Abstract
The emergence of new strains of Magnaporthe oryzae (M. oryzae) is associated with recurrent failure of resistance response mediated by single resistance (R) gene in rice. Therefore, stacking or combining of multiple R genes could improve the durability of resistance against multiple strains of M. oryzae. To achieve this, in the present study, intragenic stacking of rice blast resistance orthologue genes Pi54 and Pi54rh was performed through co-transformation approach. Both these genes were expressed under the control of independent promoters and blast susceptible indica rice line IET17021 was used for transformation. The highly virulent M. oryzae strain Mo-ei-ger1 that could knock down most of the major single blast R genes including Pi54 and exhibiting 89% virulence spectrum was used for phenotypic analysis. The stacked transgenic IET17021 lines (Pi54 + Pi54rh) have shown complete resistance to Mo-ei-ger1 strain in comparison to non-transgenic lines. These two R gene stacked indica transgenic lines could serves as a novel germplasm for rice blast resistance breeding programmes.
Electronic supplementary material
The online version of this article (10.1007/s13205-017-1062-5) contains supplementary material, which is available to authorized users.
Keywords: Rice, Magnaporthe oryzae, R genes, Rice blast disease, Indica rice, Co-transformation
Introduction
Rice blast caused by the fungus Magnaporthe oryzae is one of the major diseases affecting rice cultivation all over the world (Talbot 2003). It results in substantial loss in rice grain production, which is equivalent to feeding 60 million people annually (Nalley et al. 2016). Virulent M. oryzae isolate creates havoc on susceptible rice lines therefore; options for breeders is to manipulate the rice plant by identifying and deploying blast resistance lines (Bevitori and Ghini 2014). Flor (1971) gave gene for gene hypothesis which states that every resistance (R) gene in the plant has a corresponding avirulence (Avr) gene in the pathogen, if the interaction between R- Avr is incompatible there will be no disease and it would lead to resistance response in plant, but if the interaction is compatible it leads to susceptible reaction. Around 102 rice blast R genes have been identified and out of that 28 have been cloned and characterized (Xiao et al. 2016; Kumari et al. 2017). However, the high mutation rate in M. oryzae, largely due to transposon activity, repetitive genome, and high selection pressure in its genome which often leads to an emergence of new strains resulting in an easy breakdown of single R gene mediated resistance response in rice (Valent and Khang 2010; Singh et al. 2014). Therefore, deployment of multiple R genes having overlapping pattern of resistance has been considered as a suitable approach to improve resistance against rice blast (Dai et al. 2010; Brunner et al. 2010; Xiao et al. 2016). Stacking of multiple R genes either through transgenic or backcross breeding acts as a buffer against breakdown of one or the other gene mediated resistance (Douglas and Halpin 2010). Combining of multiple alleles or orthologues of R genes might weaken the selection pressure on respective pathogen (Brunner et al. 2010; Fukuoka et al. 2015). This approach has been used successfully in rice to develop blast resistance lines with multiple R genes against M. oryzae using molecular breeding (Fukuoka et al. 2015; Tanweer et al. 2015; Xiao et al. 2016).
The major rice blast resistance gene Pi54 was cloned from indica rice line Tetep and imparts broad spectrum resistance to multiple stains of M. oryzae (Sharma et al. 2002, 2005a, b; Rai et al. 2011). Transcriptome analysis of rice has revealed that Pi54 mediated incompatible interaction with M. oryzae triggers upregulation of various defense related genes (Gupta et al. 2012). Subsequently, two orthologues of Pi54; Pi54rh and Pi54of have been cloned and characterized from wild rice Oryza rhizomatis and Oryza officinalis, respectively (Das et al. 2012; Devanna et al. 2014). Allele mining for Pi54 also identified more promising alleles with better blast resistance (Kumari et al. 2013; Thakur et al. 2015; Vasudevan et al. 2015). Previous studies have indicated that the cloned Pi54 orthologues have varying, but overlapping spectra of resistance against different strains of M. oryzae (Das et al. 2012; Devanna et al. 2014). Therefore, the objectives of the present study were (1) stacking of Pi54 and Pi54rh through genetic co-transformation in blast susceptible indica rice line IET17021, (2) molecular characterization of putative stacked transgenic lines and (3) functional analysis of stacked transgenic lines.
