Abstract
Background
Intra-operative bleeding, post-operative brain edema and neuroinflammation are major complications in patients with surgical brain injury (SBI). Phospholipase A2 (PLA2)is the upstream enzyme which initiates the PLA2, 5-lipoxygenase (5-LOX) and leukotriene B4 (LTB4) inflammatory pathway. We hypothesizedPLA2preconditioning (PPC) prior to SBI can activate endogenous anti-inflammatory responses to protect against SBI. This study evaluated if PPC can ameliorate neurosurgical complications and elucidated PPC-mediated possible protective mechanisms in a rat SBI model.
Methods
Total105 adult male Sprague Dawley rats were used for this study. SBI was induced by partial resection of the right frontal lobe. PLA2 or 0.9% NaCl was injected via rats’ tail vein for 3 consecutive days prior to SBI. For mechanism study, a selective PLA2 inhibitor, Manoalide and 5-LOX inhibitor, Zileuton were injected intravenously with PPC to elucidate the role of PLA2 and 5-LOX in PPC-mediated anti-inflammatory effects. Brain water content (BWC) and lung water content, neurological tests, ELISA, western blot, immunohistochemistry, white blood cells (WBC) count, and spectrophotometric assay for intra-operative hemorrhage volume were evaluated.
Results
First, PPC reduced brain water content, intra-operative bleeding, and improved neurological function after SBI. Second, PPC decreased 5-LOX expression and brain leukocyte infiltration, while increasing glial fibrillary acidic protein (GFAP)expression in the peri-resection brain tissue after SBI. Third, PPC induced peripheral inflammation represented by mild pulmonary inflammation and increased peripheral blood WBC count and LTB4 level. Lastly, PPC increased blood glucose concentration and glucocorticoid levels after SBI. In addition, PPC mediated above-mentioned changes were partially reversed by administration of PLA2 inhibitor, Manoalide and 5-LOX inhibitor, Zileuton.
Conclusions
PPC conferred neuroprotection against SBI via multi-target involvement induced anti-inflammatory mechanisms.
Keywords: Surgical brain injury, sPLA2, Preconditioning, Neuroinflammation, Brain edema, Intra-operative hemorrhage
1. Introduction
An estimated 800,000cranial and spinal neurosurgeries are conducted every year in the United States(McGirt et al., 2015). Injury to brain tissue at the peri-resection site inevitably occurs during neurosurgical procedures, and this has been termed as surgical brain injury (SBI). Direct mechanical injury during surgical incisions and suction aspiration, as well as thermal injuries due to electronic drilling and electrocoagulation can lead to unavoidable trauma to the surrounding tissue. The traumatic nature of such injuries can lead to blood brain barrier (BBB) disruption, intra-operative bleeding, and direct cell death. In addition, primary and secondary neuroinflammation aggravate brain edema and worsen post-operative neurological function (Ghosh and Basu, 2012; Xu et al., 2015). Diuretics and steroids have conventionally been used to reduce post-operative complications in the clinics, but they are not always beneficial and sometimes may incur adverse effects (Un et al., 2013; Wakai et al., 2013). Neurosurgical procedures are often planned ahead of time; therefore, allow for preventative measures to be taken to reduce possible complications.
Preconditioning is described as a phenomenon in which brief exposure to noxious stimuli at small doses elicits endogenous protective response to induce tolerance against future massive or prolonged injurious insult (Abd-Elfattah and Wechsler, 1995; Przyklenk and Kloner, 1998). Various preconditioning strategies have been shown to be neuroprotective in brain injury models (Hickey et al., 2011; Pan et al., 2012). Attenuation of inflammation has been shown to be one of the mechanisms underlying preconditioning-induced neuroprotection against focal cerebral ischemia(Bowen et al., 2006). Phospholipase A2 (PLA2), 5-lipoxygenase (5-LOX) and leukotriene-B4 (LTB4) axis has been well documented to be a typical inflammatory pathway (Burnett and Levy, 2012; Hernandez et al., 2007; Xie et al., 2015). PLA2 is an important component of snake venoms responsible for the inflammatory effects of venoms (Tan et al., 2015; Vadas et al., 1989; Wei et al., 2009). Cobra venom-derived secretory PLA2 (sPLA2) catalyzes the hydrolysis of membrane phospholipids to produce the lipid mediator, arachidonic acid (AA), which is further oxygenated into LTB4 by the enzyme 5-LOX (Ghassemi and Rosenberg, 1992; Rosenberg et al., 1983; Yates et al., 1990). PLA2 isolated from Naja sputatrix venom was reported to induce pulmonary inflammation and edema when administered intravenously and intra-tracheally to rats (Cher et al., 2003). Previous studies show that PLA2 plays a role in the worsening of cerebral ischemic injury in rodent models (Bonventre et al., 1997; Clemens et al., 1996). This study was designed to investigate whether preconditioning with sPLA2 derived from Naja mossambica mossambica venom prior to SBI would provide neuroprotection against SBI induced complications via activation of the PLA2/5-LOX/LTB4 signaling pathway.
2. Materials and Methods
2.1 Animals
All animal protocols followed NIH Guide for the Care and Use of Laboratory Animals and were approved by Institutional Animal Care and Use Committee at Loma Linda University. All experiments complied with ARRIVE guidelines. Adult male Sprague Dawley rats, 260 g to 300 g (n=105) were used for the study. All animals used in the study were randomly assigned to various groups used for all the experiments. Rats (n=20) were used for Sham and (n=85) were used for SBI groups. None of the Sham rats died. A total 13 of the 85 SBI rats died (15.3%) within 24h after SBI due to excessive intra-operative bleeding or brain edema. The animal number used per group and mortality in each group is listed in Table 1. Animals were housed in humidity and temperature controlled environment with a 12h light/dark cycle and free access to food.
Table 1. Animal number and mortality per group.
The table shows animal number and mortality in each group. Outcomes for Experiment 1 were evaluated at 24h and 72h after surgery. Outcomes for Experiments 2 and 3 were evaluated at 24h after surgery.
| Groups | Total | Mortality (%) |
|---|---|---|
| Experiment 1 | ||
| 24h Outcomes | ||
| Sham | 6 | 0/6(0%) |
| SPC+SBI | 7 | 1/7 (14.2%) |
| PPC+SBI | 7 | 1/7 (14.2%) |
| 72h Outcomes | ||
| Sham | 6 | 0/6 (0%) |
| SPC+SBI | 7 | 1/7 (14.2%) |
| PPC+SBI | 7 | 1/7(14.2%) |
| Experiment 2 | ||
| Sham | 8 | 0/8 (0%) |
| SPC+SBI | 10 | 2/10(20%) |
| PPC+SBI | 10 | 2/10 (20%) |
| Experiment 3 | ||
| SPC+SBI | * | * |
| PPC+SBI | 9 | 1/9 (11.1%) |
| Vehicle+PPC+SBI | 10 | 2/10 (20%) |
| Manoalide+PPC+SBI | 9 | 1/9 (11.1%) |
| Zileuton+PPC+SBI | 9 | 1/9 (11.1%) |
| Total Animals | 105 | 13/85 (15.3%) |
Samples for SPC+SBI group were shared with Experiment 2. Vehicle refers to ethanol. SPC, saline preconditioning; SBI, surgical brain injury; PPC, PLA2 preconditioning.
2.2 Preconditioning Regime and Experimental Groups
The Experimental design for the study and animal numbers used per assay are shown in Figure 1.
Fig. 1.
