Abstract
The Lower Devonian Rhynie chert is one of the most important rock deposits yielding comprehensive information on early continental plant, animal and microbial life. Fungi are especially abundant among the microbial remains, and include representatives of all major fungal lineages except Basidiomycota. This paper surveys the evidence assembled to date of fungal hyphae, mycelial cords and reproductive units (e.g. spores, sporangia, sporocarps), and presents examples of fungal associations and interactions with land plants, other fungi, algae, cyanobacteria and animals from the Rhynie chert. Moreover, a small, chytrid-like organism that occurs singly, in chain-like, linear arrangements, planar assemblages and three-dimensional aggregates of less than 10 to  individuals in degrading land plant tissue in the Rhynie chert is formally described, and the name Perexiflasca tayloriana proposed for the organism. Perexiflasca tayloriana probably colonized senescent or atrophied plant parts and participated in the process of biological degradation. The fungal fossils described to date from the Rhynie chert constitute the largest body of structurally preserved evidence of fungi and fungal interactions from any rock deposit, and strongly suggest that fungi played important roles in the functioning of the Early Devonian Rhynie ecosystem.
individuals in degrading land plant tissue in the Rhynie chert is formally described, and the name Perexiflasca tayloriana proposed for the organism. Perexiflasca tayloriana probably colonized senescent or atrophied plant parts and participated in the process of biological degradation. The fungal fossils described to date from the Rhynie chert constitute the largest body of structurally preserved evidence of fungi and fungal interactions from any rock deposit, and strongly suggest that fungi played important roles in the functioning of the Early Devonian Rhynie ecosystem.
This article is part of a discussion meeting issue ‘The Rhynie cherts: our earliest terrestrial ecosystem revisited’.
Keywords: fungal fossil, litter layer, reproductive unit, Rhynie ecosystem, structural preservation, symbiosis
1. Introduction
The Lower Devonian Rhynie chert from Aberdeenshire, Scotland, has long been recognized as one of the most important rock deposits yielding comprehensive information on early continental plant and animal life [1–6]. More recently, the Rhynie chert has also become increasingly attractive as a source of new information on the diversity of microbial life and insights into the biology and ecology of microorganisms in ancient freshwater and terrestrial environments, inspired in part by the growing awareness of the importance of the microbial component in modern ecosystems [7,8]. The documented record of microbial life from the Rhynie chert currently comprises bacteria [9], coccoid and filamentous cyanobacteria [10–13], eukaryotic algae [14–18], peronosporomycetes [19–21], fungi belonging to all major lineages except the Basidiomycota (see below), and representatives of the enigmatic nematophytes [22,23].
Fungi (in the broadest sense of including fungus-like organisms such as Peronosporomycetes and Hyphochytridiomycetes) are remarkably abundant and diverse in the Rhynie chert. Filaments, aseptate and septate hyphae, mycelial cords and a broad spectrum of different types of small propagules (e.g. spores) and detached reproductive units (e.g. sporangia, sporocarps) are almost ubiquitous in the chert matrix, in microbial mats and litter accumulations, and within intact and decaying land plant parts [9]. Moreover, several exquisite specimens of fungi preserved in situ together with their host organisms demonstrate the existence of different types of fungal associations and interactions, including parasites on algae, land plants, other fungi and possibly animals, mycorrhizas in both sporophytes and gametophytes of land plants, and saprotrophs on decaying plant parts [8].
This paper surveys the documented fungal diversity in the Rhynie chert, thereby focusing on reproductive units, which occur in nearly every thin section of the chert. Moreover, the spectrum of fungal associations and interactions that have been documented from the Rhynie chert is reviewed. However, some of the most common fungal associations in the Rhynie chert have not been particularized to date, due probably to the fact that the microbial partners are exceedingly small. The second purpose of this paper is therefore to describe Perexiflasca tayloriana gen. et sp. nov., an excellent example of a minute, chytrid-like Rhynie chert organism that has long been known [24], but its association with partly degraded land plant tissue, although frequently encountered in litter layers, has not been detailed to date. The Rhynie chert fungal fossils suggest that fungi were instrumental to the functioning of the Rhynie ecosystem.
2. Geological setting, material and methods
The Rhynie chert Lagerstätte is located in the northern part of the Rhynie outlier in Aberdeenshire, Scotland [25,26], and includes series of chert lenses that are principally fine grained and interpreted as having accumulated on an alluvial plain associated with ephemeral ponds and lakes. The ecosystem is interpreted as a geothermal wetland [27–29], with alkaline hot springs that were part of a complex hydrothermal system [25,30]. Both aquatic and terrestrial organisms became preserved as a result of temporary flooding with silica-rich water, or by silica-rich groundwater that percolated to the surface. The Rhynie chert biota has been regarded as early (but not earliest) Pragian to earliest Emsian in age based on spore assemblages [31,32]. An age estimate based on high-precision U–Pb dating of zircon and titanite from hydrothermally altered andesite indicates an absolute age of 411.5 ± 1.3 Ma for the Rhynie chert biota [26], while another age constraint using 40Ar/39Ar in K-feldspar from a quartz-feldspar vein that is part of the hydrothermal system responsible for the formation of the Rhynie chert yields a mean age (recalculated to be U–Pb comparable) of the fossilized biota of 407.1 ± 2.2 Ma [33]. However, the andesite cannot be fixed with certainty in the stratigraphic sequence and is certainly older than the hydrothermal alteration [34]. As a result, the date estimate in [33] probably gives a more accurate age of the hydrothermal system, and hence the age of the Rhynie chert biota. An absolute age of 411.5 ± 1.3 Ma is very close to the Lochkovian/Pragian boundary (410.8 ± 2.8 Ma), while the age suggested in [33] would correspond approximately to the Pragian/Emsian boundary (407.6 ± 2.6 Ma). For additional information on the geology and palaeontology of the Rhynie chert, refer to the other papers in this volume.
All fossils described and illustrated in this paper were identified in thin sections prepared from chert blocks by cementing wafers of the chert to glass slides and then grinding the rock slices until the sections were thin enough to transmit light. The thin sections were analysed using transmitted-light microscopy; digital images were captured with a Leica DFC-480 camera and processed in Adobe Photoshop. Most of the specimens illustrated in figures 1–5 are deposited in the Bayerische Staatssammlung für Paläontologie und Geologie (SNSB-BSPG), Munich, Germany (prefix BSPG). Additional material is housed in the Abteilung Paläobotanik, Geologisch-Paläontologisches Institut, Westfälische Wilhelms-Universität, Münster, Germany (prefix P). Accession numbers for all figured materials are included in the figure captions.
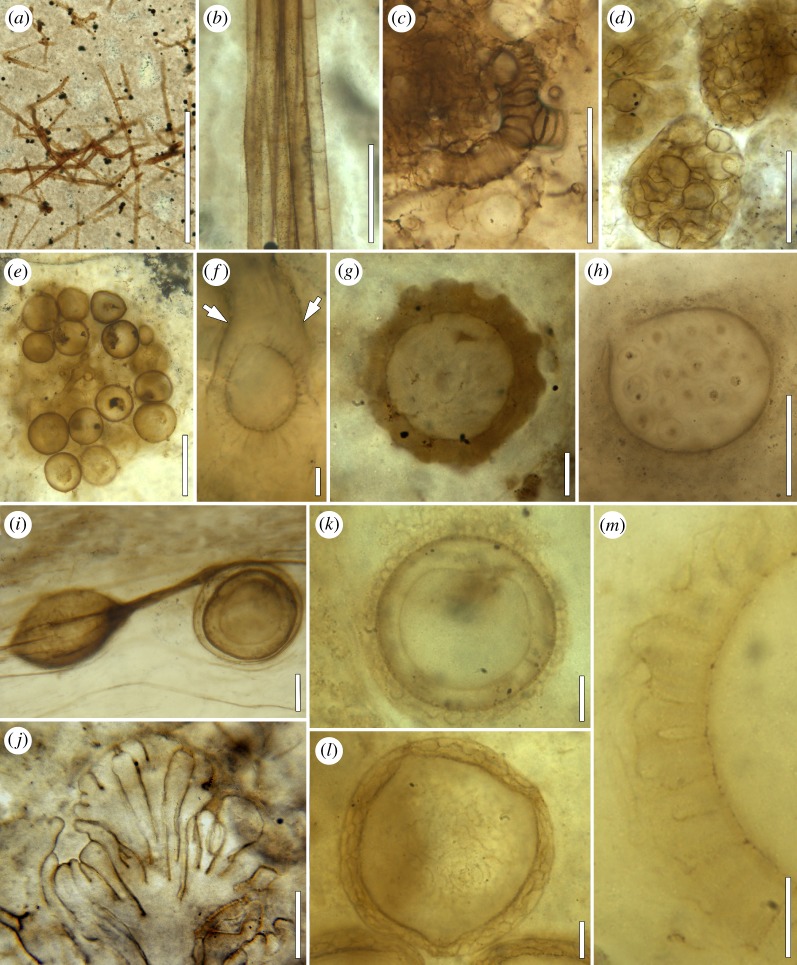
Figure 1.
Fungi and fungal interactions in the Lower Devonian Rhynie chert (explanations in the text). (a) Mycelium in chert matrix; BSPG 2008 XVI 2; bar, 500 µm. (b) Mycelial cord; BSPG 2013 V 16; bar, 100 µm. (c) Branch knot; BSPG 2015 XVII 19; bar, 50 µm. (d) Vesicle clusters in land plant cells; BSPG 1965 I 295; bar, 50 µm. (e) Cluster of chlamydospores; BSPG 2015 XVIII 8; bar, 100 µm. (f) Spiny propagule surrounded by sheath (arrows); BSPG 2008 XVI 10; bar, 10 µm. (g) Sheathed chlamydospore; BSPG 1965 I 357; bar, 10 µm. (h) Oogonium containing oospores of Frankbaronia velata; BSPG 2013 V 50; bar, 50 µm. (i) Acaulosporoid glomeromycotinan spore with sporiferous saccule; P3966; bar, 100 µm. (j) Germination shield of acaulosporoid spore; P3951; bar, 20 µm. (k) Zwergimyces vestitus; BSPG 2013 XV 38; bar, 10 µm. (l) Scepasmatocarpion fenestrulatum; BSPG 1965 I 363; bar, 10 µm. (m) Two-layered hyphal investment of H. devonica; BSPG 2013 XV 123; bar, 10 µm.
