Abstract
The Early Devonian Rhynie and Windyfield cherts remain a key locality for understanding early life and ecology on land. They host the oldest unequivocal nematode worm (Nematoda), which may also offer the earliest evidence for herbivory via plant parasitism. The trigonotarbids (Arachnida: Trigonotarbida) preserve the oldest book lungs and were probably predators that practiced liquid feeding. The oldest mites (Arachnida: Acariformes) are represented by taxa which include mycophages and predators on nematodes today. The earliest harvestman (Arachnida: Opiliones) includes the first preserved tracheae, and male and female genitalia. Myriapods are represented by a scutigeromorph centipede (Chilopoda: Scutigeromorpha), probably a cursorial predator on the substrate, and a putative millipede (Diplopoda). The oldest springtails (Hexapoda: Collembola) were probably mycophages, and another hexapod of uncertain affinities preserves a gut infill of phytodebris. The first true insects (Hexapoda: Insecta) are represented by a species known from chewing (non-carnivorous?) mandibles. Coprolites also provide insights into diet, and we challenge previous assumptions that several taxa were spore-feeders. Rhynie appears to preserve a largely intact community of terrestrial animals, although some expected groups are absent. The known fossils are (ecologically) consistent with at least part of the fauna found around modern Icelandic hot springs.
This article is part of a discussion meeting issue ‘The Rhynie cherts: our earliest terrestrial ecosystem revisited’.
Keywords: Rhynie chert, Devonian, Nematoda, Arachnida, Myriapoda, Hexapoda
1. Introduction
The Rhynie and adjacent Windyfield cherts outcrop in Aberdeenshire in Scotland. They are one of the most important windows into early life on land [1]. Fossils are preserved, often three-dimensionally, with extraordinary fidelity in the translucent chert matrix [2] and offer unparalleled insights into the external (and internal) anatomy of the animals that lived in this Early Devonian hot springs environment. Previous ecological interpretations of the Rhynie terrestrial fauna were offered by Tasch [3], Kevan et al. [4], Rolfe [5,6], Anderson & Trewin [7] and Fayers & Trewin [8]. Since these papers were published, a nematode worm [9] and several new arachnids have been described [10–13], and some previously known fossils assigned to the insects or myriapods have been reinterpreted [14,15]. Other studies focused on how the extinct trigonotarbid arachnids may have breathed [16], walked [17] and visualized their surroundings [18]. Here, we offer a modern synthesis of the terrestrial animals (primarily arthropods) known from Rhynie and their probable ecological roles. We review functional morphology, which provides evidence of where and how these animals could have lived. This is particularly important for extinct groups with no direct modern counterpart. For groups with living representatives, however, detailed comparisons of exactly how their descendants live and feed permit more accurate inferences about the Early Devonian fossils. Finally, we compare the Rhynie fauna with arthropod communities on the margins of modern hot springs.
2. Material and methods
Fossils of terrestrial animals from the Rhynie and Windyfield cherts were reviewed from the literature and compared, where possible, to closely related extant species as ecological proxies. References for individual groups are given below. The depositional setting [2] and stratigraphic age of the cherts of around 409–411 million years ([19], but see [20]) has been addressed previously in some detail. Because of the excellent preservation, it has been suggested [21] that at least the mites and springtails at Rhynie could be Recent contaminants incorporated through secondary silicification. We reject this argument (see also [22]) because: there is no known mechanism for re-mobilizing the silica in the crystalline chert; both extinct and modern-looking arthropods are preserved with similar textures; and arthropod material has also been recovered from drill cores well below the weathered surface which gave no opportunity for modern contaminants to enter the matrix.
3. Results
(a). Nematoda: Enoplia
Nematode worms living in water films in the terrestrial environment are effectively freshwater organisms, but a remarkable find from Rhynie is Palaeonema phyticum Poinar, Kerp & Hass, 2008 [9] (figure 1a) described from the stomatal chambers of the early land plant Aglaophyton majus Edwards, 1986. Trace fossils [23] and a putative body fossil [24] of marine Nematoda have been described from the Early Ordovician of China, but the Early Devonian P. phyticum remains the oldest unequivocal nematode body fossil. The worms were estimated at 0.1–1 mm in length, and assigned to an extinct family, Palaeonematidae [9], within the order Enoplia. The authors proposed this fossil was one of the oldest examples of a relationship between a terrestrial plant and an animal. Specifically, they interpreted P. phyticum as having reproduced within the plant tissue because eggs, juveniles and adults are present together. From the structure of the buccal cavity the authors also inferred that it was an epistrate feeder, using a ‘tear and swallow’ strategy. Poinar et al. [9] suggested that the Rhynie nematodes may have fed on the plant's cortical cells—which would also make it the oldest known herbivore—and that they could have supplemented their diet with microorganisms. In this context, it is worth noting an earlier comment by Challoner et al. [25] that some plant damage observed at Rhynie could have been induced by nematodes. We should mention that both free-living nematodes and (partially silicified) parasitic nematodes in the parenchyma cells of the plant Eleocharis have been observed in the modern Big Blue Hotspring at Yellowstone (Alan Channing 2017, personal communication).
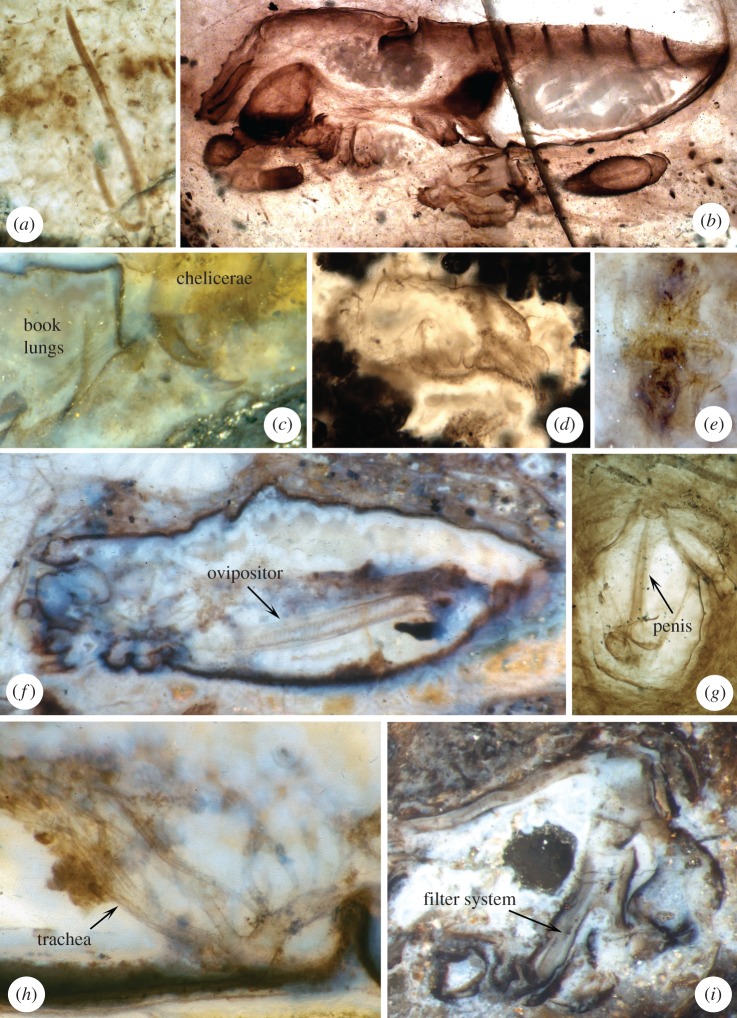
Figure 1.
Nematode worms and arachnids from Rhynie. (a) Palaeonema phyticum Poinar, Kerp & Hass, 2008 (Nematoda: Enoplia). (b,c) Palaeocharinus spp. (Arachnida: Trigonotarbida). (d,e) Two examples of mites (Arachnida: Acariformes). (f–h) Eophalangium sheari Dunlop, Anderson, Kerp & Hass, 2004 (Arachnida: Opiliones). (i) Saccogulus seldeni Dunlop, Fayers, Hass & Kerp, 2006 (Arachnida incertae sedis).
