Abstract
Prenatal alcohol exposure can impact both brain development and neurobehavioral function, including verbal learning and recall, although the relation between verbal recall and brain structure in this population has not been examined fully. We aimed to determine the structural neural correlates of verbal learning and recall in youth with histories of heavy prenatal alcohol exposure using a region of interest (ROI) approach.
As part of an ongoing multisite project, subjects (age 10–16 years) with prenatal alcohol exposure (AE, n= 81) and controls (CON, n= 81) were tested using the CVLT-C and measures of cortical volume, surface area, and thickness as well as hippocampal volume were derived from MRI. Group differences in brain and memory indices were tested with ANOVA. Multiple regression analyses tested whether brain ROIs significantly predicted memory performance.
The AE group had lower scores than the CON group on all CVLT-C variables (ps≤.001) and volume and surface area (ps<.025), although results varied by ROI. No group differences in cortical thickness were found. The relations between cortical structure and memory performance differed between group among some ROIs, particularly those in the frontal cortex, generally with smaller surface area and/or thinner cortex predicting better performance in CON but worse performance in AE.
Cortical surface area appears to be the most sensitive index to the effects of prenatal alcohol exposure, while cortical thickness appears to be the least sensitive. These findings also indicate that the neural correlates of verbal memory are altered in youth with heavy prenatal alcohol exposure compared to controls.
Keywords: Fetal Alcohol Spectrum Disorders (FASD), Prenatal Alcohol Exposure, Memory, Neurobehavioral Profile, Brain
Introduction
Prenatal alcohol exposure can lead to aberrant development, impacting physical features, growth, and organ systems. The range of detrimental outcomes caused by prenatal alcohol exposure is referred to as fetal alcohol spectrum disorders (FASD). Importantly, prenatal alcohol exposure can have particularly harmful effects on the brain (Riley, Infante, & Warren, 2011). A number of brain structural differences have been observed following such exposure. In general, those with FASD have smaller whole brain volumes compared to controls (reviewed in Lebel, Roussotte, & Sowell, 2011; Moore, Migliorini, Infante, & Riley, 2014). Furthermore, the impact of prenatal alcohol exposure on the brain translates into functional impairment, as individuals with FASD often display wide-ranging neurobehavioral deficits (Mattson, Crocker, & Nguyen, 2011).
A number of studies report verbal learning and recall deficits in children with prenatal alcohol exposure (for review, see Manji, Pei, Loomes, & Rasmussen, 2009), though there is evidence for a dose-dependent response for certain aspects of memory (Lewis et al., 2015). On word learning tasks, children with FASD not only learn fewer words (Mattson, Riley, Gramling, Delis, & Jones, 1998; Lewis et al., 2015; Mattson & Roebuck, 2002; Willoughby, Sheard, Nash, & Rovet, 2008) but also learn them at a slower rate (Mattson & Roebuck, 2002) compared to controls. In addition, alcohol-exposed children recall fewer words than control children after both short and long delays. However, once initial learning is accounted for, children with FASD often retain as many words as their peers (Crocker, Vaurio, Riley, & Mattson, 2011; Mattson & Roebuck, 2002; Lewis et al. 2015). However, it is interesting to note that in a sample of children with moderate prenatal alcohol exposure, the effects of alcohol exposure remained significant after controlling for differences in initial learning (Lewis et al., 2015). On recognition testing, children with prenatal alcohol exposure are less accurate in discriminating between words to which they were exposed during the learning trials and novel words from the same semantic categories (Crocker et al., 2011) although this impairment has not been examined in relation to initial learning. Importantly, these verbal deficits do not appear to be solely the result of general reductions in intellectual functioning (Vaurio, Riley, & Mattson, 2011).
Verbal learning and memory requires input from a number of brain regions that are affected in children with FASD. For example, individuals with prenatal alcohol exposure have smaller volumes (Chen, Coles, Lynch, & Hu, 2012; Coles et al., 2011; Rajaprakash, Chakravarty, Lerch, & Rovet, 2014) and surface areas (Rajaprakash et al., 2014) in the frontal cortex compared to controls, although research on cortical thickness has produced mixed results (Fernandez-Jaen et al., 2011; Rajaprakash et al., 2014; Robertson et al., 2016; Yang et al., 2012; Zhou et al., 2011). As cortical thickness and surface area have distinct developmental trajectories in typically developing populations (Giedd et al., 2015; Lenroot & Giedd, 2008), and at least some aspects of neurodevelopment appear to be altered in subjects with prenatal alcohol exposure (Gautam et al., 2015; Lebel et al., 2012), some of these discrepancies may be result of variance being masked or enhanced due to the age range of the sample (e.g. 8 –16 years vs. 6–30 years). The frontal cortex is involved in memory storage as well as memory retrieval in both healthy subjects and clinical populations (Biesbroek et al., 2015; Gluck, Mercado, & Myers, 2007; Wilde et al., 2011). In typically developing controls, thinner cortex and smaller volume of the frontal lobe is associated with better verbal recall performance (Østby, Tamnes, Fjell, & Walhovd, 2011; Sowell, Delis, Stiles, & Jernigan, 2001; Tamnes et al., 2013). However, the opposite pattern has been seen in children with FASD: thinner frontal cortex is associated with poorer verbal memory ability (Sowell et al., 2008).
Similarly, researchers have reported smaller overall volume of the temporal lobe (Rajaprakash et al., 2014), as well as smaller volume of inferior temporal gyrus (Chen et al., 2012) and parahippocampal gyrus (Coles et al., 2011) in young adults with FASD. Furthermore, thinner cortex in the temporal lobe has been associated with better memory performance in typically developing children and young adults (Østby et al., 2011; Sowell et al., 2001). However, in contrast to typically developing children, research on children with prenatal alcohol exposure has not found significant relationships between memory ability and either temporal lobe volume or cortical thickness (Fryer et al., 2012; Sowell et al., 2008).
Animal studies have found that the hippocampus is a brain region that is highly sensitive to prenatal alcohol exposure (Gil-Mohapel, Boehme, Kainer, & Christie, 2010). However, magnetic resonance imaging studies have been inconsistent regarding structural changes in this region among those with FASD. Many studies have found hippocampal volume is lower compared to controls (Coles, Lynch, Kable, Johnson, & Goldstein, 2010; Nardelli, Lebel, Rasmussen, Andrew, & Beaulieu, 2011; Treit et al., 2013), though not always when overall brain volume is controlled (Archibald et al., 2001; Treit et al., 2013). However, other researchers have not found consistent evidence of volume differences (Autti-Rämö et al., 2002; Coles et al., 2011; Joseph et al., 2014). Given that a wide body of work has demonstrated that the hippocampus plays an important role in memory acquisition and consolidation (reviewed in Gluck et al., 2007), the impact of prenatal alcohol exposure may be more evident in the relation between hippocampal volume and verbal memory performance. In typical young adults, smaller hippocampi are related to better memory performance (Van Petten, 2004). However, in one study of adults with prenatal alcohol exposure and dysmorphic features, greater volume of the right hippocampus was associated with more efficient encoding and better recall of verbal information (Coles et al., 2011). In children with FASD, hippocampal volume also has a positive, though modest, relationship with verbal recall scores (Willoughby et al., 2008).
Despite the amount of research examining the structural neural correlates of memory, studies have not yet examined the specific relationships between different brain morphometrics and verbal learning/recall in those with FASD. We sought to expand on previous studies examining the structural neural correlates of verbal memory in children and adolescents with heavy prenatal alcohol exposure by using a large multisite sample as part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), multivariate statistical techniques, and examining multiple neuroanatomical morphometrics. The following hypotheses were tested: (1) subjects with FASD will have significantly lower learning, recall, and recognition scores on the CVLT-C than controls, though based on previous work we expected that retention scores (i.e., recall after a short delay, accounting for initial learning) would be comparable between groups; (2) subjects in the alcohol-exposed group will show lower volume in cortical regions of interest (ROI) and that these differences would primarily be driven by effects on surface area rather than thickness (as seen in Migliorini et al. 2015); (3) subjects with prenatal alcohol exposure will have disproportionally smaller hippocampal volumes than controls; (4) the relationships between memory performance and brain morphometry will be different between subjects with FASD and controls. Specifically we expect that better performance will be associated with smaller volume, surface area, and thickness in controls; however, we predict the opposite pattern will be observed in alcohol-exposed youth (as seen in Glass et al. 2017).
Material and Methods
General Method
Data were collected as part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders Phase III (CIFASD-III), a multisite research project examining the effects of heavy prenatal alcohol exposure on children and adolescents. CIFASD sites included: the Center for Behavioral Teratology at San Diego State University, The University of Minnesota FASD Program, Emory University, and the Children’s Hospital at the University of Southern California. General CIFASD methodology has been described previously (Mattson et al., 2010). In brief, as part of a larger protocol, each subject completed a comprehensive neuropsychological battery and underwent magnetic resonance imaging (MRI). Written consent was obtained from the caregiver of each subject and assent was obtained from each child. Subjects were financially compensated for their participation. The Institutional Review Boards at each site approved the procedures.
