Abstract
Arylamine N-acetyltransferase 1 (NAT1) and 2 (NAT2) catalyze the acetylation of arylamine carcinogens. Single nucleotide polymorphisms in the NAT2 coding exon present in NAT2 haplotypes encode allozymes with reduced N-acetyltransferase activity towards the N-acetylation of arylamine carcinogens and the O-acetylation of their N-hydroxylated metabolites. NAT2 acetylator phenotype modifies urinary bladder cancer risk following exposures to arylamine carcinogens such as 4-aminobiphenyl. 4, 4’-methylene bis (2-chloroaniline) (MOCA) is a Group 1 carcinogen for which a role of the NAT2 acetylation polymorphism on cancer risk is unknown. We investigated the role of NAT2 and the genetic acetylation polymorphism on both MOCA N-acetylation and N-hydroxy-MOCA O-acetylation. MOCA N-acetylation exhibited a robust gene dose response in rabbit liver cytosol and in cryopreserved human hepatocytes derived from individuals of rapid, intermediate and slow acetylator NAT2 genotype. MOCA exhibited about 4-fold higher affinity for recombinant human NAT2 than NAT1. Recombinant human NAT2*4 (reference) and 15 variant recombinant human NAT2 allozymes catalyzed both the N-acetylation of MOCA and the O-acetylation of N-hydroxy-MOCA. Human NAT2 5, NAT2 6, NAT2 7 and NAT2 14 allozymes catalyzed MOCA N-acetylation and N-hydroxy-O-acetylation at rates much lower than the reference NAT2 4 allozyme. In conclusion, our results show that NAT2 acetylator genotype has an important role in MOCA metabolism and suggest that risk assessments related to MOCA exposures consider accounting for NAT2 acetylator phenotype in the analysis.
Keywords: 4, 4’-methylene bis (2-chloroaniline) (MOCA); N-acetyltransferase 2 (NAT2); urinary bladder cancer; arylamine carcinogens; N-acetylation; O-acetylation; acetylation polymorphism
Graphical Abstract
1.0 Introduction
As summarized in the National Toxicology Program’s (NTP) Report on Carcinogens (NTP, 2016), 4, 4’-methylene bis (2-chloroaniline) (abbreviated MOCA), also known as methylene-bis-ortho-chloroaniline (abbreviated MBOCA) is used extensively as a curing agent in the production of elastomers. MOCA is listed as a pollutant of concern by the United States Environmental Protection Agency (EPA) and is ranked among the top third of priority chemicals with most significant potential threat to human health due to their known or suspected toxicity and potential for human exposure..
MOCA recently was reclassified by the International Agency for Research on Cancer (IARC) to the Group 1 based on sufficient evidence of carcinogenicity in experimental animals. . In the dog, MOCA induces urinary bladder cancer (Stula et al., 1978). In humans, the occupational exposures to MOCA are associated with human urinary bladder cancer (Rubino et al., 1982; Ward et al., 1988; Chen et al., 2005; Liu et al., 2005; Dost et al., 2009).
Arylamine carcinogens such as 4-aminobiphenyl and benzidine undergo N-acetylation catalyzed by the arylamine N-acetyltransferases 1 (NAT1) and 2 (NAT2). MOCA undergoes N-acetylation in rabbit liver (Glowinski et al., 1978) and N-acetyl MOCA is present in urine of workers exposed to MOCA (Ducos et al., 1985; Cocker et al., 1988; Robert et al., 1995). Arylamine carcinogens are frequently substrates for N-hydroxylation, and N-hydroxy-MOCA has been identified as the predominant (80–90%) oxidized metabolite of MOCA following incubation with human liver microsomes (Morton et al., 1988; Butler et al., 1989). N-hydroxy-MOCA is produced primarily via oxidation by human CYP3A4 (Yun et al., 1992). In addition to N-acetylation, aromatic amines frequently undergo O-acetylation of their N-hydroxy metabolite which is also catalyzed by NAT1 and/or NAT2 (Hein et al., 2000). O-acetylation of the N-hydroxylated aromatic amines forms unstable N-acetoxy arylamines that can spontaneously liberate electrophilic arylnitrenium ions that bind DNA to initiate mutagenesis and carcinogenesis (Hein et al., 1992). MOCA is genotoxic (McQueen and Williams, 1990; IARC, 1993). DNA adducts are formed from reaction with N-hydroxy-MOCA and these DNA adducts have been identified in experimental tumor target organs of animals administered MOCA (DeBord et al., 1996) and in urothelial cells from a man occupationally exposed (Kaderlik et al., 1993).
Metabolites N-hydroxy-MOCA exhibits enhanced mutagenicity whereas N-acetyl-MOCA exhibits reduced mutagenicity in comparison to the parent compound MOCA (Reid et al., 1998; Hesbert et al, 1985). Inter-individual variability in N-hydroxylation and acetylation has been suggested as modifiers of MOCA cancer risks (ATSDR, 1994). As previously reviewed (Hein, 2009), humans possess two functional N-acetyltransferase isozymes identified as NAT1 and NAT2. The reference humanNAT1 4 and NAT2 4 enzymes are associated with high activity encoded by the NAT1*4 and NAT2*4 genes, respectively. Human NAT2 exhibits genetic polymorphisms leading to rapid, intermediate and slow acetylator phenotypes that result in differential efficacy and/or toxicity following administration of numerous arylamine drugs (Weber and Hein, 1985; McDonagh et al, 2014). NAT2*4 is associated with rapid acetylator phenotype whereas the most common human NAT2 haplotypes associated with slow acetylator phenotype are NAT2*5B, NAT2*6A, NAT2*7B and NAT2*14B (Hein, 2009).
