Abstract
OBJECTIVE
Create a software tool to facilitate tractography-based deep brain stimulation (DBS) electrode targeting within the patient-specific stereotactic coordinate system used in the operating room.
APPROACH
StimVision was developed with Visualization Toolkit libraries and integrates four major components: 1) medical image visualization, 2) tractography visualization, 3) DBS electrode positioning, and 4) DBS activation volume calculation with tractography intersection.
RESULTS
Initial applications of StimVision are focused on the study of subcallosal cingulate (SCC) DBS for the treatment of depression. Retrospective modeling results on SCC DBS have suggested that direct stimulation of a specific collection of tractographic pathways are necessary for therapeutic benefit; thereby creating a tractography-based DBS surgical targeting hypotheses. StimVision is the tool we created to facilitate prospective clinical evaluation of that hypothesis.
SIGNIFICANCE
Retrospective tractography-based analyses are common in DBS research; however, intraoperative software tools for interactive selection of a tractography-based DBS target are not readily available. StimVision provides an academic research tool to assist clinical implementation of new DBS targeting strategies and postoperative evaluation of targeting outcome.
Keywords: Electrode, Neurosurgery, Stereotactic, Tractography
Introduction
Stereotactic neurosurgery for deep brain stimulation (DBS) has long employed computational modeling to assist in patient-specific brain target identification for electrode placement. This includes commonly used commercial software systems from companies like Medtronic (Minneapolis, MN) or BrainLab (Munich, Germany), as well as boutique academic software tools designed to test new ideas or algorithms [e.g. 1, 2, 3, 4]. A key aspect of any neurosurgical visualization software used in the operating room (OR) for interactive target evaluation is direct integration with the stereotactic coordinate system, or surgical reference frame [5].
Many of the latest advances in scientific brain imaging databases, such as the Human Connectome Project [6], are provided in a format that is not simply transferable/customizable to a patient-specific stereotactic coordinate system in the short time available for intraoperative clinical decision support. In addition, traditional brain atlases fail to capture the individual variability that can be necessary for surgical decisions in individual patients [7]. As a result, current DBS targeting strategies typically rely on patient-specific clinical imaging data to achieve their goals [5].
Recently, DBS targeting research efforts have begun to explore the potential utility of structural connectivity metrics, such as diffusion-weighted imaging (DWI) based tractography, to assist in target identification [8]. However, DBS tractography studies are almost exclusively performed retrospectively and intraoperative software tools for the interactive selection of a tractography-based DBS target are not readily available. Therefore, we set out to create an academic research software tool, StimVision, which could enable interactive visualization and adjustment of the patient brain imaging data and tractography results, in combination with DBS electrodes and stimulation volumes, all within the context of the intraoperative stereotactic coordinate system.
Motivation for our software development effort was derived from retrospective theoretical studies on the stimulation of white matter pathways associated with therapeutic benefit from subcallosal cingulate (SCC) DBS for the treatment of depression [9]. Johansen-Berg et al. [10] used tractography to identify the anatomical connectivity of the SCC region. Lujan et al. [11] coupled SCC tractography with DBS electric field models to differentiate between therapeutic and non-therapeutic pathway activation. Then Riva-Posse et al. [12] went on to define a set of critical white matter pathways mediating successful clinical outcomes. This collection of modeling results provided a stimulation “blueprint”, including forceps minor, uncinate fasciculus, and the cingulum bundle, that could, in theory, be prospectively targeted on a patient-specific basis to maximize the probability of therapeutic benefit for SCC DBS [13]. StimVision represents an integrated software tool developed to facilitate that type of prospective clinical hypothesis testing.
