Abstract
Acute kidney injury (AKI) represents a significant clinical concern that is associated with high mortality rates and also represents a significant risk factor for the development of chronic kidney disease (CKD). This article will consider alterations in renal endothelial function in the setting of AKI that may underlie impairment in renal perfusion and how inefficient vascular repair may manifest post-AKI and contribute to the potential transition to CKD. We provide updated terminology for cells previously classified as “endothelial progenitor” that may mediate vascular repair such as pro-angiogenic cells and endothelial colony forming cells. We consider how endothelial repair may be mediated by these different cell types following vascular injury, particularly in models of AKI. We further summarize the potential ability of these different cells to mitigate the severity of AKI, improve perfusion and maintain vascular structure in pre-clinical studies.
Keywords: Rarefaction, Angiogenesis, Ischemia, Fibrosis, Inflammation
Background
Acute kidney injury (AKI) represents a significant and growing clinical concern, which affects approximately 5% of hospitalized patients and is associated with high morbidity and mortality rates. AKI is defined as a rapid loss of renal function occurring over the course of hours or days and is primarily caused by impaired renal blood flow, nephrotoxicity or sepsis leading to ischemic or hypoxic damage 1. In addition, AKI is associated with injury to the renal parenchyma, particularly tubular epithelial cells, but also vascular and interstitial cells. Repair mechanisms in the kidney typically mediate recovery of renal function and structure, which is observed in most surviving patients. However, it is now recognized that patients who have recovered from AKI are at increased risk for the development of either chronic kidney disease (CKD) or end-stage renal disease (ESRD) 2–4. This may result from repair processes following AKI that are either incomplete or maladaptive, predisposing the development of renal fibrosis 2.
Impaired perfusion in acute kidney injury
In animal models of acute kidney injury such as ischemia-reperfusion, there is typically an impairment in renal blood flow associated with lost renal function and tissue damage 5. In models of sepsis, AKI may or may not occur in the presence of reduced renal blood flow, and this has been an area of some controversy 6. The use of contrast media is another significant risk factor predisposing AKI. Contrast media is thought to promote vasoconstriction of pericytes, leading to an exacerbation of hypoxia in the renal medulla 7. Regardless of effects on total renal blood flow, it is thought that heterogeneous disturbances in microvascular flow patterns are likely evident in AKI of various etiologies 6. Such impairment at the microvascular level disrupts perfusion particularly in the renal medulla and is considered to be a significant reason why parenchymal cells in this region (primarily S3 proximal tubule cells) are the most severely affected in these models 7. Recent reviews have highlighted how impairment of renal microvascular function is of central importance in both initiating and predicting the severity of AKI and have called for development of new methods to better evaluate renal perfusion in susceptible patients 6. A better appreciation for the complicated nature of endothelial dysfunction in the setting of AKI can be obtained from a number of comprehensive review articles both by us 5, 8 as well as other investigators 9–11. For the purposes of this article, we wish to highlight central concepts regarding impaired vascular function in the development of acute kidney injury, implications for transition from acute to chronic kidney disease, and how these may relate to the potential use of endothelial-targeted cell-based therapies.
Reductions in renal blood flow (RBF) following ischemia or hypoxia have been thought to be due, in part, to rapid vasoconstriction, perhaps secondary to impaired proximal tubular sodium reabsorption and activation of tubular glomerular feedback 1. However, if glomerular filtration rate (GFR) is reduced, sustained tubular glomerular feedback likely does not contribute to sustained vasoconstriction 6. While activation of other vasoconstrictor pathways (e.g., sympathetic tone, angiotensin II, endothelin) or impairment of vasodilator pathways (e.g., prostacyclin, nitric oxide) have been suggested to reduce renal blood flow during AKI, the inability of vasodilators to reverse AKI suggests that these pathways may play only a modest (at best) contributory role to sustained loss of renal function 8.
In the last 10–15 years there has been considerable attention focused on the potential role of the post-glomerular peritubular capillaries contributing toward the development of AKI. These vessels subserve reabsorption of water and solute from the interstitium back into systemic circulation and are a characterized by low hydrostatic pressure. These vessels also deliver oxygen and nutrients to the renal tubular system and are in close apposition to these cells. Counter current flow in vasa recta allows for oxygen shunting in the renal medulla and development of an oxygen gradient producing a relatively hypoxic environment 9. Reductions in RBF can reduce GFR and reduce tubular metabolic work, but extreme impairment may further exacerbate renal hypoxia and can activate inflammatory and cell injury pathways in nearby S3 proximal tubule or thick ascending limb 1, 9, 10. Peritubular capillary damage may contribute further to impaired perfusion and may predict the severity and duration of AKI. In studies of transplant patients receiving cadaveric grafts, renal biopsies obtained immediately post-perfusion demonstrated evidence of both endothelial and vascular smooth muscle damage as indicated by reduced von Willebrand factor staining and actin cytoskeletal disorganization. The degree of vascular damage in the immediate post-transplant period was predictive of reduced graft function in the subsequent 7 days of recovery 12.
Endothelial dysfunction as a contributor to AKI
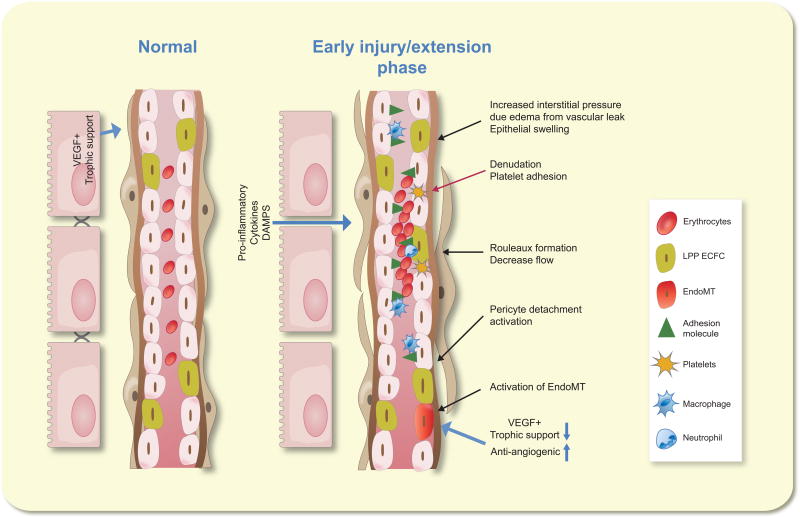
Molitoris and Sutton suggested that disturbances in microvascular flow in the kidney lead to the extension phase of AKI 13, in which tubular damage is exacerbated by events in the endothelium which may include endothelial swelling, increased expression of adhesion molecules and associated recruitment of various leukocyte populations. Endothelial leukocyte adhesion, activated in the setting of AKI, may result from increased exocytosis of Weibel-Palade bodies 14 and an immediate enhancement of surface adhesion molecules such as P-selectin 15–17 and ICAM-1 1, 18, 19,19 (Figure 1). Increased co-stimulatory factor expression is also observed on the surface of capillary endothelial cells and inhibition of B7-1 reduces monocyte infiltration in the ascending vasa recta in response to renal ischemia reperfusion 20.
Figure 1.
Alterations in endothelial function contribute to the extension phase of acute kidney injury. On the left, a peritubular capillary is shown in close apposition to tubular epithelium in a normal kidney. B) In response to injury, endothelial swelling narrows capillary space. Increased adhesion molecule expression facilitates leukocyte attachment, contributing to erythrocyte rouleaux formation and disrupting normal blood flow. Reduction in flow contributes to reduced shear stress and inhibition of NO formation, a potential trigger for endothelial mesenchymal transition (EndoMT). Addition potential contributors toward EndoMT include a reduction in trophic support from damaged tubules or injury activated perictyes. Disruption of blood flow exacerbates tissue hypoxia and further compromises epithelial injury and a reduction in renal
The initiating pro-inflammatory activity in the endothelium requires further investigation. Paracrine factors such as cytokines or other danger signals from stressed epithelium represent potential links between the epithelial and vascular compartments 10. Endothelial responses may also directly result from ischemia by altering endothelial cytoskeletal structure, resulting in reduced endothelial cell-cell contact or cell-adhesion complexes 21. Mitochondrial damage following ischemia has been documented in peritubular capillaries and a recent study showed that a compound that targets mitochondrial cardiolipin attenuated endothelial mitochondrial damage and the development of inflammation following renal ischemia reperfusion 22. Both ischemia and sepsis are known to induce glycocalyx shedding. The glycocalyx is considered an initial layer of endothelial barrier function, and its disruption may initiate downstream signaling cascades and increase access of leukocytes to endothelial adhesion molecules 21, 23. Loss of endothelial cell barrier function may also activate coagulation cascades, and potentially influence renal function by increasing interstitial edema and intra tubular pressure 23.
Leukocyte adhesion may contribute to the development of vascular rouleaux, which manifest prominently within a few hours post-ischemia in rodent models of AKI 1, 10. Vascular congestion and rouleaux have also been described in dogs following renal I/R 24 and in monkeys following LPS induced AKI 25. Further, Solez et al., demonstrated significant intravascular leukocyte accumulation in 63 of 66 cadaver kidneys from patients with established AKI for greater than 24 hours 26.
Efforts to improve renal perfusion in acute kidney injury have historically been directed toward managing fluid volume and cardiac output. While increased vasomotor tone may reduce GFR in the initial development of AKI, renal vasodilators have been shown to lack efficacy in improving renal function in established AKI 27, 28. We recently suggested that the failure of vasodilators to alleviate established AKI is based on the inability of such therapies to resolve vascular congestion/inflammation and that potential treatments should target established endothelial/leukocyte interaction 8. In support of this idea, retrograde hydrodynamic delivery of saline at high pressure into the post-glomerular vasculature of rats alleviated vascular congestion, improved capillary perfusion and resulted in more rapid recovery of serum creatinine following the establishment of AKI 29.
Effects of renal injury on peritubular capillary rarefaction and the AKI to CKD transition
The degree to which effective endothelial repair influences chronic renal function and the long-term sequelae of AKI has also received significant attention in the last 10–15 years. Several reports indicate that peritubular capillary density is reduced permanently following AKI despite the apparent recovery of function following the initial insult. In rats and mice, there is a 30–50% reduction in capillary density following AKI, which is proposed to promote tissue hypoxia and activate pathways associated with the development of interstitial fibrosis 5, 30, 31 and may also contribute to the sensitivity of post-ischemic rats to develop hypertension 32. Sustained capillary rarefaction has emerged as a potential common feature present in nearly all models of chronic renal injury associated with interstitial fibrosis 33. Capillary rarefaction is present in human CKD of a variety of different etiologies and the loss of capillaries is strongly correlated with interstitial fibrosis34. Thus it is conceivable that impaired renal microvascular perfusion may represent a potential biomarker to predict the onset and severity of CKD. Chade and colleagues have demonstrated that CT imaging can be used to detect deficits in renal perfusion associated with vascular rarefaction using a swine model of renal artery stenosis35. Similarly, MRI techniques have shown promise to identify differences in renal perfusion patterns in patients with mild CKD vs. healthy control patients 36. These observations suggest novel imaging approaches could be used to measure impaired renal perfusion and identify patients at risk of progression.
