed into competent E. coli cells. The transformed E. coli cells containing recombinant plasmid (recombinant E. coli cells) were stored as glycerol stock at − 80 °C and used in this study.
Cultivation of recombinant E. coli in a laboratory scale bioreactor
High cell density cultivation of recombinant E. coli cells expressing rhIFN-β protein was done in a laboratory scale bioreactor. The fermentation was carried out in a 3.5 L reactor (Bioflow 3000, NBS with 2–2.5 L working volume) at 37 °C, as described previously (Patel et al. 2016; Patil et al. 2016). Briefly, in a typical experiment, glycerol stock of recombinant E. coli cells was streaked on a Luria-Bertani (LB)-agar plates containing 50 μg/ml carbenicillin and incubated at 37 °C for ~ 16 h. A single colony of the bacterial cells from the plate was inoculated in 10 ml LB medium, containing 50 μg/ml carbenicillin and incubated overnight at 37 °C (200 rpm). This pre-inoculum was then inoculated into 250 ml Terrific Broth (TB) medium which served as an inoculum for the bioreactor. The whole inoculum at OD600 ~ 2 was then added in bioreactor having 2L TB medium and 4% glycerol. The culture was grown and the expression of rhIFN-β protein was induced with 1 mM IPTG when OD600 of the broth reached ~ 0.6. The cells were further grown for 8 h. For E. coli BL21(DE3) cells, carbenicillin was added in the cultivation medium, while no antibiotic was added to the medium used for growing E. coli SE1 cells. At the end, the cells were harvested and further used.
Determination of plasmid stability in recombinant E. coli cells
Stability of recombinant plasmids in the cultivated E. coli cells was determined following the method described in the literature (K. Friehs et al. 2004; Selvamani et al. 2014). Briefly, the culture (in duplicate) was collected from the bioreactor and diluted (1:10,000 times). The diluted sample was then spread on LB agar ± carbenicillin plates (in triplicate) and the plates were incubated for 16 h at 37 °C. The number of bacterial colonies formed on the plates was counted. Plasmid stability was calculated by determining the ratio between the average number of bacterial colonies in both types of plates (LB agar ± carbenicillin) (Friehs et al. 2004; Selvamani et al. 2014).
Determination of expression yield of rhIFN-β protein by SDS-PAGE and Western blot
The harvested cell mass was resuspended in ice-cold lysis buffer (50 mM Tris–HCl, pH 8.0, containing 150 mM NaCl, 2 mM β-ME, 0.1% tergitol) at a ratio of 1:10. Lysozyme (10 µg/ml) was added to the cell suspension and subjected to sonication. The suspension was treated with DNase (1 µg/ml) and stirred at 4 °C for 1 h, centrifuged to collect supernatant and cell debris fractions. Concentration of the protein in the samples was determined spectrophotometrically at 595 nm, according to the Bradford method, using bovine serum albumin as a standard (Bradford 1976). SDS-PAGE and western blot analysis of the samples were performed under denaturing conditions using the method of Laemmli (Laemmli 1970). For western blot analysis, mouse anti-hIFNβ-1b antibody was used as a primary antibody and goat anti-mouse antibody was used as a secondary antibody. The protein bands in the western blot were visualized by incubating the membrane in BCIP–NBT reagent. SDS-PAGE gels and western blots were scanned by densitometric gel scanner (BIO-RAD) and the ratio of the target protein was determined by calculating the area under the peaks. The Quantity One analysis software (BIO-RAD) was used for gel densitometry analysis.
Enzyme-linked immunosorbant assay
VeriKine™ human IFN beta ELISA kit was used to quantitate rhIFN-β protein present in the samples. The procedure followed in this assay was the same as recommended by the manufacturer.