Methods
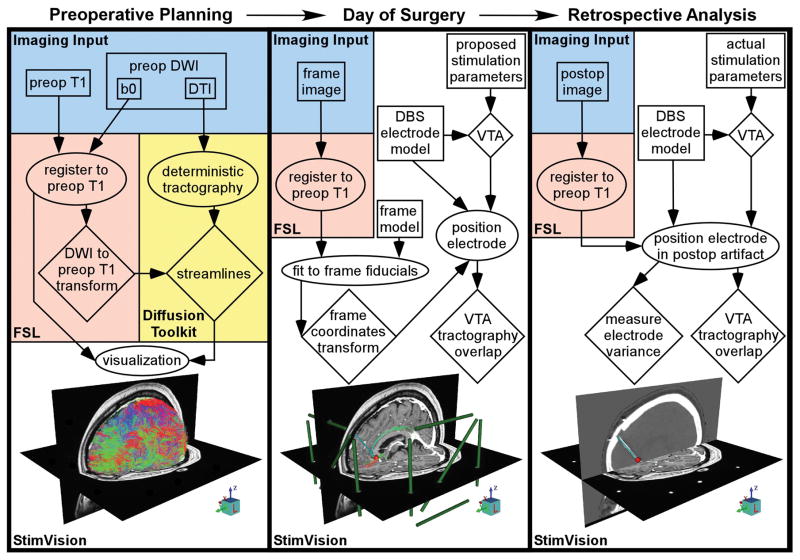
StimVision is the integration of multiple functions and software tools into a common visualization framework to facilitate the identification of a DBS electrode implant location that theoretically stimulates a predefined collection of tractographic pathways in an individual patient. To accomplish this task, StimVision consists of four major components (Fig. 1): 1) medical image visualization, 2) tractography visualization, 3) DBS electrode positioning, and 4) DBS activation volume calculation with tractography intersection. A key aspect of the software is integration of these four components within the context of the patient-specific stereotactic frame space, such that predictions on a theoretically optimal electrode position can be directly transferred to the neurosurgeon in the OR. The workflow for creating a complete patient-specific model in StimVision typically occurs over multiple days as various pieces of clinical imaging data are acquired (Fig. 1). The focus of the process is on the creation of a fully integrated model that is assembled on the morning of the surgery, after placement of the frame and just before entering the OR, which can then be used to assist in DBS electrode targeting (Fig. 2). In addition, the actual electrode implant location can be retrospectively evaluated and compared to the intended target location via integration of post-operative imaging data into the patient-specific model (Fig. 1).
Figure 1.
StimVision workflow. The patient-specific DBS model is developed in three stages. In the preoperative stage, structural and diffusion MRI data are acquired, then coregistered using the FMRIB Software Library (FSL), and loaded into StimVision. In parallel, deterministic tractography is performed via Diffusion Toolkit and the results are loaded into StimVision. In the intraoperative stage, the frame image is acquired, then coregistered in FSL, and loaded into StimVision. The frame model is then selected and fit to the fiducials in the frame image. The DBS electrode model is then selected and placed within stereotactic space. And finally, the volume of tissue activated (VTA) for a typical stimulation parameter setting is selected and the intersection of the tractography with the VTA is displayed. The DBS electrode position and trajectory is adjusted to avoid intersection with any major blood vessels, while also activating the desired tractography profile, typically with the VTA focused on a middle contact. However, following typical DBS surgical convention, the actual X, Y, Z stereotactic target point is defined at the base of the most distal contact. In the postoperative stage, an image with the implanted electrode(s) is acquired, then coregistered in FSL, and loaded into StimVision. Another DBS electrode model is then positioned based on the postoperative imaging data. Comparisons are made between the intended and actual DBS electrode positions, as well as the resulting tractography-activation profile differences. Blue regions represent the imaging input data, orange regions represent tasks done in FSL, yellow regions represent tasks done in Diffusion Toolkit, and white regions represent tasks done in StimVision. Boxes represent inputs, ellipses represent actions, and diamonds represent outputs.
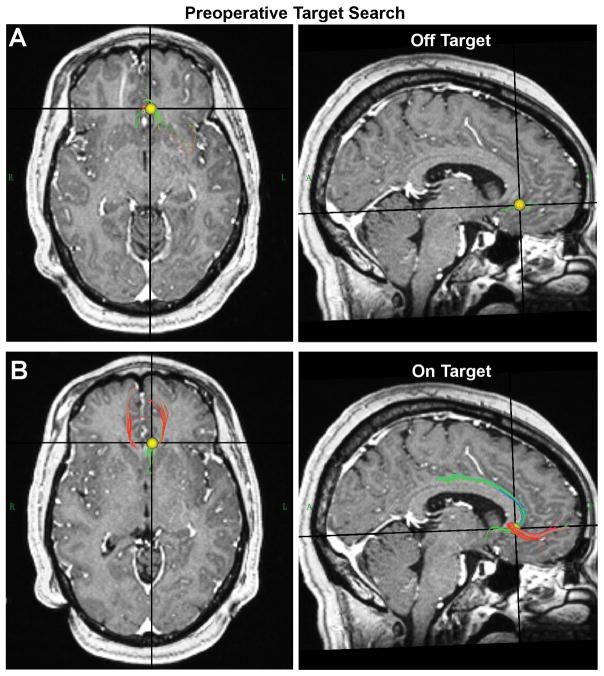
Figure 2.
