Abstract
With about 70 million cases of infertility worldwide, half of which are caused by male factors, sperm analysis is critical to determine male fertility potential. Conventional semen analysis methods involve complex and manual inspection with a microscope, these methods are labor intensive and can take several days. Due to unavailability of rapid, convenient, and user-friendly semen analysis tools, many men do not seek medical evaluation, especially in resource-constrained settings. Furthermore, since conventional methods have to be conducted in the labs, many men are unwilling to be tested as a result of social stigma in certain regions of the world. One solution can be found in at-home sperm analysis, which allows men to test their semen without the hassle of going to and paying for a clinic. Herein, we examine current at-home sperm analysis technologies and compare them to the traditional lab-based methods. In addition, we discuss emerging sperm analysis approaches and describe their limitations and future directions.
Keywords: Home-based sperm analysis, at-home sperm analysis, male fertility, sperm morphology, sperm motility
Introduction
Around 40-50% of the 70 million cases of infertility worldwide are caused by male factors [1-7]. Male infertility is caused by abnormal characteristics in several parameters, including sperm motility, morphology, velocity, semen volume, sperm concentration, and sperm count [8-17]. To determine male fertility potential, sperm analysis of these main parameters is necessary. Each of these parameters can be assessed through standard sperm analysis methods using microscopes and counting chambers. Motility is scored by evaluating each individual sperm in a given sample, counting the numbers of progressive, non-progressive, and immotile sperm, and comparing the values to find an average percentage of motility. Morphology is assessed by visual analysis through microscopy. Sperm are counted, numbered, and then assessed based on head shape, mid piece shape, and tail (principle piece) [18]. The velocity of progressive sperm is determined by measuring the speed in μm per second. Semen volume is largely measured by calculating the weight of the semen, assuming the density of 1 g/ml. It can also be quantified using direct measurement with a marked vessel, though transfer between different vessels is not recommended due to volume loss. Sperm concentration is determined by counting the number of sperm per aliquot of sample. Dilutions may need to be made in order to ensure that there are 200 sperm cells per replicated aliquot. A given volume can then be used in calculations to determine the concentration. Finally, sperm count is calculated by multiplying the sperm concentration by semen volume [18].
Since these conventional sperm analysis methods involve complex, manual inspection with a microscope, they are labor intensive and can take several days. Additionally, the results of these methods are subjective and prone to human error [8, 19]. Other methods, such as computer-assisted semen analysis (CASA), which uses algorithms to automatically track sperm, are also effective and are able to present qualitative information on sperm motility. However, CASA-based methods still have to use large, expensive, and high maintenance equipment, which hinders widespread use [20]. Both traditional methods and CASA are also limited by small field of view, which prevents large numbers of sperm being analyzed at the same time [21, 22]. Furthermore, since both methods have to be conducted in the labs, many men are unwilling to be tested as a result of social stigma in certain regions of the world [8]. Conversely, at-home analysis of male fertility is a cost effective, private and rapid solution to male fertility based inquiries, making it beneficial to men who are hesitant to seek medical evaluation. Most at-home systems will provide rudimentary analysis of a sample, giving the person an idea of whether or not to pursue further testing. In addition to men wanting to assess their fertility potential, vasectomy patients who want to confirm the success of their procedure and test for the presence or absence of sperm cells can also find at-home tests useful. Herein, we review current methods of home-based sperm analysis and compare these methods to WHO standards of semen analysis to determine which device provides the most accurate and complete analysis. We discuss the limitations of home-based sperm analysis devices and future directions are highlighted.
Standard Semen Analysis
The World Health Organization (WHO) has set standards for Sperm Analysis in WHO Laboratory Manual [18]. According to WHO, the motility of sperm cells is categorized into three types of movement, progressive motility (PR), non-progressive motility (NP), and immotility (IM). Progressive motility is defined by active motion in a large circular pattern or in a forward linear pattern and is not dependent on speed, while non-progressive motility is defined by movement without progression. Immotility is defined by no observable movement. While total motility has a lower reference limit of 40%, progressive motility has a lower reference limit of 32% [18].
The morphology of sperm is greatly varied and most of both fertile and infertile men have a range of 0-25% observed normal sperm morphology. This value is further reduced by the selection of cells by the zona pellucida, which chooses a set of morphologically similar sperm. These “zona-preferred” morphologies only contribute to 8-25% of all motile sperm. Although sperm cells are composed of head, neck, midpiece, principle piece, and endpiece, it is difficult to observe the endpiece. As a result, the sperm is considered to have a head and tail, which includes the midpiece and principle piece. Both components must be normal in order for the entire cell to be classified as normal. Generally, the head must be smooth, contoured, oval in shape, and without excessive vacuoles and the midpiece must be around the same length as the head and be in line with the major axis of the head. The principle piece must be thinner than the midpiece and about 10 times the length of the head. It can also be looped around itself, but cannot have any sharp angle, which indicates a break. Some of the more common defects include wrong sized or shaped heads, heads with vacuoles, double heads, improperly inserted midpieces, midpieces or principle pieces with abnormal width or length, broken or bent principle pieces, or any combination of these abnormalities. In addition, excessive residual cytoplasm (ERC) is another notable defect. ERC is characterized by excessive irregular cytoplasm and is often related to defective midpieces. The lower reference limit is 4% morphologically normal sperm within a single ejaculation. This rate is calculated by multiplying the normal forms by the total number of sperm within the ejaculate [18].