Results
Construction of recombinant plasmids containing rhIFN-β gene
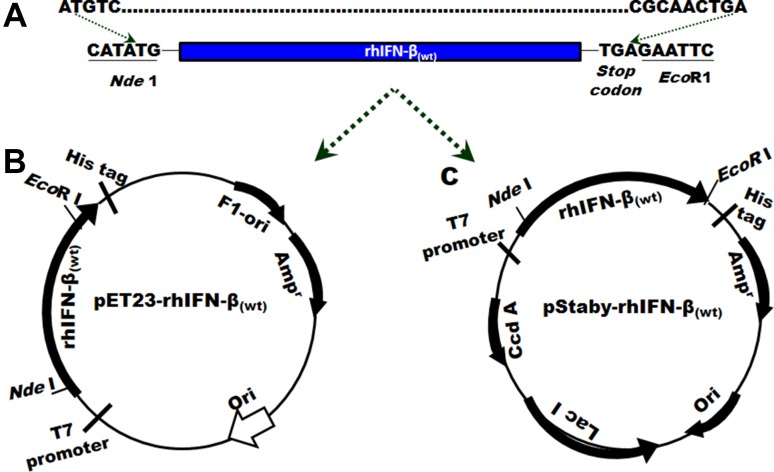
To express rhIFN-β protein in E. coli cells, a codon-optimized gene-encoding rhIFN-β protein was designed using amino acid sequence of naturally occurring rhIFN-β protein. At the 5′ end of the designed gene, the ORF is flanked by an Nde1 site and at the 3′ end the ORF is flanked by an EcoRI site which is preceded by a stop codon (Fig. 1). This gene when cloned into pET23a(+) or pStaby plasmids resulted in the expression of rhIFN-β protein.
Fig. 1.
a Schematic representation of a gene designed for the expression of rhIFN-β in E. coli cells. The gene was sub-cloned into pET23a(+) (b) and pStaby (c) plasmids
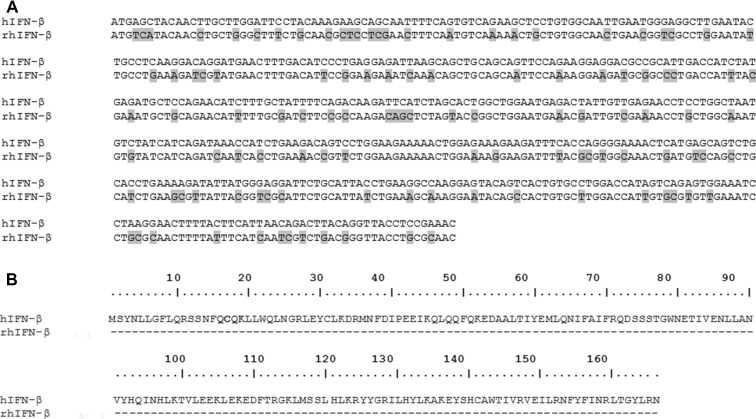
Nucleic acid sequence coding for rhIFN-β was aligned with the nucleic acid sequence of naturally occurring hIFN-β and the comparison is given in Fig. 2a. At nucleic acid level, the gene for rhIFN-β exhibits low similarity with the gene of naturally occurring hIFN-β. This indicates that the codon optimization has altered the nucleotide sequence considerably in the designed gene. However, comparison of the deduced amino acid sequences of rhIFN-β and naturally occurring hIFN-β indicated that both the proteins share 100% similarity (Fig. 2b).
Fig. 2.
Comparison of DNA sequence (a) and deduced amino acid sequence (b) of hIFN-β proteins. a Shaded area shows difference in the nucleotide residues at that particular position. b Residues in the polypeptides are numbered from the initial M residue. Dashes represent identical amino acid residues
Cultivation of recombinant E. coli in a laboratory scale bioreactor
To attain high cell density of growing cells in a short time, batch fermentation is an established way to cultivate the recombinant Escherichia coli cells. Recombinant E. coli cells expressing rhIFN-β protein were cultivated in 2.5 L medium at 37 °C in a laboratory scale bioreactor. The cultivation conditions optimized at the shake-flask level for the maximum growth of recombinant of E. coli BL21(DE3) and SE1 cells expressing rhIFN-β protein were used in bioreactor. Maximum cell mass concentration of 8.0 and 8.7 g/L was obtained with the recombinant E. coli BL21(DE3) and SE1 cells, respectively. At the end of the fermentation, cell mass was collected and used to determine plasmid stability and recombinant protein yield.
