Abstract
Objectives
In addition to traditional risk factors, excess cardiovascular disease (CVD) in rheumatoid arthritis (RA) is attributed to enhanced vascular and/or systemic inflammation. In several small studies using 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) to directly assess vascular inflammation, FDG uptake was higher in RA patients than controls. Using a substantially larger RA sample, we sought to identify RA-disease characteristics independently associated with vascular FDG uptake.
Methods
RA patients underwent cardiac FDG-PET/CT, with aortic inflammation assessed by quantification of FDG uptake in the ascending aorta, calculated as the mean and maximum (max) standardized-uptake-value (SUV) of the entire ascending aorta, and of its most diseased segment (SUV MDS). Univariable and multivariable regression models were constructed to model the associations of patient characteristics with aortic FDG uptake.
Results
Ninety-one RA patients were scanned. In multivariable models, in addition to the independent associations of hypertension and body-mass-index with increased aortic FDG uptake, the prevalence of rheumatoid nodules correlated with the SUV-mean and SUV MDS-mean measurements, while anti-CCP antibodies inversely correlated with these measures and with the SUV-max and SUV MDS-max (p<0.05). A significant association of RA disease activity with aortic FDG uptake was observed but restricted to anti-CCP seropositivity.
Conclusion
Traditional CV risk factors and RA-disease characteristics (rheumatoid nodules and DAS28-CRP in anti-CCP antibody positive individuals) were independently associated with ascending aortic FDG uptake in RA patients without clinical CVD.
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease primarily affecting the synovial joints but with important extra-articular features including significant effects on the cardiovascular system (1). Despite therapeutic advancements over the past decades, cardiovascular disease (CVD) continues to be the leading cause of excess deaths in RA with CVD mortality rates 1.5–3-fold higher than matched controls (2). Unfortunately, CV risk algorithms established for the general population generally underperform in RA (3), hence the development of strategies to better identify and manage the increased CV risk in RA has become a priority.
Chronic systemic and/or vascular inflammation are believed to contribute to the excess risk of atherosclerosis and CV events in RA. Atherosclerosis is an inflammatory process reflected in plaque by infiltrating macrophages and T cells, and systemically by mildly elevated levels of inflammatory cytokines such as tumor necrosis factor (TNF), interleukins-1 and −6 (IL-1, IL-6), and metalloproteases (MMPs) (4,5). Mild elevations of these cytokines and MMPs are potent predictors of future CV events in the general population, presumably through their promotion of plaque rupture (6–8). RA is characterized by much higher serum levels of the same molecules (5) which has led to the hypothesis that this amplified systemic inflammatory milieu accelerates atherosclerosis and plaque rupture in patients with RA. Indeed, a number of studies have shown circulating and articular measures of inflammation to be independent predictors of CV events in RA (9–11). However, an alternate or complementary hypothesis is that vascular inflammation is also enhanced in RA relative to non-RA controls but direct assessments of this hypothesis are few and consist of small sample sizes (12–14).
Most assessments of atherosclerosis in RA to date have utilized imaging techniques that identify the presence of atherosclerotic plaque and luminal stenosis, but not arterial wall inflammation. This is a crucial limitation as plaque inflammation is believed to represent one of the early and reversible steps of atherosclerosis (15,16). In addition, although assumed to be of rare occurrence, rheumatoid aortitis has been described in the spectrum of rheumatoid vasculitis independent of atherosclerotic plaque (17–19). The recent development of techniques that enable co-registration of PET with CT (or MRI) scans coupled with radioisotopes that are avidly taken up by macrophages (i.e., FDG) have enabled the direct identification of inflammation in vascular walls providing not only morphologic but physiologic information. Importantly, increased FDG uptake in the vessel wall suggests plaque instability, a risk factor for plaque rupture and CV events, but it may also represent vasculitis (20,21). Although identification of vascular inflammation in the coronaries is ultimately preferred, PET imaging of the coronary arteries remains challenging due to the small caliber of the coronary arteries as well as their motion during cardiac cycles (22). However, the known association of aortic atherosclerosis, as well as aortic FDG uptake, with CV events in the general population (20,21) makes the study of aortic inflammation of particular interest in RA.
Although vascular FDG-PET/CT studies in RA are few, Maki-Petaja et al reported a level of aortic FDG uptake in 17 RA patients with high disease activity equivalent to that of 34 non-RA subjects with stable CAD (12). A cross-sectional study of 10 psoriasis and 5 RA patients also demonstrated higher aortic FDG uptake compared with 10 healthy subjects after adjusting for CV risk factors (13). However, the small RA sample sizes in these studies were inadequate to identify RA-specific factors associated with vascular inflammation. In this cross-sectional study, we hypothesized that RA-specific features would be associated with ascending aortic FDG uptake independent of conventional CV risk factors.
METHODS
Patients
The first 91 RA participants enrolled in the RHeumatoid arthritis studY of THe Myocardium (RHYTHM) between November 2011 and March 2015 constituted the study population. RHYTHM is an ongoing study to identify and evaluate factors associated with subclinical myocardial phenotypes in RA patients without clinical CVD. Eligibility for the RA patients required fulfillment of the 2010 (23) American College of Rheumatology criteria for RA and age ≥18 years. Participants with a history of clinical CVD, defined as a self-reported physician diagnosed myocardial infarction, heart failure, coronary artery revascularization, angioplasty, peripheral arterial disease or procedures, pacemaker, or defibrillator devices, stroke and transient ischemic attack, were excluded from participation. Additional exclusion criteria included contraindications to receiving a vasodilator (adenosine or regadenoson) and an active history of cancer. Patients were consecutively recruited from the Columbia University Rheumatology Clinics and from local referring rheumatologists. All participants provided written consent prior to participation. The study was in compliance with the Helsinki Declaration and approved by the Columbia University Institutional Review Board. Enrolment for RHYTHM began in 2011 and is ongoing.
