1. Introduction
Substance use disorders have been linked with the propensity to make maladaptive decisions, and individuals with addictions persist in harmful and even destructive behaviors despite negative consequences, often against their own desires to resist. Suboptimal choices can reflect problems in decision-making, which requires the integration of various neural functions (Rangel et al., 2008). Of particular interest is reward valuation, a process by which an individual computes and compares the values of alternatives in order to select the most advantageous option (Kable and Glimcher, 2009). Reward valuation is subject to modulation by various factors, such as the timing of reward receipt (Ainslie, 1992), the risk and uncertainty involved (Huettel et al., 2006), internal signals, including autonomic (Critchley et al., 2013) and affective responses (Phelps et al., 2014), environmental cues (Wilson et al., 2004), and social influences (Sanfey, 2007). These factors produce predictable choice biases in healthy individuals (Kahneman and Tversky, 1979), but subjective valuation and how it guides choice is disordered in addiction (Monterosso et al., 2012; Platt et al., 2010). The present review focuses on departures from normative choice behavior in individuals with addictions, and how these problems are linked to abnormalities in brain function.
The review begins with an overview of the neurobiology of reward-based decision-making, focusing on mesocorticolimbic and corticostriatal circuitry, and then presents a description of three general paradigms that are used in addiction research to assess decision-making. The rest is organized around specific modulators of reward value: temporal delay, uncertainty, and internal and external factors. Each section briefly presents certain relevant tasks, information regarding how addicts perform on these tasks, and the brain structures and neural circuitry involved. Although there is a wealth of preclinical literature on this topic, the present review is limited to human neuroimaging research on decision-making. Finally, the implications and potential future directions of these areas of exploration are discussed.
2. Neurobiology of decision-making
Value-based decision-making involves a distributed network of cortical and subcortical areas. Abnormalities in brain structure and neural circuitry related to performance of addicted individuals on choice tasks have been identified. The focus has been on frontolimbic systems, specifically mesocorticolimbic and corticostriatal circuitry (Grant et al., 2000; London et al., 2015).
2.1 Reward processing
Behaviors that increase evolutionary fitness tend to be repeated, and therefore function as rewards (Hyman, 2005). Because natural rewards and drugs of abuse act on the reward circuitry of the brain in similar ways (Nesse and Berridge, 1997), drugs can exert powerful effects that bias behavior. Central to this action is the mesolimbic dopamine system, which has long been implicated in reward processing (Montague et al., 1996; O’Doherty et al., 2003; Pessiglione et al., 2006; Schultz et al., 1997) and plays a crucial role in habitual and goal-directed behaviors (Balleine et al., 2009). Within this system, midbrain dopamine neurons project to the ventral striatum, other limbic regions, such as the amygdala and hippocampus, and the prefrontal cortex (PFC) (Haber, 2003). All drugs of abuse, whether directly or indirectly, increase synaptic dopamine in the ventral striatum, and modulation of dopaminergic signaling in the mesocorticolimbic pathway is likely a central mechanism by which decision-making is biased in addiction (Dayan, 2009; London, 2016).
2.2 Reward valuation
Economists have long reasoned that, in order for different options to be compared, their values must be represented on a common scale. These subjective values should be encoded in the brain, and functional magnetic resonance imaging (fMRI) has identified brain regions where activation, indicated by the blood-oxygen level dependent (BOLD) signal, scales with subjective or objective reward values. The brain regions most consistently activated during the encoding of value are the ventral striatum and medial PFC, including the orbitofrontal cortex (OFC) (Bartra et al., 2013; Levy and Glimcher, 2012; Montague and Berns, 2002). The ventral striatum projects to the medial PFC, and activity in both regions scales with the magnitude and probability of expected rewards (Hare et al., 2008; Peters and Buchel, 2009). The OFC, densely connected with the basolateral amygdala and nucleus accumbens, is implicated in addiction through its role in evaluation of economic value, associative learning, and habit formation (London et al., 2000; Padoa-Schioppa and Assad, 2006; Schoenbaum et al., 2006).
2.3 Reward-based choice
Including these areas, a distributed network of cortical and subcortical regions contributes to reward-based decision-making (Figure 1). Reward valuation in the ventral striatum and medial PFC is modulated by uncertainty, which involves processing in the anterior cingulate cortex (ACC) and insula (Krain et al., 2006; Platt and Huettel, 2008). The ACC and insula share bidirectional connections (Reynolds and Zahm, 2005) and are implicated in a variety of functions related to decision-making, such as risk and error awareness (Magno et al., 2006; Preuschoff et al., 2008), performance monitoring and model updating (Kolling et al., 2016; Shenhav et al., 2013), and, through connections with the ventromedial PFC, the integration of visceral and affective information into choice (Medford and Critchley, 2010; Singer et al., 2009). Cognitive control relies on the dorsolateral PFC (Duncan and Owen, 2000; Miller and Cohen, 2001), which is crucial for the maintenance of goal values (Dixon and Christoff, 2014). The influence of the dorsolateral PFC on valuation is especially relevant for addictions because of its hypothesized role in self-control (Hare et al., 2009).
Figure 1. Reward-based decision-making.
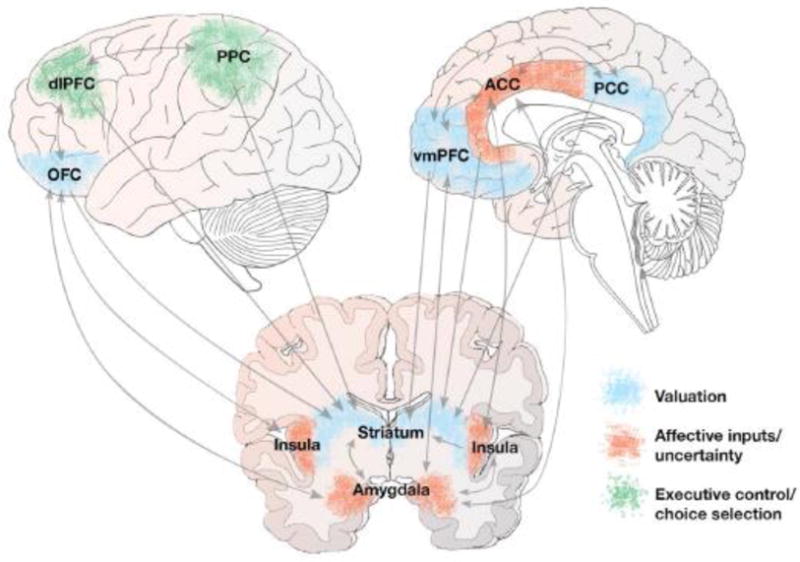
Decision-making relies on a converging network of cortical and subcortical regions. For simplicity, arrows are not drawn to indicate laterality of connections. Blue areas: reward valuation seems to primarily involve the ventral striatum, ventromedial prefrontal cortex (vmPFC), orbitofrontal cortex (OFC), and posterior cingulate cortex (PCC). Red areas: the amygdala, insula, and anterior cingulate cortex (ACC) are involved in processing uncertainty and emotional inputs into choice. Green areas: the dorsolateral cortex (dlPFC) and posterior parietal cortex (PPC) are involved in executive control and choice selection.
Much of this frontoparietal circuitry operates inefficiently in addiction (Goldstein and Volkow, 2011; London et al., 2015), and research participants with drug use disorders exhibit structural differences in this circuitry compared to healthy control subjects (Morales et al., 2015; Smith et al., 2015; Thompson et al., 2004). The present review focuses on the neural circuitry of reward valuation; brain function related to choice selection and learning is not covered, although both have implications for addiction. Very generally, acting upon the appropriate choice is thought to involve the lateral PFC and areas of the parietal cortex (Kable and Glimcher, 2009; Rangel et al., 2008), and the updating of values through learning, such as through prediction errors (Schultz, 2010), relies on dopaminergic function in the ventral striatum and midbrain (Diederen et al., 2017).
3. Decision-Making Paradigms in Addiction
Choice tasks relevant to addictions can be broadly grouped into three categories: 1) those that present direct choices for real drug, such as self-administration paradigms, 2) those that present choices for hypothetical drug rewards or drug-related cues, 3) those that test behaviors considered intricately linked to the risk of developing and/or maintaining addictions, including risk-taking and decision-making in the face of uncertainty. The present review briefly covers these three general paradigms and then continues with a consideration of modulators of value.
3.1 Drug choice
Drug choice procedures use self-administration in the laboratory to present competition between actual drug and alternative reinforcers, such as money or food (Moeller and Stoops, 2015). Notably, the choice to self-administer a substance is modulated by complex interactions (Jones and Comer, 2013), especially motivational contexts such as craving (Risinger et al., 2005), which should be considered. Drug choice procedures have been paired with neuroimaging to investigate the neural factors that influence drug choice, and have been used to evaluate a potential dopaminergic deficit.
While a deficit in striatal D2-type dopamine receptor availability, observed using positron emission tomography (PET), is a general finding across substance use disorders (Trifilieff and Martinez, 2014), striatal D2-type dopamine receptor availability is not associated with heightened cocaine choice over monetary reward in the laboratory (Martinez et al., 2004). However, in cocaine-dependent participants, amphetamine-induced dopamine release does show a negative relationship with the preference to self-administer cocaine rather than monetary reward (Martinez et al., 2007). Thus, the choice to self-administer cocaine is apparently associated with phasic striatal dopamine release. Although no difference in striatal D1 receptor availability has been observed between cocaine users and controls, D1 receptor availability in the ventral striatum is negatively associated with the choice to self-administer cocaine (Martinez et al., 2009). Compared with healthy controls, heroin users also display lower striatal D2-type receptor availability and less dopamine release, measured after methylphenidate administration, although neither is predictive of choice to self-administer heroin (Martinez et al., 2012). These results, which point to a dopaminergic deficit related to drug choice, link low dopamine release with the preference to self-administer cocaine.
3.2 Drug-related choice
Similar paradigms that use virtual rewards or drug-related stimuli can be highly informative, especially because drug-related cues can intensify decision-making deficits (Wang et al., 2012). These paradigms offer an advantage over actual drug procedures, which are ethically not possible when participants are in treatment or long-term abstinence. For example, in the drug-picture procedure, participants choose between viewing cocaine-related or affectively positive, negative, and neutral pictures (Moeller et al., 2009). Choice to view drug-related pictures is related to both current and future drug use, especially when outcomes are probabilistic rather than certain (Moeller et al., 2013a). A role of dopamine in this paradigm is inferred from the finding that cocaine users who are carriers of the 9R-allele of the dopamine transporter gene (DAT1) are more reactive to drug-related reinforcement, measured by event-related potentials, self-reported valence and arousal, simulated cocaine choice, and fMRI during exposure to cocaine-related and unrelated stimuli (Moeller et al., 2013b). Compared with the 10R-allele, the 9R-allele is related to greater expression of the dopamine transporter in the striatum (van Dyck et al., 2005), presumably leading to a shorter half-life of extracellular dopamine, and less activity at presynaptic dopamine D2-type receptors (autoreceptors) that inhibit phasic dopamine release (Ford, 2014). Greater phasic dopamine release in the striatum may explain why 9R-allele carriers show heightened cue-reactivity to drug-related reinforcement.
The authors are aware of few other paradigms that use hypothetical drug rewards as alternatives in choice tasks. Some studies using delay discounting tasks, which are described below, also have used hypothetical drug rewards as options (Bickel et al., 2011a; Coffey et al., 2003; Wesley et al., 2014). The main results point to steeper discounting of hypothetical drug rewards than monetary rewards, as would be expected.
3.3 Decision tasks
Other tasks that do not directly involve choices for drug or drug-related stimuli investigate components of decision-making that contribute to the development and maintenance of addictive disorders. These include tasks that assess risk-taking and advantageous decision-making. The following sections consider these types of choice tasks, and how varying the costs of rewards affects the computation of value, and therefore choice.
4. Intertemporal choice
Traditional economic models are based on underlying assumptions of “rationality,” according to which behavior meets a minimum set of requirements to be stable over time (Arrow, 1986). However, consistent behavioral “irrationalities” that deviate from normative economic models are observed in the laboratory (Camerer, 2014) and can have profound implications for our understanding of addiction (Monterosso et al., 2012; Platt et al., 2010). Notably, the phenomenon of delay discounting has been extensively studied, especially in light of the relatively recent merging of neuroscience with behavioral economics (Glimcher and Fehr, 2014).
4.1 Delay discounting
The delay to receipt is a consistent modulator of reward value (Loewenstein and Elster, 1992). Options received sooner in time are naturally valued more than those that are delayed, and the extent of such “delay discounting” differs among individuals (Ainslie, 1992). Contrary to predictions from traditional exponential discounting models (Samuelson, 1937), people tend to overvalue immediate rewards (Kahneman & Tversky, 1979) and reverse their preferences depending on the specific temporal dynamics (Thaler, 1981). Asked to choose between $15 immediately or $16 tomorrow, most people would choose $15; asked to choose between $15 in 99 days or $16 in 100 days, most would change their answer to the later option (Ainslie, 1975; Laibson, 1997). This time inconsistency may arise from competition between two distinct decision-making systems, an “impulsive” limbic and “executive” prefrontal system (Bechara, 2005; Bickel et al., 2007), although certain evidence points to a unified system (Hare et al., 2009; Kable and Glimcher, 2007; Luo et al., 2012; Weber and Huettel, 2008), in which inputs that differentially contribute to valuation feed into a final estimate of value (Kable and Glimcher, 2009; Monterosso and Luo, 2010). Preference reversals are especially relevant for addictions, which feature the breaking of resolutions to abstain from addictive behaviors (Monterosso and Ainslie, 2007).
