Abstract
Stem cells self-renew and produce precursor cells that differentiate to become specialized cell types. Land plants generate several types of stem cells that give rise to most organs of the plant body and whose characters determine the body organization. The moss Physcomitrella patens forms eight types of stem cells throughout its life cycle. Under gametangium-inducing conditions, multiple antheridium apical stem cells are formed at the tip of the gametophore and each antheridium apical stem cell divides to form an antheridium. We found that the gametophore apical stem cell, which typically forms leaf and stem tissues, changes to become a new type of stem cell, which we term the antheridium initial stem cell. This antheridium initial stem cell produces multiple antheridium apical stem cells, resulting in a cluster of antheridia at the tip of gametophore. This is the first report of a land plant stem cell directly producing another type of stem cell during normal development. Notably, the antheridium apical stem cells are distally produced from the antheridium initial stem cell, similar to the root cap stem cells of vascular plants, suggesting the use of similar molecular mechanisms and a possible evolutionary relationship.
This article is part of a discussion meeting issue ‘The Rhynie cherts: our earliest terrestrial ecosystem revisited’.
Keywords: Physcomitrella, stem cell, antheridium, sperm, development
1. Introduction
Stem cells are defined by their dual ability to self-renew and produce cells destined for differentiation in both animals and plants [1,2]. All land plants form different types of stem cells that initiate and contribute to the formation of various tissues and organs during development. The tissues and organs formed by stem cell progeny have diverged between land plant lineages, resulting in their morphological differences. The general behaviour of the stem cells themselves also varies between species in several ways; first, seed plants retain multiple stem cells in the shoot, root and vascular meristems, while most non-seed plants have a single apical stem cell at the tip of the shoot and root meristems [3]. Additionally, niche cells like those that maintain stem cells in angiosperms have not been identified in the bryophytes, whose stem cells are autonomous for their self-renewal [4].
Stem cells can also be distinguished by the direction in which they produce precursor cells destined for differentiation. In flowering plants, stem cells in the shoot meristem produce precursor cells in lateral or proximal directions. Columella stem cells produce columella root cap precursor cells distally, while stele stem cells proximally produce precursor cells for the central vascular tissues [1]. Monilophytes and most lycopods retain a single apical stem cell at the shoot and leaf tips; thus, their stem cells proximally produce precursor cells [5]. Root apical stem cells in monilophytes and lycopods produce a cell both distally and proximally to form the precursors of root cap cells and other root cells, respectively [6,7]. In the bryophytes, all previously described apical stem cells are located at the tip and surface of the plant bodies and proximally produce precursor cells [3,8].
The cellular and molecular mechanisms of stem cell formation and maintenance in non-seed plants have been most extensively studied in the moss Physcomitrella patens (Physcomitrella). Physcomitrella forms eight types of stem cells that produce precursors of different tissues, organs and plant bodies throughout the life cycle [3]. After spore germination, the formation of protonema apical stem cells is followed by serial cell divisions that proximally produce the subapical cells that compose protonema filaments [9]. New stem cells are initiated from these protonema cells as side branch initial cells, some of which differentiate into gametophore apical stem cells and proximally produce daughter cells, which become sources of all the cells that form the leafy shoots and gametophores [10,11]. The gametophore apical stem cell produces a precursor cell that asymmetrically divides twice to form a leaf apical stem cell and other cells to form stem tissue. Leaf apical stem cells initiated at the gametophore tips also proximally produce cells to form a leaf [10]. Epidermal cells at specific positions on the gametophore stems become rhizoid apical stem cells, which proximally produce rhizoid cells for water and nutrient conduction [12,13].
When the moss is moved from regular culture conditions at 25°C under long-day or continuous light conditions to 16°C under short-day conditions, gametangia form at gametophore tips [14]. Archegonia and antheridia are produced by archegonium apical stem cells and antheridium apical stem cells, respectively, which are located at the tip of the gametophore [15,16]. The archegonium apical stem cells divide four times to proximally produce four wedge-shaped cells, with a few more produced in antheridium development [15]. These cells periclinally divide to form inner and outer cells, which further divide several times and, respectively, differentiate to inner cells including an egg cell and archegonium cover cells or the sperm and antheridium cover cells [15,16].
