Abstract
In eukaryotes, two-pore channels (TPC1-3) comprise a family of ion channels that regulate the conductance of Na+ and Ca2+ ions across cellular membranes. TPC1-3 form endolysosomal channels, but TPC3 can also function in the plasma membrane. TPC1/3 are voltage-gated channels, but TPC2 opens in response to binding endolysosome-specific lipid phosphatidylinositol-3,5-diphosphate (PI(3,5)P2). Filoviruses, such as Ebola, exploit TPC-mediated ion release as a means of escape from the endolysosome during infection. Antagonists that block TPC1/2 channel conductance abrogate filoviral infections. TPC1/2 form complexes with the mechanistic target of rapamycin complex 1 (mTORC1) at the endolysosomal surface that couple cellular metabolic state and cytosolic nutrient concentrations to the control of membrane potential and pH. We determined the X-ray structure of TPC1 from Arabidopsis thaliana (AtTPC1) to 2.87Å resolution–one of the two first reports of a TPC channel structure. Here we summarize these findings and the implications that the structure may have for understanding endolysosomal control mechanisms and their role in human health.
Keywords: Ion channels, Structure, Membrane protein, Lysosome, Transport
Graphical abstract
Rendition of the Two-pore channel 1 (TPC1) structure (blue) bound to inhibitor trans-NED-19 (yellow) in the endolysosome (white). Ebola viruses (green) that require and co-localize with TPC1 during infection are depicted. Microtubules (red) are shown in the background. Image by Janet Iwasa, PhD.
Endolysosomes facilitate cellular trafficking, nutrient uptake and release, protein degradation, and pathogen evasion[1]. These vital cellular functions hinge on the precise control of ionic (Na+, K+, Ca2+, Cl−, H+) gradients that define the endolysosomal membrane potential[2]. Ca2+-release and uptake through ion channels and transporters enables communication[3–5] between organelles and control of vesicular movement[6]. Transporters, proton pumps, and ion channels maintain the lysosomal pH required for proteolytic functions[7]. Despite the prevalence of human disorders that exhibit lysosome dysfunction[1,8], the molecular mechanisms that control lysosome transport remain poorly understood as compared with plasma membrane transport[9].
Two-pore channels (TPCs) form a subfamily (TPC1-3) of eukaryotic voltage- and ligand-gated cation channels[10,11]. TPC1/2 function as part of the ion transport machinery that regulate the endolysosomal resting membrane potential and pH[12]. TPC1/2 knockout mice have lower cellular amino acid levels and endurance during fasting[13], suggesting that TPC-mediated control of the endolysosome membrane potential and pH regulates nutrient transport. Complexes between TPC1/2 and the mechanistic target of rapamycin complex 1 (mTORC1) on the endolysosome link cellular metabolic status and nutrient levels to regulation of amino acid efflux[13]. MTORC1 inhibits channel opening, presumably by phosphorylation, though no sites have been identified. TPC1/2-mTORC1 complexes may be therapeutic targets because greater than thirty different mTOR mutations exist in human cancers, a subset of which activate mTORC1 and facilitate tumor proliferation and chemotherapy resistance[14]. Furthermore, mutations in TPC2 disrupt Na+/Ca2+ gradients in endolysosome-like melanocytes[15,16], which may contribute to the development of skin cancer[17]. TPCs may also be important drivers of lung cancer metastasis[18]. The molecular nature of TPC1/2-mTORC1 interactions demands further investigation. Filoviruses exploit TPC1/2 ion conductance to escape the endolysosome prior to degradation in the lysosome[19]. Pharmacological block of TPC1/2 by trans-Ned-19 (NED19) and certain calcium channel blockers inhibits viral infectivity. Antagonists could prevent viral uptake through effects on membrane fusion, receptor binding, or proteolytic activation.
To approach an atomic understanding of how TPC channels control endolysosomal ion transport, we determined the structure of TPC1 from Arabidopsis thaliana (AtTPC1) by X-ray crystallography to 2.87Å resolution (Figure 1, ref. [20]). A second report determined a 3.3Å structure of AtTPC1 along with electrophysiological measurements[21]. These were the first structures of a TPC channel. A single TPC1 polypeptide contains two tandem Shaker-like domains (D1 and D2, ref. [22,23]) that dimerize to form a central quasi-tetrameric channel. Each chain contains two voltage-sensing domains (VSDs) involving transmembrane segments S1-S4 (VSD1) and S7-S10 (VSD2), two pore domains in S5-S6 (P1) and S11-S12 (P2), and activation gates following S6 and S12. D1 and D2 share 20–30% sequence identity with particular domains in voltage-gated calcium (Cav) and sodium channels (Nav), suggesting that TPCs are a family intermediate between the tetrameric channels, like voltage-gated potassium (Kv) channels, and Cavs/Navs that have four pore-forming domains in series on a single chain[24]. This heritage might explain why some inhibitors of Cav channels also block TPC channels[19,25].
Figure 1. Overview of the AtTPC1 Structure.

Views down (top) long and short channel axes, and (bottom) top-down through the central channel of AtTPC1 (PDB 5DQQ; ref. [20]). Boundaries for endolysosome/vacuole (E), membrane (M), and cytoplasm (C) are shown. Ca2+-ions, including the sites for luminal inhibition (Cai2+) and cytoplasmic activation (Caa2+), are shown as green spheres.
The structure of AtTPC1 is asymmetric. Non-equivalence of D1 and D2 and tandem architecture create asymmetry in the molecule that stems from differing lengths of the lateral helices joining VSD1 to P1 (S4-S5) and VSD2 to P2 (S10-S11). S4 partially unravels at its base, causing S4-S5 to be shorter than S10-S11 by one helical turn. The angle between S4 and S5 (87˚) is more obtuse than between S10 and S11 (38˚), leading to elongation along the dimer axis and contraction along D1-D2 that result in a rectangular 2-fold symmetric channel. Four pore domains form the central channel in a 2-fold symmetric arrangement (P1-P2-P1-P2). Discussion in sections below highlight the potential roles of TPC channel asymmetry in voltage-dependent activation and ion selectivity.
Plant TPC1 channels contain cytosolic N-terminal domains (NTD), C-terminal domains (CTD), and two EF-hand domains (EF) between the two pore subunits that confer activation by Ca2+-ions[10,26]. A di-leucine motif in the NTD directs trafficking to the endolysosome (or vacuole in plants)[27]. Both the NTDs and CTDs have roles in channel activation; when they are removed, channels no longer function[28,29].
In most eukaryotic cells, but not in plants or yeast, nicotinic acid adenine dinucleotide phosphate (NAADP) triggers the release of Ca2+-ions from acidic intracellular stores, causing temporary increases in the cytosolic Ca2+-ion concentration[11,30], influencing biological processes ranging from cell differentiation to cardiac function[31–33]. Fluorescence Ca2+-imaging experiments suggest that NAADP triggers Ca2+-release from acidic Ca2+-stores containing TPC1-3[11,34–36]. However, photoaffinity labeling experiments with 32P-labeled NAADP show that it does not directly bind TPCs[37,38]. Rather, an unidentified NAADP-binding protein may be required for activation of TPCs[39]. It is also possible that other channels and/or other organelles may be the target and functional source of Ca2+-ions. Identification of the putative NAADP-receptor will be important for determining the role of TPC channels in endolysosomal Ca2+-signaling.
