Abstract
The goals of this retrospective study were to determine the patient characteristics of dogs with high-grade primary mediastinal lymphoma and to determine outcome and associated prognostic factors. Forty-two dogs were identified; 36 received treatment and had follow up information available. The most common clinical signs included lethargy, anorexia, and polyuria/polydipsia. Hypercalcemia and pleural effusion were common findings at diagnosis. The phenotype was almost exclusively T cell, most often in association with lymphoblastic cytomorphology as defined by the WHO lymphoma classification scheme. The overall progression free survival (PFS) and overall survival (OS) were 133 and 183 days, respectively. Treatment with a CHOP protocol was associated with an improved PFS (144 days) and OS (194 days) when compared to dogs that received other medical therapies (p = 0.005 and p = 0.002, respectively); the absence of pleural effusion at diagnosis was associated with an increased OS but not PFS. These results suggest that while the prognosis for dogs with mediastinal lymphoma is poor, survival may be improved with treatment using a CHOP-based protocol.
Keywords: canine, CHOP, chemotherapy, T cell lymphoma, mediastinum
INTRODUCTION
Lymphoma is one of the most common neoplasms diagnosed in dogs and is estimated to account for 7–24% of all canine tumors and 83% of all canine hematopoietic malignancies.1 The majority of dogs diagnosed with lymphoma present with multicentric disease, and mediastinal involvement in the multicentric disease setting has been associated with the T cell phenotype. Mediastinal involvement is reported to occur in 22 to 35.9% of dogs with lymphoma, and for dogs with the T-cell phenotype, mediastinal involvement is reported to be up to 54%.2–5 Both hypercalcemia and the presence of a mediastinal mass are associated with non-indolent T-cell phenotype, and the presence of either has been associated with a worse outcome as compared to high-grade B-cell lymphoma.6–8 Anatomic localization to the mediastinum accounts for approximately 5% of cases, though this patient population has not been well described in the literature.9
Approximately 10–38% of all cases of canine lymphoma are of T-cell origin and combination chemotherapy protocols such as CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone), L-MOPP (L-asparaginase, mechlorethamine, vincristine, procarbazine, prednisone), and more recently VELCAP-TSC (vincristine, L-asparaginase, doxorubicin, cyclophosphamide, prednisolone; actinomycin-D, vincristine, procarbazine) are used to treat this disease. Despite multidrug therapy, high-grade T-cell lymphoma is generally associated with a poorer outcome as compared to B cell lymphoma in dogs, with PFS and OS reported to range from 96–200 days and 120–270 days, respectively. 4,7,10–14
Primary mediastinal lymphoma has not been well described in the veterinary literature. In people, mediastinal lymphoma can be either of T-cell or B-cell phenotype (T-lymphoblastic lymphoma or mediastinal large B-cell lymphoma, respectively), and reported treatment options include multi-agent chemotherapy protocols, immunotherapy, and mediastinal irradiation.15,16 To the authors’ knowledge, there are no published data regarding patient and tumor characteristics or survival information of dogs with primary mediastinal lymphoma. The purpose of this retrospective study was to determine the patient characteristics and clinical presentation, identify potential prognostic factors, and assess treatment response and outcome of dogs with primary mediastinal lymphoma.
MATERIALS AND METHODS
Case collection
Cases were identified retrospectively from the XXX medical record database from February 1993 to February 2015. Dogs were included if they had a diagnosis of mediastinal lymphoma documented in the medical record, which was defined as the presence of a mediastinal mass in the absence of peripheral lymphadenopathy. Dogs were excluded if a definitive diagnosis was not obtained or if peripheral lymphadenopathy was noted on examination and lymphoma confirmed cytologically. Definitive diagnosis was achieved by microscopic evaluation of aspirates or biopsies from the mediastinal mass and/or pleural effusion. At the discretion of the attending clinician either immunocytochemical, immunohistochemical or flow cytometric immunophenotyping were done to determine lymphoma lineage. Dogs that did not have immunophenotyping at diagnosis had molecular clonality PCR done subsequently if cytologic or histologic samples were available for DNA extraction and analysis. Both T and B cell molecular clonality were done in these latter instances.17,18 A lineage was assigned (T cell or B cell) if there was a clear, reproducible clonal spike in the assigned lineage, with absence of a clonal spike in the PCR assay for the other lineage. A lineage was not assigned if there was a reproducible clonal spike in both assays (cross lineage rearrangement). All dogs had thoracic radiographs performed and other staging diagnostics, including a complete blood count and chemistry panel. Full staging diagnostics were not required for inclusion. Abdominal ultrasound and / or bone marrow aspirate were performed at the discretion of the attending clinician but were not required for inclusion.