Despite the detailed descriptions of cell lineages in these processes [16], their initial steps, including the initiation of apical stem cells for both gametangia, are still unclear. The first antheridium forms in the centre of the primary gametophore apex, surrounded by leaves and axillary hairs, and subsequent antheridium primordia form beside it in Physcomitrella [16] as in other mosses [8,17], but the cellular lineages that result in these primordia have not been elucidated. The gametophore apical stem cell and leaf apical stem cells are located at the gametophore apex, but it is unknown how these cells are related to the antheridium apical stem cell lineages. Leitgeb [17] observed a moss Fontinalis antipyretica, showed three drawings of gametophore tips in two different stages and discussed that the gametophore apical stem cell generates numerous cells, some of which become antheridial initials to produce antheridia. However, the cell lineage from a gametophore apical stem cell to several antheridium initial cells was not clear. Campbell [8] showed a drawing indicating that several antheridia were formed at the tip of a gametophore in another moss Funaria hygrometrica, although the cell lineage of antheridia was not elucidated.
In the present study, we sought to determine the cellular origin of antheridium apical stem cells. To this end, we used confocal microscopy to observe the development of antheridia in gametophores under gametangium-inducing conditions. It has been interpreted in F. antipyretica that the gametophore apical stem cell changes to the antheridium apical stem cell and other antheridia are produced from the base of the first antheridium and the surrounding cells [17]. Unexpectedly, the gametophore apical stem cell became the antheridium initial stem cell, which repeatedly produced antheridium apical stem cells. Furthermore, an immediate sister cell of the first antheridium initial stem cell also became the second antheridium initial stem cell. The antheridium initial stem cells appear to be unique among the previously characterized stem cells of land plants for their ability to directly produce another type of stem cell instead of precursor cells to be differentiated. Furthermore, it is noteworthy that antheridium apical stem cells are distally produced from antheridium initial stem cells. This type of stem cell division has not previously been reported in the bryophytes, to our knowledge, but does occur in the root meristems of vascular plants. Although more detailed studies at the molecular level are necessary, these results imply that stem cells with distally producing precursor cells originated before the divergence of the vascular plants and the bryophytes or that they convergently evolved in these two lineages.
2. Material and methods
(a). Plant materials and culture conditions
Protonemata of the Cove-NIBB wild-type strain of P. patens Bruch and Schimp subsp. patens [18] were propagated for two weeks on BCDAT medium containing 1 mM CaCl2 and 0.8% (w/v) agar at 25°C under long-day conditions (16 L : 8 D) [19]. Collected protonemata were homogenized in sterilized water using a mortar and a pestle, and the suspension was spread under aseptic conditions onto a peat pellet (Jiffy-7; Jiffy Products International AS) in a transparent plastic box. The planted peat pellets were kept at 25°C under long-day conditions for one to two months and the gametophore apices were collected to observe the gametophore apical stem cells. The peat pellets were then transferred to 16°C under short-day conditions (8 L : 16 D) to induce antheridium development. The gametophore apices were collected 5 and 8 days after the transfer to observe antheridium development. Antheridium development is not strictly synchronized and different stages of development were observed in the collected materials.
(b). Fixation and pretreatment for microscopic observation
The gametophore apices were fixed overnight in 99.8% methanol at −30°C to remove chlorophyll, then returned to room temperature and rinsed with water. The fixed gametophores were placed in 10 µg ml–1 calcofluor (Fluorescent Brightener 28; Sigma-Aldrich) at 4°C for up to a week to stain their cell walls. For tissue clearing and three-dimensional reconstruction of thick plant tissue [20,21], the stained gametophores were sequentially placed in 10, 20, 40, 60, 80 and 90% 2,2′-thiodiethanol and 10 mM Mes-KOH (pH 6.5) solutions for 30 min each. The leaves of the pretreated gametophores were removed under a stereoscopic microscope to expose the apices. One to three apices were placed on a glass slide in a solution containing 2.5% 1,4-diazabicyclo[2.2.2]octane (DABCO), 90% 2,2′-thiodiethanol and 10 mM Mes-KOH (pH 6.5).
(c). Confocal laser scanning microscopy and three-dimensional analysis
To obtain three-dimensional images, Z stacks were acquired using a Zeiss LSM 510 META SP microscope (Carl Zeiss) with a 63× oil immersion lens at 0.31 µm intervals. A 405 nm diode laser was used for excitation and calcofluor fluorescence was detected using an LP420 filter. The three-dimensional reconstruction, creation of optical section and construction of three-dimensional view were performed using ImageJ (http://imageJ.nih.gov/ij/). Adobe Photoshop was used for adjusting the contrast of the optical section images.