Plasmid stability in recombinant E. coli cells
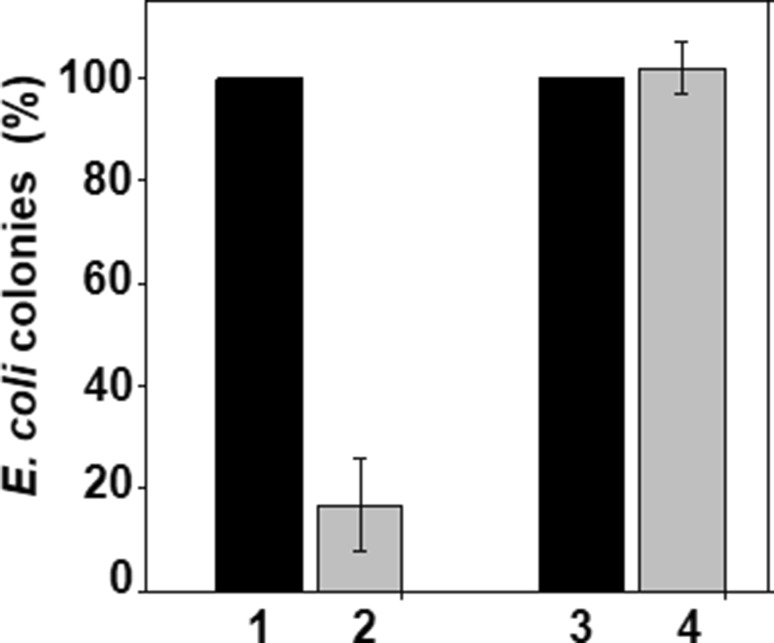
Plasmid stability was determined by following the procedure described in Materials and methods. Representative results are presented in Fig. 3. Relative percentages of recombinant E. coli cells containing recombinant plasmids differed considerably thereby indicating the difference in the stability of the recombinant plasmids in E. coli BL21(DE3) and SE1 cells. In the case of SE1 cells containing pStaby–rhIFN-β, nearly, equal amount of cells were present in both the plates (± antibiotic) (see bars 3 and 4), while the number of E. coli BL21(DE3) cells containing pET-23a(+)-rhIFN-β is significantly less in the LB plate which contained antibiotic (bar 2), suggesting less number of recombinant plasmid-containing cells in the latter case. The results also indicate that, as compared to E. coli BL21(DE3) cells, SE1 cells maintain the plasmid during cultivation. This observation is consistent with the previously published results in the literature (Peubez et al. 2010; Sodoyer et al. 2012).
Fig. 3.
Recombinant plasmid stability. Histogram depicts population of recombinant E. coli cells harboring recombinant plasmids encoding rhIFN-β protein, present in the cultivation medium after fermentation. No antibiotic was added in the medium in which E. coli SE1 cells were fermented. Bars 1 and 2 represent population of recombinant E. coli BL21 (DE3) cells and bars 3 and 4 represent population of recombinant E. coli SE1 cells. Dark bars represent total population of recombinant E. coli cells on LB-agar plate, and grey bars represent total population of recombinant E. coli cells on LB-agar plates containing antibiotic. In case of SE1, nearly, equal amount of cells were present in both plates (± antibiotic), while the number of E. coli BL21(DE3) cells is significantly less in LB plate in which antibiotic was present, indicating lower abundance of recombinant plasmid-containing cells
Determination of rhIFN-β protein yield
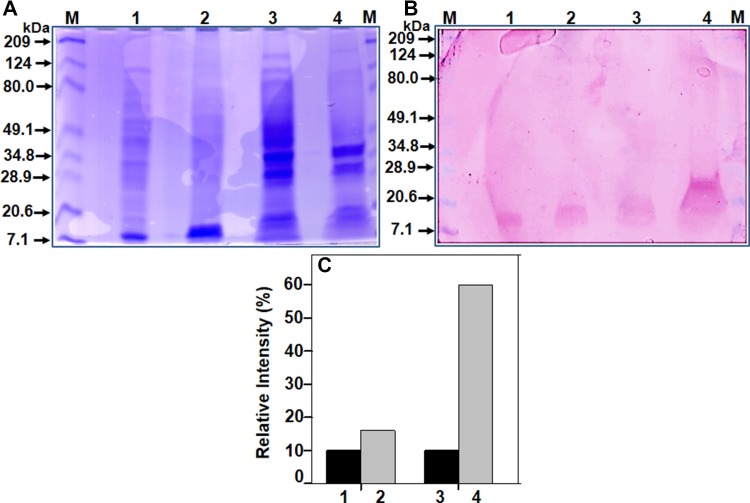
Next, the rhIFN-β protein yield in both the expression systems was compared. The cell mass obtained from the bioreactor was lysed and centrifuged to separate supernatant and cell debris fractions, and equal amount of protein was subjected to SDS-PAGE and Western blot analysis. Representative results are presented in Fig. 4. On the blot, a band of ~ 20 kDa was observed in all the samples. Higher amount of rhIFN-β protein was present in the cell debris fraction (lanes 3 and 4), suggesting that majority of the overexpressed rhIFN-β protein aggregates and forms inclusion bodies in E. coli cells. This observation is consistent with the previously published results in the literature (Ghane et al. 2008; Fazeli et al. 2011; Haji et al. 2014). Densitometry analysis suggested that the expression of rhIFN-β protein is relatively more in the SE1 cells as compared to the BL21(DE3) cells (Fig. 4c).