Materials and methods
Plant varieties and fungal culture
Seeds of indica rice cv. IET17021 used for stacking of Pi54 and Pi54rh were kindly provided by Dr. A. K. Singh, Division of Genetics, IARI, New Delhi, India. Other rice lines; TP309, TP8.3 and Tetep were available with the corresponding author. The M. oryzae isolate Mo-ei-ger1, monogenic and susceptible LTH rice lines used in the present study were available with Dr. G. Prakash, Division of Plant Pathology, IARI, New Delhi. List of primers used during this study is given in Supplementary Table 1.
Genetic transformation of rice line IET17021
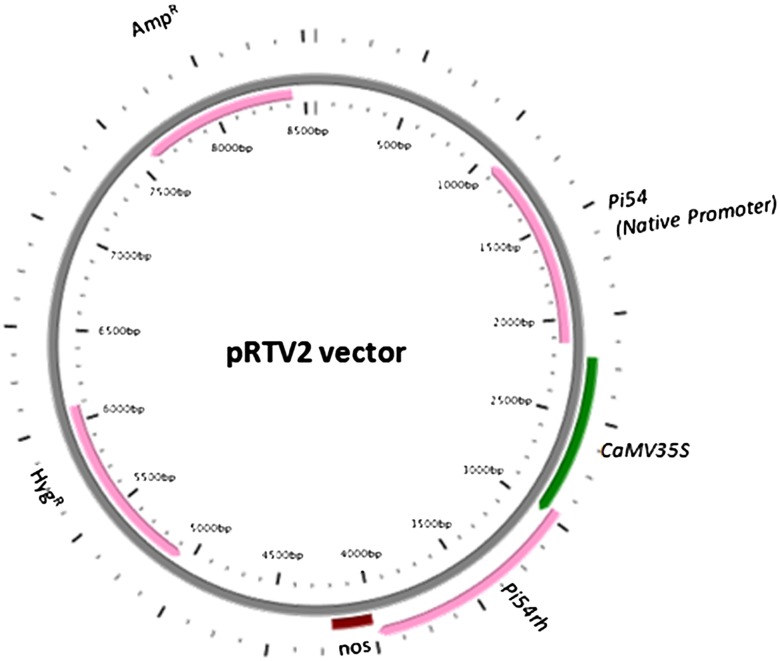
The rice transformation vector pRTV2 (Fig. 1), with stacked blast resistance orthologue genes Pi54 and Pi54rh was previously developed and also used for genetic transformation of blast susceptible japonica rice line TP309 (Kumari et al. 2017). In the present study, pRTV2 plasmid construct was used for genetic transformation of indica rice line IET17021. The rice line IET17021 is a high yielding indica rice cultivar with extra-long slender aromatic grains and is susceptible to M. oryzae. In the present study, scutellar calli derived from the mature embryos of IET17021 seeds were used for transformation following standard protocol (Sanford et al. 1987). After the transformation calli were subjected to selection on hygromycin (50 mg/l) containing MS medium for three cycles of 15 days each. Hygromycin resistant healthy calli were selected and further subjected to regeneration and rooting by following standard protocol (Rai et al. 2011). Finally, hardened and healthy rice seedlings were transferred to growth chamber having controlled conditions suitable for rice.
Fig. 1.
Schematic representation of plant transformation vector having rice blast resistance genes Pi54 and Pi54rh
Molecular analysis of putative transgenic plants
The putative transgenic plants as well as the non-transgenic (NT) IET17021 plants were subjected to molecular analysis using PCR, CAPS marker and Southern blot analysis using genomic DNA isolation by modified CTAB method (Murray and Thompson 1980). For PCR analysis, we used primers specific to hygromycin resistance (hptII) gene (HYG-F, HYG-R), those to amplify DNA fragment consisting 35S promoter and Pi54rh DNA (CVS-F1, PIR-R2) and for Pi54 gene (T7F, Pi54R1) (Supplementary Table 1). DNA of pRTV2 plasmid construct (10 ng) with hptII-Pi54-Pi54rh fragments was used as a positive control and for a negative control genomic DNA from NT-IET17021 was used. PCR positive plants were further used for Southern blot hybridization by following standard protocol (Sambrook et al. 1989). Genomic DNA (15 μg) from transgenic and NT-IET17021 plants was restriction digested with SacII for Southern blot analysis and resolved in an agarose gel (1%). The separated DNA was then transferred to HyBond nylon membrane (N + Amersham Pharmacia, UK) through capillary blotting. For hybridisation, single probe specific to CaMV35S promoter and Pi54rh gene region was PCR amplified using CVSF1 and PIRR2 primers. This probe was labelled using Digoxigenin (DIG) preparation kit (Roche Applied Science, Germany) according to the manufacturer’s guidelines. Pre-hybridization, hybridization, immunological detection and blot development was performed following the standard protocol (Sambrook et al. 1989).
