Abstract
Introduction
Alpha-1-Antitrypsin deficiency (AATD) is grouped with chronic obstructive pulmonary disease (COPD); however, this may not be appropriate. This study assessed whether AATD confers a different prognosis than COPD following lung transplantation.
Methods
We employed the UNOS database, grouping patients by diagnoses of AATD or COPD. Kaplan-Meier methods and Cox modeling were performed to determine the association of diagnosis and overall survival.
Results
Of 9569 patients, 1394 (14.6%) had a diagnosis of AATD. Patients with AATD who received a single lung transplant had reduced 1-year survival (Adjusted Hazard Ratio [AHR]: 1.68, 95% CI: 1.26, 2.23). Among patients who received a bilateral lung transplant, there was no significant difference in survival by diagnosis (AHR for AATD as compared to COPD: 0.96, 95% CI: 0.82, 1.12). After the implementation of the LAS, there was no significant difference in survival among patients who received a single (AHR: 1.15, 95% CI: 0.69, 1.95) or bilateral (AHR: 0.99, 95% CI: 0.73, 1.34) lung transplant by diagnosis.
Conclusion
Lung transplantation is increasingly employed in the care of the patient with COPD. Though recipients undergoing LTX for AATD are at increased risk of both acute rejection and airway dehiscence post-transplant, in the post-LAS era, survival rates are similar for recipients with AATD in comparison to COPD.
Keywords: Alpha-1-Antitrypsin Deficiency (AATD), Chronic Obstructive Pulmonary Disease (COPD), Clinical Outcomes, Lung Transplantation, Organ Allocation
Introduction
Alpha-1-Antitrypsin deficiency (AATD) is an inherited disorder which affects multiple organs, most notably the lung and liver. It is estimated that roughly 100,000 people in the United States are affected by this condition.1,2 In the lung, AATD leads to panacinar emphysema, a form of chronic obstructive pulmonary disease (COPD), eventually leading to pulmonary failure. Among patients with severe AATD, pulmonary failure is the leading cause of death.3 Treatment for AATD traditionally consists of supportive therapy, however augmentation with supplemental alpha-1-antitrypsin from pooled human plasma has demonstrated some efficacy in slowing the decline of lung function.2 Nonetheless, once the pulmonary disease has progressed to a certain point, only lung transplantation is available to prolong survival.4
Historically, AATD has been grouped with COPD when evaluating a patient for lung transplantation, as previous research has demonstrated that overall survival and pulmonary function is similar in patients with COPD who are either replete or deficient in alpha-1-antitrypsin.5 However, more recent data demonstrate that this grouping may not be appropriate.6 We therefore performed the following review of the United Network for Organ Sharing (UNOS) database in order to determine the association between diagnosis (AATD vs COPD) and outcomes. We hypothesized that patients with AATD have superior long-term survival following lung transplantation than patients with COPD based on recent studies.6
Methods
Patient Population
The United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research (STAR) Files were queried for patients 18 years of age or older undergoing first-time isolated deceased-donor lung transplant for a primary diagnosis of either COPD or AATD during the years 1990 to 2013. From here on, patients with a diagnosis of AATD will be referred to as the AATD group, while patients with a general diagnosis of COPD will be referred to as the COPD group. Patients were excluded if they were on extracorporeal membrane oxygenation (ECMO) at the time of transplantation or if they had unknown follow-up time. The Duke University Institutional Review Board approved this study prior to analysis.
Variables
The analysis included both candidate- and donor-related variables. With regards to pre-operative candidate variables, age, sex, race, body mass index (BMI, kg/m2), hypertension, diabetes, smoking history, previous cardiac surgery, pulmonary hypertension, functional status at transplant, steroid treatment at transplant, lung allocation score (LAS), six minute walk distance <150 feet, percent predicted forced expiratory volume in one second (FEV1), cytomegalovirus (CMV) status, Epstein-Barr virus (EBV) status, time on the waiting list prior to transplant (days), and center lung transplant volume over the study period were included. In addition, ischemic time (hours) was also included. Pulmonary hypertension was defined by the most recent pulmonary artery pressure, with hypertension being defined as >25 mmHg as previously described.7 Functional status was defined using the Karnofsky performance scale at transplant. Donor variables included age, sex, hypertension, diabetes, smoking history, positive pulmonary infection at time of transplant, CMV status, and EBV status. Outcome variables included airway dehiscence, post-operative dialysis requirement, drug-treated infection, episode of acute rejection, treatment for rejection within one year, and hospital length of stay (days). Survival was defined as the time from transplant until death or loss to follow-up. Variables with a high degree of missingness (>10%) were excluded from analysis.
Statistical Analysis
Due to the distinct differences in patients receiving a single vs bilateral transplant in this cohort, unadjusted comparisons by diagnosis were made separately by type of transplant (single vs bilateral). Patient baseline characteristics, transplant characteristics, and outcomes were compared by group. Continuous variables were compared using the Kruskal-Wallis test while categorical variables were compared using Fisher’s exact test or the chi-square test as appropriate.
In order to determine time trends in the transplantation of patients with AATD as compared to the general COPD cohort, transplantation for a diagnosis of AATD as a function of the total transplants for either COPD or AATD was determined by year. Furthermore, to ascertain trends in the use of bilateral lung transplantation among patients with AATD, the percentage of bilateral transplants as a function of all transplants performed for AATD was also determined by year. Trends were tested using the Cochran-Armitage trend test.
Kaplan-Meier methods and Cox proportional hazards regression modeling were performed to determine the unadjusted and adjusted association between diagnosis (AATD versus COPD) and overall survival. Again, due to the distinct differences between patients who receive a single versus bilateral lung transplant, these analyses were performed separately for these subgroups. Variables incorporated into the models were determined a priori based on clinical significance and included both recipient and donor characteristics. Recipient characteristics included diagnosis (AATD vs COPD), age, sex, race, BMI, smoking history, diabetes, steroid treatment at the time of transplant, functional status, FEV1 at the time of transplant, and days on the waiting list. Donor characteristics included donor age, donor diabetes, and donor pulmonary infection. Finally, ischemic time and center volume were also included. Due to abrupt changes in the patient population being transplanted secondary to the introduction of the LAS in May of 2005, we also performed a sub-analysis among patients who were transplanted after this time point. In the adjusted analyses performed after the implementation of the LAS, LAS was also included as a covariate.