Fig. 4.
Determination of expression yield of rhIFN-β protein. SDS-PAGE (a), western blot (b), and densitometry analysis (c) of fractions. After fermentation, the cell mass of recombinant E. coli expressing rhIFN-β proteins was collected by centrifugation, lysed by sonication, and centrifuged to separate clear supernatant and cell debris fractions. The samples were subjected to SDS-PAGE and Western blot analysis, by following the procedure describe in Materials and methods. Monoclonal anti-human IFN-β antibody was used as primary antibody in the Western blot analysis. Legends: Lane M—protein molecular weight marker; lane 1 and bar 1—supernatant fraction of E. coli BL21(DE3) cells; lane 2 and bar 2—supernatant fraction of E. coli SE1 cells; lane 3 and bar 3—cell debris fraction of E. coli BL21(DE3) cells, and lane 4 and bar 4—cell debris fraction of E. coli SE1 cells
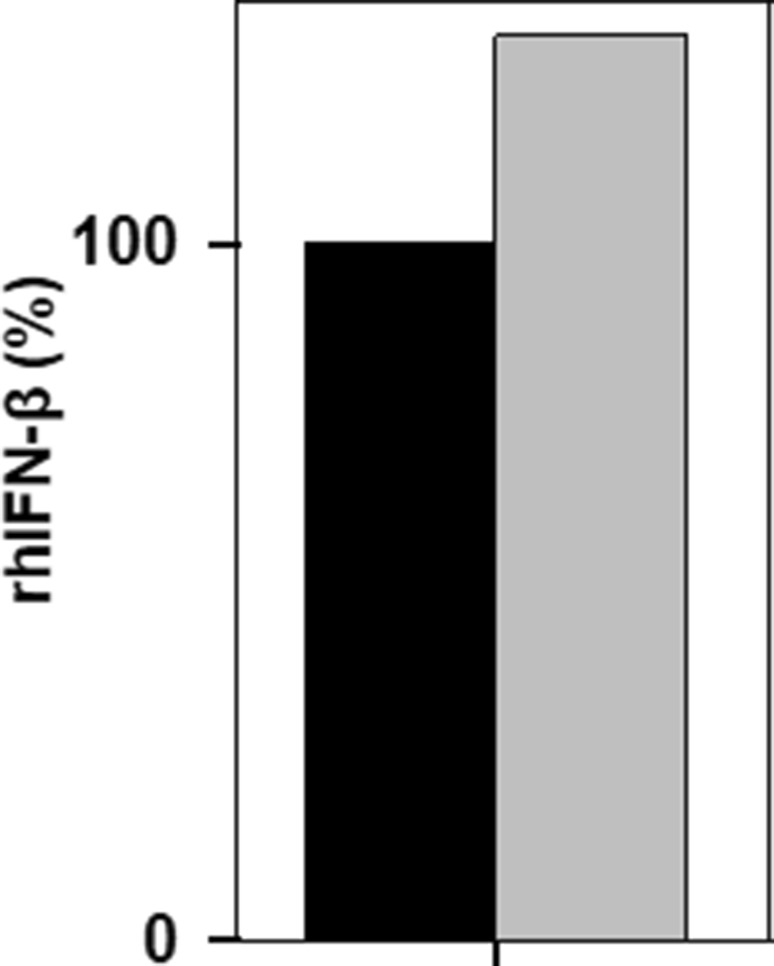
The amount of active rhIFN-β protein expressed in E. coli cells was determined by sandwich ELISA assay. ELISA is frequently used to determine the yield of recombinantly expressed proteins (Srikanth et al. 2013). Cell supernatant (which contains active rhIFN-β protein) containing equal amount of protein was used and representative results are presented in Fig. 5. The amount of active rhIFN-β present in SE1 cells fraction was higher than in BL21 (DE3) cells.