The velocity of PR sperm is varied but can be categorized into fast and slow based on whether its velocity is greater or lesser than 25 μm/sec. Semen volume is the amount of semen produced in a single ejaculate, while the concentration of sperm is the number of sperm per unit of volume. Both volume and concentration are critical due to the fact that they are used to calculate total sperm count, which refers to the total number of sperm in an entire ejaculate [18]. Although other factors contribute to and are associated with male infertility, sperm count is one of the leading cause for it [21]. According to the WHO, the lower reference limits for semen volume, sperm concentration, and sperm count in a single ejaculate are 1.5 ml, 15×106 sperm per ml, and 39×106 sperm per ejaculate, respectively (Table 1) [18].
Table 1.
Normal semen parameters and standard reference values [18].
| Parameter | Lower Reference Unit |
|---|---|
| Motility | 40% for Total Motility 32% for Progressive Motility |
| Morphology | 4% of normal forms |
| Velocity | 25 μm/sec |
| Volume | 1.5 ml |
| Concentration | 15×106 sperm per ml |
| Count | 39×106 sperm per ejaculate |
Since semen quality has a number of characteristics, male infertility can be caused by different factors or a combination of factors. Infertility trends also vary across regions. According to one study, 34.14% of the male partners of infertile couples in central India were abnormal in sperm concentration and had less than 15 million sperm/ml. 19.35% of tested men were azoospermic, meaning they lacked sperm in their semen completely. In addition, 10.70% had less than 30% motility and over 60% abnormal morphology [23]. A study done in Los Angeles, California found that 18% were abnormal in concentration with under 20 million sperm/ml, only 4% were azoospermic, 51% of men there were abnormal in motility, and 14% had abnormal morphology [24]. Comparatively, in Punjab, only 11.11% of men had a sperm concentration below 20 million sperm/ml, while 14.89% of men were azoospermic. Only 25.81% of those men had reduced sperm motility and 3.26% had abnormal morphology [25]. At another instance, in Abakaliki, Nigeria, 70% of men had sperm concentrations of below 10 million sperm/ml and, like in LA, 4% had no sperm at all (Table 2) [26]. Although the distribution of abnormal characteristics varied across areas, abnormal concentration and motility seem to be the most prominent. Comparing the results of different studies, the concentration of progressively motile sperm seems to be the most predictive factor regarding outcome, but, still, no individual parameter can be considered single best predictor of fertility [27].
Table 2.
Comparison of the percentages of men with abnormal characteristics in India, Los Angeles, Punjab, and Nigeria.
Devices for Home-based Semen Analysis
SpermCheck Fertility
The SpermCheck Fertility Test is a product that tests semen for sperm concentration at the threshold of 20 million per milliliter. The white, plastic device has a sample well and a strip that allows users to read the results (Figure 1a). The kit includes the SpermCheck device, a collection cup, a transfer device, and the SpermCheck solution bottle. As per company guidelines, the results are ready in around 10 minutes and are indicated with two lines telling whether the concentration per ml is greater or lesser than 20 million/ml. Designed as a first step to determine if further clinical evaluation is required, it retails for $39.99 USD and claims to be 98% accurate (Table 3) [28]. Although results of the SpermCheck test are quick and easy to interpret, it only indicates if sperm concentration is above or below the given threshold, preventing users from knowing the exact concentration and determining if their sperm are normal or abnormal in other parameters, such as morphology and motility.
Figure 1.
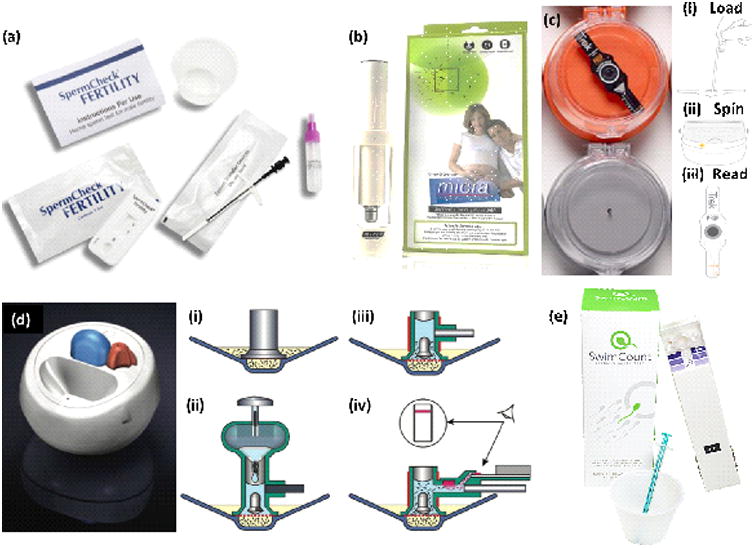
Devices for Home-Based Sperm Analysis. (a) SpermCheck Fertility Test Kit with instructions for use, SpermCheck device, collection cup, semen transfer device, and SpermCheck solution bottle. (b) Micra Sperm Test, which includes a microscope with which to analyze the sperm. (c) Trak device. (i) Few drops of sample are added on the Trak disposable test chip. (ii) Sample is centrifuged to isolate and quantify sperm cells. (iii) The height of sperm pellet is related with concentration of sperm cells. (d) Fertell Male Fertility Home Test. (i) Device with sample. (ii) Hyaluronic acid solution released to sample (iii) Sample heated up and sperm swim up through hyaluronic acid. (iv) Motile sperm react with antibody and collect on strip, producing visible red line. (e) SwimCount Sperm Quality Test with device, collection cup, and syringe. Reprinted with permissions from SpermCheck, Micra, Trak, Björndahl et al and SwimCount [28-31].
Table 3.