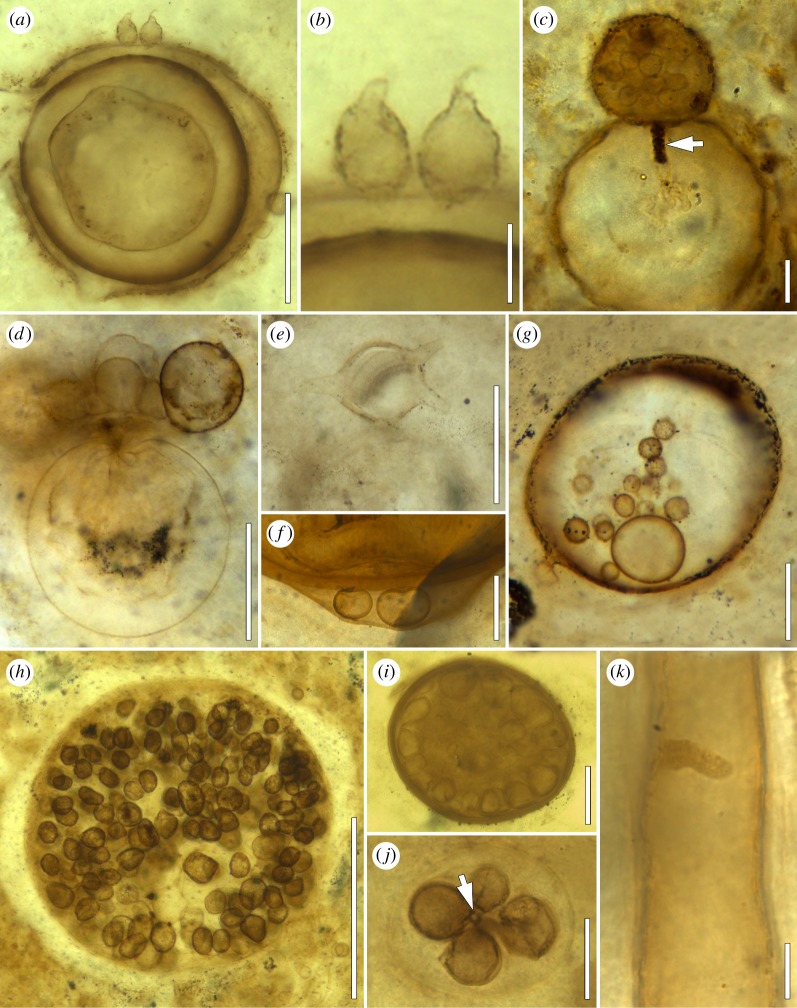
Figure 2.
Fungi and fungal interactions in the Lower Devonian Rhynie chert (explanations in the text). (a) Partially degraded, thick-walled fungal spore with chytrid zoosporangia on surface; BSPG 2013 XIV; bar, 50 µm. (b) Detail of (a), showing zoosporangia with distal discharge papilla; bar, 10 µm. (c) Chytrid zoosporangium containing zoospores on fungal spore, with prominent primary rhizoidal axis (arrow) extending into host spore lumen; BSPG 2013 V 61; bar, 10 µm. (d) Cluster of chytrid zoosporangia extending from fungal spore; BSPG 2016 VII 6; bar, 50 µm. (e) Illmanomyces corniger on fungal spore; BSPG 2013 V 8; bar, 50 µm. (f) Chytrid zoosporangia between wall layers of fungal spore; BSPG 2013 XV 5; bar, 10 µm. (g) Putative polycentric chytrid in lumen of glomeromycotinan spore; BSPG 1964 XX 24; bar, 50 µm. (h) Sporocarp in large glomeromycotinan spore; BSPG 2013 XV 37; bar, 50 µm. (i) Sporocarp with thick peridium; BSPG 2013 XV 46; bar, 50 µm. (j) Tiny fungal reproductive units extending from central hypha (arrow) in lumen of glomeromycotinan vesicle; BSPG 2013 XV 125; bar, 100 µm. (k) Callosity in fungal hypha; BSPG 2015 XIX 92; bar, 10 µm.
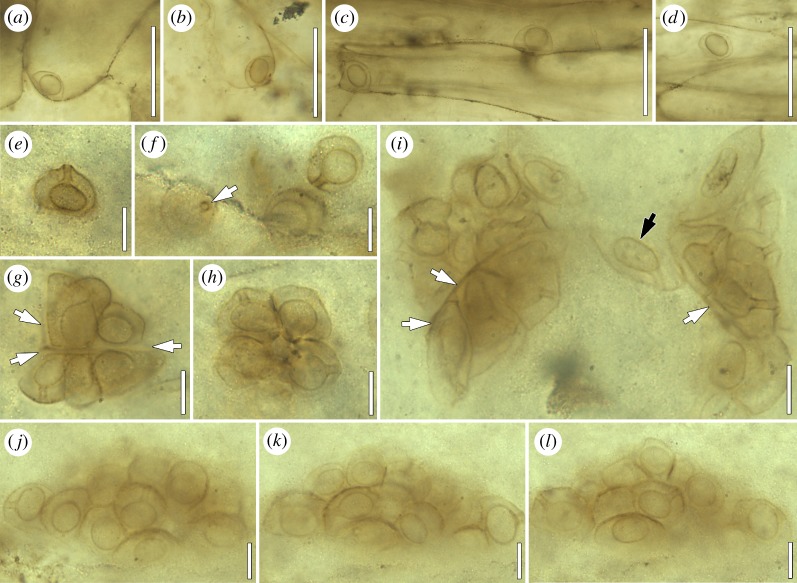
Figure 3.
Fungi and fungal interactions in the Lower Devonian Rhynie chert (explanations in the text). (a) Trewinomyces annulifer extending from surface of land plant axis; BSPG 1965 I 336; bar, 50 µm. (b) Perithecium (longitudinal section) of Paleop. devonicus in As. mackiei (Courtesy of H. Kerp & H. Hass, Münster, Germany); P3411; bar, 100 µm. (c) Zoosporangium of Paleob. milleri (Courtesy of H. Kerp & H. Hass, Münster, Germany); P2054; bar, 10 µm. (d) Monocentric chytrids on Palaeo. cranii with endobiotic apophysis and rhizomycelium (arrows); BSPG 2016 VII 5; bar = 50 µm. (e) Thallus of W. reticulata with hyphal pockets containing cyanobacteria (Courtesy of H. Kerp & H. Hass, Münster, Germany); P1323; bar, 500 µm. (f) Hyphal weft of W. reticulata enclosing cyanobacterial cells (Courtesy of H. Kerp & H. Hass, Münster, Germany); P1386; bar, 15 µm.
Figure 4.
Fungi and fungal interactions in the Lower Devonian Rhynie chert: P. tayloriana gen. et sp. nov. (a–d) Single thalli attached to the inner surface of land plant cell walls; BSPG 2013 V 13; bars 50 µm. (e) Holotype. Mature thallus in the lateral view; BSPG 2013 V 30; bar, 10 µm. (f) Thalli in the lateral and top view; arrow points to discharge tube in surface view; BSPG 2013 V 17; bar, 10 µm. (g) Thalli clustered in the corner of three land plant cells; arrows indicate remains of host cell walls; BSPG 2013 V 32; bar, 10 µm. (h) The same as figure 2g, different focal plane; bar, 10 µm. (i) Thalli in largely degraded land plant cortical tissue; white arrows indicate remains of host cell walls, black arrow points to specimen with tiny inclusions in cavity; BSPG 2013 V 31; bar, 10 µm. (j–l) Optical sections of aggregate of thalli on the inner surface of plant cell; BSPG 2013 V 17; bars, 10 µm.
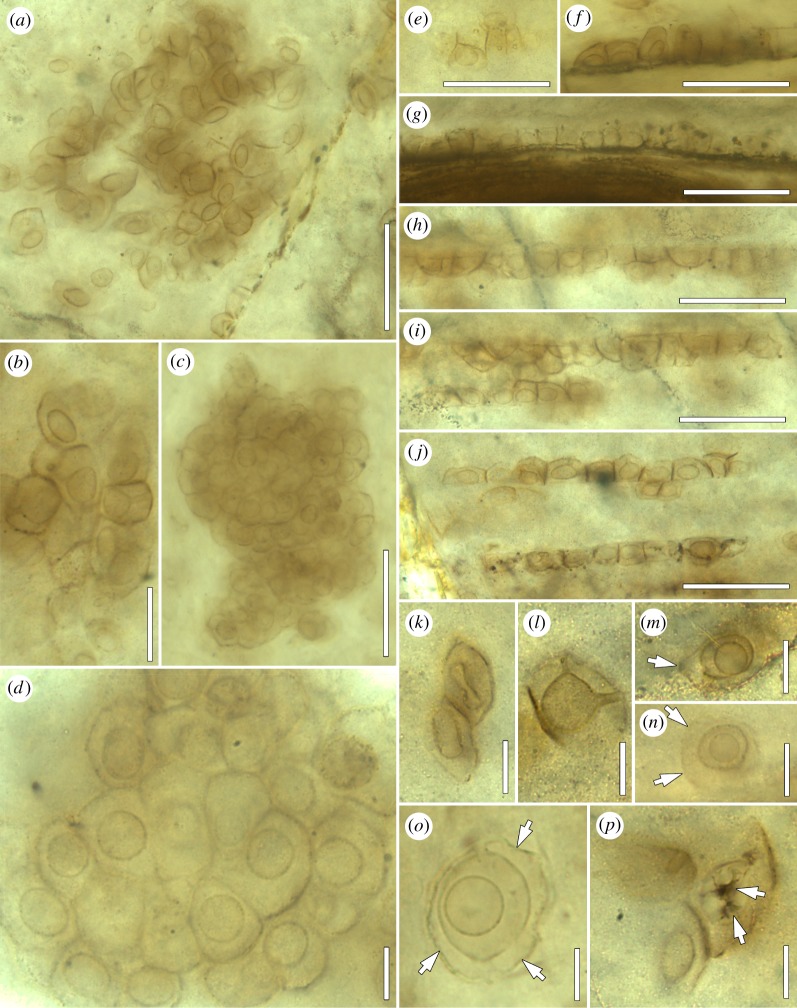
Figure 5.
Fungi and fungal interactions in the Lower Devonian Rhynie chert: P. tayloriana gen. et sp. nov. (a) Aggregate in largely degraded land plant tissue; BSPG 2013 V 16; bar, 50 µm. (b) Group of thalli, showing variability in shape; BSPG 2013 V 34; bar, 20 µm. (c) Large, three-dimensional aggregate of greater than 100 thalli in chert matrix; BSPG 1964 XX 99; bar, 50 µm. (d) Detail of figure 3c, focusing on some of the thalli; bar, 10 µm. (e) Linearly aligned thalli in top view; BSPG 2013 V 36; bar, 50 µm. (f–j) Linearly aligned thalli (lateral views) in long, narrow cells close to land plant vascular strands; BSPG 2013 V 16 (f) and 36 (g–j); bars, 50 µm. (k–p) Morphological variants. (k) Thalli with elongate to dumb bell-shaped lumen; BSPG 2013 V 30; bar, 10 µm. (l) Thallus with two discharge tubes; BSPG 2013 V 34; bar, 10 µm. (m–o) Specimens surrounded by delicate sheath with one to several pores (arrows); BSPG 2013 V 17 (m,n) and 13 (o); bars, 10 µm. (p) Specimen suggestive of the presence of small rhizomycelium (arrows); BSPG 2013 V 34; bar, 10 µm.
3. Fungi in the Rhynie chert: review of the evidence
(a). Vegetative remains
Fragments of fungal filaments and hyphae are frequently encountered throughout the Rhynie chert [9]. Moreover, sterile mycelia are preserved in situ in some sections of the chert (figure 1a). Hyphae may be aseptate or septate, thin- or thick-walled, tubular or irregular, branched or non-branched and some possess terminal or intercalary swellings; however, none are physically connected to reproductive structures that could be used to determine the systematic affinities of these fungi. Intermixed with filaments and hyphae are sometimes banded tubes that have been previously attributed to nematophytes, as well as branch knots comprised densely aggregated, profusely branched (banded) tubes (figure 1c). As to whether these structures are remnants of disintegrated Nematoplexus or other nematophyte thalli [22], or have formed outside the confines of a thallus or plexus remains unclear.