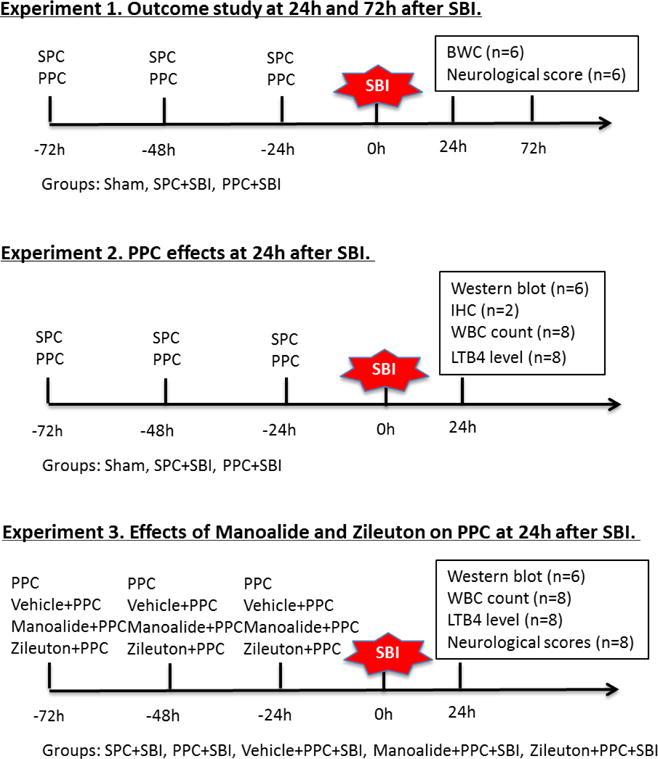
Experimental design and animal groups. Experiment 1: neurological tests were evaluated at 24h and 72h after surgery following which the animals were euthanized to collect brain samples for brain water content (BWC) measurement (n=6/group/time point). Experiment 2: animals were euthanized at 24h after surgery. Peripheral blood sample was collected during sacrifice for WBC count and LTB4 assay (n=8/group), and brain samples were harvested from the same animals for western blot (n=6/group) and immunohistochemistry (n=2/group). Experiment 3: neurological tests were evaluated at 24h following which animals were euthanized. Peripheral blood sample was collected during sacrifice for WBC count and LTB4 assay (n=8/group), after which brain samples were harvested from the same animals for western blot (n=6/group). Samples for SPC+SBI were shared with Experiment 2. Vehicle refers to ethanol. SBI, surgical brain injury; SPC, saline preconditioning, PPC, PLA2 preconditioning; BWC, brain water content, IHC, immunohistochemistry; WBC, white blood cells; LTB4, leukotriene B4.
Experiment 1 was performed to evaluate the effects of PLA2 preconditioning (PPC) on SBI-induced brain edema and neurobehavioral deficits at 24h and 72h after SBI. SBI rats were subjected to either PPC or 0.9% NaCl preconditioning (SPC) as controls. sPLA2 derived from Naja mossambica mossambica venom (Sigma Aldrich, St Louis, MO) was injected into the tail vein of rats in PPC+SBI group, once each day for 3 consecutive days prior to SBI surgery. The dose (125 mg/kg body weight) and intravenous injection route for sPLA2 administration were chosen based on previous publications(Cher et al., 2003; Tan and Arunmozhiarasi, 1989a, b). The SPC+SBI group were injected intravenously with the same volume of 0.9% NaCl for 3 consecutive days prior to SBI. Forty rats were randomly assigned into Sham, SPC+SBI and PPC+SBI groups for 24h and 72h outcome assessment (n=6/group/time point). First, we evaluated brain edema and neurobehavior at 24h after injury in the Sham, SPC+SBI and PPC+SBI groups. Next, we used a separate set of Sham, SPC+SBI and PPC+SBI animals to evaluate brain edema and neurobehavior at 72h after injury. Lung samples were collected from the same rats for measurement of lung water content at 24h and 72h after SBI.
Experiment 2 was conducted to determine the effects of PPC on cerebral 5-LOX expression, peripheral white blood cells (WBC) count and blood LTB4 levels which were measured at 24h after SBI. Preconditioning regime was performed as described in experiment 1. A separate set of 28 rats were used that were randomly assigned into 3 groups (n=8/group): Sham, SPC+SBI, PPC+SBI. Neurological tests were evaluated at 24h after surgery after which the animals were sacrificed. Brain samples were collected at 24h after surgery for western blot and immunohistological staining. Lung tissue 1cm×1cm from the apex of the right lungs was collected for staining. Blood samples were collected during sacrifice to measure peripheral WBC count and blood LTB4 levels.
Experiment 3 was designed for mechanism study. To investigate the role of PLA2 and 5-LOX, a set of 37 rats were randomly allocated into 4 groups (n=8/group): PPC+SBI, Vehicle+PPC+SBI, Manoalide+PPC+SBI and Zileuton+PPC+SBI. Data of SPC+SBI group from experiment 2 was also used. The selective PLA2 inhibitor, Manoalide(Cayman Chemicals, Ann Arbor, MI) was dissolved in ethanol to a final concentration of 4.8mM. The mixture of Manoalide and sPLA2 at 1:20 was incubated at 42°Cfor 40 min as previously described (Glaser et al., 1988; Grange et al., 1998; Lombardo and Dennis, 1985). The mixture was injected intravenously daily for 3 consecutive days prior to SBI in Manoalide+PPC+SBI group. The 5-LOX inhibitor, Zileuton (0.5 mg/kg) (Santa Cruz Biotechnology, Santa Cruz, CA) dissolved in ethanol was injected intravenously 30 min prior to each sPLA2 injection administration. The dose of Zileuton was determined according to previous studies (Gonca, 2013; Jadhav et al., 2009). The Vehicle+PPC+SBI group served as the control group and received ethanol. The mixture of same volume of ethanol and sPLA2 was injected intravenously in the Vehicle+PPC+SBI group for 3 consecutive days prior to SBI. Neurological tests were evaluated at 24h after surgery after which the animals were sacrificed. Brain samples were collected at 24h after surgery for western blot. Blood samples were collected during sacrifice to measure peripheral WBC count and blood LTB4 levels.
2.3 Surgical Brain Injury Rat Model
Rats were placed prone on a stereotactic frame after anesthetic induction with 4% isoflurane. Anesthesia was maintained using 2.5% isoflurane during surgery. A mid-line skin incision was made to expose the bregma. Craniotomy was performed to make a bone window in the right frontal bone with margins 2 mm lateral to sagittal suture and 1 mm proximal to coronal suture as previously described (Huang et al., 2015). The frontal lobe visible through the bone window was resected along margins of the bone window. The depth of resection was extended till the cranium was visualized. The resected tissue was then aspirated and 0.9% NaCl irrigation was used to obtain hemostasis. Sham rats were subjected to same surgical procedure to remove skull, but dura was kept intact. One mL of 0.9% NaCl and Buprenorphine (0.03 mg/kg) were injected subcutaneously at the end of surgery to supplement fluid loss and for post-operative analgesia, respectively.
2.4 Measurement of Brain Water Content and Lung Water Content
Animals were decapitated under deep anesthesia 24h and 72h after SBI. Brains were quickly removed and dissected in a petri dish over ice into six parts including right frontal, left frontal, right parietal, left parietal, cerebellum and brain stem. The wet weight of each part was measured immediately and weighed again after drying samples in an oven setting at 105°C for 2 days.
Next, bilateral lungs from the same animals were collected and put in an aluminum foil tray, the wet weights were weighed and recorded immediately, then the lungs were put in an oven which was set at 105°C. Forty-eight hours later, the lungs were weighed again to get the dry weights.
Percent of water content in each part was calculated using the formula [(wet weight-dry weight)/wet weight]×100 % as previously described (Yamaguchi et al., 2007).
2.5 Assessment of Neurological Function
Neurological function was assessed using modified Garcia test and beam balance test by an investigator who was blinded to the experimental groups at 24h and 72h after SBI (Yamaguchi et al., 2007). Modified Garcia test included 21-point sensorimotor assessment which consisted of seven parameters evaluating spontaneous activity, side stroking, vibrissae touch, limb symmetry, climbing, lateral turning and forelimb walking. Each parameter was given a score that ranged from 0 to 3with a total maximum score of 21 for all the parameters combined. The beam balance test used a beam measuring 2.5 cm diameter and 90 cm in length, which was elevated at 30 cm above the table surface held in place by platforms on either ends of the beam. The rats were placed in the middle of the beam and observed for 1 min. The following parameters were used to give a score that ranged from 0-5, with 5 being the best. Score 0: Fell off beam <40s; Score 1: Stayed on beam >40s; Score 2: Moved less than halfway in <40s and stayed on beam >25s; Score 3: Moved halfway to platform in <40s and stayed on beam >25s; Score 4: Reached platform in <40s; Score 5: Reached platform in <25s. Higher scores indicated better performance in the tests.