According to Bik et al. [26], the subclass Enoplia is the earliest branching clade of the Nematoda. It can be divided into a largely marine Enoplida and a terrestrial Triplonchida, the latter containing some modern plant parasites. These authors noted the paucity of nematode fossils (most come from amber), but appear to have overlooked the Rhynie species as a potential calibration point for their molecular dating. Bik et al. ([26] and references therein) also touched on the debate regarding whether nematodes originated in a marine or a terrestrial environment and—if they were marine—how often they crossed into freshwater and terrestrial habitats. Plant feeding in modern nematodes is thought to have arisen at least four times independently among the twelve currently recognized major clades [27]. Given the limited number of morphological characters, much of today's phylogenetic work is based on molecular data, and there has been no attempt to date to incorporate the extinct Rhynie family into a modern tree. If possible, this would test whether plant feeding in the fossil family is independent, or dates the origins of one of the four known transitions to plant parasitism. Bird et al. [27] reported the presence of a stylet in all the modern nematode plant parasites which the animals use to pierce plant cells, and which appears to have arisen convergently. A stylet is absent in P. phyticum [9] implying that it does not belong to one of the modern plant parasite clades, and evolved this lifestyle independently. Bird et al. [27] also noted that the modern plant parasites are often closely related to fungivorous nematodes, which suggests that (in these groups) plant-feeding evolved from fungus-feeding. Given that Rhynie has a rich fungal assemblage [28] it is interesting to speculate whether there were other nematodes in the original ecosystem which used this resource.
(b). Arachnida
(i). Trigonotarbida
Trigonotarbids are an extinct (Silurian–Permian) arachnid order. Of the five original species described from Rhynie [29]—all in the genus Palaeocharinus Hirst, 1923—only two are likely to be valid [30]; the others appear to have been diagnosed on preservational artefacts. Hirst's fossils [29,31] are about 3–4 mm in body length (figure 1b). A slightly larger (ca 6 mm) and more tuberculate Palaeocharinus species was later added [12] from the adjacent Windyfield chert. Some Rhynie trigonotarbids are up to 14 mm long [5]; however, these are only known in dorso–ventral section and it is difficult to assess whether they belong to the same genus as the smaller fossils. The exquisite preservation of the Rhynie trigonotarbids has contributed towards an excellent understanding of the group's morphology and palaeobiology. A key feature of the group is the presence of book lungs [32] (figure 1c) which confirm that they were unequivocally terrestrial, contra Tasch [3] who suggested that the entire Rhynie fauna may have been aquatic. These structures remain the oldest evidence for lungs in any animal group and re-investigation [16] confirmed that they are anatomically modern. For example, the air spaces between individual lamellae are separated from one another by tiny struts called trabeculae which prevent the lung from collapsing under its own weight. The presence of two pairs of lungs also phylogenetically places the trigonotarbids in the arachnid clade Pantetrapulmonata alongside spiders, whip spiders, whip scorpions and schizomids. That said, the Rhynie material also preserves some characters such as divided tergites, a locking mechanism between the prosoma and opisthosoma, and a tiny claw at the end of the pedipalp [33] which are shared with the rare and enigmatic living order Ricinulei.
Palaeocharinus has lateral eye tubercles bearing up to 15 individual lenses [18]. They appear to document a transition between a compound eye, as in horseshoe crabs and the extinct eurypterids, and the eyes of modern arachnids which have five lenses or fewer. It is not clear to what extent Palaeocharinus relied on vision—its median eyes look upwards at an angle and its lateral eyes look more to the side—and most modern arachnids rely more on tactile setae and/or vibrations as sensory input. The legs of Palaeocharinus have sparse setae, but do become more setose towards the tip. There is no evidence that Palaeocharinus had trichobothria (fine hairs used to detect air currents), but at least the metatarsus joint towards the ends of the legs bears groups of slit sense organs which in modern arachnids act as sensory strain gauges. Garwood & Dunlop [17] analysed details of the leg articulations in Palaeocharinus to constrain the degree to which the joints could bend in any given direction. From this, coupled with a digital reconstruction of the whole animal, they estimated the centre of mass and extrapolated a three-dimensional animation of a likely walking pattern based on comparisons with living arachnids.
These anatomical elements give the overall impression of Palaeocharinus as a short-legged, cursorial predator. The mouthparts consist of ‘clasp-knife’ chelicerae (figure 1c), with a curved fang articulating against a basal article bearing a row of teeth [34]. Like spiders, trigonotarbids may have pierced and restrained prey items with the fangs and then pressed the victim against the tooth row as part of the mastication process. None of the material shows evidence for an opening from a prosomal venom gland on the fang, which is otherwise only known in spiders. The Rhynie trigonotarbids also have a row of denticles on the pedipalp coxae as well as mesal projections from the anterior leg coxae which may have aided mastication. These are not seen in this form in any living arachnids, and could represent a remnant of something akin to the coxal gnathobases used to chew food in horseshoe crabs and eurypterids.
Most arachnids digest their prey pre-orally, regurgitating enzymes onto the food and sucking up the liquefied remains. Preoral digestion is likely to have been less effective in an aquatic environment, so again supports a mode of life on land. Two lines of evidence strongly support preoral digestion in Rhynie trigonotarbids. Rolfe [6, plate 1] figured an amorphous mass of cuticle held in the mouthparts of a trigonotarbid and there is a putative filtering device [34] between the labrum and the labium consisting of several downward-pointing opposing hairs or platelets. These suggest that particulate matter was filtered out, and so not ingested, and solid items would not be expected in the gut. Some authors [4] suggested that trigonotarbids may have been facultative spore feeders. Plant-eating by spiders is very rare, but not unknown [35], and in a few cases fungal spores were consumed. However, in the absence of direct evidence for spore feeding, such as a Palaeocharinus holding spores in its mouthparts or gut traces, we feel that the preserved morphology is more consistent with that of a predator.
(ii). Acariformes
The Rhynie mites remain the oldest unequivocal record of the order Acariformes. The other conventionally accepted mite order, Parasitiformes, is not known until the Cretaceous. The Rhynie fossils (figure 1d,e) were initially assigned [29] to a single species, Protacarus crani Hirst, 1923, with body lengths of ca 290–440 µm, and tentatively referred to the modern family Eupodidae. Subsequently, Dubinin [36] recognized four additional species. There has been an unfortunate tendency in the past to treat all Rhynie mites as an amorphous group of generalist detritivores. Hence we consider it important here to review the species' systematic placements, as well as the ecology of modern members of their respective groups. We should, however, caution that there has been no taxonomic revision of the mites since Dubinin's study, and that he based his interpretations on the (albeit excellent) published illustrations rather than direct observation of the fossils. The original species, P. crani, is currently placed in the clade Endeostigmata and the family Alycidae. Two further species are also considered to be endostigmatids: Protospeleorchestes pseudoprotacarus Dubinin, 1962 in the family Nanorchestidae and Pseudoprotacarus scoticus Dubinin, 1962 in the family Alicorhagiidae. The two remaining Rhynie mites are placed in a different clade, Prostigmata, specifically the group Eupodides and the family Tydeidae which contains Palaeotydeus devonicus Dubinin, 1962 and Paraprotacarus hirsti Dubinin, 1962.
Acariform mites can be broadly divided into two major lineages [37]: Sarcoptiformes and Trombidiformes. Endeostigmatids are sarcoptiform mites, while eupodidids are trombidiforms. The Rhynie fossils suggest that these lineages had split from one another prior to the Early Devonian, and that some extant families can potentially be traced back over 400 million years. Sarcoptiform mites are sometimes referred to as ‘chewing mites’ since they have modified mouthparts for mastication and so are able to ingest particulate food. The most abundant sarcoptiforms are the oribatid mites, which are an important component of modern soil ecosystems. None of the Rhynie mites appear to be oribatids—a group first recorded in the Mid-Devonian—and were placed instead among the endeostigmatids, which are generally interpreted as an assemblage of early branching chewing mites [38]. Krantz & Walter [37] noted that modern endeostigmatids are often found today in extreme or dry soil habitats (e.g. deserts, microbial crusts, sea shores). We speculate that their presence at Rhynie could relate to the unusual characteristics of the hot springs environment.
