Abstract
Cardiorespiratory fitness (CRF) is routinely investigated in older adults; however, the most appropriate CRF measure to use for this population has received inadequate attention. This study aimed to (i) evaluate the reliability and validity of the oxygen uptake efficiency slope (OUES) as a sub-maximal measurement of CRF, (ii) examine demographic, risk-factor, and exercise testing differences in older adults who satisfied standardized criteria for a peak oxygen consumption (V̇O2peak) test compared to those who did not and (iii) determine the difference between directly measured V̇O2peak values and OUES-predicted V̇O2peak values. One hundred ten enrollees from the Wisconsin Registry for Alzheimer’s Prevention participated in this study. Participants performed a graded maximal exercise test and wore an accelerometer for 7 days. For each participant, the OUES was calculated at 75%, 90% and 100% of exercise duration. V̇O2peak was recorded at peak effort, and one week of physical activity behavior was measured. OUES values calculated at separate relative exercise durations displayed excellent reliability (ICC=.995; p<.001), and were strongly correlated with V̇O2peak (rrange=.801–.909; p<.001). As hypothesized, participants who did not satisfy V̇O2peak criteria were significantly older than those who satisfied criteria (p=.049) and attained a directly measured V̇O2peak that was 2.31 mL·kg·min−1 less than the value that was predicted by OUES V̇O2peak (p=.003). Older adults are less likely to satisfy V̇O2peak criteria, which results in an underestimation of their CRF. Without adhering to standardized criteria, V̇O2peak measurement error may lead to misinterpretation of CRF and age-related associations. Here, we conclude that OUES is a reliable, valid measurement of CRF which does not require achievement of standardized criteria.
Keywords: Oxygen uptake efficiency slope, oxygen consumption, cardiopulmonary exercise testing, maximal exercise test, sub-maximal exercise test, Alzheimer’s disease
1. Introduction
In the aging literature there is increasing recognition for the link between physical activity behavior and prevention of Alzheimer’s disease (AD) [1–3]. Physical inactivity has the highest estimated population attributable risk for AD in the United States (21%), and third worldwide (12.7%) [4]. Moreover, cardiorespiratory fitness (CRF) a direct measure of aerobic capacity, and physical activity behavior are both positively associated with brain health in older adulthood (for reviews see: [5, 6]). Therefore, CRF and physical activity behavior are important variables to take into account when evaluating risk for future AD diagnosis. Maximal oxygen consumption (V̇O2max), the amount of oxygen consumed by a participant during maximal exercise is the gold standard measure for evaluating CRF [7]. However, obtaining a V̇O2max as defined by standardized criteria including a plateau in oxygen consumption with increased work [8] may be problematic for older adults [9] and may increase the risk of an adverse event (e.g., myocardial infarction, arrhythmia) [10]. A common solution is to relax V̇O2max criteria (plateau in oxygen consumption) and use the level of oxygen consumption at peak exercise (V̇O2peak) as the measure of CRF [11]. However, the criteria for judging the validity of a V̇O2peak test varies throughout the aging literature [12–14] and the consequences of using more liberal criteria for estimating CRF in older adults have received inadequate attention [7, 15].
One consequence of failing to set and adhere to standardized criteria is the underestimation of CRF, which may obscure reported relationships between CRF and changes in health outcomes [16]. This is a primary concern when fitness testing older adults as many outcomes of interest such as brain structure and cognitive function are known to decline with age [17–19]. Therefore, a valid measure of CRF that does not require older adults to satisfy standardized criteria would be a valuable resource for scientists investigating relationships between CRF and AD related outcomes.
The oxygen uptake efficiency slope (OUES) is defined as the relationship between oxygen consumption [absolute V̇O2 (mL·min−1)] and minute ventilation V̇E (L·min−1)] [20]. This relationship is an indirect measure of how efficiently the musculoskeletal system extracts oxygen from the cardiopulmonary system during exercise. Previous reports have shown OUES to have excellent test-retest reliability [21], be highly correlated with V̇O2peak and relatively stable throughout a graded exercise test, suggesting it could serve as a complimentary or alternative measure of CRF [20]. This is particularly relevant for participants or populations that have difficulty providing peak effort or may be challenged to satisfy objective criteria for a maximal exercise test. A majority of OUES research has been conducted in adolescents, young adults, and patients with cardiac disease (see review: [22]). For older adults, particularly those with or at-risk for dementia, OUES may offer an alternative measurement of CRF when widely used oxygen consumption criteria can not be satisfied. Thus, using this measure would decrease the likelihood of an adverse event [23] and increase the number of valid tests that can be used when determining relationships between fitness and health outcomes. However, the reliability and validity of this metric for this specific at-risk population has not been established.
There were three primary objectives to this study. (i) Evaluate the reliability and validity of OUES as a sub-maximal measurement of CRF in an older adult at-risk sample by examining the reliability between OUES sampled at 75%, 90%, and 100% of exercise duration; and examining the strength of association between OUES and an established and widely used measure of CRF (V̇O2peak). (ii) Explore demographic, risk-factor, and exercise testing differences in older adults who satisfied standardized peak effort criteria compared to those who did not. (iii) Determine the difference between directly measured V̇O2peak values and OUES-predicted V̇O2peak values. We hypothesized that (i) OUES would be significantly and strongly correlated across separate relative exercise durations, and significantly and strongly correlated with V̇O2peak, (ii) individuals who did not satisfy V̇O2peak criteria would be older than those who satisfied criteria, and (iii) the recorded V̇O2peak values for those who did not satisfy criteria would underestimate their OUES-predicted V̇O2peak.
