Abstract
Background & Aims
Short-term administration of delayed-release chenodeoxycholic acid to patients with irritable bowel syndrome with constipation (IBS-C) accelerates colonic transit and reduces symptoms. A preliminary study has shown that patients with IBS-C have reduced levels of bile acids (BAs) in feces and reduced synthesis of BA. We compared levels of primary and secondary BAs in fecal samples collected over a 48-hr period from patients with IBS-C on a diet that contained 100 g fat per day, and compared them will levels in samples from healthy volunteers (controls). We also examined the relationship between overall colonic transit and biomarkers of BAs in patients with IBS-C.
Methods
We performed a retrospective study of 45 patients with IBS-C and 184 controls. For controls, we estimated the 10th percentile of fasting serum levels of C4 (n=184) and 48-hr fecal BAs (n=46), and the 90th percentile of fasting serum level of fibroblast growth factor 19 (FGF19, n=50). Colonic transit was measured in patients using a validated scintigraphic method. Data from patients with IBS-C were analyzed using Spearman correlations to determine relationships among levels of C4, FGF19, fecal BAs, and colonic transit.
Results
Among the patients with IBS-C, 2/45 had low serum levels of C4, 4/43 had increased serum levels of FGF19, and 6/39 had low levels of BAs in feces collected over 48-hrs. Patients with IBS-C had a significant increase in proportions of fecal lithocholic acid compared with controls (P=.04) and decrease in deoxycholic acid compared to controls (P=.03). In patients with IBS-C, there were inverse relationships between serum levels of C4 and FGF19 and correlations among levels of 48-hr fecal BAs, colonic transit, and serum C4 and FGF19.
Conclusion
Approximately 15% of patients with IBS-C have reduced total BAs and level of deoxycholic acid in fecal samples collected over 48 hrs on a 100 g fat diet. In these patients, lower levels of excretion of BAs into feces correlated with slower colonic transit.
Keywords: cholic acid, chenodeoxycholic acid, 7α-hydroxy-4-cholesten-3-one, GPBAR1 (G protein coupled bile acid receptor 1)
INTRODUCTION
Bile acids (BAs) are amphipathic, detergent molecules synthesized by the liver, and they facilitate the small intestinal absorption of lipids and fat soluble vitamins.(1) Their overall structure resembles a cholesterol molecule, but the changes made by colonic bacteria change the BAs effects on colonic permeability and secretion. Lithocholic acid (LCA) and deoxycholic acid (DCA) are the major BAs present in the colon and stool. Chenodeoxycholic acid (CDCA) and DCA are the known secretory BAs.(2) Increase in the fecal excretion and change in the proportion of the various BAs in stool characterize bile acid malabsorption (BAM), resulting in diarrhea or irritable bowel syndrome with diarrhea (IBS-D),(3, 4) which are associated with increased colonic water and mucus secretion, colonic motility, and membrane permeability.(5) (6)
BAM has been reported in 10–33% of patients with IBS-D or functional diarrhea.(7) Diagnosis of BAM is based on measurement of 75SeHCAT retention, where available, or on 48 hour fecal BA excretion and fasting serum biomarkers: 7α-hydroxy-4-cholesten-3-one (C4), a direct marker of hepatic BA synthesis; and fibroblast growth factor (FGF19), an endocrine hormone released by enterocytes providing negative feedback for BA synthesis.(8, 9) Multiple studies have confirmed the presence of increased fecal BAs, C4, colonic transit, and decreased FGF19 in patients with BAM as compared to healthy volunteers and constipation controls.(10, 11) Additionally, there are increased proportions of individual secretory BAs, DCA and CDCA, in 48-hour fecal BA measurements(12, 13) in patients with IBS-D.
Less is known about the potential role of BAs in patients with constipation. In a preliminary study, Shin et al. established that, although there was no significant decrease in total fecal BAs in patients with irritable bowel syndrome with constipation (IBS-C) as compared to healthy volunteers, patients with IBS-C had decreased proportions of DCA and CDCA and increased proportion of the nonsecretory LCA as compared to healthy volunteers.(12) Fasting serum C4 was not significantly different in IBS-C and healthy volunteers, and serum C4 and FGF19 measurements did not augment the utility of measurement of total fecal BAs for discriminating among IBS-C, IBS-D and healthy volunteers.(8) An interesting report described a paradoxical increase in serum C4 in patients with slow transit constipation compared to functional constipation, suggesting a compensatory increase in BA synthesis to provide an endogenous laxative agent.(14) This intriguing observation has not been corroborated or replicated.
The aims of this study were to compare fecal BAs and serum BAM biomarkers (C4 and FGF19) in IBS-C and healthy volunteers, and to assess the relationship of these biomarkers and colonic transit in patients with IBS-C.