Both NAT1 and NAT2 are expressed in human liver and catalyze the acetylation of arylamine carcinogens such as 4-aminobiphenyl and benzidine (Hein, 2009). A gene-dosage relationship between NAT2 genotype and phenotype is clearly demonstrated for both N-acetylation and O-acetylation of arylamine carcinogens in rabbit (Hein et al., 1982; Hein and Doll, 2017) and human liver (Doll et al., 2010). The reduced N-acetyltransferase activity in the N-acetylation of arylamine carcinogens and the O-acetylation of their N-hydroxylated metabolites can be attributed to single nucleotide polymorphisms in the NAT2 coding exon present in NAT2 haplotypes (Hein et al., 1994; 1995).
A recent study reported that human rapid and intermediate N-acetylators are less susceptible to oxidative damage among workers exposed to MOCA, but concluded that the impact of NAT2 acetylator status is low, if at all, on the generation of the oxidative stress marker 8-hydroxydeoxyguaosine in the investigated group exposed to MOCA (Lin et al., 2013). The purpose of the present study was to assess the role of NAT2 and the genetic acetylation polymorphism on MOCA metabolism, including both MOCA N-acetylation and N-hydroxy-MOCA O-acetylation.
2.0 Materials and methods
2.1 N-acetylation of MOCA in rabbit liver cytosols
Genomic DNA and snap-frozen liver samples from twelve adult New Zealand White rabbits were purchased and shipped from Pel-Freeze Biologicals (Rodgers, AR). Rabbit NAT2 contains a single open reading frame of 870 nucleotides encoding a 290 amino acid protein (Blum et al., 1990). The rabbit NAT2 and β-globin genes were amplified by polymerase chain reaction (PCR) as previously described (Hein and Doll, 2017).
Individual cytosols (100,000 × g) were prepared as previously described (Fretland et al., 2003) from livers of seven rapid and five slow acetylator rabbits identified by the NAT2 genotype method described above. MOCA N-acetyltransferase activities were measured using modifications of high performance liquid chromatography assays as previously described (Walraven et al., 2006). Suitably diluted liver cytosol, acetyl coenzyme A (1000 µM), and MOCA (100 µM) were incubated at 37°C for 10 min. The reaction was stopped by the addition of 1 M perchloric acid. Following centrifugation to precipitate protein, reaction supernatants were injected (40 µl) onto an EM Science 125 mm × 4 mm Lichrocart C18 (5 µm) column fitted with a similar Lichrocart guard column (4 mm × 4 mm). Reactants and products were eluted from the column with a 10 minute linear gradient (2 ml/min) from 100% sodium perchlorate (pH 2.5) to 100% acetonitrile. Under the conditions of this assay, N,N’-diacetyl-MOCA eluted at 15.9 min, N-acetyl-MOCA at 16.5 min, and MOCA at 17.2 min. Protein concentrations in the lysates were determined using the Bio-Rad protein assay (Bio-Rad, Richmond, CA).
2.2 N-acetylation of MOCA catalyzed by human recombinant NAT1 4 and NAT2 4
NAT1*4 and NAT2*4 were expressed recombinantly in Escherichia coli JM105 as previously described (Hein et al., 1994; 1995). Briefly, bacteria harboring the NAT1*4 or NAT2*4 plasmid were grown up overnight in Luria-Bertani medium containing 100 µg/ml ampicillin at 37°C. Fresh Luria-Bertani ampicillin broth was reinoculated, and NAT1 and NAT2- expressing bacteria were grown to approximately A600=0.5. Isopropyl-β-D-thiogalactopyranoside (1 mM) was added to the broth for induction and the cultures were grown for an additional 3 h. The cells were harvested by centrifugation at 5000×g for 10 min. Cell pellets were suspended in 20 volume of homogenization buffer (20 mM sodium phosphate, pH 7.4, containing EDTA (1 mM), DTT (1 mM), and protease inhibitors aprotinin (1 µg/ml), PMSF (100 µM), and pepstatin (0.75 µM). The suspension was lysed by sonication and the lysed suspension was then subjected to centrifugation at 15,000×g for 20 min at 4°C. Collected supernatants were aliquoted and stored at −80 °C until use. MOCA N-acetyltransferase assays were conducted with MOCA concentrations ranging from 3.9 to 1000 µM in the presence of 1000 µM acetyl coenzyme A.
2.3 N-acetylation of MOCA and O-acetylation of N-hydroxy-MOCA catalyzed by human recombinant NAT2 allozymes
NAT2*4 and variant NAT2 alleles were expressed recombinantly in Escherichia coli JM105 as previously described (Hein et al., 1994; 1995). Bacterial lysates were prepared as described above and tested for their capacity to catalyze MOCA N-acetylation and N-hydroxy-MOCA O-acetylation. MOCA N-acetylation assays were conducted as described above with MOCA and acetyl coenzyme A concentrations of 1000 µM. N-hydroxy-MOCA O-acetyltransferase activities were assessed by measurement of AcCoA-dependent metabolic activation of [ring-3H] N-hydroxy-MOCA to DNA adducts as previously described (Hein and Doll, 2017). [ring-3H] N-hydroxy-MOCA was synthesized as described (Butler et al., 1989) and was a kind gift from Dr. Fred Kadubar. The O-acetylation reactions contained 20 mM sodium phosphate buffer (pH 7.4), 1 mM DTT, 1 mM EDTA, calf thymus DNA (1 mg/ml), and bacterial lysate. The [ring-3H] N-hydroxy-MOCA and AcCoA concentrations were 100 µM and 2 mM, respectively. Since N-hydroxyarylamines have been shown to bind directly to DNA at neutral pH, reaction controls without AcCoA were subtracted from the experimental determinations. Assays were carried out for 20 min and terminated by adding 1 ml of ice-cold water-saturated n-butanol. The aqueous layer was extracted with phenol, and the DNA was precipitated and washed with 80% ethanol. Since previous studies have reported equivalent levels of NAT2 immunoreactive protein expression following recombinant expression of human NAT2 alleles in Escherichia coli JM105 (Hein et al., 1994; 1995), MOCA N- and O-acetylation activities were normalized to total lysate protein measured with the Bio-Rad protein assay kit (Bio-Rad, Richmond, CA).