4.2 Delay discounting in addictions
Discounting is exaggerated in individuals with addictions, whether to alcohol, drugs, food, or gambling (Bickel et al., 2014). The ventral striatum and frontoparietal regions, specifically the medial PFC and posterior cingulate cortex, have been consistently implicated in intertemporal choice (Kable and Glimcher, 2007; McClure et al., 2004). Notably, brain function differs between drug users and healthy controls during delay discounting tasks (Table 1). In sober alcoholics, discounting correlates negatively with activity in the lateral OFC and positively with activity in the dorsal PFC and posterior parietal cortex (Boettiger et al., 2007). Alcohol use severity, however, correlates with steepness of discounting and activity in the supplementary motor cortex, insula, OFC, inferior frontal gyrus, and precuneus (Claus et al., 2011). Methamphetamine users exhibit less activity than control subjects in the precuneus, right caudate, ACC, and dorsolateral PFC during decision-making on a delay discounting task, and exhibit a positive correlation between discounting and activity in the dorsolateral PFC, posterior parietal cortex, posterior cingulate cortex, and amygdala (Hoffman et al., 2008). Control subjects show significantly greater activation in the left dorsolateral PFC and right intraparietal sulcus on hard trials than on easy trials (i.e. large versus small differences in subjective value of alternatives), but methamphetamine users show as much activation on easy trials as on hard trials, suggesting that cortical processing related to intertemporal choice may be less efficient in methamphetamine users than controls (Monterosso et al., 2007). Activity in the dorsolateral PFC may thus be crucial for mediating decisions between more difficult alternatives, and may be impaired in individuals with addictions. Indeed, in what can be assumed to be a difficult choice, cocaine users exhibit greater activity in the dorsolateral PFC when choosing future monetary reward over immediate cocaine (Wesley et al., 2014).
Table 1.
Neuroimaging of Intertemporal Choice in Individuals with Drug Use Disorders
| Reference | Imaging Method | Participants | Findings |
|---|---|---|---|
| Monterosso et al., 2007 | fMRI | 12 Methamphetamine users, 17 controls | vlPFC, dlPFC, dorsal ACC, intraparietal sulcus activation was greater for hard vs. easy choices, across groups. dlPFC and right intraparietal sulcus activation was lower during easy vs. hard trials for controls, but not in methamphetamine users. |
| Boettiger et al., 2007 | fMRI | 9 Sober alcoholics, 10 controls | Immediate reward bias was correlated with activation in PPC, dorsal PFC, and rostral parahippocampal gyrus. Delayed reward preference was correlated with lateral OFC activation. Gene score predicted behavior and dorsal PFC and PPC activation. |
| Hoffman et al., 2008 | fMRI | 19 abstinent Methamphetamine users, 17 controls | Discounting was correlated with activation in amygdala, dlPFC, PCC, PPC, across groups. Bilateral pre-cuneus, right caudate, right ACC, right dlPFC activity was lower in methamphetamine users vs. controls. Hard choices were related to activation in bilateral middle cingulate, PPC, right rostral insula, across groups. |
| Claus et al., 2011 | fMRI | 93 Non-treatment-seeking and 67 treatment-seeking alcohol drinkers with a range of alcohol use (socia drinking to severe alcohol dependence) | Alcohol use severity was positively correlated with discounting of delayed rewards and activation in supplementary motor area, insula, OFC, inferior frontal gyrus, precuneus. |
| Wesley et al., 2014 | fMRI | 25 Non-treatment-seeking cocaine users, 25 controls | Cocaine users had more activity in the dlPFC when choosing future monetary reward over immediate cocaine reward, compared to controls. Cocaine users had more activity in the dorsal striatum when choosing immediate money over future cocaine, compared to controls. |
| Ballard et al., 2015 | [18F]fallypride PET | 27 Methamphetamine users, 27 controls | Striatal D2-type binding potential was lower in methamphetamine vs. controls. Striatal binding potential was negatively correlated to discount rate in methamphetamine users. |
All studies used delay discounting tasks, and all used hypothetical money except Wesley et al., 2014.
Brain regions: ACC: anterior cingulate cortex; dlPFC: dorsolateral PFC; OFC: orbitofrontal cortex; PCC: posterior cingulate cortex; PFC: prefrontal cortex; PPC: posterior parietal cortex; vlPFC: ventrolateral PFC.
Differences in delay discounting are also related to indices of dopaminergic function. Both discounting behavior and dorsal PFC and posterior parietal cortex activation during task performance can be predicted by variation in the Val158Met polymorphism of the catechol-O-methyltransferase gene, which influences dopamine metabolism (Boettiger et al., 2007). In addition, lower striatal D2-type receptor availability, measured with PET, is related to steeper discounting in methamphetamine users (Ballard et al., 2015) and pathological gamblers (Joutsa et al., 2015). These findings demonstrate that, in controls, greater dopamine metabolism by COMT, which should reduce dopamine levels primarily in the PFC (Bilder et al., 2004), and low striatal D2-type receptor availability in methamphetamine users contribute to steeper delay discounting. Thus, optimal dopamine function is likely necessary for maintaining value in the face of delay cost.
4.3 Vulnerability to addiction and therapeutic outcome
That delay discounting is a common feature across addictions suggests that it represents an intrinsic feature of addiction and may be a tractable therapeutic target. A natural question that arises is whether steep discounting precedes or results from addiction. Chronic drug use likely affects the rate of discounting (i.e., Yi et al., 2008), but converging evidence indicates that individual differences in delay discounting also predict subsequent drug use (i.e., Sheffer et al., 2014). Notably, various behavioral approaches (Koffarnus et al., 2013), including working memory training (Bickel et al., 2011b), visualization of near future episodic events (Peters and Buchel, 2010), and orientation to the future by forward planning (Steinberg et al., 2009), reduce preference for immediate rewards. It would be important to continue investigating the extent to which reduction of delay discounting by such methods can alter therapeutic outcome.
5. Choice under uncertainty
Because choices in daily life rarely contain complete information about potential costs and benefits, most choices involve a balance between risk and reward. This balance is skewed in addicts, who engage in risky behaviors related to drug-taking and make disadvantageous choices under uncertainty in the laboratory (Gowin et al., 2013). A “risky” decision typically connotes one that involves danger or a high probability of negative outcome, but this definition of risk may differ from those used in the laboratory (Schonberg et al., 2011). Economists define a choice containing risk as one between options with different distributions of known outcomes, and laboratory tasks can distinguish between uncertainty due to risk, with known outcome probabilities, from uncertainty due to ambiguity, with unknown outcome probabilities. Decisions under uncertainty are especially relevant because they go against decision theory, which states that knowledge of probabilities should not change stated preferences (Ellsberg, 1961; Platt and Huettel, 2008). These decisions are also notoriously aversive: people make choices that go against their own benefit just to reduce uncertainty (Camerer and Weber, 1992).
This section provides an overview of brain function relevant to decision-making under risk and ambiguity in individuals with drug use disorders (Table 2). Decision-making under ambiguity is discussed by focusing on two of the most commonly used uncertainty tasks: the Iowa Gambling Task (IGT) (Bechara et al., 1994) and the Balloon Analog Risk Task (BART) (Lejuez et al., 2002). Tasks that present clear outcome contingencies are then reviewed. These typically take the form of probabilistic gambling tasks and assess uncertainty due purely to risk. Finally, studies that directly compare uncertainty under conditions of risk and ambiguity are discussed.
Table 2.
Neuroimaging of Decision-Making under Uncertainty in Individuals with Drug Use Disorders
| Reference | Imaging Method | Task | Participants | Findings |
|---|---|---|---|---|
| Bolla et al., 2003 | H215O PET | IGT | 13 Abstinent cocaine users,10 controls | Cocaine users chose risky options more often and earned less money than controls. Cocaine users had greater activity in the putamen and OFC, but less activity in the dlPFC and medial PFC, compared to controls. |
| Bolla et al., 2005 | H215O PET | IGT | 11 Abstinent marijuana users (heavy, moderate use), 11 controls | Abstinent marijuana users had less activation in the lateral OFC and dlPFC compared to controls. Brain activation and task performance of moderate marijuana users was more similar to controls than to heavy marijuana users. |
| Fein et al., 2006 | MRI | IGT | 43 Abstinent alcohols, 58 controls | Abstinent alcoholics with impairments on the task had smaller amygdala volume than controls. |
| Wesley et al., 2011 | fMRI | IGT | 16 Marijuana users, 16 controls | Marijuana users had weaker responses to losses in the ACC and medial frontal cortex, compared to controls. Marijuana users lacked a correlation between task performance and activity in the ACC, vmPFC, and rostral PFC that was demonstrated in controls. |
| Cousijn et al., 2012 | fMRI | IGT | 32 Cannabis users, 41 controls | Marijuana users had greater responses to wins vs. losses in the right OFC, superior temporal gyrus, and insula, compared to controls. Superior temporal gyrus activity correlated with higher marijuana use in the 6 months following testing. |
| Vaidya et al., 2012 | H215O PET | IGT (standard + variant with focus on punishment) | 46 Marijuana users, 34 controls | Marijuana users had worse performance than controls on the punishment variant of the IGT. Marijuana users had stronger activation than controls in the vmPFC during the standard IGT. vmPFC activation positively correlated with duration of marijuana use. |
| Crowley et al., 2010 | fMRI | BART | 20 Abstinent adolescent males at risk for substance use, 20 controls | Adolescents at risk for substance abuse had lower amygdala, insula, and ACC activation compared to controls. |
| Kohno et al., 2014 | fMRI | BART | 25 Methamphetamine users, 27 controls | Compared to controls, methamphetamine users had greater modulation of ventral striatal activation, but less modulation of dlPFC activation, by risk and reward. Methamphetamine users had greater RSFC of midbrain with the putamen, amygdala, and hippocampus, and RSFC was inversely related to dlPFC sensitivity to risk. |
| Kohno et al., 2016 | [18F]fallypride PET and fMRI | BART | 19 Methamphetamine users, 26 controls | Negative relationship between ventral striatal binding potential and RSFC of midbrain with striatum, OFC, and insula in methamphetamine users, but positive relationship in controls. Positive relationship between midbrain RSFC to ventral striatum and cognitive impulsivity in methamphetamine users, but negative relationship in controls. |
| Ersehe et al., 2005 | H215O PET | Cambridge Risk Task | 15 Chronic amphetamine, 15 chronic opiate, 15 former opiate and amphetamine users, 15 controls | Lower ACC activity was related to greater risk propensity in all drug user groups. Activation was greater in left PFC, but lower in right dlPFC of drug users vs. controls |
| Ersehe et al., 2006 | H215O PET | Cambridge Risk Task | 9 Methadone-maintained opiate users, 6 heroin users, 5 controls | Activity in the ACC and insula was related to propensity to avoid risk following loss in controls, but not in opiate users. Compared to controls, opiate users had abnormal patterns of OFC activity that was associated with risk preferences. |
| Fishbein et al., 2005a | H215O PET | Rogers Decision-Making Task | 13 Abstinent drug users, 14 controls | ACC activity was lower in drug users vs. controls. Lower ACC activity was related to greater risk propensity. |
| Bjork et al., 2008 | fMRI | Monetary game of Chicken | 17 Alcohol and cocaine users, 17 controls | ACC activity on trials that included risk was greater for controls than for drug users. Posterior mesofrontal cortical activity was lower in drug users vs. controls. |
| Gowin et al., 2014b | fMRI | Risky Gains Task | 68 Methamphetamine users, 40 controls | Methamphetamine users had lower insula activity than controls across all trials. Mid-insula activity was greater during risky vs. safe decisions in methamphetamine users. Methamphetamine users had less ACC activity following losses than controls, and were more likely to make risky decisions following losses. |
| Reske et al., 2015 | fMRI | Risky Gains Task | 158 Young adult occasional stimulant and marijuana users, 50 controls | Substance users had less activity in the ACC, PFC, insula, and dorsal striatum than controls, and attenuation in ACC activity was inversely related to past drug use. Less neural differentiation between safe and risky trials was exhibited by drug users and was driven by a lack of deactivation in the right dorsal striatum and PFC areas during safe decisions. |
Brain regions: ACC: anterior cingulate cortex; dlPFC: dorsolateral PFC; OFC: orbitofrontal cortex; PPC: posterior parietal cortex; PFC: prefrontal cortex; vmPFC: ventromedial PFC; vlPFC: ventrolateral PFC.
