Abstract
Objective
We investigated whether weight change during the early RA period was associated with subsequent mortality and evaluated for an RA-specific effect.
Methods
We identified incident RA during the Nurses’ Health Study (1976–2016) and created a comparison cohort, matching each RA case with up to 10 non-RA comparators by age and year of RA diagnosis (index date). To capture weight change around the early RA period (“peri-RA/index”), we used weight measures 2–4 years before and after index date. Cox regression estimated HRs for mortality by peri-RA/index weight change categories separately in each cohort and combined, evaluating for an RA-specific effect.
Results
Among 121,701 women, 902 developed incident RA, matched to 7,884 non-RA comparators. There were 371(41.1%) deaths in the RA cohort during mean 17.0 years follow-up after the early RA period and 2,303(29.2%) deaths among comparators during mean 18.4 years follow-up. Peri-RA weight loss >30 lb had HR of 2.78 (95%CI1.58–4.89) for mortality compared to stable weight; the comparison cohort had similar results (HR 2.16, 95%CI1.61–2.88). Weight gain >30 lb had no association with mortality among RA (HR 1.45, 95%CI0.69–3.07) or comparators (HR 1.19, 95%CI0.89–1.59). There was no interaction between RA/comparator status and weight change for mortality (pinteraction=0.68).
Conclusion
Severe weight loss during the early RA period was associated with increased subsequent mortality risk for women with and without RA. These results extend prior observations by including non-RA comparators and finding no protective association between weight gain and mortality, arguing against an RA-specific obesity paradox for mortality.
Keywords: rheumatoid arthritis, weight, obesity, mortality, epidemiology
The increased mortality risk for patients with rheumatoid arthritis (RA) compared to the general population may be due to altered immunity, medication side effects, systemic effects of inflammation, worsened physical function, accumulation of multimorbid conditions, and excess unhealthy behaviors such as smoking(1–5). The contribution of metabolic and inflammatory factors (such as obesity) to RA etiology and outcomes has received increased attention recently(6, 7). Several studies have investigated the effect of body mass index (BMI) on mortality among patients with RA. In RA cohorts, obesity has been associated with 34–67% decreased mortality compared to those with normal BMI(8–11). Follow-up studies suggested that this “obesity paradox” for mortality in RA may be explained by pathologic unintentional weight loss in the few years preceding death, rather than a biologic protective effect of obesity. According to this explanation, patients with longstanding RA who reached normal or underweight BMI have higher observed mortality and are relatively less healthy than RA patients that maintained obesity or overweight(11, 12).
In other chronic diseases and the general population, similar findings of a paradoxical protective effect for overweight or obesity, as well as increased mortality risk with unintentional weight loss, have been reported(13–16). Studies in RA evaluating obesity, weight change, and mortality have typically consisted of long duration and relatively short follow-up prior to death. Therefore, prior associations may not have been specifically related to RA, but rather may have been detecting a general phenomenon related to multimorbidities, aging, or frailty(13, 17).
We hypothesized that the early RA period is the window of time most likely to specifically contribute to weight change in RA due to systemic inflammation prior to treatment, and changes in physical activity and diet with active symptoms(18, 19). Therefore, to investigate the specific effect of weight change on mortality in RA, weight measures are needed before RA diagnosis and at the end of the early RA period to effectively capture weight changes that might occur during the entire early RA period. Furthermore, a non-RA population is required to establish that observed effects are specifically related to RA and not multimorbidities, aging, or frailty. Since weight loss often immediately precedes death, lengthy follow-up between the weight change measurement and death assessment enhances the ability to associate weight loss with an underlying chronic disease such as RA rather than as the proximal cause of death.
Within the Nurses’ Health Study (NHS), we investigated the effect of weight change during the early RA period on subsequent mortality, including a non-RA group to evaluate for the specific effect of RA. We hypothesized that severe weight loss and gain during the early RA period would be associated with increased risk of mortality compared to stable weight, but that there would be similar effects among those with and without RA.
METHODS
Study population
In 1976, 121,701 female registered nurses in the U.S. aged 30–55 enrolled in the NHS. Women in the NHS completed questionnaires at baseline and every two years, providing data on anthropometrics, behaviors, sociodemographics, diet, medications, and diseases. Follow-up in the NHS has been high, with >90% returning questionnaires each cycle(1). All aspects of this study were approved by the Partners HealthCare IRB.
Incident RA cohort
We excluded participants with prevalent RA or other connective tissue disease (CTD) prior to the NHS baseline in 1976. Women who self-reported a diagnosis of RA or other CTD after the initial questionnaire were mailed a screening questionnaire(20). For those who screened positive, medical records were obtained and reviewed by two rheumatologists to confirm RA meeting the 1987 American College of Rheumatology classification criteria(21). Details on RA characteristics at diagnosis, including date of diagnosis, serologic status, radiographic changes/erosions, and nodules, were obtained from medical record review. Seropositivity was defined as positive rheumatoid factor (RF) or cyclic citrullinated peptide (CCP) antibodies per tests sent through routine medical care. Women diagnosed with RA prior to the clinical use of CCP would not have had this tested during clinical care, so CCP was generally only available on women diagnosed later during follow-up. We only analyzed RA cases who had weight measures available at two questionnaire cycles before as well as two cycles after the date of RA diagnosis (Figure 1).
Figure 1.
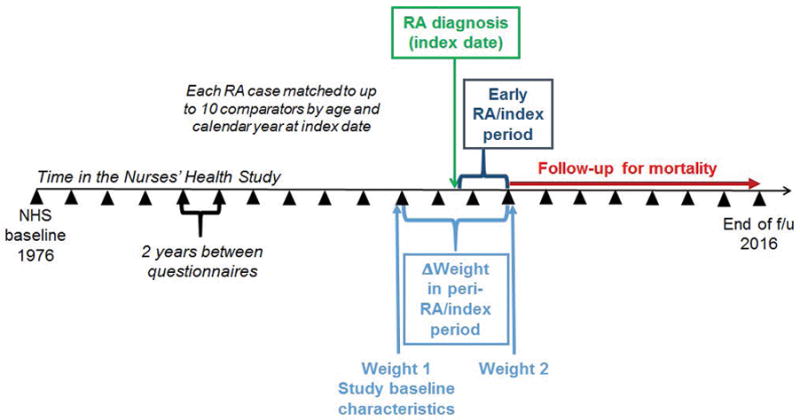
Study design schematic for weight change in the early rheumatoid arthritis period among RA cases and matched comparators. Wedges indicate questionnaire cycles during 40 years of prospective follow-up in the Nurses’ Health Study.