While hypoxia secondary to vascular dropout appears to contribute to the development of renal fibrosis following AKI, there is clearly complex interplay involving multiple cell types that culminate in the AKI to CKD transition 1, 37. For example, in recent years, it has been suggested that pericyte detachment from damaged peritubular capillary endothelial cells trigger their activation to pro-fibrotic myofibroblasts representing the primary cell type contributing toward extracellular matrix (ECM) production38.
In addition, recent evidence supports the view that persistently damaged tubular epithelium contributes to the development of fibrosis (reviewed extensively in 37, 39, 40). Interestingly, significant damage specifically in proximal tubule cells using a transgenic diphtheria toxin receptor transgenic mouse model was shown to result in persistent inflammation, activation of fibroblasts and interstitial fibrosis 41. It is thought that dedifferentiated cells and/or cells which become arrested in G2/M phase of proliferation produce pro-fibrotic mediators such as TGF-β or CTGF, interact within the interstitial environment and stimulate the production of ECM 39, 40.
The failure of epithelial cells to fully repair may represent a deficit of the tubular cell itself, however we suggest such failed recovery may also result from the lack of appropriate local perfusion secondary to capillary rarefaction. Indeed, Suzuki et al., demonstrated in a model of microembolism that hetergeneously damaged peritubular capillaries were in close apposition to areas of simplified epithelia and increased interstitial cell density42. Taken together the development of interstitial fibrosis in the AKI to CKD transition can be viewed as complex interaction of damaged vascular, epithelial and interstitial cell types, and the targeting of any of these pathways could be envisioned to promote more successful repair and improved long-term outcomes 2.
Causes of capillary rarefaction following renal injury
If reduced peritubular capillary density represents a significant contributor toward progression of CKD, then an understanding of the pathophysiological loss of capillary density following an injury may help identify potential therapeutic strategies. The cause of capillary rarefaction is not firmly established, but it likely involves endothelial mesenchymal transition (EndoMT), combined with a relative lack of compensatory endothelial proliferation43, 44 (Figure 2). These activities may result from a combination of a reduced angiogenic environment and trophic support from surrounding tubules or pericytes 45–47. Goligorsky and his colleagues have suggested inhibition of eNOS could trigger EndoMT 48and that in the setting of vascular stasis, reduced shear stress may be a trigger for EndoMT and vascular rarefaction 49. Patschan et al., suggested that EndoMT may result from endothelial cytoskeletal alterations in response to ischemia, which reduces α-tubulin expression and is associated with impaired endothelial cilia function 44. Interestingly, in animal models of AKI, EndoMT and rarefaction of capillaries is sustained despite ongoing renal tubular repair and recovery of renal function. For example, our laboratory demonstrated evidence of EndoMT for up to 7 days following I/R in rats, while Ehling et al., demonstrated the nadir of capillary density was not reached until approximately 14 days following I/R, a time point well beyond the normal re-establishment of plasma creatinine to normal values 50, 51.
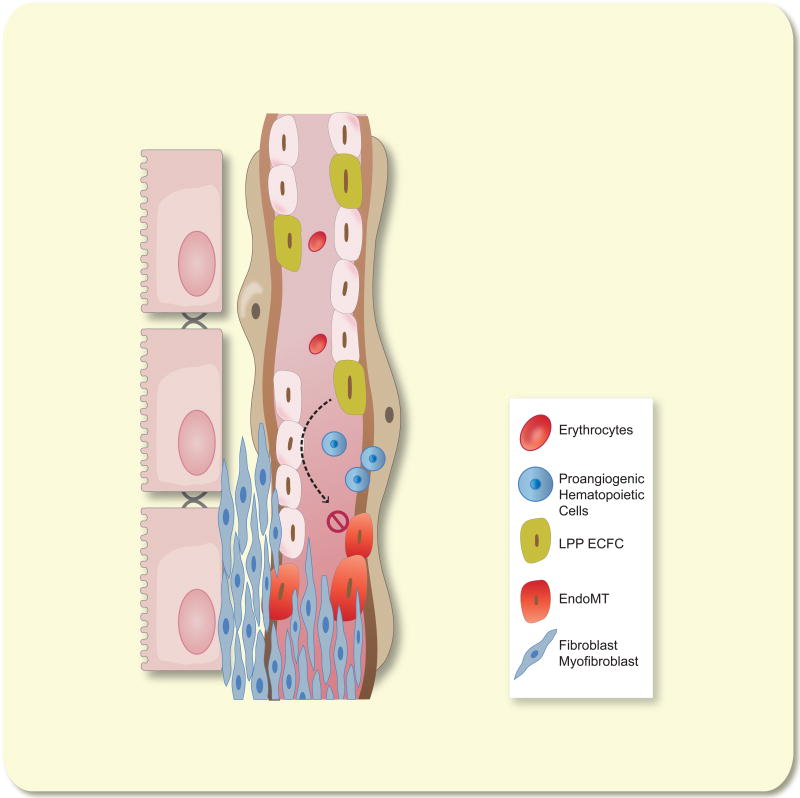
Figure 2.
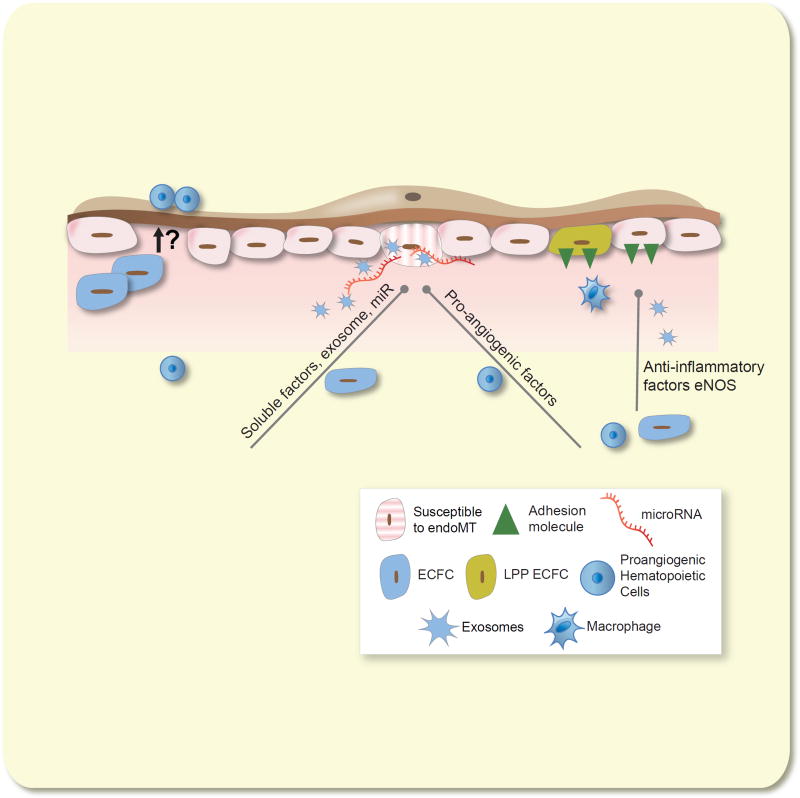
Failed vascular recovery leads to peritubular capillary rarefaction following AKI. Concurrent with resolution of GFR and tubular repair recovery of the capillary endothelium is inefficient due to a combination of EndoMT and low endothelial proliferation. Infiltration of pro-angiogenic hematopoietic cells provide pro-angiogenic stimulation but renal endothelium is unresponsive due to the lack of HPP-ECFC activity intrinsic in kidney. Expansion of fibroblasts or myofibroblasts, which derive from either pericyte activation or EndoMT, may occlude blood vessels leading to a rarefied capillary bed.
We have suggested that sustained capillary rarefaction may be attributable to the lack of resident renal endothelial progenitor activity, representing the basis for modest EC proliferation and sustained rarefaction observed following injury 50 (described further, below). Interestingly, VEGF121 treatment post I/R prevented capillary rarefaction by attenuation of EndoMT but did not influence kidney endothelial proliferation 43. Therefore, given the sustained duration of EndoMT process contributing to capillary loss following recovery from acute injury, a critical therapeutic window may be available to treat patients recovering from AKI to improve capillary survival and long term renal function.
Taken together, the issues highlighted above indicate a need for therapeutic approaches to address endothelial function to treat both the acute as well as the chronic vascular damage resulting from AKI. The remainder of this article will focus primarily on the potential role of stem and progenitor cells targeting endothelial function and the potential therapeutic applications of these cells in the setting of acute kidney injury.
Defining cells types with potential for endothelial repair potential
Numerous studies invoking a role for endothelial progenitor cells (EPC) in renal vascular repair in both acute and chronic kidney disease have been published. However, the field has been hampered by inconsistent definitions and different methodologies used to isolate or identify various cell types classified as EPCs. Because of the variety of different cell types used in these studies, we endorse previous suggestions to abandon the term “endothelial progenitor cell” in favor of more precise terminology to allow for better interpretation and comparison of the results from different studies 52. In general, cells with particular relevance to angiogenic repair can be classified into two broad categories as either hematopoietic or endothelial in origin and these distinctions are described in greater detail below.
Historically, most studies have referred to EPCs as bone marrow-derived cells, which express cell surface markers common to endothelial cells and may mediate pro-angiogenic or vascular repair activity 53 (Figure 3). Several populations of cells have been described and characterized by the nature of their biological source, isolation procedure and the presence of various cell surface markers which provide insight into their origin. Hematopoietic stem cells (HSC) give rise to multiple lineages of myeloid and lymphoid cells, many of which display pro-angiogenic activity54–57. Therefore different selection criteria may lead to isolation of upstream progenitors with the potential to differentiate into more terminally differentiated cells which may modulate vascular injury. While HSC differentiate into a variety of different myeloid and lymphoid populations, advances in confocal imaging to improve spatial localization of transplanted cells 58 and the use of lineage specific reporter transgenic murine models 59–61 or analysis of epigenetic regulation in endothelial cells 62 has eliminated any evidence that these cells are capable of differentiating into endothelial cells. Nevertheless, their involvement in processes such as vascular remodeling 63–65 make them particularly relevant in understanding potential effects in the setting of acute kidney injury.