2. Methods
2.1 Participants
All participants were recruited from the Wisconsin Registry for Alzheimer’s Prevention (WRAP). The WRAP is a longitudinal registry composed of more than 1,500 cognitively healthy adults at elevated risk AD [24]. WRAP participants are free of major medical conditions (e.g. neurological diseases, psychiatric disorders), and undergo waves of clinical and neuropsychological testing. For the present study, WRAP participants were invited to participate in a CRF test and physical activity assessment via phone calls and recruitment letters. One hundred and ten participants were deemed eligible and agreed to participate in the current study. For this study the following risk factors for AD were measured: age, family history of AD, carriers of the apolipoprotein epsilon 4 allele (APOE-e4), years of formal education, obesity, and physical activity behavior. Study participants were determined to be cognitively healthy using the Mini-Mental State Examination (MMSE ≥ 24) and were excluded from CRF testing for any of the following: documented vascular disease, type 1 or 2 diabetes mellitus, severe untreated hypertension (> 200/100 mm Hg), and the inability to safely walk on a treadmill. The University of Wisconsin Institutional Review Board approved all study procedures and written informed consent was obtained from all individual participants.
2.2 Cardiorespiratory fitness assessment
Exercise tests were conducted under controlled environmental conditions (altitude of 259 meters, 22–25°C, and 40 – 60% relative humidity) by a certified exercise physiologist along with a trained exercise specialist following a resting twelve-lead electrocardiogram (ECG) assessment. The exercise test was completed on a motor driven treadmill using a modified Balke protocol [25]. Comfortable walking speeds were determined during a warm-up period and kept constant for the duration of testing. The majority of participants walked at 3.5 miles per hour. However, if the participant indicated that this speed was uncomfortable, a slower walking speed was chosen. Following a 2-minute warm-up at 0% grade, the grade of the treadmill was increased by 2.5% every 2-minutes until the participant reached volitional exhaustion or indicated they could no longer continue.
During the exercise test, V̇O2, carbon dioxide production (V̇CO2), V̇E, and respiratory exchange ratio (RER) were calculated at 30-second intervals using a metabolic cart (TrueOne® 2400 Parvomedics, Sandy, UT) and a two-way non-rebreathing mask (Hans Rudolph, Shawnee, KS). Heart rate (HR) was continuously measured through a twelve-channel ECG device (Schiller CS-200 Exercise Stress System, Baar, Switzerland). Rating of perceived exertion (RPE) [26] and blood pressure (Welch Allyn DS-6601-300, Skaneateles Falls, NY) were recorded at 2-minute intervals throughout the exercise test. The system was calibrated volumetrically (three-liter piston syringe), and gas corrected for barometric pressure, temperature, and humidity (Davis Vantage Vue) using standard procedures within 4 hours of the exercise test to ensure accuracy.
The OUES was determined for each participant by calculating the regression slope from the linear relationship of absolute V̇O2 (mL·min−1) plotted as a function of log10 V̇E (L·min−1) (V̇O2 = log10 V̇E + b) [20]. A higher OUES value, that is, a steeper V̇O2/V̇E slope indicates more efficient oxygen extraction from the cardiopulmonary system by the working skeletal muscles [20]. Because the OUES value is calculated as a regression slope, the unit is arbitrary. Regression slope analyses included metabolic data collected during the graded exercise test and excluded the warm up and recovery stages due to irregular ventilation that is often observed during these stages. OUES was calculated at 75% (OUES75), 90% (OUES90), and 100% (OUES100) of the exercise duration. Normalized OUES values that account for body surface area (BSA) were also calculated (OUESnormalized = OUES/BSA) at the same exercise durations: OUES75normalized, OUES90normalized, and OUES100normalized [27]. Because of the delay to reach steady state (i.e., oxygen deficit) during each stage of a graded exercise test, we inspected all tests to ensure that participants completed at least one minute of the final stage of exercise.
Peak effort was determined based on American College of Sports Medicine criteria of satisfying at least two of the following: (i) RER ≥ 1.1, (ii) change in V̇O2 < 200 mL with an increase in work, (iii) RPE ≥ 17, and (iv) achieving at least 90% of age predicted maximal HR (220 - age) [28]. V̇O2peak was defined as the highest oxygen consumption value recorded at peak effort during the exercise test and is expressed both as absolute (mL·min−1) and normalized to body weight (mL·min−1·kg−1). Predicted V̇O2peak was calculated based on the following regression equation of OUES predicting V̇O2peak: V̇O2peak = β0 + β1*OUES.
2.3 Physical activity assessment
All participants wore a triaxial accelerometer (GT3X+, Actigraph, Pensacola, FL) on their hip for 7 consecutive days following the graded exercise test. Participants were instructed to wear the device during all waking hours, with the exception of when showering, swimming, or bathing. Standard accelerometry inclusion criteria consisted of at least 10 hours of valid wear time per day for a minimum of 3 weekdays and 1 weekend day [29]. Accelerometer data (in 1-second epochs) were processed using the validated Sojourn-3 Axis method, which uses an artificial neural net to identify boundaries between activities of different intensities and to estimate metabolic equivalents (METs) for each bout [30]. To calculate minutes spent in different intensity categories of physical activity, estimated METs were determined for each bout interval and were then separated into physical activity categories accordingly: <1.5 METs= sedentary, 1.5–2.99 METs= light, 3–6 METs= moderate and >6 METs= vigorous [29].
3. Statistical analyses
3.1 Reliability and Validity of OUES as a measure of CRF
The reliability between OUES values at the three separate exercise durations (i.e., OUES 75, 90, 100) was examined with two-way mixed-effects model intraclass correlation coefficients (ICC) and a one-way repeated-measures ANOVA was used to determine if OUES values were significantly different. The strength of association between OUES (absolute and normalized) and V̇O2peak (absolute and normalized) for participants who satisfied criteria for a valid V̇O2peak test was examined with bivariate Pearson correlations coefficients to establish the criterion validity.
3.2 Differences between participants who satisfied and did not satisfy V̇O2peak criteria
Independent samples t-tests and Cohen’s d effect sizes (d = M1 − M2/SDpooled) were used to compare demographic, risk-factor, and exercise testing data between participants who satisfied V̇O2peak criteria and those who did not.
3.3 OUES as a predictor of V̇O2peak in participants who satisfied and did not satisfy V̇O2peak criteria
Linear regression was used to obtain the formula for OUES predicted V̇O2peak. A one-sample t-test examined whether OUES-predicted V̇O2peak was different from the recorded V̇O2peak in participants who did not satisfy criteria for a valid V̇O2peak test. The significance level (α) for all statistical models was set at 0.05. Analyses were conducted using IBM SPSS, version 22.0.