Table 1.
Virulence analysis of Magnaporthe oryzae isolate
| Gene | Mo-ei- ger1 |
|---|---|
| Pi-1 | S |
| Pi-11(t) | S |
| Pi-12 (t) | S |
| Pi-19 | S |
| Pi-20 | R |
| Pi-3 | S |
| Pi-5 (t) | S |
| Pi-7 (t) | S |
| Pi-9 | S |
| Pi-a | S |
| Pi-b | S |
| Pi-i | S |
| Pi-k | S |
| Pi-k h | S |
| Pi-k m | S |
| Pi-kp | S |
| Pi-k S | S |
| Pi-sh | S |
| Pi-t | S |
| Pi-ta (Pi-4)* | S |
| Pita- CP1 | S |
| Pi-ta2-PI | R |
| Pita2-Re | R |
| Pi-z | S |
| Pi-z 5 (Pi-2) | S |
| Pi-z t | S |
| Pi54 | S |
| TETEP | R |
| LTH | S |
| PB1 | S |
| Genotypes susceptible | 24 |
| % virulence | 89 |
S = Disease reaction scale 4–5 type
R = Disease reaction scale 0–3 type
Pathotyping of M. oryzae strain Mo-ei-ger1
Magnaporthe oryzae isolate Mo-ei-ger1 was originally collected from Gerua Kamrup, Assam, India. The pathotype or virulence spectrum of Mo-ei-ger1 was analyzed before using it for screening of the two gene stacked transgenic plants. Rice monogenic lines harbouring 27 single blast R genes along with blast susceptible rice lines LTH and PB1 (Table 1) were challenged with M. oryzae isolate Mo-ei-ger1 following the standard phenotyping protocol described by Sharma et al. (2002). Plants inoculated with gelatine (0.2%) only were taken as mock controls and the whole experiment was proceeded under controlled growth conditions. The disease reaction was recorded 7-day post inoculation (dpi) of M. oryzae (Table 1). Pathotyping study also included indica rice line Tetep, from where Pi54 gene was originally cloned using map based cloning approach (Sharma et al. 2005a, b). Tetep is also the source of other major blast resistance genes like; Pita, Pi1 (t), Pi4 a (t), Pi4 b (t), Pi3(t), Pi-k h (Inukai et al. 1994; Jia et al. 2003; Xu et al. 2008).
Functional analysis of stacked indica transgenic rice lines
The PCR positive and Southern blot confirmed stacked transgenic plants were selected and subjected to phenotypic evaluation against blast isolate Mo-ei-ger1. Fifteen days old stacked indica transgenic rice seedlings as well as wild type non-transgenic (NT) IET17021 plants were challenged with M. oryzae strain Mo-ei-ger1 following the standard protocol described by Sharma et al. (2002). The disease response was observed and the data were recorded 7 dpi using 0–5 disease rating scale (Bonman et al. 1986). The non-transformed (NT) IET17021 was highly susceptible to Mo-ei-ger1 and visible symptoms showing half of the leaf blades damaged by enlarged lesions.