2.6 Spectrophotometric Assay for Intra-Operative Hemorrhage Volume
Intra-operative hemorrhage volume was quantified using spectrophotometric hemoglobin assay as previously described (Auriat et al., 2005; MacLellan et al., 2004). Hemoglobin assay was used to quantify the amount of intra-operative bleeding that was encountered during partial frontal lobe resection procedure. The intra-operative blood loss that occurred during resection procedure was collected by suction. The suctioned blood sample was subjected to hemoglobin assay to quantify the amount of intra-operative bleeding volume in each rat. Briefly, blood loss during frontal lobe resection procedure was collected, distilled water was added to the suctioned blood sample to reach a total volume of 50 mL. The sample was sonicated for 60 seconds on ice followed by centrifugation at 13,000 rpm for 20 min. The supernatant (0.4 mL) was added to Drabkin’s reagent (1.6 mL) (Sigma Aldrich, St. Louis, MO) and allowed to react for 15 min at room temperature. Absorbance of hemoglobin was measured at 540 nm using a spectrophotometer (Spectronix 3000, Milton-Roy, Rochester, NY). A standard curve was established in the spectrophotometer using incremental volumes of rat blood. According to the linear relationship between optical density and blood volume on the standard curve, the bleeding volume of each sample was obtained.
2.7 Immunofluorescence Staining
Brain and lung samples were harvested 24h after surgery. Rats were transcardially perfused with ice-cold phosphate buffered saline (PBS) under deep anesthesia followed by infusion with 10% formalin. The samples were immersed in 10% formalin at 4°C for 24h followed by 30% sucrose. Ten micrometer thick sections were cut using a cryostat (CM3050S; Leica Microsystems, Bannockburn, IL). Double immunofluorescence staining of brain sections was performed for 5-LOX cellular localization. Brain sections were incubated overnight at 4°C with the following primary antibodies: rabbit anti-5LOX (1:150, Cayman Chemicals, Ann Arbor, MI) co-stained with either mouse anti-Neuronal Nuclei(NeuN) (1:200, Millipore, Billerica MA), goat anti-glial fibrillary acidic protein (GFAP) (1:400, Santa Cruz Biotechnology, Santa Cruz, CA) or mouse anti-ionized calcium binding adaptor protein 1 (Iba1) (1:200, Abcam, Cambridge, MA). Brain and lung sections were also incubated overnight with following primary antibodies: anti-5LOX (1:150, Cayman Chemicals, Ann Arbor, MI), anti-GFAP (1:100, Santa Cruz Biotechnology, Inc, Dallas, TX) and anti-myeloperoxidase (MPO) (1:100, Santa Cruz Biotechnology, Dallas, TX). The sections were then incubated with corresponding FITC-conjugated or Texas Red-conjugated secondary antibodies (Jackson Immuno research, West Grove, PA) for 2h at room temperature. The slides were then cover slipped after applying vecta shield hard set mounting medium with DAPI (Vector Company) and were visualized under a fluorescence microscope (Olympus BX51).
2.8 Terminal Deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL) Staining
In situ DNA fragmentation in lung tissues was detected using TUNEL assay kit (Roche, Mannheim, Germany) as previously described (Guo et al., 2009). Briefly, slides were soaked in 0.5% Triton-100 at room temperature for 15 min, washed twice in PBS, and then incubated with TUNEL reaction mixture at 37°C for 90 min. Slides were covered with mounting medium containing DAPI and then observed under a fluorescence microscope (Olympus BX51).
2.9 Hematoxylin and Eosin Staining
After transcardial perfusion with PBS and formalin, the lungs were collected and post-fixed in formalin for 24h and then transferred to 30% sucrose till the samples sank to the bottom. For staining, lung tissue 1cmx1cm from the apex of the right lungs was collected. Routine protocol was used for H&E staining. Briefly, the dried sample slides were immersed into 95% Flex and 70% Flex for 1 min each; then rinsed in tap water and distilled water; nuclei were stained with haematoxylin for 1-2 min and then rinsed in running tap water followed by differentiation with 0.3% acid alcohol; slides were rinsed in running tap water and Scott’s tap water substitute. The cytoplasm was stained with eosin for 20 seconds to 1 min; slides were next dehydrated, cleared and mounted. Microphotographs were taken under the microscope (Olympus BX51).
2.10 Measurement of Peripheral Blood White Blood Cell (WBC) Count
Blood 200 μL was drawn from left ventricle under deep isoflurane anesthesia during sacrifice and mixed with 800 μL of citrate-phosophate-dextrose solution (Sigma Aldrich, St Louis, MO). The red blood cells (RBC) were removed by adding RBC lysis buffer at room temperature for 30 min followed by centrifugation at 1500 rpm for 5 min. The supernatant was removed and this step was repeated 3 times. The WBC pellet was obtained and resuspended with 1 mL of 0.01M PBS to make a suspension. Ten micro liters of suspension were applied to a counting slide and WBC count was determined using TC10 TM Automated Cell Counter (Bio-Rad, Hercules, CA).
2.11 Measurements of Peripheral Blood Glucocorticoid and Leukotriene B4 (LTB4)
Peripheral blood glucocorticoid concentration was measured using a rat glucocorticoid Elisa assay kit and LTB4 level was measured using a rat LTB4 Elisa kit (MyBioSource, San Diego, CA) following the vendor’s instructions. Briefly, 50 μL standard or serum samples were added to the antibody pre-coated microtiter plate. Next, conjugate100 μL was added to each well and mixed and allowed to incubate for 1h at 37°C. The wells were washed 4times after which 50 μL substrate A and 50 μL substrate B was added to each well and incubated for 15 min at 25°C in the dark followed by adding 50 μL stop solution. The absorbance was read at 450 nm wavelength within 30 min using a microtiter plate reader.
2.12 Western Blot Assay
Brain samples were collected at 24h after SBI. Rats were transcardially perfused with cold PBS (pH 7.4) under deep anesthesia after which the brains were extracted. The right frontal lobe samples were homogenized in RIPA lysis buffer (Santa Cruz Biotechnology, Dallas, TX) and protein concentration in the samples was determined using BCA assay (Bio-Rad, Hercules, CA). Equal amounts of protein (30 μg) was separated in 10% SDS-PAGE and blotted onto nitrocellulose membrane (Bio Rad, Hercules, CA). The membranes were incubated with primary antibodies including anti-5LOX (1:1000, Cayman Chemical, Ann Arbor, MI), anti-MPO (1:1000, Santa Cruz Biotechnology, Dallas, TX), anti-GFAP (1:1000, Abcam, Cambridge, MA). The same membranes were blotted with primary antibody anti-β-actin (1:5000, Santa Cruz Biotechnology, Dallas, TX) for loading controls. After incubation with appropriate secondary antibodies (1:4000, Santa Cruz Biotechnology, Dallas, TX) for 1h at room temperature, immunoblot bands were exposed on X-ray films after development with a chemiluminescence reagent kit (ECL Plus, Amersham Biosciences, Arlington Heights, IL). The bands were scanned and quantified using Image J software (NIH, Bethesda).
2.13 Statistical Analysis
Statistical analysis was performed using Sigma Plot 10. Quantitative data were expressed as the mean±SEM. One-way ANOVA for multiple comparisons followed by Student-Newman-Keuls post hoc test were used to compare differences among groups. P value less than 0.05 was regarded as statistically significant.
3. Results
3.1 PLA2 preconditioning (PPC) attenuated brain edema and neurobehavioral deficits after SBI
The brain water content (BWC) in the right frontal peri-resection site was increased significantly with lower neurological scores in SBI rats compared with sham-operated rats at 24h and 72h after injury, PPC+SBI group had significantly reduced BWC at 24h and 72h (Figs. 2A and 2B, respectively) and improved modified Garcia neurological scores at 24h and 72h after SBI (Figs. 2C and 2D, respectively) compared to SPC+SBI group. Beam balance score was significantly lower in the SBI rats compared to sham and did not show significant difference between SPC+SBI and PPC+SBI groups at 24h and 72h after SBI (Figs. 2D and 2E, respectively).
Fig. 2.