3. Results
(a). The gametophore apical stem cell repeatedly produces a precursor of the leaf apical stem cell
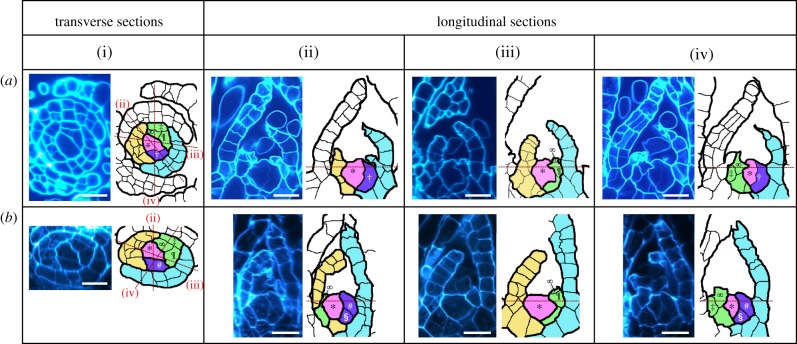
Our observations confirmed that the gametophore apical stem cell located at the tip of the gametophore (figure 1a, the pink cell with *) continuously divided to produce an immediate sister cell (figure 1a, the purple cell with †), which was a leaf and stem precursor cell under regular cultivation conditions (figure 1a). The precursor cell asymmetrically divided to form proximal and distal cells (figure 1b, the purple cells with § and #, respectively). The proximal cell proliferated to form a stem tissue basal to the leaf [3,10] (figure 1a,b, the green cells without any marks). The distal cell divided to form the distal leaf apical stem cell and a precursor cell to form stem epidermal cells (figure 1a,b, the green cell with ‡). The distal leaf apical stem cell divided to produce a distal leaf apical stem cell (figure 1a,b, the green cell with ∞) and a leaf cell (figure 1a,b, the green cell with ¶). As reported previously, the leaf apical stem cell repeatedly produced wedge-shape cells with similar morphologies, which subsequently divided to form a leaf [10]. After the cell divisions of the immediate sister cell and its daughter cells (figure 1a, the green cells), the gametophore apical stem cell divided again to produce a new precursor cell (figure 1a, the purple cell with †). This pattern of development is concordant with those in other mosses [22,23].
Figure 1.
Cell divisions of gametophore apical stem cells. Optical transverse sections (i) and longitudinal sections (ii, iii, and iv) of gametophore tips cultivated under the regular growth conditions, producing leaf apical stem cells. Longitudinal section planes corresponding to (ii), (iii) and (iv) are indicated in (i) as dashed lines. (a) Developmental stage with the gametophore apical stem cell (*) and an immediate sister cell (†). (b) Developmental stage at which the immediate sister cell has divided to form a proximal cell (§) and a distal cell (#). The distal cell divides to form the leaf apical stem cell and the precursor cell for stem epidermis (‡). The leaf apical stem cell divides to form the self-renewed leaf apical stem cell (∞) and a leaf cell (¶). The fine lines in the drawings indicate cell division planes. All cells derived from each of the precursor cells descended from the gametophore apical stem cell are indicated by the same colour. Pale blue, yellow, green and purple cells are in order of the old derivative from the gametophore apical stem cell in pink. Scale bars, 20 µm.