Information collected on the patients included signalment, body weight, presence and duration of clinical signs prior to diagnosis, substage, physical exam abnormalities, hematologic and biochemical abnormalities, diagnostic imaging results, date of diagnosis, date of corticosteroid initiation, date of chemotherapy initiation, treatment protocol, response to treatment, date of relapse, rescue therapy, and date of death. Follow up information was obtained from medical records at the VMTH and by phone calls to the referring veterinary hospitals. Complete blood count and chemistry panel abnormalities were defined as values deviating from the provided normal reference ranges and assessed prior to treatment initiation. Factors evaluated for progression-free survival and overall survival included signalment (age, weight, sex), pleural effusion, hypercalcemia, azotemia, cell morphology, and chemotherapy protocol (CHOP versus other). Response to treatment was assessed by thoracic radiographs after treatment initiation. Complete response (CR) was defined as complete resolution of the mediastinal mass and pleural effusion and partial response (PR) was defined as at least a 30% decrease in the target lesion diameter.19
Tumor sample review
When available, tumor samples were reviewed by a single clinical pathologist (XX) and categorized morphologically using criteria based on the World Health Organization (WHO) lymphoma classification scheme.20 True lymphoblastic lymphoma was defined by neoplastic lymphocytes having intermediate sized nuclei approximately 1.5–2 red blood cells (RBC) in diameter, finely stippled, immature chromatin with inapparent or inconspicuous nucleoli, and frequent nuclear membrane irregularity. Large cell lymphoma was defined as having large nuclei (> 2 RBC in diameter), immature or irregularly condensed chromatin and multiple, variably prominent nucleoli. While large cell lymphoma is not a specific WHO lymphoma type, this follows the criteria for other WHO lymphoma types that have a descriptor of “large” in their designation. Large cell lymphoma of granular lymphocyte type was defined by the presence of cytoplasmic azurophilic granules, often packeted in a peri nuclear location.
Statistical analysis
Continuous data were tested for normality using the D’Agostino-Pearson test and reported using mean or median and range; categorical data was reported as frequencies and percentages. Differences among groups were assessed using the Mann-Whitney test for continuous data and for categorical data, Fisher’s exact test was used for variables with 2 categories and the Chi-squared test used for variables with >2 categories. Progression free survival (PFS) and overall survival (OS) were calculated from the date of treatment initiation to the date of progressive disease (PD) or death due to any cause, respectively. Kaplan-Meier estimation was used to calculate PFS and OS; log-rank (Mantel-Cox) analysis was used to assess associations between patient and treatment factors and outcome. Cases were censored if they were still alive at last follow up. All deaths were considered to be attributable to disease. A p value of <0.05 was considered statistically significant. All statistical analyses were performed using commercially available software (Prism v. 6.0d, GraphPad Software, La Jolla, CA).
RESULTS
Patient population
Ninety-five patient records were screened for potential enrollment based on a medical record search for mediastinal lymphoma as the clinical diagnosis. Fifty-three cases were excluded due to the presence of peripheral lymphadenopathy. Forty-two dogs met the inclusion criteria for the study.
The mean age and weight of dogs was 7.8 years (range 2.5–12.1 years) and 33.0 kg (range 4–60 kg), respectively. There were 22 neutered males, 15 spayed females, 3 intact males, and 2 intact females. The most common represented breeds included mixed (n=10), Labrador retrievers (n=6), golden retrievers (n=4), and boxers (n=4). The remaining breeds were Australian cattle dogs (n=2), Rhodesian ridgebacks (n=2), and one each of a basset hound, Chihuahua, cocker spaniel, flat-coated retriever, German shepherd, Gordon setter, Irish setter, kelpie, miniature schnauzer, Newfoundland, old English sheepdog, Rottweiler, shih tzu, and Weimaraner.
Initial clinical signs were available for 41/42 dogs; one dog had treatment initiated at another hospital and diagnostic information was available but clinical signs were not recorded in the medical record. The most common presenting signs were lethargy (n = 29, 70.7%), anorexia (n = 24, 58.5%), polyuria/polydipsia (n = 17, 41.5%), coughing (n = 14, 34.1%), dyspnea or tachypnea (n = 10, 24.4%), and vomiting (n = 10, 24.4%). Thirty-five dogs (85.4%) were classified as substage b, as systemic signs that interfered with daily activities were present at the time of initial diagnosis. The median duration of clinical signs prior to diagnosis was 17 days (range 0–210 days).
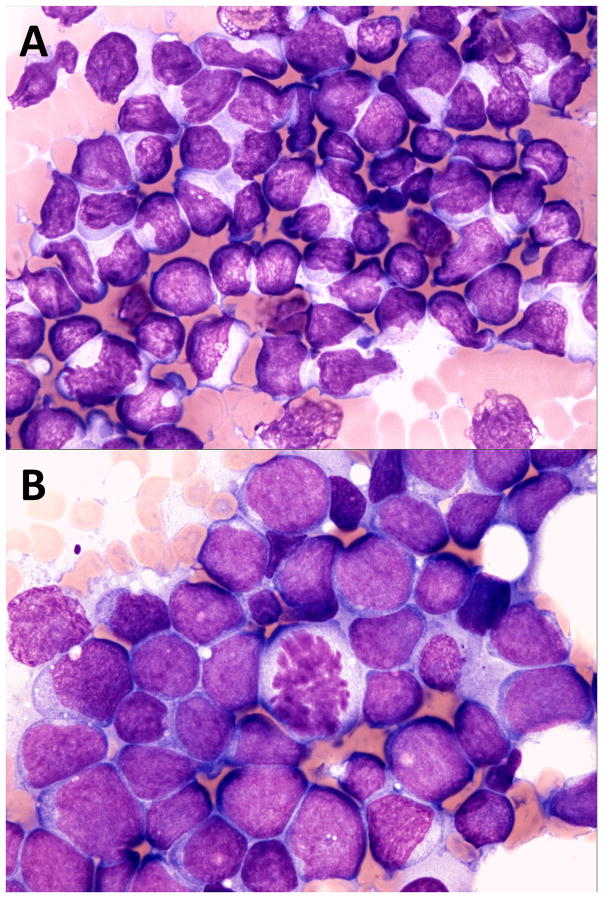
Lymphoma was confirmed with cytology in 37 dogs (36 mediastinal mass aspirates; 1 pleural effusion sample) and histopathology in 5 dogs; samples for histopathology were needle-core biopsies collected with ultrasound guidance. Tumor samples from 32 dogs were available for review; 29 cytologic samples and 3 histopathologic biopsies. The remaining samples were not available for review as the slides were missing, or the original diagnosis was made at an outside facility with the slides subsequently unavailable. Lymphomas were classified as lymphoblastic (n=18), large cell (n=13) or lymphoma of large, granular lymphocyte type (n=1).20 Representative cytology images of lymphoblastic and large cell lymphoma are shown in Figure 1. Based on cell morphology, dogs were assigned to 2 groups; lymphoblastic (n=18) versus other (n=14). The groups were compared and dogs diagnosed with lymphoblastic lymphoma were significantly more likely to be hypercalcemic (n=16; 89%) at the time of diagnosis as compared to dogs with large cell or large granular lymphoma ((n=5; 36%) (odds ratio 11.2; 95% CI 1.7 – 72.3; p = 0.01)).