Activation Mechanisms
TPC1 confers endolysosomes with electrical excitability[40]. TPC1 is primarily a voltage-gated channel, but luminal pH[40], cytosolic and luminal Ca2+-ions[41–43], and endolysosome-specific lipid PI(3,5)P2[12] regulate channel conductance. Mammalian TPC2 is voltage-insensitive and activated by PI(3,5)P2 and potentially by NAADP[11]. TPC3 is not present in humans, but the mammalian and vertebrate orthologues localize to both plasma membranes and endolysosomes[11,36,44]. On plasma membranes, fish and frog TPC3 behaves as voltage-gated, Na+-selective channels, insensitive to PI(3,5)P2 and PI(4,5)P2[44]. On endolysosomes, chicken and rat TPC3 may conduct Ca2+-ions in response to NAADP[36], whereas conflicting reports of invertebrate TPC3 NAADP-sensitivity exist[45,46].
Figure 2 summarizes the known or suggested activation/regulation mechanisms of TPCs[10,12,47–51]. Cytoplasmic Ca2+-ions are required for voltage-dependent activation of plant TPC1[10]. Gating of plant TPCs is likely a multistep process that requires coupling of the VSDs to the cytoplasmic EF domains. Each EF domain contains two Ca2+-binding sites (EF1 and EF2). Substitution of Ca2+-coordinating residues has shown that only EF2 is responsible for Ca2+-activation[26].
Figure 2. TPC Activation Mechanisms.
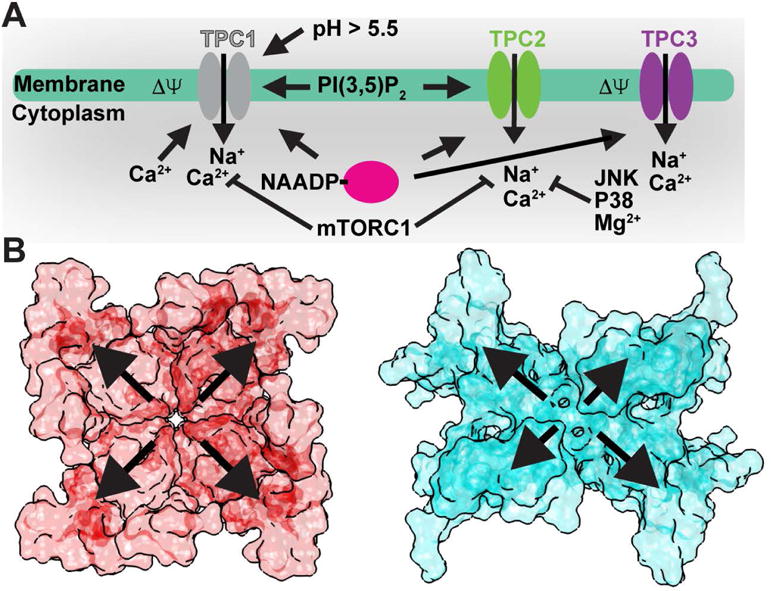
A) Summary of luminal/extracellular and cytoplasmic agents and processes that modulate TPC channel opening. B) Surface renderings of a (left) symmetrical tetrameric channel (NavAb; PDB 3RVY; ref. [56]) versus (right) the asymmetric tandem TPC1 dimer (AtTPC1; PDB 5DQQ; ref. [20]). Outward arrows highlight potential differential contributions from the sensory and pore domains to channel activation.
How do the VSDs and EFs couple structurally to facilitate activation by both membrane potential and cytoplasmic Ca2+-ions? The AtTPC1 structure captures the Ca2+-activated state of the EF2[20]. Ca2+-bound EF2 forms an intramolecular complex with the CTD via a salt-bridge between D376 of EF2 and R700 of the CTD. CTD residue S701 coordinates the activating Ca2+-ion (Caa2+; Figure 1) along with E374 and D377. The EF2-CTD interaction may serve as the ‘Ca2+-switch’, coupling Ca2+-binding in EF2 and the CTD to activation. R552 in the S10-S11 linker of VSD2 forms a salt-bridge with D685 of the CTD that could link the conformation of EF2-CTD to voltage-sensing. Removal of cytosolic Ca2+ could weaken the EF2-CTD interaction, increase dynamics in the CTD, and disrupt conformational coupling to VSD2.
The EF2-CTD interactions are probably conserved between TPCs since the NTDs and CTDs facilitate channel activation in both mammalian and plant TPCs[28,29]. In human TPC2, the CTD mutation G734E correlates with changes in skin and hair pigmentation and the defective pigment synthesis observed in melanomas[17,52]. This suggests that the EF2-CTD interaction could be important for channel function in humans.
Luminal Ca2+-ions inhibit plant TPC1 channel activation at millimolar concentrations via binding sites in the S7-S8 loop of VSD2[47]. The AtTPC1 mutation D454N, named fou2 (fatty acid oxygenation up-regulated 2), causes an increase in stress hormone jasmonate synthesis as part of the Ca2+-dependent wounding response in plants[53,54]. D454N increases channel open probability by shifting the voltage-dependence of VSD2 towards more depolarizing potentials and abolishes inhibition by luminal Ca2+-ions[47,21]. The structure of AtTPC1 and electrophysiological measurements clarified the mechanism of luminal Ca2+-inhibition by revealing two Ca2+-binding sites (site 1 and site 2) at the luminal face of VSD2[20,21]. The approximate location of these two Ca2+-sites had been predicted previously[55], though the models did not explain how either site could directly modulate voltage-sensing. The structure shows that D240 in P1, D454 in the S7-S8 loop, and E528 in S10 coordinate site 1, whereas E239 in P1 and E457 in the S7-S8 loop coordinate site 2. Site 1, not site 2, is the luminal inhibitory Ca2+ site because mutations in site 1 (D240N, D454N, E528Q), but not site 2 (D239N, E457Q), eliminate luminal Ca2+ inhibition (Cai2+; Figure 1) in electrophysiological recordings[21,47]. Site 1 mutations also shift voltage-dependent activation by nearly +50mV, such that the channel is in an open-activated state under crystallization conditions (0mV)[21]. The function of Cai2+ may be to tune the endolysosomal/vacuolar membrane potential by shifting voltage-dependent activation of TPC1. Fortuitously, this unique luminal Ca2+-site may also enable trapping of multiple conformational states of the channel by changing the Ca2+-concentration.