METHODS
Participants and Study Design
This was a single-center, retrospective study. There were 45 patients with IBS-C, defined by Rome III criteria, and 184 healthy volunteers who had participated in research studies within the last 10 years. Eligibility criteria, methods of selection, periods of recruitment, and data collection were included in prior publications.(8, 11, 12, 15) Patients with IBS-C resided in local communities in southeastern Minnesota to avoid tertiary referral bias.
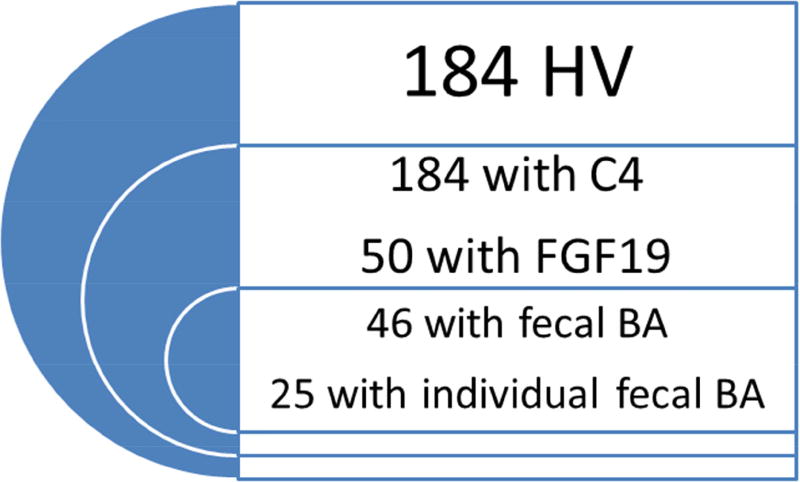
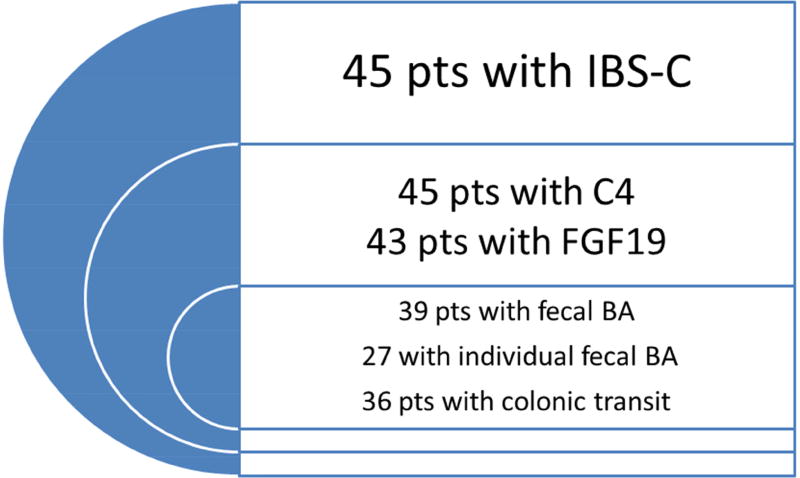
We collected previously measured fasting serum FGF19 and C4, 48 hour total and individual (CDCA, CA, DCA, LCA) fecal BA data from patients with IBS-C and healthy volunteers. From the IBS-C cohort, we also obtained 24 and 48 hour colonic transit data. Figure 1 shows the distribution of patients with available quantitative data between the two cohorts.
Figure 1.
Number of bile acid biomarkers and colonic transit measurements available for review in healthy volunteers (HV, left panel) and patients with IBS-C (right panel)
Measurement of Quantitative Data
Fasting serum C4 and FGF19
The C4 assay performed in our center (adapted from Galman et al.)(16) is based on HPLC/tandem mass spectrometry.(17)
FGF19 was measured by ELISA (FGF19 Quantikine Enzyme-Linked Immunosorbent Assay Kit, R&D Systems, Minneapolis, MN), as in previous studies.(18)
48 hour total and individual fecal bile acids
Patients were instructed to consume a 100 gram fat diet for 2 days before stool collection was started and for 2 days during stool collection. The details of extraction, measurement by LC-MS/MS, analysis and performance characteristics have been described in detail previously.(12) The 48 hour total fecal BAs (µmol/48h) and the absolute values (µmol/48h) and proportions of individual BAs (%) were measured.
24 and 48 hour colonic transit
The scintigraphic colonic transit test and its performance characteristics have been described in detail.(19) Briefly, after an overnight fast, patients ingest 111In adsorbed on activated charcoal in a methacrylate-coated, delayed-release capsule that has been shown to dissolve in the ileocolonic region. Patients are then fed 3 meals of known calorie value and standard proportions of carbohydrates, protein, and fat. Abdominal scintiscans are obtained every hour for the first 6 hours and at 8, 24, and 48 hours after ingestion of the 111In capsule. The validated colonic transit measurements are geometric center at 24 and 48 hours.