With respect to modern feeding ecologies [39], some living Alycidae are predators of nematode worms; a group now also known at Rhynie (see above). It has been speculated that other Alycidae are plant feeders, but these forms have elongate, needle-like mouthparts which are not seen in the Rhynie fossils. Alicorhagiidae are best considered omnivores, and belong to an assemblage of endeostigmatids known to ingest solid fragments of fungi and/or other small- and soft-bodied invertebrates. For example, some living alicorhagiids feed on fungi in the laboratory, but cultures do not prosper unless they have access to nematodes as prey items too [39]. Finally, Nanorchestidae are believed to be fluid-feeders and living species have been classified as microphytophages feeding on fungi and algae. As noted above, fungi are abundant at Rhynie as a potential food source and both green algae and cyanobacteria have been reported here as well [4].
Prostigmatid mites express a bewildering variety of lifestyles from free-living predators, to parasites, to plant feeders. At Rhynie the fossils' affinities dictate that we focus on Tydeidae, modern species of which include predators, scavengers and groups feeding on fungi or directly on plants [40]. In their catalogue of modern tydeids, Da Silva et al. [41] also listed them as phytophages, mycophages, pollenophages, insect parasites or scavengers, but further noted that the majority of today's species are scavengers or mycophages (i.e. fungi-feeders). Both of these feeding ecologies are compatible with the Rhynie palaeoenvironment. We should note in passing that Rolfe [6] identified structures which reminded him of the galls formed by eriophyoid mites (another prostigmatid group); however, gall formation and/or gall-producers at Rhynie have not been further investigated. In summary, the mite assemblage at Rhynie includes taxa which may have used a range of ecological niches.
(iii). Opiliones
The harvestman discovered at Rhynie [10] (figure 1f–h) was subsequently named Eophalangium sheari Dunlop, Anderson, Kerp & Hass, 2004 [11]. It is the oldest record of Opiliones. The material discovered suggests a compact body up to 6 mm long with elongate, slender legs. While trigonotarbids preserve lungs (see above), E. sheari has tracheal tubes [11], with a branching pattern similar to that of living harvestmen. In detail, a large tube extends into the prosoma—which would have contained the metabolically active limb musculature—and several smaller tubes serve the opisthosoma (figure 1h). These structures both represent the oldest direct evidence for tracheae in any arthropod and demonstrate unequivocally that E. sheari was a fully terrestrial animal.
Eophalangium sheari also preserves reproductive organs, allowing the different genders to be recognized, and inferences about their behaviour to be made. In modern harvestmen males either have an eversible spermatopositor (the suborder Cyphophthalmi) for depositing spermatophores on the substrate, or a penis (all other suborders) as an intromittent organ for direct sperm transfer. The Rhynie harvestman organ (figure 1g) terminates in a tapering tip, or stylus [11], and seems more likely to have been a penis used for direct copulation. The female of E. sheari preserves an annulated ovipositor (figure 1f) which, in comparison with living species, was presumably extended from the body to allow eggs to be laid in a specific place within the substrate. In its original description [11], E. sheari was assigned to the living suborder Eupnoi. However, the number of tendons in the male penis does not match the pattern seen in living eupnoids and the genital opening (or gonostome) appears to be open, rather than closed by a plate. Subsequently, Garwood et al. [42] resolved E. sheari closest to a new Carboniferous fossil harvestman based on this open gonostome and an anterior projection (or ocularium) bearing the median eyes. Together these two Palaeozoic fossils were assigned to a new extinct suborder, Tetrophthalmi, forming the sister group of Cyphophthalmi.
Unusually among arachnids, modern harvestmen are omnivorous; they do not practice preoral digestion. The Rhynie harvestman does, as a result, have a visible gut trace [11], but specific gut contents cannot be identified. From an overview of feeding ecology in modern harvestmen [43], it would be misleading to claim that all harvestmen are generalists. Different modern species tend to show individual food preferences. Most harvestmen are primarily predators—be it on arthropods, molluscs or worms—but may supplement their diet by scavenging on decaying animal and/or plant material. An interesting point of note [43] is that living harvestmen often prefer soft-skinned arthropods. The collembolans at Rhynie (see below) would offer a potential source of softer prey. Given E. sheari's putative sister-group position to the suborder Cyphophthalmi, it is worth noting that modern cyphophthalmids have been observed feeding on either live or dead springtails [44]. More exotic food preferences seen in living harvestmen tend to be associated with derived clades. Thus, we would caution against inferring that E. sheari regularly consumed material like decaying vegetable matter, spores or fungi.
(iv). Arachnida incertae sedis
The final putative arachnid is Saccogulus seldeni Dunlop, Fayers, Hass & Kerp, 2006. Known only from a single specimen in thin section [13] (figure 1i), it resembles in some respects the prosoma of a spider in longitudinal section, but lacks unequivocal apomorphies of Araneae. The most remarkable feature is a long internal structure interpreted as a pharynx [13], with what looks like a dense brush of hairs or platelets forming a filtering system. Similar to Palaeocharinus above, this implies an animal using preoral digestion, but in the absence of further details about its morphology or affinities its ecology remains equivocal.
(c). Myriapoda
(i). Chilopoda
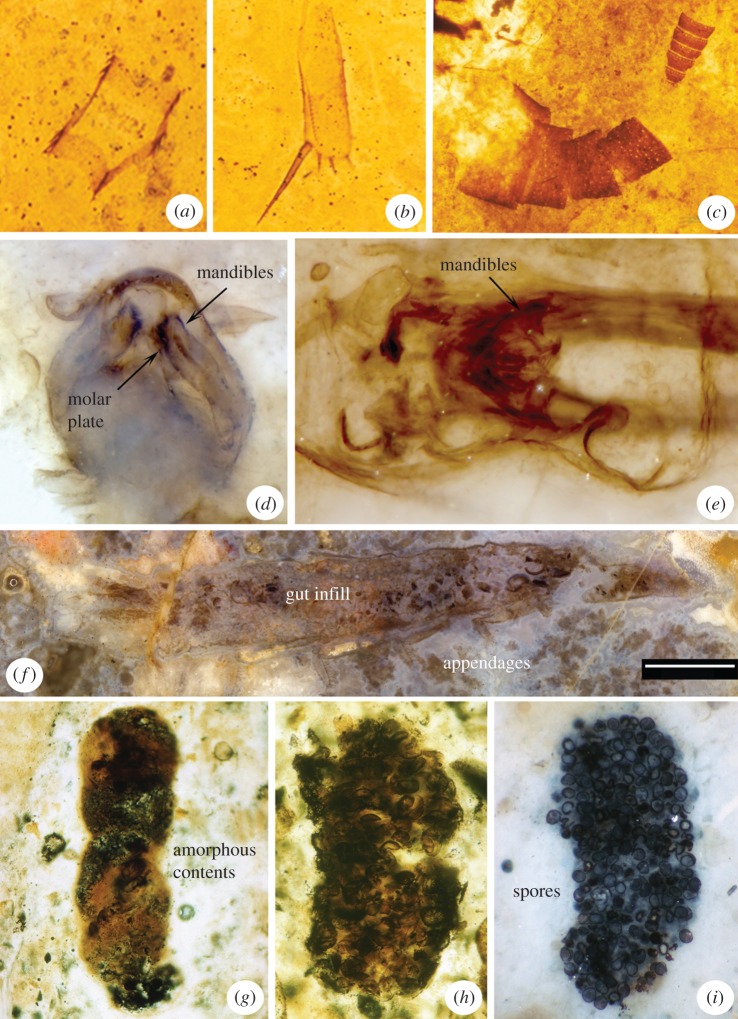
Centipedes (Chilopoda) were recorded from the Windyfield chert [7] as Crussolum sp. This genus is otherwise known from the Mid-Devonian of the USA and has been assigned to the order Scutigeromorpha, or house centipedes. The Windyfield material exhibits a distinctive cuticle type and includes isolated elements from the antennae, mouthparts and legs (figure 2a,b). A body length of about 35 mm was inferred [7]. Scutigeromorphs have a fossil record extending back to the late Silurian [45] and the presence of a forcipular apparatus in these early Palaeozoic forms, including those from Windyfield, implies biting jaws with poison glands as in living centipedes. Undheim et al. [46] noted that centipedes—and presumably also scorpions—are among the oldest terrestrial predatory arthropods to have evolved venom to subdue prey. These authors also remarked that scutigeromorph centipedes have rather delicate forcipules, largely used just to inject venom. More derived centipedes have increasingly robust mouthparts used for cutting and slicing as well. They also identified differences in venom components between scutigeromorph and scolopendromorph centipedes, which could imply a deep time split in centipede venom production and their associated feeding ecology. These Devonian fossils are in keeping with such a hypothesis.