(b). The gametophore apical stem cell becomes the antheridium initial stem cell, which produces antheridium apical stem cells
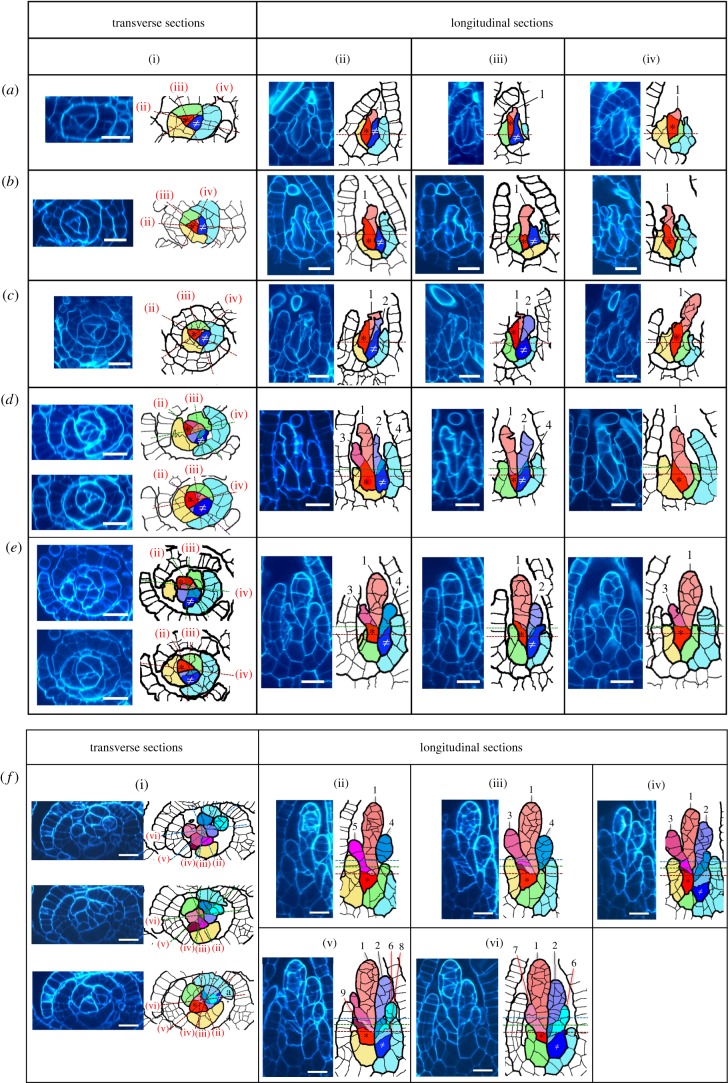
The cell division cycle of the gametophore apical stem cell changed when gametophores were moved to gametangium-inducing conditions. The apical stem cell, corresponding to the gametophore apical stem cell under regular growth conditions, divided to form a proximal cell (figure 2a, the red cell with *) and a distal cell (figure 2a, the pale red cell with ‘1’) before the cell division of the immediate sister cell (figure 2a, the blue cell with ≠). The distal cell was an antheridium apical stem cell. After the production of the first antheridium apical stem cell from the central cell (figure 2a,b), the immediate sister cell (figure 2a,b, the blue cell with ≠) divided to produce the second antheridium apical stem cell (figure 2c, the purple cell with ‘2’). Subsequently, the third antheridium apical stem cell was produced from the cell that produced the first antheridium apical stem cell (figure 2d, the red cell with *). On the other hand, the fourth antheridium apical stem cell was produced from the cell that produced the second antheridium apical stem cell (figure 2d,e the blue cell with ≠). The production of antheridium apical stem cells was also continued by both cells by turns at least until seven antheridium apical stem cells are formed (figures 2f and 3). Although paraphyses are observed in clusters of antheridia in a matured stage [16], we have not observed any paraphyses during stages shown in this study. As both cells producing antheridium apical stem cells repeatedly distally, we considered them to be self-renewed and to be stem cells of the same type. We collectively termed them ‘antheridium initial stem cells’. The antheridium apical stem cell divided six to eight times (in 28 observed antheridia, one divided six times, 21 divided seven times and six divided eight times) to produce the wedge-shaped cells that formed an antheridium (figure 4). As we observed all cell divisions of the antheridium apical stem cell in addition to previous partial description [16], we found that the antheridium apical stem cell continued to divide even after proximal segments periclinally divided.
Figure 2.
Cell divisions of antheridium initial stem cells. Optical transverse sections (i) and longitudinal sections (ii--iv) of gametophore tips cultivated under the gametangium-inducing conditions. Longitudinal section planes corresponding to (ii), (iii), (iv), (v) and (vi) are indicated in (i) as dashed lines. Gametophore tips with one (a and b), two (c), four (d and e) and seven (f) antheridium apical stem cells are shown. The eighth and ninth putative antheridium apical stem cells, which cannot be distinguished from paraphysis primordia, are also indicated (f). Leaves and antheridium clusters are shown in schematics. Axillary hairs outside of the clusters are not drawn in schematics. The fine lines in the drawings indicate cell division planes. The antheridium initial stem cell that forms the first antheridium apical stem cell is coloured red and indicated by a black asterisk (*). The other antheridium initial stem cell (≠) is coloured blue. All cells derived from each antheridium apical stem cell are indicated in the same colour. Numbers from 1 to 7 indicate the first to the seventh antheridium apical stem cells. Pale blue, yellow and green leaves are in order of the old derivative from the gametophore apical stem cell. Scale bars, 20 µm.