Subjects
Subjects were 10–16 years of age and comprised two groups: individuals with histories of heavy prenatal alcohol exposure (AE) and controls with minimal or no alcohol exposure (CON). As part of the ongoing CIFASD-III project, subjects were recruited through multiple methods, including community outreach, advertising, professional referral, and word of mouth. Subjects were included in the AE group if they had confirmed histories of heavy prenatal alcohol exposure. Positive alcohol exposure histories were confirmed retrospectively through medical records, birth records, social services records, or maternal report. When maternal report was available, heavy prenatal alcohol exposure was defined as an average of >13 drinks per week or >4 alcoholic drinks per occasion at least once per week. However, for the majority of cases (67.9%), direct maternal report was unavailable. In these cases, subjects were considered heavily exposed when collateral documentation indicated alcohol dependence or abuse during pregnancy. Additionally, children were included in the AE group if alcohol exposure was suspected and the child met criteria for a diagnosis of fetal alcohol syndrome (FAS). A member of the CIFASD dysmorphology core examined all subjects for physical features consistent with an FAS diagnosis (Jones et al., 2006; Mattson et al., 2010).
Subjects were included in the CON group if they had minimal or no prenatal alcohol exposure. When direct maternal report was available (90.12% of cases), minimal exposure was defined as no more than 1 drink per week on average and never more than 2 drinks per occasion. A dysmorphology exam indicating physical features consistent with FAS was exclusionary for the control group, even when alcohol use during pregnancy was denied. Exclusion criteria for both groups included: MRI contraindication, non-fluency in English, history of significant head injury or loss of consciousness >30 minutes, international adoptions occurring after age 5, or significant psychiatric or physical disabilities that prevented study completion.
Subjects with MRI data and neuropsychological testing collected within the same three-month period were included in the analyses. Based on the above criteria, the final sample consisted of 162 subjects: 81 AE subjects and 81 CON subjects.
Measures
California Verbal Learning Test-Children’s Version (CVLT-C)
Children were administered the CVLT-C as part of a larger neuropsychological battery. The CVLT-C is a measure designed to assess verbal learning and recall in children aged 5 to 16 (Delis, Kramer, Kaplan, & Ober, 1994). The dependent variables were Total Learning on List A (Total Learning-A), Short Delay Free Recall, Long Delay Free Recall, Retention, and Recognition Discriminability scores (see Table 1 for detailed descriptions).
Table 1.
Descriptions of California Verbal Learning Test – Children’s Version (CVLT-C) Variables
| Variable | Score Type | Description | |
|---|---|---|---|
| CVLT-C | Total Learning-A | T-score | Total correct immediate free recall responses for List A (15 items) trials 1–5. |
| Short Delay Free Recall | Z-score | Total correct free recall responses for List A after a short delay. | |
| Long Delay Free Recall | Z-score | Total correct free recall responses for List A after a long delay (20 minutes). | |
| Retention | Raw score | Raw long delay free recall, covarying by Trial A5 raw score | |
| Recognition Discriminability | Z-score | Ability of the child to discriminate target words from distractors (45 total items). [1-(False Positives+Misses)/45)*100] |
CVLT-C, California Verbal Learning Test, Children’s Version
Note: Standard administration procedures were used. List B was administered but was not analyzed in this study.
MRI data acquisition and analysis
High resolution, T1-weighted MRI scans were collected. Scanning parameters are presented in Table 2. Imaging protocols were designed to be as consistent as possible across sites, though a scanner software upgrade in San Diego resulted in parameter changes partway through the project. The scanner protocols were based on those developed by the Pediatric Imaging, Neurocognition, and Genetics (PING) project (http://pingstudy.ucsd.edu/resources/neuroimaging-cores.html), which were designed in order to minimize between-site differences. As a further precaution, site was evaluated as a potential covariate for all statistical analyses, and included in analyses when covariate inclusion criteria were met, in order to account for any location or scanner-based differences. Data were analyzed using FreeSurfer version 5.1 (http://surfer.nmr.mgh.harvard.edu/). T1-weighted anatomical scans were analyzed using the standard FreeSurfer processing stream, which includes surface-based registration and anatomical parcellation, the details of which have been described in previous publications (Dale, Fischl, & Sereno, 1999; Fischl, Liu, & Dale, 2001; Fischl et al., 2002; Fischl et al., 2004). Manual edits were made when necessary, and subjects with poor quality scan data (e.g., due to motion artifact) were excluded from analyses (n = 5, all AE subjects). Volume, surface area, and cortical thickness values for each cortical ROI (see Table 3) and subcortical volumes for the hippocampus were extracted for analysis. These ROIs were chosen based on previous literature that indicated they were related with aspects of verbal memory.
Table 2.
Neuroimaging Scan Parameters for Each Study Site
| San Diego* | Los Angeles | Atlanta | Minnesota | |
|---|---|---|---|---|
| Scanner | 3T GE Signa Excite | 3T Phillips Achieva | 3T Siemens TrioTim | 3T Siemens TrioTim |
| TR | 7.648 ms/ 7.38 ms | 6.767 ms | 2170 ms | 2170 ms |
| TE | 3.040 ms/ 2.984 ms | 3.196 ms | 4.330 ms | 4.330 ms |
| Flip Angle | 8°/8° | 8° | 7° | 7° |
| Matrix | 256 × 256/ 256 × 192 | 256 × 256 | 256 × 256 | 256 × 256 |
| Slice Thickness | 1.0 mm/1.2 mm | 1.2 mm | 1.0 mm | 1.0 mm |
| FOV | 256 mm/ 240 mm | 256 mm | 256 mm | 256 mm |
TR, Repetition time; TE, Echo time; FOV, Field of view
A scanner software upgrade in San Diego resulted in parameter changes during the study
Table 3.
Descriptions of Brain Structure Variables
| ROI | FreeSurfer Regions of Interest |
|---|---|
| Frontala | Rostral, caudal middle frontal gyrus |
| Rostral, caudal anterior cingulate gyrus | |
| Pars Opercularis | |
| Pars Orbitalis | |
| Pars Triangularis | |
| Temporala | Entorhinal cortex |
| Parahippocampal cortex | |
| Temporal pole | |
| Inferior temporal gyrus | |
| Middle temporal gyrus | |
| Superior temporal gyrus | |
| Hippocampusb | Hippocampus |
Regions were analyzed separately by hemisphere
Cortical volume, surface area, and thickness
Cortical volume data only
Statistical Analyses
All analyses were conducted using SPSS v.22. Univariate outliers were assessed using box plots. Univariate outliers were determined separately for each group and sex. Subjects with values greater than 1.5 × the interquartile range (IQR) on an observed variable were removed from the corresponding analyses. Outliers were eliminated on a measure-by-measure basis in order to conserve statistical power. Although neuronataomical outliers were assessed individually, most subjects who were outliers in one lobe were also outliers in others. Very few subjects had outliers in a single lobe or hemisphere. Multivariate outliers were assessed using Mahalanobis distance and removed if the Mahalanobis distance χ2 values were significant (p<001). The sample sizes for each analysis after removing the corresponding outliers are displayed in Table 4.
Table 4.
Number of Subjects and Covariates for each Multivariate Analysis
| Test | Covariates | n | |
|---|---|---|---|
| AE | CON | ||
| CVLT-C | Age | 76 | 73 |
| V L frontal | Site, age*, ethnicity*, ICV | 76 | 72 |
| T L frontal | Site, ethnicity, ICV | 68 | 72 |
| A L frontal | Site, ethnicity*, race*, ICV | 74 | 76 |
| V R frontal | Sex, site, ICV | 76 | 73 |
| T R frontal | Age, site, ICV | 67 | 73 |
| A R frontal | Site, ethnicity*, ICV | 73 | 77 |
| V L temporal | Site, ICV | 73 | 76 |
| T L temporal | Site, race*, ICV | 74 | 74 |
| A L temporal | Site, ICV | 74 | 79 |
| V R temporal | Site, race*, ICV | 69 | 72 |
| T R temporal | Site, race*, ICV | 72 | 75 |
| A R temporal | Site, ICV | 76 | 77 |
| V L/R Hippocampus | Site, race*, ICV | 80 | 80 |
Did not explain significant variance, removed from analyses
AE, Alcohol-Exposed Group; CON, Control Group; CVLT-C, California Verbal Learning Test, Children’s Version; V, volume; T, thickness; A, surface area; L, left hemisphere; R, right hemisphere; ICV, intracranial volume.
Subject characteristics
Subject sex, race, ethnicity, testing site, and handedness were analyzed using Pearson’s chi square tests. Age and General Cognitive Ability scores (GCA) were evaluated using analysis of variance (ANOVA).
Evaluation of covariates
Demographic variables (age, sex, race, ethnicity, and handedness) and scan site were evaluated as potential covariates. GCA was not considered as a potential covariate because covarying scores by cognitive functioning can provide either over- or underestimates of abilities (Dennis et al., 2009). For inclusion as a covariate, the variable had to be linearly related to the dependent variable(s) and had to not significantly interact with group. All covariates that met these criteria were included in the subsequent analyses.
As microcephaly is common in individuals with FASD, intracranial volume (ICV) is often used as a covariate to determine if observed differences are beyond the effects of smaller brain size. However, using ICV as a covariate can under- or over-scale results, as ICV does not have a proportionate relationship with cortical measures (Im et al., 2008). Initial analyses were run without using ICV as a covariate, and these results were interpreted as evidence of group differences in neuroanatomy. However, we acknowledge the importance of identifying whether group differences persisted after accounting for variance attributed to ICV. Therefore, significant results were rerun with inclusion of ICV as a covariate, and these results are also reported.
Neuropsychological data
Group differences on CVLT-C measures were assessed using multivariate analysis of covariance (MANCOVA). The within-subjects factors were CVLT-C scores (Total Learning-A, Short Delay Free Recall, Long Delay Free Recall, and Recognition Discriminability), and the between-subjects factor was Group (AE, CON). Sex was considered as a between subjects factor, but as there were no significant interactions or main effects, it was removed for parsimony. A significant main effect of group was followed up using ANCOVAs for each individual CVLT-C variable.