Matched non-RA comparison cohort
To evaluate for an RA-specific effect between weight change and mortality, we created a comparison cohort without RA within the NHS. To control for age and temporal trends, we matched each RA case with up to ten non-RA comparators based on age and calendar year. We defined the index date for matching as the date of RA diagnosis, as previously described(5). Participants in the NHS were eligible to be a comparator if they had never reported RA or other CTD prior to or on the index date and had weight measures at two follow-up cycles before as well as two cycles after index date. Since we matched women on the same age in years and same calendar year, exactly ten comparators with complete exposure data may not have been available for every woman with incident RA.
Weight change in the early RA/index period
All weight measures were prospectively self-reported in pounds (lb), previously validated in the NHS as highly accurate compared to measured weight (r=0.98)(22). We investigated weight change during the early RA period since this is the window when weight change is most likely to be related to RA-specific processes (Figure 1).
We chose the initial weight (Weight 1) as reported two cycles (minimum of 2 and maximum of 4 years) prior to the date of RA diagnosis since the weight measure immediately preceding RA diagnosis might have been affected by RA symptoms prior to definitive clinical diagnosis. We chose the subsequent weight (Weight 2) as reported two cycles after the date of RA diagnosis (minimum of 2 and maximum of 4 years) to capture the early RA period, which is often defined as the first two years after diagnosis(23–25). Therefore, we captured weight change in the “peri-RA” period which encompassed the entire early RA period. For comparators, we used a parallel strategy to identify weights before and after the index date to define analogous “early index” and “peri-index” periods even though these women were not diagnosed with any disease at the index date. In this study, the entire peri-RA/index period typically encompassed 6 years (Figure 1).
We created categories of weight change based on the absolute weight gained or lost comparing the initial and subsequent weights (ΔWeight=Weight 2–Weight 1). We defined stable weight as |ΔWeight| ≤10 lb, mild loss as ΔWeight <−10 to −20 lb, moderate loss as ΔWeight <−20 to −30 lb, severe loss as ΔWeight <−30 lb, mild gain as ΔWeight >10 to 20 lb, moderate gain as ΔWeight >20 to 30 lb, severe gain as ΔWeight >30 lb.
Identification of deaths
As previously described in detail, deaths were identified by systematic searches of the National Death Index and state vital records(26). This search is supplemented by family and postal authority reports. These methods ascertain >98% of deaths in the NHS(27). Women who died during the peri-RA or peri-index periods were not included in the analysis since ΔWeight could not be calculated. Complete death data were available until May 31, 2016 which was the end of the analysis.
Covariates
We considered covariates as confounders based on their association with both RA risk and mortality in prior literature. All covariates except for RA disease characteristics were assessed at the same questionnaire as Weight 1. BMI was categorized as underweight, normal, overweight, or obese(28). Age was used as a continuous variable in years. Annual household income was based on home address and US Census tract-level data as a proxy for socioeconomic status (<$40K or ≥$40K USD). Physical activity was first measured starting in 1980 using a validated survey and converted into continuous weekly hours of moderate or vigorous activity(29). Dietary factors were assessed using semi-quantitative food frequency questionnaires in 1980, 1984, 1986, and every four years until 2014 (30). Participants were classified into tertiles of the Alternate Healthy Eating Index to rank dietary quality using previously described methods(31). For multimorbidities at baseline, we used the validated Multimorbidity Weighted Index (MWI) derived using the NHS and related cohorts, composed of 74 distinct prevalent and serious conditions (such as cancer, diabetes, and cardiovascular disease) by self-report, with each condition weighted by the effect on physical health-related quality of life(32). As in previous work, we excluded: 1) conditions affecting only men, 2) premenopausal conditions not assessed in the NHS, and 3) RA or other CTD (for a total of 64 conditions in this analysis)(5). In RA-case only analyses, we further considered RA severity factors at diagnosis, including seropositivity as well as radiographic changes/erosions and nodules collected from medical records at RA diagnosis. Data on RA-specific medications were unavailable, but participants had not been diagnosed with RA yet at baseline when the initial weight was reported.
Statistical analysis
We reported descriptive statistics at baseline (time of Weight 1) according to the seven categories of weight change (stable, mild loss, moderate loss, severe loss, mild gain, moderate gain, severe gain) during the peri-RA/index period separately for the RA and comparison cohorts. We reported the proportion of women in each weight change category by cohort.
We investigated the effect of weight change during the peri-RA/index on subsequent mortality. We initially performed analyses separately in each cohort. Person-years accrued from the end of the early RA/index period (starting after the questionnaire that measured Weight 2) until end of follow-up, date of death, or date of censor, whichever came first. Loss to follow-up or self-reported RA or CTD (for comparators) were censoring events. We used Cox regression to estimate hazard ratios (HR) and 95% confidence intervals (CI) for the association of weight change in the peri-RA/index period and subsequent mortality, adjusting for age and questionnaire period. The next multivariable model adjusted for baseline BMI category. The final multivariable model included age, questionnaire period, BMI category, smoking status, income, physical activity, dietary quality, and MWI. Given that these factors are not only confounders but also mediate the effect of weight change on mortality, we did not adjust for time-varying covariates that occur after baseline in multivariable models, since it would be inappropriate to include mediators in Cox models. For the RA cohort, we additionally included severity factors at diagnosis consisting of serostatus, nodules, and radiographic changes/erosions.
We used Kaplan-Meier curves to display death-free survival according to each of the seven weight change categories after the end of the early RA/index period, defined as the questionnaire in which Weight 2 was reported. We constructed separate plots for the RA and comparator cohorts. We tested for a difference between the curves using the log-rank test.
To examine whether weight change in the peri-RA/index period had a differential effect among women with RA compared to the matched comparators, we combined both cohorts into a single analysis using an indicator variable. We tested for a multiplicative interaction between RA/comparator status and weight change for mortality using an interaction term between these variables and reported the p value.
We tested for the proportional hazards assumption by comparing nested models with and without interaction terms with follow-up time using likelihood ratio tests. The proportional hazards assumption was met in all analyses. We considered a two-sided p value <0.05 as statistically significant in all analyses. Analyses were performed using SAS version 9.4.
RESULTS
Among 121,701 women in the NHS, we identified 902 incident RA cases and 7,884 matched non-RA comparators with weights available in the peri-RA/index period. The characteristics of both cohorts at the beginning of the peri-RA/index period are presented in Table 1, stratified by weight change as defined in Figure 1. In both cohorts, those with severe weight loss (>30 lb) were older, exercised less, had worse dietary quality, were more likely to be obese, were more likely to ever have smoked, and had higher MWI than those with stable weight. Those with severe weight gain (>30 lb) were younger than those with stable weight but exercised less, had less healthy diet, were more likely to be obese, were more likely to ever have smoked, and had higher MWI. In the RA cohort, those with severe weight gain were less likely to be seropositive, have rheumatoid nodules, or have radiographic changes/erosions than those in the other weight change categories.