Figure 2.
Myriapods, hexapods and coprolites from Rhynie and Windyfield. (a,b) Limb elements of Crussolum sp. (Chilopoda: Scutigeromorpha). (c) Rhynimonstrum dunlopi Anderson & Trewin, 2003 (Arthropoda incertae sedis, possibly Diplopoda?). (d) Head capsule of Rhyniella preacursor Hirst & Maulik, 1926 (Hexapoda: Collembola). (e) Rhyniognatha hirsti Tillyard, 1928 (Hexapoda: affinities equivocal). (f) Leverhulmia mariae Anderson & Trewin, 2003 (probably Hexapoda). (g–i) Coprolites of the Lancifex Habgood, Hass & Kerp, 2004 type including amorphous (g) and spore-rich (h,i) contents.
Extant centipedes are invariably predators, leading to suggestions [7] that Crussolum may have been an active hunter in the Rhynie ecosystem. Scutigeromorphs can be characterized ecologically as fast-runners [47], preferentially searching for prey on the surface of the substrate. Extant scutigeromorphs retain large compound eyes while the more derived centipede orders show increasing trends towards leaf-litter and deeper soil habitats: for example shortening the legs and reducing, and finally losing, the eyes completely. Thus we can infer that Crussolum probably hunted largely on the substrate, rather than burrowing into it. Living scutigeromorphs have been reported to eat arachnids, springtails and various insects. A similar prey spectrum was available at Rhynie.
(ii). Myriapoda: Diplopoda
Rhynimonstrum dunlopi Anderson & Trewin, 2003 is based on enigmatic, but distinctive fragments of cuticle [7] which form annulate structures with specific patterns of pores (figure 2c). It was described as Arthropoda incertae sedis, but comparisons were made with the antennal articles of millipedes (Diplopoda). If this interpretation is correct it would imply an animal which was rather larger than the typical Rhynie arthropods. Millipedes today are almost always detritivores (see below), but in the absence of data confirming its affinities it is difficult to make further ecological inferences.
(d). Hexapoda
(i). Collembola
Rhynie hosts the oldest evidence for Hexapoda in the fossil record [14]. Springtails (Collembola) are represented by Rhyniella preacursor Hirst & Maulik, 1926 (figure 2d). The original descriptions [31] only recovered the head (including the mandibles) and thorax. Whalley & Jarzembowski [48] added an abdomen bearing the distinctive furcula (or spring) and the tube-like collophore on the underside. Rhyniella preacursor could thus be confirmed as an unequivocal, ca 1.5 mm long, collembolan. It had already evolved a mechanism to facilitate rapid jumps, which suggests the need to avoid predators; the most likely candidates being trigonotarbids, harvestmen and centipedes (see above).
Modern collembolans largely feed on fungal hyphae and decaying plant material [49], both of which would have been abundantly available at Rhynie. A few species today are predators on animals like nematodes, rotifers and other springtails. Among previous comments on food preferences of the Rhynie species, Kevan et al. [4] suggested that the lack of a well-developed molar area in the mandibles implied dietary material that did not require mastication, such as soil microorganisms or spores. They noted plant juices as another possibility, but that R. praecursor lacks piercing mandibles that would have allowed them to create puncture wounds. However, the mandibles of R. praecursor have since been shown to possess a molar plate [50] (figure 2d). A diet requiring mastication, such as detritivory, is thus plausible although it should be mentioned that the molar regions do not directly oppose each other as would be expected for a chewing animal (Joachim Haug 2017, personal communication). Further information regarding the ecomorphology of collembolan mandibles is lacking, although some extant, carnivorous species possess asymmetrical, interlocking mandibles [49] which are absent in R. praecursor. In a discussion comment in Bernays et al. [51], Ed Jarzembowski posited through comparison with extant species that the long pretarsi of R. preacursor might have enabled locomotion on the surface of water, but this speculation has not been explored further.
Hopkin [49] commented on how similar R. preacursor is to living collembolan species. He also reviewed previous suggestions about its probable affinities, favouring the proposal of the extant family Isotomidae ([50]; see also [52]). Both these studies highlighted the fact that Isotomidae possess a generalized morphology, and tend to be the dominant component within modern springtail communities. Extant members of the family are found in various litter, soil and moss habitats, and despite being more abundant in cold and damp conditions are found in a wide range of ecosystems from deserts to polar regions.
(ii). Rhyniognatha
Rhyniognatha hirsti Tillyard, 1928 is an enigmatic species [53] known from a single specimen, initially figured by Hirst & Maulik [31], preserving a pair of mandibles surrounded by harder-to-identify cuticular structures (figure 2e). Largely considered ‘insect like’ in the past, Engel & Grimaldi [14] proposed that the mouthparts were of a dicondylic nature—i.e. possessing two pivot points, a posterior condyle and anterior acetabulum—and triangular in form. From this they proposed that the animal as a whole was a pterygote (i.e. winged) insect. This tantalizing possibility has not been corroborated by any other specimens, but if correct it implies that non-insect hexapods (collembolans), apterygote insects, and some of the earliest pterygotes were all present on land by the Early Devonian. Molecular clock dating [54,55] corroborates the idea that ectognathous insects, and perhaps even pterygotes, had evolved by this point.
Irrespective of R. hirsti's affinities, the form of the mandibles—triangular, with well-differentiated incisor and molar areas—are fairly generalized and functionally comparable with those of numerous hemimetabolous insect groups (e.g. [56]), such as the Orthoptera and Blattodea. Kevan et al. [4] suggested that the mandibles are ‘reminiscent of the sharp bladed and toothed jaws of a carnivore’, while Engel & Grimaldi [14] posited that they belonged to a chewing organism, but noted that it was impossible to say whether the original diet comprised spores/pollen, leaf/stem tissue or small animals. We concur that it is impossible to reconstruct the diet of R. hirsti with any certainty, but note that the mandibles of carnivorous insects tend to have shearing cusps [57], while grasshoppers that feed on non-grass vegetation have a series of sharp pointed cusps. We tentatively suggest that R. hirsti is more like the latter condition, which hints at a non-carnivorous diet.
(iii). Leverhulmia
Leverhulmia mariae Anderson & Trewin, 2003 was initially described based on the difficult-to-study holotype specimen [7] (figure 2f), as a ca 12 mm long species assignable to Myriapoda incertae sedis. Fayers & Trewin [15] reported a new specimen, and further prepared the holotype. This revealed anterior (i.e. presumably thoracic) appendages, terminating in a pretarsus comprising a lateral pair of articulated ungues with a fixed median unguis between them. They also identified setae on the appendages as mechanoreceptors, with potential chemoreceptor structures too. In addition, they posited that the holotype represents an abdomen with a minimum of five clawless, segmented leglet pairs. Paired articulated lateral ungues imply dipluran or insect affinities, while the short median unguis is suggestive of the pretarsal arrangement of some Zygentoma (silverfish). The authors compared the abdominal leglets to the arrangement in Diplura, Zygentoma and Archaeognatha. In general, their segmented nature is suggestive of stem-, rather than crown-Hexapoda [58].