The Retention variable was not included in the MANOVA because it was highly correlated with Long Delay Free Recall scaled score, which was unsurprising given that the Retention score is the Long Delay Free Recall raw score adjusted with the raw score from learning trial A5. Instead, Retention was analyzed separately with an ANCOVA, similar to previous studies (Crocker et al., 2011; Mattson & Roebuck, 2002). The dependent variable was the Long Delay Free Recall raw score. The between-subjects factor was Group, with the raw score from learning trial A5 as well as covariates that met inclusion criteria. This technique adjusts for any group differences in initial learning that may account for the differences observed in verbal memory (Mattson & Roebuck, 2002). CVLT-C analyses were corrected for multiple comparisons using the Holm-Bonferroni method, adjusting for the five CVLT-C variables used (αaltered = .01 = .05/5; Holm, 1979).
Neuroimaging data
Twelve separate MANCOVAs were run for the cortical ROIs: 2 hemispheres (right, left) × 3 morphometrics (surface area, thickness, volume) × 2 cortical ROIs (frontal, temporal). The dependent variables for the frontal and temporal ROI MANCOVAs are shown in Table 3. An additional MANCOVA was run to examine the hippocampus, with right and left volumes as the dependent variables. For all analyses, between subjects variables were Group and Sex. Significant effects were followed up using ANCOVAs for each dependent variable. Significant MANCOVAs were also rerun adding ICV as a covariate. Results were corrected for multiple family-wise comparisons using the Holm-Bonferroni method, adjusting for analyses conducted in the bilateral frontal and temporal areas (αaltered = .025 = .05/4; Holm, 1979).
Neural correlates of memory
The relationships between CVLT-C scores and brain structures were assessed using separate multiple regression analyses. Individual CVLT-C variables were used as the dependent variables with Group, covariates that met inclusion criteria, and brain regions as predictors. Group by ROI interactions were also evaluated. Sex and its corresponding interactions with Group and ROI were included as predictors, however nonsignificant interactions were removed from the model for parsimony and sex was only retained as a covariate if statistically appropriate. Results were corrected for multiple family-wise comparisons using the Holm-Bonferroni method (adjusting for the number of subregions analyzed within each ROI (For frontal, αaltered = .007 = .05/7; for temporal, αaltered = .008 = .05/6; for hippocampus, αaltered = .025 = .05/2; Holm, 1979).
Results
Demographics
Demographic data for the AE and CON groups are presented in Table 5. Analyses comparing AE vs. CON across the entire sample indicated that there were no differences between the groups in the distribution of sex, handedness, ethnicity, race, or site (ps≥269). However, the groups did significantly differ on age (p=.022, AE<CON) and GCA (p<001, AE<CON).
Table 5.
Demographic Data for Children and Adolescents with Prenatal Alcohol Exposure (AE) and Typically Developing Controls (CON), Across and Within Sites
| AE | CON | |
|---|---|---|
| (n= 81) | (n= 81) | |
| Sex [n (% Female)] | 33 (40.7) | 40 (49.4) |
| Age [M (SD)]* | 12.9 (2.06) | 13.6 (2.16) |
| Handedness [n (% Right) | 72 (88.9) | 69 (85.2) |
| Ethnicity [n (% Hispanic)] | 15 (18.5) | 15 (18.5) |
| Race [n (% White)] | 46 (56.8) | 40 (49.4) |
| GCA [M (SD)]* | 90.6 (11.83) | 103.0 (15.57) |
| FAS [n (% Positive)] | 9 (11.1) | 0 (0.0) |
|
| ||
| Atlanta [n (% of Total)] | 12 (14.8) | 14 (17.3) |
| Sex [n (% Female)] | 5 (41.7) | 9 (64.3) |
| Age [M (SD)] | 12.5 (2.28) | 13.8 (1.94) |
| Handedness [n (% Right) | 11 (91.7) | 14 (100.0) |
| Ethnicity [n (% Hispanic)] | 1 (8.3) | 0 (0.0) |
| Race [n (% White)] | 6 (50.0) | 0 (0.0) |
| GCA [M (SD)] | 87.8 (8.45) | 91.7 (13.99) |
| FAS [n (% Positive)] | 0 (0.0) | 0 (0.0) |
|
| ||
| Los Angeles [n (% of Total)] | 15 (18.5) | 22 (27.2) |
| Sex [n (% Female)] | 5 (33.3) | 12 (54.5) |
| Age [M (SD)] | 12.7 (2.03) | 13.3 (1.91) |
| Handedness [n (% Right) | 13 (86.7) | 19 (86.4) |
| Ethnicity [n (% Hispanic)] | 1 (6.7) | 12 (54.5) |
| Race [n (% White)] | 7 (46.7) | 12 (54.5) |
| GCA [M (SD)] | 93.6 (13.37) | 96.3 (9.78) |
| FAS [n (% Positive)] | 2 (13.3) | 0 (0.0) |
|
| ||
| Minnesota [n (% of Total)] | 27 (33.3) | 27 (33.3) |
| Sex [n (% Female)] | 15 (55.6) | 12 (44.4) |
| Age [M (SD)] | 12.2 (1.68) | 14.3 (1.99) |
| Handedness [n (% Right) | 25 (92.6) | 19 (70.4) |
| Ethnicity [n (% Hispanic)] | 4 (14.8) | 0 (0.0) |
| Race [n (% White)] | 15 (55.6) | 19 (70.4) |
| GCA [M (SD)] | 93.0 (12.59) | 110.1 (16.90) |
| FAS [n (% Positive)] | 2 (7.4) | 0 (0.0) |
|
| ||
| San Diego [n (% of Total)] | 27 (33.3) | 18 (22.2) |
| Sex [n (% Female)] | 8 (29.6) | 7 (38.9) |
| Age [M (SD)]* | 13.7 (2.15) | 13.0 (2.71) |
| Handedness [n (% Right) | 23 (85.2) | 17 (94.4) |
| Ethnicity [n (% Hispanic)] | 9 (33.3) | 3 (16.7) |
| Race [n (% White)] | 18 (66.7) | 9 (50.0) |
| GCA [M (SD)]* | 87.9 (11.18) | 109.6 (12.14) |
| FAS [n (% Positive)] | 5 (18.5) | 0 (0.0) |
Significant differences between groups (p<.05)
AE, Alcohol-Exposed Group; CON, Control Group; GCA, General Cognitive Ability; FAS, Fetal Alcohol Syndrome
Analysis of Covariates
With the exception of handedness, all potential covariates were significantly correlated with at least one dependent variable. The homogeneity of regression assumption was evaluated separately for each multivariate test. Covariates that met criteria for inclusion and number of subjects for each statistical test (after removing outliers as described above) are listed in Table 4.
CVLT-C
A MANCOVA was conducted to assess memory performance on the CVLT-C, where Group was the between subjects variable, Age was a covariate, and Total Learning-A, Short Delay Free Recall, Long Delay Free Recall, and Recognition Discriminability were the dependent variables. There was a significant main effect of Group on the CVLT-C latent variable [F (4, 143)= 11.146, p <.001, η2=.238]. The AE group had significantly lower scores than the CON group on all of the CVLT-C observed variables (ps<.001). Unadjusted group means for each observed variable are presented in Table 6. Age was only significantly correlated with one observed CVLT-C variable (Recognition Discriminability), the analysis was rerun without covariates—there were no changes in results [F (5, 144)= 9.390, p <.001, η2=.207; AE<CON on all CVLT-C variables p <.001].
Table 6.
Mean Scores on the CVLT-C for Youth with Prenatal Alcohol Exposure (AE) and Controls (CON)
| Variable | AE [M (SD)] | CON [M (SD)] | p |
|---|---|---|---|
| Total Learning-A (T score) | 47.9 (10.64) | 55.0 (8.02) | <.001 |
| Short Delay Free Recall (Z score) | −.329 (.937) | .363 (.783) | <.001 |
| Long Delay Free Recall (Z score) | −.237 (.922) | .527 (.629) | <.001 |
| Recognition Discriminability (Z score) | −.079 (1.04) | .473 (.381) | <.001 |
| Long Delay Free Recall Adj. (raw) | 10.0 (1.33) | 12.3 (1.28) | <.001 |
AE, Alcohol-Exposed Group; CON, Control Group; CVLT-C, California Verbal Learning Test, Children’s Version
An ANCOVA was conducted to assess the relationship between Group and Retention. Group membership predicted Long Delay Free Recall (raw score) after covarying for scores from Learning Trial A5 (raw) and Age. The omnibus test was significant [F(3, 145)=67.474, p <.001, η2=.583]. The main effect of Group was also significant [F(1, 145)=22.274, p <.001, η2=.133]. Controlling for Age and learning on trial A5, subjects in the AE group retained significantly less information than the CON group (see Table 6). This finding differs from that in the literature, which has typically shown no differences between AE and CON on retention of learned information. It is possible that our large sample allowed us sufficient power to detect a statistically significant effect that has little practical or clinical significance. Therefore, we used a cutoff of 1.5 SD below the control mean as a threshold of clinical significance, and evaluated the proportion of children within each group who demonstrated clinically poor performance using a Pearson’s chi square analysis. By this standard, 49.4% of AE children had retention scores at least 1.5 SD below the control mean, which is significantly more than in the CON group (4.9%, χ2=39.782, p <.001).