Results
Genetic transformation of rice line IET17021 and their molecular analysis
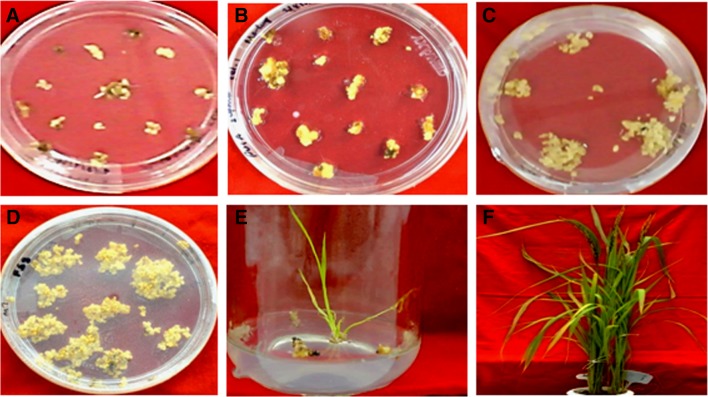
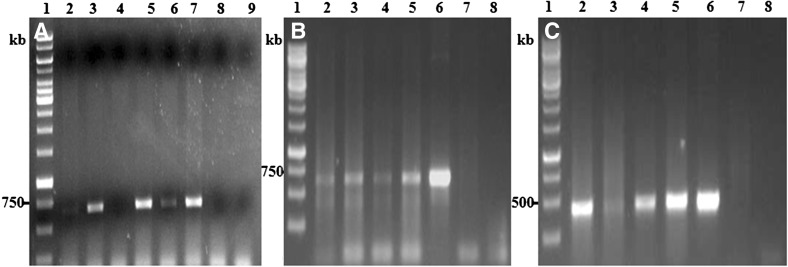
Six putative transgenic IET17021 plants were raised initially from more than hundreds of calli. Various stages of transformation of IET17021 are given in Fig. 2. Finally we could able to harvest seeds from only four independent transformants IET-1, IET-18, IET-20 and IET-23. Molecular analysis using PCR amplification confirmed the presence of transgene in these four putative transgenic lines (Fig. 3a–c). The CAPS (Cleaved Amplified Polymorphic Sequence) marker analysis of PCR positive putative transgenic plants further confirmed the presence of Pi54 along with pRTV2 gene cassette (Supplementary Figure 1a, b). Further analysis of these four transgenic plants using Southern blot confirmed the integration of the gene cassette (Supplementary Figure 2). We did not find PCR and Southern hybridization positive product in the NT-IET17021 plants.
Fig. 2.
Transformation of indica rice variety IET17021 with two gene construct pRTV2. a Calli in Ist selection; b calli in 2nd selection; c calli in 3rd selection; d, e regenerated calli; f putative transgenic plants grown at National Phytotron facility
Fig. 3.
Molecular analysis of putative transgenic plants. a Lane 1:1 kb ladder; lanes 2–6: amplified product of putative transformants using hygromycin specific primer; lane 7: amplified product of two gene construct; lane 8: NT-IET17021 wild type; lane 9: no template control; b lane 1:1 kb ladder; lanes 2–5: amplified product of putative transformants using caMv35S + Pirh specific primer; lane 6: amplified product of two gene construct; lane 7: NT-IET17021 wild type; lane 8: no template control; c lane 1: 1 kb ladder; lanes 2–5: amplified product of putative transformants using Pi54 specific primer; lane 6: amplified product of two gene construct; lane 7: NT-IET17021 wild type; lane 8: no template control
Phenotypic evaluation of stacked indica transgenic lines
Magnaporthe oryzae isolate Mo-ei-ger1 used for virulence analysis could knock down 24 of the 27 single blast R genes including Pi54. The overall virulence spectrum of this strain was 89% (Table 1). Mo-ei-ger1, however, was non virulent on rice line Tetep, from where Pi54 was originally cloned using map based cloning approach. Tetep is also the source of other major rice blast resistance genes. As expected, both the susceptible controls, LTH and PB1 showed compatible reaction while major R genes Pi-20, Pi-ta2-PI, and Pita2-Re shown incompatible interaction (Table 1). However, Mo-ei-ger1 showed incompatible interaction with Pi54rh containing transgenic line (TP8.3) and a compatible interaction with blast susceptible rice line TP309. The typical blast symptoms on TP309 included disease reaction lesions of type 4 and 5 on Bonman disease reaction scale (Supplementary Figure 3). Further, all the four Pi54-Pi54rh stacked transgenic indica rice lines; IET-1, IET-18, IET-20 and IET23 displayed resistant response against the Mo-ei-ger1 infection in comparison to non-transgenic (NT) IET17021. However, among the four transgenic lines, IET-23 was completely resistant, whereas other three lines displayed hyper sensitive (HR) response (Fig. 4).