Measures: RSFC: resting-state functional connectivity
5.1 Decision-making under ambiguity
Both the IGT and the BART incorporate elements of reward, punishment, learning, and adaptive risk-taking. On the IGT, participants progress through trials by picking cards from four different decks. Healthy participants typically learn to identify decks that deliver small immediate gains and small losses as leading to higher average gain, and alter their behavior to sample more from the advantageous decks. The IGT is thought to measure choice under ambiguity at the start of the task when stimulus-outcome contingencies are still being learned, but to involve risk alone after the contingencies have been learned (Brand et al., 2007). The ventromedial PFC is especially implicated in this sort of adaptive decision-making, as people with ventromedial PFC lesions show impairment on the task (Bechara et al., 1994), as do many substance users (Bechara et al., 2001) and at-risk populations (Ernst et al., 2002).
Findings from studies of region cerebral blood flow, measured using PET and 15O-water, suggest that in drug users performing the IGT, dysregulated striatal and ventromedial PFC/OFC activity is likely related to reward anticipation/valuation, while lower dorsolateral PFC activity contributes to dysregulated executive control inputs into reward valuation. In healthy control subjects, decision-making accompanies activation in the OFC, dorsolateral PFC, ACC, insula, inferior parietal cortex, and thalamus predominantly in the right hemisphere, and the cerebellum predominantly in the left (Ernst et al., 2002). Marijuana users perform worse than controls on a variant of the IGT that focuses on punishment, and exhibit stronger activation than controls in the ventromedial PFC during the standard IGT (Vaidya et al., 2012). Cocaine users also exhibit performance below control levels and greater activity than controls in the putamen and OFC, but less activity in the dorsolateral and medial PFC, during choice (Bolla et al., 2003). A similar pattern has been observed in abstinent marijuana users, who exhibit less activation in the lateral OFC and dorsolateral PFC compared to control subjects (Bolla et al., 2005).
Certain fMRI studies have investigated brain activity in response to wins and losses on the IGT and have the potential to elucidate differences in approach and avoidance behavior related to rewards (Melrose et al., 2015). Marijuana users may be less sensitive to negative feedback, as they exhibit weaker responses to losses in the ACC and medial frontal cortex compared to controls, and do not show a correlation between task performance and activity in the ACC, ventromedial PFC, and rostral PFC, as is demonstrated in controls (Wesley et al., 2011). In response to wins, marijuana users also respond more strongly in the right OFC, superior temporal gyrus, and insula than controls, and superior temporal gyrus activity is correlated with higher marijuana use in the 6 months following testing (Cousijn et al., 2012). These observations of dysregulated responses to outcomes suggest that addicts may have increased approach and decreased avoidance behaviors that could translate to enhanced reward-seeking and reduced sensitivity to negative outcomes.
Similar deficits are seen in the brain function of drug users performing the BART, in which the participant makes a series of choices either to pump a virtual balloon to increase monetary reward or to stop pumping and “cash out”, retaining the earnings from the trial. If the balloon pops, the rewards accrued on the trial are lost. Risk, defined as probability of explosion, increases with each pump, and participants effectively set the level of risk as they pump. In this way, risk preferences are determined in a naturalistic setting, which is enhanced by the visceral states surrounding the repeated pumping of the balloon and threat of explosion (Schonberg et al., 2011). Risk on the BART differs from that on the IGT, in which risk is defined by stimulus-outcome contingencies. The task has shown evidence of ecological validity in some studies: the average number of pumps correlates with self-reports of substance use, drinking, and smoking (Lejuez et al., 2003a; Lejuez et al., 2003b), although in some cases, substance users take less risk on the BART than controls (Ashenhurst et al., 2014; Dean et al., 2011; Kohno et al., 2014).
When participants take risk on the BART during fMRI, risk level parametrically modulates activation in a set of mesolimbic-frontal regions, including the midbrain, striatum, anterior insula, dorsolateral PFC, and ACC/medial frontal cortex (Rao et al., 2008). Moreover, in healthy controls, striatal dopamine D2-type receptor binding potential is correlated positively with modulation of activity in the ventral striatum when participants decide to cash out (take reward), but negatively with modulation of dorsolateral PFC activation during pumping (risk-taking) (Kohno et al., 2013). Moreover, fractional anisotropy of the white-matter pathways connecting the PFC, insula, and midbrain to the striatum is positively correlated with risk-taking and task performance, and with parametric modulation of activation in the anterior insula, putamen, ACC, and right medial frontal gyrus by risk (Kohno et al., 2017). These results demonstrate the importance of optimal striatal dopaminergic function and efficient mesocorticolimbic circuitry in modulating striatal and prefrontal activity for advantageous decision-making on the BART.
The BART has also been used to investigate corticostriatal circuitry in methamphetamine users. Compared to control participants, methamphetamine users exhibit greater modulation of ventral striatal activation, but less modulation of dorsolateral PFC activation, by risk and reward (Kohno et al., 2014). They also exhibit stronger resting-state functional connectivity (RSFC) of the midbrain with the putamen, amygdala, and hippocampus, and midbrain connectivity is inversely related to dorsolateral PFC sensitivity to risk during the BART (Kohno et al., 2014). This enhanced connectivity of mesostriatal and mesolimbic pathways associated with diminished sensitivity of the dorsolateral PFC is not observed in control participants. However, modulation of dorsolateral PFC activation by risk is positively related to RSFC of the dorsolateral PFC with the striatum in control participants, a pattern that is not observed in methamphetamine users (Kohno et al., 2014). In contrast, RSFC of the midbrain with the striatum, OFC, and insula is negatively related to striatal dopamine D2-type receptor availability in methamphetamine users, a pattern opposite to that seen in control subjects (Kohno et al., 2016b). RSFC of the midbrain and ventral striatum also is positively related to cognitive impulsivity in methamphetamine users, but negatively related in control participants (Kohno et al., 2016b). Thus, mesostriatal and mesolimbic circuitry may function adaptively in control subjects, but maladaptively in methamphetamine users.
In sum, converging evidence from the IGT and BART suggests that impaired brain function in mesolimbic-frontal regions of drug users contributes to aberrant decision-making under uncertainty. IGT performance points to dysregulated reward sensitivity in the striatum and ventromedial PFC/OFC and hypofunction of the dorsolateral PFC, which is less active during choice, compared to activity in healthy controls (Bolla et al., 2003; Bolla et al., 2005; Vaidya et al., 2012). Findings obtained with the BART reveal hypersensitivity to risk and reward in the ventral striatum and hyposensitivity in the dorsolateral PFC of addicts; these sensitivities are associated with baseline function of mesocorticostriatal striatal circuitry, which functions adaptively in healthy controls and exhibits abnormalities in drug users (Kohno et al., 2014). The associations between striatal dopamine D2-type receptor availability, mesocorticostriatal circuitry, and measures of impulsivity (Kohno et al., 2016a; Kohno et al., 2016b; Lee et al., 2009) underscore the crucial role of dopamine D2-type receptor signaling in advantageous decision-making and the importance of dopamine functions in dysregulated mesocorticostriatal circuitry to influence choice under ambiguity.
5.2 Decision-making under risk, without ambiguity
Tasks in which outcome probabilities are known typically take the form of probabilistic gambling tasks, such as lottery choice tasks that present choices between options with different probabilities of gains and/or losses. As trials are independent, there is no opportunity for learning, and other confounding functions, such as cognitive flexibility, working memory, reinforcement, and loss and gain sensitivity (Clark et al., 2004; Schonberg et al., 2011), can be avoided. These types of procedures can be based on economic theory and decomposed into specific constructs, such as risk, which can be parametrically varied to isolate their influence on decision-making.
It can be beneficial to investigate the neurological markers of specific risk preferences for their role in the development and maintenance of addiction. In particular, the ACC and insula have been implicated in risk tendencies that depart from classical risk neutrality. That ACC error-likelihood and expected risk signals are seen in risk-averse, but not risk-tolerant individuals, suggests that people more tolerant to risk may be less sensitive to error predictors (Brown and Braver, 2007). Risk-takers may also be less motivated by safer options than risk-averters, as neural activity in frontal, medial temporal, and striatal areas is positively correlated with risk in risk-seekers, but with expected value in risk-averters (Goh et al., 2016). A role of the ACC and insula in non-normative decision-making is further supported by the tracking of activity in these regions with reward probabilities, and also the correlation of activity in these regions with specific “irrational” risk tendencies. Specifically, activity in the ACC and insula correlates with the nonlinear transformation of probabilities (Paulus and Frank, 2006), which refers to larger risk-seeking in situations with a low probability of success and risk-avoidance in situations with a high probability of success (Kahneman and Tversky, 1979).
Neural activity in the ACC and insula is associated with avoiding loss and risk (Magno et al., 2006; Paulus et al., 2003; Preuschoff et al., 2006), and activation in these regions during decision-making under risk in is impaired substance users. While playing a monetary game called “Chicken,” in which trials offer either guaranteed reward or conflict between increasing reward and risk of penalty, patients diagnosed with both alcohol and cocaine dependence exhibit less ACC activity than controls on trials that include risk (Bjork et al., 2008). Young adults who occasionally use stimulants, and who therefore may be at risk for future substance abuse, exhibit less activity in the ACC, PFC, insula, and dorsal striatum during a risky gambling task compared to control subjects, and the attenuation in ACC activity is inversely related to past drug use (Reske et al., 2015).
Activity in the ACC and insula may play a role in thwarting maladaptive risky behavior, and these signals may malfunction in substance users. Findings from a study of current and former users of both opiates and amphetamines show that lower ACC activity is related to greater risk propensity during performance of the Cambridge Risk Task (Ersche et al., 2005); this is true as well for abstinent polydrug users performing the Rogers Decision-Making Task (Fishbein et al., 2005a). During the Risky Gains Task, methamphetamine users exhibit less ACC activity and are more likely to make risky decisions following losses compared to controls (Gowin et al., 2014b). Activity in the ACC and insula is related to propensity to avoid risk following loss in healthy controls during the Cambridge Risk Task, but this relationship is absent in opiate users, who also show abnormal OFC activity associated with risk preferences (Ersche et al., 2006). These studies in drug users demonstrate potentially deficient signaling, especially in the ACC, to inhibit risk. Such signaling may be necessary to prevent disadvantageous behavior by biasing choices to minimize cost and maximize gain (Brown and Alexander, 2017).
Findings from longitudinal studies suggest that methamphetamine users have an impaired ability to discriminate between safe and risky decisions, which may reflect altered insula signaling. For example, methamphetamine users who later relapsed displayed lower activation in the bilateral striatum, bilateral insula, left inferior frontal gyrus, and left ACC in response to winning and negative feedback on a reinforcement learning task, compared to their non-relapsing counterparts (Stewart et al., 2014a). Methamphetamine users who remained abstinent 1 year after testing displayed lower insula activation during safe decisions compared to risky decisions on the Risky Gains Task, whereas users who relapsed displayed similar insula activation during both safe and risky choices (Gowin et al., 2014a).
5.3 Risk versus Ambiguity
Certain studies have directly compared the neural circuitry involved in decisions under ambiguity and risk. Preference for choices containing ambiguity can be predicted by lateral PFC activity (Huettel et al., 2006), and greater activity in the OFC and amygdala is exhibited during ambiguous decisions than decisions with only risk (Hsu et al., 2005). In contrast, activity in the posterior parietal cortex predicts preference for choices involving risk (Huettel et al., 2006), and activity in the dorsal striatum is higher for these choices than for ambiguous choice trials (Hsu et al., 2005). Whether ambiguous choices are just a more complicated version of risky choices, or whether these two forms of uncertainty reflect distinct processes, is still an open question. However, subjective value is correlated with activation in the striatum, medial PFC, posterior parietal cortex, and amygdala during ambiguous as well as during risk trials (although the trend in the posterior parietal cortex did not reach significance for risk trials) (Levy et al., 2010). These findings suggest that, at least at the level of value representation, both types of uncertainty are represented by a unified system.
There is evidence that substance users have dysregulated circuitry involved in mediating between uncertainty due to risk and ambiguity. The learning of reward contingencies necessary to transition from ambiguous to risky choice on the IGT is delayed in alcohol-dependent patients, and this effect may be due to an impairment in properly estimating the probability distributions of alternative choices (Kim et al., 2011). Marijuana users deprived of marijuana value uncertain rewards less than controls on the Reward Uncertainty Decision-Making Task, and this uncertainty aversion is positively correlated with marijuana use (Hefner et al., 2016). That methamphetamine users have also been shown to be risk averse on the BART (Kohno et al., 2014) implicates uncertainty aversion as a potential factor in decision-making abnormalities exhibited in addiction.
6. Internal influences on choice
Internal signals, including autonomic, affective, and self-reflective processes, can alter valuation and thereby influence choice. Autonomic responses exert reciprocal influences on decision-making; cognitive processes influence bodily states, which in turn alter cognitive processes and generate interoceptive signals that contribute to affective feeling states and decision-making (Critchley et al., 2013). These internal signals are necessary for self-monitoring and self-awareness, which are crucial for adaptive decision-making (Goldstein et al., 2009). As discussed below, the integration of autonomic and affective processes with cognitive factors to influence decision-making is disrupted in individuals with addictions (Table 3).