Mycelial cords, linear aggregations of up to greater than 50 parallel-oriented hyphae, are present in many areas of the Rhynie chert (figure 1b). Hyphae within one cord may vary considerably with regard to diameter (approx. 2–greater than 10 µm), wall thickness and septation; anastomoses and intrahyphal hyphae regularly occur in larger cords. Some of the smaller (i.e. constructed of less than 10 hyphae) mycelial cords in the Rhynie chert have been shown to belong to the extramatrical mycelium of the endomycorrhizal fungus Glomites rhyniensis [35]. Mycelial cords in fungi today aid in the exploration of the environment by facilitating long distance transport of water and nutrients (e.g. [36–39]). It is therefore plausible to assume that these structures also played important roles in Rhynie chert fungi. Unfortunately, the Rhynie chert mycelial cords have not yet been systematically analysed and documented.
(b). Reproductive units
The abundance and morphological diversity of small (less than 0.5 mm) fungal propagules and reproductive units is one of the hallmark features of the Rhynie chert. However, dealing with these remains is notoriously difficult because they usually occur detached from the systems on or in which they were produced, and thus do not provide a complete range of structural features necessary to determine their systematic affinities [40]. Only a few forms occur in characteristic configurations (figure 1e) or possess special features such as elaborate surface ornaments (figure 1f) or complex wall architecture (figure 1g) that makes it possible to recognize distinctiveness and sometimes even determines affinities. Other forms can be attributed systematically with some degree of confidence based on a combination of structural features and content (figure 1h) [20,21].
Simple, spheroidal reproductive units borne terminally on hyphae are usually interpreted as glomoid glomeromycotinan spores (chlamydospores); several types were initially described and illustrated in [9] and placed in the genus Palaeomyces. Spore size and morphology are variable, and in some specimens, the wall is multi-layered. Palaeomyces is associated with several land plants, including Aglaophyton majus and Asteroxylon mackiei, as well as degraded plant material [9,41]. Other spores in As. mackiei have been described as Scutellosporites devonicus [42]. The presence of what appears to be a bulbous base in these specimens is characteristic of gigasporoid glomeromycotinan spores [43]. Moreover, a round or oval germination shield occurs within the multi-layered wall in some of the specimens. A third type of glomeromycotinan spore from the Rhynie chert develops laterally within the neck of a sporiferous saccule (figure 1i), and thus corresponds to present-day acaulosporoid AM fungi [44]. The wall of this spore has been suggested to consist of three major parts: a germination shield that can vary in morphology from plate-like with single or double lobes to tongue-shaped with infolded, distally fringed or palmate margins (figure 1j), is formed by extrusion of one of the wall components. Another acaulosporoid spore type recently discovered from the Rhynie chert is characterized by prominent fringes extending from the surface [45].
Several types of Rhynie chert fungal reproductive units have been described that all possess an ancillary covering in the form of a hyphal investment or mantle. Investment morphology varies considerably among the different types, and thus renders them easy to distinguish from one another. The investment of Zwergimyces vestitus (figure 1k) consists of interlaced hyphae extending along the circumference of the structure [46]. Variations in the organization of the mantle among the specimens suggest that mantle formation took place by repeated branching of hyphae on the surface of the developing unit and by additional hyphae extending between the pre-existing mantle hyphae [40]. On the other hand, the investment of Mycocarpon rhyniense is two-layered, with the inner layer formed by interlaced circumferential hyphae, and the outer layer of hyphal branches that are radially oriented [47]. A two-layered investment is also present in Helmutella devonica [48], with the outer layer constructed of interlaced circumferential hyphae, while the inner layer consists of radially elongate elements that are closely aligned (figure 1m). Especially interesting is that this investment morphology closely corresponds to that seen in certain Carboniferous fungal ‘sporocarps’, including Dubiocarpon and Mycocarpon (see [49,50]). The fourth investment type is similar to Z. vestitus, but differs in that the investment hyphae have club-shaped tips [51]. Finally, the investment of Scepasmatocarpion fenestrulatum occurs in the form of a pseudotissue comprised tightly interwoven hyphae [52]. Moreover, several prominent pores extend through the investment (figure 1l). Krings & Taylor [46,48] and Krings et al. [40,47] suggest that most mantled reproductive units from the Rhynie chert have systematic affinities with the Glomeromycotina or Mucoromycotina based on similar features in modern lineages known to produce spores or sporangia with hyphal investments. One form has also been compared to the so-called ‘birdsnest’ condition of certain peronosporomycte oogonia [51], while S. fenestrulatum is reminiscent of the pycnidia and cleistothecia formed by certain modern ascomycetes [52].
(c). Associations and interactions
Several different types of fungal associations and interactions with land plants, other fungi, charophytes, animals and cyanobacteria have been described from the Rhynie chert and directly compared to modern equivalents to determine the nutritional relationship (mutualistic, parasitic and saprotrophic) between the partners in the fossils.
(i). Fungi–land plants
The most significant fungal interaction in the Rhynie chert is the endomycorrhiza (paramycorrhiza sensu Strullu-Derrien & Strullu [53]) that occurs in the land plant Ag. majus [35,54–56]. The fungal partner, G. rhyniensis (Glomeromycotina), produces an extramatrical mycelium composed of hyphae and mycelial cords. Individual hyphae enter the plant through stomata in the aboveground prostrate axes and spread out in the intercellular system of the outer cortex. Within the cortex, G. rhynienis produces vesicles and glomoid spores, as well as arbuscules within a narrow zone of tissue between the outer and middle cortex. Mycorrhizal axes of Ag. majus sometimes also host a filamentous cyanobacterium, which also enters the plant through stomata and invades parenchyma cells close to and within the mycorrhizal arbuscule-zone to form intracellular coils [12]. What relationship, if any, existed between the cyanobacterium and the mycorrhizal land plant remains unresolved. The endomycorrhiza in Ag. majus from the Rhynie chert represents one of the core pieces of fossil evidence of the evolutionary history of mycorrhizal systems [57–59]. Moreover, it substantiates the hypothesis that the establishment of plant life on land concurred with, and was profoundly influenced by, the evolution of mutually beneficial symbioses between the earliest plants and certain fungi (e.g. [60–66]).
Structures suggestive of the presence of mycorrhizal associations in other Rhynie chert plants have been reported in [41,67–71]. For example, a distinct zone of fungal colonization similar to that observed in Ag. majus has been described in Rhynia gwynne-vaughanii [67]. Moreover, various fungi occur in the prostrate axes of the land plant Nothia aphylla, including one that is believed to be endomycorrhizal [69,70]. As the prostrate axes of N. aphylla lack stomata, the mycorrhizal fungus enters the plant through rhizoids. In the host cortex, the fungus forms an extensive intercellular network of hyphae, and produces vesicles and thick-walled spores. No arbuscules have been identified in N. aphylla. Finally, Strullu-Derrien et al. [71] report on two different fungi in the land plant Horneophyton lignieri. Palaeoglomus boullardii, a member of the Glomeromycotina, occurs in the upright axes within a discontinuous zone of the outer cortex where it forms vesicles, spores and arbuscule-like structures, while Palaeoendogone gwynne-vaughaniae, believed to belong to the Mucoromycotina, is present in the cortex intercellular system in the basal part of the plant and forms intracellular coils. This discovery suggests that not only Glomeromycotina but also Mucoromycotina entered into mutualistic relationships with land plants in the Rhynie ecosystem (see [72–74]).
Evidence of fungal interactions with land plants in the Rhynie chert also occurs in the form of micrometre-sized spheroidal, obpyriform or clavate vesicles, some with one or several pores or papillae in the wall, that are attached to the outer surface of land plant spores. The vesicles are usually interpreted as chytrid zoosporangia based on correspondences in overall appearance between the fossils and certain extant chytrids colonizing spores and pollen grains [9,24,75,76]. Moreover, Palaeozoosporites renaultii, a Rhynie chert fungus that consists of aseptate filaments with isotomous or sympodial branching, extends through the intercellular system in the cortex of the rooting structures of the early lycophyte As. mackiei [77]. Arising from the filaments are globous to ovoid structures interpreted as zoosporangia and resting sporangia. The fossil resembles certain present-day polycentric chytrids, but doubts remain over the precise systematic affinity.
Another interesting fungus associated with As. mackiei is the perithecial ascomycete Paleopyrenomycites devonicus, which occurs in the axes and leaf-like appendages of this early lycophyte [78,79]. The perithecia (figure 3b) are characterized by short, ostiolate necks protruding from the host epidermis through stomatal openings. Lining the interior of the perithecium are thin-walled paraphyses interspersed with asci containing ascospores. Tufts of conidiophores arising from acervuli are believed to represent the anamorphic phase of the fungus. Taylor et al. [79] suggest that Paleop. devonicus might be a pyrenomycete, perhaps a member of the Sordariomycetes; however, affinities to the Taphrinomycotina and Pezizomycetes have also been discussed [80,81]. The nutritional mode of Paleop. devonicus, whether it was a parasite or saprotroph, remains unresolved.
An exquisitely preserved saprotrophic fungus described from the Rhynie chert is Paleoblastocladia milleri [82], which occurs in the form of tufts that emerge from stomata and surface ruptures in partially degraded Ag. majus axes. Thalli are of two nearly identical morphological types that consist of branched, intramatrical rhizoids and aseptate, erect extramatrical hyphae. On the sporothallus are terminal zoosporangia (= mitosporangia), each attached to the parental hypha by a septum (figure 3c). Also associated with the sporothalli are meiosporangia, or resting sporangia, characterized by a patterned surface ornament of delicate depressions or punctae. The second thallus type of Paleob. milleri produces barrel-shaped gametangia that are smaller than the zoosporangia and organized in pairs or stacks of three. Paleoblastocladia milleri shares features with certain members of the modern Allomyces and related species in the Blastocladiomycota [83,84]. Another saprotrophic Rhynie chert fungus that occurs in the form of tufts emerging from decaying land plant axes is Trewinomyces annulifer [85]. This fungus consists of a branched, intramatrical rhizoidal system and an unbranched, erect extramatrical hypha (stalk) that bears a single, terminal sporangium (figure 3a). The overall morphology of T. annulifer resembles the extant genera Macrochytrium (Chytridiomycota) and Blastocladiella (Blastocladiomycota). However, the rhizoids are septate or pseudoseptate, a feature not known in extant zoosporic fungi, and this renders the systematic affinities of T. annulifer unresolved.
Still other fungal remains frequently associated with intact and decaying land plant axes in the Rhynie chert are intra- or intercellular vesicle clusters (figure 1d), thick-walled resting spores and sporocarps (figure 2i), wefts of hyphae and clusters of small propagules. However, the systematic affinities and nutritional modes of these fungi remain unresolved.