4. Results
4.1 Preliminary results
One hundred and ten cognitively healthy (MMSE = 29.3 ± .95) participants (mean age = 64.1 ± 5.8 years) completed the study. The supervising exercise physiologist stopped three tests due to safety concerns (arrhythmia, dyspnea, hypertension) and one participant discontinued participation due to mask discomfort. These participants were excluded from further analyses. The analytic sample included 106 participants who completed the graded exercise test to volitional exhaustion. On average, the duration of the exercise test was 12.6 ± 2.9 minutes, and the number of data points used to generate OUES at 75%, 90%, and 100% of exercise duration are as follows: 18.9 (4.3), 22.7 (5.2), 25.2 (5.8). During the final stage, seven tests ended between 1 minute and 1.49 minutes, and the remaining 99 tests were terminated between 1.5 and 2 minutes.
4.2 Primary results
4.2.1 Reliability and Validity of OUES as a measure of CRF
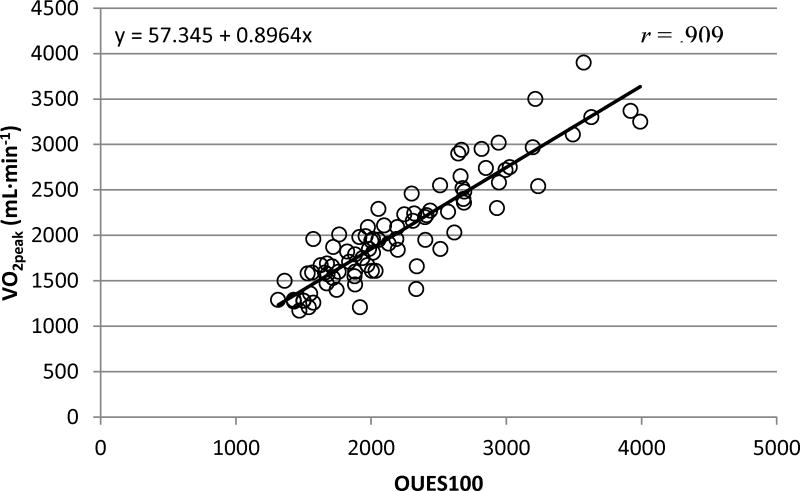
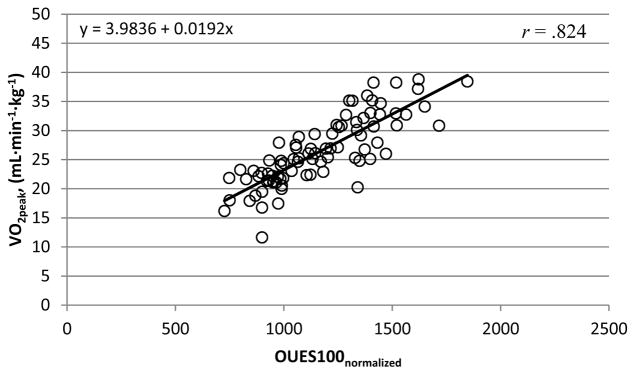
Calculated OUESs (OUES75, OUES90, OUES100) displayed excellent reliability (ICC = .995; p < .001), did not statically differ from each other (p = .063; 1.19% change) and were strongly correlated with absolute V̇O2peak (mL·min−1) (rrange = .882 – .909; p < .001; Figure 1). The reliability (ICC = .993; p < .001) remained strong for OUESnormalized values, which were also strongly correlated with normalized V̇O2peak (mL·min−1·kg−1) (rrange = .801 – .824; p < .001; Figure 3). As previous reports suggest OUES to have greater reproducibility with increasing exercise intensity [21] our prediction equations and figures report the OUES variable that was sampled from the complete exercise duration (OUES100, OUES100normalized). Prediction equations and figures for absolute and normalized OUES75 and OUES90 can be located in the supplementary material.
Figure 1. OUES100 and V̇O2peak (mL·min−1).
The linear relationship between OUES100 and V̇O2peak (mL·min−1) for participants who satisfied criteria (n=85). These data were used to obtain the OUES100 prediction equation (V̇O2peak = 57.346 + .896 * OUES100) which is represented by the solid linear slope.
Figure 3. OUES100normalized and V̇O2peak (mL·min−1·kg−1).
The linear relationship between OUES100normalized and V̇O2peak (mL·min−1·kg−1) for participants who satisfied criteria (n=85). These data were used to obtain the OUES100normalized prediction equation (V̇O2peak = 3.98 + .019 * OUES100normalized) which is represented by the solid linear slope.
4.2.2 Differences between participants who satisfied and did not satisfy V̇O2peak criteria
From the analytic sample of 106 participants, 85 participants satisfied standardized criteria for V̇O2peak and 21 participants did not. Nineteen of the 21 participants who did not satisfy V̇O2peak criteria satisfied 1 criterion (14 satisfied HR, 5 RPE, 0 RER, 0 plateau) and 2 participants did not satisfy any criterion. A total of seven participants were on beta blocker medication which did not significantly differ between those who satisfied (n = 5) and did not satisfy (n = 2) V̇O2peak criteria (p = .56). Participants who did not satisfy V̇O2peak criteria (n = 21) were significantly older than those who satisfied criteria (n = 85) (p = .049; Table 1). The groups did not differ on any other measured demographic characteristics including physical activity behavior (all p > .05; Table 1). At peak effort during the exercise test participants who did not satisfy V̇O2peak inclusion criteria had significantly lower maximal RER (d = 1.3; p < .001), RPE (d = 1.2; p < .001), and non-significantly lower HR (d = .5; p = .065) and V̇O2peak (ml/kg/min) (d = .3; p = .186) than participants who satisfied criteria (Table 2). There were no significant differences between groups for any calculated OUES variables (OUES75, OUES90, OUES100, OUES75normalized, OUES90normalized, or OUES100normalized) (drange = .02 – .15; all p > .50; Table 2).
Table 1.