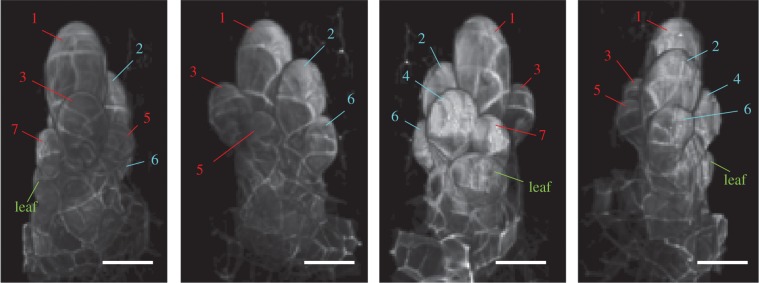
Figure 3.
Three-dimensional reconstruction of an antheridium cluster with seven developing antheridia. The first to seventh antheridia are indicated by numbers. Antheridia produced from the primary and secondary antheridium initial stem cells are coloured in red and blue, respectively. The youngest leaf primordium is also indicated. Scale bars, 20 µm.
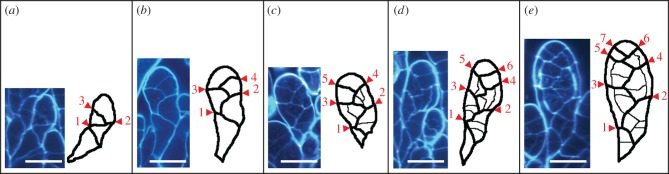
Figure 4.
Development of an antheridium from an antheridium apical stem cell. Antheridia after the third (a), fourth (b), fifth (c), sixth (d) and seventh (e) cell divisions of an antheridium apical stem cell are shown. The thicker lines between cells in the drawings indicate the cell division planes formed by cell divisions of the antheridium apical stem cell and the thinner lines indicate other cell division planes. Numbers from 1 to 7 indicate the first to the seventh cell divisions of the antheridium apical stem cell. Scale bars, 20 µm.
4. Discussion
(a). The origin of antheridium initial stem cells
After the gametophores were moved into the gametangium-inducing conditions, both the gametophore apical stem cell at the centre of the gametophore (which corresponds to the gametophore apical stem cell under regular growth conditions) and its immediate sister cell became antheridium initial stem cells. Previous studies in other mosses could not trace the cell lineage of each antheridium apical stem cell except for the first one [8,17]. In F. antipyretica, it had been speculated that the gametophore apical stem cell changes to an antheridium apical stem cell and subsequently, its basal cells and surrounding cells divide to form several antheridium initial cells from which antheridium apical stem cells are formed [17]. However, our study showed that the gametophore apical stem cell is changed to an antheridium initial stem cell, which repeatedly produces several antheridium apical stem cells instead of a single antheridium apical stem cell. Furthermore, only one additional antheridium initial stem cell is formed as a sister cell to the first antheridium initial stem cell. It is unexpected that antheridium apical stem cells are repeatedly directly produced from two cells that are antheridium initial stem cells.
While the position of the gametophore apical stem cell was clearly correlated with one of two antheridium initial stem cells, it is unclear whether the other is produced from the gametophore apical stem cell or if the leaf apical stem cell precursor, produced under the non-inductive conditions, alters its cell fate after the change in conditions. It is difficult to investigate these two possibilities, because we could not distinguish gametophores with cell fate changes from those without, and because 15 of the 51 gametophores observed did not form antheridia and may continuously produce leaves instead of antheridia even under the inductive conditions. The gametophore apical stem cell is obscured by leaves and it is not possible to observe the division patterns of cells using time-lapse techniques. The establishment of marker genes specifically expressed either in the precursor cell or the antheridium initial stem cell may help to solve this problem in the future. After approximately 12 antheridia are formed, paraphyses are formed in the antheridium cluster and archegonia are just outside the cluster separated by at least one leaf, as previously reported [16]. Cellular origins of these organs are also a future challenge.