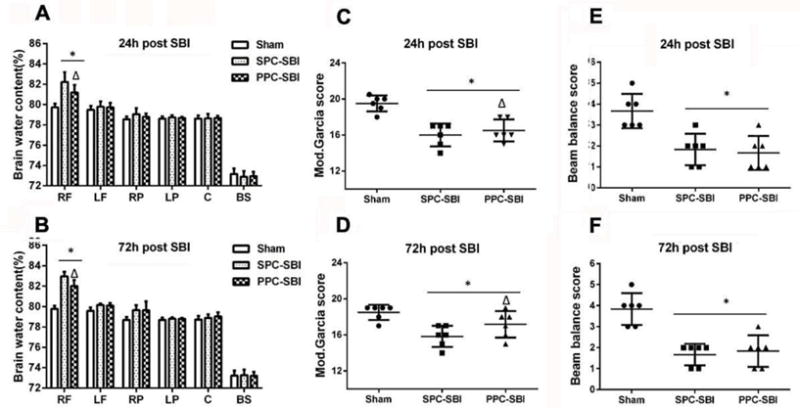
Effects of PPC on brain water content (BWC) and neurobehavioral performance at 24h and 72h post SBI. (A and B) PPC+SBI group had significantly decreased BWC in the right frontal lobe compared to SPC+SBI group at 24h and 72h post surgery. (C and D) Modified Garcia score was increased significantly in PPC+SBI group compared to SPC+SBI at 24h and 72h after SBI. (E and F). Beam balance score showed no significant differences between PPC+SBI and SPC+SBI groups. Data are expressed as mean±SEM. n=6/group. ANOVA, SNK. *P<0.05 vs Sham; Δp<0.05 vs SPC+SBI; RF=Right frontal lobe, LF=Left frontal lobe, RP=Right parietal lobe, LP=Left parietal lobe, C=cerebellum, BS= Brainstem.
3.2 PLA2 preconditioning (PPC) caused mild pulmonary inflammation
The lung water content did not show significant difference between sham-operated rats and SPC+SBI or PPC+SBI groups at 24h and 72h after surgery (Fig. 3A). No obvious morphological changes were visible in the lungs obtained from normal, SPC+SBI and PPC+SBI rats under gross examination (Fig. 3B) and with H&E staining (Fig. 3C). Double immunofluorescence staining showed that MPO-positive leukocytes co-localized with 5-LOX in lung tissue from PPC+SBI rats, and some of the MPO-positive cells were also TUNEL positive (Fig. 3D).
Fig. 3.
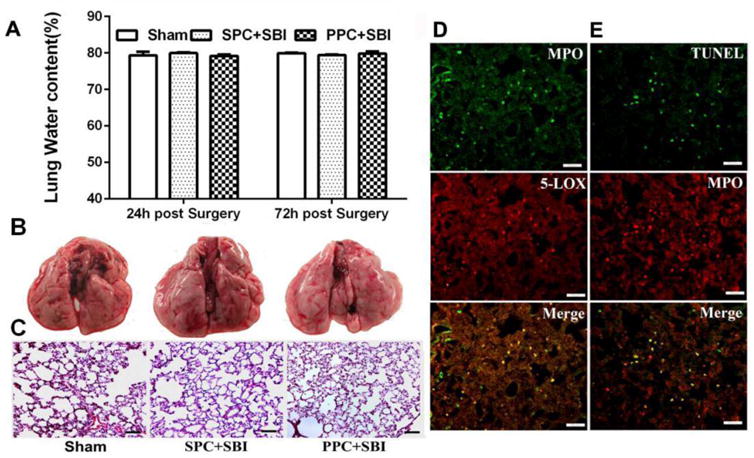
PPC induced mild pulmonary inflammation after SBI. (A)Water content of the lungs showed no significant difference within Sham, SPC+SBI and PPC+SBI groups at 24h and 72h after surgery. Data are expressed as mean±SEM. n=6/group. ANOVA, SNK. (B)Representative pictures show gross lung morphology in Sham, SPC+SBI and PPC+SBI groups at 24h after surgery. Gross lung morphology was similar in the three groups at 24h post surgery. n=6/group. (C) H&E staining of lung tissues demonstrated no obvious morphological changes in the three groups at 24h post surgery. Scale bar=100μm. n=2/group. Data not quantified. (D) PPC+SBI group lung samples showed that MPO-positive leukocytes co-localized with 5-LOX and (E) Some MPO-positive leukocytes were TUNEL-positive at 24h post SBI. Scale bar=50μm (D and E). n=2/group. Data not quantified (D and E).
3.3 PLA2 preconditioning (PPC) increased the peripheral blood white blood cell (WBC) count
The peripheral WBC count was significantly elevated in PPC+SBI rats compared to sham-operated and SPC+SBI rats (Fig. 4) at 24h after SBI.
Fig. 4.
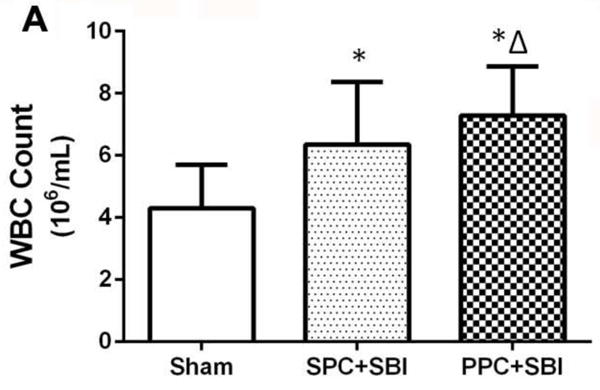
PPC increased peripheral blood WBC count measured 24h post SBI. Peripheral WBC count was increased in PPC+SBI compared with Sham and SPC+SBI groups. Data are expressed as mean±SEM. n=8/group. ANOVA, SNK. *p<0.05 vs Sham, Δp<0.05 vs SPC+SBI.
3.4 PLA2 preconditioning (PPC) increased peripheral blood glucose and glucocorticoid levels
Peripheral blood glucose level was not different between the sham-operated and SPC+SBI groups (Fig. 5A). However, blood glucose level was significantly elevated in PPC+SBI rats compared to sham-operated and SPC+SBI groups 24h after SBI (Fig. 5A). The blood glucocorticoid level was higher in PPC+SBI rats compared to sham-operated and SPC+SBI groups (Fig. 5B).
Fig. 5.
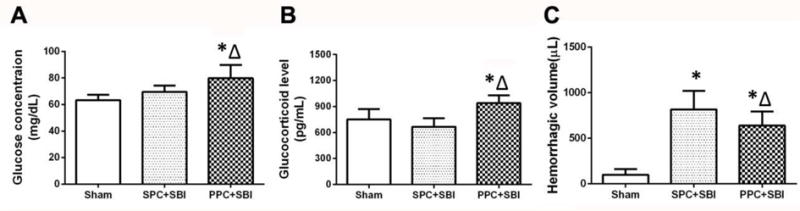
PPC effects on blood glucose, glucocorticoid levels and intra-operative hemorrhagic volume at 24h post surgery. (A)Significant elevation of blood glucose was observed in PPC+SBI group compared to SPC+SBI group. There was no difference between Sham and SPC+SBI groups.(B)PPC+SBI group had significantly increased blood glucocorticoid concentration compared to SPC+SBI rats. There was no significant difference between Sham and SPC+SBI rats. (C) PPC+SBI group had significantly decreased intra-operative hemorrhagic volume compared to SPC+SBI rats. Data are expressed as mean±SEM. n =8/group. ANOVA, SNK.*p<0.05 vs Sham, Δp<0.05 vs SPC+SBI.
3.5 PLA2 preconditioning (PPC) reduced intra-operative bleeding in SBI rats
Intra-operative hemorrhage volume quantified using hemoglobin assay showed that the intra-operative bleeding was significantly decreased in PPC+SBI rats compared to SPC+SBI rats (Fig. 5C).
3.6 PLA2 preconditioning (PPC) decreased 5-LOX and inflammatory markers in the peri-resection brain tissue after SBI
Double immunofluorescence staining showed that 5-LOX was predominantly expressed in neurons (Fig. 6A) and to a lesser extent in astrocytes (Fig. 6B) but was not expressed in microglia (Fig. 6C) at 24h after SBI. Sham group did not show any 5-LOX staining in the right frontal region (Fig. 6D). Immunofluorescence staining (Fig. 7A), western blot bands (Fig. 7B), and semi-quantitative analysis (Fig. 7C) showed that PPC+SBI group had significantly lower expression of 5-LOX and MPO in the peri-resection brain tissue compared to SPC+SBI whereas, the expression of GFAP was significantly increased in the PPC+SBI group compared to SPC+SBI rats 24h after SBI.
Fig. 6.