Participant demographics stratified by criteria
| Variable | Participants who Satisfied Criteria | Participants who did Not Satisfy Criteria |
|---|---|---|
| Sample characteristics | ||
| Sample size | 85 | 21 |
| Female, % | 66.3 | 60.9 |
| Age, years | 63.6 (6.0) | 65.9 (4.3)* |
| Weight, kg | 77.6 (15.19) | 84.2 (16.5) |
| Height, in | 66.2 (3.8) | 66.6 (3.4) |
| BMI, kg/m2 | 27.4 (5.1) | 29.6 (6.2) |
| BSA, m2 | 1.90 (.23) | 1.98 (.20) |
| MMSE, score | 29.3 (1.1) | 29.2 (.89) |
| Education, years | 16.3 (2.2) | 16.3 (2.9) |
| APOE4 +, % | 45.2 | 34.1 |
| FH +, % | 72.6 | 66.7 |
| Caucasian, % | 94.2 | 96 |
| Accelerometer data | ||
| Sedentary, minutes | 666.1 (78.2) | 654.2 (83.9) |
| Light, minutes | 167.3 (45.8) | 165.9 (50.6) |
| Moderate, minutes | 70.6 (28.5) | 81.5 (33.0) |
| Vigorous, minutes | 12.6 (11.4) | 15.3 (15.8) |
| Wear time, minutes | 916.7 (63.0) | 917.0 (67.7) |
Values indicate mean score and standard deviation. BMI = body mass index; BSA = body surface area; MMSE = mini-mental state examination; APOE4 = the epsilon 4 allele of the apolipoprotein E gene; FH = Family history of Alzheimer’s disease.
Values significantly different (p < .05) between groups (independent samples t-test).
Table 2.
Peak exercise data stratified by criteria
| Exercise Variable | Participants who Satisfied Criteria | Participants who did Not Satisfy Criteria | Cohen’s d effect size |
|---|---|---|---|
| Sample size | 85 | 21 | - |
| HR max, % | 101.3 (7.4) | 96.5 (10.9) | .52 |
| RER, value | 1.091 (.088) | .986 (.068)* | 1.3 |
| RPE, value | 17.79 (1.42) | 15.95 (1.56)* | 1.2 |
| OUES75 | 2254 (593) | 2346 (748) | .14 |
| OUES90 | 2250 (606) | 2337 (763) | .13 |
| OUES100 | 2225 (614) | 2328 (748) | .15 |
| OUES75normalized | 1185 (250) | 1192 (389) | .02 |
| OUES90normalized | 1181 (246) | 1186 (394) | .02 |
| OUES100normalized | 1167 (250) | 1181 (382) | .04 |
| Absolute V̇O2peak, mL·min−1 | 2052 (605) | 2012 (642) | .06 |
| Normalized V̇O2peak, mL·min−1·kg−1 | 26.43 (5.82) | 24.37 (8.19) | .29 |
Values indicate mean score and standard deviation. HR = heart rate; RER = respiratory exchange ratio; RPE = rating of perceived exertion; OUES = oxygen uptake efficiency slope; V̇O2peak = peak oxygen consumption.
Values significantly different (p < .05) between groups (independent samples t-test).
4.2.3 OUES as a predictor of V̇O2peak in participants who satisfied and did not satisfy V̇O2peak criteria
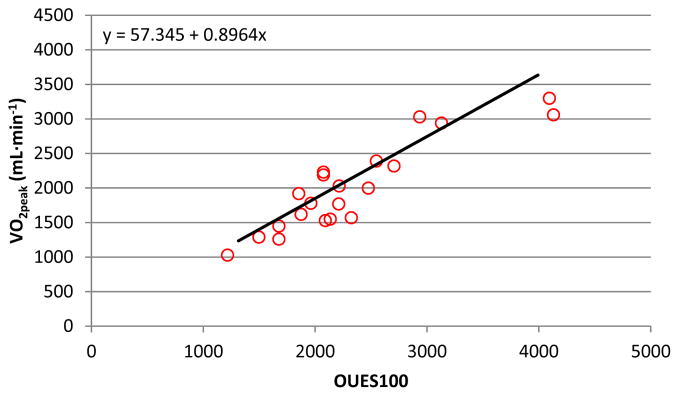
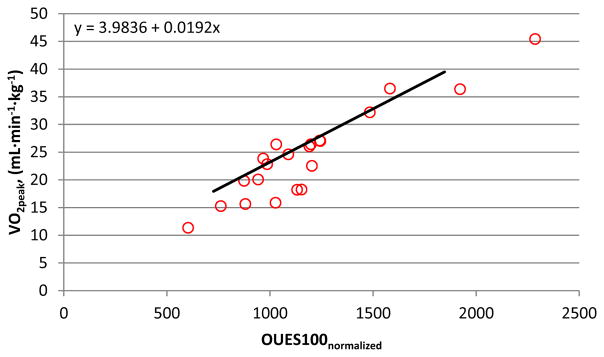
In participants who satisfied criteria (i.e. valid tests), linear regression revealed V̇O2peak (mL·min−1) = 57.3 + .896 * OUES100. This regression equation was used to predict absolute V̇O2peak for each participant based on their OUES100 value. Participants who did not satisfy criteria displayed a significantly lower recorded absolute V̇O2peak than their OUES100 predicted V̇O2peak (Absolute V̇O2peak – OUES100 Predicted V̇O2peak) (d = 1.1; p = .048; Table 3; Figure 2). Examining the normalized data, linear regression revealed V̇O2peak (mL·kg·min−1) = 3.98 + .019 * OUES100normalized. Those who did not satisfy criteria displayed a significantly lower recorded normalized V̇O2peak than their OUES100normalized predicted V̇O2peak (Normalized V̇O2peak – OUES100normalized Predicted V̇O2peak) (d = .72; p = .003; Table 3; Figure 4).
Table 3.