The proportional hazards assumption was tested for all Cox models by inspecting the plot of the Schoenfeld residuals versus the log of time. If found to be non-linear, the analysis was divided into multiple time periods. A P value of <0.05 was used to define statistical significance. All statistical analyses were performed using R version 3.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 9569 patients met final study criteria. Of these, 1394 (14.6%) had a formal diagnosis of AATD, however the proportion of the study cohort transplanted for a primary diagnosis of AATD decreased significantly over the study period (p<0.001, Figure 1). Among the entire cohort, 40.6% (n=3881) of patients received a bilateral lung transplant, but among patients with AATD, 51.3% (n=715) of patients received a bilateral lung transplant as compared to only 38.7% (n=3166) of patients with COPD. The use of bilateral transplantation for patients with AATD also increased significantly over the study period (p<0.001, Figure 2).
Figure 1.
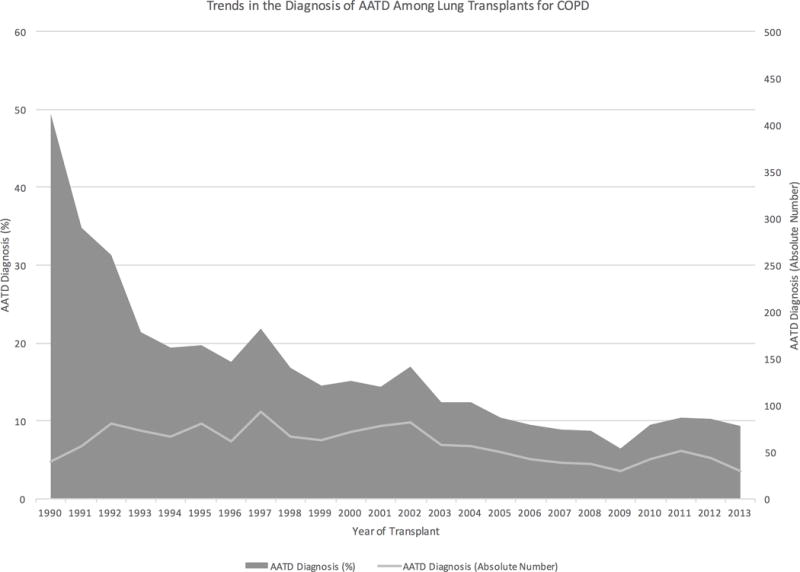
Trends in the diagnosis of alpha-1-antitrypsin deficiency among patients receiving a lung transplant for chronic obstructive pulmonary disorder from 1990–2013.
Figure 2.
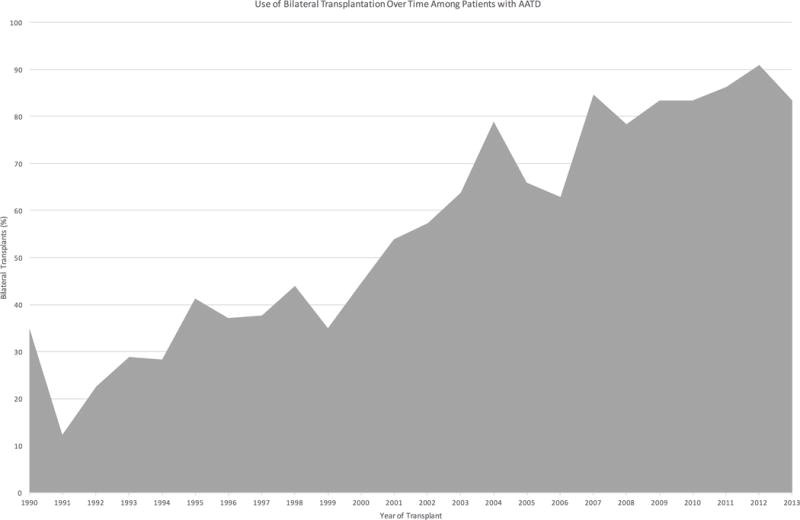
Trends in the use of bilateral lung transplantation for alpha-1-antitrypsin deficiency from 1990–2013.
Unadjusted Comparison Among Single Lung Transplants
Among patients who received a single lung transplant, patients with a diagnosis of AATD tended to be younger (median age: 51 vs 59, p<0.001) and were less likely to be female (42% vs 55%, p<0.001, Table 1). They were also significantly less likely to have a smoking history (8.4% vs 27%, p<0.001), hypertension (13% vs 18%, p<0.001), or diabetes (1.2% vs 5.1%). Among those patients with a recorded LAS, patients with AATD did not have a significantly different median LAS as compared to patients with COPD (32 vs 33, p=0.200). Lastly, patients with AATD had significantly longer waiting times prior to transplant (median days: 261 vs 234 days, p=0.002). This difference was no longer significant after the implementation of the LAS (median days: 144 vs 113, p=0.543).
Table 1.