Comparison of Home-Based Sperm Analysis Devices.
| Test | Materials | Parameters Tested | Time Until Results | Price | Accuracy |
|---|---|---|---|---|---|
| SpermCheck | Antibody reaction for color change | Concentration | 10 minutes | $39.99 | 98% |
| Micra | Microscope kit | Count, Motility, Volume | 30 minutes | $85 | Not Available |
| Trak | Centrifuge, smartphone application | Concentration | 36 minutes | $199.99 | 97% |
| Fertell | Antibody reaction for color change | Motile concentration | 1 hour | Not Available | 95.3% |
| SwimCount | Antibody reaction for color change | Motile concentration | 1 hour | €49.99 = $58.5 | 95% |
| Paper-Based | Antibody reaction for color change | Concentration, Motility, Viability | 10 minutes | Not Available | 100% |
| Microfluidic by YA Chen | Microfluidic device with resistive pulse | Motile concentration, Motility | 12 minutes | Not Available | 9% difference |
| Microfluidic by CY Chen | Microfluidic device, centrifuge | Total and motile count | 20 minutes | Not Available | 5% difference |
| YO | Smartphone | Motile concentration | 13 minutes | $49.95 | 97% |
| Smartphone | Microfluidic chip, smartphone | Concentration, Motility, Velocity, Volume, Count | Depends on phone model, mean processing time <5-s | Not Available | 97.71% |
| ReproSource | Mail-in for manual evaluation | Concentration, Motility, Morphology, more | 1-2 days | Varies depending on provider | Not Available |
| Episona | Mail-in for genetic evaluation | Genetic abnormalities | 2 weeks | $895 | Not Available |
Micra Sperm Test for Sperm Count and Motility
The Micra Sperm Test is a commercially sold product that screens for three major male fertility factors, sperm count, motility, and semen volume. The product calls for the user to collect and dilute an ejaculate (Figure 1b). This sample is then placed on the device, which has a gridded surface so that motility can be calculated by the user. The product claims to provide results quickly and easily with only thirty minutes of waiting in between ejaculation and interpreting results. The kit includes a microscope and provided slides with which the user will analyze the sperm. Also provided in the kit are instructions that allow the user to accurately interpret the data exhibited by the sperm and provide ranges for normal and abnormal sperm count, motility, and sperm volume. The device retails for approximately $85 USD. Although the Micra device is able to examine three parameters, it is prone to human error and makes it hard to receive accurate and qualitative data on a semen sample because the it requires the user to observe the sample manually.
Trak
Trak is a small portable device that uses centrifugal motion to determine sperm cell count. A sample is loaded into a chamber that centrifuges it (Figure 1c). The company claims to give an estimate of cell concentration based on the size of the cell pellet after the six-minute centrifugation is complete. The device indicates this with two marks of delineation noting 15 million/ml and 55 million/ml. On its website, it claims to show 97% accuracy when compared to standard lab evaluations. In addition, the device also comes with an app that allows users to enter data and track their sperm count. The corresponding app, made available in 2015, claims to aid the user in making more health conscious decisions that will positively affect their sperm count. The app has sections to log wellness, diet, exercise, stress, exposure to heat sources and toxins. It also lets users log the result of clinical semen analysis performed by a third party lab. That section includes areas to log the date, days abstained, the volume, viscosity and pH of semen, as well as sperm count, motility, and morphology. Becoming available in October 2016, the Trak device is under the parent company of Sandstone Diagnostics and retails for $199.99 USD for four tests [29]. Similar to other home-based sperm tests, the Trak only roughly indicates if the sperm concentration is within a certain range and fails to analyze other important parameters.
Fertell Male Fertility Home Test
Fertell Male Fertility Home Test is a device that estimates motile sperm concentration and produces an easy to interpret, visual result of a red line appearing on the device (Figure 1d). It only detects positive or negative results, based on a detection limit of 10×106/ml. The device works by separating sperm with progressive motility from liquefied semen using hyaluronic acid solution. Once the motile sperm swim up through the solution, they react with an antibody and collect at a strip, producing the red line that indicates a positive concentration. A negative result shows no line and alerts the user so that he can seek more comprehensive testing. For ease of use, a light-emitting diode (LED) provides feedback to the user at various stages to show if the device is functioning properly. The device was found to have an accuracy of 95.3% compared to a CASA test and a hyaluronate migration test (HMT) and was sold under the parent company of Genosis Ltd [30]. Again, the Fertell device is unable to provide more data than a simple positive/negative result, not giving users much information on their fertility potential and requiring them to seek further testing.
SwimCount Sperm Quality Test
SwimCount Sperm Quality Test is another home-based kit that tests the concentration of progressively motile sperm cells. The kit includes a collection cup, a syringe, instructions for use, and the device itself (Figure 1e). Users collect a sample in collection cup, wait for about thirty minutes, stir the sample ten times with the syringe, collect 0.5 ml of the sample with the syringe, and transfer it to the device. As an add-on, users may also get the SwimCount Non-Spermicide Condom to collect the sample. Then, a slider on the side must be pushed forward to activate the device, which has three chambers: the sample chamber in which the semen is deposited by the user, the separation chamber to which only progressively motile sperm can swim into, and finally the detection and result window to which the progressively motile sperm, now stained with dye, are captured onto. After another thirty minutes and pulling the slider back, the results are interpreted by the final color in the results window compared to the reference colors printed next to the window on the device. If similar to the lightest color, the concentration is below 5 million motile sperm/ml. If similar to the darkest color, the concentration is above 20 million motile sperm/ml. If similar to the middle color, the concentration is in between the other values, near at the normal level for fertile men according to WHO. SwimCount Sperm Quality Test has an accuracy of 95% compared to manual microscope methods and retails for €49.99 [31]. Like the other products, this test is also unable to provide information on parameters other than progressively motile concentration, meaning that further testing is still required.