(ii). Fungi–fungi
Abundant evidence of interfungal associations have been reported from the Rhynie chert, including mycelia and reproductive structures inside large fungal spores (figure 2g) [86–88], hyphae enveloping and subsequently penetrating glomeromycotinan vesicles [89] and small fungal propagules developing in glomeromycotinan vesicles (figure 2j) [90]. Moreover, several examples of monocentric and polycentric chytrid-like organisms have been described as colonizers of fungal hyphae and spores (figure 1a–e). Most chytrid parasites of fungal spores in the Rhynie chert are characterized by epibiotic zoosporangia and rhizomycelia extending into the host spore lumen [24,86,91]. Other chytrid-like colonizers of fungal spores are found between particular wall layers of these spores or occupying the spore lumen (figure 2f,g) [9,86]. For example, Globicultrix nugax, a polycentric thallus comprised a rhizomycelium of branched, aseptate filaments and apophysate sporangia that are exclusively terminal, occurs in the lumen of large glomeromycotinan spores [92]. The morphology and size of G. nugax has been compared to extant polycentric chytrids such as Nowakowskiella and Cladochytrium, both within the order Chytridiales. Inwardly directed pegs or papillae (termed appositions or callosities) that arise from the inner surface of the host wall and encase invading fungal hyphae or filaments (figure 2k) represent a common host response of Rhynie chert fungi to attacks by other fungi, albeit the intrusive entity is not always preserved in a recognizable form [24,86].
(iii). Fungi–charophytes
Chytrid-like organisms have also been identified as common parasites of the Rhynie chert charophyte Palaeonitella cranii (figure 3d) [93]. One of these chytrids is Milleromyces rhyniensis, which is characterized by a spheroidal, endobiotic zoosporangium with a single, prominent discharge tube extending out from the host cell wall. At the base of the zoosporangium is a small rhizomycelium. Other chytrid-like organisms associated with Palaeo. cranii include Lyonomyces pyriformis and Krispiromyces discoides, which differ from one another in thallus morphology, but are comparable with several extant chytrid parasites of freshwater algae, including members in Entophlyctis and Phlyctochytrium (see [94,95]). The host response in Palaeo. cranii consists of a massive hypertrophy of cells, which grow to approximately five times the diameter of normal cells [93]. A very similar form of hypertrophy in response to chytrid parasitism has been reported in the modern genus Chara, a relative of Palaeonitella [96].
(iv). Fungi–animals
A monocentric chytrid with epibiotic zoosporangia that is quite similar morphologically to some of the forms parasitizing Palaeo. cranii and certain fungal spores in the Rhynie chert has been described as Cultoraquaticus trewinii [97]. Zoosporangia of C. trewinii are intermixed with spiny spherules interpreted as branchiopod resting eggs attributable to the crustacean Lepidocaris rhyniensis, suggesting that chytrids played important roles in the mobilization of nutrients in early aquatic food webs. Direct evidence of fungi as parts of food webs in the Rhynie ecosystem comes from coprolites containing fragments of hyphae and fungal spores [98].
(v). Fungi–cyanobacteria
A cyanolichen-like association has been described from the Rhynie chert as Winfrenatia reticulata [99,100]. It occurs in the form of a thallus constructed of superimposed layers of parallel hyphae. The uppermost layers are folded vertically into loops that form a pattern of ridges and circular to elliptical depressions on the surface (figure 3e). Extending from the walls of the depressions are hyphae that form a three-dimensional network. As a result of hyphal branching, each depression consists of lacunae that are formed by the mycobiont. The cyanobacterial photobiont consists of coccoid cells or clusters of cells, each cluster surrounded by a prominent sheath, that occur within the lacunae of the hyphal net (figure 3f). Winfrenatia reticulata most probably colonized hard substrates such as degrading sinter surfaces and may have weathered rock surfaces, thus contributing to soil formation [101].
4. Description of Perexiflasca tayloriana gen. et sp. nov.
Fossil genus Perexiflasca gen. nov.
Mycobank: MB 819876
Type species: Perexiflasca tayloriana M. Krings, C.J. Harper & E.L. Taylor, hic designatus
Diagnosis: Simple thallus comprised spheroidal, prolate or lens-shaped (i.e. dorsiventrally compressed), thin-walled cavity enveloped in prominent, translucent sheath; single discharge tube extends from cavity through sheath to surface; thalli occur singly, in planar assemblages no more than two layers high, or in tight, three-dimensional aggregates; thallus morphology variable, determined by availability of space in place of growth; single specimens typically hemispherical or pear-shaped, linearly aligned ones more or less square with adjacent sides flattened; individuals in assemblages and aggregates highly variable in size and shape depending on position within clustering.
Etymology: The name of the genus, a combination of the Latin word perexiguus, -a, -um (= very small) and the Medieval Latin flasca (= bottle, flask), refers to the small size and characteristic feature of the fossil.
Perexiflasca tayloriana sp. nov.
Mycobank: MB 819877
Holotype: Specimen illustrated in figure 4e; in slide SNSB-BSPG 2013 V 30, SNSB-Bayerische Staatssammlung für Paläontologie und Geologie, Munich, Germany
Type locality: Rhynie, Aberdeenshire, Scotland, National Grid Reference NJ 494276
Age: Early Devonian; Pragian, 411.5 ± 1.3 Ma [26] or 407.1 ± 2.2 Ma [33]
Diagnosis: Thallus less than 22 µm wide, up to 20 µm high, near-spherical, hemispherical, lenticular to spindel-shaped, blunt, cubical, or pyramidal; spherical cavities up to 12 µm in diameter, prolate to lens-shaped ones 15 µm wide and 11 µm high; discharge tube 1.8 µm in diameter, length variable, erect or oblique relative to cavity floor; attached to cell walls or cell wall remains in degrading land plant tissue (rarely on fungal hyphae and spores), usually in litter layers, sometimes free-floating in chert matrix.
Etymology: In honour of the late Thomas N. Taylor, University of Kansas, USA, for his benchmark contributions to our understanding of the microbial component of the Rhynie ecosystem.
Remarks: Perexiflasca tayloriana was initially described (but not named) by Taylor et al. ([24]: figs 1–14) based on specimens in degrading H. lignieri rhizomes and aerial axes. The material illustrated by these authors includes several specimens with one to several prominent, papilla-like projections (referred to as ‘lobes’ in [24]) extending from the outer component. It is unclear whether these specimens also belong to P. tayloriana or represent a different organism. Support for the latter is perhaps the fact that the discharge tube in the papillate form is conical (right arrow in ([24], fig. 6), rather than tubular as in P. tayloriana. Moreover, no papilla-like projections have been observed in any of the greater than 1000 specimens that form the basis for the present study. We therefore refrained from including characters of the papillate specimens into the diagnoses.
Description: Most specimens occur in partially intact (senescent or dying) or degrading land plant axes, often attached to cell walls or cell wall remains, but they are sometimes also found attached to fungal hyphae or reproductive units, or they occur free-floating in the chert matrix. The organism appears to be widespread in the Rhynie chert, but is most frequently encountered in litter layers comprised fragmented land plant parts (axes, sporangia) in different stages of decay, fungal hyphae, fungal and land plant spores, scattered remains of other microorganisms (e.g. cyanobacteria, algal phycomata), and to a lesser extent arthropod exuvia. More than 1000 specimens (individual thalli) have been identified in approximately 120 thin sections prepared from five different chert blocks.
Specimens consist of two major parts, which we informally call ‘inner’ and ‘outer’ component. The inner component comprises a spheroidal, lens-shaped (i.e. dorsiventrally compressed), or oblong, thin-walled cavity (on average 8.7 µm in diameter if spheroidal, and up to 15 µm wide and 11 µm high if oblong), from which extends a prominent tube approximately 1.8 µm wide (e.g. figure 4e,f,h). A tube is present in greater than 80% of the specimens and can be traced readily by focusing through the fossil; the remaining less than 20% of specimens lack evidence of the tube. The cavity is usually empty; however, a few specimens contain one to several tiny, opaque inclusions up to 1.7 µm in diameter (black arrow in figure 4i). Surrounding the inner component is the translucent outer component, which is variable in shape and thickness, ranging from near-spherical (figure 4f), hemispherical (figure 4a–c,e), lenticular to spindle-shaped (figures 3a and 4i), blunt, cubical (figure 4j–f) or pyramidal (figure 4i, right side of the image). The outer surface is smooth in all specimens included in this study (but see the Remarks section). The tube that extends from the cavity traverses through the outer component and connects the cavity with the environment; a collar-like rim of more opaque material may be present around the mouth of the tube (arrow in figure 4f).
Specimens occur singly (figure 4a–e), in chain-like, linear arrangements (figure 5e–j), in planar assemblages no more than two stories high (figures 4g,i–l and 5a,b), and in three-dimensional aggregates of less than 10 to very much less than 100 tightly abutting individuals (figures 4h and 5c,d). Large assemblages sometimes adumbrate the outlines of degraded plant cells through the pattern in which the individual specimens are arranged (figure 4i); in rare instances, small fragments of actual plant cell walls are enclosed in the assemblages (white arrows in figure 4g, i). Specimen morphology is variable (figure 6a–h). Single specimens in situ (i.e. attached to substrate) are typically hemispherical or drop-/tear-shaped (figures 4a–f and 6a–c), whereas linearly aligned ones are blunt to more or less cubical, with adjacent sides flattened (figure 5e–j). The shape of specimens occurring in planar assemblages and three-dimensional aggregates depends on the position of the specimen within the clustering (figures 4g–l, 5a–d and 6d,f–h). Single, free-floating specimens are variable in morphology. Tubes are mostly oriented more or less perpendicularly to the cavity floor (figure 4e–h), but may, in clustered specimens, also be oblique (figure 4i). Tubes in clustered specimens always extend towards a portion of the outer surface that is not blocked by plant cell walls or other specimens. This is especially well recognizable in the linear arrangements where all individuals are oriented in the same direction and have only one unblocked side (figures 5e–j and 6e). Conversely, individuals located deep in the interior of three-dimensional aggregates appear to lack tubes (figures 5d and 6h).
Figure 6.
Fungi and fungal interactions in the Lower Devonian Rhynie chert: variability in thallus morphology of P. tayloriana gen. et sp. nov.; dashed lines indicate host plant cell walls; drawings are based on (a) figure 2a, (b) figure 2f, (c) figure 2e, (d) figure 2i, (e) figure 3j, (f,g) figure 2i and (h) figure 3d.
Rare (total number of specimens less than 10) variants and deviations from the normal basic morphology include specimens with a dumbbell-shaped cavity (figure 5k), others that possess what appears to be a second tube (figure 5l), and still others suggestive of the presence of a small rhizomycelium extending from the proximal side of the thallus (arrows in figure 5p). Moreover, several single specimens are enveloped in a delicate, sac-like structure that is variable in size and shape (arrows in figure 5m–o).
Discussion: The most characteristic feature of P. tayloriana is the inner component comprised a thin-walled cavity from which extends a prominent tube; cavity and tube together resemble a bellied flask, hence the genus name. It is likely that whatever developed within the cavity was liberated through the tube at maturity; specimens that lack a tube probably represent juvenile individuals. However, there is currently no evidence to determine what exactly was produced within the cavity, with the possible exception of several specimens that contain tiny inclusions (referred to as ‘refractive bodies’ in [24]) of unknown nature in the cavity (e.g. black arrow in figure 4i). Unfortunately, these inclusions are far too small to be specifically detailed.