Recipient and donor characteristics by diagnosis among patients receiving single and bilateral lung transplants.
| Among Single Lung Transplants | Among Bilateral Lung Transplants | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Overall | COPD | AATD | p-value | Overall | COPD | AATD | p-value |
| N | 5,688 | 5009 | 679 | 3,881 | 3166 | 715 | ||
| Recipient Characteristics | ||||||||
| Age (years) | 58 (53, 62) | 59 (54, 62) | 51 (45, 56) | < 0.001 | 57 (51, 62) | 58 (53, 62) | 50 (45, 56) | < 0.001 |
| Female Sex | 3,029 (53.3%) | 2,745 (54.8%) | 284 (41.8%) | < 0.001 | 1,743 (44.9%) | 1,478 (46.7%) | 265 (37.1%) | < 0.001 |
| Race | < 0.001 | < 0.001 | ||||||
| White | 5,371 (94.4%) | 4,709 (94%) | 662 (97.5%) | 3,592 (92.6%) | 2,888 (91.2%) | 704 (98.5%) | ||
| Black | 221 (3.9%) | 218 (4.4%) | 3 (0.4%) | 214 (5.5%) | 212 (6.7%) | 2 (0.3%) | ||
| Other/Unknown | 96 (1.7%) | 82 (1.6%) | 14 (2.1%) | 75 (1.9%) | 66 (2.1%) | 9 (1.3%) | ||
| Body Mass Index (kg/m2) | 23.6 (20.6, 26.8) | 23.7 (20.7, 26.9) | 22.7 (20.1, 25.9) | < 0.001 | 23.8 (20.9, 27) | 24 (21, 27.2) | 23.2 (20.7, 26) | < 0.001 |
| Hypertension | 632 (17.7%) | 581 (18.3%) | 51 (13.2%) | 0.018 | 306 (18.1%) | 277 (21.2%) | 29 (7.5%) | < 0.001 |
| Diabetes | 220 (4.7%) | 215 (5.1%) | 5 (1.2%) | < 0.001 | 242 (6.7%) | 223 (7.4%) | 19 (3.1%) | < 0.001 |
| Known Smoking History | 1,404 (24.7%) | 1,347 (26.9%) | 57 (8.4%) | < 0.001 | 2,256 (58.1%) | 2,003 (63.3%) | 253 (35.4%) | < 0.001 |
| Pulmonary Hypertension | 1,848 (42.6%) | 1,667 (42.3%) | 181 (45.2%) | 0.275 | 1,571 (45.5%) | 1,344 (46.7%) | 227 (39.7%) | 0.003 |
| Previous Cardiac Surgery | 22 (1.3%) | 20 (1.2%) | 2 (2.4%) | 0.297 | 24 (0.9%) | 24 (1.1%) | 0 (0.0%) | 0.063 |
| Steroid Treatment at Transplant | 2,220 (48%) | 2,036 (48.9%) | 184 (40.2%) | < 0.001 | 1,643 (45.7%) | 1,406 (47.3%) | 237 (38.1%) | < 0.001 |
| Functional Status at Transplant | 0.015 | <0.001 | ||||||
| ADL With No Assistance | 1,479 (32.1%) | 1,334 (32.2%) | 145 (31.8%) | 1,222 (34.0%) | 996 (33.5%) | 226 (36.3%) | ||
| ADL With Assistance | 2,912 (63.2%) | 2,610 (62.9%) | 302 (66.2%) | 1,815 (50.5%) | 1,471 (49.5%) | 344 (55.3%) | ||
| Disabled/Hospitalized | 213 (4.6%) | 204 (4.9%) | 9 (2.0%) | 554 (15.4%) | 502 (16.9%) | 52 (8.4%) | ||
| Lung Allocation Score | 32.8 (31.7, 34.1) | 32.8 (31.7, 34.1) | 32.4 (31.3, 34.3) | 0.200 | 32.8 (31.6, 34.5) | 32.9 (31.7, 34.6) | 32.4 (31.4, 34.1) | 0.003 |
| 6MWD <150 feet | 325 (11.1%) | 294 (11.3%) | 31 (9.6%) | 0.412 | 66 (5.4%) | 52 (5.8%) | 14 (4.4%) | 0.399 |
| Percent Predicted FEV1 at Transplant | 20 (16, 26) | 20 (16, 26) | 19 (15, 24) | < 0.001 | 19 (16, 25) | 20 (16, 25) | 19 (15, 24) | 0.034 |
| Recipient CMV Positive | 175 (3.1%) | 165 (3.3%) | 10 (1.5%) | < 0.001 | 201 (5.2%) | 177 (5.6%) | 24 (3.4%) | < 0.001 |
| Recipient EBV Positive | 2,233 (39.3%) | 2,081 (41.5%) | 152 (22.4%) | < 0.001 | 2,389 (61.6%) | 2,011 (63.5%) | 378 (52.9%) | < 0.001 |
| Time on Waiting List (days) | 238 (90, 483) | 234 (88, 479) | 261 (112.5, 503.5) | 0.002 | 171 (47, 507) | 150 (41, 456.5) | 299 (100, 692.5) | < 0.001 |
| Ischemic Time (hr) | 3.7 (2.9, 4.5) | 3.7 (2.9, 4.5) | 3.8 (2.9, 4.5) | 0.368 | 5.3 (4.4, 6.4) | 5.4 (4.4, 6.4) | 5.2 (4.3, 6.1) | 0.006 |
| Center Volume (over the study) | 219 (115, 312) | 230 (115, 312) | 175 (107, 312) | 0.055 | 262 (123, 430) | 262 (119, 430) | 277 (147, 430) | 0.274 |
| Donor Characteristics | ||||||||
| Donor Age (years) | 29 (20, 43) | 29 (20, 43) | 25 (19, 40) | < 0.001 | 31 (21, 45) | 31 (21, 46) | 27 (20, 42) | < 0.001 |
| Donor Female Sex | 1,831 (32.2%) | 1,663 (33.2%) | 168 (24.7%) | < 0.001 | 1,212 (31.2%) | 1,029 (32.5%) | 183 (25.6%) | < 0.001 |
| Donor Hypertension | 801 (16.4%) | 728 (16.5%) | 73 (15.2%) | 0.504 | 713 (19.2%) | 619 (20.2%) | 94 (14.6%) | 0.001 |
| Donor Diabetes | 193 (3.9%) | 178 (4%) | 15 (3.1%) | 0.387 | 221 (5.9%) | 184 (6%) | 37 (5.8%) | 0.890 |
| Donor Smoking History | 1,148 (23.6%) | 1,003 (22.8%) | 145 (30.4%) | < 0.001 | 727 (19.7%) | 590 (19.4%) | 137 (21.3%) | 0.284 |
| Donor Positive Pulmonary Infection | 1,144 (20.1%) | 1,067 (21.3%) | 77 (11.3%) | < 0.001 | 1,280 (33%) | 1,102 (34.8%) | 178 (24.9%) | < 0.001 |
| Donor CMV Positive | 3,212 (56.5%) | 2,848 (56.9%) | 364 (53.6%) | 0.199 | 2,271 (58.5%) | 1,867 (59%) | 404 (56.5%) | 0.371 |
| Donor EBV Positive | 1,016 (17.9%) | 969 (19.3%) | 47 (6.9%) | < 0.001 | 1,766 (45.5%) | 1,552 (49%) | 214 (29.9%) | < 0.001 |
ADL – Activities of Daily Living. 6MWD – Six Minute Walk Distance. FEV1 – Forced Expiratory Volume in One Second. CMV – Cytomegalovirus. EBV – Epstein-Barr Virus. Continuous variables are presented as median (interquartile range) while categorical variables are presented as frequency (percentage).