Table 1.
Age-adjusted characteristics at study baseline 2–4 years prior to index date according to weight loss during the peri-RA* or peri-index* period in the Nurses’ Health Study.
| RA cohort (n=902) | Severe Loss (>30 lb) (n=27) | Moderate Loss (>20–30 lb) (n=28) | Mild Loss (>10–20 lb) (n=87) | Stable (±10 lb) (n=580) | Mild Gain (>10–20 lb) (n=111) | Moderate Gain (>20–30 lb) (n=47) | Severe Gain (>30 lb) (n=22) |
|---|---|---|---|---|---|---|---|
| Mean age, years (SD)1 | 62.0 (8.9) | 59.3 (12.9) | 58.1 (9.1) | 55.8 (9.8) | 53.4 (9.4) | 52.3 (8.3) | 50.0 (6.8) |
| Mean household income, $1000USD (SD) | 60.6 (25.1) | 63.6 (22.0) | 66.4 (29.9) | 64.4 (24.2) | 59.2 (16.2) | 62.5 (22.0) | 58.8 (14.2) |
| Mean moderate to vigorous physical activity, hours per week (SD)2 | 1.5 (2.1) | 1.0 (1.3) | 2.7 (3.0) | 2.5 (3.1) | 2.4 (2.6) | 2.5 (2.6) | 1.1 (1.6) |
| Body mass index category, % | |||||||
| Underweight | 0.0 | 0.0 | 0.7 | 1.9 | 1.0 | 0.0 | 0.0 |
| Normal | 7.0 | 21.4 | 38.6 | 54.6 | 44.3 | 39.5 | 23.2 |
| Overweight | 24.7 | 32.6 | 38.9 | 31.5 | 36.2 | 39.6 | 43.8 |
| Obese | 68.3 | 46.0 | 21.8 | 12.0 | 18.5 | 20.9 | 33.0 |
| Alternate Healthy Eating Index tertiles, %3 | |||||||
| Tertile 1 – least healthy | 18.2 | 15.1 | 26.5 | 28.8 | 23.2 | 23.8 | 56.6 |
| Tertile 2 | 28.8 | 52.5 | 35.5 | 33.1 | 36.0 | 29.9 | 28.5 |
| Tertile 3 – most healthy | 39.1 | 30.1 | 26.9 | 29.7 | 30.9 | 39.0 | 11.9 |
| Smoking status, % | |||||||
| Never | 13.6 | 26.6 | 36.7 | 36.3 | 35.0 | 27.6 | 21.3 |
| Past | 65.2 | 49.0 | 40.8 | 42.5 | 36.8 | 41.1 | 52.2 |
| Current | 21.2 | 24.4 | 22.5 | 20.8 | 27.6 | 31.3 | 26.6 |
| Mean Multimorbidity Weighted Index (SD)4 | 6.0 (5.7) | 2.9 (2.8) | 3.5 (3.6) | 3.2 (3.8) | 2.9 (3.2) | 4.6 (4.6) | 6.4 (5.0) |
| Seropositive (RF or CCP), %*** | 61.8 | 59.8 | 64.0 | 61.2 | 57.5 | 63.0 | 29.9 |
| Rheumatoid nodules, %*** | 14.4 | 10.6 | 20.1 | 13.9 | 6.5 | 6.8 | 0.0 |
| Radiographic changes/erosions, %*** | 29.3 | 42.4 | 29.0 | 30.5 | 27.2 | 33.8 | 3.0 |
|
| |||||||
| Comparison cohort (n=7,884)** | Severe Loss (>30 lb) (n=123) | Moderate Loss (>20–30 lb) (n=181) | Mild Loss (>10–20 lb) (n=524) | Stable (±10 lb) (n=5,445) | Mild Gain (>10–20 lb) (n=1,111) | Moderate Gain (>20–30 lb) (n=320) | Severe Gain (>30 lb) (n=180) |
|
| |||||||
| Mean age, years (SD)1 | 60.1 (9.6) | 61.2 (9.2) | 60.0 (9.7) | 55.6 (9.7) | 52.8 (8.8) | 52.3 (8.6) | 51.4 (9.1) |
| Mean household income, $1000USD (SD) | 58.8 (21.0) | 63.0 (24.7) | 62.5 (25.8) | 64.5 (25.4) | 64.4 (24.5) | 61.3 (20.9) | 61.1 (21.0) |
| Mean moderate to vigorous physical activity, hours per week (SD)2 | 1.2 (1.7) | 1.6 (2.4) | 2.1 (2.6) | 2.6 (3.0) | 2.1 (2.8) | 2.2 (2.8) | 1.7 (2.6) |
| Body mass index category, % | |||||||
| Underweight | 0.0 | 0.0 | 0.2 | 2.0 | 1.2 | 0.9 | 1.4 |
| Normal | 1.7 | 12.0 | 32.3 | 58.8 | 50.6 | 37.1 | 35.2 |
| Overweight | 22.6 | 42.5 | 38.8 | 26.7 | 33.1 | 36.7 | 31.8 |
| Obese | 75.7 | 45.5 | 28.7 | 12.5 | 15.1 | 25.3 | 31.6 |
| Alternative Healthy Eating Index tertiles, %3 | |||||||
| Tertile 1 – least healthy | 34.6 | 29.7 | 27.7 | 27.6 | 27.8 | 26.3 | 32.1 |
| Tertile 2 | 23.3 | 28.6 | 31.6 | 28.3 | 31.2 | 30.1 | 24.2 |
| Tertile 3 – most healthy | 22.5 | 32.2 | 28.5 | 31.4 | 28.2 | 32.2 | 28.7 |
| Smoking status, % | |||||||
| Never | 40.8 | 48.1 | 40.9 | 44.3 | 40.8 | 39.0 | 37.0 |
| Past | 48.6 | 38.4 | 36.7 | 36.0 | 34.4 | 37.8 | 37.4 |
| Current | 10.6 | 13.4 | 22.1 | 19.4 | 24.3 | 21.9 | 25.6 |
| Mean Multimorbidity Weighted Index (SD)4 | 4.3 (4.4) | 3.9 (4.1) | 3.3 (3.8) | 2.5 (3.3) | 2.7 (3.5) | 3.2 (3.6) | 3.5 (3.9) |
Missing values are not reported.
Data on weight were obtained every two years in follow-up during the Nurses’ Health Study. The “peri-RA” period was defined as up to four years before and four years after RA diagnosis to capture early symptoms and the entire early RA period after diagnosis. The “peri-index” period was similarly defined as up to four years before and four years after index date for matched non-RA comparators.
