Abstract
The morphology of plant fossils from the Rhynie chert has generated longstanding questions about vascular plant shoot and leaf evolution, for instance, which morphologies were ancestral within land plants, when did vascular plants first arise and did leaves have multiple evolutionary origins? Recent advances combining insights from molecular phylogeny, palaeobotany and evo–devo research address these questions and suggest the sequence of morphological innovation during vascular plant shoot and leaf evolution. The evidence pinpoints testable developmental and genetic hypotheses relating to the origin of branching and indeterminate shoot architectures prior to the evolution of leaves, and demonstrates underestimation of polyphyly in the evolution of leaves from branching forms in ‘telome theory’ hypotheses of leaf evolution. This review discusses fossil, developmental and genetic evidence relating to the evolution of vascular plant shoots and leaves in a phylogenetic framework.
This article is part of a discussion meeting issue ‘The Rhynie cherts: our earliest terrestrial ecosystem revisited’.
Keywords: land plant, shoot, leaf evolution, evo–devo, plant body plan
1. Introduction
Today's biota includes ca 375 000 species of vascular plant that generate over 90% of terrestrial productivity, and variation in shoot and leaf form are major components of vascular plant biodiversity [1–3]. The earliest land plants arose about 470 million years ago and are evidenced in the fossil record as spores or spore masses [4–7]. Speculatively, these plants lacked shoots and leaves, instead having tiny fertile axes that entered reproductive development straight away or elaborated a small axis terminating in sporangium formation [8–10], and similar forms remain evident among living bryophyte relatives of the earliest land plants, which comprise ca 20 000 species [1]. Around 430 million years ago [11,12], the innovation of shoots and leaves underpinned an explosive radiation of vascular plant form analogous to the Cambrian explosion of animals. The origin of vascular plants precipitated a 10-fold increase in plant species numbers [1], promoted soil development [13] and led to an 8–20-fold atmospheric CO2 drawdown [5,14], significantly shaping Earth's geosphere and biosphere [15–17]. Many pro-vascular and early vascular plant forms in the fossil record look very different to modern vascular plants and exhibit traits that suggest stepwise changes in form from a bryophyte-like evolutionary starting point [9–11,18]. Unlike vascular plants, bryophytes have gametophyte-dominant life cycles in which the photosynthetic body of the plant is haploid; vascular plant shoots and leaves evolved in the diploid sporophyte phase of the life cycle [19]. In this review, we aim to give an overview of the stages in vascular plant shoot and leaf evolution evident in the fossil record, explain how developmental and genetic findings in bryophytes and non-seed vascular plants illuminate these steps and identify future research avenues that will tell us more about how vascular plant shoots and leaves arose. The origin of vascular plants with shoots and leaves has intrigued biologists for over 100 years, e.g. [19,20], and the new tools and fossil evidence that we have at our disposal offer the possibility to generate knowledge that will fundamentally advance our understanding of vascular plant form and evolution [10,21–23].
2. Identifying the direction of evolutionary trait change
To understand the evolution of plant form, we need to know which traits have been gained or lost through time in the plant lineages that concern us. This aim can be fully realized in studying closely related plants where divergence times are recent and traits of interest are distributed among taxa whose evolutionary relationships are well resolved. For instance, archaeology, dated molecular phylogenies and developmental genetics all support strong branch suppression in the monophyletic origin of maize from its wild relative teosinte around 9000 years ago [24–27]. However, the lineage divergence times involved in leaf evolution are ancient, spanning a period of around 440 million years [11]. Comprehensive sampling of the fossil record is not possible owing to incomplete deposition and taphonomic degradation, and extinct taxa are not open to experimentation in the way that living plants are. These features make it hard to identify the direction of trait change involved in vascular plant shoot and leaf evolution. Nevertheless, a combination of phylogenetic and fossil data illuminates some of the steps involved in the evolution of leafy forms, and these are outlined below.
3. Morphological transitions during the origin of vascular plant shoot systems
Phylogenetic evidence places bryophytes as a monophyletic sister group or paraphyletic sister grade to the vascular plants [28–30], and bryophytes all have uni-axial sporophytes terminating in reproductive sporangium formation (morphologies 1–3 in figures 1 and 2a–d), an ancestral characteristic of land plants [10,33]. The first step in shoot evolution involved the innovation of a branching habit with sporangia at the tips of each branch (morphology 4 in figure 1). Partitatheca is among the earliest branching fossils. It has small axes (ca 3 mm tall) that possess a combination of bryophyte and tracheophyte characters, including an apparent lack of vasculature, production of dyad spores, stomata and branching axes with at least one dichotomy (figures 1 and 3a) [5,9,44,45]. Aglaophyton (morphology 5 in figures 1 and 3b) shows similar composite features with no vasculature, production of trilete monad spores and a higher order of branching [31]. Cooksonia fossils (morphology 6 in figure 1; figures 3c and 4a) exemplify the earliest known vascular plants, and range in height from 1.8 mm to 6 cm [5,48–50]. Some Cooksonia fossils have axes that are considered too narrow to contain much photosynthetic tissue and, as in bryophytes, their sporophytes were most likely to have been nutritionally dependent on photosynthetic gametophytes [51]. Their repeated equally branching habit with each branch terminating in sporangium formation (figure 3c) suggests repetition of a developmental module that pre-existed in bryophytes and pro-vascular plants such as Partitatheca. Similar isotomously branching forms with terminal sporangia are manifest among vascular plants of the Rhynie chert assemblage [18], suggesting that this developmental module was a plesiomorphy of early vascular plants and their precursors (figure 1). Therefore, the earliest vascular plants had a system of equally branching axes with terminal sporangia but no leaves, and such forms are known as polysporangiophytes.
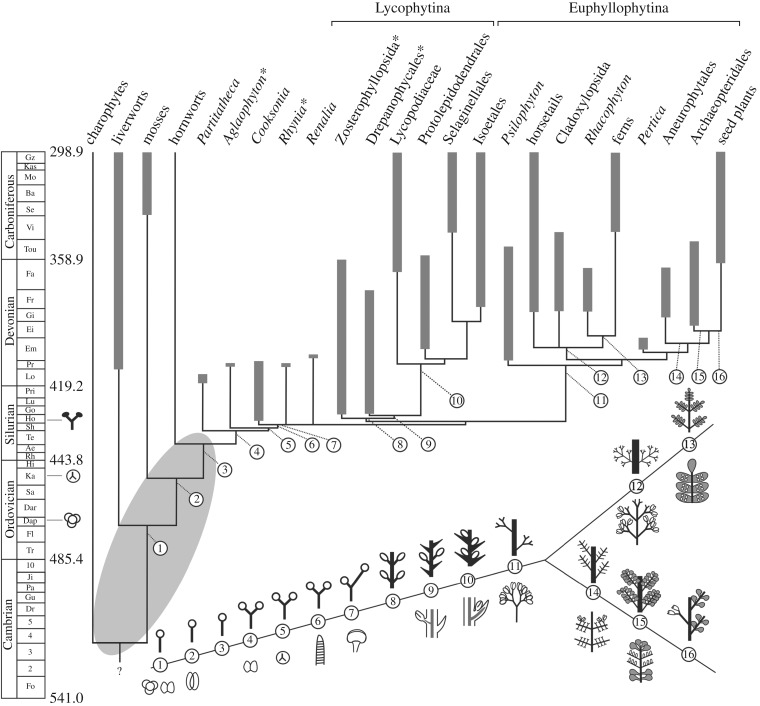
Figure 1.
Hypothetical phylogenetic tree for land plants plotted against time in the Palaeozoic, based on the stratigraphic ranges of key taxa and major groups of land plants from the fossil record (thick grey bars) with minimum implied range extensions (thin lines) (modified after Kenrick & Crane [11,31]). Starred taxa or groups were present in the Rhynie chert assemblage. The first appearances of permanent, regularly arranged cryptospores, trilete monads and an unequivocal embryophyte body are indicated against the time-scale. The timing of divergence and inter-relationships between the bryophyte and tracheophyte lineages are not yet resolved so relationships within the grey oval are uncertain; here we follow Kenrick & Crane [11,31]. The maximum age for the origin of the embryophytes is estimated around the mid Ordovician based on the first appearance of tetrahedral cryptospore tetrads [32]. Numbered illustrations indicate the phylogenetic position of key innovations in plant form, with a focus on shoot and leaf evolution. Innovations included 1–3: uni-axial, leafless sporophyte forms (see also figure 2a–d), 1: permanent tetrads and dyads, similar to those produced by some extant liverworts [32], 2: stomata (stomatophytes), 4–6: isotomous branching, 4: cryptospores, 5: trilete monads, 6: vascular tissue (tracheophytes), 7: increased branching complexity (anisotomy), 8–10: indeterminate growth with lateral insertion of bivalved sporangia, 9: non-vascularized enations, 10: vascularized lycophylls and positioning of sporangia behind leaves, 11: simple lateral branching systems with sporangia arranged in trusses, 12: complex lateral branching systems with dichotomies lateral to first or second order branches, 13: planar fronds with laminae (in grey) and sporangia positioned on abaxial surfaces, 14: increased complexity in lateral branching systems with dichotomies lateral to first or second order branches and terminal sporangia, 15: planar euphylls on lateral branching systems (in grey) with sporangia positioned on adaxial surfaces, 16: seeds arising on lateral branches.
Figure 2.
The range of shoot and leaf morphologies among major clades of living land plants (images not to scale). (a–d) Thalloid liverwort (a), leafy liverwort (b), hornwort (c) and moss (d) sporophyte forms are somewhat similar, comprising a single axis (white arrows) that terminates in sporangium formation and capsule development (pink arrows). Hornwort sporangia run most of the length of the sporophyte and are not labelled. While liverwort sporophytes are fully dependent on gametophytes for food, moss and hornwort sporophytes contain some photosynthetic tissues. (e–g) Clubmosses (e), spike mosses (f) and quillworts (g) derive from deep divergences within the lycophyte lineage as outlined in figure 1, and have lycophylls. Sporangia are borne laterally on specialized reproductive shoots termed strobili, as framed in (e). (h–m) Living monilophytes comprise horsetails (h), polypod ferns (i,j), whisk ferns (k), ophioglossid ferns (l) and filmy ferns (m), which have diverse leaf morphologies reflecting different patterns of development. White or black arrows and the frame in (m) indicate leaves or fronds, and pink arrow indicates sporangium. (n–r) A selection of leaf morphologies represented among gymnosperms. The familiar pine needle leaf form of conifers represents a narrow aspect of gymnosperm leaf morphology. (s,t) Simple and compound flowering plant leaves. Photographs contributed by (a,b) David Long, (c,d,e,m) Jeff Duckett and Silvia Pressel, (f–l,n–t) Jill Harrison and (g) Joshua Mylne.