FIGURE 1.
A, Mediastinal lymphoma in a dog, lymphoblastic cell type. Note intermediate size nuclei approximately 1.5–2.0 red blood cells (RBCs) in diameter, immature chromatin with inapparent or inconspicuous nucleoli and frequent nuclear membrane irregularity. ×60 objective magnification, Wright Giemsa stain. B, Mediastinal lymphoma in a dog, large cell type. Note large nuclei approximately 2.5–3.5 RBCs in diameter, immature chromatin and variably prominent nucleoli. ×60 objective magnification, Wright Giemsa stain
Neoplastic lymphocyte lineage was determined at diagnosis by immunophenotyping in 17 cases and molecular clonality PCR in 1 case. All 18 were confirmed to be T cell lymphoma via immunocytochemistry (n=11), immunohistochemistry (n=5), flow cytometry (n=1) and molecular clonality PCR (n=1). In 2 instances, more than one method was used (ICC or flow cytometry plus molecular clonality PCR) and results were concordant in both. Of these 18 confirmed T cell lymphomas, 16 had samples available for morphologic assessment, and 10 were classified as lymphoblastic lymphoma and 6 as large cell lymphoma. An additional 16 cases had stained cytologic smears available for DNA extraction and subsequent molecular clonality PCR. Ten of these were confirmed as T cell lymphoma, 1 as B cell lymphoma, 1 ambiguous result (clonal at both B and T cell loci) and 4 yielded DNA that was not amplifiable. Of the 10 lymphomas confirmed as T cell by molecular clonality PCR, 6 were lymphoblastic and 4 were large cell lymphomas. The single B cell lymphoma had large cell morphology. In total, 29 cases had definitive lineage assignment with 28 being T cell lymphoma and 1 being B cell lymphoma.
Results of initial blood work and staging diagnostic tests were available for 42 cases and the most common abnormalities are included in Table 1. In all cases, patients had thoracic radiographs available for review at UC-Davis VMTH and a mediastinal mass was present. Nineteen (45.2%) dogs had pleural effusion at the time of diagnosis.
Table 1.
Diagnostic results of dogs with primary mediastinal lymphoma
| Variable | Number | Frequency (%) or mean (range) |
|---|---|---|
| Hypercalcemia | n=27 | 67.5% |
| Total calcium | n=24 | 15.45 mg/dl (11.4 – 19) |
| Ionized calcium | n=12 | 1.9 mm/L (1.53 – 2.15) |
| Azotemia | n=13 | 32.5% |
| BUN | n=12 | 44 mg/dl (10 – 102) |
| Creatinine | n=12 | 2.95 mg/dl (1.7 – 7.0)* |
| Pleural effusion | n=19 | 45.2% |
| Lineage | ||
| T cell | n=28 | 96.6% |
| B cell | n=1 | 3.4% |
| Not evaluated or indeterminate | n=13 | |
| Cell morphology | ||
| Lymphoblastic | n=18 | 56.3% |
| Large cell | n=13 | 40.6% |
| Large granular lymphocyte type | n=1 | 3.1% |
| Not evaluated | n=10 | |
Median creatinine is reported as data was not normally distributed
Complete blood cell count (CBC) and chemistry panel results prior to initiating treatment were reported for 40 dogs; 2 dogs did not have lab work performed at diagnosis. Twenty-seven (67.5%) dogs were reported to be hypercalcemic. Three of these dogs were reported to be hypercalcemic at diagnosis in referring veterinarian records, but the lab work was not available for review prior to treatment initiation. Of the cases that were hypercalcemic, the mean total calcium was 15.4 mg/dL (range 11.4–19 mg/dL) for 24 dogs with results available for review, and the mean ionized calcium was 1.9 mmol/L (range 1.53–2.15 mmol/L) for 12 dogs that had ionized calcium measured. Thirteen dogs (48.1% of hypercalcemic dogs; 32.5% of all dogs) were azotemic at diagnosis; all of these dogs had concurrent hypercalcemia. The most common abnormality on the complete blood count was thrombocytopenia, occurring in 8 (20%) dogs; 7 (87.5%) of the dogs that were thrombocytopenic were also hypercalcemic. No dogs had a lymphocytosis, but 3 (7.1%) had circulating neoplastic lymphocytes reported on the CBC. Bone marrow aspirates were performed in 14 cases. Bone marrow infiltration was confirmed in 3 cases and reported to be probable or early infiltration in an additional 3 cases.