2.4 N-acetylation of MOCA in cryopreserved human hepatocytes
MOCA N-acetyltransferase activities were determined in lysates obtained from male and female cryopreserved human hepatocytes previously identified as rapid, intermediate, or slow acetylators (Doll et al., 2017). Cytosols from each hepatocyte sample were prepared as previously described (Doll et al., 2010). MOCA N-acetyltransferase activities were determined as described above with MOCA concentrations of 300 µM and acetyl coenzyme A concentrations of 1000 µM. MOCA N- acetylation activities were normalized to total lysate protein measured with the Bio-Rad protein assay kit (Bio-Rad, Richmond, CA
3.0 Results
3.1 N-acetylation of MOCA in rabbit liver cytosols
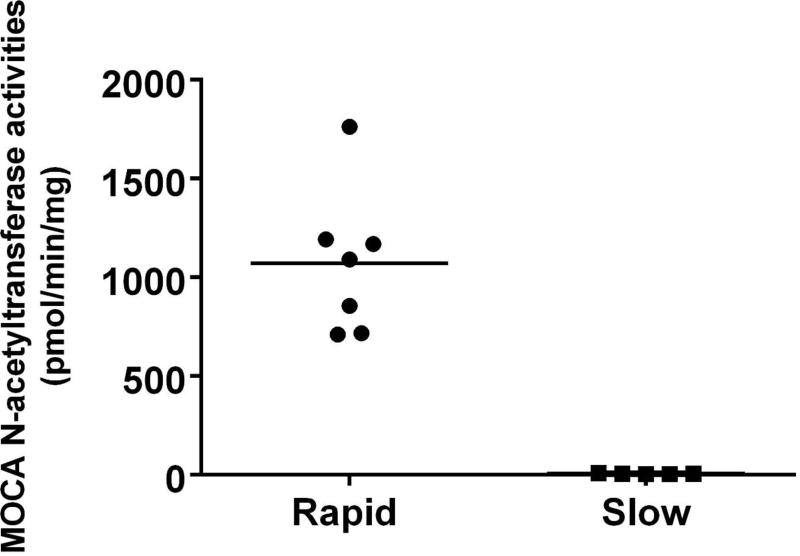
MOCA N-acetyltransferase activities were determined in liver cytosols obtained from rabbits previously identified as rapid and slow acetylators due to presence or absence of NAT2 (Hein and Doll, 2017). MOCA N-acetyltransferase activities ranged from 710 to 1763 pmoles/min/mg in the rapid acetylator liver cytosols whereas they ranged from 3.8 to 9.1 pmoles/min/mg in the slow acetylator liver cytosols (Figure 1). The MOCA N-acetylation activities were significantly (p=0.0361) higher in the rapid than slow acetylator rabbit liver cytosols.
Figure 1.
N-acetylation of MOCA in rabbit liver cytosols. MOCA N-acetyltransferase activities were determined in liver cytosols obtained from rabbits previously identified as rapid (circles) and slow (squares) acetylators due to presence or absence of NAT2 (Hein and Doll, 2017). Mean N-acetyltransferase activities are shown by the horizontal line. MOCA N-acetylation activities were significantly (p=0.0361) higher in the rapid than the slow acetylator rabbit liver cytosols.
3.2 N-acetylation of MOCA catalyzed by human recombinant NAT1 4 and NAT2 4
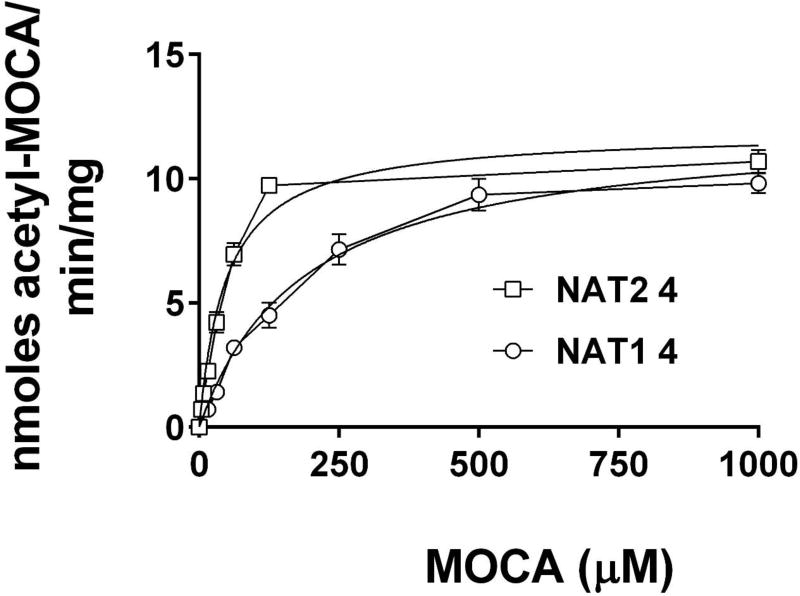
MOCA N-acetylation was concentration-dependent when catalyzed by both recombinant human NAT1 4 and NAT2 4 (Figure 2). The apparent Km for human recombinant NAT1 4 and NAT2 4 was 189 ± 25 and 48.4 ± 5.6 µM respectively in the presence of 1000 µM acetyl coenzyme A.
Figure 2.