Paper-based Semen Analysis
Paper-based devices are also beginning to emerge in various fields of biomedical engineering including microfluidics, diagnostics, POC testing [32-35]. These paper based devices overcome some of the limitations of currently available at-home sperm analysis kits, such as their need for multiple steps, subjectivity, high cost, and ability to only measure one parameter. For example, a paper-based device has been developed with the ability to measure three semen parameters, sperm viability, sperm concentration and sperm motility, in about ten minutes (Figure 2). Additionally, the paper-based device can be produced cost effectively. It is made of one laminate layer and two wax printed paper layers bound together with double-sided tape. Results are returned with a change in the device's color, which results from a reaction between yellow tetrazolium dye and the diaphorase flavoprotein enzyme, which is present in active sperm. The device determines concentration when the enzyme in the live sperm reacts with the dye, causing a colormetric change in the device from yellow to purple. Motility is determined in the same way, except that the motile sperm must successfully swim through a viscous buffer and narrow pores of a membrane filter before being able to react with the dye. This device was found to show 100% agreement with results from CASA and dye exclusion vitality assays [8]. Like the currently available home-based devices, paper-based devices only estimate the quality of semen samples and are unable to provide specific, quantitative data. Although it can evaluate three parameters (viability, concentration, and motility), it cannot evaluate other important parameters that are critical to fertility potential, such as sperm morphology.
Figure 2.
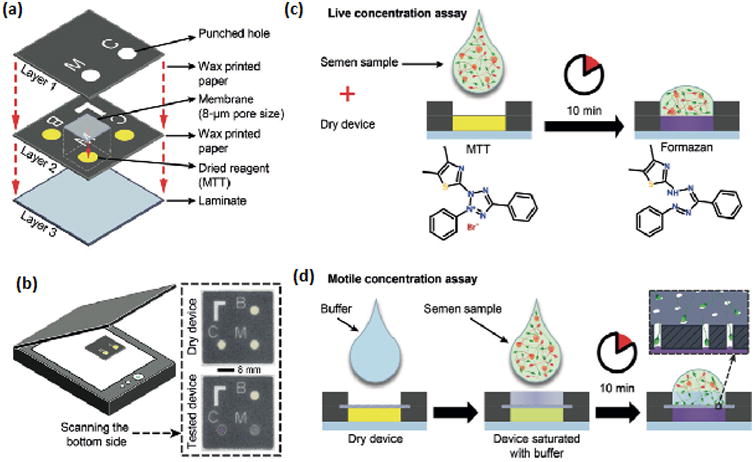
Paper-Based Device. (a) Breakdown of device. (b) Assembled device before and after sample is applied. (c) Colormetric signal is produced after semen sample is applied to dry device. (d) To test motility, sperm must pass through buffer and pores on membrane filter before producing a color change. Reprinted with permission from Nosrati et al [8].
Microfluidic Devices for Home-based Semen Analysis
Microfluidic devices are being developed for various applications in medicine including disease diagnosis, tissue culture, and cryopreservation [36-49]. Like paper-based devices, these microfluidic devices also provide a quick way to evaluate semen with a simple readout. A microfluidic device has been designed to measure motile sperm concentration and motility by producing a flow field for the sperm to swim against (Figure 3). The sperm that are able to successfully swim against the flow in a given time were then counted using resistive pulse measurement. With this method, the device only showed a 9% difference in sperm count from manual counting under a microscope. One of the main advantages of this device is that it does not require any labels or biomarkers [50]. At another instance, scientists have designed a microfluidic device that is able to quantify the total and motile sperm counts. In this device, motile sperm must swim across a phase-guide barrier to mix with a buffer (Figure 4). Afterwards, the solution is centrifuged to determine sperm concentration. Results are found rapidly, easily, and accurately in few minutes. Additionally, the results are also found to agree within 5% with those of a manual evaluation with a microscope in a Makler chamber [15]. Although these devices provide many advantages, they also have some limitations. One of the main challenges is that these microfluidic devices require external peripheral equipment, including pumps and tubing, making them less portable and more expensive and, hence, may not be suitable for at-home testing at their current stage.
Figure 3.
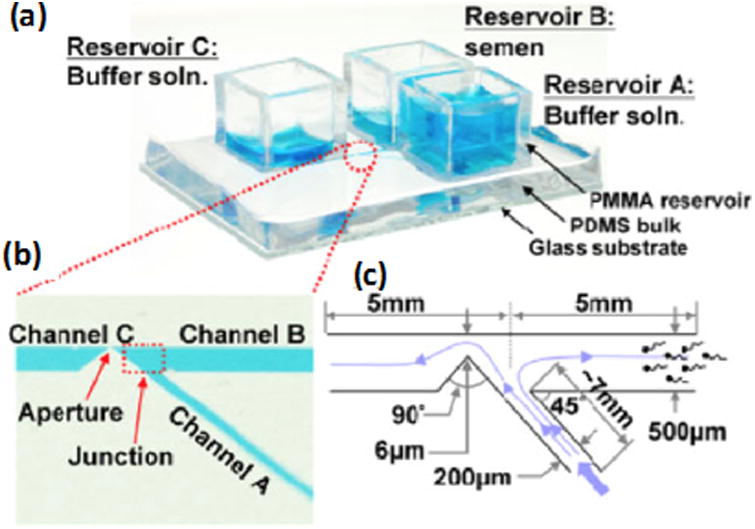
Microfluidic Device created by YA Chen et al. (a) Actual device with glass substrate, PDMS bulk, and reservoirs for semen and buffer solution. (b) Microchannels where motile sperm swim through flow of buffer. Motile sperm are able to swim through Channel B and avoid being flushed through the aperture and Channel C. (c) Microchannel dimensions. Reprinted with permission from YA Chen et al [50].