Group Differences on Neuroanatomical Variables
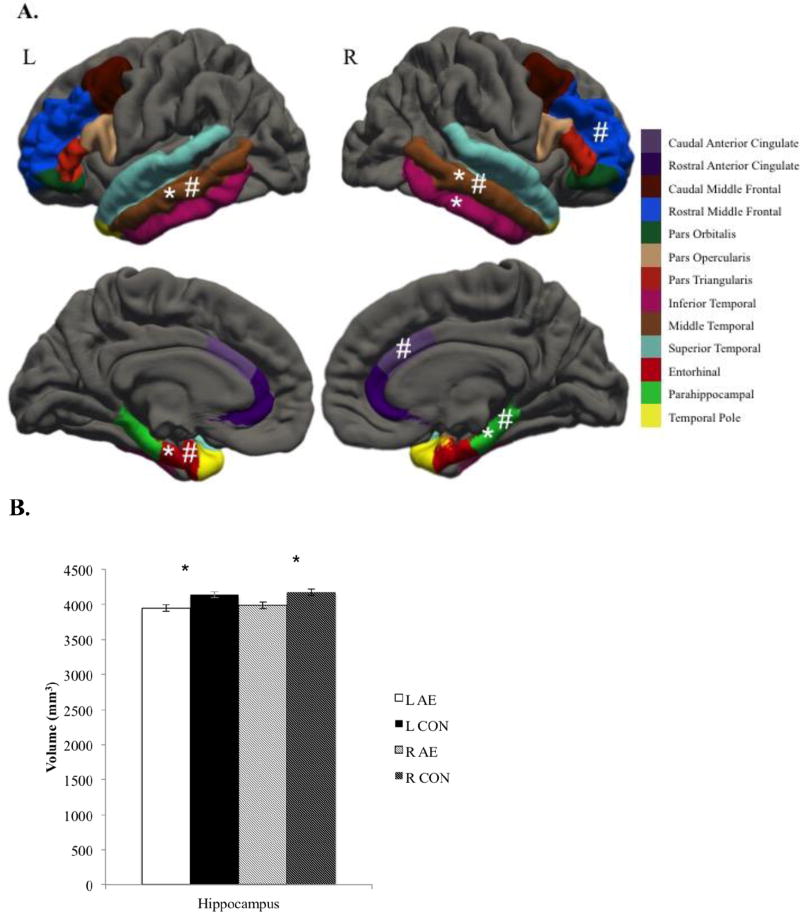
There were no differences in volume or thickness in frontal cortical ROIs and no differences in thickness for temporal cortical ROIs. However, the right frontal surface area was significantly smaller in the AE group compared to the CON group [F(7, 137)=3.097, p=.005, η2=.137], though not after controlling for ICV (p=.336). Additionally, the bilateral temporal volumes [right: F(6,130)=3.765, p=.002, η2=.148; left: F(6, 136)=2.726, p =.016, η2=.107] and surface areas [right: F(6,141)=4.271, p=.001, η2=.154; left: F(6,141)=5.089, p <.001, η2=.178] were significantly smaller in the AE group compared to the CON group. The bilateral surface area was disproportionately smaller in subjects with AE (ps<009), but differences in volume were no longer significant after controlling for ICV (ps>.08). To probe the statistically significant multivariate effects, univariate ANCOVAs were conducted on each individual ROI. Results from these ANOVAs are depicted in Figure 1A. Additionally, the AE group had significantly smaller hippocampal volume compared to the CON group [F(2, 152)=8.693 p <.001 η2=.103] in both the left (p<001) and right hippocampi (p<001). After controlling for ICV hippocampal differences did not meet our corrected significance threshold (p=.03).
Figure 1.
Group differences in volume, thickness, and surface area for regions of interest.
A) Group differences in cortical regions of interest.
L, Left; R, Right; AE, Alcohol-Exposed; CON, Control
* denotes significant group difference in volume, # denotes significant group difference in surface area. In all cases, AE < CON. There were no significant group differences in thickness.
B) Group differences in hippocampal volume.
* denotes significant group differences.
Neural Correlates of CVLT-C
Total learning
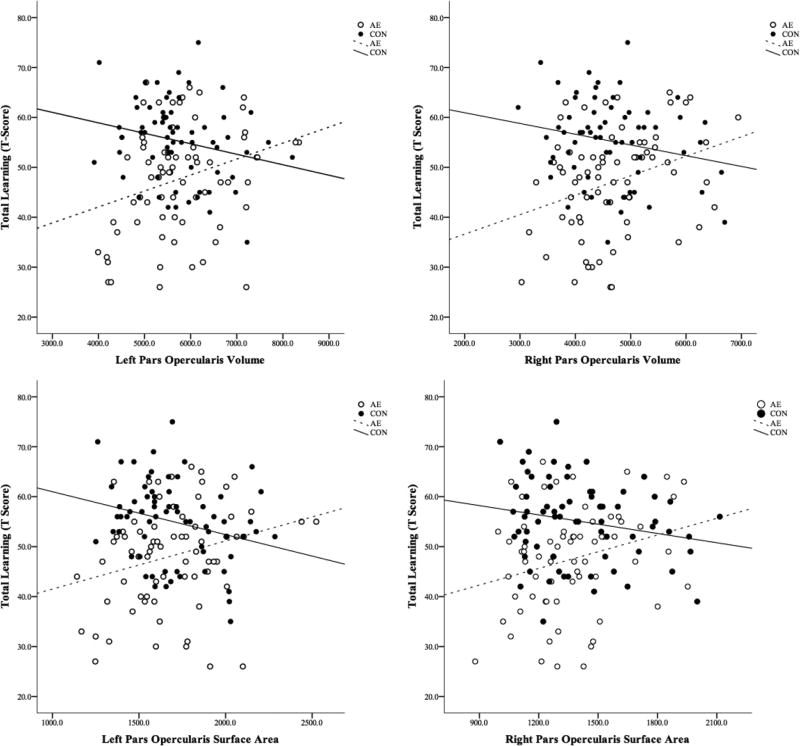
Total Learning on list A (Total Learning-A) was not significantly correlated with any demographic variables (ps≥080); therefore, no covariates were included in the regression models. There were no significant interactions between sex and any other predictors and no main effect of sex, therefore it was removed from the model for parsimony. Relations between Total Learning-A scores and brain structures are shown in Figure 2 and detailed results are available in Supplemental Table 1. Volume and surface area of the bilateral pars opercularis interacted with group to predict Total Learning-A. In general, smaller volume and surface area were related to better performance on this measure in the CON group, whereas the opposite pattern was observed in the AE group.
Figure 2.
Significant Neural Correlates of Total Learning Scores on the California Verbal Learning Test – Children’s Version (CVLT-C). Volume and area of brain regions are presented in mm3 and mm2,, respectively. CVLT-C performance is presented in T-Scores.
AE, Alcohol-Exposed; CON, Control
Short Delay Free Recall
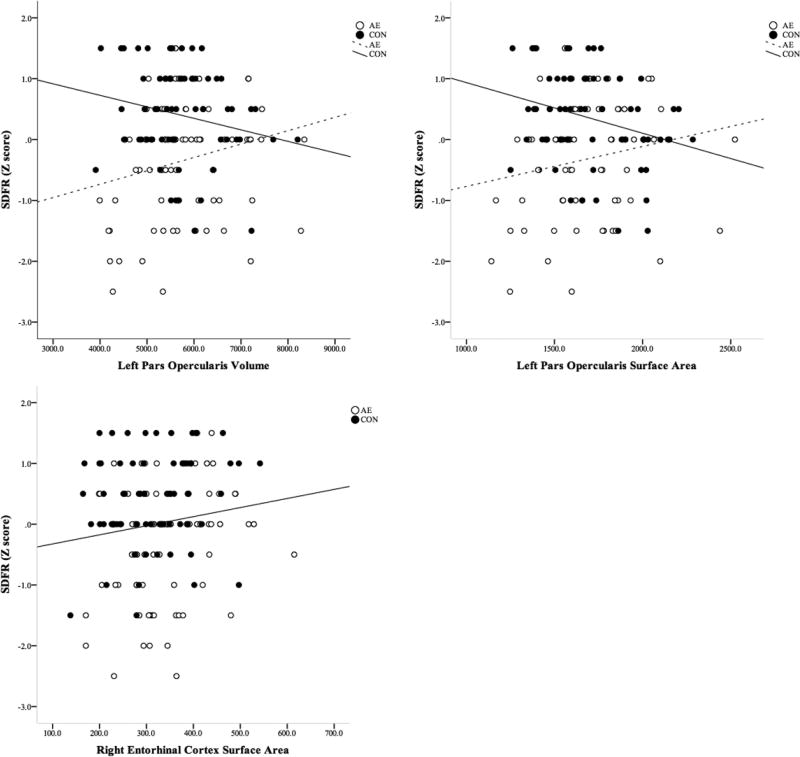
Short Delay Free Recall was not significantly correlated with any demographic variables (ps≥205), although its correlation with Sex was marginally significant (p=.056). Sex met criteria for a covariate and was included in the models. Relations between Short Delay Free Recall scores and brain structures are shown in Figure 3 and detailed results are available in Supplemental Table 2. Group interacted with the volume and surface area of the left pars opercularis to predict Short Delay Free Recall. In general, smaller size of the left pars opercularis predicted better scores in the CON group whereas the opposite was observed in the AE group. In addition, surface area of the right entorhinal cortex also predicted Short Delay Free Recall scores. On average across Group and controlling for Sex, as the surface area of the right entorhinal cortex increases, Short Delay Free Recall scores also increase.