Fig. 5.
Quantification of active rhIFN-β by ELISA. Cell supernatant containing equal amount of protein was used in the ELISA. Legends: black filled square—E. coli BL21(DE3) and grey filled square—E coli SE1
Discussion
Recombinant hIFN-β is a therapeutic protein and there is still a need for the development of cost effective and safe system for its production. Various attempts to develop expression and production protocols for achieving higher yield of rhIFN-β are still underway (Allen et al. 2015; Ashnagar et al. 2014; Moradian et al. 2013; Morowvat et al. 2015). The choice of expression system is a key in the development of new protein biopharmaceuticals and each expression system has its own advantages and disadvantages (Baneyx et al. 1999; Huang et al. 2012). Because of many reasons, commercial production of rhIFN-β proteins using E. coli expression system is preferred. However, there are still limitations associated with the production of recombinant proteins by E. coli cells, which include instability of recombinant plasmid in the expression host during cultivation resulting in low yield of target proteins (Peubez et al. 2010; Sodoyer et al. 2012). The use of antibiotics as a selection marker during cultivation of recombinant bacterial cells is also a major concern by regulatory authorities (Peubez et al. 2010; Sodoyer et al. 2012).
In this study, we have compared the expression of rhIFN-β protein in two E. coli expression systems: E. coli BL21(DE3)–pET23a(+) and E. coli SE1–pStaby. Our results clearly indicate that, compared to E. coli BL21(DE3) cells, increased stability of recombinant plasmid (encoding gene for rhIFN-β protein) was observed in E. coli SE1 cells during high-density cultivation, consequently resulting in higher yield of rhIFN-β protein (Figs. 3, 4, 5). Moreover, the recombinant E. coli SE1 cells expressing rhIFN-β protein can be cultivated in the medium without antibiotic. The pStaby-E. coli SE1 expression system is antibiotic-free expression system commercialized by Delphi Genetics, Belgium (Peubez et al. 2010; Sodoyer et al. 2012). This system exploits toxin/antitoxin post-segregational killing mechanism to maintain the stability of recombinant plasmids (encoding the gene of target recombinant protein) in the host cells (Peubez et al. 2010; Sodoyer et al. 2012). This expression system permits the growth of only those recombinant microbial cells which contain the recombinant plasmid and does not use antibiotics (as selection marker), thereby increasing the overall yield of recombinant proteins (Peubez et al. 2010; Sodoyer et al. 2012). This system, thus, offers a cost effective and safe way to produce recombinant proteins in E. coli with higher yield. In a nutshell, our results describe an effective and safe way to produce rhIFN-β protein. This can be used for the large-scale production of rhIFN-β proteins for biomedical applications.
Acknowledgements
This work was supported by a research Grant to A.H.P. from NIPER, SAS Nagar (NPLC-AHP). DharamPal is thankful to UGC, New Delhi for financial support in the form of Fellowship.
Abbreviations
- rhIFN-β
Wild-type recombinant human interferon-beta
- LB medium
Luria–Bertani medium
- IPTG
Isopropyl-1-thio β-d galactopyranoside
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TB
Terrific Broth
- WCM
Wet cell mass
Compliance with ethical standards
Conflict of interest
None.
Footnotes
Dharam Pal and Rajan K. Tripathy have contributed equally to this work.
Reference of submitted sequences
The GenBank accession number of submitted nucleotide sequences of rhIFN-β is KM514350.