Figure 4.
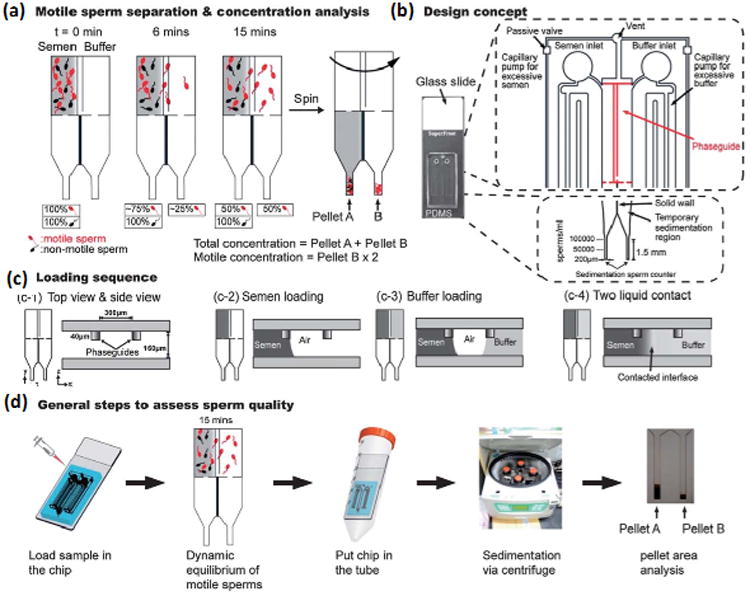
Microfluidic device created by CY Chen et al. (a) Motile sperm swimming across barrier and calculation of sperm concentrations after centrifugation. (b) Schematic of device design. (c) Semen and buffer loading sequence. (d) Steps followed to assess sperm quality. Reprinted with permission from CY Chen et al [15].
Another approach to making semen analysis more accessible with microfluidics is through one of the most common devices available, the smartphone. One commercially available smartphone-based at-home device is the YO Sperm Test, which tests for motile sperm concentration. The device works by attaching onto the phone and using the phone's camera and flash to record a video of the sperm in the sample (Figure 5a). The user must download the accompanying application, collect a sample in the provided container, add liquefying powder and wait for ten minutes. Then, they must attach the device to the phone, transfer the sample to a slide using a pipette, and then insert the slide into the device. Results are ready within three minutes and are explained in a report within the app. Users are also able to view and save the recorded video of their sperm. The YO Sperm Test claims to have over a 97% accuracy and retails for $49.95 USD for two tests [51]. Like other home tests, it is unable to perform a full analysis and can only return information on motile sperm concentration. In addition, it is only available for certain phone models and each version is specific to a particular model, meaning if a user does not have one of the offered models or purchases a new phone, they will be unable to use the test.
Figure 5.
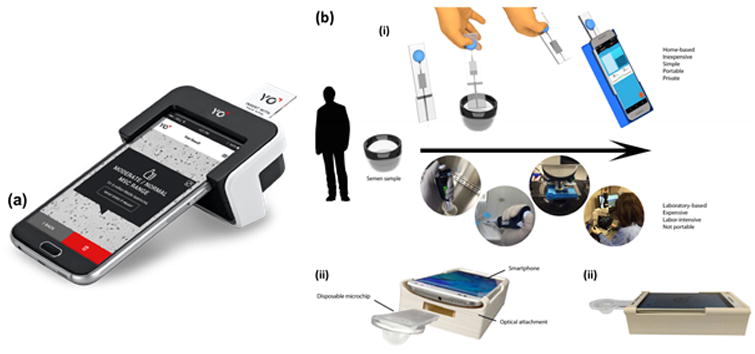
Smartphone-based devices. (a) YO Sperm Test attached to smartphone with slide inserted. Once the test is complete the application will report the concentration of motile sperm and indicate if it is within the normal range, as well as show the user a recorded video of their sperm. (b) Device by Kanakasabapathy et al. (i) A comparioson of steps of the smartphone-based device and traditional methods. Semen sample is loaded onto smartphone-based device using bulb and then the microchip is separated from the loading end and placed in the optical attachment. Sample loaded into counting chamber and microscope and then evaluated manually or with a CASA system. (ii) Actual device with smartphone, microchip, and attachment. (iii) Side view of device. Reprinted with permission from Medical Electronic Systems [51] and Kanakasabapathy et al. [52].
Another device that evaluates sperm concentration, motility, velocity, volume, and count has been developed [52]. An on-phone image analysis is performed on a semen sample with a microfluidic device and a modular wireless weight scale. It uses a lightweight optical attachment for the smartphone, which provides the appropriate lighting and positioning for proper image magnification. The attachment has a specific opening for the microchip to fit into so that it positioned correctly at the proper distance away from the smartphone. The microchip itself employs a polydimethylsiloxane (PDMS) bulb for power-free mechanical pumping that creates a negative pressure chamber (Figure 5b). Smartphone application that accompanies the device guides users through the steps to complete a test and then stores the results for long term monitoring. When compared to CASA testing, the device was found to have an accuracy of 97.71% [52]. Although this device has certain advantages, such as requiring minimal user handling and measuring multiple parameters, it still is unable to assess sperm morphology. In addition, it is sometimes prone to error when it identifies nonsperm cells as sperm [52]. Further, changing smartphone type and model may significantly skew the results.