Fig. 4.

Comparative phenotypic analysis: phenotyping of non-transgenic IET17021 line using Mo-ei-ger1 stains of M. oryzae displayed disease reaction of type 5 category, whereas transgenic IET-1, IET-18 and IET-20 showed type 1 category reaction. However, the phenotypic response of transgenic line IET-23 displayed complete resistance
Discussion
Blast disease is one of the major biotic stresses of rice crop. Considering the economic and environmental benefits of R gene mediated resistance of rice blast disease, identification and cloning of novel R genes is indispensable for tackling the constantly evolving blast pathogen M. oryzae (Wang et al. 2010). Attempts have also been made to tap the natural allelic variations of these R genes (Kumari et al. 2013; Thakur et al. 2015; Vasudevan et al. 2015; Leung et al. 2015). However, a single R gene hardly provides durable resistance as the emergence of diverse strains of M. oryzae might escape this resistance leading to resistance breakdown. Therefore, various attempts are being made to “stack” multiple R genes in a single genetic background as this would make it difficult for the pathogen to evade multiple resistance genes simultaneously (Salomon et al. 2012). Wu et al. (2015) analysed the effective combination pattern of multiple blast R genes or their alleles and found that better combination of multiple genes or alleles could provide more dynamic and durable blast resistance in rice. They also concluded that pyramiding of alleles of major blast R genes could be used as an effective strategy with stronger functional complementary and broad spectrum resistance. It could also create new source of resistant germplasm for enhanced blast resistance breeding in rice. Though pyramiding of multiple blast R genes and their alleles using molecular breeding has been successfully used in rice but there are no reports on stacking of multiple major rice blast R genes or alleles using co-transformation approach in an indica rice line (Xiao et al. 2016; Fukuoka et al. 2015; Khanna et al. 2015; Das and Rao 2015; Luo et al. 2017). Most of the studies used marker assisted selection (MAS) for crop improvement and MAS is found to be associated with linkage drag. Linkage drag could be addressed by single step co-transformation of multiple R genes (Zhu et al. 2012). Recently stacking of Pi54 and Pi54rh genes in blast susceptible japonica rice TP309 through this approach was found to enhance the resistance response against blast disease (Kumari et al. 2017). Similarly, stacking of genes coding for polyproteins in an indica rice enhanced the resistance behaviour against M. oryzae (Jha and Chattoo 2009). Therefore, the aim of the current study was to raise the transgenic indica rice lines overexpressing stacked blast resistance orthologue genes Pi54 and Pi54rh to enhance the durability of resistance against rice blast pathogen through co-transformation approach. PCR and Southern blot analysis confirmed the transgenic plants and all the four plants were showing the same banding pattern in Southern blot. Similar Southern blotting pattern among different transgenic rice lines was reported in an earlier study overexpressing Cry2ax1 gene to improve resistance against rice leaffolder disease. They further observed that the expression level of transgene varied among the lines with same banding pattern (Manikandan et al. 2016).
All the four stacked transgenic rice lines showed incompatible interaction with M. oryzae, but among them IET-23 was providing better resistance response than the rest. This variation in resistance response can be attributed to position effect of transgene integration site. The position effect leading to differential levels of gene expression has been well documented in many crops including Arabidopsis (De Bolle et al. 2003).
Therefore, in the present study we analyse the effectiveness of combining orthologue genes Pi54 and Pi54rh in the genetic background of blast susceptible indica rice line IET17021 in comparatively short duration through co-transformation. Stacking of these orthologue genes improved the resistance response of transgenic lines against M. oryzae in comparison to non-transgenic control plants. Additionally, the novel genetic resources generated in this study could be an important material for rice blast resistance breeding programmes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1. Molecular analysis of stacked transgenic lines using CAPS marker Tsp45I. a: Lane 1: 1 kb DNA ladder; Lane 2-5: amplified product of putative transformants using CAPS marker; Lane 6: amplified product of pRTV2; Lane 7: NT-IET17021; b: Lane 1: 1 kb DNA ladder; Lanes 2-5: Digested product PCR amplicon from putative transformants; Lane 6: digested amplicon pRTV2; Lane 7: digested amplicon from NT-IET17021. (TIFF 560 kb)
Supplementary Fig. 2. Southern blot hybridization using Pi54rh specific probe. Lane 1: positive control (pRTV2), Lane 2: NT-IET17021; Lanes 3-6: Putative transformants. (TIFF 1494 kb)
Supplementary Fig. 3. Phenotypic study of TP8.3 having Pi54rh and NT-TP309 challenged with M. oryzae strain Mo-ei-ger1. (TIFF 373 kb)
Acknowledgements
TRS is thankful to the Department of Biotechnology, Govt. of India and Indian Council of Agricultural Research for funding and Department of Science and Technology, Govt. of India for JC Bose National Fellowship. MK is thankful to CSIR for providing fellowship.