The same line of section in the holotype that makes the morphology of L. mariae challenging to interpret, however, does facilitate inferences about its ecology thanks to a significant length of gut infill [7,15]. This sinusoidal structure midway between the dorsal and ventral surface (figure 2f) contains fragments of various materials otherwise lacking in the host chert matrix, and runs the full preserved length of the fossil. The heterogenous material within this putative gut trace comprises ‘…macerated phytodebris, spores, amorphous organic matter and fragments of arthropod cuticle’ [15, p. 1121]. The authors reported that the phytodebris, while generally impossible to identify, contains rare examples of fragmented strands of xylem tissue. This is accompanied by scattered 50–80 µm embryophyte spores, fungal spore clusters found midway along the trace, and a minimum of two different types of arthropod cuticle, distinct from that of L. mariae. Of these, the first is found at one end of the gut trace, and comprises smooth, thick-walled cuticle in arcuate fragments. The other is found at the opposite end of the specimen, and consists of clustered almost-cylindrical fragments of smooth, thin-walled cuticle, one adorned by a short, socketed macroseta. Fayers & Trewin [15] identified a minimum bite size of 300 µm for this animal based on the size of the fragments, although no definitive mouthparts could be identified.
Anderson & Trewin [7] suggested (and we concur) that the gut contents indicate a detritivore, because the delineation of discrete packages of arthropod cuticle, macerated plant debris, spores and amorphous organic material suggests the animal was ingesting numerous food items in various states of decay. The gut contents are also comparable to the contents of coprolites found in deposits of this age, including Rhynie (see below), which are thought to originate from detritivores. Fayers & Trewin [15] also noted that extant Archaeognatha and Zygentoma are known to be detritivores. We further observe that while the plant and arthropod elements of the gut are fragmented, presumably through mastication, the same is not true of the spores [7]; presumably due to their small size. Based on the published figures, the relatively low concentration of spores suggests they were probably not a primary food source.
(e). Plant–arthropod interactions and coprolites
A potential insight into palaeoecology is arthropod-mediated plant damage and other interactions. Labandeira [59] provided a comprehensive overview, which demonstrated that from sites of equivalent age to Rhynie there is evidence of generalized palynophagy, external foliage feeding, piercing and sucking, and borings. There has been relatively little work on plant damage at Rhynie. Kevan et al. [4] reviewed and reported on surface lesions inflicted during life in Rhynie plants, and differentiated three primary type of damage. The first was suggested to be the result of metazoan penetration of plant tissues: by implication a sap-sucking arthropod, although they noted [4] this need not be unambiguously animal-mediated damage. Two further damage types could be caused by animals or fungi. Rolfe [6] concurred that the causative agent of this damage was equivocal, and could have had arthropod, fungal or other pathogenic origins. This was supported by Chaloner et al. [25, p. 178], who concluded ‘…the possibility of a sap-feeding relation involving the arthropods is probably about as far as this evidence can take us at present’. In light of this, and based on the lack of unambiguous body fossils of plant-eating arthropods (see above), we see no convincing evidence at present for arthropod-mediated herbivory in the Rhynie ecosystem.
Coprolites offer an alternative insight into palaeoecology by recording the diet of extinct organisms. Habgood et al. [60] recorded three novel coprolite ichnogenera from Rhynie. Lancifex Habgood, Hass & Kerp, 2004 was proposed for elongate structures up to about 3 mm by 1 mm (figure 2g–i). These have a diverse content, a variable degree of particle size and content degradation, are dispersed rather than clustered, and can contain mineral grains. All this points towards a detritivore. Comparisons were drawn with modern millipedes and with the similar gut contents of Leverhulmia mariae (see above), albeit then not recognized as a hexapod. Rotundafaex Habgood, Hass & Kerp, 2004 was proposed for smaller, more rounded structures, about 250 µm in diameter. Like Lancifex, these contain amorphous organic matter, fungal spores and hyphae, and plant spores. Small detritivores such as collembolans, wingless insects or small millipedes were suggested as possible producers. Finally, Bacillafaex Habgood, Hass & Kerp, 2004 was proposed for small, ca 200 µm, rod-shaped structures with amorphous organic contents. Microherbivores (possibly mites) and trigonotarbids were suggested as producers. However, the body fossils of the known mites are only about twice the size of their putative coprolites, and we should note that modern spiders (and probably trigonotarbids) are liquid-feeders and tend not to produce solid, particulate faeces. Thus we feel that the producers of Bacillafaex remain equivocal.
There is also a general hypothesis [61] that spore-feeding predated herbivory in the fossil record. Kevan et al. [4] argued that spores may have been a significant element of some Rhynie arthropods' diet, and these animals may have been central to spore dispersal. Rolfe [6] mentioned that this was not his preferred scenario for the trigonotarbids. As noted above, spores are unequivocally present in the gut of Leverhulmia mariae and in several Rhynie coprolites (figure 2h,i). In assessing their significance, we should reiterate Habgood et al.'s comments [60] that there is no distinct class of spore-rich coprolites at Rhynie as would be expected from a dedicated spore-feeder, and that where spores are found in coprolites they show little degradation. This raises questions about whether the animal actually derived any nutrients from them, or whether they were just consumed (but not processed) among the general detritus. Spore-feeding has been cited as uncommon among modern arthropods [60], and the Rhynie spores have resilient walls which could presumably resist penetration and/or digestion (Charles Wellman 2017, personal communication). We would caution against inferring spore-feeding simply because an arthropod is discovered within, or close, to a sporangium, but acknowledge that there are spore-bearing coprolites known throughout the mid to late Palaeozoic (Andrew Scott 2017, personal communication; see also [62]) which could be taken as evidence for diet.
4. Discussion
Suggested ecological relationships of the terrestrial Rhynie animals recorded to date are summarized in table 1. The only potential example of true herbivory, i.e. the consumption of living plant tissue, is the nematode. Several of the mites, as well as the collembolans, are strong candidates for having been mycophages since their living relatives have been regularly reported feeding on fungi. Potential generalist detritivores include the collembolans and Leverhulmia mariae, which may be a stem-insect (or a crown-insect potentially related to bristletails or silverfish) and uniquely reveals its diet through its gut contents. Predators at Rhynie were presumably the venomous centipede, the trigonotarbid arachnids, and the harvestman; the last may also have scavenged opportunistically on decaying organic matter. The mites also include groups which are known today to prey on nematodes, or at least use them to supplement their diet. These inferred food preferences are consistent with a simple, arthropod-dominated ecosystem with mycophages and/or detritivores as primary consumers—a mode of life reflected in the contents of the coprolites—and several predators at higher trophic levels. Top predators may have been the centipede and trigonotarbids, with body lengths of ca 35 and up to 14 mm, respectively. Arthropod herbivory appears to have been absent. To explain this, we might speculate that being a mycophage requires less specialization than being a herbivore because fungal tissue lacks cellulose and so is easier to digest. Furthermore, if herbivory is defined as animals biting off pieces of green plants, chewing them and then swallowing them, then it seems likely that all herbivores use a gut flora (fungi, bacteria, etc.) for digestion. For this reason it has been proposed [1,63] that herbivory effectively arose from detritivory by internalizing the decomposition process. Essentially, herbivores are merely farming their gut biota by feeding it pieces of chewed plant material.
Table 1.
Summary of the terrestrial arthropod fauna of the Rhynie and Windyfield chert ecosystems, and their potential ecologies.