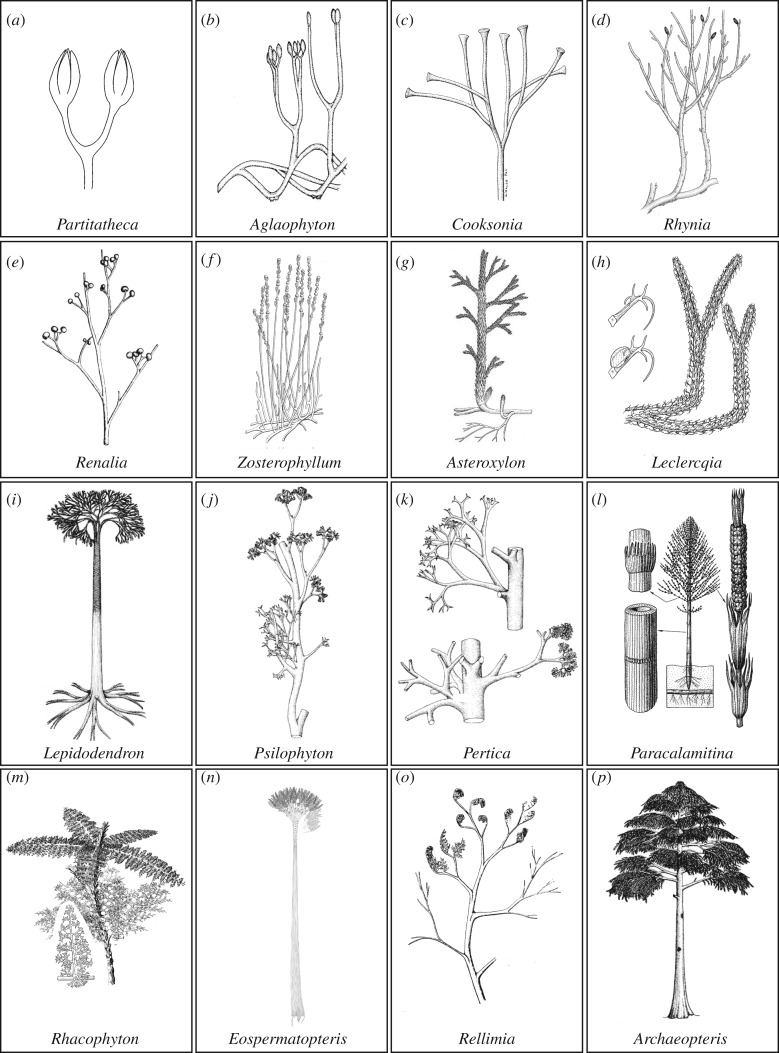
Figure 3.
(Overleaf.) Shoot system architectures of fossil pro-vascular and vascular plant lineages included in figure 1. These fossils illustrate evolutionary transitions contributing to polyphyletic leaf origins including bifurcation, sterilization, indeterminacy, overtopping, planation and webbing. (a,b) Partitatheca (a) and Aglaophyton (b) represent part of an early pro-vascular or vascular plant grade with bifurcating shoot systems and terminal sporangia. (c–e) Among basal vascular plants, increases in shoot size (Cooksonia) and developmental complexity are evidenced by sterile and reproductive branch fate acquisition (Rhynia) or unequal branch growth to produce an overtopped form (Renalia). (f–i) Fossil lycophytes have sterile indeterminate axes with lateral sporangia (Zosterophyllum) or lycophylls (g–i). Branching is isotomous (h,i) or overtopping (f,g), and some fossil isoetaleans such as Lepidodendron attained tree forms more than 30 m tall. (j,k) Stem group euphyllophytes such as Psilophyton and Pertica had overtopped shoot systems with bifurcating lateral branches that were sterile or terminated in sporangial clusters. (l–n) Monilophyte fossils include horsetail-like sphenopsids such as Paracalamitina (l), in which leaves were iterated in whorls, and sporangia differentiated from modules of the main axis or branches. Fern-like plants such as Rhacophyton (m) had partially planar lateral branches with multiple branchlets and some webbing at the distal tips. Cladoxylopsids such as Eospermatopteris (n) had a tree-like habit with terminal clusters of flattened lateral branches and multiple dichotomizing branchlets. (o,p) Progymnosperms such as Rellimia (o) and Archaeopteris (p) had planar lateral branches with multiple branchlets, and in some instances laminar tissue. Reconstructions were (a) drawn by Jennifer Morris, (b) redrawn from Edwards [34] and reproduced from Edwards [18] by permission of the Royal Society of Edinburgh, (c) reproduced from Gerrienne et al. [35] by permission of Elsevier, (d) redrawn from Edwards [36] and reproduced from Kenrick & Crane [11] with permission from Paul Kenrick, (e) redrawn from Gensel [37] and reproduced from Stewart & Rothwell [38] with permission from Cambridge University Press, (f) reproduced from Walton [39] with permission from the International Society of Plant Morphologists, (g) reproduced from Edwards [18] by permission of the Royal Society of Edinburgh, (h) reproduced from Bonamo et al. [40] by permission of the Botanical Gazette, (i–k) reproduced from Stewart & Rothwell [38] with permission from Cambridge University Press, (l) reproduced from Naugolnykh [41] with permission from Cambridge University Press, (m) reproduced from Stewart & Rothwell [38] with permission from Cambridge University Press, (n) reproduced from Stein et al. [42] with permission from Nature Publishing Group, (o) reproduced from Bonamo & Banks [43] with permission from the Botanical Society of America, (p) reproduced from Stewart & Rothwell [38] with permission from Cambridge University Press.
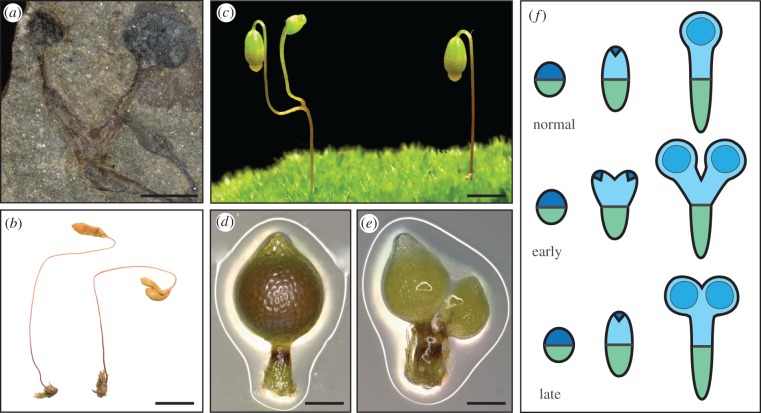
Figure 4.
Origins of a polysporangiophyte habit. (a) A pro-tracheophyte fossil Cooksonia spp. sporophyte. Scale bar, 1.8 mm. (b) A rare natural variant of Bryum radiculosum showing duplicated sporangia subtended by a portion of seta (photo by Alison Reed reproduced from Edwards & Kenrick [5]). Scale bar, 5 mm. (c) A rare natural moss variant showing sessile duplicated sporangia, as described in the classical literature [20,46] (photo by Alison Reed). Scale bar, 5 mm. (d) Wild-type sporophyte morphology in the moss Physcomitrella. Scale bar, 0.2 mm. Reproduced from Bennett et al. [47]. (e) Physcomitrella pinb mutants have a low penetrance branching phenotype. Scale bar, 0.2 mm. Reproduced from Bennett et al. [47]. (f) Embryonic development in Physcomitrella involves a transverse division to form apical (blue) and basal (green) identities. Apical (dark blue) and basal cells iterate the embryonic axis, and this embryonic development is followed by sporangium differentiation (blue circles) and intercalary proliferation. Speculatively, the branching morphologies of (b) and (c) involve early and mid-stage division and duplication of the apical cell, respectively. pin mutations in a moss-like sporophyte provide one possible entry point into the evolution of polysporangiophyte forms.
4. Stage 1. The origin of bifurcating forms
(a). Patterns of development in bryophyte sporophytes
The nature of morphological, developmental and genetic change generating polysporangiophyte branching forms has been a source of scientific speculation for over a century, and still remains an open question [5,10,11,20,30,46,49,52–60]. It is now widely accepted that polysporangiophyte forms arose from uni-axial bryophyte-like precursors [5,56–58,60]. However, the uni-axial form of liverworts, mosses and hornworts (figure 2a–d) arises by distinct embryonic trajectories both within and between lineages (table 1) [33,57]. In brief, liverwort and moss zygotes undergo a first division to form apical and basal cells, and with the exception of Riccia, the sporangium differentiates from the apical end of the embryo [33]. Axial development occurs by apical differentiation into the foot and seta in liverworts or by distinct apical cell and intercalary proliferative activities and differentiation in mosses [33]. Hornwort sporophytes show a divergent pattern of development in which the first embryonic division is vertical and subsequent transverse divisions pattern the embryo. The basal cells arising by transverse divisions differentiate into a foot region, an intercalary proliferative region and a short seta [33]. The bifurcating architectures of early polysporangiophytes are thought to reflect the activity of apical meristems with a single apical stem cell [57,58], and the transient embryonic apical cell activity of mosses may offer the closest living proxy.
Table 1.
Patterns of embryonic development in bryophytes. Thallose liverworts (TL), leafy liverworts (LL), hornworts (H) and mosses (M); data collated from Parihar [33].
| order | species | form | first embryonic division | second embryonic division | growth by apical cell | origin of sporangium | axial elongation |
|---|---|---|---|---|---|---|---|
| TL | Riccia crystallina | globoid | transverse | vertical | absent | apical + basal differentiation | none |
| TL | Marchantia polymorpha | axial | transverse | vertical | absent | apical differentiation | basal differentiation into foot and seta |
| TL | Pellia epiphylla | axial | transverse | vertical | absent | apical differentiation | apical differentiation into foot and seta |
| LL | Porella bolanderi | axial | transverse | transverse | absent | apical differentiation | apical differentiation into foot and seta |
| LL | Frullania dilatata | axial | transverse | transverse | absent | apical differentiation | basal differentiation into foot, apical differentiation into seta |
| H | Anthoceros sp. | axial | vertical | transverse | absent | apical differentiation | basal differentiation into foot, apical differentiation into intercalary meristem and seta |
| M | Sphagnum subsecundum | axial | transverse | transverse | some | apical cell division and differentiation | basal cell divisions and differentiation into foot, no seta elongation |
| M | Andreaea sp. | axial | transverse | oblique | present | apical cell division and differentiation | basal cell divisions and differentiation into foot, no seta elongation |
| M | Funaria hygrometrica | axial | transverse | oblique | present | apical cell division and differentiation | basal cell divisions and differentiation into foot and lower part of seta, apical differentiation into intercalary meristem |
| M | Physcomitrella patens | axial | transverse | oblique | present | apical cell division and differentiation | basal cell divisions and differentiation into foot and lower part of seta, apical differentiation into intercalary meristem |
(b). A note on bryophyte phylogeny and trait change inference
The extent to which inferences from mosses are transferable up the plant tree of life is unclear owing to variability in developmental patterns and currently unresolved phylogenetic relationships among bryophytes. While morphological phylogenies resolve mosses as the sister lineage to vascular plants (implying homology between mosses and early polysporangiophytes), molecular phylogenies are inconclusive or imply non-homology (reviewed in [10]). Growing support for the latter scenario will necessitate identification of developmental mechanisms that are shared among bryophytes as well as among vascular plants to understand the developmental transitions occurring as polysporangiophytes arose.