Preoperative StimVision targeting. Prior to acquisition of the frame image, it is still possible to identify a theoretical target point for stimulation using an interactive search in patient-specific anatomical space. The yellow volume is dragged over the image and the tractography streamlines that pass through the volume are displayed. A) Example location of the search volume that is off target. B) Example location of the search volume that is theoretically on target for SCC DBS.
Ethics Statement
StimVision is an academic research tool and does not have any form of official government body regulatory approval. As such, any use of StimVision is strictly limited to Institutional Review Board (IRB) approved research studies at individual academic institutions. The collection and analysis of all patient data used for this study was approved by the Emory University IRB and conducted under FDA IDEs G060028 and G130107.
Software Architecture
StimVision is written in tool command language with a graphical user interface widget toolkit (tcl/Tk) [http://www.tcl.tk/] with Visualization Toolkit (VTK) libraries [http://www.vtk.org/]. Tcl/Tk is a precompiled scripting language and VTK is an object-based visualization application programming interface that uses OpenGL for rendering. This allows the software to be cross-platform and can run on Linux, Windows, Mac OS, and Unix. The user interacts with the software through a tcl/Tk graphical user interface (GUI), which enables the import and display of images, electrode models, stimulation volume models, and tracks in an interactive VTK display window. StimVision is currently designed to work in parallel with the FMRIB Software Library (FSL) [http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/] and Diffusion Toolkit [http://www.trackvis.org/dtk/]. As such, StimVision does not do image registration or tractography within the software. Instead, StimVision reads in coregistered images from FSL and reads in tractography results from Diffusion Toolkit (Fig. 1).
Medical Image Visualization
The first steps in creation of a StimVision patient-specific DBS model are the acquisition, registration, and loading of the imaging data. For the example SCC DBS patient displayed in the figures, multi-sequence structural and diffusion MRI data were acquired in a single session approximately one month prior to surgery. T1-weighted and DWI data were acquired on a 3T Tim Trio MRI scanner with a 12-channel head array coil (Siemens Medical Solutions, Malvern, PA). DWI parameters were: FOV = 256 × 256; b value = 1000 sec/mm2; voxel resolution = 2×2×2 mm; number of slices = 64; matrix = 128 × 128; 2 averaged; 128 non-collinear directions with one non-diffusion weighted image (b=0); TR/TE = 11300/90 ms. Phase-reversal acquisition was also performed for distortion correction. High-resolution T1-weighted images were collected using a 3D magnetization-prepared rapid gradient-echo (MPRAGE) sequence with the following parameters: TR/TI/TE = 2600/900/3.02 ms; a flip angle of 8°, voxel resolution = 1×1×1 mm; number of slices = 176; matrix = 256×256. On the morning of the surgery, T1-weighted images were acquired with the frame fiducials in place. Post-surgical high-resolution CT data were acquired on a LightSpeed16 (GE Healthcare, Waukesha, WI) with resolution 0.46×0.46×0.65 mm.
All images are converted from DICOM format to NIfTI format using dcm2nii which is distributed with MRIcron [http://neuro.debian.net/pkgs/mricron.html]. The converted NIfTI images are coregistered in FSL using its Linear Image Registration Tool (FLIRT). We limit the transformation to six degrees of freedom (i.e. translation and rotation only).
Tractography Visualization
During the DICOM to NIfTI conversion process, dcm2nii generates the text files describing the b values and b vectors from the DWI datasets. The DWI data are then pre-processed using FSL including motion and eddy current correction. Whole brain deterministic tractography is performed using Diffusion Toolkit (TrackVis) to generate individual patient streamlines in diffusion space and saved as .vtk files for later import into StimVision (Fig. 1). The b0 image associated with the tractography data set is used for coregistration to the other images as described above.