Table 3.
Neuroimaging of Decision-Making with Internal or External Influences in Individuals with Drug Use Disorders
| Reference | Imaging Method | Task | Participants | Findings |
|---|---|---|---|---|
| Forman et al., 2004 | fMRI | Go/NoGo | 13 Opiate users, 20 controls | Activity in the ACC and insula was related to error recognition and was weaker in opiate users vs. controls. |
| Hester et al., 2009 | fMRI | Go/NoGo | 16 Cannabis users, 16 controls | Activity in the ACC and insula was related to error recognition and was weaker in chronic cannabis users vs. controls. |
| Moeller et al., 2014 | fMRI | Probabilistic learning choice task, Stroop task | 33 Cocaine users, 20 controls | Compared to controls and cocaine users without impaired insight, cocaine users with difficulty self-monitoring had lower emotional awareness, lower error-induced activity in the rostral ACC during Stroop task, and lower gray matter volume within the rostral ACC. |
| Fukunaga et al., 2013 | fMRI | IGT with positively- and negatively-framed conditions | 22 Substance users, 25 controls | Substance users had worse performance than controls during negatively-framed IGT conditions, and performance was related to lower risk-aversion signals in the anterior insula. Better performance in the positively-framed condition was related to activity in the ACC and insula in both groups. Only controls had a correlation between advantageous decision-making and risk-related ACC activity across decisions. |
| May et al., 2013 | fMRI | Risky gains task with positive interoceptive component | 68 Recently abstinent methamphetamine users, 40 controls | Methamphetamine users had lower anterior insula, dorsal striatum, and thalamus activity than controls. Correlation between anterior insula activity and reaction time was positive in controls, but negative in methamphetamine users. |
| Stewart et al., 2014b | fMRI | Two-choice prediction task with aversive interceptive component | 20 Methamphetamine users, 22 controls | Methamphetamine users had lower posterior insula and ACC activity than controls during breathing load, regardless of error and reward rates. Methamphetamine users had higher trait anxiety and lower anterior insula and inferior frontal gyrus activation than controls across trials. |
| Gilman et al., 2016a | fMRI | Social exclusion task (cyberball) | 20 Marijuana users, 22 controls | Both groups had activity in the ventral ACC, PFC, and insula during social exclusion, but young adult marijuana users had lower insula signaling. |
| Gilman et al., 2016b | fMRI | Social influence decision-making task | 20 Young adult marijuana users, 20 users | Marijuana users had different patterns of striatal activity (in caudate and nucleus accumbens) compared to healthy controls. |
| Gilman et al., 2016c | fMRI | Peer influence perceptual decision-making task | 20 Young adult marijuana users, 23 users | Marijuana users took more time than controls to resist group choices, and reaction times were associated with greater frontal activation. Susceptibility to group influence was associated with caudate activity in both groups, but caudate activity was greater in marijuana users. |
Brain regions: ACC: anterior cingulate cortex; CPu: striatum; dlPFC: dorsolateral PFC; OFC: orbitofrontal cortex; PPC: posterior parietal cortex; PFC: prefrontal cortex; vmPFC: ventromedial PFC; vlPFC: ventrolateral PFC.
6.1 Bodily states
Physiological responses to emotional signals are necessary for adaptive choice (Damasio, 1996). They are likely integrated with cognitive information through the insula, ACC, amygdala, and somatosensory cortex (Medford and Critchley, 2010), and integration in all these areas relies on activity in the ventromedial PFC (Bechara et al., 1994). During decision-making, abstinent drug users perform below control levels on gambling and decision-making tasks, and have lower skin conductance and smaller heart rate responses while performing both tasks (Fishbein et al., 2005b).
Indeed, substance users have been characterized on the basis of physiological responses while performing the IGT. Some are unimpaired on the task, while others display deficits as severe as those of patients with ventromedial PFC lesions (Bechara et al., 2001). Of those with deficits, some seem insensitive to positive as well as negative outcomes, as they exhibit blunted anticipatory skin conductance responses to both reward and punishment (Bechara and Damasio, 2002); others seem hypersensitive to reward, as they perform normally with regard to punishment, but display heightened physiological responses to reward magnitude (Bechara et al., 2002). These results demonstrate that faulty physiological responses, associated with dysfunction in the ventromedial PFC, are related to aberrant decision-making by drug users.
Integration of visceral experiences also relies on amygdala function (Phelps, 2006). Abstinent alcoholics with diminished IGT performance have smaller amygdala volume than controls (Fein et al., 2006), and adolescents at risk for substance abuse have less amygdala, insula, and ACC activation on the BART compared to controls (Crowley et al., 2010). These differences in activity may reflect emotional responses to the visceral motivations of the task. Integration of somatic and visceral states into the decision-making process could thus be altered and may underlie decision-making impairments seen in addiction, from reductions in sensing risk (Damasio, 1996) to overvaluation of visceral motivations (Loewenstein, 1996).
The insula has been implicated in interoception and the influence of autonomic functions on cognition (Singer et al., 2009). Disruption of interoceptive signals is considered central to decision-making deficits observed in addictions (Gray and Critchley, 2007; Verdejo-Garcia et al., 2012), especially in relation to approach and avoidance behaviors (Paulus et al., 2009). It has been proposed that an insula-dependent system integrates experience and recall of conscious pleasure derived from the interoceptive effects of drug use into the decision-making process (Naqvi and Bechara, 2010).
Studies of methamphetamine users have provided supporting data. When making decisions on a task with a positive interoceptive component, methamphetamine users exhibit less anterior insula, dorsal striatum, and thalamus activity than controls, and the correlation between anterior insula activity and reaction time is positive in controls, but negative in methamphetamine users (May et al., 2013). Methamphetamine users also exhibit less posterior insula and ACC activity than controls during a choice task with an aversive interoceptive experience (breathing load); that neural attenuations are exhibited across trials, regardless of error and reward rates, suggests that they are related to the interoceptive component of decision-making on the task (Stewart et al., 2014b). Thus, ineffective processing of interception, particularly in regions of the insula, may underlie an inability to integrate interoceptive information into decisions, especially in response to negative experiences.
6.2 Affective states and emotion regulation
Affective states are widely considered to be linked with addictive behavior, both for conferring risk and for contributing to the maintenance of drug use (Cheetham et al., 2010). Emotionally biased decisions represent one of the “irrationalities” observed in behavioral economics (Loewenstein, 1996) and may be exaggerated in addictions through impairment of neural circuitry that mediates emotional contributions to choice. For instance, choice behaviors can be associated with specific affective states, such as sadness, which enhances preference for risk (Raghunathan and Pham, 1999). The influence of affect could thus be enhanced in addictions.
Drug users display difficulties with emotion regulation, which relies on activity in the dorsal inferior frontal gyrus and amygdala (Payer et al., 2011). Compared to control participants, methamphetamine users exhibit higher trait anxiety and attenuated anterior insula and inferior frontal gyrus activity during a choice task; among methamphetamine users, attenuation in the anterior insula and inferior frontal gyrus is negatively correlated with trait anxiety (Stewart et al., 2014b). Thus, anxiety in methamphetamine users seems related to a diminished allocation of cognitive resources to the decision-making process. If executive functions related to emotional choices are disrupted, the impact of moods, emotions, or an immediate affective state on decision-making could be strengthened. Methamphetamine users also have impaired emotional recognition and processing that is linked to dopamine D2-type receptor availability in the ACC and anterior insula (Okita et al., 2016b). Further, emotion dysregulation is related to dopamine D2-type receptor availability in the amygdala of both methamphetamine users and control participants (Okita et al., 2016a). This association suggests that dopaminergic function contributes to individual differences in susceptibility to emotional influences during decision-making.
6.3 Insight
The disconnection between perception and reality frequently observed in addiction, perhaps most clear in the tendency to underestimate addiction severity, negative consequences, and the need for treatment, can bias choices towards the maintenance of destructive behaviors (Dean et al., 2015; Rinn et al., 2002). Insight necessitates an awareness of cognitive processes and involves functions such as behavior monitoring and error recognition, which are crucial for appropriate decision-making and are impaired in drug users (Forman et al., 2004; Hester et al., 2009; Moeller and Goldstein, 2014). These processes are intrinsically linked with bodily states and interoceptive awareness, as autonomic responses must be integrated with conscious self-monitoring for relevant functions, such as error recognition (Critchley et al., 2013).
Converging evidence indicates that dysfunction in the ACC of drug users contributes to poor insight. Activity in the ACC and insula related to error recognition is absent in opiate (Forman et al., 2004) and chronic cannabis (Hester et al., 2009) users. Compared to control participants and cocaine users with intact insight, cocaine users with difficulty in self-monitoring also have less emotional awareness, less error-induced activity in the rostral ACC during an inhibitory control task, and less gray matter within the rostral ACC (Moeller et al., 2014). Denial, measured by the precontemplation scale of the University of Rhode Island Change Assessment Scale (URICA), which assesses the degree to which an individual is ready to change problematic behavior, is inversely related to the strength of connectivity between the rostral ACC and frontal, limbic, and occipital areas in methamphetamine users (Dean et al., 2015). Thus, impairment in a network of brain areas, including the ACC, may contribute to impairment of insight in drug users.
7. External influences on choice
Context has a powerful and ubiquitous impact on decision-making (Tversky and Kahneman, 1981). Context dependence produces violations of rational economic models, in which preferences should be independent, regardless of irrelevant alternatives (Samuelson, 1947) or how they are framed (Arrow, 1982). Instead, preferences change depending on the availability of other options and past options, and on the framing of options (Huber et al., 1982; Tversky and Kahneman, 1989). Contextual appraisal also applies to cues in the immediate environment and the social domain, and a rich body of literature has explored both in relation to addiction.
7.1 Reference dependence
Contrary to the principles of rational choice theories, preferences for options that present risk change according to whether options are framed as gains or losses, even when the subjective values remain constant (Kahneman and Tversky, 1979). This phenomenon arises from decisions being considered in relation to a reference point and leads to systematic and predictable biases (Kuhberger, 1998). In studies in which value changes as a function of alternative options and distractors (Louie and De Martino, 2014), context-dependent neural activity related to reward valuation has been observed in the ventral striatum and parietal cortex (Cox and Kable, 2014).
Certain tasks compare choices consistent and inconsistent with the effects of framing put forth by prospect theory (Kahneman and Tversky, 1979), and find that activity in the amygdala is related to decisions consistent with framing effects and ACC activity is related to decisions that are inconsistent with framing effects (De Martino et al., 2006). Risk signals observed in the anterior insula during positively-framed messages on the IGT also have been correlated with how much the message improves choice behavior (Krawitz et al., 2010). While previously hypothesized to result from emotional biases (De Martino et al., 2006; Roiser et al., 2009), framing effects may also be related to cognitive control and engagement (Li et al., 2017). Supporting this view is the observation that activity in the dorsolateral PFC correlates with advantageous decision-making on the framing version of the IGT (Krawitz et al., 2010).
The gain/loss asymmetry of framing and the reference dependence of normalizing to the status quo could represent biologically separate systems of approach and withdrawal (Wright et al., 2012), which could influence vulnerability for addiction (Melrose et al., 2015). Susceptibility to framing effects correlates with activity in the medial and orbital PFC (De Martino et al., 2006) and the ACC (Deppe et al., 2007), and is linked with emotions (Habib et al., 2015). Moreover, a study using the IGT has indicated that better performance in the positively-framed condition is associated with activity in the ACC and insula in both healthy controls and substance users, whereas substance users perform worse than controls during negatively framed IGT conditions. Their performance also reflects lower risk-aversion signals in the anterior insula, and a correlation between advantageous decision-making and risk-related activity in the ACC across decisions is only observed in healthy controls (Fukunaga et al., 2013). Thus, impaired risk signals in the ACC and insula of substance users, especially related to negatively-framed messages, appear to contribute to disadvantageous decision-making.
The susceptibility to framing effects has therapeutic applications. For instance, conscious perspective shifts can alter value-related neural activity, as evidenced by the modification of cortical activations related to reward value and choice selection by instruction to frame food choices in terms of health or taste (Bhanji and Beer, 2012). Similarly, framing effects can bias preferences on delay discounting tasks (Lempert and Phelps, 2016; Peters and Buchel, 2010). Neural activity in the medial PFC also can predict behavioral changes in the week following persuasive messages (Falk et al., 2010). Framing effects and reference dependence warrant further investigation as related to addictions, especially considering that the status quo in addictions can be constantly shifting, causing inconsistency in decisions (Koob and Le Moal, 2001).