Wild-type AtTPC1 adopts a closed-resting state at 0mV in the presence of luminal millimolar concentrations of Ca2+-ions[21]. The functional voltage sensor VSD2 is the first case of the resting-state conformation with respect to other voltage-gated ion channel structures. The three functional gating charges (R1; R537, R2; R540, R3; R543) in S10 shift downward towards the cytosolic leaflet with respect to active-state structures (Figure 3) [56–59]. Comparing AtTPC1 resting-state and other active-state VSDs we see a striking change in the overall VSD structure. In the resting-state, the last two gating charges (R2, R3) of AtTPC1 are exposed to the cytoplasm, and the first gating charge R1 is at the well-conserved hydrophobic charge transfer center[60]. We deduce that during activation the movement of S4/S10 in VSD2 exposes the first two gating charges (R1, R2) to the endolysosomal lumen or extracellular space. This would electrically move 2 gating charges to the other side of the membrane per activation cycle per VSD2. Activation of both VSD2s would account for the estimated gating charge of ~3.9 for AtTPC1 to achieve unitary open probability[21]. Electrophysiological measurements also confirm that VSD2 is the active voltage-sensor and VSD1 is voltage-insensitive[21,61]. Movements in S7 and S9 lead to an overall closing of the solvent vestibule to the gating charges at the cytoplasmic face of VSD2, accommodating the movement of S10 during activation and opening the solvent to gating charges via the endolysosomal/extracellular face. This effectively moves gating charge across the membrane barrier and mechanically displaces the S10-S11 linker to open the channel activation gates.
Figure 3. Mechanism of Voltage-sensing.
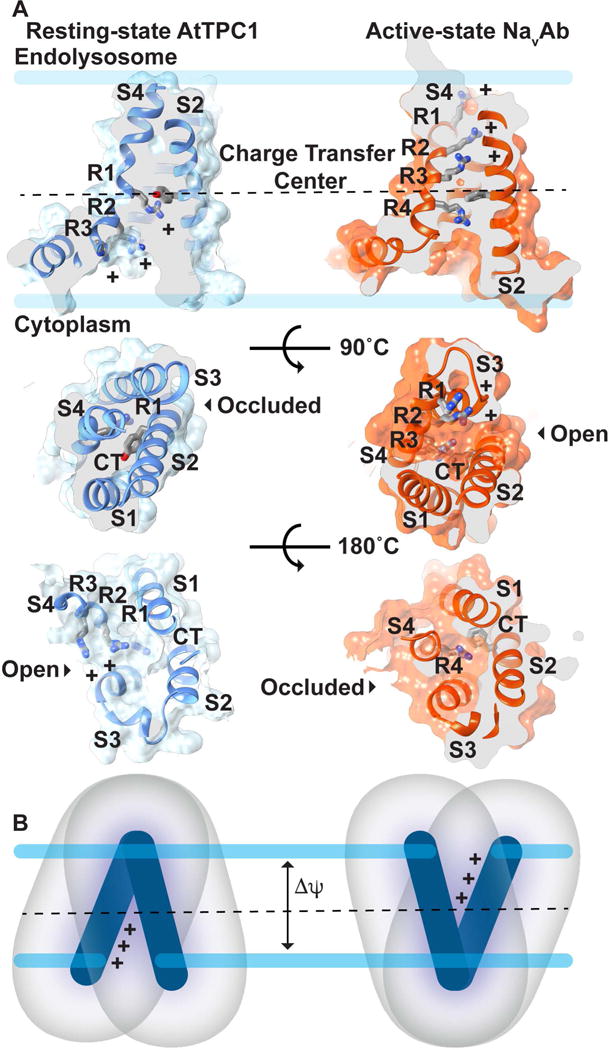
A) Comparison of (left) resting-state AtTPC1 (PDB 5DQQ; ref. [20]) and (right) active-state NavAb (PDB 3RVY; ref. [56]) structures. View through the (Top) membrane, (Middle) luminal/extracellular, and (Bottom) cytoplasmic faces of the VSD. The charge transfer center (CT) location (dash line) in S2 and gating charges (R1-R4) in S4 are shown. B) A hypothetical pathway for gating charge movement in the VSD from the cytoplasmic leaflet in the resting-state through the membrane to the luminal leaflet in the active-state.
In AtTPC1, S10 is a 310-helix. The fact that S10 maintains at least partial 310-helicity in most active-state structures[56–59,62] and our resting-state suggests that the helical pitch does not change drastically during voltage-dependent activation. Rather 310-helicity may be a conserved structural feature of voltage-sensors and required for function, as with Kv channels[57].
The M484L VSD2 mutation in human TPC2, located in S8 near the charge transfer center, is linked to melanomas and regulation of pigmentation[17,52]. The corresponding M479L mutation in AtTPC1 would disrupt the hydrogen bond with W505 in S9 and could perturb voltage-dependent activation. Structures of AtTPC1 in the active-state will shed light on the rearrangements required for channel activation.
Polyunsaturated fatty acids inhibit plant TPC1[50]. We observe a lipid bound to the interface between P1 and VSD2. This has been observed in previous structures of voltage-gated channels[63,56,64,65]. Van der Waals interactions between the lipid alkyl chain and M237 in S5 and L533 in S10 could stabilize the closed-resting state. A hydrogen bond between S277 in the S5-S6 pore loop and the fatty acid head group could also restrict pore opening. The electron density is consistent with palmitic acid, the most abundant fatty acid present in soy polar lipids–the major lipid source used for crystallization[20]. Lipid-binding to this site could restrict pore movement during voltage-dependent activation.
Ion Selectivity
A primary function of most ion channels is to selectively conduct ions through the membrane in response to excitatory stimuli. This requires a mechanism to prevent incorrect ions from entering the channel lumen. In most cation-selective channels, a domain called the selectivity filter[66], formed by the pore loops, between S5 and S6, that line the channel, imparts selectivity through structural interactions with passing ions. Selective channels coordinate passing ions either through direct or water-mediated interactions[56,66,67]. The selectivity filter in AtTPC1 is asymmetric, with diameters across P1–P1′ and P2–P2′ of ~14Å and ~5Å, respectively. Residues with negative charge are present in the selectivity filter that could coordinate ions, but they are only aligned with the conduction pathway in P2–P2′. The negative charge density in the selectivity filter may explain the cation selectivity, but AtTPC1 may be unable to distinguish cations based on hydration shell as suggested for Navs and Cavs[56,67]. AtTPC1 is 5-fold selective for Ca2+-ions over other ions[68]. The structure identifies a binding site for Ca2+ replacement Yb3+-ion in the upper selectivity filter[20]. E605 directly chelates the Yb3+-ion, suggesting that this may provide some of the modest selectivity for Ca2+-ions. This site may also explain how trivalent lanthanides and Al3+-ions inhibit plant TPC1[69].
Mammalian TPC1/2 are Na+-selective (TPC1; PNa/PK~100, TPC2; PNa/PK~30) [40], although both can conduct Ca2+ under certain conditions[70]. Indeed exchange of the less selective AtTPC1 selectivity filter in P2 for human TPC2 produces a Na+-selective channel[68]. In the TPC2 selectivity filter, a second asparagine forms an additional constriction point that contacts passing Na+-ions[68]. Unlike other Na+/Ca2+-selective channels, which use negatively charged amino acid sidechains to interact with passing cations[56,67,71], TPCs use the uncharged sidechains of two asparagine residues. Therefore, TPCs use neutral amino acid sidechains for selecting Na+/Ca2+-ions—a distinct ion selectivity mechanism from other channels.