Author contributions
TS: Conceived and designed the experiments; MK, DBN, PKS and RH involved in phenotypic experiments; MK: perform all the molecular analysis; VS: Involved in designing of work. MK, DB, VS and TRS: wrote the manuscript. All the authors read and approved the manuscript.
Compliance with ethical standards
Conflict of interest
All the authors declare no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13205-017-1062-5) contains supplementary material, which is available to authorized users.
References
- Bevitori R, Ghini R. Rice blast disease in climate change times. Rice Res Open Access. 2014 [Google Scholar]
- Bonman J, VergeldeDios T, Khin M. Physiologic specialization of Pyricularia oryzae in the Philippines. Plant Dis. 1986;70(8):767–769. doi: 10.1094/PD-70-767. [DOI] [Google Scholar]
- Brunner S, Hurni S, Streckeisen P, et al. Intragenic allele pyramiding combines different specificities of wheat Pm3 resistance alleles. Plant J. 2010;64(3):433–445. doi: 10.1111/j.1365-313X.2010.04342.x. [DOI] [PubMed] [Google Scholar]
- Dai Y, Jia Y, Correll J, Wang X, et al. Diversification and evolution of the avirulence gene AVR-Pita1 in field isolates of Magnaporthe oryzae Fungal. Genet Biol. 2010;47:973–980. doi: 10.1016/j.fgb.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Das G, Rao GJ. Molecular marker assisted gene stacking for biotic and abiotic stress resistance genes in an elite rice cultivar. Front Plant Sci. 2015 doi: 10.3389/fpls.2015.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Soubam D, Singh P, et al. A novel blast resistance gene, Pi54rh cloned from wild species of rice, Oryza rhizomatis confers broad spectrum resistance to Magnaporthe oryzae. Funct Integr Genom. 2012;12(2):215–228. doi: 10.1007/s10142-012-0284-1. [DOI] [PubMed] [Google Scholar]
- De Bolle MFC, Butaye J, Coucke WJW, Goderis WM, Wouters PFJ, Boxel N, Broekaert WF, Cammue BPA. Analysis of the influence of promoter elements and a matrix attachment region on the inter-individual variation of transgene expression in populations of Arabidopsis thaliana. Plant Sci. 2003;165:69–179. doi: 10.1016/S0168-9452(03)00156-0. [DOI] [Google Scholar]
- Devanna BN, Vijayan J, Sharma TR. The blast resistance gene Pi54of cloned from Oryza officinalis interacts with Avr-Pi54 through its novel non-LRR domains. PLoS One. 2014;9(8):e104840. doi: 10.1371/journal.pone.0104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas E, Halpin C. Gene stacking. In: Mohan Jain S, Brar DS, editors. Molecular techniques in crop improvement. 2. Heidelberg: Springer; 2010. pp. 613–629. [Google Scholar]
- Flor H. Current status of gene-for-gene concept. Annu Rev Phytopathol. 1971;9:275–296. doi: 10.1146/annurev.py.09.090171.001423. [DOI] [Google Scholar]
- Fukuoka S, Saka N, Mizukami Y, et al. Gene pyramiding enhances durable blast disease resistance in rice. Sci Rep. 2015;5:7773. doi: 10.1038/srep07773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK, Rai AK, Kanwar SS, et al. The single functional blast resistance gene Pi54 activates a complex defence mechanism in rice. J Exp Bot. 2012;63:757–772. doi: 10.1093/jxb/err297. [DOI] [PubMed] [Google Scholar]
- Inukai T, Nelson RJ, Zeigler RS, Sarkarung S, Mackill DJ, Bonman JM, Takamure I, Kinoshita T. Allelism of blast resistance genes in near-isogenic lines of rice. Phytopathology. 1994;84(11):1278–1283. doi: 10.1094/Phyto-84-1278. [DOI] [Google Scholar]