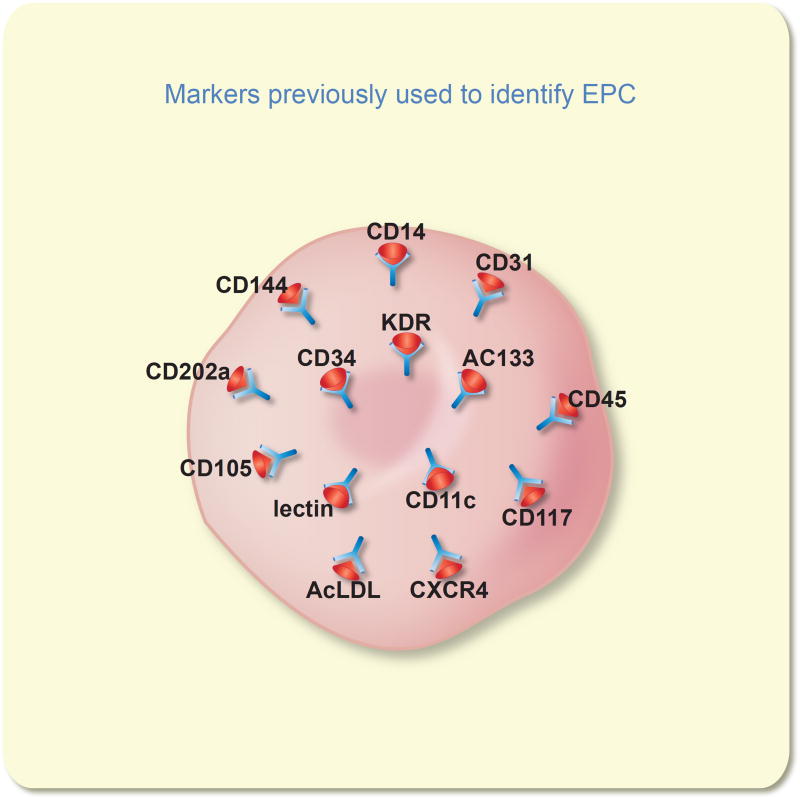
Figure 3.
Human “endothelial progenitor cells” (EPC) have been identified using monoclonal antibodies or specific ligands to detect numerous cell surface antigens. Three key antigens CD34, AC133, and KDR are the most frequently utilized as markers for human EPC. No unique antigen has been reported that can discriminate the human EPC from other cell lineages (many of the above antigens are present on blood cells).
Early outgrowth/CFU Hill cells
As originally described, EPCs are isolated from either low density mononuclear cells or CD34+ or CD133+ enriched cells, which are cultured for several days on fibronectin coated plates. The cells express markers of endothelial cells such as CD31, CD105, CD144, CD146, vWF and KDR 66. These cells have subsequently been referred to as “early outgrowth” cells or colony forming unit Hill (CFU-Hill) cells 67–70. The cells have generally been shown to be effective at promoting vascular repair via paracrine secretion of proangiogenic molecules in preclinical models of vascular repair where they typically improve or hasten remodeling 70. These cells are now all recognized to be various stages of hematopoietic cells with proangiogenic potential 70–72.
Hematopoietic pro-angiogenic cells
Putative endothelial progenitor activity from cells isolated based on their expression of surface markers of CD34, CD133 and the VEGFR2 (KDR) has been reported 68. Many studies report that these cells augment angiogenic repair in vascular injury models67, 73, 74 leading to the suggestion that these cells contain endothelial progenitor activity. However, careful examination has demonstrated that CD34/CD133/VEFR2 cells possess hematopoietic colony forming activity75, 76. While neither the CD34/CD133/VEFR2 expressing cells or CFU-Hill cells have been shown to become bone fide endothelial cells by incorporating into the endothelial layer of a repaired vessels, the ability of the CD34/CD133/VEFR2 expressing cells to promote vascular repair, and the ability to isolate these circulating cells from blood has suggested that these may serve as a potential biomarker for overall cardiovascular risk 77, 78.
Circulating Angiogenic Cells
This term has been applied to a variety of hematopoietic stem and progenitor cells that display the capacity to secrete proangiogenic factors that participate via paracrine mechanisms to promote vascular repair and regeneration. However, this term may more broadly include non-hematopoietic cells present in the circulation such as rare endothelial colony forming cells (cECFC) or even mesenchymal cells. In essence any cell that can circulate in the bloodstream that produces proangiogenic molecules may be generally referred to as a circulating angiogenic cell; thus, the term lacks specificity and as such fails to provide the reader with clarity about the cell type being examined52, 79
Myeloid angiogenic cells
This term represents a population of cells derived from peripheral blood derived cultures which express markers of myeloid progenitor cells, monocytes, or macrophages, but do not express markers of hematopoietic stem and progenitor cells and do not proliferate significantly ex vivo 80–82. Of interest, these cells do express potent angiogenic growth factors and participate in vascular remodeling and repair83. This term is a preferred choice for describing the non-ECFC types of angiogenic cells that participate in paracrine support of vascular repair according to a recent consensus statement.40
Endothelial colony forming cells
This term identifies rare circulating viable endothelial cells that give rise to colonies of endothelial cells when peripheral blood cells are cultured in vitro84, 85. While most circulating endothelial cells are undergoing apoptosis or necrosis in the bloodstream86, very rare viable cells possess this endothelial colony forming cell (ECFC) ability 85. These cells have been isolated from human, baboon, rhesus monkey, cow, sheep, dogs, pigs, and rabbit peripheral blood (reviewed in 87). Human ECFC have been delivered in numerous animal models of human disease and display postnatal vasculogenic properties by making human blood vessels in all the models in which the ECFC are delivered directly into ischemic or injured tissue 88, 89. When ECFC are delivered intravascularly into injured animals, little engraftment has been observed and the protective effects to enhance vascular recovery has been demonstrated to reside in secreted molecules released by the ECFC or contained in their exosomes 90, 91,19.
A variety of different cell types influence endothelial responses to renal injury
Based on the description above, we sought to classify studies based on whether the cells of interest could be identified as either pro-angiogenic cells, endothelial colony forming cells (ECFC) or endothelial cells (EC). The term pro-angiogenic cells is meant to convey any cell of hematopoietic origin with potential vasculogenic activity and would therefore encompass cells such as early outgrowth “endothelial” cells (eOEC) or myeloid angiogenic cells. In some cases, we were unable to be sure which of these categories was isolated by the authors of the numerous papers that have delivered “EPC” into murine models of AKI and therefore categorized the cell type as indeterminate (Table 1). It is of interest that exogenous delivery of both pro-angiogenic cells and ECFC possess protective activity in models of AKI.
Table 1.
| Author, reference, year |
Cell Type | Biological Source | Model/Biological Effect |
|---|---|---|---|
| Patschan et al100 2006 | Pro-angiogenic | Murine spleen and kidney | Unilateral I/R in FVB/NJ and Tie-2-GFP mice |
| Reduced plasma creatinine by ~50% post I/R | |||
| No evidence of homing | |||
|
| |||
| Patschan et al101 2010 | Pro-angiogenic | Murine peripheral blood, spleen and bone marrow | Unilateral I/R in male C57BL/6N mice |
| Reduced plasma creatinine by ~40–75% post-I/R | |||
| Reduced plasma creatinine by ~90% post-I/R with Epac-1 Ac pretreatment | |||
| Evidence of minimal homing | |||
|
| |||
| Patschan et al121 2012 | Murine peripheral blood and spleen | Similar models as above; pretreatment of cells with melatonin, Angiopoietin-1 and BMP-5 enhances protective response, with minimal homing | |
| Patschan et al122 2013 | |||
| Patschan et al123 2013 | |||
|
| |||
| Patschan et al124 2015 | Pro-angiogenic | Murine peripheral blood and spleen | Bilateral I/R in male C57BL/6N mice, reduced plasma creatinine, increased endothelial autophagy and decreased |
| Patschan et al442016 | EndoMT and attenuated capillary rarefaction | ||
|
| |||
| Burger et al1122015 | ECFC & ECFC exosomes | Human umbilical cord | Bilateral I/R in male NOD-SCID mice |
| Reduced plasma creatinine by ~40% post-I/R | |||
| Improved tubular morphology | |||
| No evidence of homing | |||
|
| |||
| Collett et al192017 | ECFC | Rat PMVEC and human ECFC-conditioned media | Bilateral I/R in male Sprague Dawley rats |
| Reduced plasma creatinine by ~50% post-I/R | |||
| Improved tubular morphology | |||
| Prevented loss of medullary blood flow | |||
| No evidence of homing | |||
|
| |||
| Viñas et al91 2016 | ECFC | Human umbilical cord blood ECFC derived exosomes | Bilateral I/R in male FVB mice |
| Reduced plasma creatinine by ~90% post-I/R | |||
| Improved tubular morphology | |||
|
| |||
| Brodsky et al111 2001 | EC | Human umbilical cord | Unilateral I/R in male Athymic rats |
| Reduced plasma creatinine by ~50% post-I/R | |||
| Maintained microvascular perfusion | |||
| Evidence of minimal homing | |||
|
| |||
| Cantaluppi et al115 2012 | ECFC derived microvesicles | Human peripheral blood | Unilateral I/R in male Wistar rats |
| Reduced plasma creatinine by ~80% post-I/R | |||
| Improved tubular morphology | |||
| Prevented capillary rarefaction | |||
|
| |||
| Liang et al125 2015 | EC | Human Umbilical cords (Wharton’s Jelly) | Unilateral I/R in male C57BL/6 mice |
| Reduced plasma creatinine by ~70% post-I/R | |||
| Improved tubular morphology | |||
| Potential prevention of capillary rarefaction | |||
| Evidence of minimal homing | |||
|
| |||
| Pang et al113 2017 | EC or ECFC | Human renal artery | Bilateral I/R and nephron mass reduction in NOD-SCID mice. Reduced plasma creatinine by ~50% post-I/R |
| Prevented capillary rarefaction | |||
| Minimal homing near peritubular capillaries up to 10 days | |||
|
| |||
| Patschan et al114 2017 | Indeterminate | Murine peripheral blood and spleen | Bilateral I/R in male C57BL/6 mice |
| Reduced plasma creatinine by ~10% at 1 week, did not prevent capillary rarefaction | |||
|
| |||
| Zullo et al126 2015 | Indeterminate | Murine embryonic EPC line cultured with MSCs | Improved renal function, medullary RBF and increased M1 to M2 macrophage polarization in LPS induced endoxemia in C57BL6 mice. |
ECFC-endothelial colony forming cells; EC-endothelial cells
Pro-angiogenic cells
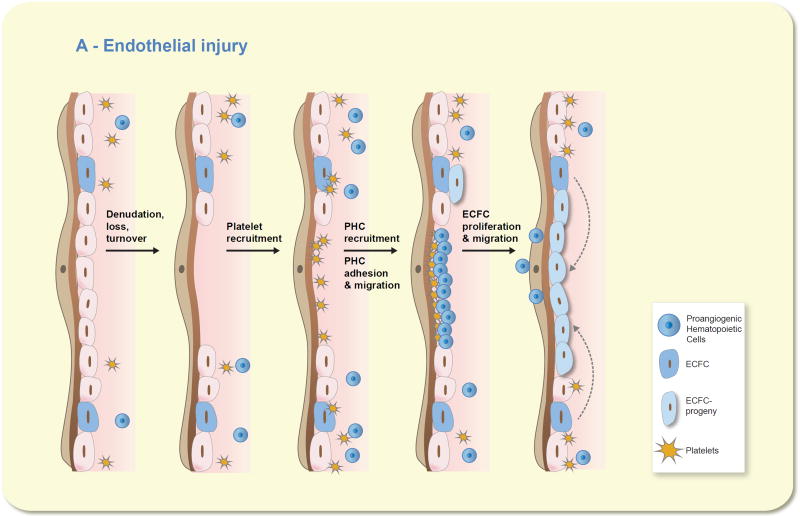
Interest in bone marrow-derived pro-angiogenic cells arises from studies demonstrating the localization of cells expressing both hematopoietic and endothelial markers at sites of vascular injury as well as studies indicating that exogenously administered pro-angiogenic cells influence outcomes in various models of vascular injury. Early outgrowth EPC or blood derived CD34+ cells home to sites of injury, improve perfusion and stimulate angiogenesis in models of ischemia of the myocardium or hindlimb 80, 92. The basis for the pro-angiogenic environment created by BM-derived cells is thought to be the production of factors such as VEGF, HGF or G-CSF 53, 92–94. Pro-angiogenic cells have been shown to limit neointima formation and improve arterial reactivity following aortic balloon injury 93. Since these cells do not differentiate into endothelial cells, the current view is that the pro-angiogenic environment created by these cells activates proliferation and migration of tissue resident HPP-ECFC (high proliferative potential-endothelial colony forming cells) to facilitate recovery from injury (Figure 4).