| taxon | suggested ecology | remarks |
|---|---|---|
| Nematoda: Enoplia | ||
| Palaeonema phyticum | fed on cortical cells of Agalophyton, supplemented by microorganisms? | |
| Arachnida: Trigonotarbida | ||
| Palaeocharinus spp. | predator on other small animals | used preoral digestion |
| Arachnida: Acariformes | ||
| Protacarus crani | predator on nematodes? | |
| Pseudoprotacarus scoticus | mycophages, plus predation on nematodes? | |
| Protospelorchestes pseudoprotacarus | mycophages and/or algal feeders? | |
| Palaeotydeus devonicus/Paraprotacarus hirsti | scavengers and/or mycophages? | |
| Arachnida: Opiliones | ||
| Eophalangium sheari | predator on other small animals, plus decaying animal and/or plant material | |
| Arachnida incertae sedis | ||
| Saccogulus seldeni | uncertain | probably used preoral digestion |
| Myriapoda: Chilopoda | ||
| Crussolum sp. | predator on other small animals | probably cursorial on the substrate |
| Myriapoda: Diplopoda? | ||
| Rhynimonstrum dunlopi | detritivore? | affinities and ecology uncertain |
| Hexapoda: Collembola | ||
| Rhyniella preacursor | mycophage/detritivore? | maybe fed on fungal hyphae and decaying plant material |
| Hexapoda: Insecta | ||
| Rhyniognatha hirsti | uncertain (non-carnivorous?) | fossil has chewing mandibles |
| Leverhulmia mariae | detritivore | gut contents include arthropod cuticle, macerated plant debris and spores |
Rhynie thus appears viable as a terrestrial ecosystem in that the animals recorded so far all have a potential source of food (fungi, detritus, other animals). Yet as with any fossil system, we do not know if all of the original assemblage has been preserved. Missing taxa at a given time period can be inferred from dated phylogenies, but as noted by Chang & James [64] for a terrestrial example (earthworms), if there is no fossil record there is nothing to calibrate molecular clocks against and estimates of cladogenesis here are likely to be inaccurate. Are significant components of the original Rhynie fauna missing? The locality is highly unusual in its chert-based preservation via siliceous sinters deposited in a hot springs environment, and is particularly well-suited to conserving small terrestrial arthropods. It is not coincidental that Rhynie hosts the oldest records of hexapods, mites and harvestmen, and potentially the oldest nematode worms as well. These are small animals, and if other microarthropods or worms were present at Rhynie there is nothing per se to suggest that they could not be preserved. Additional groups may yet be found by processing more material. If we look at the wider picture of Devonian terrestrial arthropods there are notable absences. Scorpions are known since the Silurian, and while there is debate in the literature about whether the earliest forms were aquatic or terrestrial, they might be predicted in the Rhynie ecosystem. Another group which one would expect are millipedes. Silurian body fossils with evidence for air-breathing have been described (although new data suggest these millipedes may actually be Devonian [65] and contemporary in age with Rhynie). Millipedes are only hinted at here at Rhynie through interpretations of Rhynimonstrum dunlopi fragments and some of the coprolites.
The excellent preservation, and the presence of internal organs or gut contents in some cases, suggests the Rhynie animals were killed rather rapidly (see also [66]). Yet some arthropod fragments are disarticulated, which in turn implies a degree of post-mortem transport, or at least decay. We favour a scenario in which the terrestrial arthropod community lived on the outwash aprons of hot springs and was periodically inundated by floods of hot, silica-rich water which eventually precipitated out into the cherts. The land plants could have had a sieve-like effect, trapping arthropod remains between the in situ stems. In this scenario, we might ask whether the recovered fauna shows a bias towards smaller (slower?) animals. Were larger arthropods, perhaps a few centimetres long, more easily able to escape the inundations? Parallels could be drawn with amber where larger inclusions are a rarity and most of the arthropod fossils are quite small. However, animals like the ‘missing’ millipedes are generally quite slow-moving creatures and this hypothesis does not explain why we do not see juveniles of larger arthropods.
Trewin et al. [66] used hot springs at the Yellowstone National Park of the USA as a modern analogue to understand preservation at Rhynie. Channing & Edwards [67,68] also compared the Rhynie flora to that of Yellowstone, and to other modern and fossil hot spring communities. They argued that the Rhynie plants growing on the fringes of the hot springs were physiologically specialized (typically halophytes) and noted that Rhynie plant genera are hardly ever recorded from contemporary localities elsewhere. Among the terrestrial arthropods, only the centipede Crussolum occurs outside of Rhynie. Thus from an animal perspective, was Rhynie a typical early Devonian habitat, or a specialized ecosystem hosting a more limited spectrum of (endemic?) animals compared to the wider palaeoenvironment? Comparative studies of terrestrial arthropod communities on the margins of modern hot springs ecosystems are rare. The published literature tends to focus on aquatic species (e.g. insect larvae, crustaceans, water mites) which may be of note for being thermophilous.
One study [69] collected spiders, mites and collembolans—along with various derived insect orders—from pitfall trips set close to modern hot springs in New Zealand. Details of the taxa they found are lacking, but they noted that there were fewer invertebrates found close to the hot springs themselves, probably due to the lack of diverse habitats on the sinter terraces, and that some of the scavenging arthropods appeared to raid these terraces in search of carcasses of dead animals. A more detailed account of the soil fauna around hot springs in Iceland was provided by Tuxen [70]. Records included several taxa which would not be expected at Rhynie as they are not known elsewhere from the Devonian, e.g. parasitiform mites, several groups of derived pterygote insects and terrestrial woodlice. He also recorded soft-bodied taxa, such as earthworms and gastropods which could, theoretically, occur at Rhynie but have not been found so far. Additionally, there are several interesting parallels with Rhynie. The Icelandic hot springs margins also hosted cursorial spiders (analogous to trigonotarbids), oribatid mites (which may have evolved from endostigmatids), harvestmen, centipedes, millipedes and springtails; the last including the genus Isotoma which is similar to the fossil species. Thus the terrestrial animals found at Rhynie are consistent with at least part of the fauna surrounding hot springs today for which data are available. To conclude, we would also note that Tuxen [70] found a gradation of taxa from those which occurred exclusively near hot springs through to more widely distributed taxa which happened to be more common in this habitat. That faunas can grade over quite short distances from exclusive hot springs animals to generalists in the surrounding ecosystem introduces uncertainty. How it impacts on the potential uniqueness of the Rhynie fauna—as per hypotheses about the flora (see above)—is unclear. We lack contemporary localities in the Old Red Sandstone with the same quality of preservation which could yield microarthropods and allow us to test whether Rhynie was sampling part of a more widespread Early Devonian terrestrial ecosystem.
Note added in proof
Haug & Haug (2017) recently proposed that the putative insect Rhyniognatha hirsti could in fact be a myriapod, possibly a centipede [71].
Acknowledgements
We thank Dianne Edwards and the Royal Society for inviting this contribution and Lyall Anderson, Claire Mellish, Vladimir Blagoderov, Alexander Brasier, Steven Fayers, Carsten Kamenz, Hans Kerp and the late Nigel Trewin for help procuring (or providing) images of fossil material. Alan Channing provided information on nematodes in modern hot springs, Joachim Haug commented on the springtails, Charles Wellman and Andrew Scott on spore-feeding, and two reviewers provided helpful suggestions on an earlier draft.
Data accessibility
This article has no additional data.
Authors' contributions
J.A.D. and R.J.G. wrote the paper and prepared the figures.
Competing interests
We declare we have no competing interests.
Funding
R.J.G.'s visit to Berlin was funded by the European Union's Synthesys project.