7.2 Environmental cues
Contextual cues can have major effects on drug-related choices (Chase et al., 2011; Perry et al., 2014; Redish, 2004). For instance, conditioned stimuli with enhanced salience can bias towards drug-seeking behavior (Everitt and Robbins, 2005). Thus, environmental stimuli can alter the value of certain options and induce a state of craving that heightens the value of drug-related choices compared to alternatives. Studies on the neural basis of cue-induced craving have shown greater activity in mesocorticolimbic regions (Bonson et al., 2002; Volkow et al., 2006; Wilson et al., 2004) in response to drug-related compared to neutral cues. A role of the insula has been emphasized; insula activity correlates with cue-induced drug craving in cigarette smokers (Brody et al., 2007), cocaine users (Kilts et al., 2004), and opiate users (Sell et al., 1999), and RSFC of the right insula with prefrontal networks is greater in cocaine addicts than controls (Cisler et al., 2013). Smoking addiction is disrupted by changes in cigarette craving after lesions to the insula, reinforcing its role in conscious cue-induced drug craving and, perhaps, in the pleasure derived from the bodily effects of smoking (Naqvi and Bechara, 2010).
Interestingly, suppression of craving during cigarette cues is linked to activations in limbic brain regions, specifically the left dorsal ACC, posterior cingulate cortex, and precuneus, and deactivations in primary sensory and motor cortices, specifically the cuneus, left lateral occipital gyrus, and right postcentral gyrus (Brody et al., 2007). Cocaine users also exhibit decreases in right ventral striatum and right OFC activity when instructed to inhibit cravings, compared to trials with no instruction to inhibit craving, and the decreases in activity are linked to increased activity in the lateral PFC (Volkow et al., 2010). Similar results have been found in smokers (Kober et al., 2010). Thus, modulation by the lateral PFC appears to be crucial for resisting craving, which is likely mediated by activity in the ventral striatum and OFC, as well as limbic areas.
7.3 Social factors/peer influence
Decisions made in a social environment integrate personal goals with the well-being of others, while taking into account drives such as conformity, altruism, and punishment (Adolphs, 2003; Lieberman, 2007). These drives affect the value of different options (Montague et al., 2006). Specific economic tasks, such as game theory tasks (Camerer, 2003), developed to investigate social decision-making have revealed that social decision-making processes share many neural substrates with reinforcement learning and reward valuation (Fehr and Camerer, 2007; Klucharev et al., 2009) and are associated with dopamine signaling (Yacubian and Buchel, 2009).
Addiction often is accompanied by disruptions in processes necessary for social decision-making, from understanding the self (Moeller and Goldstein, 2014) to recognizing the emotions of others (Fernandez-Serrano et al., 2010) and social functioning (Bora and Zorlu, 2017; Preller et al., 2014). Compared to healthy controls, methamphetamine users exhibit abnormal frontoparietal activity that may reflect difficulty integrating the emotional components of social information (Payer et al., 2011; Payer et al., 2008). Indeed, affective responses, which are impaired in those with addictions (Bechara and Damasio, 2002), can influence social decision-making (van ’t Wout et al., 2006). All of these factors could contribute to the development and maintenance of addictions, especially during adolescence, the most common time for the onset of addiction (Eaton et al., 2012), when social functioning is of particular significance (Pfeifer et al., 2011; Welborn et al., 2016). While peer influences on performance in delay discounting tasks have been demonstrated (Gilman et al., 2014), the authors are aware of no studies that have yet investigated brain function during game theory tasks in drug users.
Neural activity during decision-making tasks with a social component have demonstrated different patterns of striatal activity in young adult marijuana users compared to healthy controls when participants are integrating social information into decisions (Gilman et al., 2016b). The neural underpinnings of social conformity are particularly relevant, especially considering the impact of peer influences on addictions, risk-taking, and decision-making (Khavari, 1993; Van Hoorn et al., 2016). Young adult marijuana users take more time than controls to resist group choices, and reaction times are correlated with greater frontal activation (Gilman et al., 2016c). Self-reported susceptibility to group influence is associated with caudate activity in both groups, with the marijuana group exhibiting greater caudate activation than controls when presented with social influence (Gilman et al., 2016c). Both groups exhibit activation in the ventral ACC, PFC, and insula during social exclusion, but young adult marijuana users have lower insula signaling (Gilman et al., 2016a). This result is in line with evidence that insula activity is related to group conformity (Tomlin et al., 2013). These studies suggest that, during decision-making, drug users may process social information differently in striatal and frontal regions, especially in the insula.
8. Conclusions and future directions
Maladaptive decision-making may arise from disruptions in the effective computation of reward values. Clarification of how factors such as temporal delay, uncertainty, and internal and external states influence reward valuation, and how subjective value guides choice, can help explain suboptimal choice selection in addiction. “Irrational” choice behavior can reflect inconsistent judgement of delays (Ainslie, 1975; Laibson, 1997) and uncertainty (Ellsberg, 1961), or the influence of emotion (Loewenstein, 1996) and reference (Kahneman and Tversky, 1979) on evaluating utility. These biases may be exaggerated in addictions, providing a mechanism by which the underlying neurobiological processes can be better understood.
The present review is by no means comprehensive and focuses on findings in humans related to the neurobiology of valuation in addictions. A distributed network of cortical and subcortical brain regions that mediates decision-making and addictive behavior (Figure 1) has been explored. Reward-related activity in the striatum and ventromedial PFC/OFC appears to be enhanced in drug users compared to controls, whereas activity in the dorsolateral PFC, likely related to cognitive control, generally is diminished. These results are consistent with general findings in the literature of heightened reward sensitivity and impaired inhibitory functions in addictions.
The extent to which deficits in decision-making precede or result from drug use remains an unanswered question. Genetic interactions with decision-making (Boettiger et al., 2007; Moeller et al., 2013b) and converging evidence from delay discounting tasks (i.e., Sheffer et al., 2014) demonstrate that drug use can be predicted by individual differences in decision-making. Indeed, identifying an endophenotype that could predict addiction psychopathology would be beneficial (MacKillop et al., 2015). However, substance abuse itself can influence decision-making to change behavioral outcomes (i.e., Yi et al., 2008).
Several research questions warrant future work. The field of neuroeconomics can advance our understanding of addictions (Monterosso et al., 2012; Sharp et al., 2012) and combining experimental economic tasks and models with neuroscientific methods can help determine how valuation is affected by the complex components involved in making a decision. Further examining the neurobiological basis of prospect theory (Trepel et al., 2005) could provide insights into “irrationalities” of behavior and their implications for addictions. The irregular neural activity across addictions in response to ambiguity provides one such example, and the neural substrates of uncertainty aversion continue to be explored with respect to addiction.
Findings related to the lateral PFC, including the dorsolateral PFC, are especially intriguing. The lateral PFC is traditionally associated with executive functions (Dixon and Christoff, 2014). In the studies reviewed here, activity in the lateral PFC was related to choosing the later option in delay discounting tasks (McClure et al., 2004; Wesley et al., 2014), adaptive decision-making under uncertainty (Bolla et al., 2005; Kohno et al., 2014), ambiguity tolerance in risk tasks (Huettel et al., 2006), emotion regulation (Payer et al., 2011; Stewart et al., 2014b), resistance to cue-induced craving (Kober et al., 2010; Volkow et al., 2010), resistance to framing effects (Krawitz et al., 2010) and adaptive social cognition (Payer et al., 2011; Payer et al., 2008). These functions all are related to behavioral control and resisting temptation. Disruption of lateral PFC signaling thus seems to be a likely contributor to addictions. Clarifying the roles of distinct PFC circuitry is essential to improve understanding of decision-making in healthy and addicted individuals, and continued analyses of the precise mechanisms of dysfunction, especially in the lateral PFC, would be particularly useful.
The sensitivity of decision-makers to loss warrants further exploration with respect to addictions. As noted above, drug users show less activity in the ACC and insula related to loss compared to control participants (Gowin et al., 2014b; Wesley et al., 2011), and adaptive decision-making relies on loss-related ACC activity (Ersche et al., 2006; Wesley et al., 2011). Feedback to modulate decision-making likely relies on these types of adaptive signals, and their distortion could account for the impaired behavioral monitoring and responses to negative outcomes observed in drug users. Indeed, risk-aversion signals are weaker in the anterior insula of substance users than controls specifically during negatively-framed IGT conditions (Fukunaga et al., 2013). It would be beneficial to extend investigations of the effects of losses versus gains in drug users.
Another area of convergence of the aforementioned findings is the relative inability of drug users to discriminate between options that differ in cost, whether the cost is time, as in delay discounting tasks (Hoffman et al., 2008; Monterosso et al., 2007) or risk, as in tasks that present uncertainty (Gowin et al., 2014b; Reske et al., 2015). If brain activity is just as strong during easy as during hard decisions in cortical regions, such as the dorsolateral PFC, the system may be overtaxed so that resolving the conflict between competing options is more difficult. Option selection could thus be skewed towards the easier decision (i.e. the immediate option in delay discounting tasks), and this bias may be reflected in the heightened reaction times of methamphetamine users (Hoffman et al., 2008). An inadequate ability to discriminate between safe and risky options on risky decision-making tasks may also stem from a lack of risk signals (Reske et al., 2015), such as those in the ACC (Gowin et al., 2014b). Estimation of how costly a decision is, and how this estimation affects the computation of value, undoubtedly relies on a network of brain regions that may contribute to impairment.
Consistent with the general consensus of a central role for dopamine in choice and in addictions, many of the findings previously covered are related to the dopaminergic system (Ballard et al., 2015; Boettiger et al., 2007; Kohno et al., 2013; Kohno et al., 2016b; Martinez et al., 2007; Martinez et al., 2009; Moeller et al., 2013b; Okita et al., 2016a; Okita et al., 2016b). The complexity of the dopaminergic system with respect to localization and function of different dopamine receptor subtypes and mechanisms of dopamine release, and the inconsistency of results (Daw et al., 2011), necessitates clarification of how dopamine functions to modulate decision-making, especially in addictions. Undoubtedly, while not presently covered, the roles of other neurotransmitter systems warrant further investigation (Cunningham and Anastasio, 2014).
Limitations in the study of decision-making and addiction can arise from methodological variation. As choice behavior likely varies as a function of reward type, the use of different types of rewards complicate generalization across studies (Carter et al., 2010). Decision-making impairments and neural activity can also differ according to the type of addiction (Ersche et al., 2006; Rogers et al., 1999), although there is evidence of consistency across addictions to different types of drugs (Ersche et al., 2005). Further, that it can be difficult to determine whether neural responses interpreted as value signals are not related instead to other cognitive functions, such as attention or coding of outcome identity (O’Doherty, 2014), can potentially confound results. Similarly, the somewhat imprecise definitions of constructs such as risk and impulsivity necessitate caution in the generalization of results (Glimcher, 2008; Schonberg et al., 2011). For instance, delay discounting tasks capture an underlying and constant feature of addictive disorders, and still resist generalization past the quantification of the preference for an immediate over a larger delayed reward.
Continuing to move towards conceptualizing decision-making as the integration of different subsystems into a final, unified process can help depart from the Cartesian divide (Kable and Glimcher, 2009; Monterosso and Luo, 2010; Phelps et al., 2014) to refine our understanding of the fundamental processes that contribute to addiction vulnerability and maintenance. Indeed, clarifying the mechanisms of value computation and the weight attributed to higher-order versus more bottom-up processes is crucial. The extent to which choice selection by addicts reflects dysfunction in common neural mechanisms that are exaggerated or skewed, or rather a difference in the nature of what is valued, is an open question. Nonetheless, viewing value as central to the decision-making process in addictions provides a powerful framework by which addiction can help us understand the neural mechanisms of decision-making, and vice versa.
Highlights.
-
-
Individuals with addictive disorders engage in maladaptive decision-making.
-
-
Suboptimal choices can reflect impairment in subjective evaluation of rewards.
-
-
Various factors (delay, uncertainty, emotion, cues, peers) affect valuation.
-
-
Valuation relies on a distributed network of brain regions.
-
-
These regions show impaired function in addicts during decision-making.
Acknowledgments
Supported by T32 DA024635 (ZG) and K01DA037452 (SJM) grants from the National Institute on Drug Abuse and endowments from the Thomas P. and Katherine K Pike Chair in Addiction Studies (EDL) and the Margaret Greene Family Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of Interest: The authors have no conflicts of interest or financial disclosures to report.