References
- Allen J, Feng P et al (2015) Method for producing soluble recombinant interferon protein without denaturing. 2015 US20160032345A1
- Ashnagar F, Khodabandeh M, et al. Optimizing primary recovery and refolding of human interferon-β from Escherichia coli inclusion bodies. Iran J Biotech. 2014;12(4):e1157. doi: 10.15171/ijb.1157. [DOI] [Google Scholar]
- Bajaj P, Tripathy RK, et al. Expression and purification of biologically active recombinant human paraoxonase 1 from inclusion bodies of Escherichia coli. Prot Expr Purif. 2015;115:95–101. doi: 10.1016/j.pep.2015.05.011. [DOI] [PubMed] [Google Scholar]
- Baneyx F. Recombinant protein expression in Escherichia coli. Curr Opin Biotechnol. 1999;10(5):411–421. doi: 10.1016/S0958-1669(99)00003-8. [DOI] [PubMed] [Google Scholar]
- Beladiya C, Tripathy RK, et al. Expression, purification and immobilization of recombinant AiiA enzyme onto magnetic nanoparticles. Prot Expr Purif. 2015;113:56–62. doi: 10.1016/j.pep.2015.04.014. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Derynck R, Content J, et al. Isolation and structure of a human fibroblast interferon gene. Nature. 1980;285(5766):542–547. doi: 10.1038/285542a0. [DOI] [PubMed] [Google Scholar]
- Fazeli A, Shojaosadati SA, et al. Effect of parallel feeding of oxidizing agent and protein on fed-batch refolding process of recombinant interferon beta-1b. Process Biochem. 2011;46(3):796–800. doi: 10.1016/j.procbio.2010.11.007. [DOI] [Google Scholar]
- Friehs K. New trends and developments in biochemical engineering. In: Scheper T, editor. Plasmid copy number and plasmid stability. Berlin: Springer; 2004. pp. 47–82. [DOI] [PubMed] [Google Scholar]
- Ghane M, Yakhchali B, et al. Over expression of biologically active interferon beta using synthetic gene in E. coli. J Sci. 2008;19(3):203–209. [Google Scholar]
- Haji Abdolvahab M, Fazeli A, et al. The effects of dodecyl maltoside and sodium dodecyl sulfate surfactants on the stability and aggregation of recombinant interferon Beta-1b. J Interferon Cytokine Res. 2014;34(11):894–901. doi: 10.1089/jir.2013.0131. [DOI] [PubMed] [Google Scholar]
- Huang C-J, Lin H, et al. Industrial production of recombinant therapeutics in Escherichia coli and its recent advancements. J Ind Microbiol Biotechnol. 2012;39(3):383–399. doi: 10.1007/s10295-011-1082-9. [DOI] [PubMed] [Google Scholar]
- Iyengar SAR, Tripathy RK, et al. Improving storage stability of recombinant organophosphorus hydrolase. Prot Expr Purif. 2015;111:28–35. doi: 10.1016/j.pep.2015.01.012. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moradian C, Fazeli MR, et al. Over expression of the Interferon β-1b by optimizing induction conditions using response surface methodology. J Biol today’s world. 2013;2(4):217–226. [Google Scholar]
- Morowvat MH, Babaeipour V, et al. Optimization of fermentation conditions for recombinant human interferon beta production by Escherichia coli using the response surface methodology. Jundishapur J Microbiol. 2015;8(4):e16236. doi: 10.5812/jjm.8(4)2015.16236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel G, Patil MD, et al. Production of mycophenolic acid by Penicillium brevicompactum using solid state fermentation. Appl Biochem Biotechnol. 2016;182(1):97–109. doi: 10.1007/s12010-016-2313-3. [DOI] [PubMed] [Google Scholar]
- Patil MD, Shinde KD, et al. Use of response surface method for maximizing the production of arginine deiminase by Pseudomonas putida. Biotechnol Rep. 2016;10:29–37. doi: 10.1016/j.btre.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peubez I, Chaudet N, et al. Antibiotic-free selection in E. coli: new considerations for optimal design and improved production. Microb Cell Fact. 2010;9:65. doi: 10.1186/1475-2859-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reder AT, Feng X. How type I interferons work in multiple sclerosis and other diseases: some unexpected mechanisms. J Interf Cytokine Res. 2014;34(8):589–599. doi: 10.1089/jir.2013.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick RA, Goelz SE. Beta-interferon for multiple sclerosis. Exp Cell Res. 2011;317(9):1301–1311. doi: 10.1016/j.yexcr.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, et al. Molecular cloning. 2. New York: Cold Spring Harbor; 2001. [Google Scholar]
- Selvamani RSV, Telaar M, et al. Antibiotic-free segregational plasmid stabilization in Escherichia coli owing to the knockout of triosephosphate isomerase (tpiA) Microb Cell Fact. 2014;13:58. doi: 10.1186/1475-2859-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodoyer R, Courtois V, et al. Antibiotic-free selection for bio-production: moving towards a new “gold standard”. Antibiot Resist Bact. 2012 [Google Scholar]
- Srikanth P, Jain SM, et al. Procedure for establishing downstream process of recombinant platelet derived growth factor expressed in E. coli. Int J LifeSc Bt Pharm Res. 2013;2(4):64–72. [Google Scholar]