Outcome Measures
Ascending Aortic 18FDG-PET/CT uptake
FDG-PET/CT imaging of the ascending aorta was performed using reproducible, validated methods (24,25). Specifically, FDG was administered intravenously (10 mCi) after 18-hours of a carbohydrate-free diet (in order to suppress myocardial FDG uptake) and overnight fast. All patients had a blood sugar concentration of < 200 mg/dl at the time of imaging. Imaging was performed 90 mins after FDG injection. A CT scan was obtained for attenuation correction and anatomical co-registration, using a voltage of 120 kVp and MAS of 25. Thereafter, PET imaging of the chest was performed, with 10 min per bed position. The pitch was 2.8 and slice thickness was 3.27 mm. Reconstruction of attenuation-corrected images was done using the OSEM algorithm with corrections for normalization, dead time, random events, scatter, attenuation and sensitivity. Analyses were restricted to the ascending aorta, since the CT scan performed for co-registration was a cardiac CT which did not include the upper chest or neck. The area of interest was defined as the region of the aorta beginning 1 cm above the origin of the left main coronary artery and ending at the aortic arch in transaxial view. Ascending aortic inflammation was determined by 18FDG PET uptake in the arterial wall calculated as the standardized uptake value (SUV): SUV= r/ (a’/w), where r is the radioactivity activity concentration [kBq/ml] measured by the PET scanner within a region of interest (ROI), a’ is the decay-corrected amount of injected radiolabeled FDG [kBq], and w is the weight of the patient [g], which is used a surrogate for a distribution volume of tracer. ROIs were drawn around the artery in transaxial orientation to measure the SUV for each region of interest. We used several metrics to report the measured SUV. The SUV mean was defined as the average SUV of the entire ascending aorta (as defined above). Since each slice has a mean and a maximal value, we calculated the average of the mean values (SUV mean) and of the maximal values (SUV max) for all slices. The SUV of the most diseased segment (SUV MDS) was also assessed, defined as the average SUV-max (SUV MDS max) or the average MDS mean (SUV MDS mean) for three consecutive slices centered on the aortic slice with the highest SUV and the adjacent slice superior and inferior to it, providing ~1 cm of the most inflamed section of the aortic wall. Each study was read by two experienced readers using the Medview display integrated into the Corridor 4DM v. 7.0 software (Invia Medical Imaging Solutions, Ann Arbor, MI) (26). Inter-reader correlation for the SUV measurements was 0.997.
Coronary artery calcification (CAC)
CAC was ascertained with cardiac CT using a multi-detector row computed tomography system, and quantified by the Agatston method (27).
Clinical Covariates
Information on demographics and smoking was collected by standardized health questionnaires. Resting blood pressure (BP) was measured three times, and the mean value of the last two measurements was used in the analyses. Hypertension was defined as a systolic BP of ≥140 mm Hg, diastolic BP of ≥90 mm Hg, or use of antihypertensive medications. Diabetes was defined as a fasting serum glucose level of ≥126 mg/dl or use of antidiabetic medications. Body mass index (BMI) was calculated as the weight (in kg) divided by the height (in m2). Prescription and over-the-counter medications taken within the preceding 2 weeks were documented from containers supplied by the participants. RA disease duration was calculated from the date of physician diagnosis. RA activity was calculated using the Disease Activity Score in 28 joints (DAS28) with CRP level (28). Current and past use of glucocorticoids and of biologic and non-biologic DMARDs was ascertained by patient interview and medical records. Non-biologic DMARDs included methotrexate, sulfasalazine, hydroxychloroquine, and leflunomide. Biologic DMARDs included adalimumab, etanercept, infliximab, certolizumab, golimumab, tocilizumab, abatacept, tofacitinib, anakinra, and rituximab.
Laboratory Covariates
Phlebotomy was performed after an overnight fast at the same visit as the FDG PET/CT. Serum and plasma were separated by centrifugation and stored at −80°C. All analytes were measured in the Biomarkers Core Laboratory of the Columbia University Irving Institute for Clinical and Translation Research. Anti-CCP antibodies were assessed by enzyme-linked immunosorbent assay (ELISA) using the CCP3 kit with positivity defined as ≥ 60 units. Total cholesterol, high-density lipoprotein-cholesterol, and triglycerides were measured in plasma by colormetric assay using Cobas Intergra 400 Plus kits (Roche Diagnostics, Indianapolis, IN) and LDL was calculated using the Friedewald formula. IL-6 was assayed by ELISA test kits from R&D Systems (Minneapolis, MN). High-sensitivity CRP was measured by turbidimetric immunoassay from Cobas Intergra 400 Plus kits from Roche Diagnostics. Rheumatoid factor (RF) IgM antibodies were assessed by ELISA with RF defined as positive at a concentration of ≥ 40 units.
Statistical Analysis
The analyses in this report were not conceived in the original design of the RHYTHM Study, which focused on myocardial FDG uptake; rather they were performed as post-hoc analyses, hence with no a priori hypothesis or power calculation to test the aortic FDG uptake. However, the question of interest was whether RA-specific characteristics would identify patients with higher aortic FDG uptake, independent of conventional CV risk factors. Because uptake could conceivably represent discrete (or diffuse) atherosclerotic plaque or diffuse vasculitic involvement, we explored as our primary outcome several measurements of FDG uptake (SUV-mean, SUV-max, SUV MDS-mean, and SUV MDS-max). The normality of the variables of interest was determined by graphical assessment and the Kolmogorov-Smirnov test. Summary statistics for outcomes and predictor variables were examined, with comparisons made using student t-test and Wilcoxon rank-sum test for normally and non-normally distributed continuous variables, respectively. Counts and percentages were calculated for categorical variables and compared using the chi-square or Fisher’s exact test, as appropriate. Linear regression models were constructed to explore the association of RA-disease characteristics and traditional CV risk factors with ascending aortic 18FDG uptake quantified as the log transformed SUV-mean, SUV-max, SUV MDS-mean, and the SUV MDS-max. Tolerance was calculated to ensure that variables with excessive collinearity were not co-modeled; none of the variables had a tolerance <0.1. To isolate the association between CV risk factors and RA-disease characteristics with the measures of ascending aortic FDG uptake, confounders were defined as those variables associated with both the outcome (ascending aortic FDG uptake) and the independent variables (CV risk factors and RA-disease characteristics). All statistical calculations were performed using Stata 12 and SAS 9.4. In all tests, a 2-tailed α of 0.05 was defined as the level of statistical significance.
RESULTS
Patient Characteristics
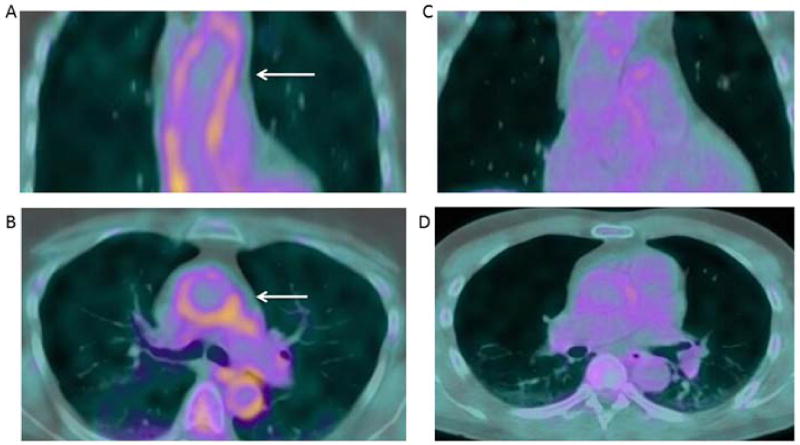
The characteristics of the study participants are summarized in Table 1. Most patients (80%) were female with established disease (median RA duration=7.2 years); however, 25% had disease duration less than 1.6 years. The majority (65%) were seropositive for RF and/or anti-CCP, and 8% had rheumatoid nodules. Seventy-seven percent were treated with a non-biologic DMARD, 40% with a biologic, and one third was currently receiving prednisone at a median daily dose of 5 mg. Forty-two percent had hypertension and 43% were current or ever smokers; only 9% had diabetes, 18% were taking statins, a third had a CAC score > 0, and the median of those with a CAC > 0 was 130 (28–573). Figure 1 shows an example of a patient with high SUV uptake compared to a patient with low uptake. For the total group of RA patients, the median ascending aortic SUV-mean, SUV-max, SUVMDS-mean and SUV MDS-max were 1.83, 2.43, 1.91, and 2.59, respectively (Table 1).