Thirty-two (76.2%) dogs had an abdominal ultrasound performed at the time of diagnosis. There were 6 dogs with changes in the spleen, liver, or intra-abdominal lymph nodes that were suggestive of possible lymphoma infiltration. Aspirates were obtained of the spleen and liver in one case, which showed extramedullary hematopoiesis and marginal lymphocytic inflammation described as a mixed population of reactive lymphocytes, respectively. Another dog had the spleen aspirated, but the report was not available for review. One dog had aspirates taken of multiple abdominal organs and a medial iliac lymph node; the liver and spleen were within normal limits but the intra-abdominal lymph node aspirate was consistent with lymphoma. Twenty-five dogs had mild abnormalities reported, suspected to be unrelated to lymphoma diagnosis per radiologist reports, with no gross evidence of infiltrative disease in any abdominal organs or lymph nodes. Four dogs had ultrasonographic evidence of mineralization in the kidneys, and all of these dogs had concurrent hypercalcemia.
Treatment and patient outcome
Three dogs were euthanized at diagnosis and 3 additional dogs were lost to follow up immediately after diagnosis. Chemotherapy protocols for the remaining 36 dogs included prednisone alone (n=3), L-asparaginase with prednisone (n=3), single agent doxorubicin with prednisone (n=1), MOPP (n=1), alternating chlorambucil and cyclophosphamide with prednisone (n=1), and CHOP (n=27). Four dogs received radiation therapy (RT) in additional to CHOP-based chemotherapy; 2 were treated in conjunction with their initial treatment and 2 were treated in the relapse setting (protocols described below).
Re-evaluation of thoracic radiographs to assess response to treatment was not consistently performed in this patient population. Of the dogs treated with a CHOP protocol (n=27), 26 had follow up thoracic imaging performed and imaging reports were available for review either from the UC Davis VMTH or from referring veterinarian communications. The overall response rate for these 26 dogs was 92.7%, with 18 dogs (69%) documented to have a complete response, 7 (27%) a partial response, and 1 (4%) progressive disease. The remaining 9 dogs that received treatment other than CHOP chemotherapy did not have consistent re-staging diagnostics to assess response to therapy.
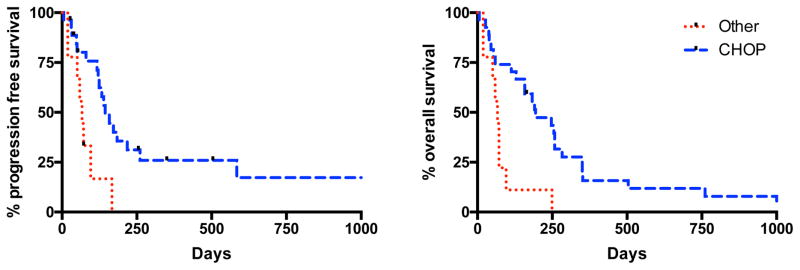
Of the 36 dogs where both treatment and follow-up information were available, the median PFS and OS were 133 and 183 days (range: 6–2096 days for both), respectively. Six dogs were lost to follow up at a median of 224 days (39–2096 days after treatment initiation) and censored at the date of last contact. The remaining 30 dogs were either deceased or euthanized and considered to be dead from disease. The only variable evaluated that was identified as significant for PFS was treatment with a CHOP-based chemotherapy versus other protocols (p=0.005, HR 2.95 (95% CI: 1.76 – 17.1); Table 2). Median PFS survival for dogs receiving CHOP protocol was 144 days (range 6–2096 days) compared to 67 days (range 19–167 days) for those receiving other treatment protocols. No other variables evaluated were significant for PFS.
Table 2.