Mail-In Semen Analysis Assays
In addition to home-based methods of sperm analysis, mail-in sperm analysis kits in which users collect a sample at home and then send it to the provider for analysis are also available. ReproSource's @Home Collection Kit contains a shipping container that is able to maintain semen quality enough for a proper evaluation within 26 hours. It also includes a preservative tube, a pipette, a biohazard bag, ice packs and cooling gels to keep the sample cold, and labels to ship it back. It offers a standard evaluation of concentration, motility, and morphology, as well as inflammatory markers and accessory gland health. Its reliability was tested by performing semen analysis on samples collected with the kit 26 hours apart, finding only a 15% coefficient of variation between the two tests. With the kit, patients are able to collect a sample at home and then send it to ReproSource, which will then send results to their physician within 1-2 days [53].
Mail-in epigenetic male fertility tests also exist. Instead of testing the basic parameters, Episona's Seed test analyzes individual genes in sperm DNA and provides clients with information on genetic abnormalities that may affect both male factor infertility risk and poor embryo development risk, which may help to determine which treatment is most appropriate for a particular case. The kits consist of instruction cards, a collection tube, a funnel, and a biohazard bag. Patients must have a kit ordered from their doctors before being able to collect a sample at home and sending it back to Episona's labs for analysis and receiving the results online. The service is currently being offered for $895 USD [54].
Limitations of Home-based Semen Analysis Methods and Future Directions
Although at-home, paper-based, and microfluidic sperm analysis products are a step ahead of the traditional methods for semen analysis, they still have many limitations. The primary issue is the fact that currently, non-conventional sperm analysis methods are best used only for indicating whether a user should or should not pursue further testing. Most can only provide information on one or a few parameters at a time. While this information can be helpful, only having data on some, but not all, factors can lead to false negatives for male infertility, since sperm can be simultaneously considered normal in one characteristic, but abnormal in another. A single parameter does not define whether an individual is fertile or infertile, but whether or not a natural pregnancy occurs within a year does. As a result, these methods are not yet a replacement for lab analysis. Formal confirmation from a fertility specialist is still recommended even after the use of a home-based test, which can actually delay getting a full clinical evaluation. Semen analysis from a clinic is much more detailed and can provide information on many more parameters simultaneously. Although not as quick or inexpensive as current home-based kits, clinics and labs can usually return results within a few days and are fairly affordable at around $100 USD for each test.
Because of these reasons, at-home testing may be more useful to vasectomy patients. As over 33 million men undergo vasectomy procedures as a safe and inexpensive contraceptive solution, it is important for them to retest their semen to ensure the surgery's success. However, the number of patients who actually follow up is very low because of the inconvenience, but the ease of home-based testing has the potential to improve patient compliance. For these patients, the main parameter to test for is sperm concentration, which should be below 100,000 sperm/ml eight to sixteen weeks after undergoing the procedure, meaning the tests that are only able to test concentration can still be valuable to them [52].
New approaches to sperm analysis are seeking ways to overcome the challenges of current technology. Lensless on-chip microscopes and imaging systems have been introduced. Researchers were able to create a lensfree on-chip microscope using digital holography that could automatically analyze sperm and process count, speed, and direction of motile sperm without the need for any bulky and expensive lenses, lasers, or other components [20, 22, 55]. Futhermore, a lensless charge-coupled device (CCD) imaging system that can both quickly analyze and sort sperm in a microfluidic channel has also been developed. Both these devices are able to quantitatively track larger numbers of sperm as a result of larger fields of view and are also portable and more compact [21]. Moving forward, even more improvements in sperm analysis and imaging can be made. Some potential advances could include improved systems that can measure multiple parameters automatically and possibly analyze sperm morphology and distinguish between normal and abnormal sperm. With the ability to automatically, accurately, and quantitatively evaluate all parameters tested in traditional analysis, but without the drawbacks of bulky, expensive equipment, lengthy wait times, lack of skilled technicians, and inconvenience, these systems would have the potential to become a common alternative to or even replacement for the current conventional sperm analysis methods.
Conclusion
At-home sperm analysis is a valuable tool for determining fertility potential, especially for couples struggling with infertility, as well as vasectomy patients. Men who are reluctant to seek conventional clinical testing due to high cost, long wait time, inconvenience, or social stigma might be more willing to use home-based sperm analysis kits, which overcome those problems. With these kits, men are able to rapidly evaluate their fertility potential with ease at a low-cost from the comfort and privacy of their own homes, unlike the traditionally used methods. Although currently available home systems only provide rudimentary results, they can give users a basic idea of their fertility potential based on few parameters and motivate them to pursue more comprehensive testing. This inability of home-based sperm analysis systems to test fertility based on all sperm functional parameters that are usually analyzed in the lab, limits their use and makes them prone to false negative results, which may actually delay men from seeking more thorough evaluation. Nevertheless, at-home sperm analysis devices are still relevant as they encourage hesitant men to take a first step in investigating their fertility potential and should continue being improved. Recent advances in microfluidics and imaging technologies should be further investigated for their application in designing more reliable home-based sperm analysis devices.
Acknowledgments
We acknowledge research support from NIH R15AI127214, Fertility & Genetics Plantation, FL, Cryos International USA, Institute for Sensing and Embedded Networking Systems Engineering (I-SENSE) Research Initiative Award, FAU Faculty Mentoring Award, Humanity in Science Award, and a start-up research support from College of Engineering and Computer Science, Florida Atlantic University, Boca Raton, FL.
Footnotes
Conflict of Interests: The authors declare no financial conflict of interest.
References
- 1.Center for disease control and prevention (CDC) Infertility FastStats. 2013 http://www.cdc.gov/nchs/fastats/fertile.htm.