Predicted V̇O2peak values
| Exercise Variable | Satisfy Criteria | Not Satisfy Criteria | Cohen’s d effect size |
|---|---|---|---|
| Sample size | 85 | 21 | - |
| Absolute values | |||
| OUES75 Predicted V̇O2peak, mL·min−1 | 2051 (534) | 2133 (674) | .13 |
| Absolute V̇O2peak – OUES75 Predicted V̇O2peak | 0.92 (285) | −121.3 (302) | .42 |
| OUES90 Predicted V̇O2peak, mL·min−1 | 2053 (547) | 2131 (689) | .13 |
| Absolute V̇O2peak – OUES90 Predicted V̇O2peak | −0.91 (261) | −119.0 (296) | .42 |
| OUES100 Predicted V̇O2peak, mL·min−1 | 2051 (550) | 2143 (700) | .15 |
| Absolute V̇O2peak – OUES100 Predicted V̇O2peak | 0.82 (252) | −130.8 (285)† | 1.1 |
| Normalized values | |||
| OUES75normalized Predicted V̇O2peak, mL·kg·min−1 | 26.4 (4.7) | 26.7 (7.4) | .05 |
| Normalized V̇O2peak –OUES75normalized Predicted V̇O2peak | 0.01 (3.5) | −2.20 (3.0)† | .68 |
| OUES90normalized Predicted V̇O2peak, mL·kg·min−1 | 26.4 (4.8) | 26.5 (7.6) | .02 |
| Normalized V̇O2peak –OUES90normalized Predicted V̇O2peak | 0.01 (3.4) | −2.15 (3.0)† | .67 |
| OUES100normalized Predicted V̇O2peak, mL·kg·min−1 | 26.4 (4.8) | 26.6 (7.4) | .03 |
| Normalized V̇O2peak –OUES100normalized Predicted V̇O2peak | 0.01 (3.3) | −2.31 (3.1)† | .72 |
OUES = oxygen uptake efficiency slope; V̇O2peak = peak oxygen consumption.
Values significantly different (p < .05) from 0 (one sample t-test).
Figure 2. OUES100 and V̇O2peak (mL·min−1).
The linear relationship between OUES100 and V̇O2peak (mL·min−1) for participants that did not satisfy criteria (n=21) with the solid linear slope (same as Figure 1) estimated from participants who satisfied criteria (n=85). Figure 2 demonstrates that data points for participants who did not satisfy criteria are located on the right of the solid slope, indicating an underestimation of their V̇O2peak. On average, these participants had a mean V̇O2peak underestimation of 130.8 mL·min−1 (Table 3).
Figure 4. OUES100normalized and V̇O2peak (mL·min−1·kg−1).
The linear relationship between OUES100normalized and V̇O2peak (mL·min−1·kg−1) for participants that did not satisfy criteria (n=21) with the solid linear slope (same as Figure 3) estimated from participants who satisfied criteria (n=85). Figure 4 demonstrates that data points for participants who did not satisfy criteria are located on the right of the solid slope, indicating an underestimation of their V̇O2peak. On average, these participants had a mean V̇O2peak underestimation of 2.31 mL·min−1·kg−1 (Table 3).
5. Discussion
5.1 Reliability and Validity of OUES as a measure of CRF
The first objective of this study was to evaluate the reliability and validity of the OUES as a sub-maximal measure of CRF in an older adult at-risk sample. Previous large scale (n ≥ 100) reports have shown moderate to strong correlations between OUES and V̇O2peak in adolescents [31] and adults [27]. These studies have also reported that the OUES has strong reliability over time in a graded exercise test [27, 31], however, there have been reported contradictions [23]. Here, we observed excellent reliability among OUESs calculated at 75%, 90%, and 100% of exercise duration before (ICC = .995) and after (ICC = .993) normalizing for BSA (OUESnormalized). Additionally, we observed strong correlations between absolute and normalized OUES and absolute and normalized V̇O2peak values (rrange = .801 – .909). These results extend previous findings by demonstrating that OUES is a reliable and valid measure of CRF that does not require peak effort in an older adult sample that is predominately at-risk for AD.
5.2 Differences between participants who satisfied and did not satisfy V̇O2peak criteria
Maximal exercise testing older adults to examine relationships between CRF and indicators of brain health has grown tremendously over the past decade. In the field of exercise and aging research the criteria used to determine V̇O2peak has been inconsistent. Previous studies have described a range of considerations when determining a valid V̇O2peak test from reporting the highest observed V̇O2 value (i.e. no specified criteria)[12, 32, 33], using one physiological variable (i.e. HR, RER)[13, 34, 35], or reporting some combination of variables [14, 36, 37]. For this project, we chose the ACSM guidelines because they provide an empirically established standard for CRF testing [28].
5.2.1 Demographics and risk-factors
There are many known risk-factors for cognitive decline and AD including both genetic and modifiable risk factors. For this study we measured the following known risk factors: age, family history of AD, carriers of the apolipoprotein epsilon 4 allele (APOE-e4), years of formal education, obesity, and physical activity [4, 38, 39]. Participants who did not satisfy V̇O2peak criteria were significantly older than those who satisfied criteria (Table 1). This is a particularly important finding as age is the single greatest risk factor for AD [38]. It has been well documented that the brain goes through pathological changes that closely track the progression of age and cognitive impairment [17]. That is, outcomes that have been shown to be negatively associated with age include but are not limited to brain structure [19], metabolism [40], and cognition [18]. The observed group difference in age (Table 1) suggests that those who did not satisfy peak effort criteria are at a greater risk for cognitive impairment and likely have greater disease associated pathology.
5.2.2 Exercise testing and physical activity behavior
Participants who did not satisfy criteria recorded significantly lower peak RER, RPE, and non-significantly lower HR and V̇O2peak compared to those who satisfied criteria (Table 2). On average, those who did not satisfy criteria reached an RER of 0.99, an RPE rating of 15.9 (hard effort), and achieved 96.5% of their age-predicted maximal HR at peak effort (Table 2). Although these participants achieved a HR that is deemed acceptable for peak effort, their RER and RPE were below standard criteria. Consistent with recommended guidelines, this finding suggests that using several criteria that are both physiological and psychological in nature is important for determining whether peak effort was attained [28].