Figure 4.
Proangiogenic hematopoietic cells (PHC) do not become endothelial cells but do stimulate resident endothelial colony forming cells (ECFC) to repair the intima. Moving from the left to the right on the image, one can visualize that any denudation injury that causes loss or turnover of the resident endothelial cells results in an area of exposed subendothelial basement membrane. Circulating platelets would be readily recruited to the exposed basement membrane and would release chemokines and growth factors to recruit circulating PHC, ranging from bone marrow progenitor cells to mature circulating monocytes and neutrophils, to assist in adhering to the basement membrane. The recruited PHC secrete a host of growth factors, chemokines, and proteolytic enzymes to stimulate the proliferation and migration of resident ECFC into the site of the injury to reconstitute the endothelial barrier and promote normal homeostatic functions through the injured vessel segment. The PHC merely migrate into the tissue where they can differentiate into mature tissue resident cells, re-enter the circulation, or undergo senescence and are cleared by macrophages in the tissues.
In kidney, selective injury to the renal vascular endothelium via infusion of concanavalin A into the renal artery was shown to induce homing of bone marrow derived CD34+/Flk1+ positive cells, which were referred to as EPCs. Mobilization of these cells was evident within 3 hours in the blood and infiltration into the kidneys established within 3–5 days 95. Mediators of this homing activity have been suggested to include secretion of cytokines such is IL-8, G-CSF or GM-SCF. Injury induced up-regulation of the chemokine SDF-1 was shown stimulate homing of pro-angiogenic cells in CXCR4 dependent fashion following I/R injury in mice 96, while others have suggested that the up-regulation of adhesion molecules on the surface of damaged endothelial cells may promote homing of these cells to injured kidney 97. Finally, uric acid, which is produced abundantly by ischemic kidney and is elevated in pigs with renal artery stenosis 98, has also been suggested as a potential homing signal, since administration of a uricase inhibitor blunted the mobilization of cells into mouse kidney following I/R 99.
Bone marrow derived pro-angiogenic cells appear to influence renal vascular function in the setting of AKI (Figure 5). Patschan and colleagues demonstrated increased levels of “EPC”-activity, defined as c-Kit+/Tie-2+ cells in a mouse model of ischemic preconditioning. When these cells were isolated from preconditioned kidneys, they conveyed protection in recipient mice subjected to I/R injury 100. Other studies using exogenous pro-angiogenic cells (derived from isolated and cultured mouse mononuclear cells), demonstrated similar effects on protection from renal injury, but did not directly assess vascular function 101, 102. Recently, administration of these cells was shown to attenuate peritubular capillary rarefaction post I/R, in part by inhibition of EndoMT 44.
Figure 5.
Both HPP-ECFC and pro-angiogenic hematopoietic cells can mitigate acute kidney injury. Both cell types have been shown to improve renal function and renal perfusion when administered prior to the establishment of renal injury. Possible mechanisms include a direct inhibition of adhesion molecule expression proposed to maintain perfusion in the early injury phase (See Figure 1). Maintenance of vascular structure likely is based on prevention of endothelial loss for which EndoMT represents a primary mechanism. Protection is likely mediated by released factors such as pro-angiogenic factors or exosomal transfer of microRNA to protect endothelial injury. Whether administered ECFC could repopulate capillary endothelium is currently subject to debate (likely depends on mode of administration). We propose that co-operative activity of both HPP-ECFC and pro-angiogenic hematopoietic cells could potentially lead to successful engraftment in the acutely injured kidney.
Other preparations of hematopoietic stem cells with putative pro-angiogenic activity have led to conflicting results on the outcome of renal injury. For example, Burger et al., isolated CD133+ cells from human cord blood, of which up to 83 percent of the cells were CD34+, 98 % were CD45+, while only 26% were KDR positive. Administration of these cells to NOD SCID mice following renal I/R worsened changes in serum creatinine, tubular damage and promoted inflammation, while the cells did not home to the kidney 103. These studies suggest that isolation based on CD133+ alone includes diverse hematopoietic progenitor cells that increases the inflammatory response to AKI rather than promoting protection.
In contrast, Li et al., studied HSPC from adult volunteers pretreated with G-CSF to promote HSC mobilization. Mobilized CD34+ cells enriched from peripheral blood mononuclear cells by magnetic separation were primarily CD45+ and only a small percentage expressed CD14, CD133, CD146, CD31 or KDR. However transplanted cells homed to kidney 24 hours following renal ischemia reperfusion injury, and improved renal function and survival in NOD SCID mice. Many cells were localized in the perivascular area between 2–3 days post-injury and expressed markers of endothelial cells such as human hCD31 or KDR, however these exogenous cells were essentially absent by 7 days post-injury 104. Interestingly, in human transplant patients, a transient elevation in recipient CD34+ cells were observed frequently along the peritubular capillary lining within 2 weeks of ischemia, while no evidence of recipient cells were observed in the vasculature after 35 or 73 days 105. Taken together, these data support the view that transient homing of bone marrow derived pro-angiogenic cells migrate to areas of vascular injury and may stimulate remodeling, but these cells do not stably integrate into the vasculature, suggesting they do not act as de facto progenitor cells.
Despite this, these cells may stimulate endothelial cell proliferation and migration, and have been shown to induce angiogenic branch formation in vitro and in vivo 53, 92–94. These cells are thought to subserve an important homeostatic function. Goligorsky and colleagues have articulated that the concept of “EPC incompetence”, based on studies demonstrating that the number or activity of bone marrow derived pro-angiogenic cells is impaired in patients with increased cardiovascular risk factors. Vascular impairment in these patients can be thought of as a result of reduced activity or mobilization of these cells to maintain vascular homoeostasis, a viewpoint consistent with the increased susceptibility of patients with CKD to develop AKI 106.
Endothelial colony forming cells
As described above, endothelial colony forming cells (ECFC), often referred to as “late outgrown endothelial cells” have been isolated following culture of blood cells on collagen following removal of non-adherent monocytes and subsequent expansion 53. ECFC express classic markers of endothelial cells including CD31 and VEGFR2, as well as other markers. In contrast to hematopoietic pro-angiogenic cells, ECFCs do not express markers such as CD45 and are capable of forming and stably integrating into functional vessels in vivo 53, 55, 107.
ECFCs can be classified based on their proliferative potential in single cell colony forming assays, in which high proliferative potential (HPP) ECFC will form large colonies (>10,000), while low proliferative potential (LPP) ECFC form small colonies (<2000). ECFCs can be isolated and expanded from blood of humans and other large species, but cannot be isolated from blood of rodents 55. However, ECFC can also be isolated from tissues of a variety species, including rodents. This observation has led to the hypothesis that a cooperative interaction between infiltrating pro-angiogenic cells of hematopoietic origin work to provide a trophic environment to stimulate local ECFC progenitor activity to stimulate vascular repair 108 (Figure 4). Interestingly, our data in rats failed to demonstrate evidence of HPP-ECFC populations in kidney; rather we found only evidence of cells capable of forming small colonies, i.e., low proliferative potential ECFC 50. These observations combined with the lack of BrdU+ capillary endothelial cells following renal I/R 43 suggest that a low degree of endogenous ECFC activity may contribute to impaired recovery and maintenance of vascular rarefaction following AKI (Figure 2).
Because ECFC represent true endothelial progenitors, there is considerable interest in exploiting these cells for potential therapeutic effects. Human cord blood represents one of the richest sources of HPP-ECFC 85 and recent studies also demonstrate that iPS cells can be differentiated into highly active HPP-ECFC 88. To date, the potential therapeutic benefit of ECFC has been less well studied in preclinical models of vascular impairment than hematopoietic pro-angiongenic cells. Nevertheless, ECFCs stimulate neovascularization in a hindlimb ischemia model 109 and attenuate the development of pulmonary hypertension in a rat model of arrested alveolar development 90
ECFC appear to effectively ameliorate the severity of injury in models of AKI (Figure 5), an observation gleaned initially from studies in which the influence of HUVEC administration was assessed in a model of I/R. HUVEC rapidly expand in culture and contain a significant population of HPP-ECFC 108. In these studies, systemic infusion of HUVEC in athymic rats following I/R injury significantly improved capillary flow rates as observed by video microscopy 110, 111. HUVEC infusion also resulted in a significant protection against the loss of renal function (e.g., by serum creatinine) and tubular injury. Surrogate non-endothelial cells had no effect on I/R induced damage, but when cells overexpressed eNOS, there was an improvement in renal blood flow leading to the suggestion endothelial supplementation influenced AKI via the nitric oxide pathway 102, 103.