Pharmacology
In animals, NED19 prevents infection by filoviruses either by altering fusion of viral membranes with the endolysosome, or by altering the binding of the viral glycoprotein to the NPC1 receptor in endosomes[19]. Serendipitously, NED19 was developed as an NAADP antagonist by virtual screening[72]. How NED19 in mammalian TPCs inhibits putative activation by NAADP is not clear. NED19 acts as a non-competitive inhibitor of NAADP activation by directly blocking mammalian TPC channels[73]. While AtTPC1 does not respond to NAADP, NED19 potently blocks channel conductance in endolysosomes (our unpublished data). The structure of AtTPC1 shows that the antagonist NED19 may prevent channel activation by clamping the pore domains to VSD2 (Figure 4). NED19 interacts with the luminal faces of P1 and P2. This suggests that NED19 may block channel opening by restricting movement of the pore helices P1 and P2.
Figure 4. TPC Pharmacology.
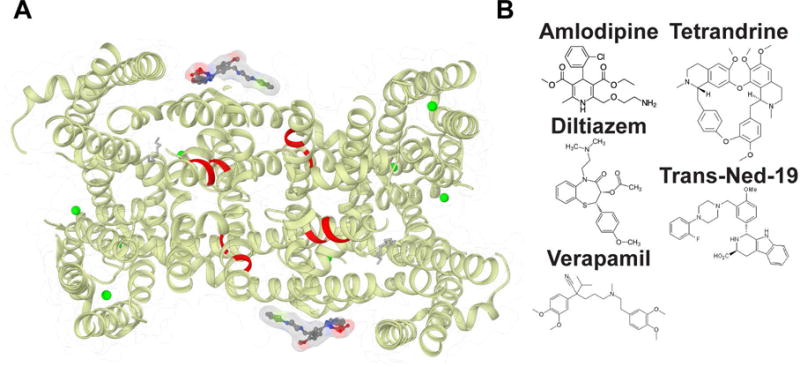
A) Predicted binding site for DHP (red) Cav blockers based on sequence homology with Cavs overlaid onto the AtTPC1 structure bound to non-DHP molecule trans-NED-19 (PDB 5DQQ; ref. [20]). B) Molecular structure of representative pharmacophores that block TPC channels.
Approved medications of the dihydropyridine (DHP), phenylalkylamine (PAA), and benzothiazepine (BTZ) classes of L-type Cav antagonists block Ebola virus trafficking and fusion in the endolysosome in a TPC1/2 dependent manner[19]. These drugs, like Tetrandrine and NED19, presumably act by blocking channel conductance. TPC channels contain conserved DHP and PPA binding sites in transmembrane segments S6 and S12 that may explain the pharmacological overlap with Cavs[25,74,75]. Recent structures of bacterial Cav channels (CavAb) bound to DHP (amlodipine and nimodipine) and PPA (verapamil) inhibitors suggest that inhibitors bind in two configurations[65]. DHP molecules bind to the pore periphery like NED19, whereas PPAs directly block the pore lumen. The pore asymmetry in TPCs may change the way these molecules, especially DHPs, link the pore and VSD domains. Determining the antagonist binding sites on TPCs may serve as a template for the development of novel therapies for filoviral infections and possibly cancer.
Tetrandrine, a bis-benzylisoquinoline alkaloid isolated from the Chinese herb Stephania tetrandra[76], potently blocks TPC channels. NED19 and Tetrandrine both show promise in treating cancers[18] and filoviral infections[19]. Given the structural similarity to verapamil, an L-type Cav antagonist, Tetrandrine likely binds TPCs in a distinct location from NED19.
Phosphoregulation by Kinases
Phosphoregulation is a hallmark of TPC channel function. Plant TPC1 is both activated (CAM-like dependent kinase) and inhibited (PKA, PKC, or a PKG-like kinase) at distinct sites by phosphorylation[77]. Cytoplasmic kinases modulate human TPC1/2 conductance and function[78–80], but only mTORC1 has been shown to directly inhibit channel currents through formation of high-affinity complexes[13].
We determined the location of phosphorylation sites in AtTPC1 in both the NTD (S22, T26, T29) and CTD (S701) (unpublished data; Figure 5). The NTD contacts EF1 via van der Waals interactions and hydrogen bonds. Since EF1 is not required for channel activation, the NTD phosphorylation sites may not directly gate the channel. However, S701 in the CTD directly contacts the activating Ca2+-ion in EF2. Phosphorylation of S701 could disrupt the interaction of the CTD with EF2, preventing Ca2+-binding, thereby mimicking the apo-Ca2+ state. This may weaken the EF2-CTD interaction, increasing flexibility in the region, and thereby perturb the structural interaction between the CTD and the S10-S11 linker in VSD2. Pinpointing the sites of phosphorylation-dependent gating will be critical for defining how TPCs integrate kinase signaling to control the resting endolysosomal membrane potential and pH.
Figure 5. Model for TPC1 Activation and Regulation.
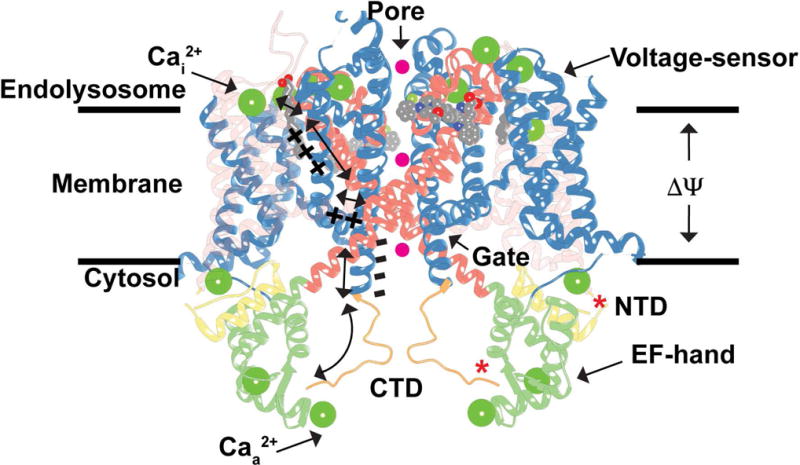
Hypothetical model for TPC1 activation by membrane potential (ΔΨ) and cytosolic Ca2+ ions (Caa2+), and inhibition by luminal Ca2+ ions (Cai2+) and phosphorylation (*) overlaid onto the AtTPC1 structure (PDB 5DQQ; ref. [20]). A possible mechanism for conformational coupling between voltage-sensor VSD2 and EF-hand domain EF2 during channel activation is shown. Hypothetical permeating ions are shown as spheres (magenta). The transparency of VSD1 is increased for clarity.
TPC-protein Interactions
Human TPC1/2 form stable complexes with mTORC1 at the endolysosomal membrane that couple changes in cellular metabolism and nutrient concentrations to control of the endolysosome membrane potential and pH[13]. MTORC1 also forms complexes with Rag GTPases, Ragulator, SLC38a9 arginine transporter, NPC1 Niemann-Pick C1 cholesterol transporter, and H+-ATPase proton pumps[81–84] as part of the endolysosomal nutrient-sensing and transport machinery. These macromolecular complexes link dynamic cytoplasmic nutrient concentrations, cellular growth signals, and stress to changes in the endolysosome transport[85,86]. Uncovering the molecular interactions within this complex will open the way to understanding how mTOR signaling regulates endolysosome physiology.