Examining physiological and psychological parameters collected at peak effort is critical when judging the validity of a V̇O2peak test. One interpretation of the current data may be that the lowest V̇O2peak values were recorded in the oldest adults because of age-related declines in CRF, which is supported in the literature [41, 42]. However, by closely examining whether or not participants satisfied standardized criteria for a valid measurement of V̇O2peak we observed that those who did not satisfy criteria provided significantly less physiological and perceptual effort, and were significantly older than the group who satisfied criteria, which may result in an underestimation of their V̇O2peak (Table 2). Interestingly, physical activity behavior did not differ between the two groups (Table 1). These data suggest it was not the least active/functional adults that were unable to provide a peak effort on the graded exercise test. This challenges the notion that more liberal criteria are appropriate for older adults under the assumption that they are less likely able to provide peak efforts due to lifestyle and functional limitations.
5.3 OUES as a predictor of V̇O2peak in participants who satisfied and did not satisfy V̇O2peak criteria
Within our sample, OUES was reliable and highly correlated with V̇O2peak. This allowed us to explore whether V̇O2peak was underestimated in participants who did not satisfy criteria. We hypothesized that those who did not provide a peak effort would exhibit a V̇O2peak that was lower than their OUES-predicted V̇O2peak. From the exercise tests that satisfied our criteria (n=85), linear regression was used to obtain the equation of OUES100normalized predicting V̇O2peak (mL·kg·min−1) (Normalized V̇O2peak = 3.98 + .019 * OUES100normalized)). Each participant’s OUES100normalized value was then used to calculate their predicted V̇O2peak (mL·kg·min−1). Supporting our hypothesis, the mean V̇O2peak of participants who did not satisfy criteria was underestimated by 2.31 mL·kg·min−1(p = .003; Table 3). Visual inspection of the linear relationship between OUES100normalized and V̇O2peak (mL·kg·min−1) revealed that a majority of data points for participants who did not satisfy criteria fell to the right of the predicted regression slope, indicating an underestimated V̇O2peak (Figure 4). Furthermore, significantly lower RER (d = 1.3) and RPE (d = 1.2) values were recorded for those participants (Table 2). Considered together, these findings suggest that the lower recorded V̇O2peak values in this group can be attributed to a failure to provide a sufficient effort rather than a physiological limitation of the participant.
5.4 Practical Implications of OUES and V̇O2peak
From the present study, we demonstrated that OUES is a reliable and valid measure of CRF in a sample predominately at-risk for AD at three separate relative exercise durations. In chronic heart failure patients the OUES method is a low-risk submaximal measure of CRF that is commonly used [23] as CRF is an established prognostic assessment in this population [43]. In older adults, the OUES method offers the same advantage of decreasing the likelihood of an adverse event during maximal exercise testing.
In contrast to OUES, it appears that V̇O2peak is only an appropriate measure to use when participants are held to the same standardized criteria, although employing less stringent criteria than are currently recommended for assessing CRF [28] may seem justifiable (safety and effort concerns). Here, we have demonstrated that on average, older adults were less likely to give a peak effort compared to their younger counterparts, which resulted in an average 2.3 mL·kg·min−1, or 9% underestimation of their predicted V̇O2peak. This degree of underestimation should caution investigators as meta-analytic evidence demonstrates that V̇O2peak declines 7 – 10% per decade in adults [41, 42]. To the greatest extent possible, participants must be held to the same standard when exercise testing. This is a critical consideration for the field of aging research as V̇O2peak is commonly the primary variable of interest in prediction equations examining the relationship between CRF and brain health.
Underestimating V̇O2peak introduces systematic error into investigations examining CRF. A practical implication of V̇O2peak measurement error in the oldest adults of a given study sample is misrepresentation of relationships between CRF and brain health outcomes; relationships which may be better explained by age. In our previous work that employed a smaller sample, we demonstrated that participants who did not satisfy peak effort criteria were statistically older (d = .69; p < .05), displayed poorer episodic memory (d = .50; p < .05), and had non-significant smaller hippocampal volumes (d = .42; p = .06) than those who satisfied criteria [36]. As expected, age was significantly and negatively associated with both outcomes of interest, memory and hippocampal volume. If these participants were included in the analytic sample the relationship between CRF and both memory and hippocampal volume would potentially be strengthened by age effects, and not their incorrectly quantified CRF level. Alternatively, failure to apply standardized criteria may obscure relationships between CRF and age-related outcomes due to measurement error leading to attenuation bias.
From the current dataset, 20% of the participants did not satisfy criteria that were used to constitute a valid V̇O2peak exercise test. A recent cross-sectional study reported the same percentage of older adult participants (~20%) that did not satisfy similar criteria [44]. Our data suggests that these participants did not provide the same effort as those who satisfied criteria, thus making their V̇O2peak value an underestimate and inappropriate to include in further analyses. If participants are unable to satisfy criteria for a valid V̇O2peak test, investigators should (i) exclude invalid V̇O2peak data altogether, or (ii) adopt an alternative CRF measurement such as OUES.
The OUES method is an attractive alternative for researchers investigating CRF in participants aged 65 and older. From our sample, it was on average the oldest adults (mean age = 65.9) that did not satisfy V̇O2peak criteria. Investigations aimed at examining CRF in a similar aging population could benefit from the OUES method as they are less likely to exclude the oldest participants that did not satisfy criteria for a V̇O2peak test. We cannot definitively state why some individuals chose to stop prior to achieving peak effort during the graded exercise test. Although those who did not satisfy peak effort criteria were older, the age ranges were similar between those who satisfied (age: 50 – 74) and did not satisfy criteria (age: 58 – 75).
Future research is needed to explore limitations of the OUES method including the role of oxygen deficit during graded exercise testing, as the test-retest reliability of steady state V̇O2 and V̇E has been shown to be variable across repeated measurements [45]. Further, the impact of disease state or medications that influence cardiac and ventilation responses to exercise require additional research. Finally, the minimum number of data points necessary for a valid exercise test using OUES is currently not known. Due to our sample’s demographic make-up (i.e., predominantly highly-educated non-Hispanic Whites) generalizability of our findings to other ethnic groups may be limited. Studies that include diverse populations are needed to determine whether our findings extend to other ethnic groups. Although standardized criteria are essential for interpreting V̇O2peak data, further research is needed to determine the most appropriate specific guidelines for measuring CRF in older adults. For instance, although V̇O2max is the gold standard CRF measure [7], recommended methods for determining V̇O2max are designed for athletes and healthy adults; a true repeated-bout V̇O2max test may present safety concerns for older adults.