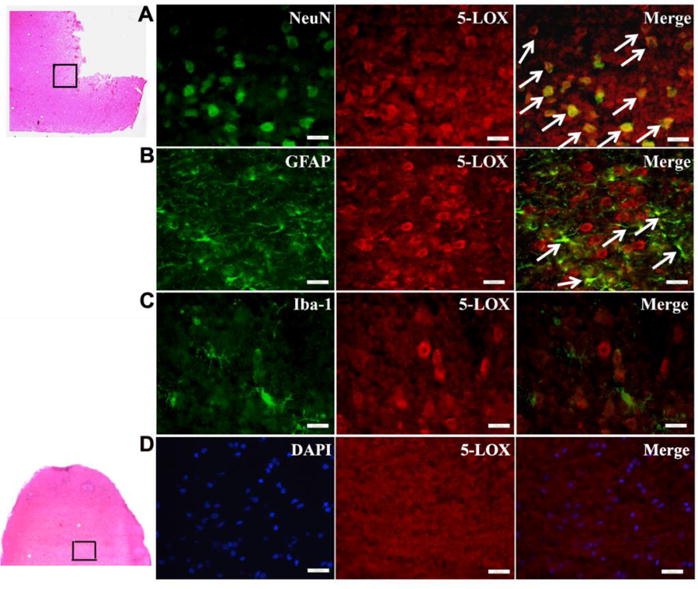
Subcellular distribution of 5-LOX in the ipsilateral hemisphere at 24h post SBI. 5-LOX is expressed mainly in NeuN-labeled neurons (A) and GFAP-labeled astrocytes (B) but not in Iba1-labeled microglia (C) 24h after SBI. Arrows indicate colocalization of 5-LOX with NeuN (A) and GFAP with Iba1 (B). Sham control group did not show 5-LOX staining in the right frontal region at 24h (D). Scale bar=25μm(A-C)and 50μm(D). n=2/group. Data not quantified.
Fig. 7.
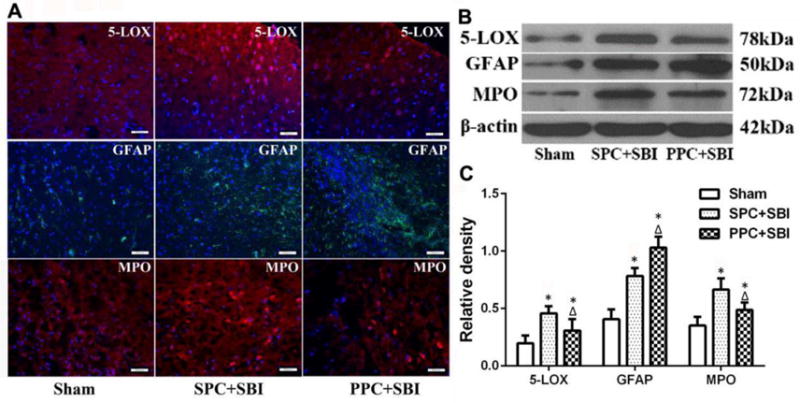
PPC effects on the expression of cerebral 5-LOX, GFAP and MPO at 24h after surgery. (A) Immunofluorescence pictures showing the expression of 5-LOX, GFAP and MPO in Sham, SPC+SBI and PPC+SBI rats. Scale bar=50μm. n=2/group. Data not quantified. Representative western blot bands (B) and quantitative analysis (C) showed the expression of 5-LOX and MPO decreased in PPC+SBI rats compared to SPC+SBI rats and GFAP expression increased in PPC+SBI rats compared to SPC+SBI rats. Data are expressed as mean±SEM. n=6/group. ANOVA, SNK. *p<0.05 vs Sham; Δp<0.05 vs SPC+SBI.
3.7 Manoalide and Zileuton partially reversed PLA2 preconditioning (PPC)-mediated changes
The peripheral WBC count (Fig. 8A) and blood LTB4 levels (Fig. 8B) were significantly increased in PPC+SBI rats compared to SPC+SBI rats 24h after SBI, which was reversed with the administration of the PLA2 inhibitor, Manoalide and 5-LOX inhibitor, Zileuton. The modified Garcia neurological score was increased in PPC+SBI rats compared to SPC+SBI group, which was reversed with Manoalide and Zileuton (Fig. 8C). The beam balance test did not show any difference between all SBI groups (Fig. 8D).
Fig. 8.
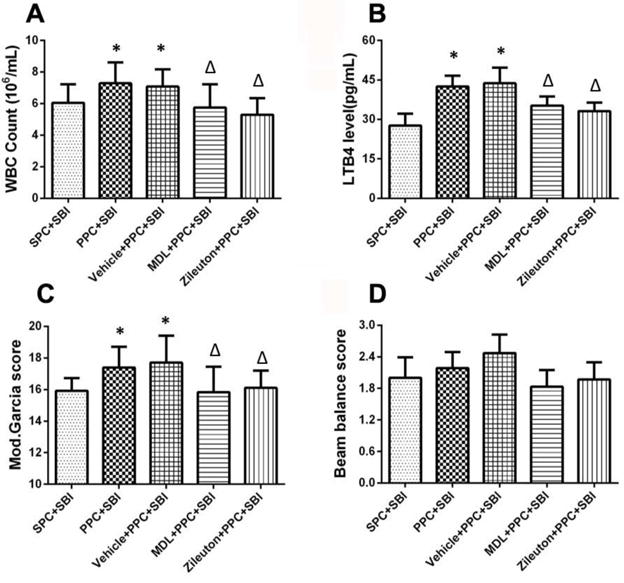
Effects of Manoalide and Zileuton on peripheral blood WBC count, LTB4 level and neurological scores 24h after surgery. Compared to SPC+SBI rats, PPC+SBI group had significantly increased WBC count (A) and blood LTB4 level (B), which was reversed with Manoalide and Zileuton. PPC+SBI group showed improved modified Garcia neurological which was reversed with Manoalide and Zileuton (C). Beam balance scores was not significantly different between groups (D). Data are expressed as mean±SEM. n=8/group. ANOVA, SNK.*p<0.05 vs SPC+SBI; Δp<0.05 vs PPC+SBI.
3.8 Manoalide and Zileuton reversed PLA2 preconditioning (PPC)-induced decrease in inflammatory markers in the peri-resection brain tissue after SBI
The expression of 5-LOX and MPO in the peri-resection brain tissue was reduced in PPC+SBI rats 24h after SBI compared to SPC+SBI as shown in the western blot bands (Fig. 9A)and semi-quantitative analyses (Fig. 9B). The expression of 5-LOX and MPO which was reduced with PPC was reversed by both Manoalide and Zileuton.
Fig. 9.
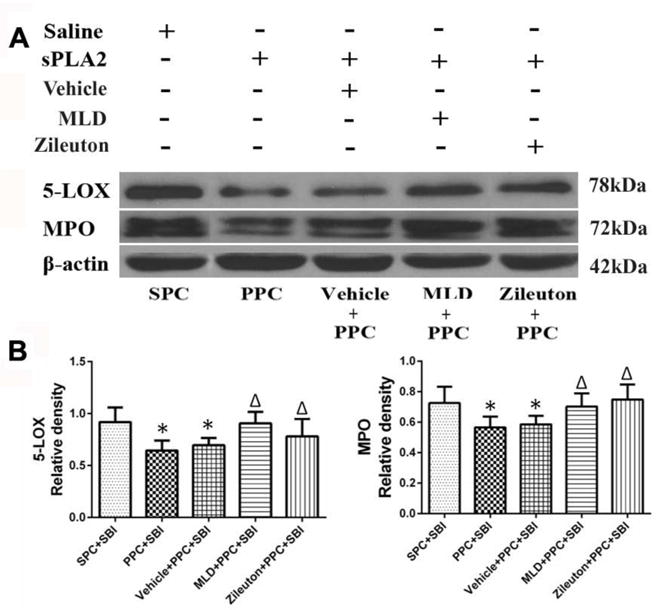
Manoalide and Zileuton reversed PPC-induced anti-inflammatory effects at 24h after surgery. (A) Representative pictures of western blot bands and (B)Quantitative analysis of the bands indicated that PPC+SBI had significantly reduced levels of 5-LOX and MPO in the peri-resection brain tissue compared to SPC+SBI rats, which were reversed by Manoalide and Zileuton. Data are expressed as mean±SEM. n=6/group. ANOVA, SNK.*p<0.05 vs SPC+SBI; Δp <0.05 vs PPC+SBI.