Figure 3.
Significant Neural Correlates of Short Delay Free Recall Scores on the California Verbal Learning Test – Children’s Version (CVLT-C). Volume and area of brain regions are presented in mm3 and mm2,, respectively. CVLT-C performance is presented in Z-Scores.
AE, Alcohol-Exposed; CON, Control
Long Delay Free Recall
Long Delay Free Recall was not significantly correlated with any demographic variables (ps≥.239), although its correlation with Sex was marginally significant (p=.052). Sex met criteria for a covariate and was included in the models. Long Delay Free Recall scores were not significantly predicted by any brain regions.
Recognition Discriminability
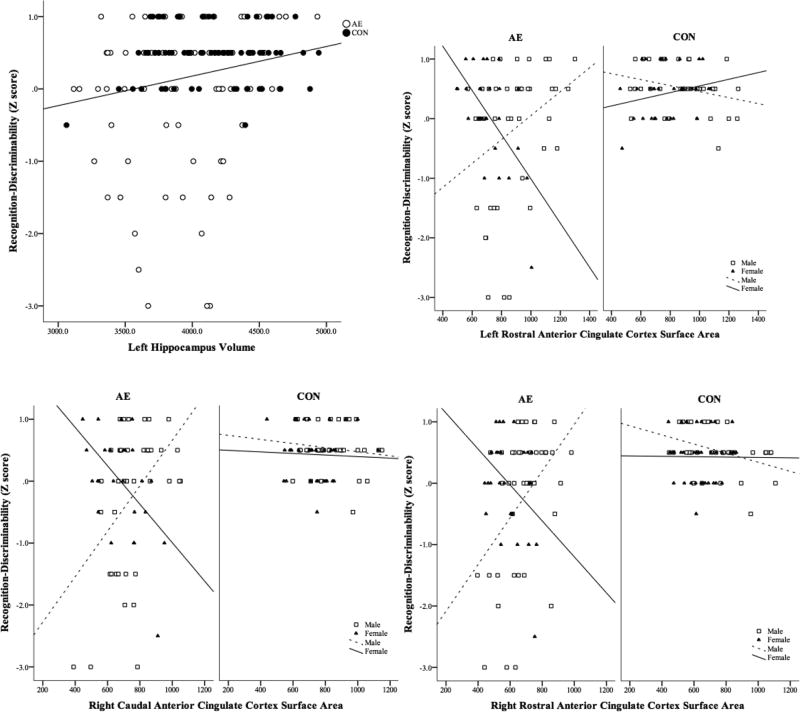
Recognition Discriminability was significantly correlated with Age (p < .001), which was included as a covariate in the regression models. Sex was included as a between subjects factor. Relations between Recognition Discriminability scores and brain structures are shown in Figure 4 and detailed results are presented in Supplemental Table 4. There was a significant positive relationship between the volume of the left hippocampus and Recognition Discriminability after accounting for the variance associated with Group, Sex, and Age.
Figure 4.
Significant Neural Correlates of Recognition Discriminability Scores on the California Verbal Learning Test – Children’s Version (CVLT-C). Volume and area of brain regions are presented in mm3 and mm2,, respectively. CVLT-C performance is presented in Z-Scores.
AE, Alcohol-Exposed; CON, Control.
In addition, the surface areas of the bilateral rostral anterior cingulate and the right caudal anterior cingulate each significantly interacted with Group and Sex to predict Recognition Discriminability performance. For males in the AE group, larger surface areas of the right and left anterior cingulate cortices predicted poorer performance, while the opposite pattern was seen in females in the AE group. No significant relationships were observed in the CON group. A similar trend was observed between the sexes in the AE group with surface areas of the right caudal anterior cingulate.
Retention
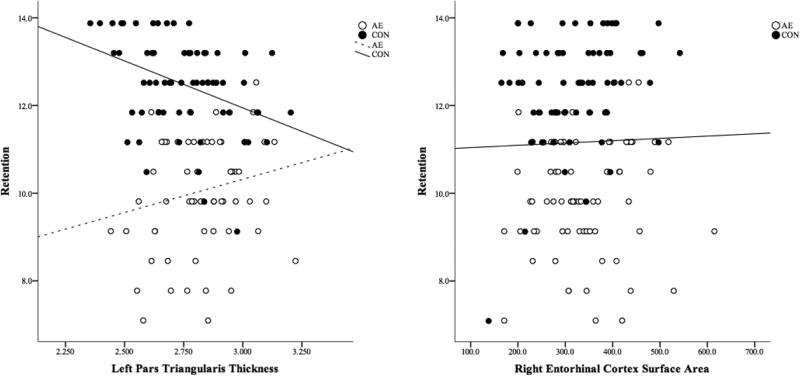
Retention was significantly correlated with age and sex (ps≤.027), and because both met criteria for inclusion as covariates, both variables were added to the models. Relations between Retention scores and brain structures are shown in Figure 5 and detailed results are displayed in Supplemental Table 5. Group and thickness of the left pars triangularis interacted to predict Retention scores. For subjects in the CON group, thinner cortex was related to better performance, while the opposite pattern is seen in the AE group. In addition, the surface area of the right entorhinal cortex predicted Retention: regardless of Group membership and after accounting for variance associated Sex and Age, larger surface area was associated with better performance.
Figure 5.
Significant Neural Correlates of Retention Scores on the California Verbal Learning Test – Children’s Version (CVLT-C). Area and thickness of brain regions are presented in mm2 and mm, respectively. CVLT-C performance is presented in adjusted raw scores.
AE, Alcohol-Exposed; CON, Control
Posthoc Analyses
Our analyses focused on the neural correlates of verbal memory in youth with histories of heavy prenatal alcohol exposure, including subjects with and without facial dysmorphology. However, the neuroanatomical effects of prenatal alcohol exposure may be more evident in children with dysmorphic features. These children are more likely to display microcephaly and may have more widespread neural anomalies. Thus, the few children with FAS (n=9) who were included in our study may have had a disproportionately large impact on our results. To address this consideration, we reanalyzed our data with the children who met criteria for FAS excluded. The exclusion of these nine children had minimal impact on our results; in fact, only six results were altered. The MANCOVAs examining group differences in the left and right temporal ROI volumes became marginally significant (ps=.052 and .019, respectively). In addition, for the regression analyses examining the relationship between the cortical structure and CVLT-C Long Delay Free Recall score, the right pars opercularis surface area × group interaction became marginally significant (p=.011); whereas, the right entorhinal thickness × group interaction achieved significance (p=.008). For the regression analyses examining the relationship between cortical structure and CVLT-C Recognition Discriminability score, the left hippocampal volume main effect became marginally significant (p=.02); whereas, the right rostral middle frontal surface area × sex interaction achieved significance (p=.01).
Discussion
Compared to typically developing controls, youth with heavy prenatal alcohol exposure displayed lower verbal memory scores and smaller volume and/or surface area of brain regions important for verbal memory, including right frontal ROIs, bilateral temporal ROIs, and bilateral hippocampi. Furthermore, alcohol-exposed youth displayed relationships between brain structure and performance on the CVLT-C that differed from those seen in controls.
As predicted, subjects in the alcohol-exposed group performed significantly worse on the CVLT-C compared to controls. However, it should be noted that while the scores of AE children in the current study were significantly different from controls, the means were still within the normal range for the CVLT-C. Consistent with previous literature, youth with FASD learned fewer words, recalled fewer words after both long and short delays, and were less accurate discriminating between novel and previously presented words (Crocker et al., 2011; Mattson et al., 1998; Mattson & Roebuck, 2002; Willoughby et al., 2008). Alcohol-exposed subjects retained significantly less information after controlling for initial learning, which deviates from some past findings (Crocker et al., 2011; Mattson & Roebuck, 2002), although it is consistent with findings from another large sample of moderately-exposed adolescent children (age range 13–16y; Lewis et al., 2015) as well as a recent study using an overlapping sample from CIFASD-III showing intact retention on the CVLT-C in younger (5–7y) but not older (10–16y) children with heavy exposure (Panczakiewicz et al. 2016). It is possible that while younger children with FASD are performing at the same level of their peers on this measure, older children and adolescents with FASD fall short of the expected age-level performance. An alternate explanation suggested by Lewis et al (2015) is that better encoding seen in their more moderately exposed sample may have made it possible to detect impairments in retention not seen in previous studies. In the current study, significant group differences were seen both learning and retention though overall performance was higher than previously reported. Future studies should consider the relation between encoding and retention in this population.
We did not identify group differences in volume or thickness of frontal ROIs, however we did observe smaller surface areas in the right frontal region. In the temporal ROIs we found lower bilateral volume and surface area in the AE group, but again no differences in thickness. To our knowledge, only two other studies have examined surface area in this population, and both have reported lower surface area in AE (Migliorini et al. 2015; Rajaprakash et al., 2014). Several studies have examined cortical thickness in this population but findings have varied widely, reporting greater thickness (Fernandez-Jaen et al., 2011; Sowell et al., 2008; Yang et al., 2012), thinning (Zhou et al., 2011), or no significant differences compared to controls (Rajaprakash et al., 2014). Further investigation into these thickness discrepancies is warranted, but two methodological factors—how thickness is measured and in-scanner motion—likely account for some of the variability in reports (Walhovd, Fjell, Giedd, Dale, & Brown, 2016). Although the FreeSurfer software itself has acceptable test-retest reliability (Iscan et al., 2015; Maclaren et al., 2014), different image analysis software packages use different techniques to measure cortical thickness, and therefore may yield different results. Additionally, analysis of multisite measurement reliability indicates that thickness measurements, while reliable, appear more affected by multisite variance than either surface area or volume (Iscan et al., 2015). In-scanner motion, an issue that can be more common in the AE population and in younger children, produces poorer delineations of cortical landmarks, which may also adversely affect thickness measurements. Finally, our findings suggest that cortical thickness may be less affected by prenatal alcohol exposure than volume or surface area, a factor that may also contribute to between study variability.