6. Summary
The decision to use OUES or V̇O2peak as the primary CRF variable is an important consideration in aging research. In this study we demonstrated that OUES is valid and complimentary to V̇O2peak in an older adult sample at-risk for AD. Further, we demonstrated the necessity of standardized criteria for V̇O2peak testing in order to avoid underestimating CRF in the oldest participants. If future investigators continue to choose V̇O2peak as their primary measure when investigating relationships between CRF and brain health, strict V̇O2peak criteria should be used to the greatest extent possible. An alternative or complimentary strategy is to use OUES, which does not require achievement of standardized criteria.
Supplementary Material
Acknowledgments
This work was supported by National Institute on Aging grants K23 AG045957 (OCO), R21 AG051858 (OCO), R01 AG027161 (SCJ), R01 AG021155 (SCJ), P50 AG033514 (SA); and by a Clinical and Translational Science Award (UL1RR025011) to the University of Wisconsin, Madison. Portions of this research were supported by the Alzheimer’s Association, the Extendicare Foundation, the Wisconsin Alumni Research Foundation, the Helen Bader Foundation, Northwestern Mutual Foundation, and from the Veterans Administration including facilities and resources at the Geriatric Research Education and Clinical Center of the William S. Middleton Memorial Veterans Hospital, Madison, WI. The authors report no conflicts of interest. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
References
- 1.Dougherty RJ, Ellingson LD, Schultz SA, Boots EA, Meyer JD, Lindheimer JB, Van Riper S, Stegner AJ, Edwards DF, Oh JM. Meeting physical activity recommendations may be protective against temporal lobe atrophy in older adults at risk for Alzheimer’s disease. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring. 2016;4:14–17. doi: 10.1016/j.dadm.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dougherty RJ, Schultz SA, Kirby TK, Boots EA, Oh JM, Edwards D, Gallagher CL, Carlsson CM, Bendlin BB, Asthana S. Moderate Physical Activity is Associated with Cerebral Glucose Metabolism in Adults at Risk for Alzheimer’s Disease. J Alzheimers Dis. 2017;58:1089–1097. doi: 10.3233/JAD-161067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med. 2009;39:3–11. doi: 10.1017/S0033291708003681. [DOI] [PubMed] [Google Scholar]
- 4.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. The Lancet Neurology. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erickson KI, Leckie RL, Weinstein AM. Physical activity, fitness, and gray matter volume. Neurobiol Aging. 2014;35:S20–S28. doi: 10.1016/j.neurobiolaging.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayes SM, Hayes JP, Cadden M, Verfaellie M. A review of cardiorespiratory fitness-related neuroplasticity in the aging brain. Front Aging Neurosci. 2013:5. doi: 10.3389/fnagi.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poole DC, Jones AM. Measurement of the maximum oxygen uptake V̇o 2max: V̇o 2peak is no longer acceptable. J Appl Physiol. 2017;122:997–1002. doi: 10.1152/japplphysiol.01063.2016. [DOI] [PubMed] [Google Scholar]
- 8.Howley ET, Bassett DR, Welch HG. Criteria for maximal oxygen uptake: review and commentary. Med Sci Sports Exerc. 1995;27:1292–1301. [PubMed] [Google Scholar]
- 9.Posner JD, Gorman KM, Klein HS, Woldow A. Exercise capacity in the elderly. The American journal of cardiology. 1986;57:C52–C58. doi: 10.1016/0002-9149(86)91027-1. [DOI] [PubMed] [Google Scholar]
- 10.Gill TM, DiPietro L, Krumholz HM. Role of exercise stress testing and safety monitoring for older persons starting an exercise program. JAMA. 2000;284:342–349. doi: 10.1001/jama.284.3.342. [DOI] [PubMed] [Google Scholar]
- 11.Hollenberg M, Ngo LH, Turner D, Tager IB. Treadmill exercise testing in an epidemiologic study of elderly subjects. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1998;53:B259–B267. doi: 10.1093/gerona/53a.4.b259. [DOI] [PubMed] [Google Scholar]
- 12.Tian Q, Studenski SA, Resnick SM, Davatzikos C, Ferrucci L. Midlife and late-life cardiorespiratory fitness and brain volume changes in late adulthood: results from the Baltimore Longitudinal Study of Aging. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2015;71:124–130. doi: 10.1093/gerona/glv041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vidoni ED, Honea RA, Billinger SA, Swerdlow RH, Burns JM. Cardiorespiratory fitness is associated with atrophy in Alzheimer’s and aging over 2 years. Neurobiol Aging. 2012;33:1624–1632. doi: 10.1016/j.neurobiolaging.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voss MW, Weng TB, Burzynska AZ, Wong CN, Cooke GE, Clark R, Fanning J, Awick E, Gothe NP, Olson EA. Fitness, but not physical activity, is related to functional integrity of brain networks associated with aging. Neuroimage. 2016;131:113–125. doi: 10.1016/j.neuroimage.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huggett DL, Connelly DM, Overend TJ. Maximal aerobic capacity testing of older adults: a critical review. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2005;60:57–66. doi: 10.1093/gerona/60.1.57. [DOI] [PubMed] [Google Scholar]
- 16.Billinger SA, Vidoni ED, Morris JK, Thyfault JP, Burns JM. Exercise Test Performance Reveals Evidence of the Cardiorespiratory Fitness Hypothesis. J Aging Phys Act. 2017;25:240–246. doi: 10.1123/japa.2015-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jack CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. The Lancet Neurology. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nilsson LG. Memory function in normal aging. Acta Neurol Scand. 2003;107:7–13. doi: 10.1034/j.1600-0404.107.s179.5.x. [DOI] [PubMed] [Google Scholar]
- 19.Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, Sullivan EV. Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85years) measured with atlas-based parcellation of MRI. Neuroimage. 2013;65:176–193. doi: 10.1016/j.neuroimage.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baba R, Nagashima M, Goto M, Nagano Y, Yokota M, Tauchi N, Nishibata K. Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J Am Coll Cardiol. 1996;28:1567–1572. doi: 10.1016/s0735-1097(96)00412-3. [DOI] [PubMed] [Google Scholar]