Cavity size and tube width (if a tube is present) are relatively uniform among the specimens, but there is considerable variation with regard to the shape and thickness of the outer component, the form of the cavity and the position and length of the tube (figure 6). Shape and thickness of the outer component, as well as the form of the cavity and the position of the tube, generally appear as functions of the surrounding in which the structure develops, while the length of the tube depends on the thickness of the outer component in the area where the tube is located.
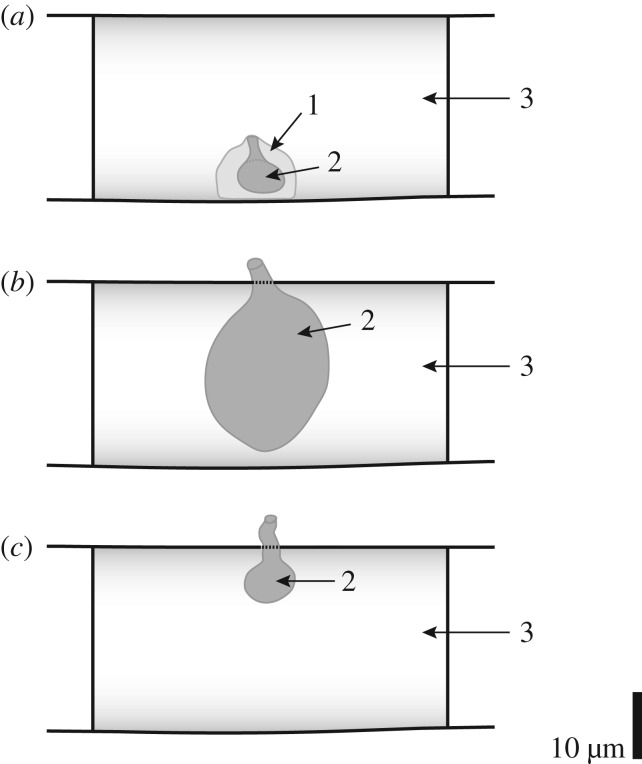
Taylor et al. [24] and Krings et al. [102] suggested that the outer component might be an algal cell or resting stage (e.g. cyst, phycoma), or perhaps a land plant cell that became detached from the source tissue during tissue degradation. The inner component was interpreted as an endobiotic zoosporangium of a holocarpic chytrid, and compared to the zoosporangia of Olpidium, a widespread chytrid parasite of plants and animals today [103,104]. Structurally similar to Olpidium are certain species in the peronosporomycete (oomycete) genus Olpidiopsis that are also parasites (e.g. of algae, fungi and other peronosporomycetes) and produce sporangia within host cells, with discharge tubes liberating the zoospores to the outside of the host cell [105]. However, the specimens described in this paper prompt a different interpretation, namely that the outer component represents an envelope or sheath produced by the organism itself. Support for this interpretation is the wide range of different morphologies, which result from the expansion of the developing structures into the available spaces in the respective places of growth. Especially interesting in this context are the strings of tightly abutting, blunt or cubical specimens that occur exclusively in close proximity to the central strands in certain largely degraded plant axes (figure 5e–j). This peculiar alignment results from the colonization of the limited space in the lumen of the narrow, elongate cortical cells adjacent to the strand. Moreover, several specimens suggest that a small rhizomycelium was produced by P. tayloriana (arrows in figure 5p). As a result, the complement of structural features displayed by P. tayloriana argues against the interpretation as a holocarpic, Olpidium- or Olpidiopsis-like organism. If P. tayloriana were like Olpidium or Olpidiopsis, one would expect to see a host cell containing an endobiotic zoosporangium that releases zoospores to the outside of the host cell via a discharge tube (figure 7b,c) (e.g. [95,104,105]). Rather, the basic morphology of P. tayloriana (figure 7a) is suggestive of a small, epibiotic chytrid zoosporangium attached to a substrate, possibly via a small rhizomycelium, perhaps comparable in basic morphology to certain present-day species in the genus Rhizophydium that are characterized by a gelatinous hull or sheath around the zoosporangium [106]. If the interpretation of the outer component of P. tayloriana as part of the organism itself is correct, then the rare variants shown in figure 5m–o might be specimens that are additionally surrounded by the contracted plasmalemma (arrows) of the host cell.
Figure 7.
Comparison of P. tayloriana gen. et sp. nov. with Olpidium and Olpidiopsis. Basic morphology of P. tayloriana (a) suggestive of epibiotic sporangium (a2) surrounded by gelatinous hull or sheath (a1) and attached to the wall of host cell (a3), whereas Olpidium (b) and Olpidiopsis (c) produce endobiotic sporangia (b2, c2) within host cells (b3, c3) and release zoospores to the outside of host cell via discharge tube.
The nature of the relationship (i.e. parasitic or saprotrophic) between P. tayloriana and land plants cannot be determined, primarily because the fossils described here most certainly represent only one of several stages in the life cycle of this organism, with information on the other stages not currently available. Moreover, the life history and biology of fungi can change based on the presence or absence of a host and the type of host (e.g. [107]). Despite these limitations, we feel confident enough to advance a hypothesis on the nature of the relationship between P. tayloriana and land plants based on the material at hand. We suggest that the organism (perhaps in the form of motile cells or spores) infected living plants or colonized senescent or atrophied plant parts and subsequently participated in the process of biological degradation. As the decomposition of the plant progressed, the P. tayloriana stage of the life cycle developed and attained maturity, and ultimately, the contents (zoospores?) were released from the cavity, leading to large numbers of new individuals that further accelerated the decomposition process. The formation of assemblages and aggregates was perhaps due to dense spacing or because the contents were discharged from the cavity in the form of coherent masses [108]. Support for this hypothesis is the fact that smaller assemblages and aggregates of specimens are usually associated with plant tissue in which some of the cell outlines are still recognizable (figures 4j–l and 5a,b), while the largest specimen clusters (figure 5c,d) occur in plant parts that no longer show cell outlines. Moreover, the presence of plant cell wall fragments in several assemblages and aggregates (arrows in figure 4g,i) indicates that aggregate formation initially required the presence of a host cell wall as a substrate. The finding that specimens are common in the Rhynie chert and occur on multiple substrates suggests that P. tayloriana was an important contributor to the degradation of organic material in the Rhynie ecosystem, and perhaps early terrestrial ecosystems in general, that has been replaced in modern ecosystems with more efficient degraders, i.e. members of the Ascomycota and Basidiomycota.
The thalli of P. tayloriana appear to have been relatively resistant to degradation based on the fact that the specimens remain intact even after complete degradation of the host tissue. This explains why specimens of P. tayloriana sometimes appear to float freely in the chert matrix or among the severely fragmented remains of decomposed plant parts. Free-floating thalli with morphologies characteristic of thalli in assemblages and aggregates suggest that specimen clusterings eventually dissociated.
5. Summary discussion and conclusion
The fossils from the Lower Devonian Rhynie chert that are reviewed and newly described in this paper (figures 1–5 and table 1) constitute the largest body of structurally (including in situ) preserved evidence of fungi and fungal interactions gathered to date from any ancient ecosystem. It comes therefore as no surprise that the Rhynie chert is today widely used as a key reference for past fungal biodiversity and interactions (e.g. [110–114]). Other rocks that have been screened more systematically for fossil fungi include Mississippian and Pennsylvanian cherts from central France, Pennsylvanian coal balls from Great Britain and North America, Permian and Triassic permineralized peat from Antarctica, and the Eocene Princeton chert from Canada (see [8] and references therein). While these deposits all have produced a variety of fungal fossils, including specimens yielding detailed information on biology and interactions (e.g. [115–119]), none come close to the quality of the Rhynie chert fossils [120].
Table 1.
Synopsis of fungal taxa and fungus-like organisms described from the Rhynie chert.
| taxon | suggested systematic affinities | occurrence/substrate | references |
|---|---|---|---|
| fungi | |||
| Cultoraquaticus trewinii Strullu-Derrien | Chytridiomycota | epibiotic on large, spheroidal structures of uncertain affinity | [97] |
| Globicultrix nugax M. Krings, Dotzler & T.N. Taylor | Chytridiomycota | endobiotic in large fungal (probably glomeromycotinan) spores | [92] |
| Glomites rhyniensis T.N. Taylor, Remy, Hass & Kerp | Mucoromycota (Glomeromycotina) | in axes of Ag. majus | [35,54,55] |
| Glomites sporocarpoides Karatygin, Snigirevskaya, K. Demchenko & Zdebska | Mucoromycota (Glomeromycotina) | in axes of R. gwynne-vaughanii and Ag. majus | [68] |
| Helmutella devonica M. Krings & T.N. Taylor | Mucoromycota incertae sedis | free-floating in chert matrix | [48] |
| Illmanomyces corniger M. Krings, T.N. Taylor | Chytridiomycota | epibiotic on fungal (probably glomeromycotinan) spores | [91] |
| Krispiromyces discoides T.N. Taylor, Hass & Remy | Chytridiomycota | epibiotic on Palaeo. cranii | [93] |
| Kryphiomyces catenulatus M. Krings & T.N. Taylor | inconclusive, perhaps Chytridiomycota | endobiotic in large fungal (probably glomeromycotinan) spores | [87] |
| Lyonomyces pyriformis T.N. Taylor, Hass & Remy | Chytridiomycota | epibiotic on Palaeo. cranii | [93] |
| Milleromyces rhyniensis T.N. Taylor, Hass & Remy | Chytridiomycota | endobiotic in Palaeo. cranii | [93] |
| Mycocarpon rhyniense M. Krings, T.N. Taylor, E.L. Taylor, H. Kerp & Dotzler | Mucoromycota incertae sedis | free-floating in chert matrix | [47] |
| Mycokidstonia sphaerialoides D. Pons et Locq. | Mucoromycota (Glomeromycotina) see 45 | free-floating in chert matrix | [109] |
| Palaeoendogone gwynne-vaughaniae Strullu-Derrien & Strullu | Mucoromycota (Mucoromycotina) | in rhizomes of H. lignieri | [71] |
| Palaeoglomus boullardii Strullu-Derrien & Strullu | Mucoromycota (Glomeromycotina) | in aerial axes of H. lignieri | [71] |
| Palaeomyces agglomeratus Kidst. & W.H. Lang | Mucoromycota incertae sedis | in aerial axes of R. gwynne-vaughanii and surrounding chert matrix | [9] |
| Palaeomyces asteroxyli Kidst. & W.H. Lang | Mucoromycota incertae sedis | in intact and decayed tissue of As. mackiei | [9] |
| Palaeomyces gordonii Kidst. & W.H. Lang (incl. P. gordonii var. major Kidst. & W.H. Lang) | Mucoromycota incertae sedis | in axes of As. mackiei, free-floating in chert matrix | [9] |
| Palaeomyces horneae Kidst. & W.H. Lang | Mucoromycota incertae sedis | in rhizomes and aerial axes of H. lignieri | [9] |
| Palaeomyces simpsonii Kidst. & W.H. Lang | Mucoromycota incertae sedis | in decayed axes of R. gwynne-vaughanii | [9] |
| Palaeomyces vestitus Kidst. & W.H. Lang | see Z. vestitus | ||
| Palaeozoosporites renaultii Strullu-Derrien | zoosporic Fungi incertae sedis, perhaps Blastocladiomycota | endobiotic in rhizomes of As. mackiei | [77] |
| Paleoblastocladia milleri Remy, T.N. Taylor & Hass | Blastocladiomycota | epibiotic on partially degraded axes of Ag. majus | [82] |
| Paleopyrenomycites devonicus T.N. Taylor, Hass, Kerp, M. Krings & Hanlin | Ascomycota | in aerial axes and lateral portions of As. mackiei | [78,79] |
| Perexiflasca tayloriana M. Krings, C.J. Harper & E.L. Taylor | inconclusive, perhaps Chytridiomycota | in intact and degraded land plant tissue, on fungal spores and hyphae, free-floating in chert matrix | this paper |