Table 1.
Patient Characteristics.
| RA (n=91) | |
|---|---|
| Demographics | |
| Age, years | 55 ± 13 |
| Female, n (%) | 73 (80) |
| Race/Ethnicity | |
| Non-Hispanic White, n (%) | 36 (40) |
| Non-Hispanic Black, n (%) | 12 (13) |
| Hispanic, n (%) | 40 (44) |
| Other, n (%) | 3 (3) |
| RA Characteristics | |
| RA duration, years | 7.2 (1.6–14.4) |
| RF > 40 units, n (%) | 49 (54) |
| CCP > 60 units, n (%) | 58 (64) |
| Shared Epitope, n (%) | 53 (58) |
| DAS28-CRP | 3.7 ± 1.2 |
| Nodules, n (%) | 7 (8) |
| CRP, mg/L | 2.2 (1.0–6.7) |
| IL-6, pg/mL | 2.3 (1.3–7.0) |
| HAQ | 1.0 (0.5–1.75) |
| Any non-biologic DMARD, n (%) | 69 (76) |
| Any biologic DMARD, n (%) | 36 (40) |
| Current prednisone, n (%) | 30 (33) |
| Conventional CV Risk Factors | |
| BMI | 28 ± 6 |
| Ever smoking, n (%) | 39 (43) |
| Current smoking, n (%) | 9 (10) |
| Hypertension, n (%) | 38 (42) |
| Diabetes, n (%) | 8 (9) |
| Total Cholesterol | 192 ± 35 |
| LDL | 110 ± 31 |
| HDL | 60 ± 19 |
| Triglycerides | 93 (77–132) |
| Statin use, n (%) | 16 (18) |
| CAC > zero, n (%), [median] (IQR) | 32 (35), [130] (28–573) |
| CAC score | 0 (0–45) |
| Median SUV-Mean | 1.83 (1.58–2.11) |
| Median SUV-Max | 2.43 (2.14–2.79) |
| Median SUV MDS-Mean | 1.91 (1.64–2.19) |
| Median SUV MDS-Max | 2.59 (2.30–3.11) |
Characteristics are expressed as n (%), as the mean ± standard deviation (SD), or as the median (interquartile range). RF, rheumatoid factor; anti-CCP, anti-citrullinated cyclic peptide; DAS28-CRP, disease activity score with C-RP; C-RP, C-reactive protein; HAQ, health assessment questionnaire; DMARD, disease modifying antirheumatic drug; BMI, body mass index; LDL, low-density lipoprotein-cholesterol; HDL, high-density lipoprotein-cholesterol; CAC, coronary artery calcium; SUV, standardized uptake value; MDS, most diseased segment.
Figure 1.
Aortic FDG Uptake in RA.
Representative FDG PET/CT imaging of patients with RA with high vs low FDG uptake are shown. Radiotracer uptake in the aorta is diffuse (arrow) with an SUV-max of 2.81 and an SUV-MDS max of 2.91(A: coronal view. B: axial view) vs low 18 F-FDG uptake with an SUV-max of 1.11 and an SUV-MDS max of 1.16 (C: coronal view. D: axial view).
Univariate analysis of the association of patient characteristics with ascending aortic 18FDG uptake
In univariate analysis (Table 2), age, BMI, hypertension, and CAC score were positively associated with the four study measures of ascending aortic FDG uptake (SUV-mean, SUV-max, SUV MDS-mean, and the SUV MDS-max), while HDL levels were negatively associated with these outcomes. Among RA characteristics, RA nodules were positively associated with SUV-mean and SUV-MDS mean.
Table 2.
Univariate association of patient characteristics with 18FDG PET aortic uptake.
| Log SUV-mean | Log SUV-max | Log SUV-MDS Mean |
Log SUV-MDS max | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Coefficient | p value | Coefficient | p value | Coefficient | p value | Coefficient | p value | |
| Demographics | ||||||||
| Age, years | 0.0037 | 0.033 | 0.0045 | 0.012 | 0.0036 | 0.038 | 0.0051 | 0.005 |
| Male | −0.027 | 0.64 | 0.029 | 0.63 | −0.028 | 0.64 | 0.062 | 0.32 |
| NH-White | −0.014 | 0.77 | −0.015 | 0.76 | −0.012 | 0.80 | −0.012 | 0.82 |
| NH-Black | −0.029 | 0.68 | −0.032 | 0.66 | −0.024 | 0.74 | −0.058 | 0.43 |
| Hispanic | 0.028 | 0.56 | 0.030 | 0.53 | 0.028 | 0.56 | 0.034 | 0.50 |
| RA Characteristics | ||||||||
| RA duration, year | 0.00087 | 0.68 | 0.00044 | 0.84 | 0.0013 | 0.53 | 0.00083 | 0.71 |
| RF>40 units | −0.072 | 0.12 | −0.058 | 0.22 | −0.063 | 0.18 | −0.055 | 0.27 |
| CCP>60 units | −0.090 | 0.061 | −0.086 | 0.083 | −0.090 | 0.064 | −0.100 | 0.053 |
| Shared Epitope | −0.045 | 0.41 | −0.046 | 0.41 | −0.060 | 0.28 | −0.050 | 0.39 |