- 21.Laethem CV, De Sutter J, Peersman W, Calders P. Intratest reliability and test–retest reproducibility of the oxygen uptake efficiency slope in healthy participants. Eur J Cardiovasc Prev Rehabil. 2009;16:493–498. doi: 10.1097/HJR.0b013e32832c88a8. [DOI] [PubMed] [Google Scholar]
- 22.Akkerman M, van Brussel M, Hulzebos E, Vanhees L, Helders PJ, Takken T. The oxygen uptake efficiency slope: what do we know? J Cardiopulm Rehabil Prev. 2010;30:357–373. doi: 10.1097/HCR.0b013e3181ebf316. [DOI] [PubMed] [Google Scholar]
- 23.Van Laethem C, Bartunek J, Goethals M, Nellens P, Andries E, Vanderheyden M. Oxygen uptake efficiency slope, a new submaximal parameter in evaluating exercise capacity in chronic heart failure patients. Am Heart J. 2005;149:175–180. doi: 10.1016/j.ahj.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Sager MA, Hermann B, La Rue A. Middle-aged children of persons with Alzheimer’s disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer’s Prevention. J Geriatr Psychiatry Neurol. 2005;18:245–249. doi: 10.1177/0891988705281882. [DOI] [PubMed] [Google Scholar]
- 25.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J. 1959;10:675–688. [PubMed] [Google Scholar]
- 26.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 27.Hollenberg M, Tager IB. Oxygen uptake efficiency slope: an index of exercise performance and cardiopulmonary reserve requiring only submaximal exercise. J Am Coll Cardiol. 2000;36:194–201. doi: 10.1016/s0735-1097(00)00691-4. [DOI] [PubMed] [Google Scholar]
- 28.Medicine ACoS. ACSM’s health-related physical fitness assessment manual. Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 29.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 30.Lyden K, Keadle SK, Staudenmayer J, Freedson PS. A method to estimate free-living active and sedentary behavior from an accelerometer. Med Sci Sports Exerc. 2014;46:386. doi: 10.1249/MSS.0b013e3182a42a2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marinov B, Mandadzhieva S, Kostianev S. Oxygen-uptake efficiency slope in healthy 7-to 18-year-old children. Pediatr Exerc Sci. 2007;19:159–170. doi: 10.1123/pes.19.2.159. [DOI] [PubMed] [Google Scholar]
- 32.Ko G, Davidson LE, Brennan AM, Lam M, Ross R. Abdominal adiposity, not cardiorespiratory fitness, mediates the exercise-induced change in insulin sensitivity in older adults. PLoS One. 2016;11:e0167734. doi: 10.1371/journal.pone.0167734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pentikäinen H, Ngandu T, Liu Y, Savonen K, Komulainen P, Hallikainen M, Kivipelto M, Rauramaa R, Soininen H. Cardiorespiratory fitness and brain volumes in men and women in the FINGER study. Age Ageing. 2017;46:310–313. doi: 10.1093/ageing/afw191. [DOI] [PubMed] [Google Scholar]
- 34.Reiter K, Nielson KA, Smith TJ, Weiss LR, Alfini AJ, Smith JC. Improved cardiorespiratory fitness is associated with increased cortical thickness in mild cognitive impairment. J Int Neuropsychol Soc. 2015;21:757–767. doi: 10.1017/S135561771500079X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sui X, LaMonte MJ, Laditka JN, Hardin JW, Chase N, Hooker SP, Blair SN. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA. 2007;298:2507–2516. doi: 10.1001/jama.298.21.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dougherty RJ, Schultz SA, Boots EA, Ellingson LD, Meyer JD, Van Riper S, Stegner AJ, Edwards DF, Oh JM, Einerson J. Relationships between cardiorespiratory fitness, hippocampal volume, and episodic memory in a population at risk for Alzheimer’s disease. Brain and behavior. 2017:7. doi: 10.1002/brb3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayes SM, Hayes JP, Williams VJ, Liu H, Verfaellie M. FMRI activity during associative encoding is correlated with cardiorespiratory fitness and source memory performance in older adults. Cortex. 2017;91:208–220. doi: 10.1016/j.cortex.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimer’s & dementia. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 39.Tanzi RE. The genetics of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006296. doi: 10.1101/cshperspect.a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proceedings of the National Academy of Sciences. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fitzgerald MD, Tanaka H, Tran ZV, Seals DR. Age-related declines in maximal aerobic capacity in regularly exercising vs. sedentary women: a meta-analysis. J Appl Physiol. 1997;83:160–165. doi: 10.1152/jappl.1997.83.1.160. [DOI] [PubMed] [Google Scholar]
- 42.Wilson TM, Tanaka H. Meta-analysis of the age-associated decline in maximal aerobic capacity in men: relation to training status. American Journal of Physiology-Heart and Circulatory Physiology. 2000;278:H829–H834. doi: 10.1152/ajpheart.2000.278.3.H829. [DOI] [PubMed] [Google Scholar]
- 43.Cahalin LP, Chase P, Arena R, Myers J, Bensimhon D, Peberdy MA, Ashley E, West E, Forman DE, Pinkstaff S. A meta-analysis of the prognostic significance of cardiopulmonary exercise testing in patients with heart failure. Heart Fail Rev. 2013;18:79–94. doi: 10.1007/s10741-012-9332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oberlin LE, Verstynen TD, Burzynska AZ, Voss MW, Prakash RS, Chaddock-Heyman L, Wong C, Fanning J, Awick E, Gothe N. White matter microstructure mediates the relationship between cardiorespiratory fitness and spatial working memory in older adults. Neuroimage. 2016;131:91–101. doi: 10.1016/j.neuroimage.2015.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myers J, Arena R, Franklin B, Pina I, Kraus WE, McInnis K, Balady GJ. Recommendations for clinical exercise laboratories. Circulation. 2009;119:3144–3161. doi: 10.1161/CIRCULATIONAHA.109.192520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.