| Scepasmatocarpion fenestrulatum M. Krings & T.N. Taylor | inconclusive, perhaps Mucoromycota or Ascomycota | free-floating in microbial mats | [52] |
| Scutellosporites devonicus Dotzler, M. Krings, T.N. Taylor & Agerer | Mucoromycota (Glomeromycotina) | in aerial axes of As. mackiei | [42] |
| Trewinomyces annulifer M. Krings, T.N. Taylor & H. Martin | inconclusive, perhaps Chytridiomycota or Blastocladiomycota | epibiotic on partially degraded land plant axes | [85] |
| Zwergimyces vestitus (Kidst. & W.H. Lang) M. Krings & T.N. Taylor | Mucoromycota incertae sedis | in intact and degraded land plant tissues, in chert litter layers | [40,46] |
| Lichen-like associations | |||
| Winfrenatia reticulata T.N. Taylor, Hass & Kerp | mycobiont: Mucoromycota photobiont: Cyanobacteria | inconclusive, probably on hard terrestrial substrate | [99,100] |
| Peronosporomycetes | |||
| Frankbaronia polyspora M. Krings, T.N. Taylor, E.L. Taylor, Kerp, Hass, Dotzler & C.J. Harper | Peronosporomycetes (Oomycota) | free-floating in microbial mats and litter layers | [20] |
| Frankbaronia velata M. Krings, T.N. Taylor, Dotzler & C.J. Harper | Peronosporomycetes (Oomycota) | free-floating in microbial mats | [21] |
| Hassiella monospora T.N. Taylor, M. Krings & Kerp | Peronosporomycetes (Oomycota) | free-floating in chert matrix | [19] |
| Nematophytes | |||
| Nematophyton taiti Kidst. & W.H. Lang | Nematophyta | inconclusive, probably on hard substrate | [9] |
| Nematoplexus rhyniensis Lyon | Nematophyta | inconclusive, probably on hard substrate | [22] |
Because of the sublime preservation, the Rhynie chert fungi have played, and continue to play a major role in shaping our perception of the diversity of fungi in ancient non-marine ecosystems and the roles that these organisms played in the biology of early plant life on land [121–126]. However, geothermal ecosystems today are remarkably rich in fungi, and plants growing in these environments often harbour diverse communities of fungi [127–130], suggesting that the fossils described to date from the Rhynie chert represent only a small segment of the fungal diversity that was actually present in the Rhynie ecosystem. The same is probably true of other microbial life (e.g. cyanobacteria, algae) in the Rhynie chert that remains generally understudied [18]. Moreover, all fungi are carbon-heterotrophic, and thus required to exploit dead organic matter and/or interact with other ecosystem constituents to obtain carbon, suggesting that the fungal interactions recorded to date from the Rhynie chert also represent only a small portion of the actual diversity [90]. The fossils of P. tayloriana detailed above, together with other, recently described minute life forms such as the cyanobacterium Rhyniosarcina devonica [13] and the alga Hagenococcus aggregatus [18], demonstrate that there is still tremendous unreported biodiversity in the Rhynie chert, and that it remains worthwhile to analyse the chert in search for new organisms.
Detailed descriptions of fossil fungi and fungal interactions represent valuable resources that can be used to not only assess past biodiversity and ecosystem complexity through the patterns and processes resulting from associations and interactions between individuals, populations, species and communities (e.g. [131–133]), but also define minimum ages for various lineages of fungi and calibrate molecular clocks (e.g. [134–136]). It is becoming increasingly clear that the Rhynie chert comprises different (micro-)facies characterized by communities of organisms that reflect once differing types of (micro-)habitats [30]. Drill core data suggest that there are greater than 50 fossiliferous chert layers [27,30,137], and the number of distinctive environments preserved in these layers is probably even larger. Future concerted research with all of the chert lenses will be necessary in order to catalogue the full complement of organisms and (micro-)habitats that existed in this Early Devonian hot spring ecosystem, and characterize the distinct communities and environments. Recent discoveries, including P. tayloriana, indicate that screening the material by using the highest possible magnification, albeit time-consuming, will open an entirely new window into the diversity of microbial life that populated the Rhynie ecosystem.
Acknowledgements
We are indebted to Hans Kerp and Hagen Hass (Münster, Germany) for continued collaboration, valuable discussion and the permission to use images from the Münster Rhynie chert collection. Special thanks to Nora Dotzler, Stefan Sónyi and Helmut Martin (all Munich, Germany) for technical assistance, and two anonymous referees for insightful comments and suggestions on the manuscript.
Data accessibility
All thin sections and original digital images of P. tayloriana are deposited in the Bayerische Staatssammlung für Paläontologie und Geologie (SNSB-BSPG), Munich, Germany. Additional material is housed in the Abteilung Paläobotanik, Geologisch-Paläontologisches Institut, Westfälische Wilhelms-Universität, Münster, Germany (prefix P).
Authors' contributions
All authors contributed equally.
Competing interests
We declare we have no competing interests.
Funding
Financial support was provided by National Science Foundation (NSF grant no. EAR-0949947) to M.K. and E.L.T., Deutsche Forschungsgemeinschaft (DFG grant no. Ke 584/13-2) to M.K. and Alexander von Humboldt-Foundation (3.1-USA/1160852 STP) to C.J.H.
References
- 1.Kidston R, Lang WH. 1917. On Old Red Sandstone plants showing structure, from the Rhynie Chert Bed, Aberdeenshire. Part I. Rhynia Gwynne-Vaughani, Kidston and Lang. Trans. R. Soc. Edinb. 51, 761–784. ( 10.1017/S0080456800008991) [DOI] [Google Scholar]
- 2.Kidston R, Lang WH. 1920. On Old Red Sandstone plants showing structure, from the Rhynie Chert Bed, Aberdeenshire. Part II. Additional notes on Rhynia Gwynne-Vaughani, Kidston and Lang; with descriptions of Rhynia major, n.sp., and Hornea lignieri, n.g. n.sp. Trans. R. Soc. Edinb. 52, 603–627. ( 10.1017/S0080456800004488) [DOI] [Google Scholar]
- 3.Kidston R, Lang WH. 1920. On Old Red Sandstone plants showing structure, from the Rhynie Chert Bed, Aberdeenshire. Part III. Asteroxylon Mackiei, Kidston and Lang. Trans. R. Soc. Edinb. 52, 643–680. ( 10.1017/S0080456800004506) [DOI] [Google Scholar]
- 4.Kidston R, Lang WH. 1921. On Old Red Sandstone plants showing structure, from the Rhynie Chert Bed, Aberdeenshire. Part IV. Restorations of the vascular cryptogams, and discussion of their bearing on the general morphology of the Pteridophyta and the origin of the organisation of land-plants. Trans. R. Soc. Edinb. 52, 831–854. ( 10.1017/S0080456800016033) [DOI] [Google Scholar]
- 5.Kerp H, Hass H. 2004. De Onder-Devonische Rhynie Chert—het oudste en meest compleet bewaard gebleven terrestrische ecosysteem. Grondboor and Hamer 58, 33–50. [Google Scholar]
- 6.Trewin NH, Rice CM (eds) 2003. The rhynie hot spings system: geology, biota and mineralisation (Transactions of the Royal Society of Edinburgh, Earth Sciences 94). Edinburgh, Scotland: The Royal Society of Edinburgh Scotland Foundation. [Google Scholar]
- 7.Dotzler N, Krings M, Kerp H, Hass H, Agerer R, Taylor TN. 2009. Mikroorganismen vor 400 Millionen Jahren, perfekt erhalten im unterdevonischen Rhynie Chert. Jahresber. 2008 Mitt. Freunde Bayer. Staatslg. Paläont. Hist. Geol. München e.V. 37, 49–62. [Google Scholar]
- 8.Taylor TN, Krings M, Taylor EL. 2015. Fossil fungi, 1st edn Amsterdam, the Netherlands: Elsevier. [Google Scholar]
- 9.Kidston R, Lang WH. 1921. On Old Red Sandstone plants showing structure, from the Rhynie Chert Bed, Aberdeenshire. Part V. The Thallophyta occurring in the peat-bed; the succession of the plants throughout a vertical section of the bed, and the conditions of accumulation and preservation of the deposit. Trans. R. Soc. Edinb. 52, 855–902. ( 10.1017/S0080456800016045) [DOI] [Google Scholar]
- 10.Croft WN, George EA. 1959. Blue-green algae from the middle Devonian of Rhynie, Aberdeenshire. Bull. Br. Mus. (Nat. Hist.), Geol. 3, 341–353. [Google Scholar]
- 11.Krings M, Kerp H, Hass H, Taylor TN, Dotzler N. 2007. A filamentous cyanobacterium showing structured colonial growth from the Early Devonian Rhynie chert. Rev. Palaeobot. Palynol. 146, 265–276. ( 10.1016/j.revpalbo.2007.05.002) [DOI] [Google Scholar]
- 12.Krings M, Hass H, Kerp H, Taylor TN, Agerer R, Dotzler N. 2009. Endophytic cyanobacteria in a 400-million-yr-old land plant: a scenario for the origin of a symbiosis? Rev. Palaeobot. Palynol. 153, 62–69. ( 10.1016/j.revpalbo.2008.06.006) [DOI] [Google Scholar]
- 13.Taylor TN, Krings M. 2015. A colony-forming microorganism with probable affinities to the Chroococcales (Cyanobacteria) from the Lower Devonian Rhynie chert. Rev. Palaeobot. Palynol. 219, 147–156. ( 10.1016/j.revpalbo.2015.04.003) [DOI] [Google Scholar]
- 14.Edwards DS, Lyon AG. 1983. Algae from the Rhynie chert. Bot. J. Linn. Soc. 86, 37–55. ( 10.1111/j.1095-8339.1983.tb00716.x) [DOI] [Google Scholar]
- 15.Dotzler N, Taylor TN, Krings M. 2007. A prasinophycean alga of the genus Cymatiosphaera in the Early Devonian Rhynie chert. Rev. Palaeobot. Palynol. 147, 106–111. ( 10.1016/j.revpalbo.2007.07.001) [DOI] [Google Scholar]
- 16.Kustatscher E, Dotzler N, Taylor TN, Krings M. 2014. Microalgae from the Lower Devonian Rhynie chert: a new Cymatiosphaera. Zitteliana A 54, 165–169. [Google Scholar]
- 17.Kustatscher E, Dotzler N, Taylor TN, Krings M. 2014. Microfossils with suggested affinities to the Pyramimonadales (Pyramimonadophyceae, Chlorophyta) from the Lower Devonian Rhynie chert. Acta Palaeobot. 54, 163–171. ( 10.2478/acpa-2014-0010) [DOI] [Google Scholar]
- 18.Krings M, Kerp H, Taylor EL, Harper CJ.. 2017. Hagenococcus aggregatus nov. gen. et sp., a microscopic, colony-forming alga from the 410-million-yr-old Rhynie chert. Nova Hedwig. 105, 205–217. [Google Scholar]
- 19.Taylor TN, Krings M, Kerp H. 2006. Hassiella monospora gen. et sp. nov., a microfungus from the 400 million year old Rhynie chert. Mycol. Res. 110, 628–632. ( 10.1016/j.mycres.2006.02.009) [DOI] [PubMed] [Google Scholar]
- 20.Krings M, Taylor TN, Taylor EL, Kerp H, Hass H, Dotzler N, Harper CJ. 2012. Microfossils from the Lower Devonian Rhynie Chert with suggested affinities to the peronosporomycetes. J. Paleontol. 86, 358–367. ( 10.1666/11-087.1) [DOI] [Google Scholar]
- 21.Krings M, Taylor TN, Dotzler N, Harper CJ. 2013. Frankbaronia velata nov. sp., a putative peronosporomycete oogonium containing multiple oospores from the Lower Devonian Rhynie chert. Zitteliana A 53, 23–30. [Google Scholar]
- 22.Lyon AG. 1962. On the fragmentary remains of an organism referable to the Nematophytales, from the Rhynie chert, ‘Nematoplexus rhyniensis' gen. et. sp. nov. Trans. R. Soc. Edinb. 65, 79–87. ( 10.1017/S0080456800012382) [DOI] [Google Scholar]
- 23.Weiss HJ.2004. (updated 2010) Enigmatic little sphere. Rhynie chert News 1 See http://www.chertnews.de/Pachytheca.html .