With regards to unadjusted outcomes by diagnosis among patients who received a single lung transplant, patients with AATD had significantly increased rates of airway dehiscence (2.1% vs 0.7%, p=0.003), and were also significantly more likely to be treated for rejection within the first year post-transplant (57.4% vs 49.3%, p=0.016, Table 2). There were no significant differences with regards to post-operative hospital length of stay (median 12 vs 13 days, p=0.977). Patients with AATD were also significantly more likely to die from an infectious process as compared to patients with COPD (23.3% vs 15.1%, p<0.001, Table 3).
Table 2.
Outcomes by diagnosis among patients receiving single and bilateral lung transplants.
| Among Single Lung Transplants | Among Bilateral Lung Transplants | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Overall | COPD | AATD | p-value | Overall | COPD | AATD | p-value |
| N | 5,688 | 5009 | 679 | 3,881 | 3166 | 715 | ||
| Airway Dehiscence | 40 (0.8%) | 30 (0.7%) | 10 (2.1%) | 0.003 | 66 (1.8%) | 44 (1.5%) | 22 (3.5%) | 0.001 |
| Post-Operative Dialysis | 141 (2.9%) | 123 (2.9%) | 18 (3.8%) | 0.315 | 147 (4%) | 126 (4.1%) | 21 (3.3%) | 0.372 |
| Drug Treated Infection | 1,325 (35.8%) | 1,163 (35.5%) | 162 (38%) | 0.328 | 721 (41.4%) | 574 (43%) | 147 (36.2%) | 0.017 |
| Acute Rejection Episode | 182 (10.4%) | 177 (10.6%) | 5 (5.6%) | 0.154 | 168 (6.6%) | 145 (6.5%) | 23 (7.1%) | 0.779 |
| Treated for Rejection within 1 Year | 1,513 (50%) | 1,365 (49.3%) | 148 (57.4%) | 0.016 | 872 (34%) | 701 (32.7%) | 171 (40.8%) | 0.002 |
| Hospital Length of Stay (days) | 13 (9, 20) | 13 (9, 20) | 12 (9, 21) | 0.977 | 16 (11, 25) | 16 (11, 26) | 15 (11, 23) | 0.006 |
Continuous variables are presented as median (interquartile range) while categorical variables are presented as frequency (percentage).
Table 3.
Cause of death by diagnosis among patients receiving single and bilateral lung transplants.
| Among Single Lung Transplants | Among Bilateral Lung Transplants | |||||||
|---|---|---|---|---|---|---|---|---|
| Cause of Death | Overall | COPD | AATD | p-value | Overall | COPD | AATD | p-value |
| N | 5,688 | 5009 | 679 | 3,881 | 3166 | 715 | ||
| Cardiovascular | 245 (4.3%) | 218 (4.4%) | 27 (4.0%) | 0.725 | 149 (3.8%) | 120 (3.8%) | 29 (4.1%) | 0.821 |
| Cerebrovascular | 62 (1.1%) | 54 (1.1%) | 8 (1.2%) | 0.969 | 29 (0.7%) | 23 (0.7%) | 6 (0.8%) | 0.940 |
| Graft Failure | 1,466 (25.8%) | 1,301 (26%) | 165 (24.3%) | 0.374 | 688 (17.7%) | 555 (17.5%) | 133 (18.6%) | 0.533 |
| Hemorrhage | 54 (0.9%) | 46 (0.9%) | 8 (1.2%) | 0.657 | 33 (0.9%) | 23 (0.7%) | 10 (1.4%) | 0.123 |
| Infection | 916 (16.1%) | 758 (15.1%) | 158 (23.3%) | < 0.001 | 331 (8.5%) | 256 (8.1%) | 75 (10.5%) | 0.045 |
| Malignancy | 216 (3.8%) | 201 (4.0%) | 15 (2.2%) | 0.028 | 70 (1.8%) | 53 (1.7%) | 17 (2.4%) | 0.262 |
| Multiple Organ Failure | 174 (3.1%) | 149 (3.0%) | 25 (3.7%) | 0.376 | 101 (2.6%) | 79 (2.5%) | 22 (3.1%) | 0.452 |
| Pulmonary Dehiscence | 10 (0.2%) | 7 (0.1%) | 3 (0.4%) | 0.107 | 9 (0.2%) | 5 (0.2%) | 4 (0.6%) | 0.066 |
| Renal Failure | 99 (1.7%) | 91 (1.8%) | 8 (1.2%) | 0.299 | 45 (1.2%) | 35 (1.1%) | 10 (1.4%) | 0.640 |
| Other/Unknown | 2,446 (43%) | 2,184 (43.6%) | 262 (38.6%) | 0.015 | 2,426 (62.5%) | 2,017 (63.7%) | 409 (57.2%) | 0.001 |
Unadjusted Comparison Among Bilateral Lung Transplants
Among patients who underwent bilateral lung transplantation, patients with AATD were again significantly younger (median age: 50 vs 58, p<0.001) and were less likely to be female (37% vs 47%, p<0.001). Again, patients with AATD were less likely to have a smoking history (35% vs 63%, p<0.001), have hypertension (7.5% vs 21%, p<0.001), or have diabetes (3.1% vs 7.4%, p<0.001). Among patients with a known LAS score, patients with AATD had significantly lower LAS scores, although not to clinical significance (median score: 32 vs 33, p=0.003).