Statistical Analysis
Since our sample sizes for both healthy volunteers and patients with IBS-C were relatively small, we elected to utilize the 10th % and 90th % for our parameter cut-offs. Altman et al. demonstrated that, for skewed samples, the 10th % and 90th % would be more appropriate than the traditional 5th % and 95th % cut-offs.(20) We calculated the 10th percentile of C4 values (ng/mL) in the 184 healthy volunteers and fecal BAs (µmol/48h) in 46 healthy volunteers to represent the lower normal limit. We appraised the 90th percentile of FGF19 in 50 healthy volunteers, as it is known that low serum FGF19 is associated with diarrhea due to BAM.
We estimated the number of patients with IBS-C with results below the 10th percentile of healthy volunteers for total fecal BAs (in 39 IBS-C patients) and serum C4 (in 45 IBS-C patients), and above the 90th percentile of healthy volunteers based on FGF19 (in 43 IBS-C patients).
For analysis of the individual BAs, we compared the proportions and absolute values of IBS-C and healthy volunteers using the Wilcoxon rank sum test.
To understand the relationships among C4, FGF19, fecal BAs and colonic transit, we performed Spearman correlations and graphed the data with best fitting regression lines using the regression wizard on SigmaPlot (Systat Software, San Jose, CA). The graphs are variations of rectangular hyperbola and hyperbolic decay, depending on which model best fit the data on scatter plots. The specific type of graph and the associated equations are described in the figures. Bonferroni correction for multiple correlation tests was not utilized because this is a hypothesis-generating, post-hoc analysis, and the results of the individual tests are more important than the collective analysis.(21)
RESULTS
Demographics
In the healthy volunteers, there was a 1.5 ratio of female to male participants (110:74) and comparable average age (42.3±13 years) to those in the IBS-C cohort. Patients with IBS-C were all female participants, overweight [average BMI: 26.2±4.2 (SD) kg/m2], and aged 43.8±9.5 years.
Serum Biomarkers and Total and Individual Fecal BAs in Healthy Volunteers
Because the patient cohort (IBS-C) consisted of only female volunteers, we calculated the healthy volunteer-based cut-offs for the combined group of both males and females, and exclusively for the female cohort.
Male and female healthy volunteer parameter cut-offs
The 10th percentile of serum C4 (n=184) was 5.05 ng/mL and the 48 hour fecal BAs (n=46) was 109.5 µmol/48h. The 90th percentile of serum FGF19 (n=50) was 267.0 pg/mL.
Table 1 demonstrates the proportions and absolute values of the individual fecal BAs (n=25) in healthy volunteers.
Table 1.
Proportion of total fecal BAs excreted and absolute values for individual BAs excreted in 48 hours (data show mean ± SD)
| Individual BA (% or µmol/48h) | Healthy volunteers (n=25) |
IBS-C (n=27) | Wilcoxon Rank Sum test |
|---|---|---|---|
| % LCA | 39 ± 14 | 51 ± 19 | Z= 2.12, p = 0.0337* |
| % CDCA | 0.0 ± 1.0 | 1.0 ± 1.3 | Z= −1.84, p = 0.07 |
| % DCA | 59 ± 14 | 48 ± 19 | Z= −2.22, p = 0.027* |
| % UDCA | 1.0 ± 2.0 | 0.0 ± 0.2 | Z= −0.81, p = 0.42 |
| % CA | 1.0 ± 1.0 | 1.0 ± 2.7 | Z= −0.30, p = 0.76 |
| LCA µmol/48h | 297.7 ± 291.4 | 204.8 ± 188.2 | Z = −1.23, p = 0.22 |
| CDCA µmol/48h | 5.56 ± 10.13 | 1.89 ± 4.0 | Z = −1.88, p = 0.06 |
| DCA µmol/48h | 500.81 ± 564.3 | 354.9 ± 691.1 | Z= −1.8, p = 0.07 |
| UDCA µmol/48h | 9.04 ± 23.11 | 0.46 ± 0.9 | Z = −1.49, p = 0.14 |
| CA µmol/48h | 6.02 ± 10.82 | 3.77 ± 8.5 | Z = −1.12, p = 0.26 |
Exclusively female healthy volunteer parameter cut-off
The 10th percentile of serum C4 (n=110) was 4.28 ng/mL and of the 48-hour fecal BAs (n=35) was 105.5 µmol/48h. The 90th percentile of serum FGF19 (n=39) was 277.4 pg/mL.
Fasting Serum C4 and FGF19 and 48 Hour Total and Individual Fecal BAs in Patients with IBS-C
Utilizing male and female healthy volunteer parameter cut-offs
Of the 39 IBS-C patients with 48-hour total fecal BAs measurements, 6 (15.4%) demonstrated low fecal BAs. The serum fasting FGF19 was increased in 4/43 (9.3%), and the C4 was decreased in 2/45 (4.4%). However, there was not complete concordance between abnormal serum biomarkers and decreased 48-hour total fecal BAs.