(c). Developmental innovations during polysporangiophyte evolution
The patterns of axial development among early polysporangiophytes remain speculative. Some authors have proposed that the bryophyte seta is homologous to the axes of polysporangiophyte forms [57,58] and others have argued that while the bryophyte seta arises from sporangial tissues, a distinct, well-established apical meristem generated early polysporangiophyte forms [52]. Rare natural liverwort and moss variants have branching sporophytes (figure 4), and such variants have received attention in light of hypotheses of polysporangiophyte evolution as they demonstrate that bryophytes can branch, and provide a potential entry point into the evolution of the polysporangiophyte habit [5,20,46]. In mosses, some variants undergo sporangial duplication (figure 4b) while others undergo a more extensive apical duplication to produce two sporangia subtended by a portion of seta (figure 4c–e) and both of these patterns are represented in the fossil record [5,20,46,61]. Speculatively these variants arise by early or later division of an embryonic apical cell, with an early duplication preceding intercalary proliferative activity and later duplication succeeding intercalary proliferation (figure 4f), and the latter form is similar to the form of early polysporangiophyte fossils.
(d). Experimental evidence for the origin of polysporangiophytes
Reverse genetic data are starting to pinpoint genes that may have been involved in the evolution of polysporangiophyte apical meristem functions. In Arabidopsis, PIN and TCP genes regulate branch initiation [62,63] and suppression of axillary bud activity [64,65] to determine plants' overall branching form. PIN-mediated polar auxin transport is conserved between Arabidopsis and moss sporophytes [66], and disruption of PIN function in a moss induces at low penetrance a branching form that closely resembles early polysporangiophyte fossils (figure 4) [47,60] and PpTCP5 disruption similarly induces branching [67]. Disrupting the function of two other gene classes in Physcomitrella can also induce sporophyte branching. Pplfy mutants have defective early embryonic divisions that impede sporophyte development, but in rare instances sporophytes are able to develop and they are branched [68]. However, in Arabidopsis, LEAFY activates the reproductive transition, and gene pathways for floral development [69], and LEAFY and PpLFY have divergent DNA binding capacities [70]. There are no PpLFY gain-of-function mutants and the downstream targets of PpLFY are not yet known, so it is hard to interpret the Physcomitrella Pplfy mutant phenotype in light of the evolution of branching. Similarly the low penetrance branching mutant phenotype of Pptel mutants is hard to interpret because TEL encodes an RNA binding protein, and the specificity of PpTEL action is not known [71]. The cellular and developmental basis of branching in the mutants above remains an open question, but the low penetrance of branching phenotypes suggests that an element of stochasticity is involved in the development of moss sporophyte branching, potentially in early embryonic cell fate specification.
5. Stage 2. The origin of indeterminate forms
(a). Patterns of axial development in early vascular plants
Early divergences in the vascular plant lineage gave rise to indeterminate forms with lateral sporangia or sporangia on simple lateral branch systems (figures 1 and 3d–f) [31,72]. Thus, a second step in the evolution of shoots with leaves involved displacement of sporangia away from their previously terminal position and the innovation of indeterminacy. Understanding of the origin(s) of indeterminacy currently rests on comparative analyses of axial development in living bryophytes and vascular plants as the cellular basis of axial elongation in extinct polysporangiophytes is unknown. However, the meristematic activities that generate axial elongation in these two groups are widely disparate. In bryophytes, axial elongation occurs with little cell proliferation (liverworts), by intercalary proliferation beneath sporangia (hornworts) or by embryonic proliferation from an apical cell coupled with later intercalary proliferation (mosses). Given phylogenetic caveats above (see section 4b), the moss proliferative pattern may be the closest living proxy to that of early polysporangiophytes, but apical cell and intercalary proliferative activities in mosses are separated temporally by developmental stage and spatially by sporangium formation (figure 4). In contrast, living vascular plants have shoot apices with juxtaposed stem cell and proliferative activities [73]. The size of the stem cell pool varies between plant groups from a single cell, as in some lycophytes and monilophytes [74,75], through to many in other lycophytes and seed plants [18,76–82], and the coordinated activity of stem cells within the stem cell pool and between the stem cell and subtending zones is required to maintain shoot apex integrity during growth [83]. Thus, comparative development suggests that the displacement of sporangia away from shoot tips and juxtaposition of stem cell and more general proliferative activities were pre-requisites for the origin of indeterminacy [10].
(b). Genetic pathways for indeterminacy and sporangium development in Arabidopsis
There is currently very little experimental evidence of mechanisms involved in the innovation of indeterminate shoot apex functions, but indeterminacy is well characterized in Arabidopsis, where two overlying genetic pathways promote cell proliferation and axial elongation. Class I KNOTTED-like homeobox (KNOX) transcription factors are necessary for meristem establishment and maintenance [84,85], acting via cytokinin biosynthesis to promote meristematic cell proliferation [86,87], and WUSCHEL-like homeobox (WOX) transcription factors act in a feedback loop with CLAVATA (CLV) genes to promote stem cell identity and maintain the size of the multicellular stem cell pool during growth [83]. The genetic basis of sporangium (in angiosperms the pollen sac and nucellus) development is less well understood than mechanisms for indeterminacy [88], but RETINOBLASTOMA cell cycle regulatory proteins suppress WUSCHEL activity to promote entry into germ line specification and meiosis during female germ line development [89], and SPOROCYTELESS MADS-like transcription factors act downstream of the floral organ identity-determining protein AGAMOUS to promote sporogenesis in both male and female germ line development [90,91].
(c). Genetic bases for the evolution of indeterminacy and sterilization
Meristematic KNOX activities are conserved within the vascular plants [92,93], and KNOX activity also promotes axial elongation in moss sporophytes [94]. While the activities of KNOX genes in liverworts and hornworts are not yet known, these data identify potential homology between mechanisms for intercalary proliferation in bryophytes and apical proliferation in vascular plant meristems (see also [10]). Physcomitrella has three globally expressed WOX13-like homologues and loss-of-function sporophytes are unable to grow, so conditional or gain-of-function mutants will be required to identify any roles in meristem activity [95] (and also see [96, 97]). CLAVATA functions remain unreported or are reportedly absent for non-flowering plants [98,99]. The patterns and position of sporangial development are very variable among non-seed plants (figures 1–3), and the extent to which pathways for sporangial development are conserved among land plants is unknown [100]. Physcomitrella knox mutant defects in sporangium development [94,101,102] suggest that KNOX genes are upstream regulators of sporangial development in mosses, and provide a potential mechanistic link between sterilization and indeterminacy during shoot evolution [10]. A comparative analysis showed that the transcriptomes of lycophyte, horsetail and flowering plant shoot apices are largely distinct, supporting the ancient divergence time of these lineages and suggesting that the innovation of indeterminate meristem functions may be polyphyletic [59,103,104].
6. Stage 3. Leaf evolution
(a). Lycophyte leaves (lycophylls)
Shoots with leaves first appeared in the fossil record following the innovation of shoots with sterile indeterminate apices, lateral branching systems and lateral sporangia (figures 1 and 3d–f). Deep evolutionary divergences within the vascular plant lineage gave rise to today's lycophyte and euphyllophyte flora (figure 1) [11,30,105,106], and living representatives of these lineages all have shoots with leaves (figure 2e–t). Early divergences within the lycophyte lineage gave rise to leafless zosterophylls (e.g. Zosterophyllum) and lycopsids with partially vascularized leaf-like enations (Asteroxylon), with an indeterminate dichotomizing habit (morphologies 8 and 9 in figures 1 and 3f,g) [9,30]. Both forms are evident in the fossil Rhynie chert flora [17,18,106]. Later lycophyte divergences gave rise to leafy lycopsids (morphology 10 in figure 1), including the extinct order Protolepidodendrales (e.g. Leclercqia) (figure 3h) and extant groups such as the Lycopodiaceae (clubmoss), sister lineage to Selaginellales (spike mosses) and Isoetalales (quillworts) [11,31]. While living lycophytes are small (figure 2e–h), isoetaleans include extinct lycopsid trees such as Lepidodendron (figure 3i) that were a major component of Carboniferous forests that later fossilized to form coal [107,108].
(b). Monilophyte fronds
The euphyllophyte stem group included leafless trimerophytes (figure 3j,k) such as Trimerophyton, Pertica and Psilophyton, which have forking lateral branches with clusters of elongated terminal sporangia [30,38,108], and the euphyllophyte divergence subsequently gave rise to living monilophytes and seed plants (figure 2h–t) [38,106,108,109]. The modern monilophyte clade comprises horsetails and ferns (figure 1), and ancient divergences within the fern lineage gave rise to leptosporangiate, marrattioid, ophioglossid and whisk ferns which have widely divergent leaf morphologies (figure 2i–l) [109–112]. Living monilophyte lineages are interspersed with extinct relatives (figures 1 and 3l–n), and fossil ferns and fern-like shoots have a wide variety of lateral branch arrangements and forms [38,105,106,108]. These are exemplified by Eospermatopteris (figure 3n), a 3 m tall tree with a crown of spirally arranged flattened first order branches giving rise to multiple dichotomizing branchlets [42], and Rhacophyton (figure 3m), a 1 m tall plant with partially planar lateral branches and multiple branchlets with some webbing at the distal tips [108,113].