- 2.Knowlton SM, Sadasivam M, Tasoglu S. Microfluidics for sperm research. Trends in biotechnology. 2015;33(4):221–229. doi: 10.1016/j.tibtech.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Garolla A, et al. Sperm selected by both birefringence and motile sperm organelle morphology examination have reduced deoxyribonucleic acid fragmentation. Fertility and sterility. 2014;101(3):647–652. doi: 10.1016/j.fertnstert.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 4.Ghaleno LR, et al. Evaluation of conventional semen parameters, intracellular reactive oxygen species, DNA fragmentation and dysfunction of mitochondrial membrane potential after semen preparation techniques: a flow cytometric study. Archives of gynecology and obstetrics. 2014;289(1):173–180. doi: 10.1007/s00404-013-2946-1. [DOI] [PubMed] [Google Scholar]
- 5.Huang H, et al. Motile Human Sperm Sorting by an Integrated Microfluidic System. J Nanomed Nanotechnol. 2014;5(199):2. [Google Scholar]
- 6.Nosrati R, et al. Rapid selection of sperm with high DNA integrity. Lab on a Chip. 2014;14(6):1142–1150. doi: 10.1039/c3lc51254a. [DOI] [PubMed] [Google Scholar]
- 7.Tung Ck, et al. Cooperative roles of biological flow and surface topography in guiding sperm migration revealed by a microfluidic model. Lab on a Chip. 2014;14(7):1348–1356. doi: 10.1039/c3lc51297e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nosrati R, et al. Paper-based quantification of male fertility potential. Clinical chemistry. 2016;62(3):458–465. doi: 10.1373/clinchem.2015.250282. [DOI] [PubMed] [Google Scholar]
- 9.Worrilow K, et al. Use of hyaluronan in the selection of sperm for intracytoplasmic sperm injection (ICSI): significant improvement in clinical outcomes—multicenter, double-blinded and randomized controlled trial. Human Reproduction. 2013;28(2):306–314. doi: 10.1093/humrep/des417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zahedi A, et al. Zeta potential vs apoptotic marker: which is more suitable for ICSI sperm selection? Journal of assisted reproduction and genetics. 2013;30(9):1181–1186. doi: 10.1007/s10815-013-0022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Safaee H, et al. Exhaustion of racing sperm in nature-mimicking microfluidic channels during sorting. PNAS. 2012 doi: 10.1002/smll.201300020. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aitken R, et al. The source and significance of DNA damage in human spermatozoa; a commentary on diagnostic strategies and straw man fallacies. Molecular human reproduction. 2013 doi: 10.1093/molehr/gat025. [DOI] [PubMed] [Google Scholar]
- 13.Aitken R, et al. On methods for the detection of reactive oxygen species generation by human spermatozoa: analysis of the cellular responses to catechol oestrogen, lipid aldehyde, menadione and arachidonic acid. Andrology. 2013 doi: 10.1111/j.2047-2927.2012.00056.x. [DOI] [PubMed] [Google Scholar]
- 14.Brown DB, et al. Evaluating a novel panel of sperm function tests for utility in predicting intracytoplasmic sperm injection (ICSI) outcome. Journal of assisted reproduction and genetics. 2013;30:461–477. doi: 10.1007/s10815-013-9960-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CY, et al. Sperm quality assessment via separation and sedimentation in a microfluidic device. Analyst. 2013;138(17):4967–4974. doi: 10.1039/c3an00900a. [DOI] [PubMed] [Google Scholar]
- 16.Lewis SE, et al. The impact of sperm DNA damage in assisted conception and beyond: recent advances in diagnosis and treatment. Reproductive biomedicine online. 2013;27(4):325–337. doi: 10.1016/j.rbmo.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Hammoud AO, et al. Impact of male obesity on infertility: a critical review of the current literature. Fertility and sterility. 2008;90(4):897–904. doi: 10.1016/j.fertnstert.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 18.WHO laboratory manual for the Examination and processing of human semen. WHO Press; Geneva, Switzerland: 2010. [Google Scholar]
- 19.Henkel R. Sperm preparation: state-of-the-art—physiological aspects and application of advanced sperm preparation methods. Asian journal of andrology. 2012;14(2):260. doi: 10.1038/aja.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su TW, et al. Compact and light-weight automated semen analysis platform using lensfree on-chip microscopy. Analytical chemistry. 2010;82(19):8307–8312. doi: 10.1021/ac101845q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, et al. Lensless imaging for simultaneous microfluidic sperm monitoring and sorting. Lab on a Chip. 2011;11(15):2535–2540. doi: 10.1039/c1lc20236g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fennell R, Asghar W. Image Sensor Road Map and Solid-State Imaging Devices. NanoWorld. 2017;1(4):10–14. [Google Scholar]
- 23.Kumar N, Choudhari AR, Singh AK. Prevalence of male factor infertility in last ten years at a rural tertiary care centre of central India: a retrospective analysis. Indian Journal of Obstetrics and Gynaecology Research. 2015;2(3):132–136. [Google Scholar]
- 24.Acacio BD, et al. Evaluation of a large cohort of men presenting for a screening semen analysis. Fertility and sterility. 2000;73(3):595–597. doi: 10.1016/s0015-0282(99)00591-9. [DOI] [PubMed] [Google Scholar]
- 25.Butt F, Akram N. Semen analysis parameters: Experiences and insight into male infertility at a tertiary care hospital in Punjab. J Pak Med Assoc. 2013;63(5):558–62. [PubMed] [Google Scholar]
- 26.Ugwuja E, Ugwu N, Ejikeme B. Prevalence of low sperm count and abnormal semen parameters in male partners of women consulting at infertility clinic in Abakaliki, Nigeria. African journal of reproductive health. 2008;12(1):67–73. [PubMed] [Google Scholar]
- 27.Tomlinson M, Lewis S, Morroll D. Sperm quality and its relationship to natural and assisted conception: British Fertility Society Guidelines for practice. Human fertility. 2013;16(3):175–193. doi: 10.3109/14647273.2013.807522. [DOI] [PubMed] [Google Scholar]
- 28.SpermCheck® Fertility. [cited 2017 September 6]; Available from: http://www.spermcheck.com/
- 29.Information obtained from webpage. [Last accessed on 10/21/2017]; https://trakfertility.com/
- 30.Björndahl L, et al. Development of a novel home sperm test. Human reproduction. 2006;21(1):145–149. doi: 10.1093/humrep/dei330. [DOI] [PubMed] [Google Scholar]
- 31.Information obtained from weblink. [Last accessed 09/18/2017]; https://www.swimcount.com.