Table 1 demonstrates the proportions and values for the individual fecal BAs. There was a significantly higher percent of LCA (p=0.034) and a lower percent of DCA (p=0.027) in patients with IBS-C.
Utilizing exclusively female healthy volunteer parameter cut-off
Even with the cut-offs for 48-hour total fecal BAs and fasting serum C4, the number of patients with IBS-C with bile acid deficiency did not change, 6/39 (15.4%) and 2/45 (4.4%) respectively. For fasting serum FGF19, there was one less patient who demonstrated bile acid deficiency [3/43 (7.0%)].
Relationships between Serum and Fecal Biomarkers and Colonic Transit in Patients with IBS-C
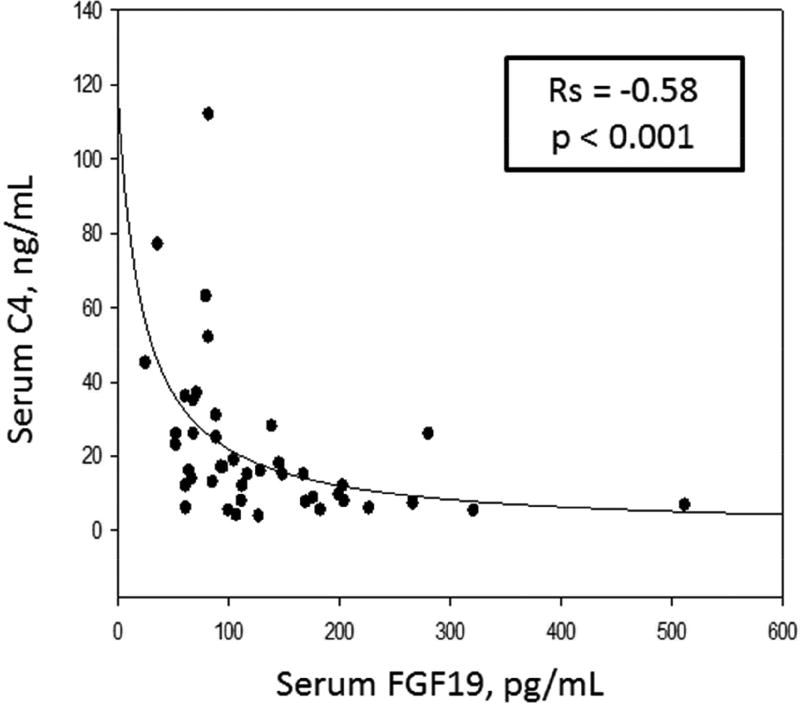
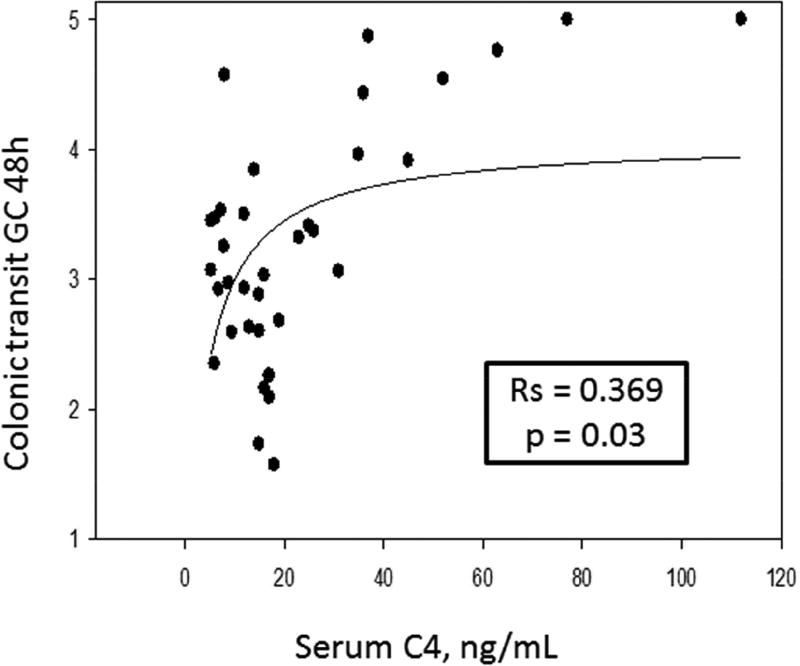
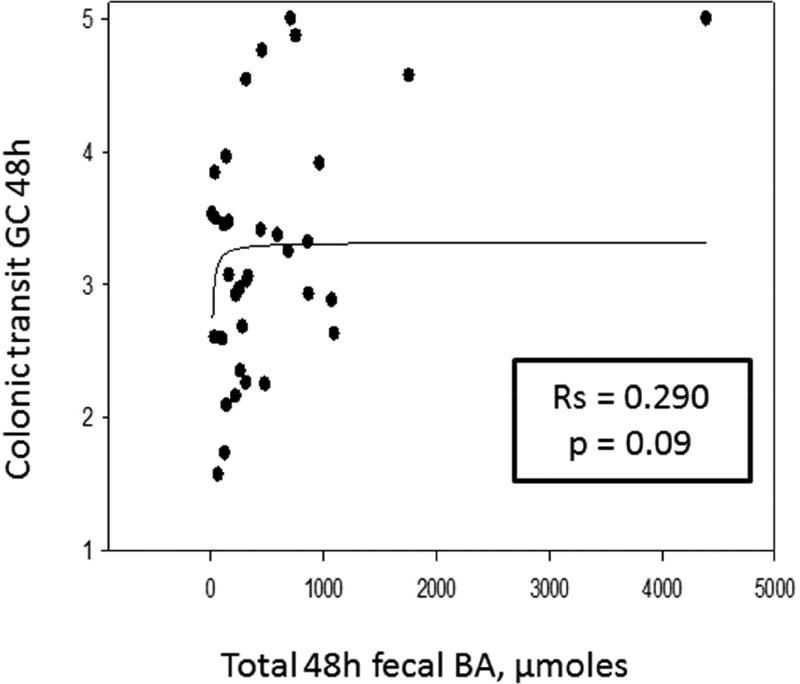
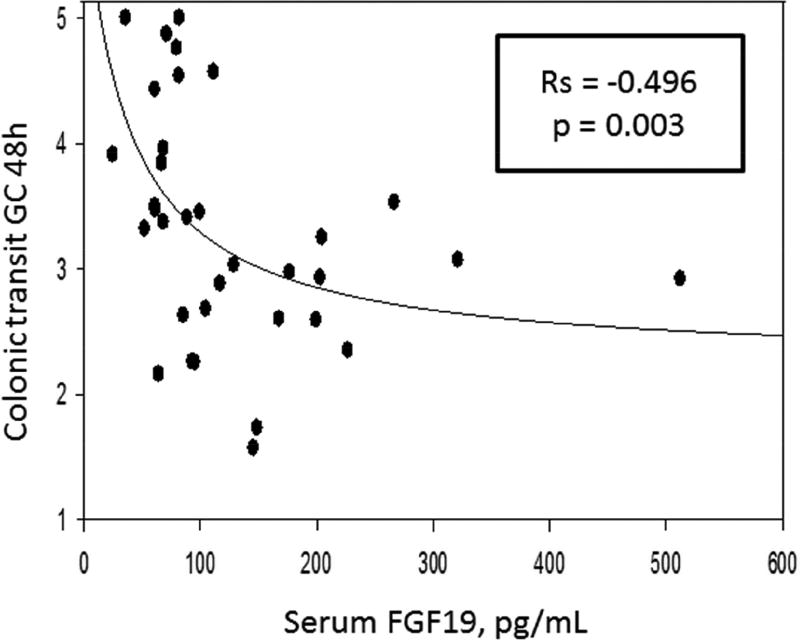
Table 2 contains the Spearman correlations, and Figures 2, 3, and 4 are the scatter plots with best fit regression lines of the data collected from patients with IBS-C. Figure 2 demonstrates a significant reciprocal relationship between fasting serum FGF19 and C4. Figure 3 shows direct correlations between serum C4 or the 48 hour fecal BAs and 48 hour colonic transit. Figure 4 shows the inverse correlation of FGF19 and colonic transit.