With regards to unadjusted outcomes among patients who received a bilateral lung transplant, patients with AATD were again significantly more likely to have increased rates of airway dehiscence (3.5% vs 1.5%, p=0.001) and were again more likely to be treated for rejection within their first year post-transplant (41% vs 33%, p=0.002). Among this cohort, patients with AATD had significantly shorter median hospital length of stays than patients with COPD (15 vs 16 days, p=0.006). Patients with AATD were again significantly more likely to die from an infectious process as compared to patients with COPD (10.5% vs 8.1%, p=0.045).
Survival Analysis
Upon investigation of overall unadjusted survival among patients transplanted with a single lung transplant, patients with AATD were found to have significantly reduced early survival (1-year survival: 74.7% vs 83.1%, p<0.001), however by 5-years, this survival difference was not significantly different (45.7% vs 48.3%, p=0.218) and by 10-years post-transplant, AATD patients had a significantly improved survival as compared to COPD patients (24.0% vs 20.1% p=0.046, Figure 3). Following adjustment for patient and transplant related factors, the proportional hazards assumption was not met when analyzing the entire time period, and therefore the adjustment was broken up into two separate intervals (early vs late). Within the first year of transplant, a diagnosis of AATD was associated with significantly worse overall survival (adjusted hazard ratio [HR]: 1.68, 95% confidence interval [CI]: 1.26, 2.23). However, among those patients who survived beyond one year, diagnosis was not associated with any significant difference in survival after one year (adjusted HR: 1.09, 95% CI: 0.92, 1.29).
Figure 3.
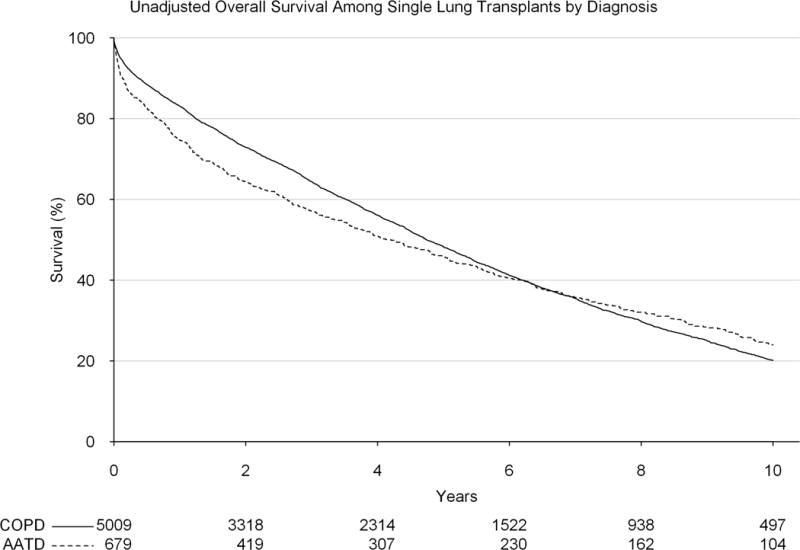
Unadjusted survival by diagnosis (alpha-1-antitrypsin deficiency vs general chronic obstructive pulmonary disorder) among patients receiving a single lung transplant over the study period.
Among patients who received a bilateral lung transplant, on unadjusted analysis, AATD was not associated with a significant early survival difference (1-year survival: 83.6% vs 85.5%, p=0.220), but significantly improved survival by 5-years (61.8% vs 56.4%, p=0.016, Figure 4). Again, due to concern for non-proportional hazards, the adjustment was broken up into an early and a late interval. However, no significant difference in survival was noted by type of transplant either within one year (adjusted HR: 1.19, 95% CI: 0.89, 1.58) or after one year (adjusted HR: 0.94, 95% CI: 0.78, 1.14).
Figure 4.
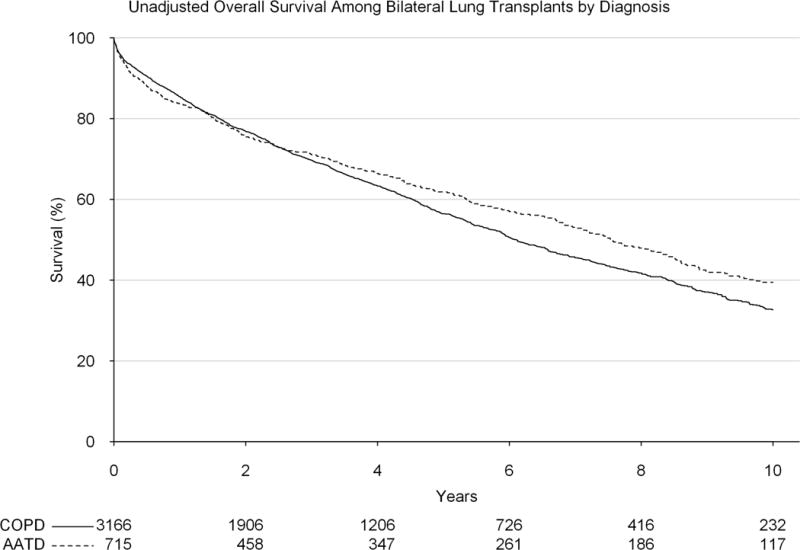
Unadjusted survival by diagnosis (alpha-1-antitrypsin deficiency vs general chronic obstructive pulmonary disorder) among patients receiving a bilateral lung transplant over the study period.