Previous studies examining brain structure in alcohol-exposed individuals have often used volume as the metric, and a number have reported group differences in the volume of the frontal (Coles et al., 2011; Rajaprakash et al., 2014; Sowell et al., 2002) or temporal (Chen et al., 2012; Coles et al., 2011; Sowell et al., 2002) regions. While we did not observe differences in frontal ROI volume, we did identify smaller volumes of temporal ROIs. Several methodological differences between our study and previous work may account for these discrepancies. First, our multisite study had a relatively large sample in comparison to many previous studies, which increased our power to detect differences but also reduced the likelihood of detecting spurious findings. Furthermore, only 11% of our sample of alcohol-exposed children met criteria for an FAS diagnosis, and exclusion of these subjects did not substantially alter our results. Microcephaly is included as part of the diagnostic criteria of FAS, therefore studies with a larger proportion of those with FAS may identify greater and more widespread volume differences across ROIs. For example, Coles et al. (2011) had a high proportion of subjects with facial dysmorphology (45% of alcohol-exposed subjects). Subjects with FAS had significantly smaller frontal lobes as compared to both controls and nondysmorphic alcohol-exposed subjects (Coles et al., 2011). Of note, in that study the controls and nondysmorphic alcohol-exposed subjects did not differ from each other in any of the examined ROIs.
Similar to previous studies, we found that subjects with prenatal alcohol exposure had significantly smaller bilateral hippocampal volume compared to controls (Astley et al., 2009; Coles et al., 2011; Nardelli et al., 2011; Roussotte et al., 2012; Treit et al., 2013) that were not disproportionate to total brain volume after correcting for multiple comparisons (Archibald et al., 2001; Astley et al., 2009; Treit et al., 2013). However, these findings are inconsistent with work that found disproportionately low volume of left hippocampus, but no differences in the right hippocampus (Willoughby et al., 2008). Additional large studies are required to determine if these results are reliable, and not an artifact of low power or statistical technique.
The bilateral pars opercularis, the left rostral anterior cingulate cortex, the bilateral rostral and caudal anterior cingulate cortex, and the left pars triangularis were differentially related to verbal memory performance. Traditionally, the pars opercularis and the pars triangularis, which comprise Broca’s area, are associated with language learning (Gluck et al., 2007). Functional imaging studies have found that, compared to IQ-matched controls, alcohol-exposed subjects have increased blood-oxygen-level-dependent (BOLD) activation in the right inferior frontal gyrus (which includes the pars opercularis and the pars triangularis) during verbal learning and recall (Sowell et al., 2007). Consistent with previous reports (Østby et al., 2011; Sowell et al., 2001; Tamnes et al., 2013), typically developing children in the current study demonstrated a negative relationship between size of specific ROIs and verbal memory ability. In contrast, we found that the alcohol-exposed group displayed a significant positive relationship between the volume of the right pars opercularis and total learning. The volume and surface area of the left pars opercularis were also significantly and positively related to short delay free recall in the alcohol-exposed group. Additionally, we observed a significant positive relationship between retention and the thickness of the left pars triangularis in the AE group. To date, researchers have not examined the relationship between the morphometry of the frontal cortex and verbal memory in subjects with FASD. These results support the hypothesis that the relationship between neuroanatomy and verbal memory is distinct in subjects with prenatal alcohol exposure as compared to typically developing controls.
Interestingly, the relationship between verbal memory and the rostral and caudal anterior cingulate is moderated by sex, but only in the alcohol-exposed group. While previous groups have found activation in the anterior cingulate in response to verbal memory tasks (Sowell et al., 2007), neither the relationship between morphometry and verbal memory nor the effect of sex have been investigated in this population. However, there is evidence that the anterior cingulate is a crucial structure for verbal memory in other pediatric clinical groups (Wilde et al., 2011). Few studies have examined the effects of sex on either neuropsychological tasks or brain development in children with FASD, an area of research that warrants replication and further investigation to determine if sex plays a significant role.
The right entorhinal cortex was significantly related to verbal memory performance. Significant functional BOLD activation of the lateral temporal lobe occurs in response to verbal working memory and verbal learning and recall tasks in both alcohol-exposed subjects and controls (O’Hare et al., 2009; Sowell et al., 2007), but medial temporal regions were activated in only typically developing subjects (Sowell et al., 2007). In both typically developing and alcohol-exposed subjects, verbal memory was positively associated with the surface area of the right entorhinal cortex. Not only do these findings contradict the hypothesis that the neural correlates in AE subjects are different from typically developing controls, these results also deviate from previous reports. Earlier studies did not find a relationship between temporal lobe and memory in either controls (Sowell et al., 2001) or children with FASD (Fryer et al., 2012). This difference is likely due to how cortical regions of interest were defined. In both studies, temporal lobe morphometrics were assessed as a whole, not divided into subregions. In addition, because previous studies only examined volume and thickness, the relationship between the right entorhinal surface area may have been confounded by the effect of cortical thickness.
We found that hippocampal volume was only a significant predictor of one memory variable: Recognition Discriminability, which is consistent with a large body of work indicating the role of the hippocampus in recognition and discriminability in both animal models and humans (Brown, Warburton, & Aggleton, 2010). Similar to our finding, work in other clinical populations has noted that the size of the hippocampus was related to ability to discriminate words (Biesbroek et al., 2015). However, previous work in both typically developing subjects (Van Petten, 2004) and subjects with FASD (Coles et al., 2011; Willoughby et al., 2008) have found that hippocampal volume was related to verbal memory more broadly. This relationship is corroborated by functional neuroimaging studies report activation of the hippocampi in response to verbal learning and recall tasks in children with prenatal alcohol exposure and controls (Sowell et al., 2007). In studies of subjects with FASD, methodological differences may account for some of these discrepancies. For example, one study used an adult sample, had a high rate of subjects with dysmophorphology, and used a different measure of verbal learning (Coles et al., 2011). While Willoughby et al. (2008) examined children and used the CVLT-C, different variables were analyzed, and many of these relationships were only marginally significant.
Limitations
We used a region of interest approach to evaluate the neural correlates of verbal memory in FASD. The regions that were included were chosen based on previous findings in clinical and typically developing populations. Important relationships may not have been identified because we did not employ a whole-brain exploratory approach. This is certainly possible given that children with alcohol-exposure do appear to have at least some distinct relationships between brain structure and verbal memory as compared to typically developing controls. For example, in the alcohol-exposed group, significant relationships between verbal memory and the frontal cortex primarily occurred in the right hemisphere. Generally, language function, including verbal memory, is left hemisphere lateralized (Gluck et al., 2007). Handedness did not appear to account for this effect, as there were a comparable amount of left-handed subjects in the two groups. Atypical lateralization has been reported in other clinical populations, such as children with a specific type of epilepsy (benign epilepsy with centrotemporal spikes; Monjauze, Broadbent, Boyd, Neville, & Baldeweg, 2011) and children born very preterm (Scheinost et al., 2015).
Conclusions
When examining group differences in the cerebral cortex, each morphometric yielded distinct results. For example, significant group differences were detected in the cortical surface area of the right frontal ROIs, but not in cortical volume or thickness. Many prior studies evaluating brain structure in FASD have only evaluated volume, which is a composite of thickness and surface area. Surface area and thickness each have distinct developmental trajectories, environmental, and biological influences (Giedd et al., 2015; Lenroot & Giedd, 2008; Panizzon et al., 2009). By only assessing cortical volume, important effects occurring at surface area or thickness levels could potentially be missed, particularly if one metric is different while the other is not (as was the case in this study) or if opposite effects are occurring in each metric. Others have noted that that surface area appears to be a sensitive metric for cortical maturation in children (Houston, Herting, & Sowell, 2014) and studies of adult brain size have noted that variation in surface area primarily drives differences seen in cortical grey matter volumes (Pakkenberg and Gundersen 1997; Im et al. 2008). MRI can not examine cellular structure to provide more in depth explanation for the biological differences that may account for our observation that surface area appears to be a more sensitive indicator of prenatal alcohol exposure in children than does thickness. However, prenatal alcohol exposure has consequences for a number of cellular processes important for cortical development, including proliferation, differentiation, migration, synaptic/dendritic formation, apoptosis and necrosis (Goodlett and Horn, 2001). Furthermore, this early alcohol exposure can have protracted effects, altering the typical course of neurodevelopment through childhood, adolescence, and into adulthood (Moore and Riley, 2015; Abbott et al., 2016).