| DAS28-CRP | 0.024 | 0.22 | 0.023 | 0.25 | 0.025 | 0.20 | 0.016 | 0.44 |
| Nodules | 0.178 | 0.039 | 0.136 | 0.13 | 0.198 | 0.023 | 0.102 | 0.28 |
| log CRP, per mg/L | 0.022 | 0.23 | 0.027 | 0.15 | 0.020 | 0.28 | 0.020 | 0.30 |
| log IL-6, per pg/mL | 0.0035 | 0.87 | 0.0017 | 0.94 | 0.011 | 0.62 | −0.0062 | 0.78 |
| HAQ, per unit | 0.017 | 0.58 | 0.024 | 0.45 | 0.022 | 0.47 | 0.031 | 0.34 |
| Non-biologic DMARDs | 0.012 | 0.83 | 0.023 | 0.69 | 0.017 | 0.77 | 0.051 | 0.40 |
| Biologic DMARDs | −0.0015 | 0.98 | 0.016 | 0.75 | 0.010 | 0.83 | 0.034 | 0.51 |
| Current prednisone | −0.014 | 0.77 | −0.018 | 0.72 | −0.010 | 0.84 | −0.018 | 0.74 |
| Conventional CV Risk Factors | ||||||||
| BMI, per unit | 0.012 | 0.004 | 0.013 | 0.002 | 0.011 | 0.007 | 0.012 | 0.005 |
| Ever smoking | 0.077 | 0.099 | 0.065 | 0.18 | 0.090 | 0.057 | 0.063 | 0.21 |
| Current smoking | 0.041 | 0.60 | 0.018 | 0.82 | 0.057 | 0.47 | −0.010 | 0.90 |
| Hypertension | 0.130 | 0.005 | 0.107 | 0.027 | 0.129 | 0.006 | 0.105 | 0.036 |
| Diabetes | 0.091 | 0.26 | 0.059 | 0.48 | 0.111 | 0.17 | 0.037 | 0.67 |
| Total cholesterol, per mg/dL | −0.0002 | 0.79 | −0.00058 | 0.40 | −0.00022 | 0.75 | −0.0010 | 0.14 |
| LDL, per mg/dL | 0.00026 | 0.72 | 0.000071 | 0.93 | 0.00028 | 0.71 | −0.00032 | 0.69 |
| HDL, per mg/dL | −0.0023 | 0.052 | −0.0030 | 0.012 | −0.0025 | 0.034 | −0.0034 | 0.007 |
| log Triglycerides, per mg/dL | 0.083 | 0.13 | 0.080 | 0.16 | 0.087 | 0.12 | 0.073 | 0.22 |
| Statin use | 0.036 | 0.56 | 0.058 | 0.36 | 0.049 | 0.43 | 0.065 | 0.32 |
| Any CAC | 0.088 | 0.069 | 0.079 | 0.12 | 0.080 | 0.10 | 0.082 | 0.11 |
| log CAC +1 | 0.020 | 0.023 | 0.019 | 0.040 | 0.019 | 0.034 | 0.020 | 0.034 |
Univariable linear regression coefficients and p values of the association of each patient and RA-specific characteristic with FDG aortic uptake measured as the log-transformed aortic FDG-PET SUV-mean, SUV-max, SUV MDS-mean, and the SUV MDS-max. SUV, standardized uptake value; MDS, most diseased segment; NH, non-Hispanic; RF, rheumatoid factor; anti-CCP, anti-citrullinated cyclic peptide; DAS28-CRP, disease activity score with C-RP; HAQ, health assessment questionnaire; DMARD, disease modifying antirheumatic drug; BMI, body mass index; LDL, low-density lipoprotein-cholesterol; HDL, high-density lipoprotein-cholesterol; CAC, coronary artery calcium.
Multivariable analysis of the association of patient characteristics with ascending aortic 18FDG uptake
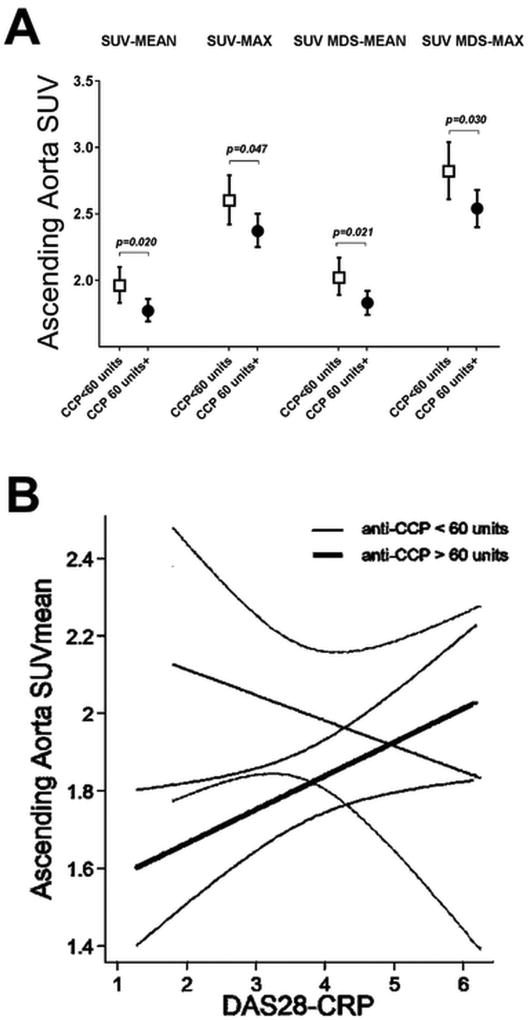
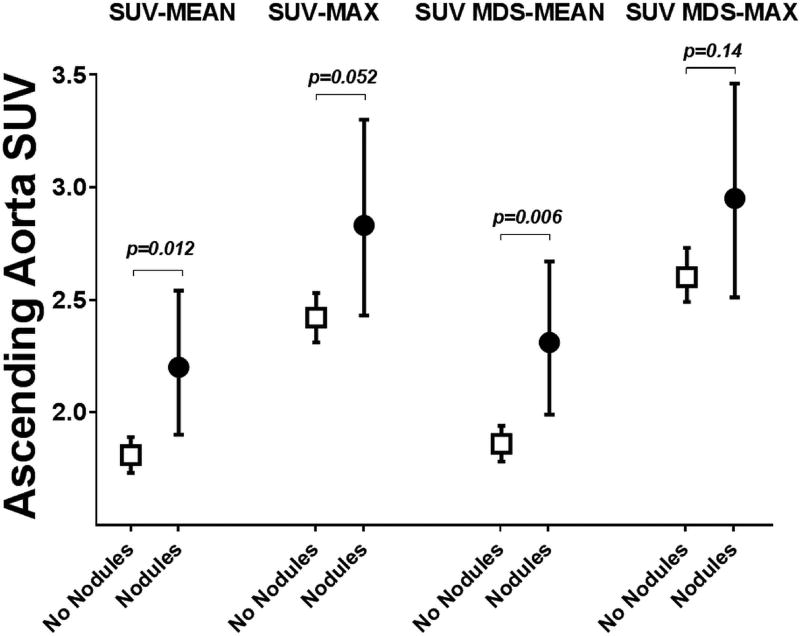
In multivariable analyses (Table 3), BMI remained significantly associated with all 4 measures of ascending aortic FDG uptake. In addition, the association between hypertension and SUV-mean and SUV MDS-mean persisted, as well as the association of age and SUV MDS-max (Table 3). Although in univariate analysis the association of anti-CCP antibody titer > 60 units with SUV ascending aortic measures did not reach statistical significance, in adjusted models the association of anti-CCP antibody titer > 60 units and all 4 measures of ascending aortic FDG uptake was statistically significant (Figure 2A). Interestingly, the association between RA-disease activity (measured by DAS28-CRP) and ascending aortic FDG uptake varied depending on anti-CCP antibody status (p interaction term= 0.047): in those with anti-CCP > 60 units, the higher the DAS28-CRP, the higher the SUV-mean and SUV MDS-mean (Figure 2B). In addition, the association of rheumatoid nodules with SUV-mean and SUV MDS-mean observed in univariate analysis remained significant in the adjusted analyses (Figure 3). No association was identified between the shared epitope and ascending aortic FDG uptake measures.