N-acetylation of MOCA catalyzed by human recombinant NAT1 4 and NAT2 4. MOCA N-acetylation was concentration-dependent when catalyzed by both recombinant human NAT1 4 (circles) and NAT2 4 (squares). Each data point represents Mean ± SEM for three determinations. Michaelis Menten kinetics constants were calculated by non-linear regression and the best fit lines are illustrated. The apparent Km for recombinant NAT1 4 and NAT2 4 was 189 ± 25 and 48.4 ± 5.6 µM respectively in the presence of 1000 µM acetyl coenzyme A.
3.3 N-acetylation of MOCA and O-acetylation of N-hydroxy-MOCA catalyzed by human recombinant NAT2 allozymes
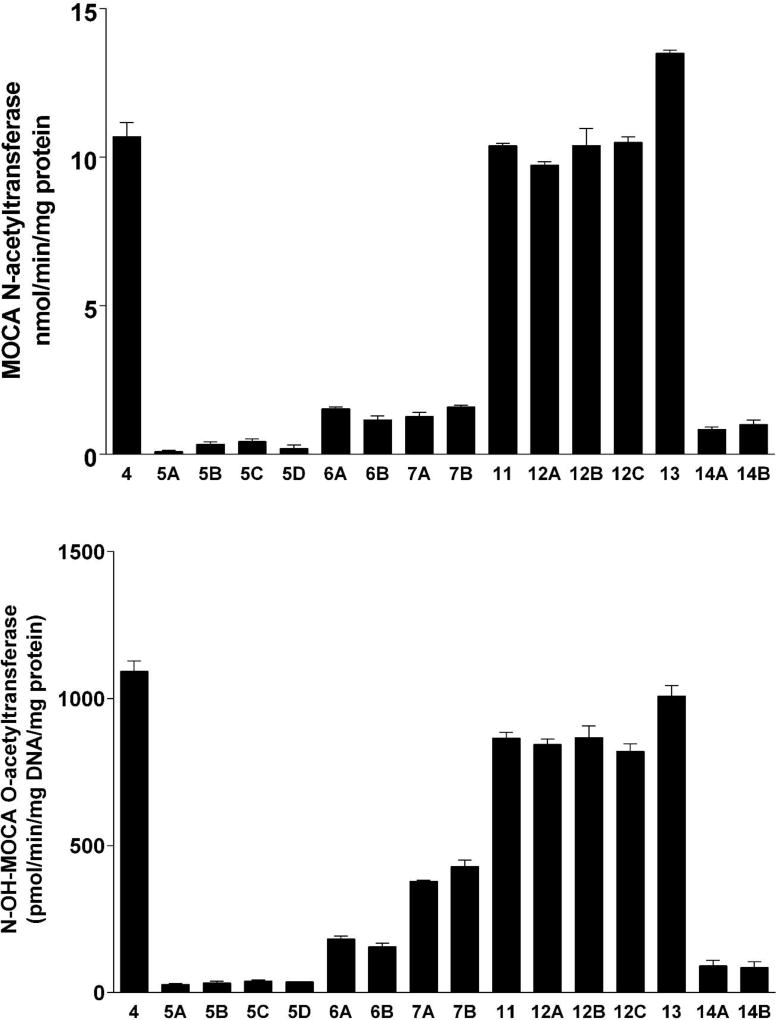
Human NAT2 4 and 15 variant NAT2 allozymes were tested for the ability to catalyze both the N-acetylation of MOCA and the O-acetylation of N-hydroxy-MOCA. Human NAT2 5, NAT2 6, NAT2 7 and NAT2 14 allozymes catalyzed MOCA N-acetylation at rates much lower than the reference NAT2 4 or the NAT2 11, NAT2 12, NAT2 13 allozymes (Figure 3).
Figure 3.
N-acetylation of MOCA (top) and O-acetylation of N-hydroxy-MOCA (bottom) catalyzed by human recombinant NAT2 allozymes. Each bar represents Mean ± SEM for three to five determinations. MOCA N-acetyltransferase activities differed significantly across the allozymes following one way analysis of variance (p<0.0001). NAT2 allozymes 5A, 5B, 5C, 5D, 6A, 6B, 7A, 7B, 14A, and 14B catalyzed MOCA N-acetylation at rates much lower than NAT2 4 reference. N-hydroxy-MOCA O-acetyltransferase activities differed significantly across the allozymes following one way analysis of variance (p<0.0001). NAT2 allozymes 5A, 5B, 5C, 5D, 6A, 6B, 7A, 7B, 13, 14A, and 14B catalyzed N-hydroxy- MOCA O-acetylation at rates much lower than NAT2 4 reference. Rates of MOCA N-acetylation and N-hydroxy-MOCA O-acetylation were highly correlated following linear regression. (r2) = 0.931; p<0.0001.