Each RA case was matched with up to 10 women without RA or other connective tissue disease on age and calendar year at index date to form the comparison cohort.
RA factors were obtained on review of medical records at the time of clinical diagnosis.
Value is not age-adjusted.
Physical activity was first assessed in 1980.
Alternative Healthy Eating Index was first assessed in 1984.
The previously validated Multimorbidity Weighted Index is composed of prevalent/serious conditions, each condition weighted by the effect on physical health-related quality of life. We did not include rheumatoid arthritis and other connective tissue diseases, male conditions, and premenopausal female conditions that were not assessed so the MWI included a total of 64 conditions for these analyses.
RA, rheumatoid arthritis; SD, standard deviation; US, United States.
Weight change during the peri-RA/index period
Table 2 shows the proportion of women in each cohort that were in each category of weight change during the peri-RA/index period. Stable weight was most common in both cohorts (64.3% for RA and 69.1% for comparators). More women with RA lost weight (15.8%, 3.0% with severe weight loss) than comparators (10.6%, 1.6% with severe weight loss). A similar proportion of women in both cohorts gained weight during the peri-RA/index period (any gain: 19.9% among women with RA and 20.5% of comparators; severe gain: 2.4% in the RA cohort vs. 2.3% in the comparator cohort).
Table 2.
Percentage of women in each category of weight change during the peri-RA or peri-index period.
| RA cohort (n=902) | Comparison cohort (n=7,884) | |
|---|---|---|
|
|
||
| Severe Loss (>30 lb) | 3.0% | 1.6% |
| Moderate Loss (>20–30 lb) | 3.1% | 2.3% |
| Mild Loss (>10–20 lb) | 9.7% | 6.7% |
| Stable (±10 lb) | 64.3% | 69.1% |
| Mild Gain (>10–20 lb) | 12.3% | 14.1% |
| Moderate Gain (>20–30 lb) | 5.2% | 4.1% |
| Severe Gain (>30 lb) | 2.4% | 2.3% |
RA, rheumatoid arthritis.
Mortality rates after the early RA or matched index period
In the RA cohort, there were 371 (41.2%) deaths during 16,007 person-years of follow-up after the early RA period (mean follow-up per woman of 17.0 (SD 8.8) years, Table 3). In the comparison cohort, there were 2,303 (29.2%) deaths during 150,074 person-years of follow-up after the early index period (mean follow-up per woman of 18.4 (SD 9.4) years). The RA cohort had higher absolute mortality rate (1,770–6,909 deaths/100,000 person-years) across all weight change categories than comparators (1,305–3,717 deaths/100,000 person-years).
Table 3.
Hazard ratios for mortality according to peri-RA* or peri-index* weight change, analyzed separately in the RA and comparison cohorts.
| Deaths/Person-years | Mortality rate** | Age-adjusted HR (95% CI)1 | Age and pre-RA/index BMI-adjusted HR (95% CI)2 | Multivariable HR (95% CI)3 | Multivariable and RA severity- adjusted HR (95% CI)4 | |
|---|---|---|---|---|---|---|
| RA cohort (n=902) | ||||||
| Severe Loss (>30 lb) | 19/275 | 6,909 | 3.51 (2.12–5.80) | 3.10 (1.80–5.33) | 2.78 (1.58–4.89) | 2.71 (1.54–4.77) |
| Moderate Loss (>20–30 lb) | 16/376 | 4,251 | 1.47 (0.86–2.52) | 1.36 (0.77–2.40) | 1.35 (0.76–2.38) | 1.32 (0.75–2.34) |
| Mild Loss (>10–20 lb) | 41/1,274 | 3,219 | 1.72 (1.22–2.42) | 1.71 (1.21–2.41) | 1.78 (1.25–2.54) | 1.75 (1.22–2.49) |
| Stable (±10 lb) | 218/10,530 | 2,070 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Mild Gain (>10–20 lb) | 51/2,229 | 2,288 | 1.26 (0.92–1.73) | 1.27 (0.93–1.74) | 1.21 (0.88–1.67) | 1.25 (0.90–1.74) |
| Moderate Gain (>20–30 lb) | 18/870 | 2,069 | 1.30 (0.79–2.14) | 1.33 (0.81–2.19) | 1.05 (0.63–1.75) | 1.08 (0.64–1.80) |
| Severe Gain (>30 lb) | 8/452 | 1,770 | 1.58 (0.76–3.28) | 1.44 (0.69–3.01) | 1.45 (0.69–3.07) | 1.57 (0.74–3.32) |
| Comparison cohort (n=7,884)*** | ||||||
| Severe Loss (>30 lb) | 55/1,480 | 3,717 | 2.98 (2.27–3.91) | 2.42 (1.81–3.22) | 2.16 (1.61–2.88) | - |
| Moderate Loss (>20–30 lb) | 69/2,311 | 2,986 | 1.87 (1.47–2.38) | 1.70 (1.33–2.18) | 1.50 (1.17–1.92) | - |
| Mild Loss (>10–20 lb) | 209/8,205 | 2,547 | 1.46 (1.26–1.68) | 1.39 (1.20–1.61) | 1.24 (1.06–1.43) | - |
| Stable (±10 lb) | 1,532/104,883 | 1,461 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | - |
| Mild Gain (>10–20 lb) | 296/22,892 | 1,293 | 1.14 (1.00–1.29) | 1.13 (1.00–1.28) | 1.09 (0.96–1.24) | - |
| Moderate Gain (>20–30 lb) | 94/6,625 | 1,419 | 1.34 (1.09–1.65) | 1.29 (1.05–1.59) | 1.20 (0.97–1.48) | - |
| Severe Gain (>30 lb) | 48/3,679 | 1,305 | 1.42 (1.06–1.89) | 1.36 (1.01–1.81) | 1.19 (0.89–1.59) | - |
Data on weight were obtained every two years in follow-up during the Nurses’ Health Study. The “peri-RA” period was defined as up to four years before and four years after RA diagnosis to capture early symptoms and the entire early RA period after diagnosis. The “peri-index” period was similarly defined as up to four years before and four years after index date for matched non-RA comparators.
Per 100,000 person-years
The comparison cohort was formed by matching each RA case with up to 10 women without RA based on same age and calendar year at the index date of RA diagnosis.
Adjusted for age (continuous, years) and calendar year.
Additionally adjusted for pre-RA/index date BMI (<18.5, 18.5–24.9, 25–29.9, 30–34.9, ≥35 kg/m2).
Additionally adjusted for cigarette smoking (never, past, current), physical activity (hours of moderate or vigorous exercise per week, continuous), census-tract household family income (<$40K, $40K+ per year), and Alternative Healthy Index dietary score (tertiles), and the Multimorbidity Weighted Index (continuous).