Survival Analysis Post-Lung Allocation Score Implementation
Among patients who were transplanted after the implementation of the LAS, there was no significant difference in early (1-year survival: 84.2% vs 86.9%, p=0.606) or late (5-year survival 51.1% vs 51.7%, p=0.947) unadjusted survival by diagnosis (AATD vs COPD) among patients undergoing a single lung transplant (Figure 5). The same was found after adjustment (adjusted HR: 1.15, 95% CI: 0.69, 1.95). Among bilateral lung transplants performed after the implementation of the LAS, there was also no significant difference in early (1-year survival: 88.7% vs 86.5%, p=0.327) or late (5-year survival: 62.7% vs 56.9%, p=0.218) unadjusted survival (Figure 6). The same was found after adjustment (adjusted HR: 0.99, 95% CI: 0.73, 1.34).
Figure 5.
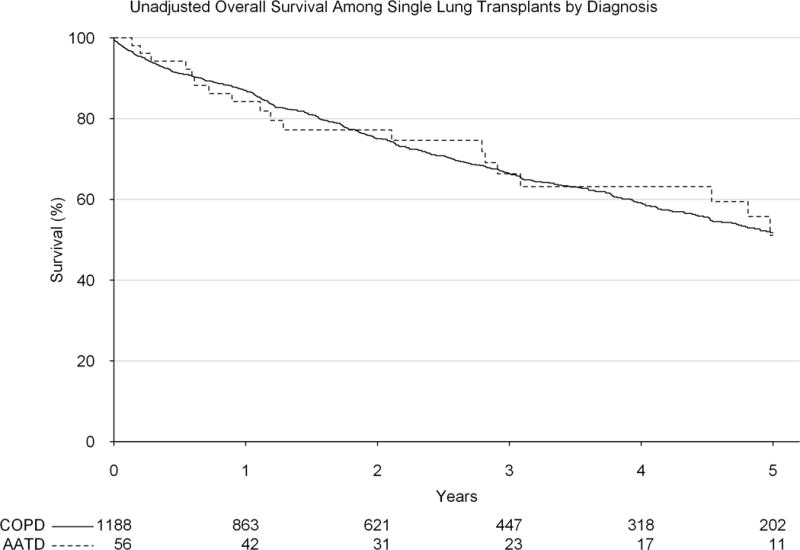
Unadjusted survival by diagnosis (alpha-1-antitrypsin deficiency vs general chronic obstructive pulmonary disorder) among patients receiving a single lung transplant since the implementation of the lung allocation score.
Figure 6.
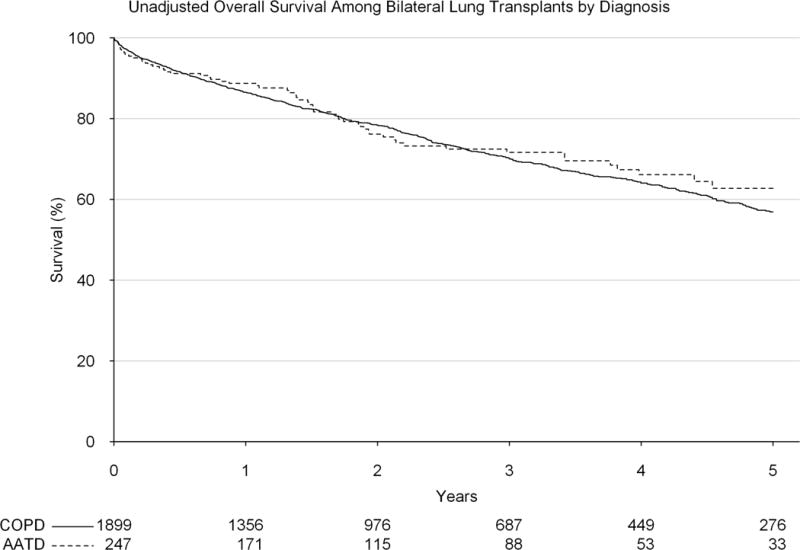
Unadjusted survival by diagnosis (alpha-1-antitrypsin deficiency vs general chronic obstructive pulmonary disorder) among patients receiving a bilateral lung transplant since the implementation of the lung allocation score.
Discussion
Although only identified in 1963, AATD has become a widely studied disease, affecting roughly 1.0–2.5% of all Americans suffering from COPD.1,8 AATD often leads to basal panacinar emphysema, which although distinct from the apical centrilobular emphysema of COPD, can still lead to severe pulmonary disease.8,9 Roughly 10% of patients will go on to require a lung or liver transplant, and lung transplantation has been demonstrated to be associated with significant improvements in overall survival in this population as compared to medical therapy.6,9 In this study, we evaluated the outcomes associated with lung transplantation in patients with a diagnosis of AATD as compared to patients with a general diagnosis of COPD. We found significant differences in the baseline characteristics of patients with AATD as compared to patients with COPD, and furthermore, significantly higher rates of airway dehiscence and rejection. Furthermore, when investigating differences in overall survival, AATD patients had significantly reduced early survival when receiving single lung transplants as compared to patients with COPD, however this difference appears to have disappeared since the implementation of the LAS. Otherwise, patients with AATD appear to have similar long-term outcomes following lung transplantation as the general COPD cohort.