Table 3.
Multivariable associations of patient characteristics with FDG uptake in the Ascending Aorta.
| Extended MV Model | Reduced MV Model | |||
|---|---|---|---|---|
|
|
||||
| Coefficient | p value | Coefficient | p value | |
| log SUV-mean | ||||
| Age, year | 0.00092 | 0.62 | ||
| BMI, kg/m2 | 0.0089 | 0.045 | 0.01 | 0.006 |
| Hypertension | 0.095 | 0.048 | 0.097 | 0.025 |
| Ever smoking | 0.037 | 0.41 | ||
| HDL, mg/dL | −0.0014 | 0.27 | ||
| log Triglycerides | −0.031 | 0.63 | ||
| RF>40 units | −0.08 | 0.11 | ||
| CCP>60 units | −0.06 | 0.24 | −0.101 | 0.02 |
| DAS28-CRP | −0.0016 | 0.93 | ||
| Nodules | 0.195 | 0.016 | 0.195 | 0.012 |
| R2 | 0.194 | 0.0022* | 0.211 | 0.0001*1 |
| log SUV- max | ||||
| Age, year | 0.0022 | 0.28 | 0.0031 | 0.073 |
| BMI, kg/m2 | 0.0103 | 0.035 | 0.012 | 0.002 |
| Hypertension | 0.056 | 0.26 | ||
| Ever smoking | 0.029 | 0.55 | ||
| HDL, mg/dL | −0.0022 | 0.13 | ||
| log Triglycerides | −0.057 | 0.4 | ||
| RF>40 units | −0.07 | 0.18 | ||
| CCP>60 units | −0.058 | 0.28 | −0.091 | 0.047 |
| logCRP | −0.002 | 0.92 | ||
| Nodules | 0.149 | 0.074 | 0.158 | 0.052 |
| R2 | 0.161 | 0.0069* | 0.177 | 0.0004*2 |
| log SUV MDS-mean | ||||
| Age, year | 0.0006 | 0.75 | ||
| BMI, kg/m2 | 0.0077 | 0.091 | 0.0098 | 0.011 |
| Hypertension | 0.1 | 0.05 | 0.097 | 0.028 |
| Ever smoking | 0.055 | 0.24 | ||
| Diabetes | −0.028 | 0.75 | ||
| HDL, mg/dL | −0.0019 | 0.17 | ||
| log Triglycerides | −0.028 | 0.67 | ||
| RF>40 units | −0.071 | 0.16 | ||
| CCP>60 units | −0.064 | 0.22 | −0.102 | 0.021 |
| DAS28-CRP | 0.00086 | 0.96 | ||
| Nodules | 0.212 | 0.012 | 0.215 | 0.006 |
| R2 | 0.185 | 0.0039* | 0.207 | 0.0001*3 |
| log SUV MDS-max | ||||
| Age, year | 0.0027 | 0.19 | 0.0038 | 0.038 |
| BMI, kg/m2 | 0.0089 | 0.074 | 0.011 | 0.009 |
| Hypertension | 0.066 | 0.23 | ||
| Ever smoking | 0.034 | 0.5 | ||
| Diabetes | −0.068 | 0.46 | ||
| HDL, mg/dL | −0.0029 | 0.057 | ||
| log Triglycerides | −0.067 | 0.34 | ||
| RF>40 units | −0.06 | 0.27 | ||
| CCP>60 units | −0.0057 | 0.18 | −0.105 | 0.03 |
| DAS28-CRP | −0.0057 | 0.79 | ||
| Nodules | 0.128 | 0.15 | 0.125 | 0.14 |
| R2 | 0.142 | 0.016* | 0.158 | 0.0010*4 |
Multivariable linear regression coefficients and p values of the association of patient and RA-specific characteristic with FDG aortic uptake measured as the log-transformed aortic FDG-PET SUV-mean, SUV-max, SUV-MDS mean, and the SUV-MDS max.
Root Mean Square Error (RMSE) of 0.19;
RMSE of 0.20;
RMSE of 0.19;
RMSE of 0.21.
SUV, standardized uptake value; MDS, most diseased segment; SUV, standardized uptake value; MDS, most diseased segment; BMI, body mass index; HDL, high-density lipoprotein-cholesterol; RF, rheumatoid factor; anti-CCP, anti-citrullinated cyclic peptide; DAS28-CRP, disease activity score with C-RP.
Figure 2.
Adjusted Associations of anti-CCP level with Measures of FDG Uptake in the Ascending Aorta.
A. Means and 95% confidence intervals depicted. Adjusted for age, BMI, hypertension, and the presence of rheumatoid nodules. B. Interaction plot depicting the association between RA-disease activity (measured by DAS28-CRP) and aortic SUV uptake vary by anti-CCP antibody level (interaction term p=0.047).
Figure 3.
Adjusted Associations of Rheumatoid Nodules with Measures of FDG Uptake in the Ascending Aorta.
Means and 95% confidence intervals depicted. Adjusted for age, BMI, hypertension, and anti-CCP.
DISCUSSION
The key finding in this study is that several RA-specific features (anti-CCP seropositivity and rheumatoid nodules) are associated with ascending aortic inflammation, as measured by 18F-FDG PET/CT, independent of conventional cardiovascular risk factors. Additionally, we have shown that the association between RA disease activity and ascending aortic inflammation was restricted to patients with anti-CCP seropositivity. Finally, this study supports the ability of PET imaging as a tool to detect aortic vessel wall inflammation in RA patients without clinical CVD; our study serving as the largest study to date to investigate this vascular outcome in RA using this imaging modality.