Table 2.
Spearman correlations between the serum and fecal biomarkers and colonic transit
| GC24 | GC48 | Fecal BA | FGF19 | |
|---|---|---|---|---|
| C4 | Rs = 0.363, p=0.03* | Rs = 0.369, p=0.03* | Rs = 0.362, p = 0.02* | Rs = −0.58, p<0.001* |
| FGF19 | Rs = −0.267, p=0.126 | Rs = −0.496, p=0.003* | Rs = −0.235, p=0.16 | |
| Fecal BAs | Rs = 0.357, p = 0.036* | Rs = 0.290, p = 0.09 | Rs = −0.235, p=0.160 |
significant correlations
Figure 2.
Inverse relationship between fasting serum C4 and FGF19 in patients with IBS-C. Graph represents a 2-parameter hyperbolic decay: Y=ab/(b+x).
Figure 3.
Direct correlation of serum C4 (left panel) and fecal BA excretion (right panel) and colonic transit (geometric center, GC) at 48 hours. Graphs represent 2-parameter rectangular hyperbola: y=ax/(b+x).
Figure 4.
Inverse relationship between fasting serum FGF19 and colonic transit (geometric center, GC) at 48 hours. Graphs represent a 3-parameter hyperbolic decay: y=y0 + ab/(b+x).