4. Discussion
This study evaluated the possible effects of PLA2 preconditioning (PPC) on attenuating neurosurgical complications in the SBI rat model. We first performed outcome study to determine the effects of PPC on SBI-induced brain edema, neurological deficits and intra-operative bleeding. The unavoidable mechanical and heat injury during neurosurgical procedures leads to primary and secondary inflammatory processes which contributes to progression of brain edema that worsens post-operative neurological function (McIntosh et al., 1998). Intra-operative bleeding occurs inevitably during brain surgeries; the resulting hematoma formation and blood loss can often increase mortality. Additionally, hemoglobin, heme, and iron released from the lysed red blood cells can aggravate inflammation through mechanisms including microglial activation, leukocyte infiltration, toll-like receptor activation, and danger associated molecular pattern regulation (Namas et al., 2009; Wagener et al., 1997; Wagener et al., 2001). Here, we observed that preconditioning with minor dose of PLA2 for 3 consecutive days prior to SBI decreased intra-operative hemorrhagic volume during surgical resection, reduced brain edema and improved post-operative neurological function in SBI rats.
Next, we examined the effects of PPC on SBI-induced neuroinflammation. Previous studies showed that following traumatic brain injury (TBI), the direct external insult and successive calcium influx activated PLA2, increased the expression of 5-LOX in glial cells and in the infiltrating neutrophils. Various cell types have been reported to express 5-LOX including peripheral blood leukocytes, mast cells, epithelial cells, alveolar macrophages and microglia (Maneshi et al., 2015; Ostrow and Sachs, 2005). Likewise, we observed that 5-LOX was predominantly expressed in neurons and to a lesser extent in astrocytes in the peri-resection brain tissue 24h after SBI. Our results showed that PPC decreased 5-LOX expression and leukocyte infiltration in the peri-resection brain tissue after SBI. The enzyme 5-LOX induces the production of LTB4, which is a potent chemoattractant that can induce the adhesion and activation of leukocytes on the endothelium (Barone et al., 1995) and further result in BBB disruption (Baba et al., 1991). Furthermore, while neurons and astrocytes lack 5-LOX under normal conditions, the small number of infiltrated leukocytes after brain injury canincreaseLTB4 production from neurons and astrocytes via transcellular mechanism, which further augments leukocyte infiltration (Farias et al., 2007; Kim et al., 2010). Leukotriene B4 was first reported to be involved in the pathogenesis of spinal cord injury through the amplification of leukocyte infiltration (Saiwai et al., 2010). Previous reports indicate that LTB4 increased markedly at 4h and peaked at 24h in rat TBI model (Schuhmann et al., 2003). Although we did not measure LTB4 levels in the brain after SBI, the expression of 5-LOX and leukocyte marker MPO in the peri-resection site after SBI were reduced by PPC. We suggest that PPC reduced SBI-induced neuroinflammation by attenuating PLA2-5LOX-LTB4 cascade in the brain. In order to test this hypothesis, selective pharmacological inhibitor for PLA2, Manoalide and 5-LOX inhibitor, Zileuton were administered with PPC in order to block the activities of PLA2 and 5-LOX. We observed that both interventions reversed the effect of PPC by reducing peripheral WBC count and blood LTB4 levels. The inhibitors also reversed PPC-mediated reduction in 5-LOX and MPO expression in the peri-resection brain tissue after SBI. This suggests that neuroprotective effects of PPC were at least in part mediated via the activation of PLA2-5LOX-LTB4 cascade.
We then evaluated the peripheral inflammatory effects of PPC in SBI rats. Results showed that PPC induced mild peripheral inflammation represented by mild pulmonary inflammation and increased peripheral blood WBC count and LTB4 level. The presence of inflammatory mediators 5-LOX and MPO in the lungs indicated that pulmonary inflammation was induced by PPC, but without adversely affecting the lung water content which did not show significant difference between the PPC+SBI and SPC+SBI groups. The exogenously administered sPLA2 triggers sPLA2-5LOX-LTB4 cascade in the presence of blood Ca2+. The elevated blood LTB4promotes neutrophil recruitment and pro-inflammatory factors expression, including IL-1, IL-6 and TNF-α, which further aggravate inflammation by vicious cycles in the periphery (Kim et al., 2010; Maneshi et al., 2015; Ostrow and Sachs, 2005). Thus, when injected intravenously as preconditioning agent, sPLA2 activates and attracts a large number of leukocytes in the periphery. Therefore, with the mobilization and retention of peripheral leukocytes, fewer leukocytes invade into the brain tissue in the PPC+SBI rats following SBI. In other words, PPC exhausted the peripheral leukocytes by activating peripheral sPLA2-5LOX-LTB4 cascade and thereby, reduced brain infiltration of leukocytes after SBI.
We also observed that PLA2 preconditioning increased the expression of astroglial marker GFAP in SBI rats. Under normal conditions, the intimate interaction of astrocytic end feet with brain micro-vessel stabilizes the BBB and prevents uncontrolled entry of peripheral immune cells to the brain (Bush et al., 1999). Although prolonged astrogliosis can contribute to cytotoxin release, inflammation, and scar formation (Chou et al., 2014), astrogliosis has been reported to have anti-inflammatory activities as well as neuronal protective function by secreting trophic factors (Streit et al., 1999). At the early stage of inflammation, astrocytic proliferation can provide neuroprotection by strengthening BBB integrity and nurturing neighboring neurons via paracrine secretion of neurotrophins.
Lastly, we observed that blood glucose and glucocorticoid levels were increased with PPC. Glucocorticoids are known to have nonspecific anti-inflammatory effects, and the primary mechanism involves synthesis of lipocortin-1 which not only reduces pro-inflammatory eicosanoid production by suppressing PLA2 but also inhibits leukocyte activation (Goppelt-Struebe et al., 1989). Furthermore, glucocorticoids can regulate genes that encode inflammatory proteins and thereby, suppress inflammation. For instance, glucocorticoid-glucocorticoid receptor complex can up-regulate anti-inflammatory protein expression in the nucleus by trans-activation, and down-regulate pro-inflammatory protein expression in the cytosol by trans-repression (Caldenhoven et al., 1995; Rhen and Cidlowski, 2005; Rugstad, 1988). Thus, we postulate that PPC promotes glucocorticoid release as one of the endogenous protective mechanisms to combat against subsequent inflammatory insults.
In summary, our findings demonstrate that PPC improved neurological function after SBI by reducing intra-operative bleeding, brain edema and neuroinflammation. The possible mechanisms include 1) PPC reduced intra-operative hemorrhage and therefore, may reduce hematoma formation and heme-induced inflammation; 2) PPC induced the activation of peripheral PLA2-5LOX-LTB4 cascade and consequently reduced brain leukocyte infiltration and neuroinflammation after SBI; 3) PPC increased astrocyte proliferation and increased blood glucocorticoid level which potentiate PPC-mediated neuroprotection. Hence, this study suggests that multi-targets were involved in PPC-mediated protection against neuroinflammation in the SBI rat model.
Our study had some limitations. First, it is possible that PPC may have up-regulated various other endogenous protective factors that contributed to the development of resistance against the ensuing massive injury inflicted by SBI. We did not explore alternate pathways that could have been involved in PPC-mediated protection after SBI in this study. Second, the exact mechanisms underlying the reduced intra-operative bleeding with PLA2 preconditioning was not explored. Previous studies have reported that sPLA2 has antiplatelet (Huang et al., 1997; Huang and Chiang, 1994; Teng et al., 1986), anticoagulant (Faure and Saul, 2011; Kini and Evans, 1988), hemolytic (Arce-Bejarano et al., 2014; Francis et al., 1997) and hemorrhagic properties (Francis et al., 1997). It is possible that the hemorrhagic properties of PLA2 primes the body to upregulate pro-coagulant factors, which may have contributed to reduced intra-operative bleeding in PPC+SBI rats. Additionally, we only evaluated the early effects of PPC in this study. Behavioral studies were conducted early post injury at 24h and 72h after SBI. Such early findings may not be predictive of long-term changes, and this is one of the limitations of our study. We chose these time points since previous studies have shown that SBI rats exhibit significant neurological deficits at these time points. However, it is necessary to carry on experiments at 7 days or longer to investigate the long-term effects of PPC for clinical translation of our findings. These limitations of the current study warrant further investigation in future.