Cardiovascular disease continues to be the leading cause of death in RA patients. The excess risk compared with controls is only partially explained by traditional cardiovascular risk factors, with RA itself being independently associated with CVD (29). Inflammation is known to play an important role in all phases of atherosclerosis, from initiation to atherothrombosis (15,16). Chronically higher levels of circulating inflammatory cytokines in RA, relative to non-RA patients with atherosclerosis, may fuel plaque inflammation and rupture leading to increased risk of CV events; however infiltration of plaque itself with increased numbers of inflammatory cells is hypothesized to play a key role in this increased risk.
A novel imaging modality, the 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET), has emerged in the last decade as a marker of atherosclerosis in the general population as it allows the quantification of 18F-2-deoxy-D-glucose uptake (vascular inflammation) within the atheroma and overall in the arterial wall (24,30). Standardized uptake values (SUVs), the outcome variables in our study, are a measure of radiotracer uptake normalized for injected dose and patient weight. SUVs are most commonly used in the assessment of 18 F-FDG PET/CT oncology studies for the diagnosis and staging of cancer. In addition to the anatomical imaging that CT and MRI offer, multimodal imaging with FDG-PET/CT provides molecular and functional data allowing the detection of signature pathological mechanisms. More recently, FDG PET/CT has been used in cardiac imaging for the diagnosis and follow-up of patients with inflammatory conditions of the heart including sarcoidosis, endocarditis and pacemaker infections. In vivo measures of FDG PET uptake are reproducible, positively correlate with metabolically active macrophages and macrophage infiltration into the vessel wall, and are modifiable by different pharmacological interventions targeted at reducing atherosclerotic inflammation, such as statins (31–34). More importantly, increased arterial FDG uptake is known to predict plaque expansion and rupture, leading to CV events including myocardial infarction and strokes (20,21). FDG-PET/CT is increasingly used as a primary outcome in randomized controlled trials of anti-atherogenic drugs because arterial inflammation is believed to represent one of the early and reversible steps of atherosclerosis (35,36). Several studies have also shown an association between FDG signal and CV risk factors or risk scores (21,37–40). Moreover, vascular FDG PET imaging has proven to improve the prediction of CVD beyond the traditionally used Framingham risk stratification score (41). All of these features make the use of FDG PET vascular imaging compelling in the study of CVD in RA, an illness in which systemic and articular inflammation are the sine qua non.
Aortic FDG PET imaging in two prior small studies of RA patients without clinical CVD revealed significantly increased subclinical aortic FDG uptake when compared with controls (12,13). Rose et al reported an SUV-mean of 1.49 in RA patients; similarly, we identified an ascending aortic SUV-mean of 1.83 in our RA cohort. In the general population it is documented that vessel wall inflammation leads to endothelial dysfunction, aortic stiffening, accelerated atherosclerosis, plaque destabilization, and adverse CV events (20,21,37). Indeed, Maki-Petaja et al (12) showed in a study of 17 RA patients that a decrease in aortic stiffness in response to anti-TNF treatment was paralleled by a decrease in aortic FDG uptake. Additionally, in a study of 23 RA subjects Bernelot Moens et al showed that RA patients in long-term clinical remission did not have increased arterial wall inflammation compared to controls. However, those on TNF-inhibitors had increased aortic uptake compared to subjects on non-biologic DMARDs, an association explained by longer RA disease duration (42). Hence, identification of vessel wall inflammation in RA may detect those patients at highest risk for plaque instability/rupture and CV events. It is also possible in RA that aortic wall inflammation could represent rheumatoid vasculitis (aortitis), which the FDG PET/CT technology would not be able to differentiate from plaque inflammation. The prevalence of aortitis in RA had been considered low based on autopsy studies from the 1970s and 1980s (17,18) but the challenges in diagnosing it preclude establishing its true prevalence. Thus, while most studies of vascular FDG uptake utilize maximal SUV uptake values (32,33) as the primary outcome because of their focus on atherosclerotic plaque, we also explored mean SUV values for both the MDS and the whole ascending aorta as potential outcomes that might be more reflective of diffuse homogenous inflammation as might be envisioned with vasculitis. We speculated that conventional CV risk factors might be related to the four measures of FDG uptake differently from RA-specific characteristics. However, this did not appear to be the case, as a conventional CV risk factor (BMI) and an RA-specific characteristic (anti-CCP titer > 60) were both independently associated with all four measures of aortic FDG uptake. Likewise, another CV risk factor (hypertension) and another RA-specific characteristic (nodules) were both independently associated with SUV mean and SUV-MDS mean but not with SUV-max or SUV-MDS max. It should be noted, however, that SUV-max has been reported to have significantly improved reproducibility as compared to SUV-mean since the SUV-max value within a region of interest (ROI) is typically invariant with respect to small spatial shifts of the ROI, while the SUV-mean is only accurate if the lesion of interest has a uniform true SUV value (43).
That a component of ascending aortic FDG uptake in our RA patients is due to plaque is suggested by the independent association of BMI and hypertension with aortic FDG uptake; moreover, this association is consistent with reports in the general population in which arterial FDG uptake correlated with burden of CV risk factors (40). In non-RA patients, aortic FDG uptake has also been associated with baseline CAC as well as its progression (44, 45); however, in our RA patients, the association of CAC with ascending aortic FDG uptake lost statistical significance in the adjusted analysis, which could have been due to the exclusion of patients with clinical CVD in our studies in which the median overall CAC score was zero.
Interestingly, among the RA-specific characteristics examined, we found a positive correlation between rheumatoid nodules and FDG uptake even after adjustment for CV risk factors. Surprisingly, in univariate analyses we observed a negative correlation between anti-CCP antibody status and ascending aortic FDG PET uptake. This apparent counterintuitive association was explained, at least in part, in the multivariable analyses by an interaction with disease activity. Thus, in patients with active disease, CCP seropositivity was positively associated with SUV uptake. Interestingly, citrullinated proteins have been demonstrated within aortic atherosclerotic plaque of autopsied non-RA patients, and anti-citrullinated protein antibodies (ACPAs) in RA subjects have been associated with an increase in aortic plaque burden (46), suggesting a role for ACPAs in the acceleration of atherosclerosis in RA.