Additionally adjusted additionally adjusted for RA serostatus (seropositive, seronegative), rheumatoid nodules, and radiographic changes/erosions according to medical record review at RA diagnosis. This model was not applicable to the comparison cohort.
BMI, body mass index; CI, confidence interval; HR, hazard ratio; RA, rheumatoid arthritis; SD, standard deviation.
Mortality in the RA cohort by weight change in the peri-RA period
In the age-adjusted model, severe weight loss in the peri-RA period had HR for subsequent mortality of 3.51 (95%CI2.12–5.80) compared to stable weight. There was no statistically significant association between moderate loss or any weight gain category in the peri-RA period (compared to stable weight) and mortality. However, mild weight loss in the peri-RA period had a HR for mortality of 1.72 (95%CI1.22–1.42) compared to stable weight. These associations persisted when adjusting for baseline BMI, although the HR for severe loss was attenuated (HR 3.10, 95%CI1.80–5.33). In the multivariable model adjusting for baseline BMI, smoking, physical activity, diet quality, income, and multimorbidities, severe loss (HR 2.78, 95%CI1.58–4.89) and mild loss (HR 1.78, 95%CI1.25–2.54) remained statistically significantly associated with increased subsequent mortality compared to stable weight. These associations were similar when further adjusting for RA severity factors of serostatus, rheumatoid nodules, and erosions (severe loss: HR 2.71, 95%CI1.54–4.77 compared to stable weight).
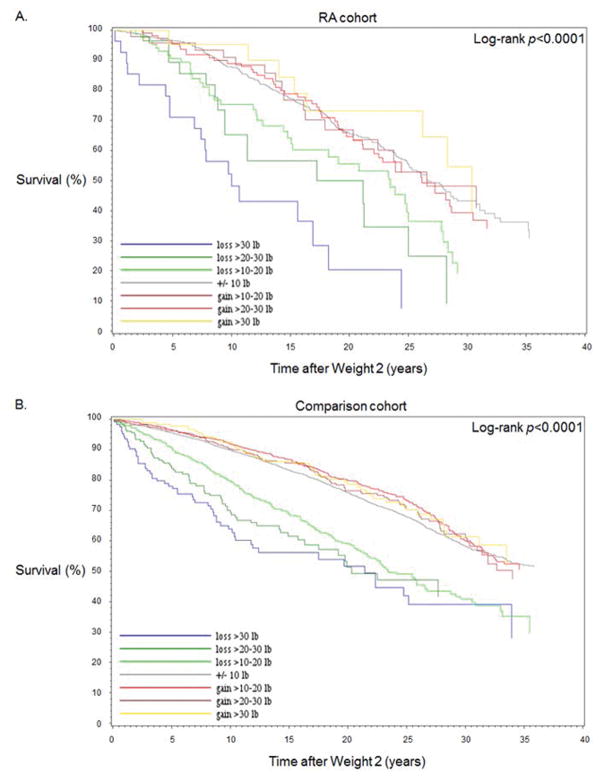
Figure 2A displays the Kaplan-Meier curves for women with RA for death-free survival after the end of the early RA period. The survival curves according to weight change categories were statistically different (p<0.0001). Women with severe weight loss had the worst survival, followed by moderate weight loss and mild loss. The curves for stable weight and all weight gain categories were relatively similar. The median survival among women with RA and severe weight loss was 8.0 years (interquartile range [IQR] 4.8–12.0). Women with stable weight had median survival of 16.2 years (IQR 10.8–24.0) after the end of the early RA period.
Figure 2.
Kaplan-Meier survival curves for mortality after the early RA or index period according to peri-RA or peri-index weight change among A) the RA cohort (n=902) and B) the comparison cohort (n=7,884).
Mortality in the comparison cohort by weight change in the peri-index period
In the age- and BMI-adjusted analysis performed only among matched comparators, there were statistically significant associations between both weight loss and weight gain in the peri-index period for subsequent mortality compared to stable weight (severe loss HR 2.42, 95%CI1.81–3.22; severe gain HR 1.36, 95%CI1.01–1.81). However, when further adjusting for smoking, physical activity, diet quality, income, and multimorbidities, only severe and moderate weight loss remained significantly associated with mortality. Severe loss in the peri-RA period had HR for subsequent mortality of 2.16 (95%CI1.61–2.88) in the multivariable model.
Figure 2B displays the Kaplan-Meier curves for matched comparators for death-free survival after the end of the early index period. The survival curves according to weight change categories were statistically different for comparators (p<0.0001). Similar to the RA Kaplan-Meier curves, the comparator survival curves for stable weight and weight gain categories were overlapping, while weight loss categories showed a graded effect across severity of weight loss. The median survival for comparators with severe weight loss was 8.8 years (IQR 4.4–14.0). Women with stable weight had median survival of 17.9 years (IQR 11.3–26.0) after the end of the early index period. Comparators had longer survival than women with RA in all analogous weight change categories.
Combined analysis of women with RA and matched comparators
Figure 3 displays the multivariable HRs for the combined analysis of RA and matched comparators (consisting of a total of 8,786 women) with stable weight comparators as the reference group. In this analysis, the 95%CIs were wide at the extreme weight change categories due to low sample size, particularly for women with RA. Women with RA had higher HR for mortality than comparators in every weight change category. The highest HR for mortality for both RA and comparators was in the severe weight loss category. Women with RA and severe weight loss in the peri-RA period had HR for mortality of 2.92 (95%CI1.83–4.66) compared to comparators with stable weight. Comparators with severe weight loss had HR for mortality of 2.21 (95%CI1.66–2.95) compared to comparators with stable weight. There was no statistical interaction for RA/comparator status and weight change category for mortality (p=0.68).
Figure 3.
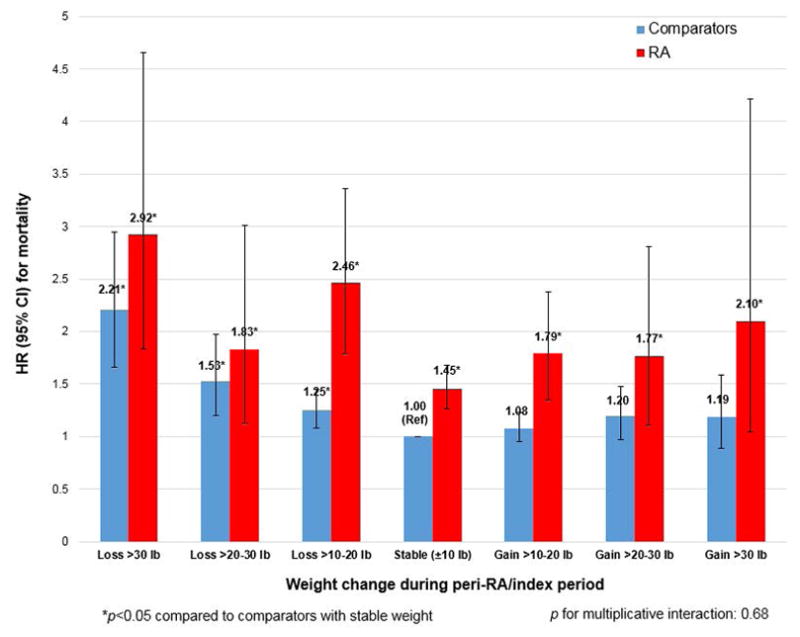
Multivariable hazard ratios for mortality according to peri-RA or peri-index weight change, combining the RA and comparison cohorts into a single analysis (n=8,786).