In addition to altered brain structure, we found that alcohol-exposed children displayed distinct relationships between structure and verbal memory performance as compared to typically developing children. Disrupted neurodevelopment may have yielded compensatory brain structure-function relationships in alcohol-exposed children, which could account for these effects—in other words; children with histories of prenatal alcohol exposure may require larger neural substrates to overcome neural damage, while controls are able to use smaller substrates more efficiently. Studies using animal models of FASD found altered neuroplasticity as a result of prenatal alcohol exposure, which has had negative effects on performance on learning and memory tasks (Medina, 2011). On the other hand, it is possible that, although children with FASD have the same underlying brain-behavior relationship as controls, it appears different due to an altered developmental timeframe, such as a developmental lag or arrest in the alcohol-exposed group. Several MRI studies have noted different trajectories of brain development in FASD (e.g. Gautam et al., 2015; Lebel et al., 2012) and some neurobehavioral studies have noted that deficits are more pronounced in adolescents than in children (Crocker, Vaurio, Riley, & Mattson, 2009; Gross et al., 2016). The alcohol-exposed children displayed a number of relationships between verbal memory and brain structure that were not only distinct from controls, but were in the opposite direction. At this point the neurobiological basis for this finding is unclear. Additional research is required, perhaps using longitudinal samples, to determine whether atypical relationships or an altered developmental trajectory may account for these results.
Although many studies have examined the neuroanatomy of children with prenatal alcohol exposure, the neural developmental trajectory in this population is not well characterized. In addition, while verbal memory deficits compared to controls have consistently been reported in subjects with FASD, the underlying neural substrates have not been thoroughly investigated. The current study sought to assess the development of specific brain regions in children with FASD and determine how this development relates to verbal learning and memory functioning. A better understanding of brain development and neural correlates in children with FASD may help researchers and clinicians make better predictions about how these relationships will progress over time.
Supplementary Material
Acknowledgments
All or part of this work was done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol and Alcohol Abuse (NIAAA). Additional information about CIFASD can be found at www.cifasd.org.
The authors thank the families that participate in our research studies and acknowledge the support of the Center for Behavioral Teratology.
Funding: This work was supported by the National Institute on Alcohol Abuse and Alcoholism [grant numbers U01 AA014834 and U01 AA017122]. Additional support provided by U01 AA014815, and U01 AA014811 and K99/R00 AA022661.
Abbreviations
- FASD
Fetal alcohol spectrum disorder
- FAS
Fetal alcohol syndrome
- CVLT-C
California Verbal Learning Test, Children’s Version
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: The authors have no potential conflicts of interest to disclose.
Compliance with Ethical Standards
Ethical approval: The work described in this paper was approved by the institutional review boards at San Diego State University, the University of California, and all participating data collection locations.
Informed consent: Informed consent and assent from subjects and their parents was obtained prior to participation.
References
- Abbott CW, Kozanian OO, Kanaan J, Wendel KM, Huffman KJ. The impact of prenatal ethanol exposure on neuroanatomical and behavioral development in mice. Alcoholism: Clinical and Experimental Research. 2016;40(1):122–133. doi: 10.1111/acer.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Developmental Medicine and Child Neurology. 2001;43:148–154. doi: 10.1097/00004703-200110000-00024. [DOI] [PubMed] [Google Scholar]
- Astley SJ, Aylward EH, Carmichael Olson H, Kerns K, Brooks A, Coggins TE, Richards T. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research. 2009;33(10):1671–1689. doi: 10.1111/j.1530-0277.2009.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autti-Rämö Ilona, Autti Taina, Korkman Marit, Kettunen Satu, Salonen Oili, Valanne Leena. MRI findings in children with school problems who had been exposed prenatally to alcohol. Developmental Medicine and Child Neurology. 2002;44(2):98–106. doi: 10.1017/s0012162201001748. [DOI] [PubMed] [Google Scholar]
- Biesbroek JM, van Zandvoort MJE, Kappelle LJ, Schoo L, Kuijf HJ, Velthuis BK, Postma A. Distinct Anatomical Correlates of Discriminability and Criterion Setting in Verbal Recognition Memory Revealed by Lesion-Symptom Mapping. Human Brain Mapping. 2015;36:1292–1303. doi: 10.1002/hbm.22702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Warburton E, Aggleton JP. Recognition memory: material, processes, and substrates. Hippocampus. 2010;20(11):1228–1244. doi: 10.1002/hipo.20858. [DOI] [PubMed] [Google Scholar]
- Chen X, Coles CD, Lynch ME, Hu X. Understanding specific effects of prenatal alcohol exposure on brain structure in young adults. Human Brain Mapping. 2012;33(7):1663–1676. doi: 10.1002/hbm.21313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Goldstein FC, Lynch ME, Chen X, Kable JA, Johnson KC, Hu X. Memory and brain volume in adults prenatally exposed to alcohol. Brain and Cognition. 2011;75(1):67–77. doi: 10.1016/j.bandc.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Lynch ME, Kable JA, Johnson KC, Goldstein FC. Verbal and nonverbal memory in adults prenatally exposed to alcohol. Alcoholism: Clinical and Experimental Research. 2010;34(5):897–906. doi: 10.1111/j.1530-0277.2010.01162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker N, Vaurio L, Riley EP, Mattson SN. Comparison of adaptive behavior in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Alcoholism: Clinical and Experimental Research. 2009;33(11):2015–2023. doi: 10.1111/j.1530-0277.2009.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker N, Vaurio L, Riley EP, Mattson SN. Comparison of verbal learning and memory in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Alcoholism: Clinical and Experimental Research. 2011;35(6):1114–1121. doi: 10.1111/j.1530-0277.2011.01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Delis Dean C, Kramer Joel H, Kaplan, Edith, Ober Beth A. Manual for the California Verbal Learning Test - Children’s Version. San Antonio: The Psychological Corporation; 1994. [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15(3):331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Jaen A, Fernandez-Mayoralas DM, Quinones Tapia D, Calleja-Perez B, Garcia-Segura JM, Arribas SL, Munoz Jareno N. Cortical thickness in fetal alcohol syndrome and attention deficit disorder. Pediatric Neurology. 2011;45(6):387–391. doi: 10.1016/j.pediatrneurol.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions on Medical Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Dale AM. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Dale AM. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fryer Susanna L, Mattson Sarah N, Jernigan Terry L, Archibald Sarah L, Jones Kenneth Lyons, Riley Edward P. Caudate Volume Predicts Neurocognitive Performance in Youth with Heavy Prenatal Alcohol Exposure. Alcoholism-Clinical and Experimental Research. 2012;36(11):1932–1941. doi: 10.1111/j.1530-0277.2012.01811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam P, Lebel C, Narr KL, Mattson SN, May PA, Adnams CM, Sowell ER. Volume changes and brain-behavior relationships in white matter and subcortical gray matter in children with prenatal alcohol exposure. Human Brain Mapping. 2015;36(6):2318–2329. doi: 10.1002/hbm.22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Alexander-Bloch A, Schmitt E, Gogtay N, Rapoport JL. Child psychiatry branch of the National Institute of Mental Health longitudinal structural magnetic resonance imaging study of human brain development. Neuropsychopharmacology. 2015;40(1):43–49. doi: 10.1038/npp.2014.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Mohapel J, Boehme F, Kainer L, Christie BR. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: Insights from different rodent models. Brain Research Reviews. 2010;64(2):283–303. doi: 10.1016/j.brainresrev.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Glass L, Moore EM, Akshoomoff N, Jones KL, Riley EP, Mattson SN. Academic difficulties in children with prenatal alcohol exposure: Presence, profile, and neural correlates. Alcoholism: Clinical and Experimental Research. 2017 doi: 10.1111/acer.13366. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck MA, Mercado E, Myers CE. Learning and memory: From brain to behavior. 1. Worth Publishers; 2007. [Google Scholar]
- Goodlett CR, Horn KH. Mechanisms of alcohol-induced damage to the developing nervous system. Alcohol Research and Health. 2001;25(3):175–184. [PMC free article] [PubMed] [Google Scholar]
- Gross LA, Glass L, Goh P, Coles CD, Jones KL, Kable JA CIFASD. Specificity of memory performance in children and adolescents with prenatal alcohol exposure. 2016 Manuscript in preparation. [Google Scholar]
- Holm, Sture A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6(2):65–70. [Google Scholar]
- Houston SM, Herting MM, Sowell ER. The Neurobiology of Childhood Structural Brain Development: Conception Through Adulthood. Curr Top Behav Neurosci. 2014;16:3–17. doi: 10.1007/7854_2013_265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Lee J-M, Lyttelton O, Kim SH, Evans AC, Kim SI. Brain Size and Cortical Structure in the Adult Human Brain. Cerebral Cortex. 2008;18(9):2181–2191. doi: 10.1093/cercor/bhm244. [DOI] [PubMed] [Google Scholar]
- Iscan Z, Jin TB, Kendrick A, Szeglin B, Lu H, Trivedi M, Adams P. Test-retest reliability of freesurfer measurements within and between sites: Effects of visual approval process. Human brain mapping. 2015;36(9):3472–3485. doi: 10.1002/hbm.22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Robinson LK, Bakhireva LN, Marintcheva G, Storojev V, Strahova A, Chambers CD. Accuracy of the diagnosis of physical features of fetal alcohol syndrome by pediatricians after specialized training. Pediatrics. 2006;118(6):E1734–E1738. doi: 10.1542/peds.2006-1037. [DOI] [PubMed] [Google Scholar]