Recent results from Burger et al., support the suggestion that ECFC have renal protective properties. Using human cord-blood derived HPP-ECFC injected immediately following ischemia reperfusion, AKI was attenuated in SCID mice as assessed by creatinine, tubular necrosis, macrophage infiltration and oxidative stress 112. In contrast to results obtained with bone marrow derived pro-angiogenic cells, ECFCs showed very little evidence of homing into the kidney. Similarly, our group recently demonstrated that rat pulmonary microvascular endothelial cells (PMVEC), which have a high level of HPP-ECFC, failed to home to the kidney but protected Sprague Dawley rats from I/R induced AKI 19. In contrast, studies by Pang et al., demonstrated that a small number of EC/ECFC from isolated from human renal artery migrate into the renal peritubular region of SCID mice following severe I/R injury 113, suggesting that the fate of transplanted EC/ECFC following kidney injury is not clearly understood.
It is of interest that low proliferative potential ECFC (from pulmonary artery) had no protective effect on ischemia reperfusion injury 19, indicating that the protective effects are a function of the proliferative capacity of ECFC. Interestingly, Patschan et al., found only minimal protection from AKI by late outgrowth cells obtained from mouse peripheral blood and spleen114. These cells expressed markers of EC such as VE-cadherin, but since ECFC do not circulate in mice 114, these cells likely had low proliferative potential relative to other studies demonstrating protective effects of ECFC.
The lack of homing of ECFC suggests that secreted factors may promote resistance to AKI. Exosomes derived from ECFC conditioned media improve renal function post ischemia and prevent endothelial injury to hypoxia in vitro 112. ECFC derived exosomes contain a high level of microRNA486-5p which may mediate these protective effects by decreasing endothelial PTEN (Phosphatase and tensin homolog) and increasing Akt 91. Other investigators have demonstrated microvesicles (MV) from conditioned media of human blood derived EC protected Wistar rats from AKI, while micro-vesicles derived from fibroblasts did not. Treatment of MV with Dicer or antimir 126 or 296 blocked the protective effect of microvessicles115. The authors proposed a so-called “horizontal transfer” of information of miRNA from micro-vesicles into the endothelium as the basis by which protection is mediated 115, 116. We recently demonstrated that rat PMVEC or conditioned media of human ECFC protected against early alterations in renal hemodynamics following ischemia reperfusion injury, by preventing the loss of renal medullary blood flow. Human ECFC conditioned media also prevented the immediate up-regulation of endothelial ICAM-1 and the rapid infiltration of T-lymphocytes into the kidney within hours of ischemia reperfusion 19.
Despite protective effects of both bone marrow derived pro-angiogenic cells and ECFC in acute kidney injury, whether these strategies can be used to effectively reverse the rarefaction of vessels associated with AKI and prevent progressive CKD has not been explored. The paradigm of cooperative interaction 108 (and Figure 4) highlights the role of local ECFC in the vascular repair response which could be stimulated by pro-angiogenic cell-treatment.
Future Directions
We hypothesize that a limited endogenous kidney ECFC proliferative potential contributes to impaired vascular repair and sustained rarefaction following injury. Whether cell-based therapies may have a future in treating patients with AKI is not yet clear. While preclinical data using ECFC, pro-angiogenic cells or their conditioned media indicate the potential to preserve vascular function in AKI, translating these observations to the clinic represents a significant hurdle. We propose cell-based therapies in AKI, or any therapeutic study in AKI, should not be restricted to acute hospital outcomes but incorporate the potential development of subsequent CKD following discharge of AKI survivors, which may take months or years to manifest. We envision that agents with little obvious acute protective effect in the recovery period may provide long-term benefit and that such goals should be considered in clinical study design.
From a revascularization point of view, the inability of ECFC to home to the kidney following I/R hampers efforts to facilitate vascular remodeling and is a hurdle that must be overcome. By extension, supplementing with high proliferative ECFC may result in improved vascularization, if retention and integration could be achieved. Future studies should investigate how improved methods of administration of cells could help overcome barriers toward homing of ECFC and facilitate repair. It is possible that a combination of bone marrow derived pro-angiogenic cells with ECFC may help to revascularize the kidney. In support of this hypothesis, co-culture of these two cell types promoted cooperative formation of capillary-like structures in Matrigel plugs, while co-adoptive transfer of both cell types in hind limb ischemia promotes greater in vivo neo-angiogenesis when compared to a single cell transfers alone 117. It is of interest that Chade et al., have demonstrated that administration of endothelial progenitors described as a mix of “early” and “late” outgrowth cells directly into the renal artery of swine with renal artery stenosis improved in renal vascular structure, renal function and an attenuated renal fibrosis 118.
New approaches such as the use of stromal vascular fraction (SVF), which contain both ECFC like cells and pro-angiogenic trophic cells could be used as SVF has been shown in animal models to attenuate the development of AKI and progressive CKD following I/R 119. Similarly, we demonstrated that human adipose derived stromal cells could prevent the loss of renal peritubular capillaries and limit renal fibrosis up to 1 week after recovery from renal ischemia reperfusion in rats 120. Therefore, a combination of different cells with cooperative activities could be envisioned to improve vascular repair or prevent capillary loss following kidney injury as a means to enable better long term renal function.
Finally, it will be important to more fully understand the biological basis for low level of ECFC proliferative potential in kidney. At the current time, these progenitor cells can only be effectively studied and identified ex vivo using clonal analysis. No unique set of markers has yet been identified for HPP ECFC, although efforts are clearly underway to identify key genes regulating ECFC activity 88. If proper markers can be identified, it may enable efforts to study the nature of ECFC activity in the kidney, the pathways that subserve the high proliferative potential, and even perhaps to devise strategies to influence progenitor activity of the renal EC themselves.
Acknowledgments
Support for studies by the authors reviewed in this paper are summarized as follows: DPB was supported by NIH grant DK-063114 and Fortune Fry Fund and Bridge Funding from the Indiana University Research Foundation and pilot funding from the George M O’Brien Center P30-DK79312 (Molitoris, program director). Support for JAC was by NIH T32 HL079995-10 (K. March, PI) and an NIH/CATS postdoctoral fellowship TL1R001107 (A.Shekar, PI). MCY was partially supported by funds from the National Institutes of Health grant DK106846 and National Center for Advancing Translational Sciences grant TR000006 and the Riley Children’s Hospital Foundation.
Footnotes
Conflict of Interest: The authors report no conflict of interest.
References
- 1.Basile DP, Anderson M, Sutton TA. Pathophysiology of acute kidney injury. Comprehensive Physiology. 2012;2:1303–1353. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basile DP, Bonventre JV, Mehta R, Nangaku M, Unwin R, Rosner MH, Kellum JA, Ronco C. Group, tAXW: Progression after AKI: Understanding Maladaptive Repair Processes to Predict and Identify Therapeutic Treatments. Journal of the American Society of Nephrology. 2016;27:687–697. doi: 10.1681/ASN.2015030309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute Kidney Injury and Chronic Kidney Disease as Interconnected Syndromes. New England Journal of Medicine. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney International. 2007;72:151–156. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 6.Matejovic M, Ince C, Chawla LS, Blantz R, Molitoris BA, Rosner MH, Okusa MD, Kellum JA, Ronco C. Renal Hemodynamics in AKI: In Search of New Treatment Targets. Journal of the American Society of Nephrology. 2016;27:49–58. doi: 10.1681/ASN.2015030234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fahling M, Seeliger E, Patzak A, Persson PB. Understanding and preventing contrast-induced acute kidney injury. Nat Rev Nephrol. 2017;13:169–180. doi: 10.1038/nrneph.2016.196. [DOI] [PubMed] [Google Scholar]
- 8.Basile D, Yoder M. Renal endothelial dysfunction in acute kidney ischemia reperfusion. Cardiovascular & Haematological Disorders-Drug Targets. 2014;14:3–14. doi: 10.2174/1871529x1401140724093505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans RG, Ince C, Joles JA, Smith DW, May CN, O’Connor PM, Gardiner BS. Haemodynamic influences on kidney oxygenation: The clinical implications of integrative physiology. Clinical and Experimental Pharmacology and Physiology. 2012 doi: 10.1111/1440-1681.12031. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 10.Molitoris BA. Therapeutic translation in acute kidney injury: the epithelial/endothelial axis. The Journal of Clinical Investigation. 2014;124:2355–2363. doi: 10.1172/JCI72269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brodsky SV, Goligorsky MS. ENDOTHELIUM UNDER STRESS: LOCAL AND SYSTEMIC MESSAGES. Seminars in Nephrology. 2012;32:192–198. doi: 10.1016/j.semnephrol.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon O, Hong S-M, Sutton TA, Temm CJ. Preservation of peritubular capillary endothelial integrity and increasing pericytes may be critical to recovery from postischemic acute kidney injury. Am J Physiol Renal Physiol. 2008;295:F351–359. doi: 10.1152/ajprenal.90276.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molitoris BA, Sutton TA. Endothelial injury and dysfunction: role in the extension phase of acute renal failure. Kidney International. 2004;66:496–499. doi: 10.1111/j.1523-1755.2004.761_5.x. [DOI] [PubMed] [Google Scholar]