DISCUSSION
Our study demonstrates there is a subgroup of ~15% patients with IBS-C who has decreased 48 hour fecal BAs. Of the fasting serum biomarkers, 4.3% of patients with IBS-C had low serum C4 and 9.3% had elevated serum FGF19. When adjusting the control group to include only female participants to match the IBS-C patient cohort of exclusively females, the prevalence of bile acid deficiency did not alter significantly. There was a significant direct correlation between fecal BAs excretion and serum C4 and colonic transit, and there was an inverse correlation between serum FGF19 and colonic transit. As demonstrated in Figure 2, serum FGF19 and C4 demonstrated a significant inverse relationship. Though demonstrated in multiple prior studies evaluating bile acid diarrhea in patients with IBS-D, this has not previously been described in patients with IBS-C. These observations demonstrate that a portion of patients with IBS-C may suffer from colonic bile acid deficiency as the underlying pathophysiology. Importantly, when such patients with IBS-C are refractory to standard treatment of constipation, they may be candidates for treatment with strategies that increase intra-colonic bile acid concentrations such as bile acid supplementation or inhibitors of the ileal bile acid transporter (IBAT), as discussed below.
The observations in this study have practical and clinical implications for future studies as well as insights relative to prior clinical trials. For example, Rao et al. provided supplemental chenodeoxycholate to patients with IBS-C and functional constipation and showed improvement in colonic transit time, frequency and consistency of stool.(22) In addition, when elobixibat, an inhibitor of the ileal bile acid transporter which is responsible for ~95% of BA reabsorption in the terminal ileum, was administered to patients with functional constipation, it also improved colonic transit, stool consistency and frequency.(23) Elobixibat resulted in increased fasting serum C4 and decreased FGF19, secondary to increased BA synthesis after increased fecal BA loss.(24, 25) Thus, there are potential therapeutic approaches to correct the abnormal biochemical parameters, given that the patients’ fasting serum C4 was increased to >10 ng/mL with elobixibat,(24) which could correct the low C4 (≤ 5.05 ng/mL) in a subgroup of patients with IBS-C in our current study.
Previous studies have demonstrated decreased fecal BAs in a subset of patients with IBS-C compared to patients with IBS-D, but were unable to demonstrate statistical significance when compared to healthy volunteers.(11, 12) When evaluating the percentage of major, individual primary and secondary BAs, our data revealed a significant increase in LCA (p=0.034) and decrease in the proportion of the secretory BA, DCA (p=0.027), in patients with IBS-C compared to healthy volunteers. This difference in individual BA proportions has been replicated in other studies.(12) In constipated children, there was no difference in the proportion of DCA, but there was increased sulfonated CDCA. The sulfonation removes the active secretory component of the BA.(26)
There are two plausible hypotheses regarding constipation and fecal BA measurements. First, decreased proportion of secretory fecal BAs results in constipation. At face value, our data would support this hypothesis in up to 15% of patients with IBS-C because they had low total fecal BA and DCA excretion. A second hypothesis is that increased colonic transit time in constipation results in increased passive absorption of fecal BAs and, hence, lower excretion of secretory BAs such as DCA. The precise contribution of colonic transit to the fecal excretion of individual BAs has been the subject of few previous papers. Within the colon, there is passive absorption of unconjugated, di-hydroxy BAs, which include DCA (3,12 di-α hydroxylated) and UDCA (3α, 7β di-hydroxylated).(27) When constipated patients were given senna to accelerate colonic transit, it was observed that the biliary DCA (which must have been reabsorbed through the colonic mucosa) was decreased.27 Conversely, healthy volunteers who received loperamide to decelerate colonic transit had increased biliary DCA.(28) Similarly, in patients with acromegaly, octreotide retarded colonic transit, yielding an increase in serum DCA.(29)
CONCLUSION
The current data are consistent with the hypothesis that a subset of patients with IBS-C has decreased total fecal BAs and percentage of fecal secretory BAs, which is significantly correlated to a decrease in colonic transit. The serum biomarkers, C4 and FGF19, were not always concordant with total fecal BAs. Based on our data, we believe 48-hour total and percentage of fecal secretory BAs will be considered the gold standard until further studies are completed. Given the potential limitations of a retrospective analysis, future studies are planned to confirm these findings in a prospective cohort of patients with functional constipation and IBS-C.
Acknowledgments
The authors thank Mrs. Cindy Stanislav for excellent secretarial assistance.