(b). The antheridium initial stem cell repeatedly produces another type of stem cells: antheridium apical stem cells
Eight types of stem cells have been reported in Physcomitrella and the change of the stem cell characteristics correspond to the progression of the life cycle [3]. The common feature of the stem cells is that one daughter retains the stem cell identity, while the other differentiates and does not self-renew. The antheridium initial stem cell that we describe here represents a newly discovered type of stem cell in Physcomitrella that, in contrast with other stem cells, repeatedly produces antheridium apical stem cells instead of cells that differentiate without self-renewal. The repeated production of the antheridium apical stem cells results in multiple antheridia at each gametophore tip. The early developmental stages of antheridia have not been well studied in other mosses and it is not known whether the development we observed in Physcomitrella is common throughout the mosses. Although Leitgeb [17] showed a single section of the gametohpore apex of F. antipyretica, which is concordant with the existence of two antheridium initial stem cells, we should analyse the three-dimensional structure in detail before concluding the similarity of development between two mosses.
(c). Antheridium apical stem cells are distally produced from the antheridium initial stem cell
After cell division of a stem cell, one daughter cell is fated to be a stem cell and the other to be differentiated. Cell fates of two cells should be determined by internal and external cues, and which cell becomes a stem cell is strictly regulated during development. All eight of the previously characterized types of Physcomitrella stem cells produce daughter cells in a proximal direction from the plant body; however, the antheridium initial stem cells produce their progeny distally. While stem cells that produce cells distally are not found in other bryophytes to the best of our knowledge, vascular plants commonly use this distal production. In these lineages, distal stem cell divisions produce precursor cells that are involved in the formation of tissues with adaptive morphology: root cap stem cells that produce root cap tissues to protect the root meristem. Future studies should therefore aim to infer the origin and evolution of such stem cell types.
Acknowledgements
We would like to thank all members of the Hasebe laboratory for their useful discussion and technical support, especially Ruan de Villiers, Yosuke Tamada and Masaki Ishikawa for their careful reading of the manuscript. We also thank the Model Research Facility and the Data Integration and Analysis Facility of the National Institute for Basic Biology, and Katsuji Yoshioka of the Cancer Research Institute of Kanazawa University for experimental facilities.
Data accessibility
This article has no additional data.
Authors' contributions
R.K. and M.H. designed the research; T.M. and R.K. developed the confocal laser scanning microscopic observation method for thick tissue of Physcomitrella; Y.Y. and R.K. collected data; R.K., Y.Y. and M.H. analysed data and developed figures; R.K., T.M. and M.H. wrote the paper. All authors discussed the results and commented on the paper.
Competing interests
We declare we have no competing interests.
Funding
This work was partially funded by a MEXT KAKENHI grant to M.H., T.M. and R.K. (16H06378).
References
- 1.Heidstra R, Sabatini S. 2014. Plant and animal stem cells: similar yet different. Nat. Rev. Mol. Cell Biol. 15, 301–312. ( 10.1038/nrm3790) [DOI] [PubMed] [Google Scholar]
- 2.Sparks EE, Imai A, Hasebe M, Benfey P. 2014. Developmental regulation and de novo formation of stem cells in plants. In Stem cells: from basic research to therapy, vol. 1 (eds Calegari F, Waskow C), pp. 405–434. New York, NY: CRC Press. [Google Scholar]
- 3.Kofuji R, Hasebe M. 2014. Eight types of stem cells in the life cycle of the moss Physcomitrella patens. Curr. Opin. Plant Biol. 17, 13–21. ( 10.1016/j.pbi.2013.10.007) [DOI] [PubMed] [Google Scholar]