References
- Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 2003;4(3):165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82(4):463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Ainslie G. Picoeconomics: The Strategic Interaction of Successive Motivational States Within the Person. Cambridge University Press; Cambridge England; New York: 1992. [Google Scholar]
- Arrow KJ. Risk perception in psychology and economics. Econ Inq. 1982;20(1):1–9. [Google Scholar]
- Arrow KJ. Rationality of self and others in an economic system. J Bus. 1986;59(4):S385–S399. [Google Scholar]
- Ashenhurst JR, Bujarski S, Jentsch JD, Ray LA. Modeling behavioral reactivity to losses and rewards on the Balloon Analogue Risk Task (BART): moderation by alcohol problem severity. Exp Clin Psychopharmacol. 2014;22(4):298–306. doi: 10.1037/a0036837. [DOI] [PubMed] [Google Scholar]
- Ballard ME, Mandelkern MA, Monterosso JR, Hsu E, Robertson CL, Ishibashi K, Dean AC, London ED. Low dopamine D2/D3 receptor availability is associated with steep discounting of delayed rewards in methamphetamine dependence. Int J Neuropsychopharmacol. 2015;18(7) doi: 10.1093/ijnp/pyu119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Daw ND, O’Doherty J. Multiple forms of value learning and the function of dopamine. In: Glimcher PW, editor. Neuroeconomics: Decision Making and the Brain. Elsevier/Academic Press; Amsterdam; Boston: 2009. p. 538. [Google Scholar]
- Bartra O, McGuire JT, Kable JW. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage. 2013;76(1):412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8(11):1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40(10):1675–1689. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39(4):376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40(10):1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Bhanji JP, Beer JS. Taking a different perspective: mindset influences neural regions that represent value and choice. Soc Cogn Affect Neurosci. 2012;7(7):782–793. doi: 10.1093/scan/nsr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Koffarnus MN, Moody L, Wilson AG. The behavioral- and neuro-economic process of temporal discounting: A candidate behavioral marker of addiction. Neuropharmacology. 2014;76(Pt B):518–527. doi: 10.1016/j.neuropharm.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Landes RD, Christensen DR, Jackson L, Jones BA, Kurth-Nelson Z, Redish AD. Single- and cross-commodity discounting among cocaine addicts: the commodity and its temporal location determine discounting rate. Psychopharmacology (Berl) 2011a;217(2):177–187. doi: 10.1007/s00213-011-2272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 2007;90(Suppl 1):S85–91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biol Psychiatry. 2011b;69(3):260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29(11):1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Momenan R, Smith AR, Hommer DW. Reduced posterior mesofrontal cortex activation by risky rewards in substance-dependent patients. Drug Alcohol Depend. 2008;95(1–2):115–128. doi: 10.1016/j.drugalcdep.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D’Esposito M, Fields HL. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. J Neurosci. 2007;27(52):14383–14391. doi: 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19(3):1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. 2005;26(2):480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26(3):376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Bora E, Zorlu N. Social cognition in alcohol use disorder: a meta-analysis. Addiction. 2017;112(1):40–48. doi: 10.1111/add.13486. [DOI] [PubMed] [Google Scholar]
- Brand M, Recknor EC, Grabenhorst F, Bechara A. Decisions under ambiguity and decisions under risk: correlations with executive functions and comparisons of two different gambling tasks with implicit and explicit rules. J Clin Exp Neuropsychol. 2007;29(1):86–99. doi: 10.1080/13803390500507196. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, London ED, Monterosso JR, Tiffany ST, Korb A, Gan JJ, Cohen MS. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62(6):642–651. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JW, Alexander WH. Foraging value, risk avoidance, and multiple control signals: How the ACC controls value-based decision-making. J Cogn Neurosci. 2017:1–18. doi: 10.1162/jocn_a_01140. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Risk prediction and aversion by anterior cingulate cortex. Cogn Affect Behav Neurosci. 2007;7(4):266–277. doi: 10.3758/cabn.7.4.266. [DOI] [PubMed] [Google Scholar]
- Camerer C. Behavioral Game Theory: Experiments in Strategic Interaction. Russell Sage Foundation; Princeton University Press; Princeton, N.J.: 2003. [Google Scholar]
- Camerer C, Weber M. Recent developments in modeling preferences - uncertainty and ambiguity. J Risk Uncertainty. 1992;5(4):325–370. [Google Scholar]
- Camerer CF. Behavioral economics. Curr Biol. 2014;24(18):R867–871. doi: 10.1016/j.cub.2014.07.040. [DOI] [PubMed] [Google Scholar]
- Carter RM, Meyer JR, Huettel S. Functional neuroimaging of intertemporal choice models: a review. J Neurosci Psychol Econ. 2010;3(1):27–45. [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, Hogarth L. The neural basis of drug stimulus processing and craving: An activation likelihood estimation meta-analysis. Biol Psychiatry. 2011;70(8):785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Yucel M, Lubman DI. The role of affective dysregulation in drug addiction. Clin Psychol Rev. 2010;30(6):621–634. doi: 10.1016/j.cpr.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Elton A, Kennedy AP, Young J, Smitherman S, Andrew James G, Kilts CD. Altered functional connectivity of the insular cortex across prefrontal networks in cocaine addiction. Psychiatry Res. 2013;213(1):39–46. doi: 10.1016/j.pscychresns.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain Cogn. 2004;55(1):41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- Claus ED, Kiehl KA, Hutchison KE. Neural and behavioral mechanisms of impulsive choice in alcohol use disorder. Alcohol Clin Exp Res. 2011;35(7):1209–1219. doi: 10.1111/j.1530-0277.2011.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11(1):18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Cousijn J, Goudriaan AE, Ridderinkhof KR, van den Brink W, Veltman DJ, Wiers RW. Approach-bias predicts development of cannabis problem severity in heavy cannabis users: results from a prospective fMRI study. PLoS One. 2012;7(9):e42394. doi: 10.1371/journal.pone.0042394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox KM, Kable JW. BOLD subjective value signals exhibit robust range adaptation. J Neurosci. 2014;34(49):16533–16543. doi: 10.1523/JNEUROSCI.3927-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Eccles J, Garfinkel SN. Interaction between cognition, emotion, and the autonomic nervous system. Handb Clin Neurol. 2013;117:59–77. doi: 10.1016/B978-0-444-53491-0.00006-7. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Dalwani MS, Mikulich-Gilbertson SK, Du YP, Lejuez CW, Raymond KM, Banich MT. Risky decisions and their consequences: neural processing by boys with Antisocial Substance Disorder. PLoS One. 2010;5(9):e12835. doi: 10.1371/journal.pone.0012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Anastasio NC. Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction. Neuropharmacology. 2014;76(Pt B):460–478. doi: 10.1016/j.neuropharm.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ. Model-based influences on humans’ choices and striatal prediction errors. Neuron. 2011;69(6):1204–1215. doi: 10.1016/j.neuron.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P. Dopamine, reinforcement learning, and addiction. Pharmacopsychiatry. 2009;42(Suppl 1):S56–65. doi: 10.1055/s-0028-1124107. [DOI] [PubMed] [Google Scholar]
- De Martino B, Kumaran D, Seymour B, Dolan RJ. Frames, biases, and rational decision-making in the human brain. Science. 2006;313(5787):684–687. doi: 10.1126/science.1128356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean AC, Kohno M, Morales AM, Ghahremani DG, London ED. Denial in methamphetamine users: associations with cognition and functional connectivity in brain. Drug Alcohol Depend. 2015;151:84–91. doi: 10.1016/j.drugalcdep.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean AC, Sugar CA, Hellemann G, London ED. Is all risk bad? Young adult cigarette smokers fail to take adaptive risk in a laboratory decision-making test. Psychopharmacology (Berl) 2011;215(4):801–811. doi: 10.1007/s00213-011-2182-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppe M, Schwindt W, Pieper A, Kugel H, Plassmann H, Kenning P, Deppe K, Ringelstein EB. Anterior cingulate reflects susceptibility to framing during attractiveness evaluation. Neuroreport. 2007;18(11):1119–1123. doi: 10.1097/WNR.0b013e3282202c61. [DOI] [PubMed] [Google Scholar]
- Diederen KM, Ziauddeen H, Vestergaard MD, Spencer T, Schultz W, Fletcher PC. Dopamine modulates adaptive prediction error coding in the human midbrain and striatum. J Neurosci. 2017;37(7):1708–1720. doi: 10.1523/JNEUROSCI.1979-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon ML, Christoff K. The lateral prefrontal cortex and complex value-based learning and decision making. Neurosci Biobehav Rev. 2014;45:9–18. doi: 10.1016/j.neubiorev.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23(10):475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Eaton DK, Kann L, Kinchen S, Shanklin S, Flint KH, Hawkins J, Harris WA, Lowry R, McManus T, Chyen D, Whittle L, Lim C, Wechsler H, Centers for Disease Control and Prevention (CDC) Prevention Youth risk behavior surveillance - United States, 2011. MMWR Surveill Summ. 2012;61(4):1–162. [PubMed] [Google Scholar]
- Ellsberg D. Risk, ambiguity, and the Savage axioms. Q J Econ. 1961:643–669. [Google Scholar]
- Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, London ED. Decision-making in a risk-taking task: a PET study. Neuropsychopharmacology. 2002;26(5):682–691. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Fletcher PC, Lewis SJ, Clark L, Stocks-Gee G, London M, Deakin JB, Robbins TW, Sahakian BJ. Abnormal frontal activations related to decision-making in current and former amphetamine and opiate dependent individuals. Psychopharmacology (Berl) 2005;180(4):612–623. doi: 10.1007/s00213-005-2205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Fletcher PC, Roiser JP, Fryer TD, London M, Robbins TW, Sahakian BJ. Differences in orbitofrontal activation during decision-making between methadone-maintained opiate users, heroin users and healthy volunteers. Psychopharmacology (Berl) 2006;188(3):364–373. doi: 10.1007/s00213-006-0515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Falk EB, Berkman ET, Mann T, Harrison B, Lieberman MD. Predicting persuasion-induced behavior change from the brain. J Neurosci. 2010;30(25):8421–8424. doi: 10.1523/JNEUROSCI.0063-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr E, Camerer CF. Social neuroeconomics: the neural circuitry of social preferences. Trends Cogn Sci. 2007;11(10):419–427. doi: 10.1016/j.tics.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Fein G, Landman B, Tran H, McGillivray S, Finn P, Barakos J, Moon K. Brain atrophy in long-term abstinent alcoholics who demonstrate impairment on a simulated gambling task. Neuroimage. 2006;32(3):1465–1471. doi: 10.1016/j.neuroimage.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Serrano MJ, Lozano O, Perez-Garcia M, Verdejo-Garcia A. Impact of severity of drug use on discrete emotions recognition in polysubstance abusers. Drug Alcohol Depend. 2010;109(1–3):57–64. doi: 10.1016/j.drugalcdep.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Fishbein D, Eldreth DL, Hyde C, Matochik JA, London ED, Contoreggi C, Kurian V, Kimes AS, Breeden A, Grant S. Risky decision making and the anterior cingulate cortex in abstinent drug abusers and nonusers. Brain Res Cogn Brain Res. 2005a;23(1):119–136. doi: 10.1016/j.cogbrainres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Fishbein D, Hyde C, Eldreth D, London ED, Matochik J, Ernst M, Isenberg N, Steckley S, Schech B, Kimes A. Cognitive performance and autonomic reactivity in abstinent drug abusers and nonusers. Exp Clin Psychopharmacol. 2005b;13(1):25–40. doi: 10.1037/1064-1297.13.1.25. [DOI] [PubMed] [Google Scholar]
- Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13–22. doi: 10.1016/j.neuroscience.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Dougherty GG, Casey BJ, Siegle GJ, Braver TS, Barch DM, Stenger VA, Wick-Hull C, Pisarov LA, Lorensen E. Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biol Psychiatry. 2004;55(5):531–537. doi: 10.1016/j.biopsych.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Fukunaga R, Bogg T, Finn PR, Brown JW. Decisions during negatively-framed messages yield smaller risk-aversion-related brain activation in substance-dependent individuals. Psychol Addict Behav. 2013;27(4):1141–1152. doi: 10.1037/a0030633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Curran MT, Calderon V, Schuster RM, Evins AE. Altered neural processing to social exclusion in young adult marijuana users. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016a;1(2):152–159. doi: 10.1016/j.bpsc.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Curran MT, Calderon V, Stoeckel LE, Evins AE. Impulsive social influence increases impulsive choices on a temporal discounting task in young adults. PLoS One. 2014;9(7):e101570. doi: 10.1371/journal.pone.0101570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Lee S, Kuster JK, Lee MJ, Kim BW, van der Kouwe A, Blood AJ, Breiter HC. Variable activation in striatal subregions across components of a social influence task in young adult cannabis users. Brain Behav. 2016b;6(5):e00459. doi: 10.1002/brb3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Schuster RM, Curran MT, Calderon V, van der Kouwe A, Evins AE. Neural mechanisms of sensitivity to peer information in young adult cannabis users. Cogn Affect Behav Neurosci. 2016c;16(4):646–661. doi: 10.3758/s13415-016-0421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher PW. Understanding risk: a guide for the perplexed. Cogn Affect Behav Neurosci. 2008;8(4):348–354. doi: 10.3758/CABN.8.4.348. [DOI] [PubMed] [Google Scholar]
- Glimcher PW, Fehr E. Neuroeconomics: Decision Making and the Brain. 2nd. Academic Press; London: 2014. [Google Scholar]