(c). Seed plant leaves
The modern seed plant lineage (figure 2n–t) arose from progymnosperms (figure 3o,p) such as Aneurophyton and Archaeopteris (figure 1) [30,108]. Aneurophyton has three orders of spiralling lateral branches from which leaves or distinct fertile axes with adaxial sporangia arise, and Archaeopteris has planar lateral branching systems with spirally arranged simple leaves or fertile terminal axes (figure 3p) [108]. While fossil and phylogenetic data do not fully resolve ancestor–descendant relationships in vascular plant evolution, they demonstrate that lycophytes, monilophytes and seed plants all have leafless fossil precursors and therefore that there were multiple independent origins of vascular plant leaves [105]. Polyphyletic modification of lateral branching systems is considered to have given rise to euphyllophyte leaves in as many as seven to nine independent instances, one in seed plants with the remainder in living and extinct monilophytes [105]. However, the extent of homology in developmental traits such as leaf initiation pattern, determinacy, dorsiventrality and lamination is currently unclear.
(d). Patterns of leaf development in living vascular plants
Polyphyletic leaf origins are reflected in diverse patterns of leaf development among living vascular plants, reviewed by group in: Ambrose, lycophytes [114]; Tomescu et al., horsetails [75]; Schneider and Vasco et al., ferns [111,112]; Stevenson, gymnosperms [115]; and Tsukaya, angiosperms [116]. Shared properties of vascular plant leaf development include initiation in a regular phyllotactic pattern at a distance from stem cells that propagate the shoot axis, establishment of proximodistal, mediolateral and dorsiventral axes of symmetry, vein insertion, laminar development, proliferation and growth, but the sequence and extent to which these events occur and are combined vary, leading to diversity in leaf form [105]. The apical functions of different vascular plant lineages are also distinct [76,117,118]. While many vascular plants generate branches subapically (horsetails) [119], on axes at a distance from leaves (some ferns) [111] or in leaf axils (seed plants) [120], lycophytes and other ferns have shoot apices that periodically bifurcate to generate the overall branching form [76–78,114], and a requisite for bifurcation may affect the position of leaf primordia. Patterns of lycophyll development have been identified in a living exemplar of the lycophyte lineage, Selaginella kraussiana (figure 2f). A clonal analysis showed that two epidermal cells initiate each lycophyll, and that mediolateral cell divisions precede divisions that generate leaf dorsiventrality and tissue layers [77]. However, lycophylls arise from multiple cell layers in Lycopodiaceae (figure 2e) and Isoetaceae (figure 2g) and patterns of cell proliferation are also divergent among lycophytes [114]. In horsetails, apical cell derivatives divide to attain leaf or intercalary meristem fate [75]. The small leaves (figure 2h) have a single vein and emerge in a ring beneath the intercalary proliferative regions that generate the modular shoot axis [75]. Monilophyte fronds (figure 2i,j) are typified by a shoot-like, tip-down pattern of development with lamina developing by edge-in divisions, and these features may be monilophyte synapomorphies [74,111]. However, there were multiple origins of fronds or leafy forms within the monilophytes and these are reflected in shape diversity [58,112]. Whisk ferns (figure 2k) have very small bifid leaves subtending sporangia, ophioglossid ferns (figure 2l) have a single entire leaf, and filmy ferns (figure 2m) have leaves comprising partially webbed bifurcating axes, with lamina a single cell layer thick [112]. Gymnosperm leaves (figure 2n–q) are similarly diverse and range from small and scale-like to large multipinnate forms [115].
(e). Pathways for leaf development in Arabidopsis
Pathways for leaf development are well characterized in flowering plants, exemplified by Arabidopsis in which leaves initiate in regular phyllotactic patterns from the peripheral (proliferative) zone of multicellular meristems [121,122]. The position of leaf initiation emerges as an outcome of short-range polar auxin transport principally in the outermost cell layer of the meristem [123]. PIN auxin transporters dynamically direct auxin to maxima on the apical dome, and maximum formation is necessary and sufficient for leaf emergence [64,123–127]. Mechanical forces also contribute to leaf emergence [128], and cell wall loosening by pectin methylesterase or expansin enzymes is sufficient to trigger emergence [129,130]. The recruitment of a pool of meristematic cells into determinate leaf development pathways involves downregulation of meristematic KNOX gene activity and maintenance of a KNOX off state by ARP transcription factors [84,131,132]. Leaf primordium dorsiventrality is partially inherited from radial symmetries within the shoot axis as primordia emerge for the apical dome [133,134]. HD-zipIII genes are expressed centrally in the shoot axis and adaxially within leaves, and KANADI and YABBY genes are expressed peripherally in the shoot axis and/or abaxially within leaves; loss-of-function mutants respectively generate adaxialized or abaxialized leaves [133,135,136]. ARP genes are expressed adaxially, and Antirrinum arp mutants also have abaxialized leaves, demonstrating that juxtaposed tissue layers with distinct dorsal and ventral identities are necessary for laminar outgrowth [131,137,138]. Once leaf primordia are established, cell proliferation and growth contribute to leaf shape determination, and many pathways regulating these processes have been identified as an outcome of sophisticated interdisciplinary approaches to understanding how planar forms are attained in plants (e.g. [139]).
(f). Hypotheses of leaf evolution
The leaf evolution literature has widely recognized lycophylls and euphylls as leaves with distinct evolutionary origins [72,105,106,140–146], and disparity in their size, initiation and venation patterns led to the ‘microphyll’ (lycophyll and horsetail leaves) and ‘megaphyll’ (fern and seed plant fronds and leaves) concepts [73]. The telome theory of leaf evolution proposed that transformative evolutionary processes of unequal branching (overtopping), rearrangement of lateral branches into a single plane (planation) and infilling of spaces between branches with laminar tissue (webbing) generated euphyllophyte leaves [142]. Lycophylls were proposed to have arisen by reduction from a more elaborate precursor state similar to euphyll precursors by a process of evolutionary loss (reduction) [142], as enations by epidermal outgrowth from the stem [46] or by sterilization of lateral branches terminating in sporangia [143]. Zimmermann's hypotheses are dated by the phylogenetic framework and fossil evidence used to infer the direction and nature of character change during leaf evolution [147,148], and more recent literature has moved away from ‘microphyll’ and ‘megaphyll’ terminology as it under-represents the degree of polyphyly in vascular plant leaf evolution [105]. Nevertheless, the telome, enation and sterilization hypotheses highlight developmental processes that may have been generally important in leaf evolution.
(g). Testing hypotheses of leaf evolution
Evo–devo studies of leaf evolution have only recently started [149,150] and so far have largely focused on leaves with widely disparate origins, for instance comparing lycophyll, fern frond and Arabidopsis leaf development pathways [92,151–154]. Analyses of polar auxin transport and/or PIN functions in a lycophyte and a moss suggest that PIN-mediated auxin transport is an ancient and conserved regulator of branch and/or organ position [47,155]. Analyses of HD-ZipIII transcription factor function showed that dorsiventral HD-zipIII and YABBY expression patterns in leaf initiation are conserved among seed plants, supporting dorsiventrality as a seed plant homology [156,157]. However, HD-zipIII activities segregated distinctly among paralogues during gene family evolution, with lycophyte paralogues having functions distinct from seed plant orthologues, and roles for HD-zipIIIs and YABBY in ferns remain to be identified [152,153,157]. An analysis of ARP transcription factor function showed that ARP proteins were independently recruited to suppress KNOX activities during leaf initiation in lycophylls and flowering plant leaves [92]. In contrast, KNOX activities are persistent in fern fronds [92,154,158], in line with their late transition to determinate fate [159]. The approaches above support the notion of wide divergence times in vascular plant leaf evolution. Testing more specific hypotheses of character state transition and homology in leaf evolution will necessitate the use of further species in which a particular feature is present or absent and it is possible to do genetics.
(h). Why have leaves evolved multiple times?
The evidence reviewed above demonstrates that vascular plant leaves have evolved multiple times from branching shoot systems, and that branching forms diversified extensively in lycophyte, monilophyte and seed plant lineages prior to origins of determinate, dorsiventral leaves. Initial constraints to leaf evolution probably involved high atmospheric global temperatures, low stomatal densities and low capacities for water uptake prior to root evolution and the evolution of efficient vascular transport in leaves [16,160,161]. Under these conditions, high incident light absorption would have ‘cooked’ fully webbed leaves or led to vascular embolism in plants' stems [16]. Polyphyletic leaf origins were coupled with declining atmospheric CO2 levels, declining global temperatures, increasing stomatal and vein densities in leaves, the evolution of extensive rooting systems and increasing plant competition for space to acquire environmental resources [15–17,162]. In other words, the selection pressures that favour shoots with leaves in today's environment arose at a relatively late stage of plant body plan evolution.
7. Conclusion and avenues for future research
(a). Stages 1 and 2 of shoot and leaf evolution
Combined palaeobotanical, developmental and genetic data are starting to reveal the basis of trait change during polysporangiophyte evolution and pinpoint questions that will reveal a much fuller picture of shoot and leaf evolution if answered. Specific questions for the fossil record include:
— what was the apical organization of Cooksonioid forms,
— are there apical cells,
— what was the cellular basis of bifurcation,
— is there any evidence of intercalary proliferation,
— did vegetative axes develop independently of sporangia, and
— if so, at which evolutionary juncture did such a capacity arise?
By demonstrating the apical activities of polysporangiophytes, answers to these questions will reveal proliferative capacities that predated the origin of indeterminate vascular plant meristems. Fossils of the Rhynie chert could play a key role because they show diversity in relevant traits with high-quality cellular preservation, and occupy key phylogenetic positions.
Developmental and genetic questions include:
— are apical cell and intercalary proliferation coordinated in mosses,
— how do the branching morphologies of Physcomitrella pinb, tel, lfy and tcp5 mutants arise during development,
— which genes regulate apical cell activity,
— how does apical cell activity cease in bryophytes,
— which genes regulate intercalary proliferation in bryophytes,
— which genes regulate sporangium development,
— are the pathways above conserved among bryophytes, and
— is there conservation between bryophytes and vascular plants?
By answering the above questions, developmental and genetic studies in bryophytes have the potential to reveal mechanisms for apical activity that predated the evolution of polysporangiophytes, i.e. the ancestral mechanisms for axial development, bifurcation and sporangium development. Such mechanisms are likely to have been modified during the radiation of branching lycophyte and euphyllophyte forms.