- 14.Yasuda K, Vasko R, Hayek P, Ratliff B, Bicer H, Mares J, Maruyama S, Bertuglia S, Mascagni P, Goligorsky MS. Functional consequences of inhibiting exocytosis of Weibel-Palade bodies in acute renal ischemia. American Journal of Physiology - Renal Physiology. 2012;302:F713–F721. doi: 10.1152/ajprenal.00541.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boesen EI, Crislip GR, Sullivan JC. Use of ultrasound to assess renal reperfusion and P-selectin expression following unilateral renal ischemia. American Journal of Physiology - Renal Physiology. 2012;303:F1333–F1340. doi: 10.1152/ajprenal.00406.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zizzi HC, Zibari GB, Granger DN, Singh I, Cruz LD, Abreo F, McDonald JC, Brown MF. Quantification of P-selectin expression after renal ischemia and reperfusion. Journal of Pediatric Surgery. 1997;32:1010–1013. doi: 10.1016/s0022-3468(97)90388-2. [DOI] [PubMed] [Google Scholar]
- 17.Farrar CA, Wang Y, Sacks SH, Zhou W. Independent Pathways of P-Selectin and Complement-Mediated Renal Ischemia/Reperfusion Injury. The American Journal of Pathology. 2004;164:133–141. doi: 10.1016/S0002-9440(10)63104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly KJ, Williams WW, Jr, Colvin RB, Bonventre JV. Antibody to intercellular adhesion molecule 1 protects the kidney against ischemic injury. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:812–816. doi: 10.1073/pnas.91.2.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collett JA, Mehrotra P, Crone A, Shelley WC, Yoder MC, Basile DP. Endothelial colony forming cells ameliorate endothelial dysfunction via secreted factors following ischemia-reperfusion injury. American Journal of Physiology - Renal Physiology. 2017 doi: 10.1152/ajprenal.00643.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Greef K, Ysebaert D, Dauwe S, Persy V, Vercauteren S, Mey D, De Broe M. Anti B7-1 blocks mononuclear cell adherence in vasa recta after ischemia. Kidney International. 2001;60:1415–1427. doi: 10.1046/j.1523-1755.2001.00944.x. [DOI] [PubMed] [Google Scholar]
- 21.Sutton TA, Mang HE, Campos SB, Sandoval RM, Yoder MC, Molitoris BA. Injury of the renal microvascular endothelium alters barrier function after ischemia. American Journal of Physiology - Renal Physiology. 2003;285:F191–198. doi: 10.1152/ajprenal.00042.2003. [DOI] [PubMed] [Google Scholar]
- 22.Liu S, Soong Y, Seshan SV, Szeto HH. Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation, and fibrosis. American Journal of Physiology - Renal Physiology. 2014;306:F970–F980. doi: 10.1152/ajprenal.00697.2013. [DOI] [PubMed] [Google Scholar]
- 23.Sutton TA. Alteration of microvascular permeability in acute kidney injury. Microvascular Research. 2009;77:4–7. doi: 10.1016/j.mvr.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandal AK, Taylor CA, Bell RD, Hillman NM, Jarnot MD, Cunningham JD, Phillips LG. Erythrocyte deformation in ischemic acute tubular necrosis and amelioration by splenectomy in the dog. Lab Invest. 1991;65:566–576. [PubMed] [Google Scholar]
- 25.Lerolle N, Nochy D, Guérot E, Bruneval P, Fagon J-Y, Diehl J-L, Hill G. Histopathology of septic shock induced acute kidney injury: apoptosis and leukocytic infiltration. Intensive Care Medicine. 2010;36:471–478. doi: 10.1007/s00134-009-1723-x. [DOI] [PubMed] [Google Scholar]
- 26.Solez K, Kramer E, RH H. AMERICAN JOURNAL OF PATHOLOGY. AMER SOC INVESTIGATIVE PATHOLOGY, INC 428 EAST PRESTON ST, BALTIMORE, MD 21202-3993: 1974. PATHOLOGY OF ACUTE RENAL-FAILURE-LEUKOCYTE ACCUMULATION IN VASA RECTA; pp. A31–A32. [Google Scholar]
- 27.Le Dorze M, Legrand M, Payen D, Ince C. The role of the microcirculation in acute kidney injury. Current Opinion in Critical Care. 2009;15:503–508. doi: 10.1097/MCC.0b013e328332f6cf. [DOI] [PubMed] [Google Scholar]
- 28.Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Critical Care. 2013;17:1–15. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collett JA, Corridon PR, Mehrotra P, Kolb AL, Rhodes GJ, Miller CA, Molitoris BA, Pennington JG, Sandoval RM, Atkinson SJ, Campos-Bilderback SB, Basile DP, Bacallao RL. Hydrodynamic Isotonic Fluid Delivery Ameliorates Moderate-to-Severe Ischemia-Reperfusion Injury in Rat Kidneys. Journal of the American Society of Nephrology. 2017 doi: 10.1681/ASN.2016040404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basile DP, Donohoe DL, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. American Journal of Physiology. 2001;281:F887–F899. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 31.Horbelt M, Lee S-Y, Mang HE, Knipe NL, Sado Y, Kribben A, Sutton TA. Acute and chronic microvascular alterations in a mouse model of ischemic acute kidney injury. Am J Physiol Renal Physiol. 2007;293:F688–695. doi: 10.1152/ajprenal.00452.2006. [DOI] [PubMed] [Google Scholar]
- 32.Pechman KR, De Miguel C, Lund H, Leonard EC, Basile DP, Mattson DL. Recovery from renal ischemia-reperfusion injury is associated with altered renal hemodynamics, blunted pressure natriuresis, and sodium-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1358–1363. doi: 10.1152/ajpregu.91022.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing progressive nephropathy. Current Opinion in Nephrology and Hypertension. 2004;13:1–13. doi: 10.1097/00041552-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Eardley KS, Kubal C, Zehnder D, Quinkler M, Lepenies J, Savage CO, Howie AJ, Kaur K, Cooper MS, Adu D, Cockwell P. The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int. 2008;74:495–504. doi: 10.1038/ki.2008.183. [DOI] [PubMed] [Google Scholar]
- 35.Chade AR, Kelsen S. Reversal of renal dysfunction by targeted administration of VEGF into the stenotic kidney: a novel potential therapeutic approach. American Journal of Physiology - Renal Physiology. 2012;302:F1342–F1350. doi: 10.1152/ajprenal.00674.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossi C, Artunc F, Martirosian P, Schlemmer H-P, Schick F, Boss A. Histogram Analysis of Renal Arterial Spin Labeling Perfusion Data Reveals Differences Between Volunteers and Patients With Mild Chronic Kidney Disease. Investigative Radiology. 2012;47:490–496. doi: 10.1097/RLI.0b013e318257063a. [DOI] [PubMed] [Google Scholar]
- 37.Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. Journal of the American Society of Nephrology. 2015 doi: 10.1681/ASN.2015010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin S, Chang F, Schrimpf C, Chen Y, Wu C, Wu V, Chiang W, Kuhnert F, Kuo C, Chen Y, Wu K, Tsai T, duffield J. Targetting endothelium pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol. 2011;178:911–923. doi: 10.1016/j.ajpath.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grgic I, Campanholle G, Bijol V, Wang C, Sabbisetti VS, Ichimura T, Humphreys BD, Bonventre JV. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82:172–183. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16:535–543. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takaori K, Nakamura J, Yamamoto S, Nakata H, Sato Y, Takase M, Nameta M, Yamamoto T, Economides AN, Kohno K, Haga H, Sharma K, Yanagita M. Severity and Frequency of Proximal Tubule Injury Determines Renal Prognosis. Journal of the American Society of Nephrology. 2015 doi: 10.1681/ASN.2015060647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki T, Kimura M, Asano M, Fujigaki Y, Hishida A. Role of atrophic tubules in development of interstitial fibrosis in microembolism-induced renal failure in rat. American Journal of Pathology. 2001;158:75–85. doi: 10.1016/S0002-9440(10)63946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Basile DP, Friedrich JL, Spahic J, Knipe NL, Mang HE, Leonard EC, Ashtiyani SC, Bacallao RL, Molitoris BA, Sutton TA. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. American Journal of Physiology - Renal Physiology. 2011;300:F721–733. doi: 10.1152/ajprenal.00546.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patschan D, Schwarze K, Henze E, Patschan S, Müller GA. The endothelial-to-mesenchymal transition and endothelial cilia in EPC-mediated postischemic kidney protection. American Journal of Physiology - Renal Physiology. 2016;310:F679–F687. doi: 10.1152/ajprenal.00306.2015. [DOI] [PubMed] [Google Scholar]
- 45.Lin S-L, Chang F-C, Schrimpf C, Chen Y-T, Wu C-F, Wu V-C, Chiang W-C, Kuhnert F, Kuo CJ, Chen Y-M, Wu K-D, Tsai T-J, Duffield JS. Targeting Endothelium-Pericyte Cross Talk by Inhibiting VEGF Receptor Signaling Attenuates Kidney Microvascular Rarefaction and Fibrosis. The American journal of pathology. 2011;178:911–923. doi: 10.1016/j.ajpath.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schrimpf C, Xin C, Campanholle G, Gill SE, Stallcup W, Lin SL, Davis GE, Gharib SA, Humphreys BD, Duffield JS. Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. Journal of the American Society of Nephrology: JASN. 2012;23:868–883. doi: 10.1681/ASN.2011080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basile DP, Fredrich K, Chelladurai B, Leonard EC, Parrish AR. Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am J Physiol Renal Physiol. 2008;294 doi: 10.1152/ajprenal.00596.2007. [DOI] [PubMed] [Google Scholar]
- 48.O’Riordan E, Mendelev N, Patschan S, Patschan D, Eskander J, Cohen-Gould L, Chander P, Goligorsky MS. Chronic NOS inhibition actuates endothelial-mesenchymal transformation. Am J Physiol Heart Circ Physiol. 2007;292:H285–294. doi: 10.1152/ajpheart.00560.2006. [DOI] [PubMed] [Google Scholar]