- 24.Taylor TN, Remy W, Hass H. 1992. Fungi from the lower Devonian Rhynie chert: Chytridiomycetes. Am. J. Bot. 79, 1233–1241. ( 10.2307/2445050) [DOI] [PubMed] [Google Scholar]
- 25.Rice CM, Trewin NH, Anderson LI. 2002. Geological setting of the Early Devonian Rhynie cherts, Aberdeenshire, Scotland: an early terrestrial hot spring system. J. Geol. Soc. 159, 203–214. ( 10.1144/0016-764900-181) [DOI] [Google Scholar]
- 26.Parry SF, Noble SR, Crowley QG, Wellman CH. 2011. A high precision U-Pb age constraint on the Rhynie chert Konservat-Lagerstätte: time scale and other implications. J. Geol. Soc. 168, 863–872. ( 10.1144/0016-76492010-043) [DOI] [Google Scholar]
- 27.Channing A, Edwards D. 2009. Yellowstone hot spring environments and the palaeo-ecophysiology of Rhynie chert plants: towards a synthesis. Plant Ecol. Divers. 2, 111–143. ( 10.1080/17550870903349359) [DOI] [Google Scholar]
- 28.Channing A, Edwards D. 2009. Silicification of higher plants in geothermally influenced wetlands: yellowstone as a Lower Devonian Rhynie analog. Palaios 24, 505–521. ( 10.2110/palo.2008.p08-131r) [DOI] [Google Scholar]
- 29.Channing A, Edwards D. 2013. Wetland megabias: ecological and ecophysiological filtering dominates the fossil record of hot spring floras. Palaeontology 56, 523–556. ( 10.1111/pala.12043) [DOI] [Google Scholar]
- 30.Trewin NH, Fayers SR. 2016. Macro to micro aspects of the plant preservation in the Early Devonian Rhynie cherts, Aberdeenshire, Scotland. Earth Environ. Sci. Trans. R. Soc. Edinb. 106, 67–80. ( 10.1017/S1755691016000025) [DOI] [Google Scholar]
- 31.Wellman CH. 2006. Spore assemblages from the Lower Devonian ‘Lower Old Red Sandstone’ deposits of the Rhynie outlier, Scotland. Trans. R. Soc. Edinb. Earth Sci. 97, 167–211. ( 10.1017/S0263593300001449) [DOI] [Google Scholar]
- 32.Wellman CH, Kerp H, Hass H. 2006. Spores of the Rhynie chert plant Aglaophyton (Rhynia) major (Kidston and Lang) D.S. Edwards, 1986. Rev. Palaeobot. Palynol. 142, 229–250. ( 10.1016/j.revpalbo.2006.04.009) [DOI] [Google Scholar]
- 33.Mark DF, Rice CM, Fallick AE, Trewin NH, Lee MR, Boyce A, Lee JKW. 2011. 40Ar/39Ar dating of hydrothermal activity, biota and gold mineralization in the Rhynie hot-spring system, Aberdeenshire, Scotland. Geochim. Cosmochim. Acta 75, 555–569. ( 10.1016/j.gca.2010.10.014) [DOI] [Google Scholar]
- 34.Mark DF, Rice CM, Trewin NH. 2013. Discussion on ‘A high-precision U-Pb age constraint on the Rhynie Chert Konservat-Lagerstätte: time scale and other implications'. J. Geol. Soc. 170, 701–703. ( 10.1144/jgs2011-110) [DOI] [Google Scholar]
- 35.Taylor TN, Remy W, Hass H, Kerp H. 1995. Fossil arbuscular mycorrhiza from the Early Devonian. Mycologia 87, 560–573. ( 10.2307/3760776) [DOI] [Google Scholar]
- 36.Thompson W, Rayner ADM. 1983. Extent development and functioning of mycelial cord systems in soil. Trans. Br. Mycol. Soc. 81, 333–345. ( 10.1016/S0007-1536(83)80085-0) [DOI] [Google Scholar]
- 37.Cairney JWG. 1992. Translocation of solutes in ectomycorrhizal and saprotrophic rhizomorphs. Mycol. Res. 96, 135–141. ( 10.1016/S0953-7562(09)80928-3) [DOI] [Google Scholar]
- 38.Jennings DH. 1994. Translocation in mycelia. In The mycota, vol. 1. Growth, differentiation and sexuality (eds Wessels JGH, Meinhardt F), pp. 163–173. Berlin, Germany:Springer. [Google Scholar]
- 39.Tlalka M, Bebber DP, Darrah P, Watkinson SC. 2008. Mycelial networks: nutrient uptake, translocation, and role in ecosystems. In Ecology of saprotrophic basidiomycetes (eds Boddy L, Frankland J, van West P), pp. 43–62. Amsterdam, the Netherlands: Academic Press. [Google Scholar]
- 40.Krings M, Taylor TN, Dotzler N, Harper CJ. 2016. Morphology and ontogenetic development of Zwergimyces vestitus, a fungal reproductive unit enveloped in a hyphal mantle from the Lower Devonian Rhynie chert. Rev. Palaeobot. Palynol. 228, 47–56. ( 10.1016/j.revpalbo.2016.01.005) [DOI] [Google Scholar]
- 41.Sharma BD, Bohra DR, Harsh R. 1993. Vesicular arbuscular mycorrhizae association in Lower Devonian plants of the Rhynie chert. Phytomorphology 43, 105–110. [Google Scholar]
- 42.Dotzler N, Krings M, Taylor TN, Agerer R. 2006. Germination shields in Scutellospora (Glomeromycota: Diversisporales, Gigasporaceae) from the 400 million-year-old Rhynie chert. Mycol. Progr. 5, 178–184. ( 10.1007/s11557-006-0511-z) [DOI] [Google Scholar]
- 43.Souza T. 2015. Handbook of arbuscular mycorrhizal fungi. Cham, Heidelberg, New York, Dordrecht, London: Springer. [Google Scholar]
- 44.Dotzler N, Walker C, Krings M, Hass H, Kerp H, Taylor TN, Agerer R. 2009. Acaulosporoid glomeromycotan spores with a germination shield from the 400-million-year-old Rhynie chert. Mycol. Progr. 8, 9–18. ( 10.1007/s11557-008-0573-1) [DOI] [Google Scholar]
- 45.Krings M, Walker C, Harper CJ, Martin H, Sónyi S, Kustatscher E, Taylor TN.. 2017. Unusual fungal reproductive units from the Lower Devonian Rhynie chert. Zitteliana 89, 29–37. [Google Scholar]
- 46.Krings M, Taylor TN. 2013. Zwergimyces vestitus (Kidston et W.H. Lang) nov. comb., a fungal reproductive unit enveloped in a hyphal mantle from the Lower Devonian Rhynie chert. Rev. Palaeobot. Palynol. 190, 15–19. ( 10.1016/j.revpalbo.2012.11.008) [DOI] [Google Scholar]
- 47.Krings M, Taylor TN, Taylor EL, Kerp H, Dotzler N. 2014. First record of a fungal ‘sporocarp’ from the Lower Devonian Rhynie chert. Palaeobiodiv. Palaeoenviron. 94, 221–227. ( 10.1007/s12549-013-0135-7) [DOI] [Google Scholar]
- 48.Krings M, Taylor TN. 2014. A mantled fungal reproductive unit from the Lower Devonian Rhynie chert that demonstrates Carboniferous ‘sporocarp’ morphology and development. N. Jb. Geol. Paläontol., Abh. 273, 197–205. ( 10.1127/0077-7749/2014/0423) [DOI] [Google Scholar]
- 49.Stubblefield SP, Taylor TN, Miller CE, Cole GT. 1983. Studies of Carboniferous fungi. II. The structure and organization of Mycocarpon, Sporocarpon, Dubiocarpon and Coleocarpon (Ascomycotina). Am. J. Bot. 70, 1482–1498. ( 10.2307/2443347) [DOI] [Google Scholar]
- 50.Krings M, Taylor TN, Dotzler N. 2013. Fossil evidence of the zygomycetous fungi. Persoonia 30, 1–10. ( 10.3767/003158513X664819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krings M, Taylor TN. 2015. Mantled fungal reproductive units in land plant tissue from the Lower Devonian Rhynie chert. Bull. Geosci. 90, 1–6. ( 10.3140/bull.geosci.1519) [DOI] [Google Scholar]
- 52.Krings M, Taylor TN. 2015. A fungal reproductive unit from the Lower Devonian Rhynie chert (Aberdeenshire, Scotland) that demonstrates an unusual hyphal investment pattern. Scot. J. Geol. 51, 131–139. ( 10.1144/sjg2014-026) [DOI] [Google Scholar]
- 53.Strullu-Derrien C, Strullu DG. 2007. Mycorrhization of fossil and living plants. C.R. Palevol 6, 483–494. ( 10.1016/j.crpv.2007.09.006) [DOI] [Google Scholar]
- 54.Remy W, Taylor TN, Hass H, Kerp H. 1994. Four hundred-million-year-old vesicular arbuscular mycorrhizae. Proc. Natl Acad. Sci. USA 91, 11 841–11 843. ( 10.1073/pnas.91.25.11841) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor TN, Kerp H, Hass H. 2005. Life history biology of early land plants: Deciphering the gametophyte phase. Proc. Natl Acad. Sci. USA 102, 5892–5897. ( 10.1073/pnas.0501985102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Remy W, Hass H. 1996. New information on gametophytes and sporophytes of Aglaophyton major and inferences about possible environmental adaptations. Rev. Palaeobot. Palynol. 90, 175–193. ( 10.1016/0034-6667(95)00082-8) [DOI] [Google Scholar]
- 57.Harrier LA. 2001. The arbuscular mycorrhizal symbiosis: a molecular review of the fungal dimension. J. Exp. Bot. 52(Suppl. 1), 469–478. ( 10.1093/jxb/52.suppl_1.469) [DOI] [PubMed] [Google Scholar]
- 58.Helgason T, Fitter A. 2005. The ecology and evolution of the arbuscular mycorrhizal fungi. Mycologist 19, 96–101. ( 10.1017/S0269-915X(05)00302-2) [DOI] [Google Scholar]
- 59.Parniske M. 2008. Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat. Rev. Microbiol. 6, 763–775. ( 10.1038/nrmicro1987) [DOI] [PubMed] [Google Scholar]
- 60.Pirozynski KA, Malloch DW. 1975. The origin of land plants: a matter of mycotrophism. Biosystems 6, 153–164. ( 10.1016/0303-2647(75)90023-4) [DOI] [PubMed] [Google Scholar]