Univariate analysis for 36 dogs with primary mediastinal lymphoma that were not euthanized or lost to follow up after diagnosis
| Parameter | Median PFS (days) | P-value | HR (95% CI) | Median OS (days) | P-value | HR (95% CI) | |
|---|---|---|---|---|---|---|---|
| Age | <7.8 yr (n=16) | 133 | 0.218 | 0.61 | 171 | 0.302 | 0.72 |
| ≥7.8 yr (n=20) | 121 | (0.28 – 1.32) | 144 | (0.35 – 1.34) | |||
|
| |||||||
| Weight | <33.0 kg (n=19) | 139 | 0.815 | 0.91 | 158 | 0.508 | 0.81 |
| ≥33.0 kg (n=17) | 133 | (0.42 – 1.99) | 158 | (0.40 – 1.55) | |||
|
| |||||||
| Sex (intact & neutered) | Female (n=13) | 133 | 0.162 | 0.56 | 183 | 0.266 | 0.69 |
| Male (n=23) | 124 | (0.26 – 1.24) | 158 | (0.35 – 1.31) | |||
|
| |||||||
| Hypercalcemia | No (n=12) | 121 | 0.481 | 1.34 | 101 | 0.883 | 1.05 |
| Yes (n=23) | 144 | (0.57 – 3.40) | 183 | (0.52 – 2.15) | |||
|
| |||||||
| Presence of effusion | No (n=20) | 133 | 0.143 | 0.57 | 183 | 0.018 | 0.48 |
| Yes (n=16) | 117 | (0.22– 1.21) | 70 | (0.19 – 0.81) | |||
|
| |||||||
| CHOP protocol | No (n=9) | 67 | 0.005 | 2.95 | 67 | 0.002 | 2.89 |
| Yes (n=27) | 144 | (1.76 – 17.1) | 194 | (2.02 – 17.2) | |||
|
| |||||||
| Lymphoblastic LSA | No (n=12) | 144 | 0.5 | 1.34 | 194 | 0.902 | 0.95 |
| Yes (n=15) | 133 | (0.54 – 3.53) | 183 | (0.44 – 2.06) | |||
|
| |||||||
| Azotemia | No (n=24) | 124 | 0.926 | 1.04 | 158 | 0.975 | 0.99 |
| Yes (n=10) | 172 | (0.45 – 2.42) | 191 | (0.45 – 2.19) | |||
PFS = progression free survival; HR = hazard ratio; OS = overall survival
Prognostic factors evaluated that were associated with improved OS included treatment with a CHOP-based protocol (p=0.002, HR 2.89 (95% CI: 2.02 – 17.2)) and the absence of pleural effusion at diagnosis (p=0.018, HR 0.48 (95% CI: 0.19 – 0.82); Table 2). Median OS for dogs receiving a CHOP protocol was 194 days (range 6–2096 days) compared to 67 days (range 19–249 days) for those receiving another protocol (Figure 3). Median OS for dogs without pleural effusion was 183 days (range 39–2096 days) compared to 70 days (range 6–283 days) for dogs with pleural effusion. When evaluating only dogs treated with a CHOP based protocol, presence of pleural effusion at diagnosis remained prognostic for OS (p = 0.018, HR 0.43 (95% CI: 0.13 – 0.76) but not PFS.
Four dogs received radiation therapy (RT) in addition to CHOP-based chemotherapy. Two of these were treated with radiation during their initial CHOP-based chemotherapy protocol. One received a dose of cranial half body radiation and the other received both cranial and caudal half body low-dose rate irradiation after the first cycle of CHOP as described by Lurie et al.21 The patient that was treated with cranial and caudal half body RT did not have a documented relapse and died at day 1779 of an unknown cause. The dog treated with cranial half body radiation experienced a PFS of 281 days and OS of 352 days. Two other patients were treated in the rescue setting after progressive disease during the initial CHOP protocol. A definitive treatment protocol (16 × 3Gy) to the cranial thoracic region was administered to one dog, and this patient experienced a PFS of 117 days and OS of 258 days. The other patient was treated with a similar protocol, but had numerous treatment delays and only received 10 doses. This dog experienced a PFS of 133 days and an OS of 183 days.
There were 18 dogs with disease progression or relapse either during therapy (n=13) or after completion of therapy (n=5) that had follow up information available. These dogs were treated with a variety of rescue protocols, including RT as described previously (n=2; also received chemotherapy) and various rescue chemotherapy protocols (n = 14). There were 2 dogs that were euthanized at the time of relapse. Eight dogs were confirmed to develop progression of lymphoma outside the thoracic cavity in the peripheral lymph nodes (n=6), peri-aortic lymph nodes (n=1) or nasopharyngeal region (n=1). Seven of these dogs had thoracic radiographs performed that confirmed recurrence or progression of a cranial mediastinal mass at the time of relapse.
DISCUSSION
The objective of this study was to determine patient demographics, clinical signs, prognostic factors, and outcome with treatment for dogs with primary mediastinal lymphoma. To the authors’ knowledge, this publication is the first to describe a uniform cohort of dogs with primary mediastinal lymphoma. Results of this study indicate that primary mediastinal lymphoma in dogs is a disease characterized by nonspecific clinical signs, a T-cell phenotype most often associated with WHO lymphoblastic morphology, frequent humoral hypercalcemia of malignancy, and pleural effusion. The median PFS and OS for dogs with primary mediastinal lymphoma in this study (133 and 183 days, respectively) is similar to previous reports of dogs with multicentric high-grade T-cell lymphoma treated with multi-agent protocols (PFS 96–200 days; OS 120–270 days). 4,7,10–14
Previous publications have reported that dogs with mediastinal masses in the multicentric lymphoma disease setting have a worse prognosis than dogs without mediastinal involvement, however these studies did not evaluate phenotype.6,22 When subsets of patients with a T-cell phenotype have been evaluated, mediastinal involvement has not been reported to be a negative prognostic factor. 4,5,14 Only a single dog in this study was determined to have a B-cell phenotype, and the outcome data is consistent with previously published reports on high-grade T-cell lymphoma, indicating that primary mediastinal lymphoma typically represents a variant of high-grade T-cell lymphoma.
Hypercalcemia was not found to be prognostic for PFS or OS in this study. Previous studies have reported hypercalcemia to be prognostic for survival, however, more recent studies evaluating only high-grade T cell lymphoma have not established the presence of hypercalcemia as a negative predictor of outcome for this disease.4–6,14,23 This study provides further evidence that hypercalcemia is not likely an independent negative prognostic factor for lymphoma outcome but rather a marker of high grade, T cell phenotype.