- 32.Asghar W, et al. Toxicology Study of Single-walled Carbon Nanotubes and Reduced Graphene Oxide in Human Sperm. Scientific Reports. 2016;6 doi: 10.1038/srep30270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asghar W, et al. Selection of functional human sperm with higher DNA integrity and fewer reactive oxygen species. Advanced healthcare materials. 2014;3(10):1671–1679. doi: 10.1002/adhm.201400058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rappa KL, et al. Sperm processing for advanced reproductive technologies: Where are we today? Biotechnology advances. 2016;34(5):578–587. doi: 10.1016/j.biotechadv.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Sher M, et al. Paper-based analytical devices for clinical diagnosis: recent advances in the fabrication techniques and sensing mechanisms. Expert Review of Molecular Diagnostics. 2017 doi: 10.1080/14737159.2017.1285228. just-accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asghar W, et al. Electrical fingerprinting, 3D profiling and detection of tumor cells with solid-state micropores. Lab on a Chip. 2012;12(13):2345–2352. doi: 10.1039/c2lc21012f. [DOI] [PubMed] [Google Scholar]
- 37.Asghar W, et al. Engineering long shelf life multi-layer biologically active surfaces on microfluidic devices for point of care applications. Scientific reports. 2016;6 doi: 10.1038/srep21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Safavieh M, et al. Advances in Candida detection platforms for clinical and point-of-care applications. Critical reviews in biotechnology. 2016:1–18. doi: 10.3109/07388551.2016.1167667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shafiee H, et al. Paper and flexible substrates as materials for biosensing platforms to detect multiple biotargets. Scientific reports. 2015;5:8719. doi: 10.1038/srep08719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asghar W, et al. Preserving human cells for regenerative, reproductive, and transfusion medicine. Biotechnology journal. 2014;9(7):895–903. doi: 10.1002/biot.201300074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miki K, Clapham DE. Rheotaxis guides mammalian sperm. Current Biology. 2013;23(6):443–452. doi: 10.1016/j.cub.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seiringer M, et al. Efficacy of a sperm-selection chamber in terms of morphology, aneuploidy and DNA packaging. Reproductive biomedicine online. 2013;27(1):81–88. doi: 10.1016/j.rbmo.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Tasoglu S, et al. Manipulating biological agents and cells in micro-scale volumes for applications in medicine. Chemical Society Reviews. 2013 doi: 10.1039/c3cs60042d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tasoglu S, et al. Exhaustion of Racing Sperm in Nature-Mimicking Microfluidic Channels During Sorting. Small. 2013;9(20):3374–3384. doi: 10.1002/smll.201300020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hafeez A, et al. GPU-based real-time detection and analysis of biological targets using solid-state nanopores. Medical & biological engineering & computing. 2012;50(6):605–615. doi: 10.1007/s11517-012-0893-9. [DOI] [PubMed] [Google Scholar]
- 46.Islam M, et al. Cell Elasticity-based Microfluidic Label-free Isolation of Metastatic Tumor Cells. British Journal of Medicine & Medical Research. 2014;4(11):2129–2140. [Google Scholar]
- 47.Coarsey CT, et al. Strategies in Ebola virus disease (EVD) diagnostics at the point of care. Critical Reviews in Microbiology. 2017:1–20. doi: 10.1080/1040841X.2017.1313814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanakasabapathy MK, et al. Rapid, label-free CD4 testing using a smartphone compatible device. Lab on a Chip. 2017;17(17):2910–2919. doi: 10.1039/c7lc00273d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adenmosun OO, Asghar W, Kumi-Diaka J. Sickle Cell Sperm Selection with Hb-S Mab: A Future Application for Intracytoplasmic Genotypically Selected Sperm Injection (IGSI) Archives of Clinical Microbiology. 2017 [Google Scholar]
- 50.Chen YA, et al. Analysis of sperm concentration and motility in a microfluidic device. Microfluidics and Nanofluidics. 2011;10(1):59–67. [Google Scholar]
- 51.Information obtained from weblink. [Last accessed 09/18/2017]; http://www.yospermtest.com.
- 52.Kanakasabapathy MK, et al. An automated smartphone-based diagnostic assay for point-of-care semen analysis. Science translational medicine. 2017;9(382):eaai7863. doi: 10.1126/scitranslmed.aai7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Information obtained from weblink. [Last accessed 09/18/2017]; http://reprosource.com.
- 54.Information obtained from weblink. [Last accessed 09/18/2017]; https://www.episona.com.
- 55.Sobieranski AC, et al. Portable lensless wide-field microscopy imaging platform based on digital inline holography and multi-frame pixel super-resolution. Light: Science & Applications. 2015;4(10):e346. doi: 10.1038/lsa.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]