The human TPC2 NTD contains a Rab GTPase-binding motif, not conserved in human TPC1. Rab-binding regulates trafficking and channel function[87]. The interaction of plant TPCs with Rab GTPases has not been tested, but the structure of AtTPC1 shows that binding to NTD could affect channel opening by influencing the structure of the nearby EF domains. While it is not known yet whether plant TPCs form protein-protein interactions via the NTD or CTD, the TPC1/2 CTD may form a hub for binding of apoptotic machinery[88].
We conclude that the structure AtTPC1 provides key insights into the mechanisms of voltage-sensing, ion selectivity, channel activation, and the phosphoregulation of TPC channels. It provides a template for the design of experiments probing the regulatory mechanisms of TPC channels.
Acknowledgments
This work was supported by NIH grant GM24485 (to R.M.S) and a Postdoctoral Independent Research Grant (to A.F.K) from the University of California, San Francisco Program for Breakthrough Biomedical Research, which is partially funded by the Sandler Foundation. Beamline 8.3.1 at the Advanced Light Source is operated by the University of California Office of the President, Multicampus Research Programs and Initiatives grant MR-15-328599. The Berkeley Center for Structural Biology is supported in part by the National Institutes of Health, National Institute of General Medical Sciences, and the Howard Hughes Medical Institute. The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under Contract No. DE-AC02-05CH11231. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-76SF00515. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, and by the National Institutes of Health, National Institute of General Medical Sciences (including P41GM103393). Figures were made with Chimera developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIGMS P41-GM103311).
Abbreviations
- mTORC1
Mechanistic target of rapamycin complex 1
- TPC
Two-pore channel
- PI(3,5)P2
Phosphatidylinositol-3,5-diphosphate
- AtTPC1
Arabidopsis thaliana TPC1
- NED19
Trans-Ned-19
- VSD
Voltage-sensing domain
- P1
Pore domain in S5-S6
- P2
Pore domain in S11-S12
- Cav
Voltage-gated calcium channel
- Nav
Voltage-gated sodium channel
- Kv
Voltage-gated potassium channel
- NTD
N-terminal domain
- CTD
C-terminal domain
- EF
EF-hand domain
- NAADP
Nicotinic acid adenine dinucleotide phosphate
- PI(4,5)P2
Phosphatidylinositol-4,5-diphosphate
- DHP
Dihydropyridine
- PAA
Phenylalkylamine
- BTZ
Benzothiazepine
- Caa2+
Activating Ca2+-ion
- Cai2+
Inhibitory Ca2+-ion
- fou2
Fatty acid oxygenation up-regulated 2
- SLC38a9
Sodium-coupled neutral amino acid transporter 9
- NPC1
Niemann-Pick C1
- PKA
Protein kinase A
- PKC
Protein kinase C
- PKG
Protein kinase G
- H+-ATPase
Proton Pump
- 32P
Phosphorus-32
References
- 1.De Duve C, Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- 2.Xu H, Ren D. Lysosomal Physiology. Annu Rev Physiol. 2015;77:57–80. doi: 10.1146/annurev-physiol-021014-071649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X, Cheng X, Yu L, Yang J, Calvo R, Patnaik S, Hu X, Gao Q, Yang M, Lawas M, Delling M, Marugan J, Ferrer M, Xu H. MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat Commun. 2016;7:12109. doi: 10.1038/ncomms12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garrity AG, Wang W, Collier CM, Levey SA, Gao Q, Xu H. The endoplasmic reticulum, not the pH gradient, drives calcium refilling of lysosomes. eLife. 2016;5:e15887. doi: 10.7554/eLife.15887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kilpatrick BS, Yates E, Grimm C, Schapira AH, Patel S. Endo-lysosomal TRP mucolipin-1 channels trigger global ER Ca2+ release and Ca2+ influx. J Cell Sci. 2016;129:3859–3867. doi: 10.1242/jcs.190322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Rydzewski N, Hider A, Zhang X, Yang J, Wang W, Gao Q, Cheng X, Xu H. A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat Cell Biol. 2016;18:404–417. doi: 10.1038/ncb3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mindell JA. Lysosomal acidification mechanisms. Annu Rev Physiol. 2012;74:69–86. doi: 10.1146/annurev-physiol-012110-142317. [DOI] [PubMed] [Google Scholar]
- 8.Rajawat YS, Hilioti Z, Bossis I. Aging: Central role for autophagy and the lysosomal degradative system. Ageing Res Rev. 2009;8:199–213. doi: 10.1016/j.arr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Hille B. Ion Channels of Excitable Membranes, Third Edition. 3rd. Sinauer Associates; Sunderland, Mass: 2001. [Google Scholar]
- 10.Hedrich R, Neher E. Cytoplasmic calcium regulates voltage-dependent ion channels in plant vacuoles. Nature. 1987;329:833–836. [Google Scholar]
- 11.Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang K-T, Lin P, Xiao R, Wang C, Zhu Y, Lin Y, Wyatt CN, Parrington J, Ma J, Evans AM, Galione A, Zhu MX. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Zhang X, Dong X-P, Samie M, Li X, Cheng X, Goschka A, Shen D, Zhou Y, Harlow J, Zhu MX, Clapham DE, Ren D, Xu H. TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012;151:372–383. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cang C, Zhou Y, Navarro B, Seo Y, Aranda K, Shi L, Battaglia-Hsu S, Nissim I, Clapham DE, Ren D. mTOR Regulates Lysosomal ATP-Sensitive Two-Pore Na+ Channels to Adapt to Metabolic State. Cell. 2013;152:778–790. doi: 10.1016/j.cell.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grabiner BC, Nardi V, Birsoy K, Possemato R, Shen K, Sinha S, Jordan A, Beck AH, Sabatini DM. A diverse array of cancer-associated MTOR mutations are hyperactivating and can predict rapamycin sensitivity. Cancer Discov. 2014;4:554–563. doi: 10.1158/2159-8290.CD-13-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambrosio AL, Boyle JA, Aradi AE, Christian KA, Pietro SMD. TPC2 controls pigmentation by regulating melanosome pH and size. Proc Natl Acad Sci. 2016;113:5622–5627. doi: 10.1073/pnas.1600108113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellono NW, Escobar IE, Oancea E. A melanosomal two-pore sodium channel regulates pigmentation. Sci Rep. 2016;6:26570. doi: 10.1038/srep26570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sulem P, Gudbjartsson DF, Stacey SN, Helgason A, Rafnar T, Jakobsdottir M, Steinberg S, Gudjonsson SA, Palsson A, Thorleifsson G, Pálsson S, Sigurgeirsson B, Thorisdottir K, Ragnarsson R, Benediktsdottir KR, Aben KK, Vermeulen SH, Goldstein AM, Tucker MA, Kiemeney LA, Olafsson JH, Gulcher J, Kong A, Thorsteinsdottir U, Stefansson K. Two newly identified genetic determinants of pigmentation in Europeans. Nat Genet. 2008;40:835–837. doi: 10.1038/ng.160. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen ONP, Grimm C, Schneider LS, Chao Y-K, Atzberger C, Bartel K, Watermann A, Ulrich M, Mayr D, Wahl-Schott C, Biel M, Vollmar AM. Two-pore channel function is crucial for migration of invasive cancer cells. Cancer Res. 2017 doi: 10.1158/0008-5472.CAN-16-0852. canres.0852.2016. [DOI] [PubMed] [Google Scholar]