The only patient variable identified in this study to be prognostic at the time of diagnosis for OS, but not PFS, was the presence of pleural effusion. PFS was not significantly different between dogs presenting with pleural effusion and those without, and this suggests that mediastinal lymphoma with concurrent effusion is not necessarily a more aggressive or treatment-refractory tumor. The difference between survival times for these dogs could be related to the fact that dogs requiring repeated thoracocentesis to manage clinical signs associated with their cancer may lead owners to opt for euthanasia sooner than dogs without effusion. When these patients relapse, it may occur in a more emergent setting, thus owners may elect euthanasia over attempts to reinduce remission with chemotherapy or radiation. It is possible that this may not be an independent effect on outcome as a multivariate analysis was not performed, and therefore could be impacted by other confounding variables that were not elucidated in this study.
Another goal of this study was to characterize the lineage and cell morphology of primary mediastinal lymphoma in dogs and determine any associations with hypercalcemia, pleural effusion, or a worse outcome. All but one lymphoma in this study was of T cell origin. While dogs with a lymphoblastic subtype were significantly more likely to be hypercalcemic, there was no difference noted in outcome between dogs with lymphoblastic lymphoma compared to the other subtypes observed. The predominance of the WHO lymphoblastic morphologic type in this study is similar to a previous study evaluating lymphoma in the boxer breed. Interestingly, in that study, 6 dogs were also described as having mediastinal T cell lymphoblastic lymphoma.24
In people, the most common mediastinal non-Hodgkin lymphomas are primary mediastinal large B cell lymphoma and lymphoblastic T cell lymphoma.18,19 The variant of lymphoma described in the dogs in this publication is likely similar to T cell lymphoblastic lymphoma/leukemia in humans, which is characterized by the presence of a mediastinal mass in up to 91% of patients. 25,26 Standardized treatment protocols have not been established for either primary mediastinal large B-cell lymphoma or lymphoblastic T-cell lymphoma in people, however CHOP-based chemotherapy protocols (combined with Rituxamab +/− etoposide for B-cell malignancies) with or without RT are generally recommended.16,27 The role of RT for people with primary mediastinal lymphoma remains unclear. Optimal protocols for RT have yet to be elucidated with either cranial irradiation or targeted (local) radiotherapy being utilized in several reports.25 Some studies have shown survival benefit in subsets of patients with T cell lymphoblastic lymphoma while others have not.16,28,29 With the advent of monoclonal antibody therapy, some studies have suggested that in patients with diffuse large B cell mediastinal lymphoma, rituximab may reduce the benefit of RT in patients that achieve a metabolic complete remission with chemotherapy alone, while others have suggested it may still play a role.30–32
The dogs treated with RT in this study were few in number and treated with varying protocols, but it is interesting to note that two of these dogs did have prolonged progression free and overall survival compared to the rest of the dogs in this study. The two patients that received half body radiation in combination with a CHOP chemotherapy protocol experienced the longest PFS (281,1779 days) and OS (352 days with cranial half body RT, 1779 days with cranial and caudal half body RT). Two additional dogs were treated with RT to the mediastinum during relapse as rescue therapy. The primary concern with mediastinal RT in humans, especially children or young adults, is the development of serious late term cardiopulmonary toxicities and secondary malignancies. This likely represents less of a concern for canine patients given the shorter life expectancy of dogs. Further investigation into the role of RT in the treatment of dogs with primary mediastinal lymphoma is needed to better understand the potential impact on patient outcome.
There are inherent limitations in this study due to its retrospective design, including incomplete staging, inconsistent patient follow-up and variable treatment protocols. To better assess the effect of treatment protocol on outcome, the treatment groups were categorized as CHOP-based versus other. The numbers in treatment groups other than CHOP were small, therefore it was impossible to accurately compare the efficacy of CHOP to other chemotherapy protocols, such as MOPP. A prospective trial with larger numbers would be required to better determine the most efficacious chemotherapy protocols.
Staging diagnostics were not standardized and were incomplete for some dogs, potentially underestimating the stage of disease for some dogs in this study. Not all dogs had an abdominal ultrasound performed, and there were patients with possible intra-abdominal disease that did not have aspirates performed to confirm organ involvement. This led to the inclusion of dogs that had either confirmed (n=1) or possible (n= 5) intra-abdominal disease. These dogs were included in this study as they lacked peripheral lymphadenopathy, their presenting clinical signs were attributable to the presence of a large mediastinal mass, and questionable prognostic value of hepatic and splenic aspirates in lymphoma staging.33
Progression free survival is generally the preferred endpoint for evaluating a population of dogs undergoing treatment for a certain disease given that OS is influenced by owner elected euthanasia prior to death from disease. While PFS was reported in this study, it is important to note the accuracy is inherently flawed given this is a disease characterized by internal lesions without peripheral lymphadenopathy. Thus, relapse could have occurred sooner than what was reported. Response and relapse were also difficult to document retrospectively given lack of standardization with re-staging diagnostics and many dogs were censored from PFS analysis for this reason. Primary care veterinarians evaluated many of the dogs at relapse and for euthanasia, so relapse may be under-reported in this study, and the location reported may not be representative of all dogs with primary mediastinal lymphoma.
In summary, primary mediastinal lymphoma in dogs is characterized by nonspecific clinical signs, a T cell phenotype most often with WHO lymphoblastic morphology, frequent hypercalcemia, and pleural effusion. Prognosis for this disease is poor, but survival is improved when patients are treated with a multi-agent chemotherapy protocol.
FIGURE 2.