Preoperative Target Search
Given that there are typically multiple days (to weeks) of time available between acquisition of the preoperative MRI data and the day of surgery, StimVision provides an opportunity to identify a preliminary target stimulation location within anatomical space in preparation for the surgery (Fig. 2). An adjustable volume (default = 3 mm in radius) can be interactively dragged through brain space by the user to visualize the tractography overlap. Once an empirically determined optimal is defined by the user, the center of that volume represents the start point for the positioning of a central contact in the preoperative planning performed on the day of surgery.
DBS Electrode Positioning
On the day of surgery, after all of the preoperative imaging data (anatomical MRI, diffusion MRI, and frame image) has been coregistered and imported into StimVision, the neurosurgical stereotactic coordinate system can be established. This requires selection of the appropriate frame fiducial model and fitting of that model to the fiducials visible in the frame image. StimVision is currently capable of representing the Leksell (Elekta Instrument, Stockholm, Sweden) and CRW (Integra LifeSciences, Plainsboro, NJ) frame systems. A virtual model of the frame fiducials is displayed over the patient imaging data and the GUI provides click buttons to manipulate the frame model into correspondence with the image frame fiducials (Fig. 1). Once the frame model is appropriately positioned in StimVision, the stereotactic coordinates of the anterior and posterior commissures are checked against the commercial neurosurgical navigation system used in the OR as a validation step.
Establishing the stereotactic coordinate system in StimVision enables interactive positioning of the DBS electrode within the patient-specific brain volume such that any selected position can be directly transferred to the surgical plan in the OR. Contact 1 of the DBS electrode is snapped to the center of the initial preoperative search volume (Fig. 2), which can now be defined via specific X, Y, Z coordinates in stereotactic space. With a target point defined, the entry point and trajectory are defined by the surgeon to avoid passing through sulci and vessels. These decisions dictate the arc and ring angles. If necessary, fine adjustment of the X, Y, Z position of the electrode, as well as the arc and ring trajectory angles can be further evaluated with submillimeter precision via clicking in the GUI.
Activation Volume Calculation
An important aspect of StimVision is the ability to calculate and display activation volumes generated by the DBS electrode as a function of the selected stimulation parameter settings. StimVision currently uses the most up to date models for the “volume of tissue activated” or VTA, as defined by the McIntyre Lab, which are currently calculated via artificial neural network predictor functions [14]. The VTA was originally designed to estimate the spatial extent of activation for large diameter myelinated axons surrounding the DBS electrode [15]. Over time the computational methodology for calculating the VTA has evolved, and will continue to evolve as DBS simulations become more anatomically and electrically accurate. Therefore, StimVision is designed to be agnostic to the specific VTA methodology. Nonetheless, the current iteration of VTA models available in StimVision are capable of representing the spread of stimulation from monopolar, as well as multipolar, stimulation configurations, for a wide range of pulse widths and amplitudes used in clinical DBS [14]. Simulating a VTA provides the opportunity to visualize the tractography streamlines that intersect with the VTA and evaluate how that collection of streamlines is altered by changes in the stimulation parameter settings, electrode position, and/or electrode design (Figs. 3, 4).
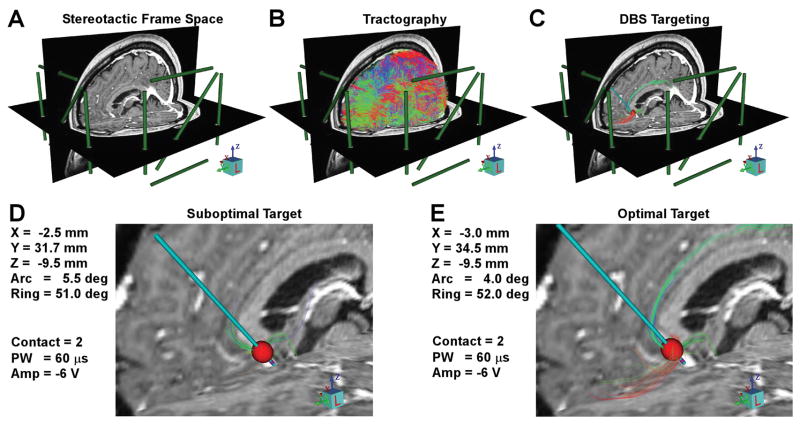
Figure 3.