- 19.Sakurai Y, Kolokoltsov AA, Chen C-C, Tidwell MW, Bauta WE, Klugbauer N, Grimm C, Wahl-Schott C, Biel M, Davey RA. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science. 2015;347:995–998. doi: 10.1126/science.1258758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kintzer AF, Stroud RM. Structure, inhibition and regulation of two-pore channel TPC1 from Arabidopsis thaliana. Nature. 2016;531:258–264. doi: 10.1038/nature17194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo J, Zeng W, Chen Q, Lee C, Chen L, Yang Y, Cang C, Ren D, Jiang Y. Structure of the voltage-gated two-pore channel TPC1 from Arabidopsis thaliana. Nature. 2016;531:196–201. doi: 10.1038/nature16446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rietdorf K, Funnell TM, Ruas M, Heinemann J, Parrington J, Galione A. Two-pore Channels Form Homo- and Heterodimers. J Biol Chem. 2011;286:37058–37062. doi: 10.1074/jbc.C111.289835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hooper R, Churamani D, Brailoiu E, Taylor CW, Patel S. Membrane topology of NAADP-sensitive two-pore channels and their regulation by N-linked glycosylation. J Biol Chem. 2011;286:9141–9149. doi: 10.1074/jbc.M110.189985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahman T, Cai X, Brailoiu GC, Abood ME, Brailoiu E, Patel S. Two-pore channels provide insight into the evolution of voltage-gated Ca2+ and Na+ channels. Sci Signal. 2014;7:ra109–ra109. doi: 10.1126/scisignal.2005450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White PJ. Calcium channels in higher plants. Biochim Biophys Acta BBA - Biomembr. 2000;1465:171–189. doi: 10.1016/s0005-2736(00)00137-1. [DOI] [PubMed] [Google Scholar]
- 26.Schulze C, Sticht H, Meyerhoff P, Dietrich P. Differential contribution of EF-hands to the Ca2+-dependent activation in the plant two-pore channel TPC1. Plant J. 2011;68:424–432. doi: 10.1111/j.1365-313X.2011.04697.x. [DOI] [PubMed] [Google Scholar]
- 27.Larisch N, Schulze C, Galione A, Dietrich P. An N-Terminal Dileucine Motif Directs Two-Pore Channels to the Tonoplast of Plant Cells. Traffic. 2012;13:1012–1022. doi: 10.1111/j.1600-0854.2012.01366.x. [DOI] [PubMed] [Google Scholar]
- 28.Larisch N, Kirsch SA, Schambony A, Studtrucker T, Böckmann RA, Dietrich P. The function of the two-pore channel TPC1 depends on dimerization of its carboxy-terminal helix. Cell Mol Life Sci. 2016:1–17. doi: 10.1007/s00018-016-2131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Churamani D, Hooper R, Rahman T, Brailoiu E, Patel S. The N-terminal region of two-pore channel 1 regulates trafficking and activation by NAADP. Biochem J. 2013;453:147–151. doi: 10.1042/BJ20130474. [DOI] [PubMed] [Google Scholar]
- 30.Cancela JM, Churchill GC, Galione A. Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature. 1999;398:74–76. doi: 10.1038/18032. [DOI] [PubMed] [Google Scholar]
- 31.Galione A. A primer of NAADP-mediated Ca2+ signalling: From sea urchin eggs to mammalian cells. Cell Calcium. 2015;58:27–47. doi: 10.1016/j.ceca.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Morgan AJ, Davis LC, Ruas M, Galione A. TPC: the NAADP discovery channel? Biochem Soc Trans. 2015;43:384–389. doi: 10.1042/BST20140300. [DOI] [PubMed] [Google Scholar]
- 33.Patel S. Function and dysfunction of two-pore channels. Sci Signal. 2015;8:re7–re7. doi: 10.1126/scisignal.aab3314. [DOI] [PubMed] [Google Scholar]
- 34.Zong X, Schieder M, Cuny H, Fenske S, Gruner C, Rötzer K, Griesbeck O, Harz H, Biel M, Wahl-Schott C. The two-pore channel TPCN2 mediates NAADP-dependent Ca2+-release from lysosomal stores. Pflugers Arch. 2009;458:891–899. doi: 10.1007/s00424-009-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brailoiu E, Churamani D, Cai X, Schrlau MG, Brailoiu GC, Gao X, Hooper R, Boulware MJ, Dun NJ, Marchant JS, Patel S. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol. 2009;186:201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogunbayo OA, Zhu Y, Shen B, Agbani E, Li J, Ma J, Zhu MX, Evans AM. Organelle-specific Subunit Interactions of the Vertebrate Two-pore Channel Family. J Biol Chem. 2015;290:1086–1095. doi: 10.1074/jbc.M114.610493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walseth TF, Lin-Moshier Y, Jain P, Ruas M, Parrington J, Galione A, Marchant JS, Slama JT. Photoaffinity Labeling of High Affinity Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP)-Binding Proteins in Sea Urchin Egg. J Biol Chem. 2012;287:2308–2315. doi: 10.1074/jbc.M111.306563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin-Moshier Y, Walseth TF, Churamani D, Davidson SM, Slama JT, Hooper R, Brailoiu E, Patel S, Marchant JS. Photoaffinity Labeling of Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP) Targets in Mammalian Cells. J Biol Chem. 2012;287:2296–2307. doi: 10.1074/jbc.M111.305813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruas M, Davis LC, Chen C-C, Morgan AJ, Chuang K-T, Walseth TF, Grimm C, Garnham C, Powell T, Platt N, Platt FM, Biel M, Wahl-Schott C, Parrington J, Galione A. Expression of Ca2+-permeable two-pore channels rescues NAADP signalling in TPC-deficient cells. EMBO J. 2015;34:1743–1758. doi: 10.15252/embj.201490009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cang C, Bekele B, Ren D. The voltage-gated sodium channel TPC1 confers endolysosomal excitability. Nat Chem Biol. 2014;10:463–469. doi: 10.1038/nchembio.1522. [DOI] [PubMed] [Google Scholar]