- 4.Sato Y, Sugimoto N, Hirai T, Imai A, Kubo M, Hiwatashi Y, Nishiyama T, Hasebe M. 2017. Cells reprogramming to stem cells inhibit the reprogramming of adjacent cells in the moss Physcomitrella patens. Sci. Rep. 7, 1909 ( 10.1038/s41598-017-01786-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imaichi R. 2008. Meristem organization and organ diversity. In Biology and evolution of ferns and lycophytes (eds Ranker TA, Haufler CH), pp. 75–103. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 6.Gifford EM, Foster AS. 1989. Morphology and evolution of vascular plants, 3rd edn New York, NY: W. H. Freeman and Company. [Google Scholar]
- 7.Gunning BES, Hughes JE, Hardham AR. 1978. Formative and proliferative cell divisions, cell differentiation, and developmental changes in the meristem of Azolla roots. Planta 143, 121–144. ( 10.1007/BF00387786) [DOI] [PubMed] [Google Scholar]
- 8.Campbell DH. 1895. The structure and development of mosses and ferns. London, UK: The Macmillan Company. [Google Scholar]
- 9.Cove DJ, Knight CD. 1993. The moss Physcomitrella patens, a model system with potential for the study of plant reproduction. Plant Cell 5, 1483–1488. ( 10.1105/tpc.5.10.1483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison CJ, Roeder AHK, Meyerowitz EM, Langdale JA. 2009. Local cues and asymmetric cell divisions underpin body plan transitions in the moss Physcomitrella patens. Curr. Biol. 19, 461–471. ( 10.1016/j.cub.2009.02.050) [DOI] [PubMed] [Google Scholar]
- 11.Aoyama T, Hiwatashi Y, Shigyo M, Kofuji R, Kubo M, Ito M, Hasebe M. 2012. AP2-type transcription factors determine stem cell identity in the moss Physcomitrella patens. Development 139, 3120–3129. ( 10.1242/dev.076091) [DOI] [PubMed] [Google Scholar]
- 12.Jang G, Yi K, Pires ND, Menand B, Dolan L. 2011. RSL genes are sufficient for rhizoid system development in early diverging land plants. Development 138, 2273–2281. ( 10.1242/dev.060582) [DOI] [PubMed] [Google Scholar]
- 13.Sakakibara K, Nishiyama T, Sumikawa N, Kofuji R, Murata T, Hasebe M. 2003. Involvement of auxin and a homeodomain-leucine zipper I gene in rhizoid development of the moss Physcomitrella patens. Development 130, 4835–4846. ( 10.1242/dev.00644) [DOI] [PubMed] [Google Scholar]
- 14.Hohe A, Rensing SA, Mildner M, Lang D, Reski R. 2002. Day length and temperature strongly influence sexual reproduction and expression of a novel MADS-box gene in the moss Physcomitrella patens. Plant Biol. 4, 595–602. [Google Scholar]
- 15.Kofuji R, Yoshimura T, Inoue H, Sakakibara K, Hiwatashi Y, Kurata T, Aoyama T, Ueda K, Hasebe M. 2009. Gametangia development in the moss Physcomitrella patens. In Annual plant reviews, Vol. 36: the moss Physcomitrella patens (eds Knight C, Perroud P-F, Cove D), pp. 167–181 Oxford, UK: Wiley-Blackwell. [Google Scholar]
- 16.Landberg K, Pederson ER, Viaene T, Bozorg B, Friml J, Jönsson H, Thelander M, Sundberg E. 2013. The moss Physcomitrella patens reproductive organ development is highly organized, affected by the two SHI/STY genes and by the level of active auxin in the SHI/STY expression domain. Plant Physiol. 162, 1406–1419. ( 10.1104/pp.113.214023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leitgeb H. 1868. Beiträge zur Entwicklungsgeschichte der Pflanzenorgane II. Entwicklung der Antheridien bei Fontinalis antipyretica. Sitzungsber. Akad. Wiss. Wien., Math.-Nat. Cl. Abt. 1, 58: 525–537. [Google Scholar]
- 18.Ashton NW, Cove DJ. 1977. The isolation and preliminary characterisation of auxotrophic and analogue resistant mutants in the moss Physcomitrella patens. Mol. Gen. Genet. 154, 87–95. [Google Scholar]
- 19.Nishiyama T, Hiwatashi Y, Sakakibara K, Kato M, Hasebe M. 2000. Tagged mutagenesis and gene-trap in the moss, Physcomitrella patens by shuttle mutagenesis. DNA Res. 7, 9–17. [DOI] [PubMed] [Google Scholar]
- 20.Aoyagi Y, Kawakami R, Osanai H, Hibi T, Nemoto T. 2015. A rapid optical clearing protocol using 2,2′-thiodiethanol for microscopic observation of fixed mouse brain. PLoS ONE 10, e0116280 ( 10.1371/journal.pone.0116280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasegawa J, Sakamoto Y, Nakagami S, Aida M, Sawa S, Matsunaga S. 2016. Three-dimensional imaging of plant organs using a simple and rapid transparency technique. Plant Cell Physiol. 57, 462–472. ( 10.1093/pcp/pcw027) [DOI] [PubMed] [Google Scholar]
- 22.Berthier J. 1973. Recherches sur la structure et le développement de l'apex du gamétophyte feuillé des Mousses. Rev. Bryol. Lichénol. 38, 421–551. [Google Scholar]
- 23.Frey W. 1971. Blattentwicklung bei Laubmoosen. Nova Hedwigia 20, 463–556. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.