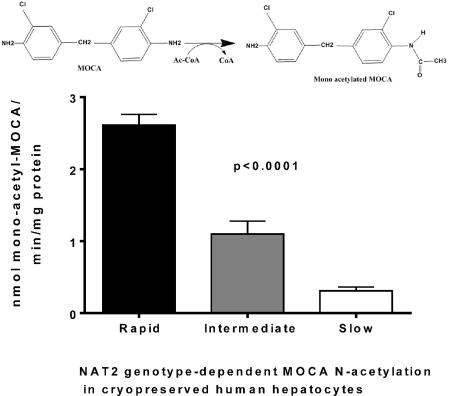
3.4 N-acetylation of MOCA in cryopreserved human hepatocytes
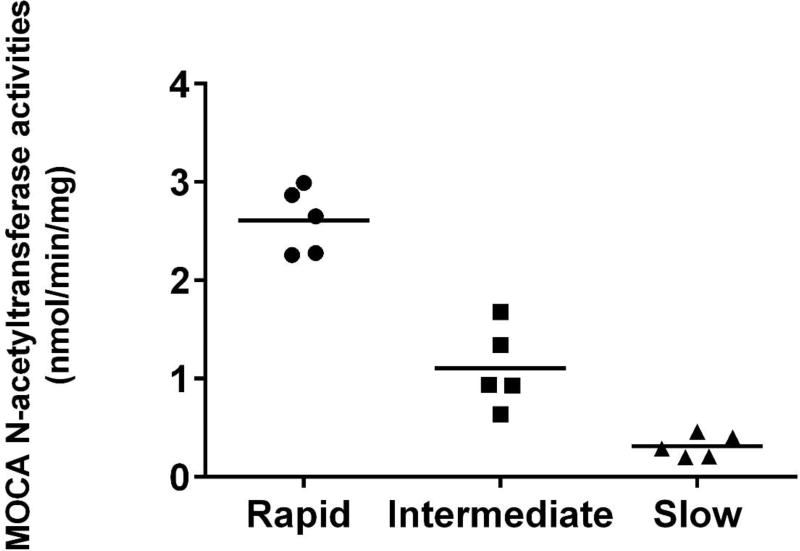
MOCA N-acetyltransferase activities were determined in lysates obtained from cryopreserved human hepatocytes previously identified as rapid, intermediate, or slow acetylators (Doll et al., 2017). A robust and highly significant (p<0.0001) gene dose-response relationship was observed for MOCA N-acetylation, with highest levels in rapid NAT2 acetylators, lower levels in intermediate NAT2 acetylators, and lowest levels in slow NAT2 acetylators (Figure 4). Differences in MOCA N-acetyltransferase activities between males and females within each acetylator phenotype were analyzed for significance by unpaired t test. The activities did not differ significantly between males and females among rapid (p=0.348) or slow (p=0.416) acetylator cryopreserved human hepatocytes. MOCA N-acetyltransferase activities in male and female intermediate acetylator cryopreserved human hepatocytes also were highly similar (only one male sample precluded statistical analysis of the difference).
Figure 4.
N-acetylation of MOCA in cryopreserved human hepatocytes. MOCA N-acetyltransferase activities were determined in lysates obtained from cryopreserved human hepatocytes previously identified as rapid, intermediate, or slow acetylators (Doll et al., 2017). A robust and highly significant gene dose-response relationship was observed for MOCA N-acetylation, with highest levels in rapid NAT2 acetylators (circles), lower levels in intermediate NAT2 acetylators (squares) and lowest levels in slow NAT2 acetylators (triangles). Mean N-acetyltransferase activities are shown by the horizontal line which differed significantly following one way analysis of variance (p<0.0001) and subsequent Tukey’s multiple comparisons test (p<0.005). The NAT2 genotypes of the rapid acetylators were NAT2*4/*4 (two male and two female samples) and NAT2*4/*13 (one female sample). The NAT2 genotypes of the intermediate acetylators were NAT2*4/*5B (one male and two female samples), NAT2*4/*5A (one female sample) and NAT2*4/*6A (one female sample). The NAT2 genotypes of the slow acetylators were NAT2*5B/*5B (one male and one female samples), NAT2*5B/*6A (one female sample), NAT2*5A/*6A (one male sample) and NAT2*6A/*6A (one male sample).
4.0 Discussion
MOCA is N-acetylated by both recombinant rat NAT1 and NAT2, but to a much greater extent by rat NAT1 than by rat NAT2 (Walraven et al., 2006). Since the substrate specificity of rat NAT1 and NAT2 mirrors human NAT2 and NAT1, it was not unexpected that human NAT2 would exhibit a higher affinity for MOCA than human NAT1 (apparent Km of 48 versus 189 µM). The N-acetylation of MOCA in human liver cytosol which expresses both human NAT1 and NAT2 reported an apparent Km for MOCA of 65 µM (Glowinski et al., 1978). These findings suggest that MOCA N-acetylation is selectively but not specifically catalyzed by human NAT2. MOCA N-acetylation will thus be impacted by the relative expression of human NAT1 and NAT2 in various organs.
Unlike rat NAT2, rabbit NAT2 has similar substrate selectively with human NAT2 (Weber and Hein, 1985). Thus, the rabbit model of the N-acetylation polymorphism was considered the more appropriate animal model to investigate the role of NAT2 genotype on MOCA metabolism. To our knowledge, MOCA metabolism in the rabbit has yet to be investigated or reported, but our findings clearly show NAT2 genotype-dependent MOCA N-acetylation as has previously been shown for N-acetylation of other arylamine carcinogens such as 2-aminofluorene (Hein et al., 1982), 4-aminobiphenyl and benzidine (Hein and Doll, 2017).
The reference recombinant human NAT2 4 and 15 variant NAT2 allozymes each catalyzed the N-acetylation of MOCA and the O-acetylation of N-hydroxy-MOCA but at substantially reduced rates for NAT2 allozymes of the NAT2*5, NAT2*6, NAT2*7 and NAT2*14 clusters. This result is markedly similar to previous findings for the N-acetylation of 2-aminofluorene (Hein et al., 1994; 1995) and the O-acetylation of N-hydroxy-2-aminofluorene and N-hydroxy-4-aminobiphenyl (Hein et al., 1995) and are consistent with associations of the NAT2*5, NAT2*6 NAT2*7 and NAT2*14 haplotypes with slow acetylator phenotype (Hein and Doll, 2012).
Cryopreserved human hepatocytes have been used for in vitro investigations into the metabolism of arylamine carcinogens such as 4-aminobiphenyl and benzidine including investigations into the role of NAT2 genotype (Hein and Doll, 2012; 2017; Doll et al., 2010; 2017; Doll and Hein, 2017). Our findings clearly showed that MOCA N-acetylation was NAT2 genotype dependent in both rabbit and human liver. These findings are consistent with the greater affinity of MOCA for human NAT2 than NAT1, and the large differences in N-acetylation of MOCA and the O-acetylation of N-hydroxy-MOCA between NAT2 allozymes associated with rapid acetylator versus slow acetylator phenotype. Previous studies have reported the absence of gender differences in aromatic amine activation human hepatocytes (Williams et al., 2016). Significant gender differences in MOCA N-acetylation were not observed in the cryopreserved human hepatocytes used in our study.