Intraoperative StimVision targeting. A) Establishing stereotactic frame space allows the model results to be directly transferred to the surgical plan. B) Whole brain deterministic tractography is used to provide patient-specific representation of major white matter pathways. C) Integrating advanced models of the volume of tissue activated (VTA) by DBS electrodes with the patient-specific tractography enables identification of target regions in the brain where pathways of interest converge. D) An example position of the DBS electrode in the SCC region generates a tractography activation profile with relatively limited recruitment of the cingulum bundle and uncinate fasciculus. E) Theoretically optimized positioning generates strong recruitment of forceps minor and the cingulum bundle, as well as some recruitment of the uncinate fasciculus.
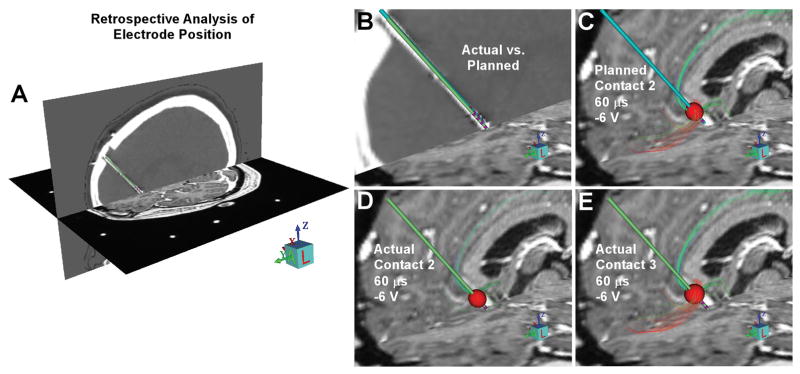
Figure 4.
Postoperative StimVision targeting evaluation. A) Postoperative imaging data is coregistered to the frame space image to enable evaluation of the actual electrode location relative to the intraoperative plan. B) The planned electrode position (blue electrode) and the actual electrode position (green electrode), as defined by postoperative CT acquired 3 weeks after the surgery. The actual electrode ended slightly deeper and more lateral than planned. C) VTA-tractography overlap for the planned electrode position at contact 2, catching the target pathways. D) VTA-tractography overlap for the actual electrode position at contact 2, missing the target pathways. E) VTA-tractography overlap for the actual electrode position at contact 3, catching the target pathways.
Results
The SCC region contains a confluence of axonal pathways linking multiple brain regions [16, 17]. Direct stimulation of specific pathways have been identified as critical in mediating therapeutic benefit from SCC DBS, including forceps minor and the cingulum bundle [12, 13]. Given the relatively strong anisotropy associated with these pathways, patient-specific representations of them can be consistently reconstructed via tractography results calculated from high quality DWI datasets. Therefore, SCC DBS represents a reasonable testing ground for the initial application of the StimVision software. In our experience, StimVision has proven useful in the patient-specific preoperative assessment of both anatomic and tractographic results, as well as identification of target locations for electrode placement. Under current practice this has been an interactive process where the clinical team visually evaluates a range of different electrode locations and empirically identifies a target. Then intraoperatively, the final target location for SCC DBS electrode placement can be defined directly within the patient-specific stereotactic space (Fig. 3). We have consistently noted, in both prospective and retrospective analyses of SCC DBS with StimVision, that relatively small changes (2–3 mm) in stimulation location can generate large differences in the pathway activation patterns, and hence the definition of the optimal electrode location (Figs. 3, 4). This reinforces the need to use the best image coregistration processes available, with independent verification/validation checks of accuracy whenever possible.
StimVision is currently being used to assist in patient-specific DBS electrode targeting in ongoing prospective clinical trials of SCC DBS for the treatment of depression (NCT01984710 and NCT00367003). These trials are not designed to directly evaluate outcome differences between traditional anatomical-based targeting and new tractography-based targeting strategies. However, preliminary clinical results do suggest that tractography-based targeting with StimVision is generating consistent pathway activation patterns across patients [18].