DISCUSSION
During 40 years of prospective follow-up, we found that severe weight loss defined as >30 lb around RA diagnosis, and a similar matched period among women without RA, was associated with increased mortality compared to stable weight. We studied weight change during the period around RA diagnosis, since weight change during this period is more likely to be related to intrinsic disease processes rather than general aging processes. While the absolute risk of death for women with RA was higher than women without RA, there was a similar trend between the relative risk of weight change on mortality for both groups. Therefore, reports associating BMI categories and weight loss with mortality risk among RA cohorts may have been detecting general population effects rather than RA-specific effects.
Studies have established that patients with RA have increased mortality compared to the general population(1–4). Recent investigations have focused on either RA-specific or potentially modifiable factors contributing to this mortality gap(5, 33–36). For example, smoking has been associated with worsened RA severity factors such as disease activity and erosions, which may in turn contribute to excess mortality. Our group recently reported that smoking contributes more to mortality risk among patients with RA than matched non-RA comparators(5). Since patients with RA have excess cardiovascular disease than the general population, cardiometabolic risk factors, such as obesity, may be related to outcomes including mortality(37). In contrast to the generally adverse effects of obesity on many health outcomes, obesity has been paradoxically associated with decreased risk of bone erosions in RA compared to normal BMI(7). This finding led to other investigations in which obesity has been associated with expected findings of worsened disease activity and functional status(7, 38, 39). These discrepant results prompted further investigations of downstream events including total and cause-specific mortality in RA(8, 9, 11, 12).
These prior studies investigating the association of weight change and BMI categories with mortality were typically performed in RA-only cohorts of longstanding duration with relatively limited follow-up to death. Therefore, it was unclear whether the associations reported were related specifically to RA or were describing typical patterns that might emerge in any population that was frail and advanced in age. Patients with normal BMI measured near the end of life may have reached this state due to pathologic weight loss and have higher than expected mortality compared to patients who maintained obesity or overweight. Therefore, near the end of life, “normal” BMI may be relatively less healthy than overweight or obese individuals that remained that way by not losing weight(17). This hypothesis is further supported by increased mortality of patients with underweight BMI since elderly patients often unintentionally become underweight due to pathologic states rather than healthy, intentional weight loss through diet and physical activity(13). Therefore, studying weight change has been considered a method to correct the “obesity paradox” of lower than expected mortality for those with obesity, particularly near the end of life(11).
Two previous studies have used weight loss as a method to correct the obesity paradox for mortality in RA(11, 12). Baker and colleagues found that BMI loss of ≥1 kg/m2 was associated with 2-fold increased mortality compared to those that did not lose weight in an RA cohort(11). Patients who reached underweight after previously being obese had the highest risk of death(11). A follow-up study investigated cause-specific mortality and found that weight loss was associated with cardiovascular and cancer mortality, while being underweight was associated with increased respiratory mortality(12). Both studies were performed using data from the Veterans Affairs so were composed of mostly older men (mean age 63.5 and 63.4 years in each study), many with established RA (median 7.4 and 8.2 years), and relatively limited follow-up for mortality (median 5.5 and 3.2 years)(11, 12).
Our study differs from these prior studies in several important ways. First, we included matched comparators who had identical measures as those with RA. Second, since we identified incident RA during follow-up in the NHS we could investigate weight change during the early RA period and create a similar period for comparators. Therefore, we captured weight change around RA diagnosis when disease-specific processes were most likely to have contributed to weight change. Since pathologic weight loss often precedes death, we designed our study around RA diagnosis rather than observing weight changes near the end of life which may be related to pathologic underlying processes leading to death rather than RA-specific processes(13). Third, we investigated weight gain in addition to weight loss. While pathologic weight loss is well known to portend high risk of death in studies of the general population as well as RA, weight gain is less studied particularly in RA. Weight gain may have had a unique relationship with mortality in RA as dietary changes, medication side effects, and physical activity changes might result in weight gain during the early RA period(40). Prior studies investigating weight loss included both weight stability and gain in the reference group so the relationship between weight loss, stability, and weight gain was previously unclear(11, 12). Lastly, our study had lengthy follow-up that commenced after the end of the early RA period.
We found that severe weight loss (>30 lb) similarly increased mortality for both RA and non-RA. In both cohorts, this association was attenuated, but not completely explained, by adjustment for baseline BMI, smoking, physical activity, dietary quality, income, and multimorbidities. In the RA cohort, adjustment for RA severity factors at diagnosis including serostatus, nodules, and radiographic change/erosions, did not explain the increased risk. As expected for a chronic disease, more women with RA had weight loss of 10 lb or more compared to the comparators (15.8% among RA; 10.6% among comparators). Some of those with weight loss may have had rheumatoid cachexia explaining weight loss, which has previously been associated with worse RA outcomes and increased mortality(41). However, those with severe weight loss in the comparison cohort also had similar mortality so rheumatoid cachexia does not fully explain the increased mortality in the severe weight loss group among those with RA.
While the obesity paradox for mortality may argue that weight gain could be protective for mortality, we hypothesized that those with severe weight gain would have increased mortality, perhaps more evident among patients with RA since factors such as glucocorticoid use and decreased physical activity would presumably differentially affect those with RA. However, we found that the proportion of those who experienced weight gain was similar in both cohorts (19.9% for RA; 20.5% for comparators). Further, we found no statistical association of weight gain with mortality compared to stable weight in the early RA period. While the point estimate (HR 1.45) for severe weight gain may suggest a modestly increased mortality risk in the RA cohort, the confidence interval was wide and was not statistically significant. It is possible that we were underpowered to detect a true effect of weight gain on mortality risk in RA, but it is unlikely that weight gain in the early RA period confers a protective effect on mortality. In the comparator cohort, there was a statistically significant association of weight gain with mortality that demonstrated a dose effect in the age and BMI-adjusted analysis. However, this effect was no longer detectable when adjusting for confounders, despite adequate power. Overall, these results do not suggest that weight gain is strongly associated with subsequent risk of mortality independent of known mortality risk factors.