Enhanced risk for urinary bladder cancer among slow acetylators occupationally exposed to arylamine carcinogens was proposed by Cartwright et al., (1982) and has been the subject of subsequent reviews (Hein, 2006; Rothman et al., 2007). A more recent study of MOCA-exposed workers reported that rapid and intermediate N-acetylators are less susceptible to oxidative damage from MOCA, but concluded that the impact of NAT2 acetylator status is low, if at all, on the generation of the oxidative stress marker 8-hydroxydeoxyguaosine in the investigated group exposed to MOCA (Lin et al., 2013). One explanation for this finding could be primary or at least substantial acetylation by NAT1 versus NAT2. Based on the results of the present study, this would appear to be unlikely in tissues such as the liver where both NAT1 and NAT2 are expressed. N-acetylation in the skin is catalyzed by human NAT1 (Lichter et al., 2017) which may suggest predominantly dermal exposures to MOCA in the previous group (Lin et al., 2013). Toxicokinetic investigations of MOCA and N-acetyl-MOCA levels stratified by NAT2 genotype would provide a more direct measure into the role of NAT2 genotype on MOCA metabolism. Alternatively, 8-hydroxydeoxyguanosine plasma levels may not be an appropriate biomarker for MOCA genotoxicity particularly since significant differences in plasma 8-hydroxydeoxyguanosine levels were not observed between MOCA exposed and non-exposed groups (Chen et al., 2007). Earlier studies which measured MOCA and its urinary metabolites in exposed workers reported that N-acetylation of MOCA to N-acetyl-MOCA was an important deactivation pathway in humans, that the urinary ratio of N-acetyl-MOCA to MOCA varied from 0 to 100%, and that the di-acetylated MOCA metabolite was less than 3% of the total MOCA metabolites (Robert et al., 1995). Our studies are quite consistent with the previous findings (Robert et al., 1995) since we found that N-acetyl-MOCA levels in human liver were NAT2 genotype-dependent and N,N-diacetyl- MOCA was not detected.
In conclusion, our results suggest that NAT2 acetylator phenotype has an important role in MOCA metabolism, and by implication its carcinogenicity further suggests that risk assessments related to MOCA exposures consider accounting for NAT2 acetylator phenotype.
Highlights.
A role of the NAT2 acetylation polymorphism in MOCA cancer risk is unknown.
MOCA exhibited about 4-fold higher affinity for recombinant human NAT2 than NAT1.
MOCA N-acetylation exhibited NAT2 gene dose response in rabbit and human liver.
Slow acetylator NAT2 allozymes exhibited reduced MOCA N-and O-acetylation.
Risk assessments related to MOCA exposures should include NAT2 acetylator phenotype.
Acknowledgments
This work was partially supported by USPHS grants P20GM113226 and P42ES023716. We thank Timothy Moeller and Bioreclamation IVT (Baltimore, MD) for their valuable contributions.
Abbreviations
- MOCA
4, 4’-methylene bis (2-chloroaniline)
- MBOCA
methylene-bis-ortho-chloroaniline
- NAT1
N-acetyltransferase 1
- NAT2
N-acetyltransferase 2
- NTP
United States National Toxicology Program
- EPA
United States Environmental Protection Agency
- ATSDR
United States Agency for Toxic Substances and Disease Registry
- IARC
International Agency for Research on Cancer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blum M, Heim M, Meyer UA. Nucleotide sequence of rabbit NAT2 encoding polymorphic liver arylamine N-acetyltransferase (NAT) Nucleic Acids Res. 1990;18:5295. doi: 10.1093/nar/18.17.5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MA, Guengerich FP, Kadlubar FF. Metabolic oxidation of the carcinogens 4-aminobiphenyl and 4,4'-methylene-bis(2-chloroaniline) by human hepatic microsomes and by purified rat hepatic cytochrome P-450 monooxygenases. Cancer Res. 1989;49:25–31. [PubMed] [Google Scholar]
- Cartwright RA, Glashan RW, Rogers HJ, Ahmad RA, Barham-Hall D, Higgins E, Kahn MA. Role of N-acetyltransferase phenotypes in bladder carcinogenesis: a pharmacogenetic epidemiological approach to bladder cancer. Lancet. 1982;2:842–845. doi: 10.1016/s0140-6736(82)90810-8. [DOI] [PubMed] [Google Scholar]
- Chen HI, Liou SH, Ho SF, Wu KY, Sun CW, Chen MF, Cheng LC, Shih TS, Loh CH. Oxidative DNA damage estimated by plasma 8-hydroxydeoxyguanosine (8-OHdG): influence of 4, 4'-methylenebis (2-chloroaniline) exposure and smoking. J Occup Health. 2007;49:389–398. doi: 10.1539/joh.49.389. [DOI] [PubMed] [Google Scholar]
- Chen HI, Liou SH, Loh CH, Uang SN, Yu YC, Shih TS. Bladder cancer screening and monitoring of 4,4'-methylenebis(2-chloroaniline) exposure among workers in Taiwan. Urology. 2005;66:305–310. doi: 10.1016/j.urology.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Cocker J, Boobis AR, Davies DS. Determination of the N-acetyl metabolites of 4,4'-methylene dianiline and 4,4'-methylene-bis(2-chloroaniline) in urine. Biomedical & environmental mass spectrometry. 1988;17:161–167. doi: 10.1002/bms.1200170303. [DOI] [PubMed] [Google Scholar]