We also found that there appears to have been significant reductions in the use of lung transplantation for AATD over the study period as a function of transplantation for all patients with COPD. However, this is less likely to be a result of a reduction in the use of transplantation for AATD, which has remained relatively stable over the study period in terms of absolute numbers, as much as there has been an increase in the use of lung transplantation in patients with COPD, a finding also demonstrated in the Registry of the International Society for Heart and Lung Transplantation.10 Nonetheless, the use of lung transplantation in patients with AATD as compared to the overall cohort of patients with COPD appears to be much more common than the general incidence of AATD in the overall population (1–2% of all COPD cases).11 Furthermore, our inability to demonstrate an increase in the overall rate of lung transplantation for AATD over time may be secondary to improvements in the medical treatment of AATD due to the increasing use of alpha-1-antitrypsin augmentation therapy. In a survey of patients with AATD performed in 2003, roughly 75% of patients with obstructive lung disease were currently using augmentation therapy.9 We also found significant increases in the use of bilateral lung transplants for patients with AATD over the study period, however this is likely a function of the increasing use of bilateral lung transplantation overall for patients with COPD, and is not likely distinct to the AATD cohort.10
Our demonstration of significant differences in the rates of airway anastomotic leaks, both among single and bilateral lung transplants by diagnosis appears to be a new finding. There are many hypothetical reasons why this may occur. Alpha-1-antitrypsin is an inhibiting agent for numerous proteinases including neutrophil elastase, proteinase 3, and kallikreins 7 and 14.11–13 The imbalance between elastase and anti-elastase due to the loss of neutrophil elastase inhibition in this cohort is thought to be the primary etiology behind AATD-induced pulmonary disease.11 It is possible that this imbalance of elastase continues to be a concern following lung transplantation, and reduces the ability of the bronchus to heal properly following anastomosis. This hypothesis is supported by reports of wound healing issues in patients with AATD, which improve with alpha-1-antitrypsin augmentation.14 Unfortunately, based on the granularity of the UNOS database, we cannot determine which patients were on augmentation therapy at the time of transplant, or which patients were continued on this therapy through transplant, but this finding may indicate that augmentation therapy may be important in the perioperative period in these patients. Further granularity in this regard may improve the ability of the UNOS database to answer this question more definitively in the future. Increased rates of complications related to wound healing in the AATD group are also in the setting of decreased rates of steroid use and diabetes compared to the COPD group, both of which likely put the COPD group at higher risk of wound complications. This may suggest that recipients with a diagnosis of AATD and a history of diabetes or steroid dependency might be at an even higher risk of wound complications, an insight that may give reason to particularly emphasize modifiable risk factors in this setting.
We also found significantly increased rates of rejection within one year among both single and bilateral lung transplant patients with AATD as compared to the overall COPD cohort. This also appears to be a novel finding as compared to previous reports.5 Again we could not determine exactly why this finding was seen, but it may be secondary to the differences in baseline characteristics seen between the AATD and COPD groups. Patients with AATD tended to be younger and were more likely to be white, both factors found to be associated with significantly higher rates of rejection in previous studies.15 Conversely, it may be secondary to a yet unknown process intrinsic to patients with AATD.
Lastly, there is substantial controversy in the literature regarding the overall survival differences among patients who undergo lung transplantation for AATD versus COPD. In a single institution study, Banga and colleagues found no significant differences in early or late survival,5 however a study by de Perrot and colleagues found that patients with AATD suffered impaired survival post-transplant.16 Conversely, a Swedish study by Tanash and colleagues found AATD recipients performed significantly better than non-AATD patients.6 The differences in these small single institution studies is likely secondary to variations in the use of augmentation therapy and practice patterns by site. In our investigation of a large national database, we found that overall, patients with AATD receiving a single lung transplant had reduced early survival as compared to the overall COPD cohort, but that these patients then had similar long-term survival. However, these differences disappeared after the implementation of the LAS in May 2005. Among patients receiving a bilateral lung transplant, these differences were severely diminished, and again disappeared entirely after the implementation of the LAS.
The differences in survival in the overall cohort as compared to the post-LAS cohort are intriguing and likely multifactorial. The LAS has been demonstrated to have substantially reduced waiting times for transplant, without any significant impact on post-transplant overall survival.17 However, as the LAS does not differentiate between diagnosis with regards to AATD (both AATD and COPD are grouped as a “Group A” Diagnosis), it is possible that there were differences in the severity of illness by diagnosis prior to the implementation of the LAS which led to reduced early survival for patients with AATD, which have been corrected since that time. Alternatively, although augmentation therapy was first implemented in the 1980s, its growing use may reduce the dissimilarities in patients with AATD presenting for lung transplant as compared to other patients with COPD, thus leading to more similar short and long-term survivals.8 Finally, these observations may be attributed to overall improvements in the approach to lung transplantation more broadly, but have impacted specific disease processes more profoundly than others. The approach to over- and under-sizing of lung allografts has been shown to contribute to post-transplant outcomes in both restrictive and obstructive diseases, a finding that has resulted in changes in approach for many centers and may contribute to temporal variation in outcomes.18
Although we present here the largest study to date on the use of lung transplantation in patients with AATD as compared to patients with other forms of COPD, there are important limitations which bear consideration. First, the diagnosis of AATD in the UNOS database relies on the interpretation of the data manager, and is not authenticated by genetic testing. Second, as a retrospective review of a national database, there as always exists the potential for unobserved confounding which could not be accounted for in our adjusted analyses. Third, as discussed above, the UNOS database does not record the use of augmentation therapy for patients with AATD, so we could not determine how many patients were on augmentation therapy at the time of transplant or which patients had augmentation therapy continued through transplant, nor could we incorporate these variables into our adjustment.
In conclusion, there are significant differences with regards to baseline characteristics and post-operative complications between patients with AATD and the general COPD cohort. Furthermore, there are significant differences in overall survival by diagnosis among patients treated with a single lung transplant, however these differences do not seem to occur in patients receiving a bilateral lung transplant. Lastly, it does not appear that these differences in survival have been sustained since the implementation of the LAS. Consequently, our findings demonstrate that there are still areas for improvement regarding the transplantation of patients with AATD, specifically related to a better understanding of why these patients suffer from higher rates of airway dehiscence and rejection. Nonetheless, as survival appears to be similar by diagnosis since the implementation of the LAS, the combining of these two diagnoses for research and organ allocation purposes is not contraindicated.
Acknowledgments
The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government. In addition, this work was supported by the NIH funded Cardiothoracic Surgery Trials Network (B.C.G. and M.G.H.), 5U01HL088953-05.
Funding Sources: Institutional Funding was the primary funding source for this study. In addition, this work was supported by the NIH-funded Cardiothoracic Surgery Trials Network (B.C.G., and M.G.H.), 5U01HL088953-05.