Our study has limitations to consider. The NHS is composed of only women who were mostly white and working at baseline in 1976. It is unclear how generalizable these results might be to other contemporary populations. Overall, our results associating severe weight loss with increased mortality are similar to the two prior studies investigating weight loss and mortality that were performed among mostly men with RA(11, 12). We constructed the weight change period around RA diagnosis as a time point relevant to all patients diagnosed with RA and chose the baseline prior to RA diagnosis to protect against reverse causation with RA symptoms affecting the initial weight among patients with RA. It is still possible that early symptoms may have affected weight at the initial assessment among those who later developed RA. While we had detailed data available for adjustment, residual confounding from factors such as severity of multimorbidities, rather than just their presence, may have affected the weight loss association. We did not have data available about RA characteristics after diagnosis such as medication use, disease activity, or bone erosions/deformities. When adjusting for the RA severity factors that were available at diagnosis, we found similar results. Since these RA characteristics are not relevant to comparators without RA, we would have been unable to investigate the effect of these factors in the combined analysis of RA and matched comparators. While BMI and weight have been widely used in epidemiologic analyses, patients with RA may have different body compositions than non-RA comparators that we were unable to analyze(42). The reason for weight change was unavailable so we cannot determine whether women were intending to lose weight through diet and exercise. We suspect that severe weight loss may have been due to pathologic processes for most women. Therefore, these results should not be interpreted as discouraging healthy weight loss through diet and exercise.
In conclusion, we found that severe weight loss during the early RA period was associated with increased subsequent mortality risk for both RA and matched comparators. Compared to stable weight during the early RA period, we found that weight gain was not associated with subsequent mortality risk either for RA or matched comparators. These results emphasize the need to consider a non-RA population when investigating the relationship between an exposure and an outcome that are not specifically related to the disease population of interest. These results extend on prior reports on the effect of weight loss on mortality in RA and argue that findings were related to general population aging effects rather than specifically to RA. While weight control in the early RA period may be important for disease processes such as bone erosions or disease activity, the relationship between weight change and mortality in the early RA period is likely similar to that observed in the general population.
Acknowledgments
Funding/Support: This work was supported by the Rheumatology Research Foundation Disease-Targeted Innovative Award (Dr. Choi) and Scientist Development Awards (Dr. Sparks and Dr. Barbhaiya). This work was also supported by the National Institutes of Health (grant numbers K24 AR052403, P60 AR047782, L30 AR066953, L30 AR070514, R01 AR049880, UM1 CA186107, K23 AR069688, K01 AR064351, and T32 AR007530). The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank the participants of the NHS for their dedicated participation in this longitudinal study as well as the NHS staff members at the Channing Division of Network Medicine (Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School). We also thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual contact, and all authors approved the final version to be published. Dr. Sparks had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Sparks, Chang, Nguyen, Zhang, Choi, Karlson
Acquisition of data. Sparks, Barbhaiya, Tedeschi, Costenbader, Karlson
Analysis and interpretation of data. Sparks, Chang, Nguyen, Barbhaiya, Tedeschi, Lu, Costenbader, Zhang, Choi, Karlson
References
- 1.Sparks JA, Chang SC, Liao KP, Lu B, Fine AR, Solomon DH, et al. Rheumatoid Arthritis and Mortality Among Women During 36 Years of Prospective Follow-Up: Results From the Nurses’ Health Study. Arthritis Care Res (Hoboken) 2016;68(6):753–62. doi: 10.1002/acr.22752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez A, Maradit Kremers H, Crowson CS, Nicola PJ, Davis JM, 3rd, Therneau TM, et al. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum. 2007;56(11):3583–7. doi: 10.1002/art.22979. [DOI] [PubMed] [Google Scholar]
- 3.England BR, Sayles H, Michaud K, Caplan L, Davis LA, Cannon GW, et al. Cause-Specific Mortality in Male US Veterans With Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2016;68(1):36–45. doi: 10.1002/acr.22642. [DOI] [PubMed] [Google Scholar]
- 4.Dadoun S, Zeboulon-Ktorza N, Combescure C, Elhai M, Rozenberg S, Gossec L, et al. Mortality in rheumatoid arthritis over the last fifty years: systematic review and meta-analysis. Joint Bone Spine. 2013;80(1):29–33. doi: 10.1016/j.jbspin.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Sparks JA, Chang SC, Nguyen UDT, Barbhaiya M, Tedeschi SK, Lu B, et al. Smoking behavior changes in the early rheumatoid arthritis period and risk of mortality during 36 years of prospective follow-up. Arthritis Care Res (Hoboken) 2017 doi: 10.1002/acr.23269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin B, Yang M, Fu H, Ma N, Wei T, Tang Q, et al. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose-response meta-analysis. Arthritis Res Ther. 2015;17:86. doi: 10.1186/s13075-015-0601-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vidal C, Barnetche T, Morel J, Combe B, Daien C. Association of Body Mass Index Categories with Disease Activity and Radiographic Joint Damage in Rheumatoid Arthritis: A Systematic Review and Metaanalysis. J Rheumatol. 2015;42(12):2261–9. doi: 10.3899/jrheum.150224. [DOI] [PubMed] [Google Scholar]
- 8.Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165(14):1624–9. doi: 10.1001/archinte.165.14.1624. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64(10):1471–9. doi: 10.1002/acr.21627. [DOI] [PubMed] [Google Scholar]