(b). Stage 3 of leaf evolution
There are fewer specific questions relating to stage 3 of leaf evolution because the phylogenetic relationships between early diverging lycophyte, monilophyte and seed plant lineages are not well resolved and mutants have not yet revealed phenotypes that are intermediate between living and fossil forms. Analyses of apical, branch and laminar development in early diverging lycophyte, euphyllophyte, monilophyte and seed plant fossils will be required to identify character transitions involved in vascular plant leaf evolution and reveal structural homologies among vascular plant branch and organ systems. While many genes with roles in flowering plant leaf development have been identified, there are few reverse, and no forward genetic data from other vascular plant lineages. The establishment of a fern genetic model [99,163] will go some way to breaking up the wide evolutionary distance between bryophyte [164] and flowering plant [139] models of planar development, but in-depth understanding of leaf evolution and development will require far broader sampling among lycophytes, monilophytes and seed plants [165]. Identifying the developmental and genetic basis of shoot and leaf evolution will be important in future efforts to engineer novel architectural trait combinations to maintain or improve plant productivity in the face of future global change.
Acknowledgements
We thank Mihai Tomescu and Marcus Heisler for useful discussions about leaf homology and Zoe Nemec Venza and two reviewers for comments on manuscript drafts. We thank Jeff Duckett, Silvia Pressel and David Long for plant photos. C.J.H. thanks Liam Dolan, Dianne Edwards and Paul Kenrick for their invitation to speak at the Rhynie chert discussion meeting.
Data accessibility
This article has no additional data.
Authors' contributions
C.J.H. wrote the manuscript with help from J.L.M. C.J.H. and J.L.M. prepared the figures together.
Competing interests
We declare we have no competing interests.
Funding
We thank the Royal Society (UF130563) and NERC (Standard Grant NE/N003438/1) for funding.
References
- 1.Royal Botanic Gardens Kew, Missouri Botanical Garden. 2013 The plant list, version 1.1. London, UK: Royal Botanic Gardens, Kew. See http://www.theplantlist.org (accessed 19 June 2017).
- 2.Belnap J. 2012. Biogeochemistry: unexpected uptake. Nat. Geosci. 5, 443–444. ( 10.1038/ngeo1514) [DOI] [Google Scholar]
- 3.Elbert W, Weber B, Burrows S, Steinkamp J, Büdel B, Andreae MO, Pöschl U. 2012. Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat. Geosci. 5, 459–462. ( 10.1038/ngeo1486) [DOI] [Google Scholar]
- 4.Brown RC, Lemmon BE. 2011. Spores before sporophytes: hypothesizing the origin of sporogenesis at the algal–plant transition. New Phytol. 190, 875–881. ( 10.1111/j.1469-8137.2011.03709.x) [DOI] [PubMed] [Google Scholar]
- 5.Edwards D, Kenrick P. 2015. The early evolution of land plants, from fossils to genomics: a commentary on Lang (1937) ‘On the plant-remains from the Downtonian of England and Wales’. Phil. Trans. R. Soc. B 370, 20140343 ( 10.1098/rstb.2014.0343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harholt J, Moestrup Ø, Ulvskov P. 2016. Why plants were terrestrial from the beginning. Trends Plant Sci. 21, 96–101. ( 10.1016/j.tplants.2015.11.010) [DOI] [PubMed] [Google Scholar]
- 7.Wellman CH, Strother PK. 2015. The terrestrial biota prior to the origin of land plants (embryophytes): a review of the evidence. Palaeontology 58, 601–627. ( 10.1111/pala.12172) [DOI] [Google Scholar]
- 8.Wellman CH, Osterloff PL, Mohiuddin U. 2003. Fragments of the earliest land plants. Nature 425, 282–285. ( 10.1038/nature01884) [DOI] [PubMed] [Google Scholar]
- 9.Edwards D, Morris JL, Richardson JB, Kenrick P. 2014. Cryptospores and cryptophytes reveal hidden diversity in early land floras. New Phytol. 202, 50–78. ( 10.1111/nph.12645) [DOI] [PubMed] [Google Scholar]
- 10.Harrison CJ. 2017. Development and genetics in the evolution of land plant body plans. Phil. Trans. R. Soc. B 372, 20150490 ( 10.1098/rstb.2015.0490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenrick P, Crane PR. 1997. The origin and early evolution of plants on land. Nature 389, 33–39. ( 10.1038/37918) [DOI] [Google Scholar]
- 12.Becker R, Gradstein F, Hammer O. 2012. The Devonian Period. Geol. Time Scale. 2, 559–601. ( 10.1016/B978-0-444-59425-9.00022-6) [DOI] [Google Scholar]
- 13.Kenrick P, Strullu-Derrien C. 2014. The origin and early evolution of roots. Plant Physiol. 166, 570–580. ( 10.1104/pp.114.244517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berner RA. 2009. 2006 GEOCARBSULF: a combined model for Phanerozoic atmospheric O2 and CO2. Geochim. Cosmochim. Acta 70, 5653–5664. ( 10.1016/j.gca.2005.11.032) [DOI] [Google Scholar]
- 15.Beerling DJ, Osborne CP, Chaloner WG. 2001. Evolution of leaf-form in land plants linked to atmospheric CO2 decline in the Late Palaeozoic era. Nature 410, 352–354. ( 10.1038/35066546) [DOI] [PubMed] [Google Scholar]
- 16.Beerling DJ. 2005. Leaf evolution: gases, genes and geochemistry. Ann. Bot. 96, 345–352. ( 10.1093/aob/mci186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue J, et al. 2015. Stepwise evolution of Paleozoic tracheophytes from South China: contrasting leaf disparity and taxic diversity. Earth Sci. Rev. 148, 77–93. ( 10.1016/j.earscirev.2015.05.013) [DOI] [Google Scholar]
- 18.Edwards D. 2004. Embryophytic sporophytes in the Rhynie and Windyfield cherts. Trans. R. Soc. Edinburgh Earth Sci. 94, 397–410. ( 10.1017/S0263593300000778) [DOI] [Google Scholar]
- 19.Hofmeister WFB. 1862. On the germination, development, and fructification of the higher Cryptogamia: and on the fructification of the coniferae. London, UK: Robert Hardwicke. [Google Scholar]
- 20.Leitgeb H. 1876. Ueber verzweigte Moossporogonien [On branched moss sporophytes]. Mitt. Naturwiss. Ver. Steiermark 13, 3–20 (in German). [Google Scholar]
- 21.Graham LE, Cook ME, Busse JS. 2000. The origin of plants: body plan changes contributing to a major evolutionary radiation. Proc. Natl Acad. Sci. USA 97, 4535–4540. ( 10.1073/pnas.97.9.4535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cronk QC. 2001. Plant evolution and development in a post-genomic context. Nat. Rev. Genet. 2, 607–619. ( 10.1038/35084556) [DOI] [PubMed] [Google Scholar]
- 23.Pires ND, Dolan L. 2012. Morphological evolution in land plants: new designs with old genes. Phil. Trans. R. Soc. B 367, 508–518. ( 10.1098/rstb.2011.0252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang R-L, Stec A, Hey J, Lukens L, Doebley J. 1999. The limits of selection during maize domestication. Nature 398, 236–239. ( 10.1038/18435) [DOI] [PubMed] [Google Scholar]
- 25.Piperno DR, Ranere AJ, Holst I, Iriarte J, Dickau R. 2009. Starch grain and phytolith evidence for early ninth millennium BP maize from the Central Balsas River Valley, Mexico. Proc. Natl Acad. Sci. USA 106, 5019–5024. ( 10.1073/pnas.0812525106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Heerwaarden J, Doebley J, Briggs WH, Glaubitz JC, Goodman MM, Gonzalez J, Ross-Ibarra J. 2011. Genetic signals of origin, spread, and introgression in a large sample of maize landraces. Proc. Natl Acad. Sci. USA 108, 1088–1092. ( 10.1073/pnas.1013011108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Studer A, Zhao Q, Ross-Ibarra J, Doebley J. 2011. Identification of a functional transposon insertion in the maize domestication gene tb1. Nat. Genet. 43, 1160–1163. ( 10.1038/ng.942) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox CJ, Li B, Foster PG, Embley M, Civáň P. 2014. Conflicting phylogenies for early land plants are caused by composition biases among synonymous substitutions. Syst. Biol. 63, 272–279. ( 10.1093/sysbio/syt109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wickett NJ, et al. 2014. Phylotranscriptomic analysis of the origin and early diversification of land plants. Proc. Natl Acad. Sci. USA 111, E4859–E4868. ( 10.1073/pnas.1323926111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerrienne P, Servais T, Vecoli M. 2016. Plant evolution and terrestrialization during Palaeozoic times—the phylogenetic context. Rev. Palaeobot. Palynol. 227, 4–18. ( 10.1016/j.revpalbo.2016.01.004) [DOI] [Google Scholar]
- 31.Kenrick P, Crane PR. 1997. The origin and early diversification of land plants. A cladistic study. Washington, DC: Smithsonian Institute Press. [Google Scholar]
- 32.Rubinstein CV, Gerrienne P, de la Puente G, Astini R, Steemans P. 2010. Early Middle Ordovician evidence for land plants in Argentina (eastern Gondwana). New Phytol. 188, 365–369. ( 10.1111/j.1469-8137.2010.03433.x) [DOI] [PubMed] [Google Scholar]