Kaplan-Meier curve for progression free survival (left) and overall survival (right) in dogs with primary mediastinal lymphoma treated with a CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone)-based chemotherapy protocol vs those that were not
Acknowledgments
This work was completed at and supported by the Center for Companion Animal Health, School of Veterinary Medicine, University of California, Davis. Dr. Burton is supported by the National Cancer Institute of the National Institutes of Health under Award Number K12CA138464. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- CBC
complete blood count
- CHOP
cyclophosphamide, doxorubicin, vincristine, prednisone
- CR
complete response
- ICC
immunocytochemistry
- L-MOPP
L-asparaginase, mechlorethamine, vincristine, procarbazine, prednisone
- OS
overall survival
- PCR
polymerase chain reaction
- PFS
progression free survival
- PR
partial response
- PD
progressive disease
- RBC
red blood cell
- RT
radiation therapy
- VELCAP-TSC
vincristine, L-asparaginase, doxorubicin, cyclophosphamide, prednisolone
- actinomycin-D
vincristine, procarbazine
- VMTH
Veterinary Medical Teaching Hospital
- WHO
World Health Organization
References
- 1.Vail DM, Pinkerton ME, Young KM. Hematopoietic Tumors. In: Withrow SJ, Vail DM, Page RL, editors. Withrow & MacEwen’s Small Animal Clinical Oncology. 4. St. Louis, MO: Elsevier; 2013. pp. 608–678. [Google Scholar]
- 2.Geyer NE, Reichle JK, Valdes-Martinez A, et al. Radiographic appearance of confirmed pulmonary lymphoma in cats and dogs. Vet Radiol Ultrasound. 2010;51(4):386–390. doi: 10.1111/j.1740-8261.2010.01683.x. [DOI] [PubMed] [Google Scholar]
- 3.Starrak GS, Berry CR, Page RL, Johnson JL, Thrall DE. Correlation between thoracic radiographic changes and remission/survival duration in 270 dogs with lymphosarcoma. Vet Radiol Ultrasound. 1997;38(6):411–418. doi: 10.1111/j.1740-8261.1997.tb00863.x. [DOI] [PubMed] [Google Scholar]
- 4.Rebhun RB, Kent MS, Borrofka SA, Frazier S, Skorupski K, Rodriguez CO. CHOP chemotherapy for the treatment of canine multicentric T-cell lymphoma. Vet Comp Oncol. 2011;9(1):38–44. doi: 10.1111/j.1476-5829.2010.00230.x. [DOI] [PubMed] [Google Scholar]
- 5.Avery PR, Burton J, Bromberek JL, et al. Flow cytometric characterization and clinical outcome of CD4+ T-cell lymphoma in dogs: 67 cases. J Vet Intern Med. 2014;28(2):538–546. doi: 10.1111/jvim.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg MP, Matus RE, Patnaik AK. Prognostic factors in dogs with lymphoma and associated hypercalcemia. J Vet Intern Med. 1991;5(5):268–271. doi: 10.1111/j.1939-1676.1991.tb03133.x. [DOI] [PubMed] [Google Scholar]
- 7.Vail DM, Kisseberth WC, Obradovich JE, et al. Assessment of potential doubling time (Tpot), argyrophilic nucleolar organizer regions (AgNOR), and proliferating cell nuclear antigen (PCNA) as predictors of therapy response in canine non-Hodgkin’s lymphoma. Exp Hematol. 1996;24(7):807–815. [PubMed] [Google Scholar]
- 8.Ruslander DA, Gebhard DH, Tompkins MB, Grindem CB, Page RL. Immunophenotypic characterization of canine lymphoproliferative disorders. In Vivo. 1997;11(2):169–172. [PubMed] [Google Scholar]
- 9.Villamil JA, Henry CJ, Hahn AW, Bryan JN, Tyler JW, Caldwell CW. Hormonal and sex impact on the epidemiology of canine lymphoma. J Cancer Epidemiol. 2009;2009:591753. doi: 10.1155/2009/591753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chun R, Garrett LD, Vail DM. Evaluation of a high-dose chemotherapy protocol with no maintenance therapy for dogs with lymphoma. J Vet Intern Med. 2000;14(2):120–124. doi: 10.1892/0891-6640(2000)014<0120:eoahcp>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Siedlecki CT, Kass PH, Jakubiak MJ, Dank G, Lyons J, Kent MS. Evaluation of an actinomycin-D-containing combination chemotherapy protocol with extended maintenance therapy for canine lymphoma. Can Vet J. 2006;47(1):52–59. [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman IH, Moore AS, Frimberger AE. Treatment of canine non-indolent T cell lymphoma using the VELCAP-TSC protocol: A retrospective evaluation of 70 dogs (2003–2013) Vet J. 2016;211:39–44. doi: 10.1016/j.tvjl.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Curran K, Thamm DH. Retrospective analysis for treatment of naive canine multicentric lymphoma with a 15-week, maintenance-free CHOP protocol. Vet Comp Oncol. 2016;14(Suppl 1):147–155. doi: 10.1111/vco.12163. [DOI] [PubMed] [Google Scholar]
- 14.Brodsky EM, Maudlin GN, Lachowicz JL, Post GS. Asparaginase and MOPP treatment of dogs with lymphoma. J Vet Intern Med. 2009;23(3):578–584. doi: 10.1111/j.1939-1676.2009.0289.x. [DOI] [PubMed] [Google Scholar]