- 41.Rybalchenko V, Ahuja M, Coblentz J, Churamani D, Patel S, Kiselyov K, Muallem S. Membrane Potential Regulates Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP) Dependence of the pH- and Ca2+-sensitive Organellar Two-pore Channel TPC1. J Biol Chem. 2012;287:20407–20416. doi: 10.1074/jbc.M112.359612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pitt SJ, Lam AKM, Rietdorf K, Galione A, Sitsapesan R. Reconstituted human TPC1 is a proton-permeable ion channel and is activated by NAADP or Ca2+ Sci Signal. 2014;7:ra46. doi: 10.1126/scisignal.2004854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lagostena L, Festa M, Pusch M, Carpaneto A. The human two-pore channel 1 is modulated by cytosolic and luminal calcium. Sci Rep. 2017;7:43900. doi: 10.1038/srep43900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cang C, Aranda K, Ren D. A Non-inactivating High-voltage-activated Two-Pore Na+ Channel that Supports Ultra-long Action Potentials and Membrane Bistability. Nat Commun. 2014;5:5015. doi: 10.1038/ncomms6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruas M, Rietdorf K, Arredouani A, Davis LC, Lloyd-Evans E, Koegel H, Funnell TM, Morgan AJ, Ward JA, Watanabe K, Cheng X, Churchill GC, Zhu MX, Platt FM, Wessel GM, Parrington J, Galione A. Purified TPC Isoforms Form NAADP Receptors with Distinct Roles for Ca2+ Signaling and Endolysosomal Trafficking. Curr Biol. 2010;20:703–709. doi: 10.1016/j.cub.2010.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brailoiu E, Hooper R, Cai X, Brailoiu GC, Keebler MV, Dun NJ, Marchant JS, Patel S. An ancestral deuterostome family of two-pore channels mediates nicotinic acid adenine dinucleotide phosphate-dependent calcium release from acidic organelles. J Biol Chem. 2010;285:2897–2901. doi: 10.1074/jbc.C109.081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beyhl D, Hörtensteiner S, Martinoia E, Farmer EE, Fromm J, Marten I, Hedrich R. The fou2 mutation in the major vacuolar cation channel TPC1 confers tolerance to inhibitory luminal calcium. Plant J. 2009;58:715–723. doi: 10.1111/j.1365-313X.2009.03820.x. [DOI] [PubMed] [Google Scholar]
- 48.Carpaneto A, Cantù AM, Gambale F. Redox agents regulate ion channel activity in vacuoles from higher plant cells. FEBS Lett. 1999;442:129–132. doi: 10.1016/s0014-5793(98)01642-1. [DOI] [PubMed] [Google Scholar]
- 49.Scholz-Starke J, Angeli AD, Ferraretto C, Paluzzi S, Gambale F, Carpaneto A. Redox-dependent modulation of the carrot SV channel by cytosolic pH. FEBS Lett. 2004;576:449–454. doi: 10.1016/j.febslet.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 50.Gutla PVK, Boccaccio A, De Angeli A, Gambale F, Carpaneto A. Modulation of plant TPC channels by polyunsaturated fatty acids. J Exp Bot. 2012;63:6187–6197. doi: 10.1093/jxb/ers272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Genazzani AA, Mezna M, Dickey DM, Michelangeli F, Walseth TF, Galione A. Pharmacological properties of the Ca2+-release mechanism sensitive to NAADP in the sea urchin egg. Br J Pharmacol. 1997;121:1489–1495. doi: 10.1038/sj.bjp.0701295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kocarnik JM, Park SL, Han J, Dumitrescu L, Cheng I, Wilkens LR, Schumacher FR, Kolonel L, Carlson CS, Crawford DC, Goodloe RJ, Dilks HH, Baker P, Richardson D, Matise TC, Ambite JL, Song F, Qureshi AA, Zhang M, Duggan D, Hutter C, Hindorff L, Bush WS, Kooperberg C, Marchand LL, Peters U. Pleiotropic and Sex-Specific Effects of Cancer GWAS SNPs on Melanoma Risk in the Population Architecture Using Genomics and Epidemiology (PAGE) Study. PLOS ONE. 2015;10:e0120491. doi: 10.1371/journal.pone.0120491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonaventure G, Gfeller A, Proebsting WM, Hörtensteiner S, Chételat A, Martinoia E, Farmer EE. A gain-of-function allele of TPC1 activates oxylipin biogenesis after leaf wounding in Arabidopsis. Plant J Cell Mol Biol. 2007;49:889–898. doi: 10.1111/j.1365-313X.2006.03002.x. [DOI] [PubMed] [Google Scholar]
- 54.Bonaventure G, Gfeller A, Rodríguez VM, Armand F, Farmer EE. The fou2 gain-of-function allele and the wild-type allele of Two Pore Channel 1 contribute to different extents or by different mechanisms to defense gene expression in Arabidopsis. Plant Cell Physiol. 2007;48:1775–1789. doi: 10.1093/pcp/pcm151. [DOI] [PubMed] [Google Scholar]
- 55.Dadacz-Narloch B, Beyhl D, Larisch C, López-Sanjurjo EJ, Reski R, Kuchitsu K, Müller TD, Becker D, Schönknecht G, Hedrich R. A novel calcium binding site in the slow vacuolar cation channel TPC1 senses luminal calcium levels. Plant Cell. 2011;23:2696–2707. doi: 10.1105/tpc.111.086751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 58.Wu J, Yan Z, Li Z, Qian X, Lu S, Dong M, Zhou Q, Yan N. Structure of the voltage-gated calcium channel Cav1.1 at 3.6 Å resolution. Nature. 2016;537:191–196. doi: 10.1038/nature19321. [DOI] [PubMed] [Google Scholar]
- 59.Zhang X, Ren W, DeCaen P, Yan C, Tao X, Tang L, Wang J, Hasegawa K, Kumasaka T, He J, Wang J, Clapham DE, Yan N. Crystal structure of an orthologue of the NaChBac voltage-gated sodium channel. Nature. 2012;486:130–134. doi: 10.1038/nature11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tao X, Lee A, Limapichat W, Dougherty DA, MacKinnon R. A Gating Charge Transfer Center in Voltage Sensors. Science. 2010;328:67–73. doi: 10.1126/science.1185954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jaślan D, Mueller TD, Becker D, Schultz J, Cuin TA, Marten I, Dreyer I, Schönknecht G, Hedrich R. Gating of the two-pore cation channel AtTPC1 in the plant vacuole is based on a single voltage-sensing domain. Plant Biol Stuttg Ger. 2016;18:750–760. doi: 10.1111/plb.12478. [DOI] [PubMed] [Google Scholar]
- 62.Shen H, Zhou Q, Pan X, Li Z, Wu J, Yan N. Structure of a eukaryotic voltage-gated sodium channel at near-atomic resolution. Science. 2017:eaal4326. doi: 10.1126/science.aal4326. [DOI] [PubMed] [Google Scholar]
- 63.Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 64.Ahuja S, Mukund S, Deng L, Khakh K, Chang E, Ho H, Shriver S, Young C, Lin S, Johnson JP, Wu P, Li J, Coons M, Tam C, Brillantes B, Sampang H, Mortara K, Bowman KK, Clark KR, Estevez A, Xie Z, Verschoof H, Grimwood M, Dehnhardt C, Andrez J-C, Focken T, Sutherlin DP, Safina BS, Starovasnik MA, Ortwine DF, Franke Y, Cohen CJ, Hackos DH, Koth CM, Payandeh J. Structural basis of Nav1.7 inhibition by an isoform-selective small-molecule antagonist. Science. 2015;350:aac5464. doi: 10.1126/science.aac5464. [DOI] [PubMed] [Google Scholar]