- 49.Goligorsky MS. Microvascular rarefaction: The decline and fall of blood vessels. Organogenesis. 2010;6:1–10. doi: 10.4161/org.6.1.10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Basile DP, Zeng P, Friedrich JL, Leonard E, Yoder MC. Low proliferative potential and impaired angiogenesis of cultured rat kidney endothelial cells. Microcirculation. 2012;19:598–609. doi: 10.1111/j.1549-8719.2012.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ehling J, Bábíčková J, Gremse F, Klinkhammer BM, Baetke S, Knuechel R, Kiessling F, Floege J, Lammers T, Boor P. Quantitative Micro-Computed Tomography Imaging of Vascular Dysfunction in Progressive Kidney Diseases. Journal of the American Society of Nephrology: JASN. 2016;27:520–532. doi: 10.1681/ASN.2015020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Medina RJ, Barber CL, Sabatier F, Dignat-George F, Melero-Martin JM, Khosrotehrani K, Ohneda O, Randi AM, Chan JKY, Yamaguchi T, Van Hinsbergh VWM, Yoder MC, Stitt AW Endothelial Progenitors. A Consensus Statement on Nomenclature. STEM CELLS Translational Medicine. 2017 doi: 10.1002/sctm.16-0360. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basile DP, Yoder MC. Circulating and Tissue Resident Endothelial Progenitor Cells. Journal of Cellular Physiology. 2014;229:10–16. doi: 10.1002/jcp.24423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wara AK, Croce K, Foo S, Sun X, Icli B, Tesmenitsky Y, Esen F, Rosenzweig A, Feinberg MW. Bone marrow-derived CMPs and GMPs represent highly functional proangiogenic cells: implications for ischemic cardiovascular disease. Blood. 2011;118:6461–6464. doi: 10.1182/blood-2011-06-363457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rose JA, Erzurum S, Asosingh K. Biology and flow cytometry of proangiogenic hematopoietic progenitors cells. Cytometry Part A. 2015;87:5–19. doi: 10.1002/cyto.a.22596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Estes ML, Mund JA, Mead LE, Prater DN, Cai S, Wang H, Pollok KE, Murphy MP, An CST, Srour EF, Ingram DA, Case J. Application of polychromatic flow cytometry to identify novel subsets of circulating cells with angiogenic potential. Cytometry Part A. 2010;77A:831–839. doi: 10.1002/cyto.a.20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Purhonen S, Palm J, Rossi D, Kaskenpää N, Rajantie I, Ylä-Herttuala S, Alitalo K, Weissman IL, Salven P. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proceedings of the National Academy of Sciences. 2008;105:6620–6625. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Göthert JR, Gustin SE, van Eekelen JAM, Schmidt U, Hall MA, Jane SM, Green AR, Göttgens B, Izon DJ, Begley CG. Genetically tagging endothelial cells in vivo: bone marrow-derived cells do not contribute to tumor endothelium. Blood. 2004;104:1769–1777. doi: 10.1182/blood-2003-11-3952. [DOI] [PubMed] [Google Scholar]
- 60.Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–2086. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perry TE, Song M, Despres DJ, Kim SM, San H, Yu Z-X, Raghavachari N, Schnermann J, Cannon RO, III, Orlic D. Bone marrow-derived cells do not repair endothelium in a mouse model of chronic endothelial cell dysfunction. Cardiovascular Research. 2009;84:317–325. doi: 10.1093/cvr/cvp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohtani K, Vlachojannis GJ, Koyanagi M, Boeckel J-N, Urbich C, Farcas R, Bonig H, Marquez VE, Zeiher AM, Dimmeler S. Epigenetic Regulation of Endothelial Lineage Committed Genes in Pro-Angiogenic Hematopoietic and Endothelial Progenitor Cells: Novelty and Significance. Circulation Research. 2011;109:1219–1229. doi: 10.1161/CIRCRESAHA.111.247304. [DOI] [PubMed] [Google Scholar]
- 63.De Palma M, Naldini L. Angiopoietin-2 TIEs Up Macrophages in Tumor Angiogenesis. Clinical Cancer Research. 2011;17:5226–5232. doi: 10.1158/1078-0432.CCR-10-0171. [DOI] [PubMed] [Google Scholar]
- 64.Willenborg S, Lucas T, van Loo G, Knipper JA, Krieg T, Haase I, Brachvogel B, Hammerschmidt M, Nagy A, Ferrara N, Pasparakis M, Eming SA. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood. 2012;120:613–625. doi: 10.1182/blood-2012-01-403386. [DOI] [PubMed] [Google Scholar]
- 65.Laurent J, Touvrey C, Botta F, Kuonen F, Ruegg C. Emerging paradigms and questions on pro-angiogenic bone marrow derived myelomonocytic cells. Int J Dev Biol. 2011;55:527–534. doi: 10.1387/ijdb.103228jl. [DOI] [PubMed] [Google Scholar]
- 66.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of Putative Progenitor Endothelial Cells for Angiogenesis. Science. 1997;275:964–966. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 67.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone Marrow Origin of Endothelial Progenitor Cells Responsible for Postnatal Vasculogenesis in Physiological and Pathological Neovascularization. Circulation Research. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 68.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MAS, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 69.Hill JM, Zalos G, Halcox JPJ, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating Endothelial Progenitor Cells, Vascular Function, and Cardiovascular Risk. New England Journal of Medicine. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 70.Fadini GP, Agostini C, Avogaro A. Autologous stem cell therapy for peripheral arterial disease: Meta-analysis and systematic review of the literature. Atherosclerosis. 2010;209:10–17. doi: 10.1016/j.atherosclerosis.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 71.Yoder MC. Endothelial progenitor cell: a blood cell by many other names may serve similar functions. Journal of Molecular Medicine. 2013;91:285–295. doi: 10.1007/s00109-013-1002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Asahara T, Kawamoto A, Masuda H. Concise Review: Circulating Endothelial Progenitor Cells for Vascular Medicine. STEM CELLS. 2011;29:1650–1655. doi: 10.1002/stem.745. [DOI] [PubMed] [Google Scholar]
- 73.Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K-i, Eguchi H, Onitsuka I, Matsui K, Imaizumi T. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. The Journal of Clinical Investigation. 2000;105:1527–1536. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim H, Cho H-J, Kim S-W, Liu B, Choi YJ, Lee J, Sohn Y-D, Lee M-Y, Houge MA, Yoon Y-s. CD31(+) cells represent highly angiogenic and vasculogenic cells in bone marrow: novel role of non-endothelial CD31(+) cells in neovascularization and their therapeutic effects on ischemic vascular disease. Circulation research. 2010;107:602–614. doi: 10.1161/CIRCRESAHA.110.218396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS, Ingram DA. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Experimental hematology. 2007;35:1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 76.Timmermans F, Plum J, Yöder MC, Ingram DA, Vandekerckhove B, Case J. Endothelial progenitor cells. identity defined? Journal of Cellular and Molecular Medicine. 2009;13:87–102. doi: 10.1111/j.1582-4934.2008.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hayek SS, MacNamara J, Tahhan AS, Awad M, Yadalam A, Ko Y-A, Healy S, Hesaroieh I, Ahmed H, Gray B, Sher SS, Ghasemzadeh N, Patel R, Kim J, Waller EK, Quyyumi AA. Circulating Progenitor Cells Identify Peripheral Arterial Disease in Patients With Coronary Artery Disease: Novelty and Significance. Circulation Research. 2016;119:564–571. doi: 10.1161/CIRCRESAHA.116.308802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hayek SS, Klyachkin Y, Asfour A, Ghasemzadeh N, Awad M, Hesaroieh I, Ahmed H, Gray B, Kim J, Waller EK, Quyyumi AA, Abdel-Latif AK. Bioactive Lipids and Circulating Progenitor Cells in Patients with Cardiovascular Disease. STEM CELLS Translational Medicine. 2017;6:731–735. doi: 10.5966/sctm.2016-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fadini GP, Losordo D, Dimmeler S. Critical re-evaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circulation Research. 2012;110:624–637. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rehman J, Li J, Orschell CM, March KL. Peripheral Blood Endothelial Progenitor Cells Are Derived From Monocyte/Macrophages and Secrete Angiogenic Growth Factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 81.Joshi S, Singh AR, Zulcic M, Durden DL. A Macrophage-Dominant PI3K Isoform Controls Hypoxia-Induced HIF1α and HIF2α Stability and Tumor Growth, Angiogenesis, and Metastasis. Molecular Cancer Research. 2014;12:1520–1531. doi: 10.1158/1541-7786.MCR-13-0682. [DOI] [PubMed] [Google Scholar]
- 82.He L, Marneros AG. Doxycycline inhibits polarization of macrophages to the proangiogenic M2-type and subsequent neovascularization. Journal of Biological Chemistry. 2014 doi: 10.1074/jbc.M113.535765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Newman AC, Hughes CCW. Macrophages and angiogenesis: a role for Wnt signaling. Vascular Cell. 2012;4:13. doi: 10.1186/2045-824X-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. Journal of Clinical Investigation. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 86.Woywodt A, Blann AD, Kirsch T, Erdbruegger U, Banzet N, Haubitz M, Dignat-George F. Isolation and enumeration of circulating endothelial cells by immunomagnetic isolation: proposal of a definition and a consensus protocol. Journal of Thrombosis and Haemostasis. 2006;4:671–677. doi: 10.1111/j.1538-7836.2006.01794.x. [DOI] [PubMed] [Google Scholar]
- 87.Yoder MC. Is Endothelium the Origin of Endothelial Progenitor Cells? Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:1094–1103. doi: 10.1161/ATVBAHA.109.191635. [DOI] [PubMed] [Google Scholar]
- 88.Prasain N, Lee MR, Vemula S, Meador JL, Yoshimoto M, Ferkowicz MJ, Fett A, Gupta M, Rapp BM, Saadatzadeh MR, Ginsberg M, Elemento O, Lee Y, Voytik-Harbin SL, Chung HM, Hong KS, Reid E, O’Neill CL, Medina RJ, Stitt AW, Murphy MP, Rafii S, Broxmeyer HE, Yoder MC. Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells. Nat Biotech. 2014;32:1151–1157. doi: 10.1038/nbt.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Newey SE, Tsaknakis G, Khoo CP, Athanassopoulos T, Camicia R, Zhang Y, Grabowska R, Harris AL, Roubelakis MG, Watt SM. The Hematopoietic Chemokine CXCL12 Promotes Integration of Human Endothelial Colony Forming Cell-Derived Cells into Immature Vessel Networks. Stem Cells and Development. 2014;23:2730–2743. doi: 10.1089/scd.2014.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alphonse RS, Vadivel A, Fung M, Shelley WC, Critser PJ, Ionescu L, O’Reilly M, Ohls RK, McConaghy S, Eaton F, Zhong S, Yoder M, Thébaud B. Existence, Functional Impairment, and Lung Repair Potential of Endothelial Colony-Forming Cells in Oxygen-Induced Arrested Alveolar GrowthCLINICAL PERSPECTIVE. Circulation. 2014;129:2144–2157. doi: 10.1161/CIRCULATIONAHA.114.009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Viñas JL, Burger D, Zimpelmann J, Haneef R, Knoll W, Campbell P, Gutsol A, Carter A, Allan DS, Burns KD. Transfer of microRNA-486-5p from human endothelial colony forming cell-derived exosomes reduces ischemic kidney injury. Kidney International. 2016;90:1238–1250. doi: 10.1016/j.kint.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 92.Khakoo AY, Finkel T. Endothelial Progenitor Cells. Annual Review of Medicine. 2004;56:79–101. doi: 10.1146/annurev.med.56.090203.104149. [DOI] [PubMed] [Google Scholar]