References
- 1.Shear WA. 1991. The early development of terrestrial ecosystems. Nature 351, 283–289. ( 10.1038/351283a0) [DOI] [Google Scholar]
- 2.Trewin NH. 1994. Depositional environment and preservation of biota in the Lower Devonian hot-springs of Rhynie, Aberdeenshire, Scotland. Trans. R. Soc. Edinb. Earth Sci. 84, 433–442. ( 10.1017/S0263593300006234) [DOI] [Google Scholar]
- 3.Tasch P. 1957. Flora and fauna of the Rhynie Chert: a palaeoecological reevaluation of published evidence. Bull. Uni. Wichita 32, 3–24. [Google Scholar]
- 4.Kevan PG, Chaloner WG, Saville DBO. 1975. Interrelationships of early terrestrial arthropods and plants. Palaeontology 18, 391–417. [Google Scholar]
- 5.Rolfe WDI. 1980. Early invertebrate terrestrial faunas. In The terrestrial environment and the origin of land vertebrates (ed. Panchen AL.), pp. 117–157. London, UK: Academic Press. [Google Scholar]
- 6.Rolfe WDI. 1985. Early terrestrial arthropods: a fragmentary record. Phil. Trans. R. Soc. Lond. B 309, 207–218. ( 10.1098/rstb.1985.0080) [DOI] [Google Scholar]
- 7.Anderson LI, Trewin NH. 2003. An Early Devonian arthropod fauna from the Windyfield cherts, Aberdeenshire, Scotland. Palaeontology 46, 467–509. ( 10.1111/1475-4983.00308) [DOI] [Google Scholar]
- 8.Fayers SR, Trewin NH. 2004. A review of the palaeoenvironments and biota of the Windyfield chert. Trans. R. Soc. Edinb. Earth Sci. 94, 325–339. ( 10.1017/S0263593300000729) [DOI] [Google Scholar]
- 9.Poinar GO, Kerp H, Hass H. 2008. Palaeonema phyticum gen. n., sp. n. (Nematoda: Palaeonematidae fam. n.), a Devonian nematode associated with early land plants. Nematology 10, 9–14. ( 10.1163/156854108783360159) [DOI] [Google Scholar]
- 10.Dunlop JA, Anderson LI, Kerp H, Hass H. 2003. Preserved organs of Devonian harvestmen. Nature 425, 916 ( 10.1038/425916a) [DOI] [PubMed] [Google Scholar]
- 11.Dunlop JA, Anderson LI, Kerp H, Hass H. 2004. A harvestman (Arachnida: Opiliones) from the Early Devonian Rhynie cherts, Aberdeenshire, Scotland. Trans. R. Soc. Edinb. Earth Sci. 94, 341–354. ( 10.1017/S0263593300000730) [DOI] [Google Scholar]
- 12.Fayers SR, Dunlop JA, Trewin NH. 2005. A new Early Devonian trigonotarbid arachnid from the Windyfield chert, Rhynie Scotland. J. Syst. Palaeont. 2, 269–284. ( 10.1017/S147720190400149X) [DOI] [Google Scholar]
- 13.Dunlop JA, Fayers SR, Hass H, Kerp H. 2006. A new arthropod from the early Devonian Rhynie chert Aberdeenshire (Scotland), with a remarkable filtering device in the mouthparts. Paläont. Z. 80, 296–306. ( 10.1007/BF02988443) [DOI] [Google Scholar]
- 14.Engel MS, Grimaldi DA. 2004. New light shed on the oldest insect. Nature 427, 627–630. ( 10.1038/nature02291) [DOI] [PubMed] [Google Scholar]
- 15.Fayers SR, Trewin NH. 2005. A hexapod from the Early Devonian Windyfield chert, Rhynie, Scotland. Palaeontology 48, 1117–1130. ( 10.1111/j.1475-4983.2005.00501.x) [DOI] [Google Scholar]
- 16.Kamenz C, Dunlop JA, Scholtz G, Kerp H, Hass H. 2008. Microanatomy of Early Devonian book lungs. Biol. Lett. 4, 212–215. ( 10.1098/rsbl.2007.0597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garwood RJ, Dunlop JA. 2014. The walking dead: blender as a tool for paleontologists with a case study on extinct arachnids. J. Paleont. 88, 735–746. ( 10.1666/13-088) [DOI] [Google Scholar]
- 18.Miether ST, Dunlop JA. 2016. Lateral eye evolution in the arachnids. Arachnology 17, 103–119. ( 10.13156/arac.2006.17.2.103) [DOI] [Google Scholar]
- 19.Parry SF, Noble SR, Crowley QG, Wellman CH. 2011. A high precision U–Pb age constraint on the Rhynie Chert Konservat-Lagerstätte: time scale and other implications. J. Geol. Soc. Lond. 168, 863–872. ( 10.1144/0016-76492010-043) [DOI] [Google Scholar]
- 20.Mark DF, Rice CM, Trewin NH. 2013. Discussion on ‘A high precision U–Pb age constraint on the Rhynie Chert Konservat-Lagerstätte: time scale and other implications’. J. Geol. Soc. Lond. 170, 701–703. ( 10.1144/jgs2011-110) [DOI] [Google Scholar]
- 21.Crowson RA. 1985. Comments on the Insecta of the Rhynie Chert. Entomol. Gener. 11, 97–98. ( 10.1127/entom.gen/11/1985/97) [DOI] [Google Scholar]
- 22.Greenslade PMJ. 1988. Reply to R. A. Crowson's ‘Comments on Insecta of the Rhynie Chert’ (1985 Entomol. Gener. 11(1/2): 097–098). Entomol. Gener. 13, 115–117. ( 10.1127/entom.gen/13/1988/115) [DOI] [Google Scholar]
- 23.Baliński A, Sun Y, Dzik J. 2013. Traces of marine nematodes from 470 million years old Early Ordovician rocks in China. Nematology 15, 567–574. ( 10.1163/15685411-00002702) [DOI] [Google Scholar]
- 24.Muir LA, Ng T-W, Li X-F, Xang Y-D, Lin J-P. 2014. Palaeoscolecidan worms and a possible nematode from the Early Ordovician of South China. Palaeoworld 23, 15–24. ( 10.1016/j.palwor.2013.06.003) [DOI] [Google Scholar]
- 25.Chaloner WG, Scott AC, Stephenson J, Jarzembowski EA, Alexander RM, Collinson ME. 1991. Fossil evidence for plant–arthropod interactions in the Palaeozoic and Mesozoic. Phil. Trans. R. Soc. Lond. B 333, 177–186. ( 10.1098/rstb.1991.0066) [DOI] [Google Scholar]
- 26.Bik HM, Lambshead PJD, Thomas WK, Lunt DH. 2010. Moving towards a complete molecular framework of the Nematoda: a focus on the Enoplida and early-branching clades. BMC Evol. Biol. 10, 353 ( 10.1186/1471-2148-10-353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bird DK, Jones JT, Opperman CH, Kikuchi T, Danchin EGJ. 2015. Signatures of adaptation to plant parasitism in nematode genomes. Parasitology 142, S71–S84. ( 10.1017/S0031182013002163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor TN, Klavins SD, Krings M, Taylor EL, Kerp H, Hass H. 2004. Fungi from the Rhynie chert: a view from the dark side. Trans. R. Soc. Edinb. Earth Sci. 94, 457–473. ( 10.1017/S026359330000081X) [DOI] [Google Scholar]
- 29.Hirst S. 1923. On some arachnid remains from the Old Red Sandstone (Rhynie Chert bed, Aberdeenshire). Ann. Mag. Nat. Hist. 12, 455–474. ( 10.1080/00222932308632963) [DOI] [Google Scholar]
- 30.Dunlop JA. 1994 Paleobiology of the trigonotarbid arachnids. PhD thesis, University of Manchester, UK.