- 65.Tang L, El-Din TMG, Swanson TM, Pryde DC, Scheuer T, Zheng N, Catterall WA. Structural basis for inhibition of a voltage-gated Ca2+ channel by Ca2+ antagonist drugs. Nature. 2016;537:117–121. doi: 10.1038/nature19102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 67.Tang L, Gamal El-Din TM, Payandeh J, Martinez GQ, Heard TM, Scheuer T, Zheng N, Catterall WA. Structural basis for Ca2+ selectivity of a voltage-gated calcium channel. Nature. 2014;505:56–61. doi: 10.1038/nature12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo J, Zeng W, Jiang Y. Tuning the ion selectivity of two-pore channels. Proc Natl Acad Sci. 2017 doi: 10.1073/pnas.1616191114. 201616191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kawano T, Kadono T, Fumoto K, Lapeyrie F, Kuse M, Isobe M, Furuichi T, Muto S. Aluminum as a specific inhibitor of plant TPC1 Ca2+ channels. Biochem Biophys Res Commun. 2004;324:40–45. doi: 10.1016/j.bbrc.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 70.Schieder M, Rötzer K, Brüggemann A, Biel M, Wahl-Schott CA. Characterization of Two-pore Channel 2 (TPCN2)-mediated Ca2+ Currents in Isolated Lysosomes. J Biol Chem. 2010;285:21219–21222. doi: 10.1074/jbc.C110.143123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saotome K, Singh AK, Yelshanskaya MV, Sobolevsky AI. Crystal structure of the epithelial calcium channel TRPV6. Nature. 2016;534:506–511. doi: 10.1038/nature17975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Naylor E, Arredouani A, Vasudevan SR, Lewis AM, Parkesh R, Mizote A, Rosen D, Thomas JM, Izumi M, Ganesan A, Galione A, Churchill GC. Identification of a chemical probe for NAADP by virtual screening. Nat Chem Biol. 2009;5:220–226. doi: 10.1038/nchembio.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pitt SJ, Funnell TM, Sitsapesan M, Venturi E, Rietdorf K, Ruas M, Ganesan A, Gosain R, Churchill GC, Zhu MX, Parrington J, Galione A, Sitsapesan R. TPC2 Is a Novel NAADP-sensitive Ca2+ Release Channel, Operating as a Dual Sensor of Luminal pH and Ca2+ J Biol Chem. 2010;285:35039–35046. doi: 10.1074/jbc.M110.156927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hockerman GH, Johnson BD, Abbott MR, Scheuer T, Catterall WA. Molecular Determinants of High Affinity Phenylalkylamine Block of l-type Calcium Channels in Transmembrane Segment IIIS6 and the Pore Region of the α1Subunit. J Biol Chem. 1997;272:18759–18765. doi: 10.1074/jbc.272.30.18759. [DOI] [PubMed] [Google Scholar]
- 75.Peterson BZ, Tanada TN, Catterall WA. Molecular Determinants of High Affinity Dihydropyridine Binding in L-type Calcium Channels. J Biol Chem. 1996;271:5293–5296. doi: 10.1074/jbc.271.10.5293. [DOI] [PubMed] [Google Scholar]
- 76.Zhang L, Geng Y, Duan W, Wang D, Fu M, Wang X. Ionic liquid-based ultrasound-assisted extraction of fangchinoline and tetrandrine from Stephaniae tetrandrae. J Sep Sci. 2009;32:3550–3554. doi: 10.1002/jssc.200900413. [DOI] [PubMed] [Google Scholar]
- 77.Bethke PC, Jones RL. Reversible protein phosphorylation regulates the activity of the slow-vacuolar ion channel. Plant J. 1997;11:1227–1235. [Google Scholar]
- 78.Gómez-Suaga P, Luzón-Toro B, Churamani D, Zhang L, Bloor-Young D, Patel S, Woodman PG, Churchill GC, Hilfiker S. Leucine-rich repeat kinase 2 regulates autophagy through a calcium-dependent pathway involving NAADP. Hum Mol Genet. 2012;21:511–525. doi: 10.1093/hmg/ddr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jha A, Ahuja M, Patel S, Brailoiu E, Muallem S. Convergent regulation of the lysosomal two-pore channel-2 by Mg2+, NAADP, PI(3,5)P2 and multiple protein kinases. EMBO J. 2014;33:501–511. doi: 10.1002/embj.201387035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Horton JS, Wakano CT, Speck M, Stokes AJ. Two-pore channel 1 interacts with citron kinase, regulating completion of cytokinesis. Channels. 2015;9:21–29. doi: 10.4161/19336950.2014.978676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag Complex Targets mTORC1 to the Lysosomal Surface and Is Necessary for Its Activation by Amino Acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rebsamen M, Pochini L, Stasyk T, de Araújo MEG, Galluccio M, Kandasamy RK, Snijder B, Fauster A, Rudashevskaya EL, Bruckner M, Scorzoni S, Filipek PA, Huber KVM, Bigenzahn JW, Heinz LX, Kraft C, Bennett KL, Indiveri C, Huber LA, Superti-Furga G. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature. 2015;519:477–481. doi: 10.1038/nature14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang S, Tsun Z-Y, Wolfson RL, Shen K, Wyant GA, Plovanich ME, Yuan ED, Jones TD, Chantranupong L, Comb W, Wang T, Bar-Peled L, Zoncu R, Straub C, Kim C, Park J, Sabatini BL, Sabatini DM. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science. 2015;347:188–194. doi: 10.1126/science.1257132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Castellano BM, Thelen AM, Moldavski O, Feltes M, van der Welle REN, Mydock-McGrane L, Jiang X, van Eijkeren RJ, Davis OB, Louie SM, Perera RM, Covey DF, Nomura DK, Ory DS, Zoncu R. Lysosomal cholesterol activates mTORC1 via an SLC38A9-Niemann-Pick C1 signaling complex. Science. 2017;355:1306–1311. doi: 10.1126/science.aag1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perera RM, Zoncu R. The Lysosome as a Regulatory Hub. Annu Rev Cell Dev Biol. 2016;32:223–253. doi: 10.1146/annurev-cellbio-111315-125125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Laplante M, Sabatini DM. mTOR Signaling in Growth Control and Disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin-Moshier Y, Keebler MV, Hooper R, Boulware MJ, Liu X, Churamani D, Abood ME, Walseth TF, Brailoiu E, Patel S, Marchant JS. The Two-pore channel (TPC) interactome unmasks isoform-specific roles for TPCs in endolysosomal morphology and cell pigmentation. Proc Natl Acad Sci. 2014;111:13087–13092. doi: 10.1073/pnas.1407004111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lam AKM, Galione A, Lai FA, Zissimopoulos S. Hax-1 identified as a two-pore channel (TPC)-binding protein. FEBS Lett. 2013;587:3782–3786. doi: 10.1016/j.febslet.2013.10.031. [DOI] [PubMed] [Google Scholar]