- Jha S, Chattoo BB. Transgene stacking and coordinated expression of plant defensins confer fungal resistance in rice. Rice. 2009;2(4):143–154. doi: 10.1007/s12284-009-9030-2. [DOI] [Google Scholar]
- Jia Y, Bryan GT, Farrall L, Valent B. Natural variation at the Pi-ta rice blast resistance locus. Phytopathology. 2003;93(11):1452–1459. doi: 10.1094/PHYTO.2003.93.11.1452. [DOI] [PubMed] [Google Scholar]
- Khanna A, Sharma V, Ellur R, et al. Development and evaluation of near-isogenic lines for major blast resistance gene (s) in Basmati rice. Theor Appl Genet. 2015;128(7):1243–1259. doi: 10.1007/s00122-015-2502-4. [DOI] [PubMed] [Google Scholar]
- Kumari A, Das A, Devanna BN, et al. Mining of rice blast resistance gene Pi54 shows effect of single nucleotide polymorphisms on phenotypic expression of the alleles. Eur J Plant Pathol. 2013;137(1):55–65. doi: 10.1007/s10658-013-0216-5. [DOI] [Google Scholar]
- Kumari M, Rai AK, Devanna BN, et al. Co-transformation mediated stacking of blast resistance genes Pi54 and Pi54rh in rice provides broad spectrum resistance against Magnaporthe oryzae. Plant Cell Rep. 2017 doi: 10.1007/s00299-017-2189-x. [DOI] [PubMed] [Google Scholar]
- Leung H, Raghavan C, Zhou B, et al. Allele mining and enhanced genetic recombination for rice breeding. Rice. 2015;8(1):34. doi: 10.1186/s12284-015-0069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Huang M, Guo T, et al. Marker-assisted selection for rice blast resistance genes Pi2 and Pi9 through high-resolution melting of a gene-targeted amplicon. Plant Breed. 2017;136(1):67–73. doi: 10.1111/pbr.12447. [DOI] [Google Scholar]
- Manikandan R, Balakrishnan N, Sudhakar D, Udayasuriyan V. Transgenic rice plants expressing synthetic cry2AX1 gene exhibits resistance to rice leaffolder (Cnaphalocrosis medinalis) 3 Biotech. 2016;6(1):10. doi: 10.1007/s13205-015-0315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalley L, Tsiboe F, Durand-Morat A, et al. Economic and environmental impact of rice blast pathogen (Magnaporthe oryzae) alleviation in the United States. PLoS One. 2016;11(12):e0167295. doi: 10.1371/journal.pone.0167295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai AK, Kumar SP, Gupta SK, et al. Functional complementation of rice blast resistance gene Pi-kh (Pi54) conferring resistance to diverse strains of Magnaporthe oryzae. J Plant Biochem Biotechnol. 2011;20(1):55–65. doi: 10.1007/s13562-010-0026-1. [DOI] [Google Scholar]
- Salomon D, Sessa G, Altman A, Hasegawa PM (2012) Biotechnological strategies for engineering plants with durable resistance to fungal and bacterial pathogens. In: Plant biotechnology and agriculture-prospects for the 21st century, pp 329–342
- Sambrook J, Fritsch E, Maniatis T. Molecular cloning, a laboratory manual index. Cold Spring Harbor: Cold Spring Harbor Press; 1989. [Google Scholar]
- Sanford JC, Klein TM, Wolf ED, et al. Delivery of substances into cells and tissues using a particle bombardment process. Part Sci Technol. 1987;5(1):27–37. doi: 10.1080/02726358708904533. [DOI] [Google Scholar]
- Sharma T, Chauhan RS, Singh BM, et al. RAPD and pathotype analyses of Magnaporthe grisea populations from the north-western Himalayan region of India. J Phytopathol. 2002;150(11–12):649. doi: 10.1046/j.1439-0434.2002.00812.x. [DOI] [Google Scholar]
- Sharma T, Madhav MS, Singh BM, et al. High-resolution mapping, cloning and molecular characterization of the Pi-kh gene of rice, which confers resistance to Magnaporthe grisea. Mol Genet Genom. 2005;274(6):569–578. doi: 10.1007/s00438-005-0035-2. [DOI] [PubMed] [Google Scholar]