This study has translational significance since PPC can easily be applied prior to neurological surgeries and extended to other kinds of surgeries. We did not observe any adverse effects with the dose regime of PPC that was used in this study. However, it is crucial to personalize preconditioning protocol based on individual differences in order to attain beneficial effects without inducing toxic or adverse effects.
Highlights.
Preconditioning with PLA2 is proposed to combat against neuroinflammation.
PLA2 preconditioning improved outcomes in surgical brain injury rat model.
Anti-inflammatory mechanism of PLA2 preconditioning involved PLA2-5LOX-LTB4 cascade.
Acknowledgments
This study was supported by NIH grants (NS084921, NS082184 and 23 NS43338), Central University Basic Scientific Research Professional Expenses Special Fund in China (101201221612427),Guangdong Medical Scientific Research Fund in China (A2016010) and Guangdong Administration of Traditional Chinese Medicine in China (2017294).
Abbreviations
- SBI
surgical brain injury
- PLA2
phospholipase A2
- PPC
PLA2 preconditioning
- 5-LOX
5-lipoxygenase
- AA
arachidonic acid
- LTB4
leukotriene-B4
- BWC
brain water content
- WBC
white blood cells
- GFAP
glial fibrillary acidic protein
- MPO
myeloperoxidase
- NeuN
Neuronal Nuclei
- Iba1
ionized calcium binding adaptor molecule 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: none.
Author Contributions
John H. Zhang designed the project. Y.W., O.A. and D.W. carried out the experiments. Y.W., P.S. and L.H. wrote the manuscript. All authors discussed the results and reviewed the manuscript.
Competing Interests
The authors declare no competing financial interests.
References
- Abd-Elfattah AS, Wechsler AS. Myocardial preconditioning: a model or a phenomenon? J Card Surg. 1995;10:381–8. doi: 10.1111/j.1540-8191.1995.tb00666.x. [DOI] [PubMed] [Google Scholar]
- Arce-Bejarano R, et al. Intravascular hemolysis induced by the venom of the Eastern coral snake, Micrurus fulvius, in a mouse model: identification of directly hemolytic phospholipases A2. Toxicon. 2014;90:26–35. doi: 10.1016/j.toxicon.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Auriat A, et al. 17 beta-estradiol pretreatment reduces bleeding and brain injury after intracerebral hemorrhagic stroke in male rats. Journal of Cerebral Blood Flow and Metabolism. 2005;25:247–256. doi: 10.1038/sj.jcbfm.9600026. [DOI] [PubMed] [Google Scholar]
- Baba T, et al. Intracarotid infusion of leukotriene C4 selectively increases blood-brain barrier permeability after focal ischemia in rats. J Cereb Blood Flow Metab. 1991;11:638–43. doi: 10.1038/jcbfm.1991.115. [DOI] [PubMed] [Google Scholar]
- Barone FC, et al. Time-related changes in myeloperoxidase activity and leukotriene B4 receptor binding reflect leukocyte influx in cerebral focal stroke. Mol Chem Neuropathol. 1995;24:13–30. doi: 10.1007/BF03160109. [DOI] [PubMed] [Google Scholar]
- Bonventre JV, et al. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–5. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- Bowen KK, et al. Prevention of inflammation is a mechanism of preconditioning-induced neuroprotection against focal cerebral ischemia. Neurochemistry International. 2006;49:127–135. doi: 10.1016/j.neuint.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Burnett BP, Levy RM. 5-Lipoxygenase metabolic contributions to NSAID-induced organ toxicity. Adv Ther. 2012;29:79–98. doi: 10.1007/s12325-011-0100-7. [DOI] [PubMed] [Google Scholar]
- Bush TG, et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- Caldenhoven E, et al. Negative cross-talk between RelA and the glucocorticoid receptor: a possible mechanism for the antiinflammatory action of glucocorticoids. Mol Endocrinol. 1995;9:401–12. doi: 10.1210/mend.9.4.7659084. [DOI] [PubMed] [Google Scholar]
- Cher CD, et al. Pulmonary inflammation and edema induced by phospholipase A2: global gene analysis and effects on aquaporins and Na+/K+-ATPase. J Biol Chem. 2003;278:31352–60. doi: 10.1074/jbc.M302446200. [DOI] [PubMed] [Google Scholar]
- Chou SH, et al. Monitoring biomarkers of cellular injury and death in acute brain injury. Neurocrit Care. 2014;21(Suppl 2):S187–214. doi: 10.1007/s12028-014-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens JA, et al. Reactive glia express cytosolic phospholipase A2 after transient global forebrain ischemia in the rat. Stroke. 1996;27:527–35. doi: 10.1161/01.str.27.3.527. [DOI] [PubMed] [Google Scholar]
- Farias SE, et al. Transcellular biosynthesis of cysteinyl leukotrienes in rat neuronal and glial cells. J Neurochem. 2007;103:1310–8. doi: 10.1111/j.1471-4159.2007.04830.x. [DOI] [PubMed] [Google Scholar]
- Faure G, Saul F. Structural and Functional Characterization of Anticoagulant, FXa-binding Viperidae Snake Venom Phospholipases A2. Acta Chim Slov. 2011;58:671–7. [PubMed] [Google Scholar]
- Francis BR, et al. Toxins isolated from the venom of the Brazilian coral snake (Micrurus frontalis frontalis) include hemorrhagic type phospholipases A2 and postsynaptic neurotoxins. Toxicon. 1997;35:1193–203. doi: 10.1016/s0041-0101(97)00031-7. [DOI] [PubMed] [Google Scholar]
- Ghassemi A, Rosenberg P. Effects of snake venom phospholipase A2 toxins (beta-bungarotoxin, notexin) and enzymes (Naja naja atra, Naja nigricollis) on aminophospholipid asymmetry in rat cerebrocortical synaptosomes. Biochem Pharmacol. 1992;44:1073–83. doi: 10.1016/0006-2952(92)90370-x. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Basu A. Network medicine in drug design: implications for neuroinflammation. Drug Discov Today. 2012;17:600–7. doi: 10.1016/j.drudis.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Glaser KB, et al. Inactivation of phospholipase A2 by manoalide. Localization of the manoalide binding site on bee venom phospholipase A2. Biochem Pharmacol. 1988;37:3639–46. doi: 10.1016/0006-2952(88)90396-6. [DOI] [PubMed] [Google Scholar]
- Gonca E. The effects of zileuton and montelukast in reperfusion-induced arrhythmias in anesthetized rats. Curr Ther Res Clin Exp. 2013;75:27–32. doi: 10.1016/j.curtheres.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goppelt-Struebe M, et al. Glucocorticoids inhibit prostaglandin synthesis not only at the level of phospholipase A2 but also at the level of cyclo-oxygenase/PGE isomerase. Br J Pharmacol. 1989;98:1287–95. doi: 10.1111/j.1476-5381.1989.tb12676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange E, et al. Manoalide, a phospholipase A2 inhibitor, inhibits arachidonate incorporation and turnover in brain phospholipids of the awake rat. Neurochem Res. 1998;23:1251–7. doi: 10.1023/a:1020788031720. [DOI] [PubMed] [Google Scholar]
- Guo J, et al. Self-assembling peptide nanofiber scaffold promotes the reconstruction of acutely injured brain. Nanomedicine. 2009;5:345–51. doi: 10.1016/j.nano.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Hernandez V, et al. Effects of naturally occurring dihydroflavonols from Inula viscosa on inflammation and enzymes involved in the arachidonic acid metabolism. Life Sci. 2007;81:480–8. doi: 10.1016/j.lfs.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Hickey E, et al. Lipopolysaccharide-induced preconditioning against ischemic injury is associated with changes in toll-like receptor 4 expression in the rat developing brain. Pediatr Res. 2011;70:10–4. doi: 10.1203/PDR.0b013e31821d02aa. [DOI] [PubMed] [Google Scholar]