- 33.Parihar NS. 1967. Bryophyta. Allahabad, India: Indian Universities Press. [Google Scholar]
- 34.Edwards DS. 1986. Aglaophyton major, a non-vascular land-plant from the Devonian Rhynie Chert. Bot. J. Linnean Soc. 93, 173–204. ( 10.1111/j.1095-8339.1986.tb01020.x) [DOI] [Google Scholar]
- 35.Gerrienne P, Bergamaschi S, Pereira E, Rodrigues M-AC, Steemans P. 2001. An Early Devonian flora, including Cooksonia, from the Paraná Basin (Brazil). Rev. Palaeobot. Palynol. 116, 19–38. ( 10.1016/S0034-6667(01)00060-4) [DOI] [Google Scholar]
- 36.Edwards DS. 1980. Evidence for the sporophytic status of the Lower Devonian plant Rhynia gwynne-vaughanii Kidston and Lang. Rev. Palaeobot. Palynol. 29, 177–188. ( 10.1016/0034-6667(80)90057-3) [DOI] [Google Scholar]
- 37.Gensel PG. 1976. Renalia hueberi, a new plant from the Lower Devonian of Gaspé. Rev. Palaeobot. Palynol. 22, 19–37. ( 10.1016/0034-6667(76)90009-9) [DOI] [Google Scholar]
- 38.Stewart WN, Rothwell GW. 1993. Paleobotany and the evolution of plants. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 39.Walton J. 1964. On the morphology of Zosterophyllum and some other early Devonian plants. Phytomorphology 14, 155–160. [Google Scholar]
- 40.Bonamo P, Banks H, Grierson J. 1988. Leclercqia, Haskinsia, and the role of leaves in delineation of Devonian lycopod genera. Bot. Gazette. 149, 222–239. ( 10.1086/337711) [DOI] [Google Scholar]
- 41.Naugolnykh SV. 2002. Paracalamitina striata, a newly reconstructed equisetophyte from the Permian of Angaraland. J. Paleontol. 76, 377–385. ( 10.1017/S0022336000041755) [DOI] [Google Scholar]
- 42.Stein WE, Mannolini F, Hernick LV, Landing E, Berry CM. 2007. Giant cladoxylopsid trees resolve the enigma of the Earth's earliest forest stumps at Gilboa. Nature 446, 904–907. ( 10.1038/nature05705) [DOI] [PubMed] [Google Scholar]
- 43.Bonamo PM, Banks HP. 1967. Tetraxylopteris schmidtii: its fertile parts and its relationships within the Aneurophytales. Am. J. Bot. 54, 755–768. ( 10.2307/2440953) [DOI] [Google Scholar]
- 44.Edwards D, Richardson JB, Axe L, Davies KL. 2012. A new group of Early Devonian plants with valvate sporangia containing sculptured permanent dyads. Bot. J. Linnean Soc. 168, 229–257. ( 10.1111/j.1095-8339.2011.01207.x) [DOI] [Google Scholar]
- 45.Morris JL, Edwards D, Richardson JB, Axe L. 2012. New dyad-producing plants from the Lower Devonian (Lochkovian) of the Welsh Borderland. Bot. J. Linnean Soc. 169, 569–595. ( 10.1111/j.1095-8339.2012.01231.x) [DOI] [Google Scholar]
- 46.Bower F. 1935. Primitive land plants. London, UK: Macmillan. [Google Scholar]
- 47.Bennett TA, et al. 2014. Plasma membrane-targeted PIN proteins drive shoot development in a moss. Curr. Biol. 24, 2776–2785. ( 10.1016/j.cub.2014.09.054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lang WH. 1937. On the plant-remains from the Downtonian of England and Wales. Phil. Trans. R. Soc. Lond. B 227, 245–291. ( 10.1098/rstb.1937.0004) [DOI] [Google Scholar]
- 49.Gonez P, Gerrienne P. 2010. A new definition and a lectotypification of the genus Cooksonia Lang 1937. Int. J. Plant Sci. 171, 199–215. ( 10.1086/648988) [DOI] [Google Scholar]
- 50.Gerrienne P, Dlicher D, Bergamaschi S, Milagres I, Periera E, Rodrigues M. 2006. An exceptional specimen of the early land plant Cooksonia paranensis, and a hypothesis on the life cycle of the earliest eutracheophytes. Rev. Palaeobot. Palynol. 142, 123–130. ( 10.1016/j.revpalbo.2006.05.005) [DOI] [Google Scholar]
- 51.Boyce C. 2009. How green was Cooksonia? The importance of size in understanding the early evolution of physiology in the vascular plant lineage. Paleobiology 34, 179–194. ( 10.1666/0094-8373(2008)034%5B0179:HGWCTI%5B2.0.CO;2) [DOI] [Google Scholar]
- 52.Kato M, Akiyama H. 2005. Interpolation hypothesis for origin of the vegetative sporophyte of land plants. Taxon 54, 443–450. ( 10.2307/25065371) [DOI] [Google Scholar]
- 53.Niklas KJ, Kutschera U. 2009. The evolutionary development of plant body plans. Funct. Plant Biol. 36, 682–695. ( 10.1071/FP09107) [DOI] [PubMed] [Google Scholar]
- 54.Yin-Long Q, B TA, McManus HA. 2012. Evolution of the life cycle in land plants. J. System. Evol. 50, 171–194. ( 10.1111/j.1759-6831.2012.00188.x) [DOI] [Google Scholar]
- 55.Goffinet B, Buck WR. 2012. The evolution of body form in bryophytes. Ann. Plant Rev. 45, 51–89. ( 10.1002/9781118305881.ch2) [DOI] [Google Scholar]
- 56.Ligrone R, Duckett JG, Renzaglia KS. 2012. Major transitions in the evolution of early land plants: a bryological perspective. Ann. Bot. 109, 851–871. ( 10.1093/aob/mcs017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ligrone R, Duckett JG, Renzaglia KS. 2012. The origin of the sporophyte shoot in land plants: a bryological perspective. Ann. Bot. 110, 935–941. ( 10.1093/aob/mcs176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tomescu AM, Wyatt SE, Hasebe M, Rothwell GW. 2014. Early evolution of the vascular plant body plan – the missing mechanisms. Curr. Opin. Plant Biol. 17, 126–136. ( 10.1016/j.pbi.2013.11.016) [DOI] [PubMed] [Google Scholar]
- 59.Harrison CJ. 2015. Shooting through time: new insights from transcriptomic data. Trends Plant Sci. 20, 468–470. ( 10.1016/j.tplants.2015.06.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harrison CJ. 2017. Auxin transport in the evolution of branching forms. New Phytol. 215, 545–551. ( 10.1111/nph.14333) [DOI] [PubMed] [Google Scholar]
- 61.Morris JL, Richardson JB, Edwards D. 2011. Lower Devonian plant and spore assemblages from Lower Old Red Sandstone strata of Tredomen Quarry, South Wales. Rev. Palaeobot. Palynol. 165, 183–208. ( 10.1016/j.revpalbo.2011.03.003) [DOI] [Google Scholar]
- 62.Wang Q, Kohlen W, Rossmann S, Vernoux T, Theres K. 2014. Auxin depletion from the leaf axil conditions competence for axillary meristem formation in Arabidopsis and tomato. Plant Cell 26, 2068–2079. ( 10.1105/tpc.114.123059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y, Wang J, Shi B, Yu T, Qi J, Meyerowitz EM, Jiao Y. 2014. The stem cell niche in leaf axils is established by auxin and cytokinin in Arabidopsis. Plant Cell 26, 2055–2067. ( 10.1105/tpc.114.123083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galweiler L, Guan C, Muller A, Wisman E, Mendgen K, Yephremov A, Palme K. 1998. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282, 2226–2230. ( 10.1126/science.282.5397.2226) [DOI] [PubMed] [Google Scholar]
- 65.Aguilar-Martinez J, Poza-Carrion C, Cubas P. 2007. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 19, 458–472. ( 10.1105/tpc.106.048934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fujita T, Sakaguchi H, Hiwatashi Y, Wagstaff SJ, Ito M, Deguchi H, Sato T, Hasebe M. 2008. Convergent evolution of shoots in land plants: lack of auxin polar transport in moss shoots. Evol. Dev. 10, 176–186. ( 10.1111/j.1525-142X.2008.00225.x) [DOI] [PubMed] [Google Scholar]
- 67.Ortiz-Ramirez C, Hernandez-Coronado M, Thamm A, Catarino B, Wang M, Dolan L, Feijó JA, Becker JD. 2016. A transcriptome atlas of Physcomitrella patens provides insights into the evolution and development of land plants. Mol. Plant 9, 205–220. ( 10.1016/j.molp.2015.12.002) [DOI] [PubMed] [Google Scholar]
- 68.Tanahashi T, Sumikawa N, Kato M, Hasebe M. 2005. Diversification of gene function: homologs of the floral regulator FLO/LFY control the first zygotic cell division in the moss Physcomitrella patens. Development 132, 1727–1736. ( 10.1242/dev.01709) [DOI] [PubMed] [Google Scholar]
- 69.Weigel D, Alvarez J, Smyth DR, Yanofsky MF, Meyerowitz EM. 1992. LEAFY controls floral meristem identity in Arabidopsis. Cell 69, 843–859. ( 10.1016/0092-8674(92)90295-N) [DOI] [PubMed] [Google Scholar]
- 70.Sayou C, et al. 2014. A promiscuous intermediate underlies the evolution of LEAFY DNA binding specificity. Science 343, 645–648. ( 10.1126/science.1248229) [DOI] [PubMed] [Google Scholar]
- 71.Vivancos J, Spinner L, Mazubert C, Charlot F, Paquet N, Thareau V, Dron M, Nogué F, Charon C. 2012. The function of the RNA-binding protein TEL1 in moss reveals ancient regulatory mechanisms of shoot development. Plant Mol. Biol. 78, 323–336. ( 10.1007/s11103-011-9867-9) [DOI] [PubMed] [Google Scholar]
- 72.Kenrick P. 2002. The telome theory. In Developmental genetics and plant evolution (eds Cronk QCB, Bateman RM, Hawkins JA), pp. 365–387. London, UK: Taylor and Francis. [Google Scholar]
- 73.Gifford EM, Foster AS. 1989. Morphology and evolution of vascular plants. New York, NY: W. H. Freeman. [Google Scholar]