- DeBord DG, Cheever KL, Werren DM, Reid TM, Swearengin TF, Savage RE., Jr Determination of 4,4'-methylene-bis(2-chloroaniline)-DNA adduct formation in rat liver and human uroepithelial cells by the 32P postlabeling assay. Fundamental and applied toxicology. 1996;30:138–144. doi: 10.1006/faat.1996.0050. [DOI] [PubMed] [Google Scholar]
- Doll MA, Salazar-Gonzalez RA, Bodduluri S, Hein DW. Arylamine N-acetyltransferase 2 genotype-dependent N-acetylation of isoniazid in cryopreserved human hepatocytes. Acta pharmaceutica Sinica. B. 2017;7:517–522. doi: 10.1016/j.apsb.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll MA, Zang Y, Moeller T, Hein DW. Codominant expression of N-acetylation and O-acetylation activities catalyzed by N-acetyltransferase 2 in human hepatocytes. J Pharmacol Exp Ther. 2010;334:540–544. doi: 10.1124/jpet.110.168567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dost A, Straughan JK, Sorahan T. Cancer incidence and exposure to 4,4'-methylene-bis-ortho-chloroaniline (MbOCA) Occupational medicine (Oxford, England) 2009;59:402–405. doi: 10.1093/occmed/kqp093. [DOI] [PubMed] [Google Scholar]
- Ducos P, Maire C, Gaudin R. Assessment of occupational exposure to 4,4'-methylene-bis-(2-chloroaniline) "MOCA" by a new sensitive method for biological monitoring. Int Arch Occup Environ Health. 1985;55:159–167. doi: 10.1007/BF00378378. [DOI] [PubMed] [Google Scholar]
- Fretland AJ, Devanaboyina US, Doll MA, Zhao S, Xiao GH, Hein DW. Metabolic activation of 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine in Syrian hamsters congenic at the N-acetyltransferase 2 (NAT2) locus. Toxicol Sci. 2003;74:253–259. doi: 10.1093/toxsci/kfg133. [DOI] [PubMed] [Google Scholar]
- Glowinski IB, Radtke HE, Weber WW. Genetic variation in N-acetylation of carcinogenic arylamines by human and rabbit liver. Mol Pharmacol. 1978;14:940–949. [PubMed] [Google Scholar]
- Hein DW. N-acetyltransferase 2 genetic polymorphism: effects of carcinogen and haplotype on urinary bladder cancer risk. Oncogene. 2006;25:1649–1658. doi: 10.1038/sj.onc.1209374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein DW. N-acetyltransferase SNPs: emerging concepts serve as a paradigm for understanding complexities of personalized medicine. Expert Opin Drug Metab Toxicol. 2009;5:353–366. doi: 10.1517/17425250902877698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein DW, Doll MA. Accuracy of various human NAT2 SNP genotyping panels to infer rapid, intermediate and slow acetylator phenotypes. Pharmacogenomics. 2012;13:31–41. doi: 10.2217/pgs.11.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein DW, Doll MA. Rabbit N-acetyltransferase 2 genotyping method to investigate role of acetylation polymorphism on N- and O-acetylation of aromatic and heterocyclic amine carcinogens. Arch Toxicol. 2017;91:3185–3188. doi: 10.1007/s00204-017-1997-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein DW, Doll MA, Fretland AJ, Leff MA, Webb SJ, Xiao GH, Devanaboyina US, Nangju NA, Feng Y. Molecular genetics and epidemiology of the NAT1 and NAT2 acetylation polymorphisms. Cancer Epidemiol Biomarkers Prev. 2000;9:29–42. [PubMed] [Google Scholar]
- Hein DW, Doll MA, Rustan TD, Ferguson RJ. Metabolic activation of N-hydroxyarylamines and N-hydroxyarylamides by 16 recombinant human NAT2 allozymes: effects of 7 specific NAT2 nucleic acid substitutions. Cancer Res. 1995;55:3531–3536. [PubMed] [Google Scholar]
- Hein DW, Ferguson RJ, Doll MA, Rustan TD, Gray K. Molecular genetics of human polymorphic N-acetyltransferase: enzymatic analysis of 15 recombinant wild-type, mutant, and chimeric NAT2 allozymes. Hum Mol Genet. 1994;3:729–734. doi: 10.1093/hmg/3.5.729. [DOI] [PubMed] [Google Scholar]
- Hein DW, Rustan TD, Doll MA, Bucher KD, Ferguson RJ, Feng Y, Furman EJ, Gray K. Acetyltransferases and susceptibility to chemicals. Toxicol Lett. 1992;64–65:123–130. doi: 10.1016/0378-4274(92)90181-i. [DOI] [PubMed] [Google Scholar]
- Hein DW, Smolen TN, Fox RR, Weber WW. Identification of genetically homozygous rapid and slow acetylators of drugs and environmental carcinogens among established inbred rabbit strains. J Pharmacol Exp Ther. 1982;223:40–44. [PubMed] [Google Scholar]
- Hesbert A, Bottin MC, De Ceaurriz J. Mutagenicity of 4,4'-methylene-bis-(2-chloroaniline) "MOCA" and its N-acetyl derivatives in S. typhimurium. Int Arch Occup Environ Health. 1985;55:169–174. doi: 10.1007/BF00378379. [DOI] [PubMed] [Google Scholar]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans. Vol. 57. Lyon, France: International Agency for Research on Cancer; 1993. 4,4′-Methylene bis(2-chloroaniline) (MOCA) pp. 271–303. [Google Scholar]