Discussion
The last decade has seen impressive advances in the basic science of human brain mapping methods and the creation of human connectome mapping studies [e.g. 6]. Those scientific concepts, and their results, are beginning to make their way into clinical research studies, often with desires of future utility in direct clinical diagnostic applications. However, prior to clinical adoption of connectome-based surgical targeting concepts, research software tools are needed that can facilitate prospective clinical testing of the general ideas and generate prototype workflows for intraoperative clinical implementation. StimVision represents our first iteration of a DBS-focused tractography-based surgical targeting platform to aid clinical research studies. These concepts and methods are also complementary to other recent academic DBS software tools designed to enable retrospective tractography analysis from implanted DBS electrodes [19, 20].
In many ways experimental DBS and research connectomics represent logical partners. Scientific understanding of DBS mechanisms has evolved to recognize that the disorders treated by DBS are most likely the result of abnormal activity in specific subsets of brain circuitry [21]. Structural connectivity metrics, such as tractography, are available to provide patient-specific maps that give insight into that abnormal circuitry [8]. And, when tractography maps are coupled with DBS models of directly stimulated pathways, important components of the circuitry can be dissected [12]. However, both DBS and tractography are blunt instruments with wide ranging limitations for brain circuitry analysis. Therefore, our experience suggests that current clinical applications of tractography-based DBS targeting should be focused on therapies that stimulate relatively large white matter pathways, with known anatomical bases, which can be robustly reconstructed via tractography with high confidence. In addition, it should be noted that the surgical targeting strategies facilitated by StimVision are experimental and for the most part clinically untested.
The recent work of Riva-Posse et al. [13] demonstrates the clinical potential of prospective tractography-based DBS targeting for the treatment of depression. That work showed that connectomic targeting can produce outcomes that rival traditional targeting. However, connectomic targeting is a new concept based on complex models, so definition of the “optimal” connectomic target remains an unknown. Scientific definition of best practices and/or minimum requirements for the imaging data and the model analyses are only beginning to be addressed. Therefore, we created StimVision to provide a platform for the DBS community to explore these questions.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 MH102238 and R01 MH106173) and the Hope for Depression Research Foundation.
Footnotes
Conflict of Interest Statement: Cameron C. McIntyre is a paid consultant for Boston Scientific Neuromodulation, and is a shareholder in the following companies: Surgical Information Sciences, Inc.; Autonomic Technologies, Inc.; Cardionomic, Inc.; Enspire DBS, Inc.; Neuros Medical, Inc. Helen S. Mayberg is a paid consultant with licensed intellectual property to St Jude Medical. Robert E. Gross is a paid consultant for the following companies: Medtronic; St Jude Medical; Neuropace; SanBio; Neuralstem. Robert E. Gross has received research grants from the following companies: Medtronic; Neuropace; SanBio. The remaining authors have no relevant financial relationships.
Authorship Statement: Angela M. Noecker and Cameron C. McIntyre designed the software, with input from Ki Sueng Choi, Patricio Riva-Posse, Robert E. Gross and Helen S. Mayberg. Angela M. Noecker coded the software. Ki Sueng Choi processed the patient data. Cameron C. McIntyre prepared the manuscript and all authors approved the final paper.
References
- 1.Nowinski WL, Belov D, Benabid AL. An algorithm for rapid calculation of a probabilistic functional atlas of subcortical structures from electrophysiological data collected during functional neurosurgery procedures. Neuroimage. 2003;18(1):143–55. doi: 10.1006/nimg.2002.1299. [DOI] [PubMed] [Google Scholar]
- 2.Finnis KW, Starreveld YP, Parrent AG, Sadikot AF, Peters TM. Three-dimensional database of subcortical electrophysiology for image-guided stereotactic functional neurosurgery. IEEE Trans Med Imaging. 2003;22(1):93–104. doi: 10.1109/TMI.2002.806567. [DOI] [PubMed] [Google Scholar]