- 74.Sanders HL, Darrah PR, Langdale JA. 2011. Sector analysis and predictive modelling reveals iterative shoot-like development in fern fronds. Development 138, 2925–2934. ( 10.1242/dev.065888) [DOI] [PubMed] [Google Scholar]
- 75.Tomescu AM, Escapa IH, Rothwell GW, Elgorriaga A, Cúneo NR. 2017. Developmental programmes in the evolution of Equisetum reproductive morphology: a hierarchical modularity hypothesis. Ann. Bot. 119, 489–505. ( 10.1093/aob/mcw273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Philipson WR. 1990. The significance of apical meristems in the phylogeny of land plants. Plant Syst. Evol. 173, 17–38. ( 10.1007/BF00937760) [DOI] [Google Scholar]
- 77.Harrison CJ, Rezvani M, Langdale JA. 2007. Growth from two transient apical initials in the meristem of Selaginella kraussiana. Development 134, 881–889. ( 10.1242/dev.001008) [DOI] [PubMed] [Google Scholar]
- 78.Harrison CJ, Langdale JA. 2010. Response to ‘The developmental pattern of shoot apices in Selaginella kraussiana (Kunze) A. Braun’. Int. J. Plant Sci. 171, 690–692. ( 10.1086/653134) [DOI] [Google Scholar]
- 79.Yin X, Meicenheimer RD. 2017. Anisotomous dichotomy results from an unequal bifurcation of the original shoot apical meristem in Diphasiastrum digitatum (Lycopodiaceae). Am. J. Bot. 104, 782–786. ( 10.3732/ajb.1700021) [DOI] [PubMed] [Google Scholar]
- 80.Gola EM, Jernstedt JA. 2011. Impermanency of initial cells in Huperzia lucidula (Huperziaceae) shoot apices. Int. J. Plant Sci. 172, 847–855. ( 10.1086/660878) [DOI] [Google Scholar]
- 81.Korn RW. 2001. Analysis of shoot apical organization in six species of the Cupressaceae based on chimeric behavior. Am. J. Bot. 88, 1945–1952. ( 10.2307/3558421) [DOI] [PubMed] [Google Scholar]
- 82.Irish VF, Sussex IM. 1992. A fate map of the Arabidopsis embryonic shoot apical meristem. Development 115, 745–753. [Google Scholar]
- 83.Heidstra R, Sabatini S. 2014. Plant and animal stem cells: similar yet different. Nat. Rev. Mol. Cell Biol. 15, 301–312. ( 10.1038/nrm3790) [DOI] [PubMed] [Google Scholar]
- 84.Long JA, Moan EI, Medford JI, Barton MK. 1996. A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379, 66–69. ( 10.1038/379066a0) [DOI] [PubMed] [Google Scholar]
- 85.Scofield S, Dewitte W, Murray JA. 2007. The KNOX gene SHOOT MERISTEMLESS is required for the development of reproductive meristematic tissues in Arabidopsis. Plant J. 50, 767–781. ( 10.1111/j.1365-313X.2007.03095.x) [DOI] [PubMed] [Google Scholar]
- 86.Jasinski S, Piazza P, Craft J, Hay A, Woolley L, Rieu I, Phillips A, Hedden P, Tsiantis M. 2005. KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Curr. Biol. 15, 1560–1565. ( 10.1016/j.cub.2005.07.023) [DOI] [PubMed] [Google Scholar]
- 87.Yanai O, Shani E, Dolezal K, Tarkowski P, Sablowski R, Sandberg G, Samach A, Ori N. 2005. Arabidopsis KNOXI proteins activate cytokinin biosynthesis. Curr. Biol. 15, 1566–1571. ( 10.1016/j.cub.2005.07.060) [DOI] [PubMed] [Google Scholar]
- 88.Schmidt A, Schmid MW, Grossniklaus U. 2015. Plant germline formation: common concepts and developmental flexibility in sexual and asexual reproduction. Development 142, 229–241. ( 10.1242/dev.102103) [DOI] [PubMed] [Google Scholar]
- 89.Zhao XA, et al. 2017. RETINOBLASTOMA RELATED1 mediates germline entry in Arabidopsis. Science 356, eaaf6532 ( 10.1126/science.aaf6532) [DOI] [PubMed] [Google Scholar]
- 90.Ito T, Wellmer F, Yu H, Das P, Ito N, Alves-Ferreira M, Riechmann JL, Meyerowitz EM. 2004. The homeotic protein AGAMOUS controls microsporogenesis by regulation of SPOROCYTELESS. Nature 430, 356–360. ( 10.1038/nature02733) [DOI] [PubMed] [Google Scholar]
- 91.Chen G-H, Sun J-Y, Liu M, Liu J, Yang W-C. 2014. SPOROCYTELESS is a novel embryophyte-specific transcription repressor that interacts with TPL and TCP proteins in Arabidopsis. J. Genet. Genomics 41, 617–625. ( 10.1016/j.jgg.2014.08.009) [DOI] [PubMed] [Google Scholar]
- 92.Harrison CJ, Corley SB, Moylan EC, Alexander DL, Scotland RW, Langdale JA. 2005. Independent recruitment of a conserved developmental mechanism during leaf evolution. Nature 434, 509–514. ( 10.1038/nature03410) [DOI] [PubMed] [Google Scholar]
- 93.Ambrose BA, Vasco A. 2016. Bringing the multicellular fern meristem into focus. New Phytol. 210, 790–793. ( 10.1111/nph.13825) [DOI] [PubMed] [Google Scholar]
- 94.Sakakibara K, Nishiyama T, Deguchi H, Hasebe M. 2008. Class 1 KNOX genes are not involved in shoot development in the moss Physcomitrella patens but do function in sporophyte development. Evol. Develop. 10, 555–566. ( 10.1111/j.1525-142X.2008.00271.x) [DOI] [PubMed] [Google Scholar]
- 95.Sakakibara K, et al. 2014. WOX13-like genes are required for reprogramming of leaf and protoplast cells into stem cells in the moss Physcomitrella patens. Development 141, 1660–1670. ( 10.1242/dev.097444) [DOI] [PubMed] [Google Scholar]
- 96.Nardmann J, Werr W. 2012. The invention of WUS-like stem cell-promoting functions in plants predates leptosporangiate ferns. Plant Mol. Biol. 78, 123–134. ( 10.1007/s11103-011-9851-4) [DOI] [PubMed] [Google Scholar]
- 97.Nardmann J, Werr W. 2013. Symplesiomorphies in the WUSCHEL clade suggest that the last common ancestor of seed plants contained at least four independent stem cell niches. New Phytol. 199, 1081–1092. ( 10.1111/nph.12343) [DOI] [PubMed] [Google Scholar]
- 98.Floyd SK, Bowman JL. 2007. The ancestral developmental toolkit of land plants. Int. J. Plant Sci. 168, 1–35. ( 10.1086/509079) [DOI] [Google Scholar]
- 99.Plackett AR, Di Stillio VS, Langdale JA. 2015. Ferns: the missing link in shoot evolution and development. Front. Plant Sci. 6, 972 ( 10.3389/fpls.2015.00972) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Harrison CJ, Alvey E, Henderson IR. 2010. Meiosis in flowering plants and other green organisms. J. Exp. Bot. 61, 2863–2875. ( 10.1093/jxb/erq191) [DOI] [PubMed] [Google Scholar]
- 101.Singer SD, Ashton NW. 2007. Revelation of ancestral roles of KNOX genes by a functional analysis of Physcomitrella homologues. Plant Cell Rep. 26, 2039–2054. ( 10.1007/s00299-007-0409-5) [DOI] [PubMed] [Google Scholar]
- 102.Sakakibara K, Ando S, Yip HK, Tamada Y, Hiwatashi Y, Murata T, Deguchi H, Hasebe M, Bowman JL. 2013. KNOX2 genes regulate the haploid-to-diploid morphological transition in land plants. Science 339, 1067–1070. ( 10.1126/science.1230082) [DOI] [PubMed] [Google Scholar]
- 103.Frank MH, Edwards MB, Schultz ER, McKain MR, Fei Z, Sørensen I, Rose JKC, Scanlon MJ. 2015. Dissecting the molecular signatures of apical cell-type shoot meristems from two ancient land plant lineages. New Phytol. 207, 893–904. ( 10.1111/nph.13407) [DOI] [PubMed] [Google Scholar]
- 104.Banks JA. 2015. The evolution of the shoot apical meristem from a gene expression perspective. New Phytol. 207, 486–487. ( 10.1111/nph.13525) [DOI] [PubMed] [Google Scholar]
- 105.Tomescu AMF. 2008. Megaphylls, microphylls and the evolution of leaf development. Trends Plant Sci. 14, 5–12. ( 10.1016/j.tplants.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 106.Galtier J. 2010. The origins and early evolution of the megaphyllous leaf. Int. J. Plant Sci. 171, 641–661. ( 10.1086/653130) [DOI] [Google Scholar]
- 107.Bateman RM. 1994. Evolutionary-developmental change in the growth architecture of fossil rhizomorphic lycopsids: scenarios constructed on cladistic foundations. Biol. Rev. 69, 527–597. ( 10.1111/j.1469-185X.1994.tb01249.x) [DOI] [Google Scholar]
- 108.Taylor EL, Taylor TN, Krings M. 2009. Paleobotany: the biology and evolution of fossil plants. New York, NY: Academic Press. [Google Scholar]
- 109.Rothwell GW, Nixon KC. 2006. How does the inclusion of fossil data change our conclusions about the phylogenetic history of euphyllophytes? Inter. J. Plant Sci. 167, 737–749. ( 10.1086/503298) [DOI] [Google Scholar]
- 110.Pryer KM, Schneider H, Smith AR, Cranfill R, Wolf PG, Hunt JS, Sipes SD. 2001. Horsetails and ferns are a monophyletic group and the closest living relatives to seed plants. Nature 409, 618–622. ( 10.1038/35054555) [DOI] [PubMed] [Google Scholar]
- 111.Schneider H. 2013. Evolutionary morphology of ferns (monilophytes). In The evolution of plant form (eds Ambrose BA, Purugganan M), pp. 1–32. Oxford, UK: Wiley-Blackwell. [Google Scholar]
- 112.Vasco A, Moran RC, Ambrose BA. 2013. The evolution, morphology, and development of fern leaves. Front. Plant Sci. 4, 345 ( 10.3389/fpls.2013.00345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leclercq S.1951. Etude morphologique et anatomique d'une fougère du Dévonien Supérieur, le Rhacophyton zygopteroides nov. sp. [Morphology and anatomy of an Upper Devonian fern, Rhacophyton zygopteroides nov. sp.]. Liège: H. Vaillant-Carmanne. (In French.)