- 15.Martelli M, Di Rocco A, Russo E, Perrone S, Foa R. Primary mediastinal lymphoma: diagnosis and treatment options. Expert Rev Hematol. 2015;8(2):173–186. doi: 10.1586/17474086.2015.994604. [DOI] [PubMed] [Google Scholar]
- 16.Bassan R, Maino E, Cortelazzo S. Lymphoblastic lymphoma: an updated review on biology, diagnosis, and treatment. Eur J Haematol. 2016;96(5):447–460. doi: 10.1111/ejh.12722. [DOI] [PubMed] [Google Scholar]
- 17.Zwingenberger AL, Vernau W, Shi C, et al. Development and characterization of 5 canine B-cell lymphoma cell lines. Leuk Res. 2012;36(5):601–606. doi: 10.1016/j.leukres.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keller SM, Moore PF. A novel clonality assay for the assessment of canine T cell proliferations. Vet Immunol Immunopathol. 2012;145(1–2):410–419. doi: 10.1016/j.vetimm.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Vail DM, Michels GM, Khanna C, Selting KA, London CA Veterinary Cooperative Oncology G. Response evaluation criteria for peripheral nodal lymphoma in dogs (v1.0)--a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol. 2010;8(1):28–37. doi: 10.1111/j.1476-5829.2009.00200.x. [DOI] [PubMed] [Google Scholar]
- 20.Valli VE, San Myint M, Barthel A, et al. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet Pathol. 2011;48(1):198–211. doi: 10.1177/0300985810379428. [DOI] [PubMed] [Google Scholar]
- 21.Lurie DM, Gordon IK, Theon AP, Rodriguez CO, Suter SE, Kent MS. Sequential low-dose rate half-body irradiation and chemotherapy for the treatment of canine multicentric lymphoma. J Vet Intern Med. 2009;23(5):1064–1070. doi: 10.1111/j.1939-1676.2009.0353.x. [DOI] [PubMed] [Google Scholar]
- 22.Moore AS, Cotter SM, Rand WM, et al. Evaluation of a discontinuous treatment protocol (VELCAP-S) for canine lymphoma. J Vet Intern Med. 2001;15(4):348–354. [PubMed] [Google Scholar]
- 23.Ponce F, Magnol JP, Ledieu D, et al. Prognostic significance of morphological subtypes in canine malignant lymphomas during chemotherapy. Vet J. 2004;167(2):158–166. doi: 10.1016/j.tvjl.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Lurie DM, Milner RJ, Suter SE, Vernau W. Immunophenotypic and cytomorphologic subclassification of T-cell lymphoma in the boxer breed. Vet Immunol Immunopathol. 2008;125(1–2):102–110. doi: 10.1016/j.vetimm.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Cortelazzo S, Ponzoni M, Ferreri AJ, Hoelzer D. Lymphoblastic lymphoma. Crit Rev Oncol Hematol. 2011;79(3):330–343. doi: 10.1016/j.critrevonc.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Dugas M, Messerer D, Hasford J, et al. German multicenter study group for adult ALL (GMALL): recruitment in comparison to ALL incidence and its impact on study results. Ann Hematol. 2003;82(2):83–87. doi: 10.1007/s00277-002-0585-x. [DOI] [PubMed] [Google Scholar]
- 27.Dunleavy K, Pittaluga S, Maeda LS, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;368(15):1408–1416. doi: 10.1056/NEJMoa1214561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellin F, Jerkeman M, Hagberg H, Relander T. Treatment outcome in T-cell lymphoblastic lymphoma in adults - a population-based study from the Swedish Lymphoma Registry. Acta Oncol. 2014;53(7):927–934. doi: 10.3109/0284186X.2014.889850. [DOI] [PubMed] [Google Scholar]
- 29.Hoelzer D, Gokbuget N, Digel W, et al. Outcome of adult patients with T-lymphoblastic lymphoma treated according to protocols for acute lymphoblastic leukemia. Blood. 2002;99(12):4379–4385. doi: 10.1182/blood-2002-01-0110. [DOI] [PubMed] [Google Scholar]
- 30.Giri S, Bhatt VR, Pathak R, Bociek RG, Vose JM, Armitage JO. Role of radiation therapy in primary mediastinal large B-cell lymphoma in rituximab era: A US population-based analysis. Am J Hematol. 2015;90(11):1052–1054. doi: 10.1002/ajh.24172. [DOI] [PubMed] [Google Scholar]
- 31.Jackson MW, Rusthoven CG, Jones BL, Kamdar M, Rabinovitch R. Improved Survival With Radiation Therapy in Stage I–II Primary Mediastinal B Cell Lymphoma: A Surveillance, Epidemiology, and End Results Database Analysis. Int J Radiat Oncol Biol Phys. 2016;94(1):126–132. doi: 10.1016/j.ijrobp.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 32.Jackson MW, Rusthoven CG, Jones BL, Kamdar M, Rabinovitch R. Improved survival with combined modality therapy in the modern era for primary mediastinal B-cell lymphoma. Am J Hematol. 2016;91(5):476–480. doi: 10.1002/ajh.24325. [DOI] [PubMed] [Google Scholar]
- 33.Nerschbach V, Eberle N, Joetzke AE, et al. Splenic and hepatic ultrasound and cytology in canine lymphoma: effects of findings on stage migration and assessment of prognosis. Vet Comp Oncol. 2014 doi: 10.1111/vco.12127. [DOI] [PubMed] [Google Scholar]