- Goh JO, Su YS, Tang YJ, McCarrey AC, Tereshchenko A, Elkins W, Resnick SM. Frontal, striatal, and medial temporal sensitivity to value distinguishes risk-taking from risk-aversive older adults during decision making. J Neurosci. 2016;36(49):12498–12509. doi: 10.1523/JNEUROSCI.1386-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13(9):372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12(11):652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, Harle KM, Stewart JL, Wittmann M, Tapert SF, Paulus MP. Attenuated insular processing during risk predicts relapse in early abstinent methamphetamine-dependent individuals. Neuropsychopharmacology. 2014a;39(6):1379–1387. doi: 10.1038/npp.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, Mackey S, Paulus MP. Altered risk-related processing in substance users: imbalance of pain and gain. Drug Alcohol Depend. 2013;132(1–2):13–21. doi: 10.1016/j.drugalcdep.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, Stewart JL, May AC, Ball TM, Wittmann M, Tapert SF, Paulus MP. Altered cingulate and insular cortex activation during risk-taking in methamphetamine dependence: losses lose impact. Addiction. 2014b;109(2):237–247. doi: 10.1111/add.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38(8):1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Gray MA, Critchley HD. Interoceptive basis to craving. Neuron. 2007;54(2):183–186. doi: 10.1016/j.neuron.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26(4):317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Habib M, Cassotti M, Moutier S, Houde O, Borst G. Fear and anger have opposite effects on risk seeking in the gain frame. Front Psychol. 2015;6:253. doi: 10.3389/fpsyg.2015.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(5927):646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hare TA, O’Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28(22):5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner KR, Starr MJ, Curtin JJ. Altered subjective reward valuation among drug-deprived heavy marijuana users: aversion to uncertainty. J Abnorm Psychol. 2016;125(1):138–150. doi: 10.1037/abn0000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009;34(11):2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman WF, Schwartz DL, Huckans MS, McFarland BH, Meiri G, Stevens AA, Mitchell SH. Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology (Berl) 2008;201(2):183–193. doi: 10.1007/s00213-008-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310(5754):1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- Huber J, Payne JW, Puto C. Adding asymmetrically dominated alternatives: violations of regularity and the similarity hypothesis. J Cons Res. 1982;9(1):90–98. [Google Scholar]
- Huettel SA, Stowe CJ, Gordon EM, Warner BT, Platt ML. Neural signatures of economic preferences for risk and ambiguity. Neuron. 2006;49(5):765–775. doi: 10.1016/j.neuron.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162(8):1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Jones JD, Comer SD. A review of human drug self-administration procedures. Behav Pharmacol. 2013;24(5–6):384–395. doi: 10.1097/FBP.0b013e3283641c3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutsa J, Voon V, Johansson J, Niemela S, Bergman J, Kaasinen V. Dopaminergic function and intertemporal choice. Transl Psychiatry. 2015;5:e520. doi: 10.1038/tp.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10(12):1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neurobiology of decision: consensus and controversy. Neuron. 2009;63(6):733–745. doi: 10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47(2) [Google Scholar]
- Khavari KA. Interpersonal influences in college students’ initial use of alcohol and drugs–the role of friends, self, parents, doctors, and dealers. Int J Addict. 1993;28(4):377–388. doi: 10.3109/10826089309039635. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161(2):233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Kim YT, Sohn H, Jeong J. Delayed transition from ambiguous to risky decision making in alcohol dependence during Iowa Gambling Task. Psychiatry Res. 2011;190(2–3):297–303. doi: 10.1016/j.psychres.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Klucharev V, Hytonen K, Rijpkema M, Smidts A, Fernandez G. Reinforcement learning signal predicts social conformity. Neuron. 2009;61(1):140–151. doi: 10.1016/j.neuron.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A. 2010;107(33):14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Jarmolowicz DP, Mueller ET, Bickel WK. Changing delay discounting in the light of the competing neurobehavioral decision systems theory: a review. J Exp Anal Behav. 2013;99(1):32–57. doi: 10.1002/jeab.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M, Ghahremani DG, Morales AM, Robertson CL, Ishibashi K, Morgan AT, Mandelkern MA, London ED. Risk-taking behavior: dopamine D2/D3 receptors, feedback, and frontolimbic activity. Cereb Cortex. 2013;25(1):236–245. doi: 10.1093/cercor/bht218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M, Morales AM, Ghahremani DG, Hellemann G, London ED. Risky decision making, prefrontal cortex, and mesocorticolimbic functional connectivity in methamphetamine dependence. JAMA Psychiatry. 2014;71(7):812–820. doi: 10.1001/jamapsychiatry.2014.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M, Morales AM, Guttman Z, London ED. A neural network that links brain function, white-matter structure and risky behavior. Neuroimage. 2017;149:15–22. doi: 10.1016/j.neuroimage.2017.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M, Nurmi EL, Laughlin CP, Morales AM, Gail EH, Hellemann GS, London ED. Functional genetic variation in dopamine signaling moderates prefrontal cortical activity during risky decision making. Neuropsychopharmacology. 2016a;41(3):695–703. doi: 10.1038/npp.2015.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M, Okita K, Morales AM, Robertson CL, Dean AC, Ghahremani DG, Sabb FW, Rawson RA, Mandelkern MA, Bilder RM, London ED. Midbrain functional connectivity and ventral striatal dopamine D2-type receptors: link to impulsivity in methamphetamine users. Mol Psychiatry. 2016b;21(11):1554–1560. doi: 10.1038/mp.2015.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolling N, Wittmann MK, Behrens TE, Boorman ED, Mars RB, Rushworth MF. Value, search, persistence and model updating in anterior cingulate cortex. Nat Neurosci. 2016;19(10):1280–1285. doi: 10.1038/nn.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Krain AL, Wilson AM, Arbuckle R, Castellanos FX, Milham MP. Distinct neural mechanisms of risk and ambiguity: a meta-analysis of decision-making. Neuroimage. 2006;32(1):477–484. doi: 10.1016/j.neuroimage.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Krawitz A, Fukunaga R, Brown JW. Anterior insula activity predicts the influence of positively framed messages on decision making. Cogn Affect Behav Neurosci. 2010;10(3):392–405. doi: 10.3758/CABN.10.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhberger A. The influence of framing on risky decisions: a meta-analysis. Organ Behav Hum Decis Process. 1998;75(1):23–55. doi: 10.1006/obhd.1998.2781. [DOI] [PubMed] [Google Scholar]
- Laibson D. Golden eggs and hyperbolic discounting. Q J Econ. 1997;112(2):443–478. [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29(47):14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Jones HA, Richards JB, Strong DR, Kahler CW, Read JP. The Balloon Analogue Risk Task (BART) differentiates smokers and nonsmokers. Exp Clin Psychopharmacol. 2003a;11(1):26–33. doi: 10.1037//1064-1297.11.1.26. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Zvolensky MJ, Pedulla CM. Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. J Adolesc. 2003b;26(4):475–479. doi: 10.1016/s0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown RA. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J Exp Psychol Appl. 2002;8(2):75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Lempert KM, Phelps EA. The malleability of intertemporal choice. Trends Cogn Sci. 2016;20(1):64–74. doi: 10.1016/j.tics.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DJ, Glimcher PW. The root of all value: a neural common currency for choice. Curr Opin Neurobiol. 2012;22(6):1027–1038. doi: 10.1016/j.conb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy I, Snell J, Nelson AJ, Rustichini A, Glimcher PW. Neural representation of subjective value under risk and ambiguity. J Neurophysiol. 2010;103(2):1036–1047. doi: 10.1152/jn.00853.2009. [DOI] [PubMed] [Google Scholar]
- Li R, Smith DV, Clithero JA, Venkatraman V, Carter RM, Huettel SA. Reason’s enemy is not emotion: engagement of cognitive control networks explains biases in gain/loss framing. J Neurosci. 2017;37(13):3588–3598. doi: 10.1523/JNEUROSCI.3486-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Annu Rev Psychol. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Loewenstein G. Out of control: visceral influences on behavior. Organ Behav Hum Decis Process. 1996;65(3):272–292. [Google Scholar]
- Loewenstein G, Elster J. Choice over Time. Russell Sage Foundation; New York: 1992. [Google Scholar]
- London ED. Impulsivity, stimulant abuse, and dopamine receptor signaling. Adv Pharmacol. 2016;76:67–84. doi: 10.1016/bs.apha.2016.01.002. [DOI] [PubMed] [Google Scholar]
- London ED, Ernst M, Grant S, Bonson K, Weinstein A. Orbitofrontal cortex and human drug abuse: functional imaging. Cereb Cortex. 2000;10(3):334–342. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- London ED, Kohno M, Morales AM, Ballard ME. Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging. Brain research. 2015;1628(Pt A):174–185. doi: 10.1016/j.brainres.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie K, De Martino B. The neurobiology of context-dependent valuation and choice. In: Fehr E, Glimcher PW, editors. Neuroeconomics. Second. Academic Press; San Diego: 2014. pp. 455–476. [Google Scholar]
- Luo S, Ainslie G, Pollini D, Giragosian L, Monterosso JR. Moderators of the association between brain activation and farsighted choice. Neuroimage. 2012;59(2):1469–1477. doi: 10.1016/j.neuroimage.2011.08.004. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Gray JC, Bidwell LC, Bickel WK, Sheffer CE, McGeary JE. Genetic influences on delay discounting in smokers: examination of a priori candidates and exploration of dopamine-related haplotypes. Psychopharmacology (Berl) 2015;232(20):3731–3739. doi: 10.1007/s00213-015-4029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magno E, Foxe JJ, Molholm S, Robertson IH, Garavan H. The anterior cingulate and error avoidance. J Neurosci. 2006;26(18):4769–4773. doi: 10.1523/JNEUROSCI.0369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, Perez A, Frankle WG, Cooper T, Kleber HD, Fischman MW, Laruelle M. Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004;29(6):1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164(4):622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- Martinez D, Saccone PA, Liu F, Slifstein M, Orlowska D, Grassetti A, Cook S, Broft A, Van Heertum R, Comer SD. Deficits in dopamine D(2) receptors and presynaptic dopamine in heroin dependence: commonalities and differences with other types of addiction. Biol Psychiatry. 2012;71(3):192–198. doi: 10.1016/j.biopsych.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Narendran R, Foltin RW, Broft A, Hwang DR, Perez A, Abi-Dargham A, Fischman MW, Kleber HD, Laruelle M. Dopamine D1 receptors in cocaine dependence measured with PET and the choice to self-administer cocaine. Neuropsychopharmacology. 2009;34(7):1774–1782. doi: 10.1038/npp.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May AC, Stewart JL, Migliorini R, Tapert SF, Paulus MP. Methamphetamine dependent individuals show attenuated brain response to pleasant interoceptive stimuli. Drug Alcohol Depend. 2013;131(3):238–246. doi: 10.1016/j.drugalcdep.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306(5695):503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struct Funct. 2010;214(5–6):535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose AJ, Hsu E, Monterosso J. The potent but inconsistent motivations characteristic of addiction. In: Wilson SJ, editor. The Wiley Handbook on the Cognitive Neuroscience of Addiction Wiley. Blackwell; Oxford: 2015. p. 440. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Beebe-Wang N, Woicik PA, Konova AB, Maloney T, Goldstein RZ. Choice to view cocaine images predicts concurrent and prospective drug use in cocaine addiction. Drug Alcohol Depend. 2013a;130(1–3):178–185. doi: 10.1016/j.drugalcdep.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Goldstein RZ. Impaired self-awareness in human addiction: deficient attribution of personal relevance. Trends Cogn Sci. 2014;18(12):635–641. doi: 10.1016/j.tics.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Konova AB, Parvaz MA, Tomasi D, Lane RD, Fort C, Goldstein RZ. Functional, structural, and emotional correlates of impaired insight in cocaine addiction. JAMA Psychiatry. 2014;71(1):61–70. doi: 10.1001/jamapsychiatry.2013.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Dunning JP, Alia-Klein N, Woicik PA, Hajcak G, Telang F, Wang GJ, Volkow ND, Goldstein RZ. Enhanced choice for viewing cocaine pictures in cocaine addiction. Biol Psychiatry. 2009;66(2):169–176. doi: 10.1016/j.biopsych.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Parvaz MA, Shumay E, Beebe-Wang N, Konova AB, Alia-Klein N, Volkow ND, Goldstein RZ. Gene × abstinence effects on drug cue reactivity in addiction: multimodal evidence. J Neurosci. 2013b;33(24):10027–10036. doi: 10.1523/JNEUROSCI.0695-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Stoops WW. Cocaine choice procedures in animals, humans, and treatment-seekers: can we bridge the divide? Pharmacol Biochem Behav. 2015;138:133–141. doi: 10.1016/j.pbb.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Berns GS. Neural economics and the biological substrates of valuation. Neuron. 2002;36(2):265–284. doi: 10.1016/s0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- Montague PR, Dayan P, Sejnowski TJ. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J Neurosci. 1996;16(5):1936–1947. doi: 10.1523/JNEUROSCI.16-05-01936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, King-Casas B, Cohen JD. Imaging valuation models in human choice. Annu Rev Neurosci. 2006;29:417–448. doi: 10.1146/annurev.neuro.29.051605.112903. [DOI] [PubMed] [Google Scholar]