- 31.Hirst S, Maulik S. 1926. On some arthropod remains from the Rhynie chert (Old Red Sandstone). Geol. Mag. 63, 69–71. ( 10.1017/S0016756800083692) [DOI] [Google Scholar]
- 32.Claridge MF, Lyon AG. 1961. Lung-books in the Devonian Palaeocharinidae (Arachnida). Nature 191, 1190–1191. ( 10.1038/1911190b0) [DOI] [Google Scholar]
- 33.Dunlop JA, Kamenz C, Talarico G. 2009. A fossil trigonotarbid with a ricinuleid-like pedipalpal claw. Zoomorphology 128, 305–313. ( 10.1007/s00435-009-0090-z) [DOI] [Google Scholar]
- 34.Dunlop JA. 1994. Filtration mechanisms in the mouthparts of tetrapulmonate arachnids (Trigonotarbida, Araneae, Amblypygi, Uropygi, Schizomida). Bull. Brit. Arachnol. Soc. 9, 267–273. [Google Scholar]
- 35.Nyffler M, Olsen EJ, Symondson WOC. 2016. Plant-eating by spiders. J. Arachnol. 44, 15–27. ( 10.1636/P15-45.1) [DOI] [Google Scholar]
- 36.Dubinin VB. 1962. Class Acaromorpha: mites or gnathosomic chelicerate arthropods. In Fundamentals of paleontology (ed. Rodendorf BB.), pp. 447–473. Moscow, Russia: Academy of Science. [Google Scholar]
- 37.Krantz GW, Walter DE (eds). 2009. A manual of acarology, 3rd edn Lubbock, TX: Texas Tech University Press. [Google Scholar]
- 38.Pepato AR, Klimov PB. 2015. Origin and higher-level diversification of acariform mites—evidence from nuclear ribosomal genes, extensive taxon sampling, and secondary structure alignment. BMC Evol. Biol. 15, 128 ( 10.1186/s12862-015-0458-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walter DE. 1988. Predation and mycophagy by endeostigmatid mites. Exp. Appl. Acarol. 4, 159–166. ( 10.1007/BF01193873) [DOI] [Google Scholar]
- 40.Hessein NA, Perring TN. 1986. Feeding habits of the Tydeidae with evidence of Homeopronematus anconai (Acari: Tydeidae) predation on Aculops lycopersici (Acari: Eriophyidae). Int. J. Acarol. 12, 215–221. ( 10.1080/01647958608683467) [DOI] [Google Scholar]
- 41.Da Silva GL, Metzelthin JH, Da Silva OS, Ferla NJ. 2016. Catalogue of the mite family Tydeidae (Acari: Prostigmata) with the world key to the species. Zootaxa 4135, 1–68. ( 10.11646/zootaxa.4135.1.1) [DOI] [PubMed] [Google Scholar]
- 42.Garwood RJ, Sharma PP, Dunlop JA, Giribet G. 2014. A Paleozoic stem group to mite harvestmen revealed through integration of phylogenetics and development. Curr. Biol. 24, 1–7. ( 10.1016/j.cub.2014.03.039) [DOI] [PubMed] [Google Scholar]
- 43.Acosta LE, Machado G. 2007. Diet and foraging. In Harvestmen. The biology of Opiliones (eds Pinto-da-Rocha R, Machado G, Giribet G), pp. 309–338. Cambridge, MA: Harvard University Press. [Google Scholar]
- 44.Juberthie C. 1964. Recherches sur la Biologie des Opilions. Ann. Spéléol. Paris 19, 1–237. [Google Scholar]
- 45.Shear WA, Jeram AJ, Selden PA. 1998. Centiped legs (Arthropoda, Chilopoda, Scutigeromorpha) from the Silurian and Devonian of Britain and the Devonian of North America. Am. Mus. Novit. 3231, 1–16. [Google Scholar]
- 46.Undheim EAB, Jones A, Klauser KR, Holland JW, Pineda SS, King GF, Fry BG. 2014. Clawing through evolution: toxin diversification and convergence in the ancient lineage Chilopoda (centipedes). Mol. Biol. Evol. 31, 2124–2148. ( 10.1093/molbev/msu162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voigtländer K. 2011. Ecology of Chilopoda. In The Myriapoda revised and updated from Traité de Zoologie. Vol. 1 Chilopoda (ed. Minelli A.), pp. 309–325. Leiden, Netherlands: Brill. [Google Scholar]
- 48.Whalley P, Jarzembowski EA. 1981. A new assessment of Rhyniella, the earliest known insect from the Devonian of Rhynie, Scotland. Nature 291, 317 ( 10.1038/291317a0) [DOI] [Google Scholar]
- 49.Hopkin SP. 1997. Biology of the springtails. Insecta: Collembola. Oxford, UK: Oxford University Press. [Google Scholar]
- 50.Greenslade P, Whalley PES. 1986. The systematic position of Rhyniella praecursor Hirst & Maulik (Collembola), the earliest known hexapod. In Second International Seminar on Apterygota, pp. 319–323. Siena, Italy: University of Siena.
- 51.Bernays EA, Jarzembowski EA, Malcolm SB. 1991. Evolution of insect morphology in relation to plants. Phil. Trans. R. Soc. Lond. B 333, 257–264. ( 10.1098/rstb.1991.0075) [DOI] [Google Scholar]
- 52.Sánchez-García A, Engel MS. 2016. Springtails from the Early Cretaceous amber of Spain (Collembola: Entomobryomorpha), with an annotated checklist of fossil Collembola. Am. Mus. Novit. 3862, 1–47. ( 10.1206/3862.1) [DOI] [Google Scholar]
- 53.Tillyard RJ. 1928. Some remarks on the Devonian fossil insects from the Rhynie chert beds, Old Red Sandstone. Trans. R. Ent. Soc. Lond. 76, 65–71. ( 10.1111/j.1365-2311.1928.tb01188.x) [DOI] [Google Scholar]
- 54.Rota-Stabelli O, Daley AC, Pisani D. 2013. Molecular timetrees reveal a Cambrian colonization of land and a new scenario for ecdysozoan evolution. Curr. Biol. 23, 392–398. ( 10.1016/j.cub.2013.01.026) [DOI] [PubMed] [Google Scholar]
- 55.Misof B, et al. 2014. Phylogenomics resolves the timing and pattern of insect evolution. Science 346, 763–767. ( 10.1126/science.1257570) [DOI] [PubMed] [Google Scholar]
- 56.Weihmann T, Kleinteich T, Gorb S., Wipfler B. 2015. Functional morphology of the mandibular apparatus in the cockroach Periplaneta americana (Blattodea, Blattidae)—a model species for omnivore insects. Arthropod Syst. Phyl. 73, 477–488. [Google Scholar]
- 57.Chapman RF. 1998. The insects: structure and function. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 58.Grimaldi DA. 2010. 400 million years on six legs: on the origin and early evolution of Hexapoda. Arthropod Struct. Dev. 39, 191–203. ( 10.1016/j.asd.2009.10.008) [DOI] [PubMed] [Google Scholar]
- 59.Labandeira CC. 2013. Deep-time patterns of tissue consumption by terrestrial arthropod herbivores. Naturwissenschaften 100, 355–364. ( 10.1007/s00114-013-1035-4) [DOI] [PubMed] [Google Scholar]
- 60.Habgood KS, Hass H, Kerp H. 2004. Evidence for an early terrestrial food web: coprolites from the Early Devonian Rhynie chert. Trans. R. Soc. Edinb. Earth Sci. 94, 371–389. ( 10.1017/S0263593300000754) [DOI] [Google Scholar]
- 61.Scott AC, Stephenson J, Chalanor WG. 1992. Interaction and coevolution of plants during the Palaeozoic and Mesozoic. Phil. Trans. R. Soc. Lond. B 335, 129–165. ( 10.1098/rstb.1992.0016) [DOI] [Google Scholar]
- 62.Scott AC. 1977. Coprolites containing plant material from the Carboniferous of Britain. Palaeontology 20, 59–68. [Google Scholar]
- 63.Edwards D, Selden PA. 1992. The development of early terrestrial ecosystems. Bot. J. Scot. 46, 337–366. ( 10.1080/03746600508684794) [DOI] [Google Scholar]
- 64.Chang C-H, James S. 2011. A critique of earthworm molecular phylogenetics. Pedobiologia 54(Suppl. 29), S3–S9. ( 10.1016/j.pedobi.2011.07.015) [DOI] [Google Scholar]
- 65.Suarez SE, Brookfield M, Catlos EJ, Stockli DF. 2016. U–Pb age of the oldest air-breathing animal. Geol. Soc. Am. Abstr. Programs 48, 7 ( 10.1130/abs/2016AM-283259) [DOI] [Google Scholar]
- 66.Trewin NH, Fayers SR, Kelman R. 2003. Subaqueous silicification of the contents of small ponds in an Early Devonian hot-springs complex, Rhynie, Scotland. Can. J. Earth Sci. 40, 1697–1712. ( 10.1139/e03-065) [DOI] [Google Scholar]
- 67.Channing A, Edwards D. 2009. Yellowstone hot spring environments and the palaeo-ecophysiology of Rhynie chert plants: towards a synthesis. Plant Ecol. Divers. 2, 111–143. ( 10.1080/17550870903349359) [DOI] [Google Scholar]
- 68.Channing A, Edwards D. 2013. Wetland megabias: ecological and ecophysiological filtering dominates the fossil record of hot spring floras. Palaeontology 56, 523–556. ( 10.1111/pala.12043) [DOI] [Google Scholar]
- 69.Boothroyd IKG, Browne GN. 2006. Invertebrates of geothermally influenced aquatic and terrestrial ecosystems: longitudinal and lateral linkages In Proc. 28th NZ Geothermal Workshop 2006, Auckland Paper 212, 4 p. [Google Scholar]
- 70.Tuxen SL. 1944. The animals living near the hot springs. In The hot springs of Iceland: their animal communities and their zoogeographical significance (SL Tuxen), pp. 136–166. (The Zoology of Iceland, vol 1, part 11) Copenhagen, Denmark: Munksgaard. [Google Scholar]
- 71.Haug C, Haug JT. 2017. The presumed oldest flying insect: more likely a myriapod? PeerJ. 5, e3402 ( 10.7717/peerj.3402) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.