- 93.Kiernan TJ, Boilson BA, Witt TA, Dietz AB, Lerman A, Simari RD. Vasoprotective effects of human CD34+ cells: towards clinical applications. Journal of Translational Medicine. 2009;7:66–66. doi: 10.1186/1479-5876-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ahrens I, Domeij H, Topcic D, Haviv I, Merivirta R-M, Agrotis A, Leitner E, Jowett JB, Bode C, Lappas M, Peter K. Successful In Vitro Expansion and Differentiation of Cord Blood Derived CD34+ Cells into Early Endothelial Progenitor Cells Reveals Highly Differential Gene Expression. PLoS ONE. 2011;6:e23210. doi: 10.1371/journal.pone.0023210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hohenstein B, Kuo M-C, Addabbo F, Yasuda K, Ratliff B, Schwarzenberger C, Eckardt K-U, Hugo CPM, Goligorsky MS. Enhanced progenitor cell recruitment and endothelial repair after selective endothelial injury of the mouse kidney. American Journal of Physiology - Renal Physiology. 2010;298:F1504–F1514. doi: 10.1152/ajprenal.00025.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Togel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67:1772–1784. doi: 10.1111/j.1523-1755.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 97.Reinders MEJ, Rabelink TJ, Briscoe DM. Angiogenesis and Endothelial Cell Repair in Renal Disease and Allograft Rejection. Journal of the American Society of Nephrology. 2006;17:932–942. doi: 10.1681/ASN.2005121250. [DOI] [PubMed] [Google Scholar]
- 98.Chade AR, Zhu X-Y, Krier JD, Jordan KL, Textor SC, Grande JP, Lerman A, Lerman LO. Endothelial Progenitor Cells Homing and Renal Repair in Experimental Renovascular Disease. STEM CELLS. 2010;28:1039–1047. doi: 10.1002/stem.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Patschan D, Patschan S, Gobe GG, Chintala S, Goligorsky MS. Uric Acid Heralds Ischemic Tissue Injury to Mobilize Endothelial Progenitor Cells. Journal of the American Society of Nephrology. 2007;18:1516–1524. doi: 10.1681/ASN.2006070759. [DOI] [PubMed] [Google Scholar]
- 100.Patschan D, Krupincza K, Patschan S, Zhang Z, Hamby C, Goligorsky MS. Dynamics of mobilzation and homing of endothelial progenitor cells after acute renal ischemia: modulation by ischemic preconditioning. Am J Physiol. 2006;291:F176–F185. doi: 10.1152/ajprenal.00454.2005. [DOI] [PubMed] [Google Scholar]
- 101.Patschan D, Patschan S, Wessels JT, Becker JU, David S, Henze E, Goligorsky MS, Müller GA. Epac-1 activator 8-O-cAMP augments renoprotective effects of allogeneic murine EPCs in acute ischemic kidney injury. American Journal of Physiology - Renal Physiology. 2010;298:F78–F85. doi: 10.1152/ajprenal.00485.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Patschan D, Patschan S, Muller GA. Endothelial Progenitor Cells in Acute Ischemic Kidney Injury: Strategies for Increasing the Cells' Renoprotective Competence. International Journal of Nephrology. 2011:2011. doi: 10.4061/2011/828369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Burger D, Gutsol A, Carter A, Allan DS, Touyz RM, Burns KD. Human cord blood CD133+ cells exacerbate ischemic acute kidney injury in mice. Nephrology Dialysis Transplantation. 2012 doi: 10.1093/ndt/gfs110. [DOI] [PubMed] [Google Scholar]
- 104.Li B, Cohen A, Hudson TE, Motlagh D, Amrani DL, Duffield JS. Mobilized Human Hematopoietic Stem/Progenitor Cells Promote Kidney Repair After Ischemia/Reperfusion Injury. Circulation. 2010;121:2211–2220. doi: 10.1161/CIRCULATIONAHA.109.928796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kwon O, Miller S, Li N, Khan A, Kadry Z, Uemura T. Bone Marrow-derived Endothelial Progenitor Cells and Endothelial Cells May Contribute to Endothelial Repair in the Kidney Immediately After Ischemia-Reperfusion. Journal of Histochemistry and Cytochemistry. 2010;58:687–694. doi: 10.1369/jhc.2010.956011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goligorsky MS, Kuo M-C, Patschan D, Verhaar MC. Review article: Endothelial progenitor cells in renal disease. Nephrology. 2009;14:291–297. doi: 10.1111/j.1440-1797.2009.01112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hirschi KK, Ingram DA, Yoder MC. Assessing Identity, Phenotype, and Fate of Endothelial Progenitor Cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783–2786. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 109.Schwarz TM, Leicht SF, Radic T, Rodriguez-Arabaolaza I, Hermann PC, Berger F, Saif J, Böcker W, Ellwart JW, Aicher A, Heeschen C. Vascular Incorporation of Endothelial Colony-Forming Cells Is Essential for Functional Recovery of Murine Ischemic Tissue Following Cell Therapy. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32:e13–e21. doi: 10.1161/ATVBAHA.111.239822. [DOI] [PubMed] [Google Scholar]
- 110.Yamamoto T, Tada T, Brodsky SV, Tanaka H, Noiri E, Kajiya F, Goligorsky MS. Intravital videomicroscopy of peritubular capillaries in renal ischemia. American Journal of Physiology - Renal Fluid & Electrolyte Physiology. 2002;282:F1150–1155. doi: 10.1152/ajprenal.00310.2001. [DOI] [PubMed] [Google Scholar]
- 111.Brodsky SV, Yamamoto T, Tada T, Kim B, Chen J, Kajiya F, Goligorsky MS. Endothelial dysfunction in ischemic acute renal failure: rescue by transplanted endothelial cells. American Journal of Physiology - Renal Fluid & Electrolyte Physiology. 2002;282:F1140–1149. doi: 10.1152/ajprenal.00329.2001. [DOI] [PubMed] [Google Scholar]
- 112.Burger D, Viñas JL, Akbari S, Dehak H, Knoll W, Gutsol A, Carter A, Touyz RM, Allan DS, Burns KD. Human Endothelial Colony-Forming Cells Protect against Acute Kidney Injury. The American Journal of Pathology. 2015;185:2309–2323. doi: 10.1016/j.ajpath.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 113.Pang P, Abbott M, Chang SL, Abdi M, Chauhan N, Mistri M, Ghofrani J, Fucci Q-A, Walker C, Leonardi C, Grady S, Halim A, Hoffman R, Lu T, Cao H, Tullius SG, Malek S, Kumar S, Steele G, Kibel A, Freedman BS, Waikar SS, Siedlecki AM. Human vascular progenitor cells derived from renal arteries are endothelial-like and assist in the repair of injured renal capillary networks. Kidney International. 2017;91:129–143. doi: 10.1016/j.kint.2016.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Patschan D, Schwarze K, Tampe B, Zeisberg M, Patschan S, Müller GA. Endothelial Colony Forming Cells (ECFCs) in murine AKI - implications for future cell-based therapies. BMC Nephrology. 2017;18:53. doi: 10.1186/s12882-017-0471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cantaluppi V, Gatti S, Medica D, Figliolini F, Bruno S, Deregibus MC, Sordi A, Biancone L, Tetta C, Camussi G. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012;82:412–427. doi: 10.1038/ki.2012.105. [DOI] [PubMed] [Google Scholar]
- 116.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney International. 78:838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 117.Yoon C-H, Hur J, Park K-W, Kim J-H, Lee C-S, Oh I-Y, Kim T-Y, Cho H-J, Kang H-J, Chae I-H, Yang H-K, Oh B-H, Park Y-B, Kim H-S. Synergistic Neovascularization by Mixed Transplantation of Early Endothelial Progenitor Cells and Late Outgrowth Endothelial Cells. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 118.Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO. Endothelial Progenitor Cells Restore Renal Function in Chronic Experimental Renovascular Disease. Circulation. 2009;119:547–557. doi: 10.1161/CIRCULATIONAHA.108.788653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou L, Xu L, Shen J, Song Q, Wu R, Ge Y, Xin H, Zhu J, Wu J, Jia R. Preischemic Administration of Nonexpanded Adipose Stromal Vascular Fraction Attenuates Acute Renal Ischemia/Reperfusion Injury and Fibrosis. Stem Cells Translational Medicine. 2016 doi: 10.5966/sctm.2015-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Collett J, Mehrotra P, Crone A, Merfeld-Clauss S, March KL, Traktuev D, Basile D. Human adipose stromal cells ameliorate ischemia-reperfusion renal injury by reducing local inflammation and attenuating capillary rarefaction. Journal of Cellular and Molecular Medicine, In press. 2017 doi: 10.1111/jcmm.13071. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Patschan D, Hildebrandt A, Rinneburger J, Wessels JT, Patschan S, Becker JU, Henze E, Kruger A, Muller GA. The hormone melatonin stimulates renoprotective effects of "early outgrowth" endothelial progenitor cells in acute ischemic kidney injury. Am J Physiol Renal Physiol. 2012;302:F1305–1312. doi: 10.1152/ajprenal.00445.2011. [DOI] [PubMed] [Google Scholar]
- 122.Patschan D, Rinneburger J, Idrizi N, Backhaus R, Schwarze K, Henze E, Patschan S, Müller GA. Angiopoietin-1 treated early endothelial outgrowth cells (eEOCs) are activated in vitro and reduce renal damage in murine acute ischemic kidney injury (iAKI) BMC Nephrology. 2013;14:227–227. doi: 10.1186/1471-2369-14-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Patschan D, Schwarze K, Lange A, Meise N, Henze E, Becker JU, Patschan S, Muller GA. Bone morphogenetic protein-5 and early endothelial outgrowth cells (eEOCs) in acute ischemic kidney injury (AKI) and 5/6-chronic kidney disease. Am J Physiol Renal Physiol. 2013;305:F314–322. doi: 10.1152/ajprenal.00677.2012. [DOI] [PubMed] [Google Scholar]
- 124.Patschan D, Schwarze K, Henze E, Patschan S, Muller GA. Endothelial autophagy and Endothelial-to-Mesenchymal Transition (EndoMT) in eEPC treatment of ischemic AKI. Journal of nephrology. 2015 doi: 10.1007/s40620-015-0222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liang CJ, Shen WC, Chang FB, Wu VC, Wang SH, Young GH, Tsai JS, Tseng YC, Peng YS, Chen YL. Endothelial Progenitor Cells Derived From Wharton’s Jelly of Human Umbilical Cord Attenuate Ischemic Acute Kidney Injury by Increasing Vascularization and Decreasing Apoptosis, Inflammation, and Fibrosis. Cell Transplant. 2015;24:1363–1377. doi: 10.3727/096368914X681720. [DOI] [PubMed] [Google Scholar]
- 126.Zullo JA, Nadel EP, Rabadi MM, Baskind MJ, Rajdev MA, Demaree CM, Vasko R, Chugh SS, Lamba R, Goligorsky MS, Ratliff BB. The Secretome of Hydrogel-Coembedded Endothelial Progenitor Cells and Mesenchymal Stem Cells Instructs Macrophage Polarization in Endotoxemia. Stem Cells Transl Med. 2015;4:852–861. doi: 10.5966/sctm.2014-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]