- Sharma T, Shanker P, Singh BM, et al. Molecular mapping of rice blast resistance gene Pi-kh in the rice variety Tetep. J Plant Biochem Biotechnol. 2005;14(2):127. doi: 10.1007/BF03263240. [DOI] [Google Scholar]
- Singh PK, Thakur S, Rathour R, et al. Transposon-based high sequence diversity in Avr-Pita alleles increases the potential for pathogenicity of Magnaporthe oryzae populations. Funct Integr Genom. 2014;14:419–429. doi: 10.1007/s10142-014-0369-0. [DOI] [PubMed] [Google Scholar]
- Talbot NJ. On the trail of a cereal killer: exploring the biology of Magnaporthe grisea. Annu Rev Microbiol. 2003;57(1):177–202. doi: 10.1146/annurev.micro.57.030502.090957. [DOI] [PubMed] [Google Scholar]
- Tanweer FA, Rafii MY, Sijam K, et al. Introgression of blast resistance genes (putative Pi-b and Pi-kh) into elite rice cultivar MR219 through marker-assisted selection. Front Plant Sci. 2015 doi: 10.3389/fpls.2015.01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur S, Singh PK, Das A, et al. Extensive sequence variation in rice blast resistance gene Pi54 makes it broad spectrum in nature. Front Plant Sci. 2015 doi: 10.3389/fpls.2015.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valent B, Khang CH. Recent advances in rice blast effector research. Curr Opin Plant Biol. 2010;13(4):434–441. doi: 10.1016/j.pbi.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Vasudevan K, Gruissem W, Bhullar NK. Identification of novel alleles of the rice blast resistance gene Pi54. Sci Rep. 2015 doi: 10.1038/srep15678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wen JW, Liu WP et al (2010) Interaction studies between rice and Pyricularia grisea in Jilin Province, PR China. In: Proc. of the 5th Intern. Rice Blast Conf., USA. USDA-DBNRRC
- Wu Y, Xio N, Yu L, et al. Combination patterns of major R genes determine the level of resistance to the M. oryzae in rice (Oryza sativa L.) PLoS One. 2015;10(6):e0126130. doi: 10.1371/journal.pone.0126130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao N, Wu Y, Pan C, et al. Improving of rice blast resistances in japonica by pyramiding major R genes. Front Plant Sci. 2016;7:1918. doi: 10.3389/fpls.2016.01918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Hayashi N, Wang CT, Kato H, Fujimura T, Kawasaki S. Efficient authentic fine mapping of the rice blast resistance gene Pik-h in the Pik cluster, using new Pik-h-differentiating isolates. Mol Breed. 2008;22(2):289–299. doi: 10.1007/s11032-008-9175-5. [DOI] [Google Scholar]
- Zhu S, Li Y, Vossen JH, et al. Functional stacking of three resistance genes against Phytophthora infestans in potato. Transgenic Res. 2012;21(1):89–99. doi: 10.1007/s11248-011-9510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Molecular analysis of stacked transgenic lines using CAPS marker Tsp45I. a: Lane 1: 1 kb DNA ladder; Lane 2-5: amplified product of putative transformants using CAPS marker; Lane 6: amplified product of pRTV2; Lane 7: NT-IET17021; b: Lane 1: 1 kb DNA ladder; Lanes 2-5: Digested product PCR amplicon from putative transformants; Lane 6: digested amplicon pRTV2; Lane 7: digested amplicon from NT-IET17021. (TIFF 560 kb)
Supplementary Fig. 2. Southern blot hybridization using Pi54rh specific probe. Lane 1: positive control (pRTV2), Lane 2: NT-IET17021; Lanes 3-6: Putative transformants. (TIFF 1494 kb)
Supplementary Fig. 3. Phenotypic study of TP8.3 having Pi54rh and NT-TP309 challenged with M. oryzae strain Mo-ei-ger1. (TIFF 373 kb)