- 3.Miocinovic S, Noecker AM, Maks CB, Butson CR, McIntyre CC. Cicerone: stereotactic neurophysiological recording and deep brain stimulation electrode placement software system. Acta Neurochir Suppl. 2007;97(Pt 2):561–7. doi: 10.1007/978-3-211-33081-4_65. [DOI] [PubMed] [Google Scholar]
- 4.D’Haese PF, Pallavaram S, Li R, Remple MS, Kao C, Neimat JS, Konrad PE, Dawant BM. CranialVault and its CRAVE tools: a clinical computer assistance system for deep brain stimulation (DBS) therapy. Med Image Anal. 2012;16(3):744–53. doi: 10.1016/j.media.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan FR, Henderson JM. Deep brain stimulation surgical techniques. Handb Clin Neurol. 2013;116:27–37. doi: 10.1016/B978-0-444-53497-2.00003-6. [DOI] [PubMed] [Google Scholar]
- 6.Van Essen DC, Smith SM, Barch DM, Behrens TE, Yacoub E, Ugurbil K WU-Minn HCP Consortium. The WU-Minn Human Connectome Project: an overview. Neuroimage. 2013;80:62–79. doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nestor KA, Jones JD, Butson CR, Morishita T, Jacobson CE, 4th, Peace DA, Chen D, Foote KD, Okun MS. Coordinate-based lead location does not predict Parkinson’s disease deep brain stimulation outcome. PLoS One. 2014;9(4):e93524. doi: 10.1371/journal.pone.0093524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coenen VA, Schlaepfer TE, Allert N, Mädler B. Diffusion tensor imaging and neuromodulation: DTI as key technology for deep brain stimulation. Int Rev Neurobiol. 2012;107:207–34. doi: 10.1016/B978-0-12-404706-8.00011-5. [DOI] [PubMed] [Google Scholar]
- 9.Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, Wint D, Craighead MC, Kozarsky J, Chismar R, Moreines JL, Mewes K, Posse PR, Gutman DA, Mayberg HS. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012;69(2):150–8. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, Lozano AM, Mayberg HS. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18(6):1374–83. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lujan JL, Chaturvedi A, Choi KS, Holtzheimer PE, Gross RE, Mayberg HS, McIntyre CC. Tractography-activation models applied to subcallosal cingulate deep brain stimulation. Brain Stimul. 2013;6(5):737–9. doi: 10.1016/j.brs.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riva-Posse P, Choi KS, Holtzheimer PE, McIntyre CC, Gross RE, Chaturvedi A, Crowell AL, Garlow SJ, Rajendra JK, Mayberg HS. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014;76(12):963–9. doi: 10.1016/j.biopsych.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riva-Posse P, Choi KS, Holtzheimer PE, Crowell AL, Garlow SJ, Rajendra JK, McIntyre CC, Gross RE, Mayberg HS. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol Psychiatry. doi: 10.1038/mp.2017.59. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaturvedi A, Luján JL, McIntyre CC. Artificial neural network based characterization of the volume of tissue activated during deep brain stimulation. J Neural Eng. 2013;10(5):056023. doi: 10.1088/1741-2560/10/5/056023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butson CR, McIntyre CC. Role of electrode design on the volume of tissue activated during deep brain stimulation. J Neural Eng. 2006;3(1):1–8. doi: 10.1088/1741-2560/3/1/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehman JF, Greenberg BD, McIntyre CC, Rasmussen SA, Haber SN. Rules ventral prefrontal cortical axons use to reach their targets: implications for diffusion tensor imaging tractography and deep brain stimulation for psychiatric illness. J Neurosci. 2011;31(28):10392–402. doi: 10.1523/JNEUROSCI.0595-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vergani F, Martino J, Morris C, Attems J, Ashkan K, Dell’Acqua F. Anatomic connections of the subgenual cingulate region. Neurosurgery. 2016;79(3):465–72. doi: 10.1227/NEU.0000000000001315. [DOI] [PubMed] [Google Scholar]
- 18.Choi KS, Riva-Posse P, McIntyre CC, Noecker AM, Gross RE, Mayberg HS. Org Hum Brain Mapp. Geneva, Switzerland: 2016. Target selection toolbox development for deep brain stimulation surgery. [Google Scholar]
- 19.Horn A, Kühn AA. Lead-DBS: a toolbox for deep brain stimulation electrode localizations and visualizations. Neuroimage. 2015;107:127–35. doi: 10.1016/j.neuroimage.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Lauro PM, Vanegas-Arroyave N, Huang L, Taylor PA, Zaghloul KA, Lungu C, Saad ZS, Horovitz SG. DBSproc: An open source process for DBS electrode localization and tractographic analysis. Hum Brain Mapp. 2016;37(1):422–33. doi: 10.1002/hbm.23039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lozano AM, Lipsman N. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron. 2013;77(3):406–24. doi: 10.1016/j.neuron.2013.01.020. [DOI] [PubMed] [Google Scholar]