- 10.Mikuls TR, Fay BT, Michaud K, Sayles H, Thiele GM, Caplan L, et al. Associations of disease activity and treatments with mortality in men with rheumatoid arthritis: results from the VARA registry. Rheumatology (Oxford) 2011;50(1):101–9. doi: 10.1093/rheumatology/keq232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker JF, Billig E, Michaud K, Ibrahim S, Caplan L, Cannon GW, et al. Weight Loss, the Obesity Paradox, and the Risk of Death in Rheumatoid Arthritis. Arthritis Rheumatol. 2015;67(7):1711–7. doi: 10.1002/art.39136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.England BR, Baker JF, Sayles H, Michaud K, Caplan L, Davis LA, et al. Body Mass Index, Weight Loss, and Cause-Specific Mortality in Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2017 doi: 10.1002/acr.23258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alley DE, Metter EJ, Griswold ME, Harris TB, Simonsick EM, Longo DL, et al. Changes in weight at the end of life: characterizing weight loss by time to death in a cohort study of older men. Am J Epidemiol. 2010;172(5):558–65. doi: 10.1093/aje/kwq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oga EA, Eseyin OR. The Obesity Paradox and Heart Failure: A Systematic Review of a Decade of Evidence. J Obes. 2016;2016:9040248. doi: 10.1155/2016/9040248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim NH, Lee J, Kim TJ, Kim NH, Choi KM, Baik SH, et al. Body Mass Index and Mortality in the General Population and in Subjects with Chronic Disease in Korea: A Nationwide Cohort Study (2002–2010) PLoS One. 2015;10(10):e0139924. doi: 10.1371/journal.pone.0139924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Somes GW, Kritchevsky SB, Shorr RI, Pahor M, Applegate WB. Body mass index, weight change, and death in older adults: the systolic hypertension in the elderly program. Am J Epidemiol. 2002;156(2):132–8. doi: 10.1093/aje/kwf019. [DOI] [PubMed] [Google Scholar]
- 18.Albrecht K, Richter A, Callhoff J, Huscher D, Schett G, Strangfeld A, et al. Body mass index distribution in rheumatoid arthritis: a collaborative analysis from three large German rheumatoid arthritis databases. Arthritis Res Ther. 2016;18:149. doi: 10.1186/s13075-016-1043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller R, Kull M, Polluste K, Aart A, Eglit T, Lember M, et al. The metabolic profile in early rheumatoid arthritis: a high prevalence of metabolic obesity. Rheumatol Int. 2017;37(1):21–7. doi: 10.1007/s00296-016-3464-9. [DOI] [PubMed] [Google Scholar]
- 20.Karlson EW, Sanchez-Guerrero J, Wright EA, Lew RA, Daltroy LH, Katz JN, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol. 1995;5(4):297–302. doi: 10.1016/1047-2797(94)00096-c. [DOI] [PubMed] [Google Scholar]
- 21.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 22.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1(6):466–73. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Contreras-Yanez I, Pascual-Ramos V. Window of opportunity to achieve major outcomes in early rheumatoid arthritis patients: how persistence with therapy matters. Arthritis Res Ther. 2015;17:177. doi: 10.1186/s13075-015-0697-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramagli A, Corbacho I, Linhares F, de Abreu P, Teijeiro R, Garau M, et al. Characteristics of Patients With Early-Onset Arthritis in Latin America: Description of the REPANARC Cohort. J Clin Rheumatol. 2015;21(6):283–8. doi: 10.1097/RHU.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 25.Ajeganova S, Andersson ML, Frostegard J, Hafstrom I. Disease factors in early rheumatoid arthritis are associated with differential risks for cardiovascular events and mortality depending on age at onset: a 10-year observational cohort study. J Rheumatol. 2013;40(12):1958–66. doi: 10.3899/jrheum.130365. [DOI] [PubMed] [Google Scholar]
- 26.Bao Y, Han J, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, et al. Association of nut consumption with total and cause-specific mortality. N Engl J Med. 2013;369(21):2001–11. doi: 10.1056/NEJMoa1307352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140(11):1016–9. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 28.de Onis M, Habicht JP. Anthropometric reference data for international use: recommendations from a World Health Organization Expert Committee. Am J Clin Nutr. 1996;64(4):650–8. doi: 10.1093/ajcn/64.4.650. [DOI] [PubMed] [Google Scholar]
- 29.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25(1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, et al. Validity of a Dietary Questionnaire Assessed by Comparison With Multiple Weighed Dietary Records or 24-Hour Recalls. Am J Epidemiol. 2017;185(7):570–84. doi: 10.1093/aje/kww104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Y, Sparks JA, Malspeis S, Costenbader KH, Hu FB, Karlson EW, et al. Long-term dietary quality and risk of developing rheumatoid arthritis in women. Ann Rheum Dis. 2017 doi: 10.1136/annrheumdis-2016-210431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei MY, Kawachi I, Okereke OI, Mukamal KJ. Diverse Cumulative Impact of Chronic Diseases on Physical Health-Related Quality of Life: Implications for a Measure of Multimorbidity. Am J Epidemiol. 2016;184(5):357–65. doi: 10.1093/aje/kwv456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viatte S, Plant D, Han B, Fu B, Yarwood A, Thomson W, et al. Association of HLA-DRB1 haplotypes with rheumatoid arthritis severity, mortality, and treatment response. JAMA. 2015;313(16):1645–56. doi: 10.1001/jama.2015.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62(6):1583–91. doi: 10.1002/art.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez A, Icen M, Kremers HM, Crowson CS, Davis JM, 3rd, Therneau TM, et al. Mortality trends in rheumatoid arthritis: the role of rheumatoid factor. J Rheumatol. 2008;35(6):1009–14. [PMC free article] [PubMed] [Google Scholar]
- 36.Chehata JC, Hassell AB, Clarke SA, Mattey DL, Jones MA, Jones PW, et al. Mortality in rheumatoid arthritis: relationship to single and composite measures of disease activity. Rheumatology (Oxford) 2001;40(4):447–52. doi: 10.1093/rheumatology/40.4.447. [DOI] [PubMed] [Google Scholar]
- 37.Kremers HM, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50(11):3450–7. doi: 10.1002/art.20612. [DOI] [PubMed] [Google Scholar]
- 38.Ajeganova S, Andersson ML, Hafstrom I, Group BS. Association of obesity with worse disease severity in rheumatoid arthritis as well as with comorbidities: a long-term followup from disease onset. Arthritis Care Res (Hoboken) 2013;65(1):78–87. doi: 10.1002/acr.21710. [DOI] [PubMed] [Google Scholar]
- 39.Stavropoulos-Kalinoglou A, Metsios GS, Panoulas VF, Nevill AM, Jamurtas AZ, Koutedakis Y, et al. Underweight and obese states both associate with worse disease activity and physical function in patients with established rheumatoid arthritis. Clin Rheumatol. 2009;28(4):439–44. doi: 10.1007/s10067-008-1073-z. [DOI] [PubMed] [Google Scholar]
- 40.Baker JF, Sauer BC, Cannon GW, Teng CC, Michaud K, Ibrahim S, et al. Changes in Body Mass Related to the Initiation of Disease-Modifying Therapies in Rheumatoid Arthritis. Arthritis Rheumatol. 2016;68(8):1818–27. doi: 10.1002/art.39647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Summers GD, Metsios GS, Stavropoulos-Kalinoglou A, Kitas GD. Rheumatoid cachexia and cardiovascular disease. Nat Rev Rheumatol. 2010;6(8):445–51. doi: 10.1038/nrrheum.2010.105. [DOI] [PubMed] [Google Scholar]
- 42.Ngeuleu A, Allali F, Medrare L, Madhi A, Rkain H, Hajjaj-Hassouni N. Sarcopenia in rheumatoid arthritis: prevalence, influence of disease activity and associated factors. Rheumatol Int. 2017;37(6):1015–20. doi: 10.1007/s00296-017-3665-x. [DOI] [PubMed] [Google Scholar]