Funding: Grant R01-DK92179 from National Institutes of Health (MC)
Abbreviations
- BA
Bile Acid
- BAM
Bile Acid Malabsorption
- BMI
Body Mass Index
- C4
7α-hydroxy-4-cholesten-3-one
- CDCA
chenodeoxycholic acid
- CA
cholic acid
- DCA
deoxycholic acid
- ELISA
Enzyme-Linked Immunosorbent Assay
- FGF19
Fibroblast Growth Factor 19
- GC
geometric center
- GPBAR1 (TGR5)
G protein-coupled bile acid receptor 1
- HV
Healthy volunteers
- HPLC
High Performance Liquid Chromatography
- IBAT
Ileal bile acid transporter
- IBS-C
irritable bowel syndrome with constipation
- IBS-D
irritable bowel syndrome with diarrhea
- In
Indium
- LCA
Lithocholic Acid
- LC-MS/MS
Liquid chromatography tandem-mass spectrometry
- SD
Standard Deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflicts of interest.
Authors’ contributions:
Priya Vijayvargiya, MD - research fellow and patient care, authorship of manuscript
Irene Busciglio, BS - study coordination and database management
Duane Burton, MHA - database management, colonic transit measurement
Leslie Donato, PhD* - laboratory supervision, authorship of manuscript
Alan Lueke, MS* - lab measurements of serum C4 and fecal bile acids, authorship of manuscript
Michael Camilleri, MD - principal investigator, patient care, study concept and design, analysis and interpretation of data, drafting and revising manuscript, study supervisor
References
- 1.Wildt S, Norby Rasmussen S, Lysgard Madsen J, Rumessen JJ. Bile acid malabsorption in patients with chronic diarrhoea: clinical value of SeHCAT test. Scandinavian journal of gastroenterology. 2003;38(8):826–30. doi: 10.1080/00365520310004461. [DOI] [PubMed] [Google Scholar]
- 2.Chadwick VS, Gaginella TS, Carlson GL, Debongnie JC, Phillips SF, Hofmann AF. Effect of molecular structure on bile acid-induced alterations in absorptive function, permeability, and morphology in the perfused rabbit colon. The Journal of laboratory and clinical medicine. 1979;94(5):661–74. [PubMed] [Google Scholar]
- 3.Pattni S, Walters JR. Recent advances in the understanding of bile acid malabsorption. British medical bulletin. 2009;92:79–93. doi: 10.1093/bmb/ldp032. [DOI] [PubMed] [Google Scholar]
- 4.Fromm H, Malavolti M. Bile acid-induced diarrhoea. Clinics in gastroenterology. 1986;15(3):567–82. [PubMed] [Google Scholar]
- 5.Mekhjian HS, Phillips SF. Perfusion of the canine colon with unconjugated bile acids. Effect on water and electrolyte transport, morphology, and bile acid absorption. Gastroenterology. 1970;59(1):120–9. [PubMed] [Google Scholar]
- 6.Camilleri M. Bile Acid diarrhea: prevalence, pathogenesis, and therapy. Gut and liver. 2015;9(3):332–9. doi: 10.5009/gnl14397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wedlake L, A'Hern R, Russell D, Thomas K, Walters JR, Andreyev HJ. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Alimentary pharmacology & therapeutics. 2009;30(7):707–17. doi: 10.1111/j.1365-2036.2009.04081.x. [DOI] [PubMed] [Google Scholar]
- 8.Camilleri M, Shin A, Busciglio I, Carlson P, Acosta A, Bharucha AE, et al. Validating biomarkers of treatable mechanisms in irritable bowel syndrome. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2014;26(12):1677–85. doi: 10.1111/nmo.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vijayvargiya P, Camilleri M, Shin A, Saenger A. Methods for diagnosis of bile acid malabsorption in clinical practice. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11(10):1232–9. doi: 10.1016/j.cgh.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pattni SS, Brydon WG, Dew T, Johnston IM, Nolan JD, Srinivas M, et al. Fibroblast growth factor 19 in patients with bile acid diarrhoea: a prospective comparison of FGF19 serum assay and SeHCAT retention. Alimentary pharmacology & therapeutics. 2013;38(8):967–76. doi: 10.1111/apt.12466. [DOI] [PubMed] [Google Scholar]
- 11.Wong BS, Camilleri M, Carlson P, McKinzie S, Busciglio I, Bondar O, et al. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2012;10(9):1009–15.e3. doi: 10.1016/j.cgh.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin A, Camilleri M, Vijayvargiya P, Busciglio I, Burton D, Ryks M, et al. Bowel functions, fecal unconjugated primary and secondary bile acids, and colonic transit in patients with irritable bowel syndrome. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11(10):1270–5.e1. doi: 10.1016/j.cgh.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duboc H, Rainteau D, Rajca S, Humbert L, Farabos D, Maubert M, et al. Increase in fecal primary bile acids and dysbiosis in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2012;24(6):513–20. e246–7. doi: 10.1111/j.1365-2982.2012.01893.x. [DOI] [PubMed] [Google Scholar]