In non-RA patients, several groups have shown that statin therapy results in a significant reduction in arterial FDG PET uptake after 3 months (31–33). In ankylosing spondylitis, similar results were seen in a small study evaluating the change in aortic FDG uptake following treatment with atorvastatin (34). However, we did not find an association between the use of statins and aortic FDG uptake in our cross-sectional study possibly due to the fact that only 16 of the patients in our cohort were on statins. Additionally, a reduction in aortic FDG uptake has been described in a small study of 17 RA patients following 8-week treatment with anti-tumor necrosis factor-α (12). Although in another study, the use of TNF blockers in 13 RA subjects was associated with increased arterial uptake compared to 10 RA patients on non-biologics DMARDs, the association was confounded by RA disease duration (42). However we did not see an association between the use of TNF-inhibitors and the extent of ascending aortic FDG uptake in our cross-sectional study. An interesting question is whether any RA therapy that can induce disease remission will also reduce vascular inflammation. To address this question, the Treatments Against RA and Effect on FDG PET/CT (TARGET) Trial (NCT0237402) will evaluate the effect of two different DMARD regimens (anti-TNF plus methotrexate vs triple therapy with methotrexate, sulfasalazine and hydroxychloroquine) on aortic and carotid inflammation and will correlate these findings with changes in RA disease activity (https://clinicaltrials.gov/ct2/show/NCT02374021?term=TARGET+RA&rank=1).
Strengths of our study include the comprehensive phenotyping of the RA cohort for cardiovascular and RA characteristics, including both structural and physiological measures of atherosclerosis. This, coupled with our large sample size, allowed us to evaluate for the first time the independent contribution of conventional CV risk factors and disease-specific characteristics with increased ascending aortic FDG uptake in patients with RA.
Limitations of our study include its cross-sectional nature, which precludes establishment of causation for our findings, and the evaluation of whether changes in the ascending aortic FDG uptake might parallel changes in RA-disease features (i.e. disease activity, inflammatory markers, treatment modifications). Also, while the consistency in the covariates retained in our final models across the different SUV measures make our findings less likely to be due to chance, results should be interpreted with caution.
In conclusion, in addition to conventional CV risk factors, ascending aortic FDG uptake in RA was associated with the presence of rheumatoid nodules and disease activity in those with anti-CCP antibody seropositivity. Our study results suggest that both traditional CV risk factors and RA-disease characteristics are independently associated with ascending aortic wall inflammation and likely the development of atherosclerosis in RA. These findings support the use of FDG PET imaging as an additional research modality for assessing CV risk in RA patients.
Supplementary Material
Acknowledgments
The authors would like to thank the RHYTHM study staff (Afshin Zartoshti, Janine Rose, Rachel Broderick, Rachelle Morgenstern, Thania Perez) for their hard work and dedication, and the participants in the RHYTHM study who graciously agreed to participate in this research. We also thank Drs. Alice Chu, Anna Broder, Teja Kapoor, Edward Dwyer, Jane Kang and others for recommending their patients for this study.
Financial Support
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases under Award Number AR-050026 (JMB) and AR050026-08S1 (JMB, LG-P), the Rheumatology Research Foundation under Award Number CU15-0082 (JMB), and by the National Institutes of Health National Center for Translational Science Clinical and Translational Science Award (CTSA) grant UL1 TR000040, of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Symmons DP, Jones MA, Scott DL, Prior P. Long-term mortality outcome in patients with rheumatoid arthritis: early presenters continue to do well. J Rheumatol. 1998 Jun;25(6):1072–1077. [PubMed] [Google Scholar]
- 2.Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Cathey MA, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994 Apr;37(4):481–494. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 3.Crowson CS, Matteson EL, Roger VL, Therneau TM, Gabriel SE. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012 Aug 1;110(3):420–424. doi: 10.1016/j.amjcard.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012 Sep;32(9):2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006 Feb;83(2):456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 6.Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Danesh J, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010 Jan 9;375(9709):132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000 Apr 18;101(15):1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002 Nov 14;347(20):1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 9.Myasoedova E, Crowson CS, Kremers HM, Roger VL, Fitz-Gibbon PD, Gabriel SE, et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011 Mar;70(3):482–487. doi: 10.1136/ard.2010.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Innala L, Moller B, Ljung L, Magnusson S, Smedby T, Wållberg-Jonsson S, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther. 2011;13(4):R131. doi: 10.1186/ar3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon DH, Reed GW, Kremer JM, Curtis JR, Farkouh ME, Greenberg JD, et al. Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Arthritis Rheumatol. 2015 Jun;67(6):1449–55. doi: 10.1002/art.39098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maki-Petaja KM, Elkhawad M, Cheriyan J, Joshi FR, Ostör AJ, Wilkinson IB, et al. Anti-tumor necrosis factor alpha therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation. 2012;126:2473–80. doi: 10.1161/CIRCULATIONAHA.112.120410. [DOI] [PubMed] [Google Scholar]
- 13.Rose S, Sheth NH, Baker JF, Ogdie A, Raper A, Mehta NN, et al. A comparison of vascular inflammation in psoriasis, rheumatoid arthritis, and healthy subjects by FDG-PET/CT: a pilot study. Am J Cardiovasc Dis. 2013;3(4):273–278. [PMC free article] [PubMed] [Google Scholar]
- 14.Emami H, Vijayakumar J, Subramanian S, Vucic E, Singh P, Tawakol A, et al. Arterial 18F-FDG uptake in rheumatoid arthritis correlates with synovial activity. JACC Cardiovasc Imaging. 2014 Sep;7(9):959–60. doi: 10.1016/j.jcmg.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 16.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 17.Reimer KA, Rodgers RF, Oyasu R. Rheumatoid arthritis with rheumatoid heart disease and granulomatous aortitis. JAMA. 1976 Jun 7;235(23k0):2510–2. doi: 10.1001/jama.235.23.2510. [DOI] [PubMed] [Google Scholar]
- 18.Gravallese EM, Corson JM, Coblyn JS, Pinkus GS, Weinblatt ME. Rheumatoid aortitis: a rarely recognized but clinically significant entity. Medicine (Baltimore) 1989 Mar;68(2):95–106. [PubMed] [Google Scholar]
- 19.Kaneko S, Yamashita H, Sugimori Y, Takahashi Y, Kaneko H, Mimori A, et al. Rheumatoid arthritis-associated aortitis: a case report and literature review. Springerplus. 2014 Sep 9;3:509. doi: 10.1186/2193-1801-3-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rominger A, Saam T, Wolpers S, Cyran CC, Schmidt M, Hacker M, et al. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med. 2009 Oct;50(10):1611–1620. doi: 10.2967/jnumed.109.065151. [DOI] [PubMed] [Google Scholar]