- Huang L, et al. Phosphoinositide 3-Kinase Gamma Contributes to Neuroinflammation in a Rat Model of Surgical Brain Injury. J Neurosci. 2015;35:10390–401. doi: 10.1523/JNEUROSCI.0546-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MZ, et al. Role of enzymatic activity in the antiplatelet effects of a phospholipase A2 from Ophiophagus hannah snake venom. Life Sci. 1997;61:2211–7. doi: 10.1016/s0024-3205(97)00923-5. [DOI] [PubMed] [Google Scholar]
- Huang TF, Chiang HS. Effect on human platelet aggregation of phospholipase A2 purified from Heloderma horridum (beaded lizard) venom. Biochim Biophys Acta. 1994;1211:61–8. doi: 10.1016/0005-2760(94)90139-2. [DOI] [PubMed] [Google Scholar]
- Jadhav V, et al. Cyclo-oxygenase-2 mediates hyperbaric oxygen preconditioning-induced neuroprotection in the mouse model of surgical brain injury. Stroke. 2009;40:3139–42. doi: 10.1161/STROKEAHA.109.549774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, et al. Signaling Pathways in the Activation of Mast Cells Cocultured with Astrocytes and Colocalization of Both Cells in Experimental Allergic Encephalomyelitis. Journal of Immunology. 2010;185:273–283. doi: 10.4049/jimmunol.1000991. [DOI] [PubMed] [Google Scholar]
- Kini RM, Evans HJ. Correlation between the enzymatic activity, anticoagulant and antiplatelet effects of phospholipase A2 isoenzymes from Naja nigricollis venom. Thromb Haemost. 1988;60:170–3. [PubMed] [Google Scholar]
- Lombardo D, Dennis EA. Cobra venom phospholipase A2 inhibition by manoalide. A novel type of phospholipase inhibitor. J Biol Chem. 1985;260:7234–40. [PubMed] [Google Scholar]
- MacLellan CL, et al. Delayed onset of prolonged hypothermia improves outcome after intracerebral hemorrhage in rats. Journal of Cerebral Blood Flow and Metabolism. 2004;24:432–440. doi: 10.1097/00004647-200404000-00008. [DOI] [PubMed] [Google Scholar]
- Maneshi MM, et al. A Threshold Shear Force for Calcium Influx in an Astrocyte Model of Traumatic Brain Injury. J Neurotrauma. 2015;32:1020–9. doi: 10.1089/neu.2014.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGirt MJ, et al. Lumbar Surgery in the Elderly Provides Significant Health Benefit in the US Health Care System: Patient-Reported Outcomes in 4370 Patients From the N2QOD Registry. Neurosurgery. 2015;77(Suppl 4):S125–35. doi: 10.1227/NEU.0000000000000952. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, et al. The Dorothy Russell Memorial Lecture. The molecular and cellular sequelae of experimental traumatic brain injury: pathogenetic mechanisms. Neuropathol Appl Neurobiol. 1998;24:251–67. doi: 10.1046/j.1365-2990.1998.00121.x. [DOI] [PubMed] [Google Scholar]
- Namas R, et al. The acute inflammatory response in trauma/hemorrhage and traumatic brain injury: current state and emerging prospects. Libyan J Med. 2009;4:97–103. doi: 10.4176/090325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrow LW, Sachs F. Mechanosensation and endothelin in astrocytes–hypothetical roles in CNS pathophysiology. Brain Res Brain Res Rev. 2005;48:488–508. doi: 10.1016/j.brainresrev.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Pan LN, et al. Toll-like receptor 3 agonist Poly I:C protects against simulated cerebral ischemia in vitro and in vivo. Acta Pharmacol Sin. 2012;33:1246–53. doi: 10.1038/aps.2012.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przyklenk K, Kloner RA. Ischemic preconditioning: exploring the paradox. Prog Cardiovasc Dis. 1998;40:517–47. doi: 10.1016/s0033-0620(98)80002-9. [DOI] [PubMed] [Google Scholar]
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med. 2005;353:1711–23. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- Rosenberg P, et al. Dissociation of pharmacological and enzymatic activities of snake venom phospholipases A2 by modification of carboxylate groups. Biochem Pharmacol. 1983;32:3525–30. doi: 10.1016/0006-2952(83)90298-8. [DOI] [PubMed] [Google Scholar]
- Rugstad HE. Antiinflammatory and immunoregulatory effects of glucocorticoids: mode of action. Scand J Rheumatol Suppl. 1988;76:257–64. doi: 10.3109/03009748809102977. [DOI] [PubMed] [Google Scholar]
- Saiwai H, et al. The LTB4-BLT1 axis mediates neutrophil infiltration and secondary injury in experimental spinal cord injury. Am J Pathol. 2010;176:2352–66. doi: 10.2353/ajpath.2010.090839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmann MU, et al. Temporal profiles of cerebrospinal fluid leukotrienes, brain edema and inflammatory response following experimental brain injury. Neurol Res. 2003;25:481–91. doi: 10.1179/016164103101201896. [DOI] [PubMed] [Google Scholar]
- Streit WJ, et al. Reactive microgliosis. Prog Neurobiol. 1999;57:563–81. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Tan CH, et al. Venom-gland transcriptome and venom proteome of the Malaysian king cobra (Ophiophagus hannah) BMC Genomics. 2015;16:687. doi: 10.1186/s12864-015-1828-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan NH, Arunmozhiarasi A. The anticoagulant activity of Malayan cobra (Naja naja sputatrix) venom and venom phospholipase A2 enzymes. Biochem Int. 1989a;19:803–10. [PubMed] [Google Scholar]
- Tan NH, Arunmozhiarasi A. Isolation and characterization of an acidic lethal phospholipase Az from Malayan cobra (Naja naja sputatrix) venom. Biochem Int. 1989b;18:785–92. [PubMed] [Google Scholar]
- Teng CM, et al. Effect of cobra venom phospholipase A2 on platelet aggregation in comparison with those produced by arachidonic acid and lysophophatidylcholine. Thromb Res. 1986;44:875–86. doi: 10.1016/0049-3848(86)90033-2. [DOI] [PubMed] [Google Scholar]
- Un KC, et al. Systemic progesterone for modulating electrocautery-induced secondary brain injury. Journal of Clinical Neuroscience. 2013;20:1329–1330. doi: 10.1016/j.jocn.2012.10.032. [DOI] [PubMed] [Google Scholar]
- Vadas P, et al. The proinflammatory effect of intra-articular injection of soluble human and venom phospholipase A2. Am J Pathol. 1989;134:807–11. [PMC free article] [PubMed] [Google Scholar]
- Wagener FA, et al. Heme induces the expression of adhesion molecules ICAM-1, VCAM-1, and E selectin in vascular endothelial cells. Proc Soc Exp Biol Med. 1997;216:456–63. doi: 10.3181/00379727-216-44197. [DOI] [PubMed] [Google Scholar]
- Wagener FA, D TG, et al. Heme is a potent inducer of inflammation in mice and is counteracted by heme oxygenase. Blood. 2001;98:1802–1811. doi: 10.1182/blood.v98.6.1802. [DOI] [PubMed] [Google Scholar]
- Wakai A, et al. Mannitol for acute traumatic brain injury. Cochrane Database Syst Rev. 2013:CD001049. doi: 10.1002/14651858.CD001049. [DOI] [PubMed] [Google Scholar]
- Wei JF, et al. Induction of mast cell accumulation, histamine release and skin edema by N49 phospholipase A2. BMC Immunol. 2009;10:21. doi: 10.1186/1471-2172-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie CF, et al. Anti-inflammatory Activity of Magnesium Isoglycyrrhizinate Through Inhibition of Phospholipase A2/Arachidonic Acid Pathway. Inflammation. 2015;38:1639–1648. doi: 10.1007/s10753-015-0140-2. [DOI] [PubMed] [Google Scholar]
- Xu FF, et al. Comparison between self-assembling peptide nanofiber scaffold (SAPNS) and fibrin sealant in neurosurgical hemostasis. Cts-Clinical and Translational Science. 2015;8:490–494. doi: 10.1111/cts.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, et al. Matrix metalloproteinase inhibition attenuates brain edema in an in vivo model of surgically-induced brain injury. Neurosurgery. 2007;61:1067–75. doi: 10.1227/01.neu.0000303203.07866.18. discussion 1075–6. [DOI] [PubMed] [Google Scholar]
- Yates SL, et al. Leukotriene and prostaglandin production in rat brain synaptosomes treated with phospholipase A2 neurotoxins and enzymes. Prostaglandins. 1990;39:425–38. doi: 10.1016/0090-6980(90)90123-d. [DOI] [PubMed] [Google Scholar]