- 14.Abrahamsson H, Ostlund-Lindqvist AM, Nilsson R, Simren M, Gillberg PG. Altered bile acid metabolism in patients with constipation-predominant irritable bowel syndrome and functional constipation. Scandinavian journal of gastroenterology. 2008;43(12):1483–8. doi: 10.1080/00365520802321212. [DOI] [PubMed] [Google Scholar]
- 15.Kenyon AL Stacy M, Meeusen Jeffrey W, Camilleri Michael, Donato Leslie J. Measurement of 7-alpha-hydroxy-cholestene-3-one in Serum Using LC-MS/MS for the Screening of Bile Acid Malabsorption in IBS-D. 2017 Manuscript in progress. [Google Scholar]
- 16.Galman C, Arvidsson I, Angelin B, Rudling M. Monitoring hepatic cholesterol 7alpha-hydroxylase activity by assay of the stable bile acid intermediate 7alpha-hydroxy-4-cholesten-3-one in peripheral blood. Journal of lipid research. 2003;44(4):859–66. doi: 10.1194/jlr.D200043-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Camilleri M, Nadeau A, Tremaine WJ, Lamsam J, Burton D, Odunsi S, et al. Measurement of serum 7alpha-hydroxy-4-cholesten-3-one (or 7alphaC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2009;21(7):734–e43. doi: 10.1111/j.1365-2982.2009.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walters JR, Tasleem AM, Omer OS, Brydon WG, Dew T, le Roux CW. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2009;7(11):1189–94. doi: 10.1016/j.cgh.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 19.Deiteren A, Camilleri M, Bharucha AE, Burton D, McKinzie S, Rao AS, et al. Performance characteristics of scintigraphic colon transit measurement in health and irritable bowel syndrome and relationship to bowel functions. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2010;22(4):415–23. e95. doi: 10.1111/j.1365-2982.2009.01441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altman DG, Bland JM. Quartiles, quintiles, centiles, and other quantiles. BMJ (Clinical research ed) 1994;309(6960):996. doi: 10.1136/bmj.309.6960.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong RA. When to use the Bonferroni correction. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians (Optometrists) 2014;34(5):502–8. doi: 10.1111/opo.12131. [DOI] [PubMed] [Google Scholar]
- 22.Rao AS, Wong BS, Camilleri M, Odunsi-Shiyanbade ST, McKinzie S, Ryks M, et al. Chenodeoxycholate in females with irritable bowel syndrome-constipation: a pharmacodynamic and pharmacogenetic analysis. Gastroenterology. 2010;139(5):1549–58. 58.e1. doi: 10.1053/j.gastro.2010.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chey WD, Camilleri M, Chang L, Rikner L, Graffner H. A randomized placebo-controlled phase IIb trial of a3309, a bile acid transporter inhibitor, for chronic idiopathic constipation. The American journal of gastroenterology. 2011;106(10):1803–12. doi: 10.1038/ajg.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong BS, Camilleri M, McKinzie S, Burton D, Graffner H, Zinsmeister AR. Effects of A3309, an ileal bile acid transporter inhibitor, on colonic transit and symptoms in females with functional constipation. The American journal of gastroenterology. 2011;106(12):2154–64. doi: 10.1038/ajg.2011.285. [DOI] [PubMed] [Google Scholar]
- 25.Simren M, Bajor A, Gillberg PG, Rudling M, Abrahamsson H. Randomised clinical trial: The ileal bile acid transporter inhibitor A3309 vs. placebo in patients with chronic idiopathic constipation--a double-blind study. Alimentary pharmacology & therapeutics. 2011;34(1):41–50. doi: 10.1111/j.1365-2036.2011.04675.x. [DOI] [PubMed] [Google Scholar]
- 26.Hofmann AF, Loening-Baucke V, Lavine JE, Hagey LR, Steinbach JH, Packard CA, et al. Altered bile acid metabolism in childhood functional constipation: inactivation of secretory bile acids by sulfation in a subset of patients. Journal of pediatric gastroenterology and nutrition. 2008;47(5):598–606. doi: 10.1097/MPG.0b013e31816920a6. [DOI] [PubMed] [Google Scholar]
- 27.Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Archives of internal medicine. 1999;159(22):2647–58. doi: 10.1001/archinte.159.22.2647. [DOI] [PubMed] [Google Scholar]
- 28.Marcus SN, Heaton KW. Intestinal transit, deoxycholic acid and the cholesterol saturation of bile--three inter-related factors. Gut. 1986;27(5):550–8. doi: 10.1136/gut.27.5.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas LA, Veysey MJ, Murphy GM, Russell-Jones D, French GL, Wass JA, et al. Octreotide induced prolongation of colonic transit increases faecal anaerobic bacteria, bile acid metabolising enzymes, and serum deoxycholic acid in patients with acromegaly. Gut. 2005;54(5):630–5. doi: 10.1136/gut.2003.028431. [DOI] [PMC free article] [PubMed] [Google Scholar]