- Monterosso J, Ainslie G. The behavioral economics of will in recovery from addiction. Drug Alcohol Depend. 2007;90(Suppl 1):S100–111. doi: 10.1016/j.drugalcdep.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso J, Piray P, Luo S. Neuroeconomics and the study of addiction. Biol Psychiatry. 2012;72(2):107–112. doi: 10.1016/j.biopsych.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007;28(5):383–393. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Luo S. An argument against dual valuation system competition: cognitive capacities supporting future orientation mediate rather than compete with visceral motivations. J Neurosci Psychol Econ. 2010;3(1):1–14. doi: 10.1037/a0016827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales AM, Kohno M, Robertson CL, Dean AC, Mandelkern MA, London ED. Midbrain dopamine D2/D3 receptor availability and drug craving are associated with mesocorticolimbic gray matter volume in methamphetamine users. Mol Psychiatry. 2015;20(6):658. doi: 10.1038/mp.2015.59. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214(5–6):435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesse RM, Berridge KC. Psychoactive drug use in evolutionary perspective. Science. 1997;278(5335):63–66. doi: 10.1126/science.278.5335.63. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. The problem with value. Neurosci Biobehav Rev. 2014;43:259–268. doi: 10.1016/j.neubiorev.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38(2):329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- Okita K, Ghahremani DG, Payer DE, Robertson CL, Dean AC, Mandelkern MA, London ED. Emotion dysregulation and amygdala dopamine D2-type receptor availability in methamphetamine users. Drug Alcohol Depend. 2016a;161:163–170. doi: 10.1016/j.drugalcdep.2016.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Ghahremani DG, Payer DE, Robertson CL, Mandelkern MA, London ED. Relationship of alexithymia ratings to dopamine D2-type receptors in anterior cingulate and insula of healthy control subjects but not methamphetamine-dependent individuals. Int J Neuropsychopharmacol. 2016b;19(5) doi: 10.1093/ijnp/pyv129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441(7090):223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Frank LR. Anterior cingulate activity modulates nonlinear decision weight function of uncertain prospects. Neuroimage. 2006;30(2):668–677. doi: 10.1016/j.neuroimage.2005.09.061. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19(4):1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schulteis G. The role of interoception and alliesthesia in addiction. Pharmacol Biochem Behav. 2009;94(1):1–7. doi: 10.1016/j.pbb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payer DE, Lieberman MD, London ED. Neural correlates of affect processing and aggression in methamphetamine dependence. Arch Gen Psychiatry. 2011;68(3):271–282. doi: 10.1001/archgenpsychiatry.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payer DE, Lieberman MD, Monterosso JR, Xu J, Fong TW, London ED. Differences in cortical activity between methamphetamine-dependent and healthy individuals performing a facial affect matching task. Drug Alcohol Depend. 2008;93(1–2):93–102. doi: 10.1016/j.drugalcdep.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry CJ, Zbukvic I, Kim JH, Lawrence AJ. Role of cues and contexts on drug-seeking behaviour. Br J Pharmacol. 2014;171(20):4636–4672. doi: 10.1111/bph.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442(7106):1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Buchel C. Overlapping and distinct neural systems code for subjective value during intertemporal and risky decision making. J Neurosci. 2009;29(50):15727–15734. doi: 10.1523/JNEUROSCI.3489-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Buchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66(1):138–148. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Moore WE, 3rd, Oswald TM, Mazziotta JC, Iacoboni M, Dapretto M. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69(5):1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Lempert KM, Sokol-Hessner P. Emotion and decision making: multiple modulatory neural circuits. Annu Rev Neurosci. 2014;37:263–287. doi: 10.1146/annurev-neuro-071013-014119. [DOI] [PubMed] [Google Scholar]
- Platt ML, Huettel SA. Risky business: the neuroeconomics of decision making under uncertainty. Nat Neurosci. 2008;11(4):398–403. doi: 10.1038/nn2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt ML, Watson KK, Hayden BY, Shepherd SV, Klein JT. Neuroeconomics: implications for understanding the neurobiology of addiction. In: Kuhn CM, Koob GF, editors. Advances in the Neuroscience of Addiction. CRC Press/Taylor & Francis; Boca Raton (FL): 2010. [PubMed] [Google Scholar]
- Preller KH, Herdener M, Schilbach L, Stampfli P, Hulka LM, Vonmoos M, Ingold N, Vogeley K, Tobler PN, Seifritz E, Quednow BB. Functional changes of the reward system underlie blunted response to social gaze in cocaine users. Proc Natl Acad Sci U S A. 2014;111(7):2842–2847. doi: 10.1073/pnas.1317090111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoff K, Bossaerts P, Quartz SR. Neural differentiation of expected reward and risk in human subcortical structures. Neuron. 2006;51(3):381–390. doi: 10.1016/j.neuron.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28(11):2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunathan R, Pham MT. All negative moods are not equal: motivational influences of anxiety and sadness on decision making. Organ Behav Hum Decis Process. 1999;79(1):56–77. doi: 10.1006/obhd.1999.2838. [DOI] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9(7):545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H, Korczykowski M, Pluta J, Hoang A, Detre JA. Neural correlates of voluntary and involuntary risk taking in the human brain: an fMRI Study of the Balloon Analog Risk Task (BART) Neuroimage. 2008;42(2):902–910. doi: 10.1016/j.neuroimage.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD. Addiction as a computational process gone awry. Science. 2004;306(5703):1944–1947. doi: 10.1126/science.1102384. [DOI] [PubMed] [Google Scholar]
- Reske M, Stewart JL, Flagan TM, Paulus MP. Attenuated neural processing of risk in young adults at risk for stimulant dependence. PLoS One. 2015;10(6):e0127010. doi: 10.1371/journal.pone.0127010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Zahm DS. Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J Neurosci. 2005;25(50):11757–11767. doi: 10.1523/JNEUROSCI.3432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn W, Desai N, Rosenblatt H, Gastfriend DR. Addiction denial and cognitive dysfunction: a preliminary investigation. J Neuropsychiatry Clin Neurosci. 2002;14(1):52–57. doi: 10.1176/jnp.14.1.52. [DOI] [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffmann RG, Bloom AS, Garavan H, Stein EA. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage. 2005;26(4):1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20(4):322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Roiser JP, de Martino B, Tan GC, Kumaran D, Seymour B, Wood NW, Dolan RJ. A genetically mediated bias in decision making driven by failure of amygdala control. J Neurosci. 2009;29(18):5985–5991. doi: 10.1523/JNEUROSCI.0407-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson P. A note on measurement of utility. Rev Econ Stud. 1937;4:155–161. [Google Scholar]
- Samuelson PA. Foundations of Economic Analysis. Harvard University Press; Cambridge Mass.: 1947. [Google Scholar]
- Sanfey AG. Social decision-making: insights from game theory and neuroscience. Science. 2007;318(5850):598–602. doi: 10.1126/science.1142996. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006;29(2):116–124. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg T, Fox CR, Poldrack RA. Mind the gap: bridging economic and naturalistic risk-taking with cognitive neuroscience. Trends Cogn Sci. 2011;15(1):11–19. doi: 10.1016/j.tics.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct. 2010;6:24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Sell LA, Morris J, Bearn J, Frackowiak RS, Friston KJ, Dolan RJ. Activation of reward circuitry in human opiate addicts. Eur J Neurosci. 1999;11(3):1042–1048. doi: 10.1046/j.1460-9568.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- Sharp C, Monterosso J, Montague PR. Neuroeconomics: a bridge for translational research. Biol Psychiatry. 2012;72(2):87–92. doi: 10.1016/j.biopsych.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffer CE, Christensen DR, Landes R, Carter LP, Jackson L, Bickel WK. Delay discounting rates: A strong prognostic indicator of smoking relapse. Addict Behav. 2014;39(11):1682–1689. doi: 10.1016/j.addbeh.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron. 2013;79(2):217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13(8):334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Smith DG, Jones PS, Williams GB, Bullmore ET, Robbins TW, Ersche KD. Overlapping decline in orbitofrontal gray matter volume related to cocaine use and body mass index. Addict Biol. 2015;20(1):194–196. doi: 10.1111/adb.12081. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Graham S, O’Brien L, Woolard J, Cauffman E, Banich M. Age differences in future orientation and delay discounting. Child Dev. 2009;80(1):28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- Stewart JL, Connolly CG, May AC, Tapert SF, Wittmann M, Paulus MP. Striatum and insula dysfunction during reinforcement learning differentiates abstinent and relapsed methamphetamine-dependent individuals. Addiction. 2014a;109(3):460–471. doi: 10.1111/add.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, May AC, Poppa T, Davenport PW, Tapert SF, Paulus MP. You are the danger: attenuated insula response in methamphetamine users during aversive interoceptive decision-making. Drug Alcohol Depend. 2014b;142:110–119. doi: 10.1016/j.drugalcdep.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler R. Some empirical evidence on dynamic inconsistency. Economics Letters. 1981;8(3):201–207. [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24(26):6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlin D, Nedic A, Prentice DA, Holmes P, Cohen JD. The neural substrates of social influence on decision making. PLoS One. 2013;8(1):e52630. doi: 10.1371/journal.pone.0052630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepel C, Fox CR, Poldrack RA. Prospect theory on the brain? Toward a cognitive neuroscience of decision under risk. Brain Res Cogn Brain Res. 2005;23(1):34–50. doi: 10.1016/j.cogbrainres.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Trifilieff P, Martinez D. Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacology. 2014;76(Pt B):498–509. doi: 10.1016/j.neuropharm.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. The framing of decisions and the psychology of choice. Science. 1981;211(4481):453–458. doi: 10.1126/science.7455683. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. Rational choice and the framing of decisions. In: Karpak B, Zionts S, editors. Multiple Criteria Decision Making and Risk Analysis Using Microcomputers. Springer; Berlin Heidelberg, Berlin, Heidelberg: 1989. pp. 81–126. [Google Scholar]
- Vaidya JG, Block RI, O’Leary DS, Ponto LB, Ghoneim MM, Bechara A. Effects of chronic marijuana use on brain activity during monetary decision-making. Neuropsychopharmacology. 2012;37(3):618–629. doi: 10.1038/npp.2011.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van ’t Wout M, Kahn RS, Sanfey AG, Aleman A. Affective state and decision-making in the Ultimatum Game. Exp Brain Res. 2006;169(4):564–568. doi: 10.1007/s00221-006-0346-5. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, Laruelle M, Baldwin RM, Innis RB, Gelernter J. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. J Nucl Med. 2005;46(5):745–751. [PubMed] [Google Scholar]
- Van Hoorn J, Van Dijk E, Guroglu B, Crone EA. Neural correlates of prosocial peer influence on public goods game donations during adolescence. Soc Cogn Affect Neurosci. 2016;11(6):923–933. doi: 10.1093/scan/nsw013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Clark L, Dunn BD. The role of interoception in addiction: a critical review. Neurosci Biobehav Rev. 2012;36(8):1857–1869. doi: 10.1016/j.neubiorev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Jayne M, Ma Y, Pradhan K, Wong C, Swanson JM. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2010;49(3):2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26(24):6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GB, Zhang XL, Zhao LY, Sun LL, Wu P, Lu L, Shi J. Drug-related cues exacerbate decision making and increase craving in heroin addicts at different abstinence times. Psychopharmacology (Berl) 2012;221(4):701–708. doi: 10.1007/s00213-011-2617-5. [DOI] [PubMed] [Google Scholar]
- Weber BJ, Huettel SA. The neural substrates of probabilistic and intertemporal decision making. Brain research. 2008;1234:104–115. doi: 10.1016/j.brainres.2008.07.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welborn BL, Lieberman MD, Goldenberg D, Fuligni AJ, Galvan A, Telzer EH. Neural mechanisms of social influence in adolescence. Soc Cogn Affect Neurosci. 2016;11(1):100–109. doi: 10.1093/scan/nsv095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley MJ, Hanlon CA, Porrino LJ. Poor decision-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiatry Res. 2011;191(1):51–59. doi: 10.1016/j.pscychresns.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley MJ, Lohrenz T, Koffarnus MN, McClure SM, De La Garza R, 2nd, Salas R, Thompson-Lake DG, Newton TF, Bickel WK, Montague PR. Choosing money over drugs: the neural underpinnings of difficult choice in chronic cocaine users. J Addict. 2014;2014:189853. doi: 10.1155/2014/189853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7(3):211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright ND, Symmonds M, Hodgson K, Fitzgerald TH, Crawford B, Dolan RJ. Approach-avoidance processes contribute to dissociable impacts of risk and loss on choice. J Neurosci. 2012;32(20):7009–7020. doi: 10.1523/JNEUROSCI.0049-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacubian J, Buchel C. The genetic basis of individual differences in reward processing and the link to addictive behavior and social cognition. Neuroscience. 2009;164(1):55–71. doi: 10.1016/j.neuroscience.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Yi R, Johnson MW, Giordano LA, Landes RD, Badger GJ, Bickel WK. The effects of reduced cigarette smoking on discounting future rewards: an initial evaluation. Psychol Rec. 2008;58(2):163–174. doi: 10.1007/bf03395609. [DOI] [PMC free article] [PubMed] [Google Scholar]