- Joseph J, Warton C, Jacobson SW, Jacobson JL, Molteno CD, Eicher A, Meintjes EM. Three-dimensional surface deformation-based shape analysis of hippocampus and caudate nucleus in children with fetal alcohol spectrum disorders. Human Brain Mapping. 2014;35(2):659–672. doi: 10.1002/hbm.22209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Roussotte F, Sowell ER. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychology Review. 2011;21(2):102–118. doi: 10.1007/s11065-011-9163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel, Catherine, Mattson Sarah N, Riley Edward P, Jones Kenneth L, Adnams Colleen M, May Philip A, Sowell Elizabeth R. A Longitudinal Study of the Long-Term Consequences of Drinking during Pregnancy: Heavy In Utero Alcohol Exposure Disrupts the Normal Processes of Brain Development. Journal of Neuroscience. 2012;32(44):15243–15251. doi: 10.1523/JNEUROSCI.1161-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. The changing impact of genes and environment on brain development during childhood and adolescence: initial findings from a neuroimaging study of pediatric twins. Development and Psychopathology. 2008;20(4):1161–1175. doi: 10.1017/S0954579408000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CE, Thomas KG, Dodge NC, Molteno CD, Meintjes EM, Jacobson JL, Jacobson SW. Verbal learning and memory impairment in children with fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research. 2015;39(4):724–732. doi: 10.1111/acer.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclaren J, Han Z, Vos SB, Fischbein N, Bammer R. Reliability of brain volume measurements: A test-retest dataset. Scientific data. 2014;1 doi: 10.1038/sdata.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji S, Pei J, Loomes C, Rasmussen C. A review of the verbal and visual memory impairments in children with foetal alcohol spectrum disorders. Developmental Neurorehabilitation. 2009;12(4):239–247. doi: 10.1080/17518420902980118. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: Neuropsychological and behavioral features. Neuropsychology Review. 2011;21(2):81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Foroud T, Sowell ER, Jones KL, Coles CD, Fagerlund Å CIFASD the. Collaborative initiative on fetal alcohol spectrum disorders: Methodology of clinical projects. Alcohol. 2010;44(7–8):635–641. doi: 10.1016/j.alcohol.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling LJ, Delis DC, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12(1):146–153. doi: 10.1037/0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Roebuck TM. Acquisition and retention of verbal and nonverbal information in children with heavy prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 2002;26(6):875–882. doi: 10.1111/j.1530-0277.2002.tb02617.x. [DOI] [PubMed] [Google Scholar]
- Medina AE. Fetal Alcohol Spectrum Disorders and Abnormal Neuronal Plasticity. The Neuroscientist. 2011;17(3):274–287. doi: 10.1177/1073858410383336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliorini R, Moore EM, Glass L, Infante MA, Tapert SF, Jones KL, Riley EP. Anterior cingulate cortex surface area relates to behavioral inhibition in adolescents with and without heavy prenatal alcohol exposure. Behavioural brain research. 2015;292:26–35. doi: 10.1016/j.bbr.2015.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monjauze C, Broadbent H, Boyd SG, Neville BGR, Baldeweg T. Language deficits and altered hemispheric lateralization in young people in remission from BECTS. Epilepsia. 2011;52(8):e79–e83. doi: 10.1111/j.1528-1167.2011.03105.x. [DOI] [PubMed] [Google Scholar]
- Moore EM, Migliorini R, Infante MA, Riley EP. Fetal Alcohol Spectrum Disorders: Recent Neuroimaging Findings. Curr Dev Disord Rep. 2014;1(3):161–172. doi: 10.1007/s40474-014-0020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Riley EP. What happens when children with fetal alcohol spectrum disorders become adults? Curr Dev Disord Rep. 2015;2(3):219–227. doi: 10.1007/s40474-015-0053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli A, Lebel C, Rasmussen C, Andrew G, Beaulieu C. Extensive deep gray matter volume reductions in children and adolescents with fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research. 2011;35(8):1404–1417. doi: 10.1111/j.1530-0277.2011.01476.x. [DOI] [PubMed] [Google Scholar]
- O’Hare ED, Lu LH, Houston SM, Bookheimer SY, Mattson SN, O’Connor MJ, Sowell ER. Altered frontal-parietal functioning during verbal working memory in children and adolescents with heavy prenatal alcohol exposure. Human Brain Mapping. 2009;30(10):3200–3208. doi: 10.1002/hbm.20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østby Y, Tamnes CK, Fjell AM, Walhovd KB. Morphometry and connectivity of the fronto-parietal verbal working memory network in development. Neuropsychologia. 2011;49:3854–3862. doi: 10.1016/j.neuropsychologia.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJG. Neocortical neuron number in humans: effect of sex and age. Journal of Comparative Neurology. 1997;384(2):312–320. doi: 10.1002/(SICI)1096-9861(19970728)384:2<312::AID-CNE10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Panczakiewicz A, Doyle LR, Coles CD, Kable JA, Sowell ER, Wozniak JR, Jones KL, Riley EP, Mattson SN the CIFASD. Age and prenatal alcohol exposure: Effects on verbal learning and memory. Manuscript in preparation 2016 [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Xian H. Distinct genetic influences on cortical surface area and cortical thickness. Cerebral Cortex. 2009 doi: 10.1093/cercor/bhp026. bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaprakash M, Chakravarty MM, Lerch JP, Rovet J. Cortical morphology in children with alcohol-related neurodevelopmental disorder. Brain Behav. 2014;4(1):41–50. doi: 10.1002/brb3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EP, Infante MA, Warren KR. Fetal alcohol spectrum disorders: an overview. Neuropsychology Review. 2011;21(2):73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson FC, Narr KL, Molteno CD, Jacobson JL, Jacobson SW, Meintjes EM. Prenatal alcohol exposure is associated with regionally thinner cortex during the preadolescent period. Cerebral Cortex. 2016;26(7):3083–3095. doi: 10.1093/cercor/bhv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussotte Florence F, Sulik Kathleen K, Mattson Sarah N, Riley Edward P, Jones Kenneth L, Adnams Colleen M, Sowell Elizabeth R. Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Human Brain Mapping. 2012;33(4):920–937. doi: 10.1002/hbm.21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Lacadie C, Vohr BR, Schneider Karen C, Papademetris X, Constable RT, Ment LR. Cerebral Lateralization is Protective in the Very Prematurely Born. Cerebral Cortex. 2015;25:1858–1866. doi: 10.1093/cercor/bht430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Lu LH, O’Hare ED, McCourt ST, Mattson SN, O’Connor MJ, Bookheimer SY. Functional magnetic resonance imaging of verbal learning in children with heavy prenatal alcohol exposure. NeuroReport. 2007;18(7):635–639. doi: 10.1097/WNR.0b013e3280bad8dc. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Kan E, Thompson PM, Riley EP, Toga AW. Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cerebral Cortex. 2008;18(1):136–144. doi: 10.1093/cercor/bhm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cerebral Cortex. 2002;12(8):856–865. doi: 10.1093/cercor/12.8.856. [DOI] [PubMed] [Google Scholar]
- Sowell Elizabeth R, Delis Dean C, Stiles Joan, Jernigan Terry L. Improved memory functioning and frontal lobe maturation between childhood and adolescence: A structural MRI study. Journal of the International Neuropsychological Society. 2001;7(3):312–322. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Walhovd KB, Grydeland H, Holland D, Østby Y, Dale AM, Fjell AM. Longitudinal Working Memory Development is Relatead to Structural Maturation of Frontal and Parietal Cortices. Journal of Cognitive Neuroscience. 2013;25(10):1611–1623. doi: 10.1162/jocn_a_00434. [DOI] [PubMed] [Google Scholar]
- Treit S, Lebel C, Baugh L, Rasmussen C, Andrew G, Beaulieu C. Longitudinal MRI reveals altered trajectory of brain development during childhood and adolescence in fetal alcohol spectrum disorders. Journal of Neuroscience. 2013;33(24):10098–10109. doi: 10.1523/JNEUROSCI.5004-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petten Cyma. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Vaurio, Linnea, Riley Edward P, Mattson Sarah N. Neuropsychological Comparison of Children with Heavy Prenatal Alcohol Exposure and an IQ-Matched Comparison Group. Journal of the International Neuropsychological Society. 2011;17(3):463–473. doi: 10.1017/S1355617711000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Giedd J, Dale AM, Brown TT. Through Thick and Thin: a Need to Reconcile Contradictory Results on Trajectories in Human Cortical Development. Cerebral Cortex. 2016:1–10. doi: 10.1093/cercor/bhv301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde EA, Newsome MR, Bigler ED, Pertab J, Merkley TL, Hanten G, Levin HS. Brain Imaging Correlates of Verbal Working Memory in Children Following Traumatic Brain Injury. International Journal of Psychophysiology. 2011;82(1):86–96. doi: 10.1016/j.ijpsycho.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby KA, Sheard ED, Nash K, Rovet J. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. Journal of the International Neuropsychological Society. 2008;14(6):1022–1033. doi: 10.1017/S1355617708081368. [DOI] [PubMed] [Google Scholar]
- Yang YL, Roussotte F, Kan E, Sulik KK, Mattson SN, Riley EP, Sowell ER. Abnormal Cortical Thickness Alterations in Fetal Alcohol Spectrum Disorders and Their Relationships with Facial Dysmorphology. Cerebral Cortex. 2012;22(5):1170–1179. doi: 10.1093/cercor/bhr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Yaling, Roussotte Florence, Kan Eric, Sulik Kathleen K, Mattson Sarah N, Riley Edward P, Sowell Elizabeth R. Abnormal Cortical Thickness Alterations in Fetal Alcohol Spectrum Disorders and Their Relationships with Facial Dysmorphology. Cerebral Cortex. 2012;22(5):1170–1179. doi: 10.1093/cercor/bhr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Lebel C, Lepage C, Rasmussen C, Evans A, Wyper K, Beaulieu C. Developmental cortical thinning in fetal alcohol spectrum disorders. NeuroImage. 2011;58(1):16–25. doi: 10.1016/j.neuroimage.2011.06.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.