Abbreviations
- AATD
Alpha-1-Antitrypsin Deficiency
- COPD
Chronic Obstructive Pulmonary Disease
- UNOS
United Network for Organ Sharing
- ECMO
Extra-Corporeal Membrane Oxygenation
- BMI
Body Mass Index
- LAS
Lung Allocation Score
- FEV1
Forced Expiratory Volume in one second
- CMV
Cytomegalovirus
- EBV
Epstein-Barr Virus
Footnotes
DR. MICHAEL S MULVIHILL (Orcid ID : 0000-0002-8122-1483)
Brian C Gulack designed research/study, wrote manuscript, performed research, analyzed data
Michael S Mulvihill wrote manuscript, performed research
Asvin M Ganapathi performed research
Paul J Speicher performed research, analyzed data
Godefroy Chery performed research
Laurie D Snyder designed research study, analyzed data, critical review
R Duane Davis designed research study, analyzed data, critical review
Matthew G Hartwig designed research study, analyzed data, critical review
Conflicts of Interest: The authors have no conflicts of interest to report.
References
- 1.American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. American journal of respiratory and critical care medicine. 2003;168(7):818–900. doi: 10.1164/rccm.168.7.818. [DOI] [PubMed] [Google Scholar]
- 2.Stoller JK, Aboussouan LS. A review of alpha1-antitrypsin deficiency. American journal of respiratory and critical care medicine. 2012;185(3):246–259. doi: 10.1164/rccm.201108-1428CI. [DOI] [PubMed] [Google Scholar]
- 3.Larsson C. Natural history and life expectancy in severe alpha1-antitrypsin deficiency, Pi Z. Acta medica Scandinavica. 1978;204(5):345–351. doi: 10.1111/j.0954-6820.1978.tb08452.x. [DOI] [PubMed] [Google Scholar]
- 4.Tanash HA, Riise GC, Hansson L, Nilsson PM, Piitulainen E. Survival benefit of lung transplantation in individuals with severe alpha1-anti-trypsin deficiency (PiZZ) and emphysema. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2011;30(12):1342–1347. doi: 10.1016/j.healun.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Banga A, Gildea T, Rajeswaran J, Rokadia H, Blackstone EH, Stoller JK. The natural history of lung function after lung transplantation for alpha(1)-antitrypsin deficiency. American journal of respiratory and critical care medicine. 2014;190(3):274–281. doi: 10.1164/rccm.201401-0031OC. [DOI] [PubMed] [Google Scholar]
- 6.Tanash HA, Riise GC, Ekstrom MP, Hansson L, Piitulainen E. Survival benefit of lung transplantation for chronic obstructive pulmonary disease in Sweden. The Annals of thoracic surgery. 2014;98(6):1930–1935. doi: 10.1016/j.athoracsur.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 7.Hayes D, Jr, Higgins RS, Kirkby S, et al. Impact of pulmonary hypertension on survival in patients with cystic fibrosis undergoing lung transplantation: an analysis of the UNOS registry. Journal of cystic fibrosis: official journal of the European Cystic Fibrosis Society. 2014;13(4):416–423. doi: 10.1016/j.jcf.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Stockley RA. Alpha1-antitrypsin review. Clin Chest Med. 2014;35(1):39–50. doi: 10.1016/j.ccm.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Strange C, Stoller JK, Sandhaus RA, Dickson R, Turino G. Results of a survey of patients with alpha-1 antitrypsin deficiency. Respiration; international review of thoracic diseases. 2006;73(2):185–190. doi: 10.1159/000088061. [DOI] [PubMed] [Google Scholar]
- 10.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report—2012. The Journal of Heart and Lung Transplantation. 2012;31(10):1073–1086. doi: 10.1016/j.healun.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Janciauskiene SM, Bals R, Koczulla R, Vogelmeier C, Kohnlein T, Welte T. The discovery of alpha1-antitrypsin and its role in health and disease. Respiratory medicine. 2011;105(8):1129–1139. doi: 10.1016/j.rmed.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Gooptu B, Lomas DA. Conformational pathology of the serpins: themes, variations, and therapeutic strategies. Annual review of biochemistry. 2009;78:147–176. doi: 10.1146/annurev.biochem.78.082107.133320. [DOI] [PubMed] [Google Scholar]
- 13.Luo LY, Jiang W. Inhibition profiles of human tissue kallikreins by serine protease inhibitors. Biological chemistry. 2006;387(6):813–816. doi: 10.1515/BC.2006.103. [DOI] [PubMed] [Google Scholar]
- 14.Cathomas M, Schuller A, Candinas D, Inglin R. Severe postoperative wound healing disturbance in a patient with alpha-1-antitrypsin deficiency: the impact of augmentation therapy. International wound journal. 2015 doi: 10.1111/iwj.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mangi AA, Mason DP, Nowicki ER, et al. Predictors of acute rejection after lung transplantation. The Annals of thoracic surgery. 2011;91(6):1754–1762. doi: 10.1016/j.athoracsur.2011.01.076. [DOI] [PubMed] [Google Scholar]
- 16.de Perrot M, Chaparro C, McRae K, et al. Twenty-year experience of lung transplantation at a single center: Influence of recipient diagnosis on long-term survival. The Journal of thoracic and cardiovascular surgery. 2004;127(5):1493–1501. doi: 10.1016/j.jtcvs.2003.11.047. [DOI] [PubMed] [Google Scholar]
- 17.Kozower BD, Meyers BF, Smith MA, et al. The impact of the lung allocation score on short-term transplantation outcomes: a multicenter study. The Journal of thoracic and cardiovascular surgery. 2008;135(1):166–171. doi: 10.1016/j.jtcvs.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 18.Eberlein M, Reed RM, Bolukbas S, Parekh KR, Arnaoutakis GJ, Orens JB, et al. Lung size mismatch and survival after single and bilateral lung transplantation. Ann Thorac Surg. 2013;96(2):457–63. doi: 10.1016/j.athoracsur.2013.04.064. [DOI] [PubMed] [Google Scholar]