- 114.Ambrose BA. 2013. The morphology and development of lycophytes. Ann. Plant Rev. 45, 91–114. ( 10.1002/9781118305881.ch3) [DOI] [Google Scholar]
- 115.Stevenson DWM. 2013. Gymnosperms. Ann. Plant Rev. 45, 141–161. ( 10.1002/9781118305881.ch5) [DOI] [Google Scholar]
- 116.Tsukaya H. 2014. Comparative leaf development in angiosperms. Curr. Opin. Plant Biol. 17, 103–109. ( 10.1016/j.pbi.2013.11.012) [DOI] [PubMed] [Google Scholar]
- 117.Popham RA. 1951. Principal types of vegetative shoot apex organization in vascular plants. Ohio J. Sci. 51, 249–270. [Google Scholar]
- 118.Piazza P, Jasinski S, Tsiantis M. 2005. Evolution of leaf developmental mechanisms. New Phytol. 167, 693–710. ( 10.1111/j.1469-8137.2005.01466.x) [DOI] [PubMed] [Google Scholar]
- 119.Golub SJ, Wetmore RH. 1948. Studies of development in the vegetative shoot of Equisetum arvense L. The shoot apex. Am. J. Bot. 35, 755–767. ( 10.2307/2438157) [DOI] [Google Scholar]
- 120.Domagalska MA, Leyser O. 2011. Signal integration in the control of shoot branching. Nat. Rev. Mol. Cell Biol. 12, 211–221. ( 10.1038/nrm3088) [DOI] [PubMed] [Google Scholar]
- 121.Braybrook SA, Kuhlemeier C. 2010. How a plant builds leaves. Plant Cell 22, 1006–1018. ( 10.1105/tpc.110.073924) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tsukaya H. 2013. Leaf development. Arabidopsis Book 11, e0163 ( 10.1199/tab.0163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Reinhardt D, Mandel T, Kuhlemeier C. 2000. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12, 507–518. ( 10.1105/tpc.12.4.507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bhatia N, Bozorg B, Larsson A, Ohno C, Jönsson H, Heisler MG. 2016. Auxin acts through MONOPTEROS to regulate plant cell polarity and pattern phyllotaxis. Curr. Biol. 26, 3202–3208. ( 10.1016/j.cub.2016.09.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Reinhardt D, Pesce E-R, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C. 2003. Regulation of phyllotaxis by polar auxin transport. Nature 426, 255–260. ( 10.1038/nature02081) [DOI] [PubMed] [Google Scholar]
- 126.Jönsson H, Heisler MG, Shapiro BE, Meyerowitz EM, Mjolness E. 2006. An auxin-driven polarized transport model for phyllotaxis. Proc. Natl Acad. Sci. USA 103, 1633–1638. ( 10.1073/pnas.0509839103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Smith RS, Guyomarc'h S, Mandel T, Reinhard D, Kuhlemeier C, Prusinkiewicz P. 2006. A plausible model of phyllotaxis. Proc. Natl Acad. Sci. USA 103, 1301–1306. ( 10.1073/pnas.0510457103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hamant O, et al. 2008. Developmental patterning by mechanical signals in Arabidopsis. Science 322, 1650–1655. ( 10.1126/science.1165594) [DOI] [PubMed] [Google Scholar]
- 129.Fleming AJ, Calderas D, Wehrli E, McQueen-Mason S, Kuhlemeier C. 1999. Analysis of expansin-induced morphogenesis on the apical meristem of tomato. Planta 208, 166–174. ( 10.1007/s004250050546) [DOI] [Google Scholar]
- 130.Peaucelle A, Braybrook SA, Le Guillou L, Bron E, Kuhlemeier C, Höfte H. 2011. Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Curr. Biol. 21, 1720–1726. ( 10.1016/j.cub.2011.08.057) [DOI] [PubMed] [Google Scholar]
- 131.Byrne ME, et al. 2000. ASYMMETRIC LEAVES1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 408, 967–971. ( 10.1038/35050091) [DOI] [PubMed] [Google Scholar]
- 132.Lodha M, Marco CF, Timmermans MC. 2013. The ASYMMETRIC LEAVES complex maintains repression of KNOX homeobox genes via direct recruitment of Polycomb-repressive complex2. Genes Dev. 27, 596–601. ( 10.1101/gad.211425.112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Emery JF, Floyd SK, Alvarez J, Eshed Y, Hawker NP, Izhaki A, Baum SF, Bowman JL. 2003. Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr. Biol. 13, 1768–1774. ( 10.1016/j.cub.2003.09.035) [DOI] [PubMed] [Google Scholar]
- 134.Merelo P, et al. 2016. Regulation of MIR165/166 by class II and class III homeodomain leucine zipper proteins establishes leaf polarity. Proc. Natl Acad. Sci. USA 113, 11 973–11 978. ( 10.1073/pnas.1516110113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sawa S, Watanabe K, Goto K, Kanaya E, Morita EH, Okada K. 1999. FILAMENTOUS FLOWER, a meristem and organ identity gene of Arabidopsis, encodes a protein with a zinc finger and HMG-related domains. Genes Dev. 13, 1079–1088. ( 10.1101/gad.13.9.1079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Eshed Y, Izhaki A, Baum SF, Floyd SK, Bowman JL. 2004. Asymmetric leaf development and blade expansion in Arabidopsis are mediated by KANADI and YABBY activities. Development 131, 2997–3006. ( 10.1242/dev.01186) [DOI] [PubMed] [Google Scholar]
- 137.Waites R, Selvadurai HRN, Oliver IR, Hudson A. 1998. The PHANTASTICA gene encodes a MYB transcription factor involved in growth and dorsoventrality of lateral organs in Antirrhinum. Cell 93, 779–789. ( 10.1016/S0092-8674(00)81439-7) [DOI] [PubMed] [Google Scholar]
- 138.Tsiantis M, Schneeberger R, Golz JF, Freeling M, Langdale JA. 1999. The maize rough sheath2 gene and leaf development programs in monocot and dicot plants. Science 284, 154–156. ( 10.1126/science.284.5411.154) [DOI] [PubMed] [Google Scholar]
- 139.Kuchen EE, et al. 2012. Generation of leaf shape through early patterns of growth and tissue polarity. Science 335, 1092–1096. ( 10.1126/science.1214678) [DOI] [PubMed] [Google Scholar]
- 140.Boyce CK, Knoll AH. 2002. Evolution of developmental potential and the multiple independent origin of leaves in Paleozoic vascular plants. Paleobiology 28, 70–100. ( 10.1666/0094-8373(2002)028%3C0070:EODPAT%3E2.0.CO;2) [DOI] [Google Scholar]
- 141.Stein WE, Boyer JS. 2006. Evolution of land plant architecture: beyond the telome theory. Paleobiology 32, 450–482. ( 10.1666/04036.1) [DOI] [Google Scholar]
- 142.Zimmermann W. 1951. Main results of the ‘telome theory’. Palaeobotanist 1, 456–470. [Google Scholar]
- 143.Crane P, Kenrick P. 1997. Diverted development of reproductive organs: a source of morphological innovation in land plants. Plant Syst. Evol. 206, 161–174. ( 10.1007/BF00987946) [DOI] [Google Scholar]
- 144.Beerling DJ, Fleming AJ. 2007. Zimmermann's telome theory of megaphyll leaf evolution: a molecular and cellular critique. Curr. Opin. Plant Biol. 10, 4–12. ( 10.1016/j.pbi.2006.11.006) [DOI] [PubMed] [Google Scholar]
- 145.Sanders H, Rothwell GW, Wyatt SE. 2009. Key morphological alterations in the evolution of leaves. Int. J. Plant Sci. 170, 860–868. ( 10.1086/600135) [DOI] [Google Scholar]
- 146.Boyce CK. 2010. The evolution of plant development in a paleontological context. Curr. Opin. Plant Biol. 13, 102–107. ( 10.1016/j.pbi.2009.10.001) [DOI] [PubMed] [Google Scholar]
- 147.Zimmermann W. 1959. Die Phylogenie der Pflanzen [The phylogeny of plants]. Stuttgart, Germany: Fischer; (In German.) [Google Scholar]
- 148.Donoghue MJ, Kadereit JW. 1992. Walter Zimmermann and the growth of phylogenetic theory. Syst. Biol. 41, 74–85. ( 10.1093/sysbio/41.1.74) [DOI] [Google Scholar]
- 149.Tsiantis M, Hay A. 2003. Comparative plant development: the time of the leaf? Nat. Rev. Genet. 4, 169–180. ( 10.1038/nrg1002) [DOI] [PubMed] [Google Scholar]
- 150.Tsukaya H. 2010. Leaf development and evolution. J. Plant Res. 123, 3–6. ( 10.1007/s10265-009-0285-x) [DOI] [PubMed] [Google Scholar]
- 151.Kim M, McCormick S, Timmermans M, Sinha N. 2003. The expression domain of PHANTASTICA determines leaflet placement in compound leaves. Nature 424, 438–443. ( 10.1038/nature01820) [DOI] [PubMed] [Google Scholar]
- 152.Floyd SK, Bowman JL. 2006. Distinct developmental mechanisms reflect the independent origins of leaves in vascular plants. Curr. Biol. 16, 1911–1917. ( 10.1016/j.cub.2006.07.067) [DOI] [PubMed] [Google Scholar]
- 153.Prigge MJ, Clarke SE. 2006. Evolution of the class III HD-Zip gene family in land plants. Evol. Develop. 8, 350–361. ( 10.1111/j.1525-142X.2006.00107.x) [DOI] [PubMed] [Google Scholar]
- 154.Sano R, Juarez CM, Hass B, Sakakibara K, Ito M, Banks JA, Hasebe M. 2005. KNOX homeobox genes potentially have similar function in both diploid unicellular and multicellular meristems, but not in haploid meristems. Evol. Develop. 7, 69–78. ( 10.1111/j.1525-142X.2005.05008.x) [DOI] [PubMed] [Google Scholar]
- 155.Sanders HL, Langdale JA. 2013. Conserved transport mechanisms but distinct auxin responses govern shoot patterning in Selaginella kraussiana. New Phytol. 198, 419–428. ( 10.1111/nph.12183) [DOI] [PubMed] [Google Scholar]
- 156.Sarojam R, Sappl PG, Goldshmidt A, Efroni I, Floyd SK, Eshed Y, Bowman JL. 2010. Differentiating Arabidopsis shoots from leaves by combined YABBY activities. Plant Cell 22, 2113–2130. ( 10.1105/tpc.110.075853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Floyd SK, Zalewski CS, Bowman JL. 2006. Evolution of class III homeodomain-leucine zipper genes in streptophytes. Genetics 173, 373–388. ( 10.1534/genetics.105.054239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bharathan G, Goliber TE, Moore C, Kessler S, Pham T, Sinha NR. 2002. Homologies in leaf form inferred from KNOXI gene expression during development. Science 296, 1858–1860. ( 10.1126/science.1070343) [DOI] [PubMed] [Google Scholar]
- 159.Steeves TA, Sussex IM. 1989. Patterns in plant development. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 160.Osborne C, Beerling D, Lomax B, Chaloner W. 2004. Biophysical constraints on the origin of leaves inferred from the fossil record. Proc. Natl Acad. Sci. USA 101, 10 360–10 362. ( 10.1073/pnas.0402787101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shougang H, Beck CB, Deming W. 2003. Structure of the earliest leaves: adaptations to high concentrations of atmospheric CO2. Int. J. Plant Sci. 164, 71–75. ( 10.1086/344557) [DOI] [Google Scholar]
- 162.Feild TS, et al. 2011. Fossil evidence for Cretaceous escalation in angiosperm leaf vein evolution. Proc. Natl Acad. Sci. USA 108, 8363–8366. ( 10.1073/pnas.1014456108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Plackett AR, Huang L, Sanders HL, Langdale JA. 2014. High-efficiency stable transformation of the model fern species Ceratopteris richardii via microparticle bombardment. Plant Physiol. 165, 3–14. ( 10.1104/pp.113.231357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Solly JE, Cunniffe NJ, Harrison CJ. 2017. Regional growth rate differences specified by apical notch activities regulate liverwort thallus shape. Curr. Biol. 27, 16–26. ( 10.1016/j.cub.2016.10.056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rensing SA. 2017. Why we need more non-seed plant models. New Phytol. 216, 355–360. ( 10.1111/nph.14464) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.