- Kaderlik KR, Talaska G, DeBord DG, Osorio AM, Kadlubar FF. 4,4'-Methylene-bis(2-chloroaniline)-DNA adduct analysis in human exfoliated urothelial cells by 32P-postlabeling. Cancer Epidemiol Biomarkers Prev. 1993;2:63–69. [PubMed] [Google Scholar]
- Lichter J, Bock U, Lotz C, Groeber F, Blömeke B. Functional expression of N-acetyltransferase 1 in differentiated human skin keratinocytes. Br J Dermatol. 2017 doi: 10.1111/bjd.15091. [DOI] [PubMed] [Google Scholar]
- Lin IS, Fan PL, Chen HI, Loh CH, Shih TS, Liou SH. Rapid and intermediate N-acetylators are less susceptible to oxidative damage among 4,4'-methylenebis(2-chloroaniline) (MBOCA)-exposed workers. Int J Hyg Environ Health. 2013;216:515–520. doi: 10.1016/j.ijheh.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Liu CS, Liou SH, Loh CH, Yu YC, Uang SN, Shih TS, Chen HI. Occupational bladder cancer in a 4,4 -methylenebis(2-chloroaniline) (MBOCA)-exposed worker. Environ Health Perspect. 2005;113:771–774. doi: 10.1289/ehp.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonagh EM, Boukouvala S, Aklillu E, Hein DW, Altman RB, Klein TE. PharmGKB summary: very important pharmacogene information for N-acetyltransferase 2. Pharmacogenet Genomics. 2014;24:409–425. doi: 10.1097/FPC.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen CA, Williams GM. Review of the genotoxicity and carcinogenicity of 4,4'-methylene-dianiline and 4,4'-methylene-bis-2-chloroaniline. Mutat Res. 1990;239:133–142. doi: 10.1016/0165-1110(90)90034-9. [DOI] [PubMed] [Google Scholar]
- Morton KC, Lee MS, Siedlik P, Chapman R. Metabolism of 4,4'-methylene-bis-2-chloroaniline (MOCA) by rats in vivo and formation of N-hydroxy MOCA by rat and human liver microsomes. Carcinogenesis. 1988;9:731–739. doi: 10.1093/carcin/9.5.731. [DOI] [PubMed] [Google Scholar]
- NTP (National Toxicology Program) Report on Carcinogens. Fourteenth. Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service; 2016. http://ntp.niehs.nih.gov/go/roc14/National Toxicology Program, Report on Carcinogens, 2014. [Google Scholar]
- Reid TM, DeBord DG, Cheever KL, Savage RE., Jr Mutagenicity of N-OH-MOCA (4-amino-4'-hydroxylamino-bis-3,3'-dichlorodiphenylmethane) and PBQ (2-phenyl-1,4-benzoquinone) in human lymphoblastoid cells. Toxicol Lett. 1998;95:205–210. doi: 10.1016/s0378-4274(98)00039-3. [DOI] [PubMed] [Google Scholar]
- Robert A, Ducos P, Francin JM. Determination of urinary 4,4'-methylenedianiline and its acetylated metabolites by solid-phase extraction and HPLC analysis with UV and electrochemical detection. Int Arch Occup Environ Health. 1995;68:44–51. doi: 10.1007/BF01831632. [DOI] [PubMed] [Google Scholar]
- Rothman N, Garcia-Closas M, Hein DW. Commentary: Reflections on G. M. Lower and colleagues' 1979 study associating slow acetylator phenotype with urinary bladder cancer: meta-analysis, historical refinements of the hypothesis, and lessons learned. Int J Epidemiol. 2007;36:23–28. doi: 10.1093/ije/dym026. [DOI] [PubMed] [Google Scholar]
- Rubino GF, Scansetti G, Piolatto G, Pira E. The carcinogenic effect of aromatic amines: an epidemiological study on the role of o-toluidine and 4,4'-methylene bis (2-methylaniline) in inducing bladder cancer in man. Environmental research. 1982;27:241–254. doi: 10.1016/0013-9351(82)90079-2. [DOI] [PubMed] [Google Scholar]
- Stula EF, Barnes JR, Sherman H, Reinhardt CF, Zapp JA., Jr Urinary bladder tumors in dogs from 4,4'-methylene-bis (2-chloroaniline) (MOCA) Journal of environmental pathology and toxicology. 1978;1:31–50. [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for 4,4-Methylenebis(2-Chloroaniline) (MBOCA) Atlanta, GA: 1994. [PubMed] [Google Scholar]
- Walraven JM, Doll MA, Hein DW. Identification and characterization of functional rat arylamine N-acetyltransferase 3: Comparisons with rat arylamine N-acetyltransferases 1 and 2. Journal of Pharmacology and Experimental Therapeutics. 2006;319:369–375. doi: 10.1124/jpet.106.108399. [DOI] [PubMed] [Google Scholar]
- Ward E, Halperin W, Thun M, Grossman HB, Fink B, Koss L, Osorio AM, Schulte P. Bladder tumors in two young males occupationally exposed to MBOCA. American journal of industrial medicine. 1988;14:267–272. doi: 10.1002/ajim.4700140304. [DOI] [PubMed] [Google Scholar]
- Weber WW, Hein DW. N-acetylation pharmacogenetics. Pharmacol Rev. 1985;37:25–79. [PubMed] [Google Scholar]
- Williams GM, Duan JD, Iatropoulos MJ, Perrone CE. Sex differences in DNA damage produced by the carcinogen 2-acetylaminofluorene in cultured human hepatocytes compared to rat liver and cultured rat hepatocytes. Arch Toxicol. 2016;90:427–432. doi: 10.1007/s00204-014-1415-3. [DOI] [PubMed] [Google Scholar]
- Yun CH, Shimada T, Guengerich FP. Contributions of human liver cytochrome P450 enzymes to the N-oxidation of 4,4'-methylene-bis(2-chloroaniline) Carcinogenesis. 1992;13:217– 222. doi: 10.1093/carcin/13.2.217. [DOI] [PubMed] [Google Scholar]