- 21.Paulmier B, Duet M, Khayat R, Pierquet-Ghazzar N, Laissy JP, Faraggi M, et al. Arterial wall uptake of fluorodeoxyglucose on PET imaging in stable cancer disease patients indicates higher risk for cardiovascular events. J Nucl Cardiol. 2008 Mar-Apr;15(2):209–217. doi: 10.1016/j.nuclcard.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Wells RG, Ruddy TD. The dream of imaging coronary artery inflammation with FDG PET/CT imaging. J Nucl Cardiol. 2016 Jun;:3. doi: 10.1007/s12350-016-0549-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Hawker G, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010 Sep;62(9):2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 24.Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Fischman AJ, et al. In vivo 18Ffluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48:1818–24. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 25.Rudd JH, Myers KS, Bansilal S, Machac J, Rafique A, Fayad ZA, et al. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol. 2007;50:892–6. doi: 10.1016/j.jacc.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 26.Ficaro EP1, Lee BC, Kritzman JN, Corbett JR. Corridor4DM: the Michigan method for quantitative nuclear cardiology. J Nucl Cardiol. 2007 Jul;14(4):455–65. doi: 10.1016/j.nuclcard.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Vimonte M, Detrano R. Quantification of coronary artery calcium from electron beam tomograms. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 28.Wells G, Becker JC, Teng J, Dougados M, Schiff M, van Riel PL, et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009;68(6):954–960. doi: 10.1136/ard.2007.084459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warrington KJ, Kent PD, Frye RL, Lymp JF, Kopecky SL, Weyand CM, et al. Rheumatoid arthritis is an independent risk factor for multi-vessel coronary artery disease: a case control study. Arthritis Res Ther. 2005;7(5):R984–91. doi: 10.1186/ar1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Weissberg PL, et al. Imaging atherosclerotic plaque inflammation with 18F-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105:2708–11. doi: 10.1161/01.cir.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- 31.Wu Y-W, Kao H-L, Huang C-L, Chen MF, Lin LY, Yang WS, et al. The effects of 3-month atorvastatin therapy on arterial inflammation, calcification, abdominal adipose tissue and circulating biomarkers. European Journal of Nuclear Medicine and Molecular Imaging. 2012;39(3):399–407. doi: 10.1007/s00259-011-1994-7. [DOI] [PubMed] [Google Scholar]
- 32.Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Imaizumi T, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006 Nov 7;48(9):1825–1831. doi: 10.1016/j.jacc.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 33.Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Shankar SS, et al. Intensification of Statin Therapy Results in a Rapid Reduction in Atherosclerotic Inflammation Results of a Multicenter Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography Feasibility Study. J Am Coll Cardiol. 2013;62(10):909–917. doi: 10.1016/j.jacc.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 34.van der Valk FM, Bernelot Moens SJ, Verweij SL, Strang AC, Nederveen AJ, Stroes ES, et al. Increased arterial wall inflammation in patients with ankylosing spondylitis is reduced by statin therapy. Ann Rheum Dis. 2016 Oct;75(10):1848–51. doi: 10.1136/annrheumdis-2016-209176. [DOI] [PubMed] [Google Scholar]
- 35.Abdelbaky A, Corsini E, Figueroa AL, Fontanez S, Subramanian S, Tawakol A, et al. Focal arterial inflammation precedes subsequent calcification in the same location: a longitudinal FDG-PET/CT study. Circ Cardiovasc Imaging. 2013 Sep;6(5):747–754. doi: 10.1161/CIRCIMAGING.113.000382. [DOI] [PubMed] [Google Scholar]
- 36.Hjortnaes J, New SE, Aikawa E. Visualizing novel concepts of cardiovascular calcification. Trends Cardiovasc Med. 2013 Apr;23(3):71–79. doi: 10.1016/j.tcm.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim TN, Kim S, Yang SJ, Yoo HJ, Seo JA, Choi KM, et al. Vascular inflammation in patients with impaired glucose tolerance and type 2 diabetes: analysis with 18F-fluorodeoxyglucose positron emission tomography. Circ Cardiovasc Imaging. 2010 Mar;3(2):142–148. doi: 10.1161/CIRCIMAGING.109.888909. [DOI] [PubMed] [Google Scholar]
- 38.Bural GG, Torigian DA, Chamroonrat W, Houseni M, Chen W, Alavi A, et al. FDG-PET is an effective imaging modality to detect and quantify age-related atherosclerosis in large arteries. Eur J Nucl Med Mol Imaging. 2008 Mar;35(3):562–569. doi: 10.1007/s00259-007-0528-9. [DOI] [PubMed] [Google Scholar]
- 39.Joly L, Djaballah W, Koehl G, Mandry D, Dolivet G, Benetos A, et al. Aortic inflammation, as assessed by hybrid FDG-PET/CT imaging, is associated with enhanced aortic stiffness in addition to concurrent calcification. European Journal of Nuclear Medicine and Molecular Imaging. 2009;36(6):979–985. doi: 10.1007/s00259-008-1047-z. [DOI] [PubMed] [Google Scholar]
- 40.Rudd JH, Myers KS, Bansilal S, Machac J, Woodward M, Fayad ZA, et al. Relationships among regional arterial inflammation, calcification, risk factors, and biomarkers: a prospective fluorodeoxyglucose positron-emission tomography/computed tomography imaging study. Circ Cardiovasc Imaging. 2009 Mar;2(2):107–115. doi: 10.1161/CIRCIMAGING.108.811752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Tawakol A, et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. J Am Coll Cardiol Img. 2013;6:1250–9. doi: 10.1016/j.jcmg.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Bernelot Moens SJ, van der Valk FM, Strang AC, Kroon J, Smits LP, Stroes ES, et al. Unexpected arterial wall and cellular inflammation in patients with rheumatoid arthritis in remission using biological therapy: a cross-sectional study. Arthritis Res Ther. 2016 May 21;18(1):115. doi: 10.1186/s13075-016-1008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kinahan PE, Fletcher JW. Positron emission tomography-computed tomography standardized uptake values in clinical practice and assessing response to therapy. Semin Ultrasound CT MR. 2010 Dec;31(6):496–505. doi: 10.1053/j.sult.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdelbaky A, Corsini E, Figueroa AL, Fontanez S, Subramanian S, Ferencik M, et al. Focal arterial inflammation precedes subsequent calcification in the same location: a longitudinal FDG-PET/CT study. Circ Cardiovasc Imaging. 2013;6:747–54. doi: 10.1161/CIRCIMAGING.113.000382. [DOI] [PubMed] [Google Scholar]
- 45.Cho SG, Park KS, Kim J, Kang SR, Kwon SY, Bom HS, et al. Prediction of coronary artery calcium progression by FDG uptake of large arteries in asymptomatic individuals. Eur J Nucl Med Mol Imaging. 2017 Jan;44(1):129–140. doi: 10.1007/s00259-016-3523-1. [DOI] [PubMed] [Google Scholar]
- 46.Sokolove J, Brennan MJ, Sharpe O, Lahey LJ, Kao AH, Robinson WH, et al. Brief report: citrullination within the atherosclerotic plaque: a potential target for the anti-citrullinated protein antibody response in rheumatoid arthritis. Arthritis Rheum. 2013 Jul;65(7):1719–24. doi: 10.1002/art.37961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.