- 61.Bonfante P, Selosse MA.. 2010. A glimpse into the past of land plants and of their mycorrhizal affairs: from fossils to evo-devo. New Phytol. 186, 267–270. ( 10.1111/j.1469-8137.2010.03196.x) [DOI] [PubMed] [Google Scholar]
- 62.Brundrett MC. 2002. Coevolution of roots and mycorrhizas of land plants. New Phytol. 154, 275–304. ( 10.1046/j.1469-8137.2002.00397.x) [DOI] [PubMed] [Google Scholar]
- 63.Humphreys CP, Franks PJ, Rees M, Bidartondo MI, Leake JR, Beerling DJ.. 2010. Mutualistic mycorrhiza-like symbiosis in the most ancient group of land plants. Nat. Commun. 1, 103 ( 10.1038/ncomms1105) [DOI] [PubMed] [Google Scholar]
- 64.Bidartondo MI, Read DJ, Trappe JM, Merckx V, Ligrone R, Duckett JG. 2011. The dawn of symbiosis between plants and fungi. Biol. Lett. 7, 574–577. ( 10.1098/rsbl.2010.1203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Delaux PM, et al. 2015. Algal ancestor of land plants was preadapted for symbiosis. Proc. Natl Acad. Sci. USA 112, 13 390–13 395. ( 10.1073/pnas.1515426112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weiblen GD, Treiber EL. 2015. Evolutionary origins and diversification of mutualism. In Mutualism (ed. Bronstein JL.), pp. 37–56. Oxford, UK: Oxford University Press. [Google Scholar]
- 67.Boullard B, Lemoigne Y. 1971. Les champignons endophytes du Rhynia gwynne-vaughanii K. et L. Étude morphologique et déductions sur leur biologie. Botaniste 54, 49–89. [Google Scholar]
- 68.Karatygin IV, Snigirevskaya NS, Demchenko KN. 2006. Species of the genus Glomites as plant mycobionts in Early Devonian ecosystems. Paleontol. J. 40, 572–579. ( 10.1134/S0031030106050121) [DOI] [Google Scholar]
- 69.Krings M, Taylor TN, Hass H, Kerp H, Dotzler N, Hermsen E.J. 2007. An alternative mode of early land plant colonization by putative endomycorrhizal fungi. Plant Signal. Behav. 2, 125–126. ( 10.4161/psb.2.2.3970) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krings M, Taylor TN, Hass H, Kerp H, Dotzler N, Hermsen EJ. 2007. Fungal endophytes in a 400-million-yr-old land plant: infection pathways, spatial distribution, and host responses. New Phytol. 174, 648–657. ( 10.1111/j.1469-8137.2007.02008.x) [DOI] [PubMed] [Google Scholar]
- 71.Strullu-Derrien C, Kenrick P, Pressel S, Duckett JG, Rioult JP, Strullu DG.. 2014. Fungal associations in Horneophyton ligneri from the Rhynie chert (c. 407 million year old) closely resemble those in extant lower land plants: novel insights into ancestral plant–fungus symbioses. New Phytol. 203, 964–979. ( 10.1111/nph.12805) [DOI] [PubMed] [Google Scholar]
- 72.Rimington WR, Pressel S, Duckett JG, Bidartondo MI. 2015. Fungal associations of basal vascular plants: reopening a closed book? New Phytol. 205, 1394–1398. ( 10.1111/nph.13221) [DOI] [PubMed] [Google Scholar]
- 73.Rimington WR, Pressel S, Field KJ, Strullu-Derrien C, Duckett JG, Bidartondo MI. 2017. Reappraising the origin of mycorrhizas. In Molecular mycorrhizal symbioses (ed. Martin F.), pp. 21–32. Hoboken, NJ: Wiley Blackwell. [Google Scholar]
- 74.Strullu-Derrien C, Kenrick P, Selosse MA. 2017. Origins of the mycorrhizal symbioses. In Molecular mycorrhizal symbiosis, pp. 3–20. Hoboken, NJ: Wiley Blackwell. [Google Scholar]
- 75.Harvey R, Lyon AG, Lewis PN. 1969. A fossil fungus from Rhynie chert. Trans. Br. Mycol. Soc. 53, 155–156. ( 10.1016/S0007-1536(69)80025-2) [DOI] [Google Scholar]
- 76.Illman WI. 1984. Zoosporic fungal bodies in the spores of the Devonian fossil vascular plant, Horneophyton. Mycologia 76, 545–547. ( 10.2307/3793338) [DOI] [Google Scholar]
- 77.Strullu-Derrien C, Wawrzyniak Z, Goral T, Kenrick P. 2015. Fungal colonization of the rooting system of the early land plant Asteroxylon mackiei from the 407-Myr-old Rhynie chert (Scotland, UK). Bot. J. Linn. Soc. 179, 201–213. ( 10.1111/boj.12307) [DOI] [Google Scholar]
- 78.Taylor TN, Hass H, Kerp H. 1999. The oldest fossil ascomycetes. Nature 399, 648 ( 10.1038/21349) [DOI] [PubMed] [Google Scholar]
- 79.Taylor TN, Hass H, Kerp H, Krings M, Hanlin RT. 2005. Perithecial ascomycetes from the 400 million year old Rhynie chert: An example of ancestral polymorphism. Mycologia 97, 269–285. ( 10.1080/15572536.2006.11832862) [DOI] [PubMed] [Google Scholar]
- 80.Taylor JW, Berbee ML. 2006. Dating divergences in the Fungal tree of life: review and new analyses. Mycologia 98, 838–849. ( 10.1080/15572536.2006.11832614) [DOI] [PubMed] [Google Scholar]
- 81.Lücking R, Huhndorf S, Pfister DH, Plata ER, Lumbsch HT. 2009. Fungi evolved right on track. Myologia 101, 810–822. ( 10.3852/09-016) [DOI] [PubMed] [Google Scholar]
- 82.Remy W, Taylor TN, Hass H. 1994. Early Devonian fungi: a blastocladalean fungus with sexual reproduction. Am. J. Bot. 81, 690–702. ( 10.2307/2445647) [DOI] [Google Scholar]
- 83.Emerson R. 1941. An experimental study of the life cycles and taxonomy of Allomyces . Lloydia 4, 77–144. [Google Scholar]
- 84.Emerson R, Robertson JA. 1974. Two new members of the Blastocladiaceae. I. Taxonomy, with an evaluation of genera and interrelationships in the family. Am. J. Bot. 61, 303–317. ( 10.2307/2441610) [DOI] [Google Scholar]
- 85.Krings M, Taylor TN, Martin H. 2016. An enigmatic fossil fungus from the 410 Ma Rhynie chert that resembles Macrochytrium (Chytridiomycota) and Blastocladiella (Blastocladiomycota). Mycologia 108, 303–312. ( 10.3852/15-224) [DOI] [PubMed] [Google Scholar]
- 86.Hass H, Taylor TN, Remy W. 1994. Fungi from the Lower Devonian Rhynie chert: mycoparasitism. Am. J. Bot. 81, 29–37. ( 10.2307/2445559) [DOI] [PubMed] [Google Scholar]
- 87.Krings M, Dotzler N, Longcore JE, Taylor TN. 2010. An unusual microfungus in a fungal spore from the Lower Devonian Rhynie chert. Palaeontology 53, 753–759. ( 10.1111/j.1475-4983.2010.00959.x) [DOI] [Google Scholar]
- 88.Krings M, Taylor TN, Kerp H, Walker C. 2015. Deciphering interfungal relationships in the 410-million-yr-old Rhynie chert: Sporocarp formation in glomeromycotan spores. Geobios 48, 449–458. ( 10.1016/j.geobios.2015.09.003) [DOI] [Google Scholar]
- 89.Krings M, Taylor TN. 2014. Deciphering interfungal relationships in the 410-million-yr-old Rhynie chert: an intricate interaction between two mycelial fungi. Symbiosis 64, 53–61. ( 10.1007/s13199-014-0302-2) [DOI] [Google Scholar]
- 90.Harper CJ, Krings M, Dotzler N, Taylor EL, Taylor TN. 2017. Deciphering interfungal relationships in the 410-million-yr-old Rhynie chert: Morphology and development of vesicle-colonizing microfungi. Geobios 50, 9–22. ( 10.1016/j.geobios.2016.11.003) [DOI] [Google Scholar]
- 91.Krings M, Taylor TN. 2014. An unusual fossil microfungus with suggested affinities to the Chytridiomycota from the Lower Devonian Rhynie chert. Nova Hedwig. 99, 403–412. ( 10.1127/0029-5035/2014/0205) [DOI] [Google Scholar]
- 92.Krings M, Dotzler N, Taylor TN. 2009. Globicultrix nugax nov. gen. et nov. spec. (Chytridiomycota), an intrusive microfungus in fungal spores from the Rhynie chert. Zitteliana A 48/49, 165–170. [Google Scholar]
- 93.Taylor TN, Hass H, Remy W. 1992. Devonian fungi: Interactions with the green alga Palaeonitella. Mycologia 84, 901–910. ( 10.2307/3760288) [DOI] [Google Scholar]
- 94.Sparrow FK., Jr 1960. Aquatic phycomycetes, 2nd edn Ann Arbor, MI: University of Michigan Press. [Google Scholar]
- 95.Karling JS. 1977. Chytridiomycetarum iconographia: An illustrated and brief descriptive guide to the chytridiomycetous genera with a supplement of the hyphochytridiomycetes. Monticello, NY: Lubrecht and Cramer. [Google Scholar]
- 96.Karling JS. 1928. Studies in the Chytridiales III. A parasitic chytrid causing cell hypertrophy in Chara. Am. J. Bot. 15, 485–496. ( 10.2307/2435797) [DOI] [Google Scholar]
- 97.Strullu-Derrien C, Goral T, Longcore JE, Olesen J, Kenrick P, Edgecombe GD.. 2016. A new chytridiomycete fungus intermixed with crustacean resting eggs in a 407-Million-year-old continental freshwater environment. PLoS ONE 11, e0167301 ( 10.1371/journal.pone.0167301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Habgood KS, Hass H, Kerp H. 2003. Evidence for an early terrestrial food web: coprolites from the Early Devonian Rhynie chert. Trans. R. Soc. Edinb. Earth Sci. 94, 371–389. ( 10.1017/S0263593300000754) [DOI] [Google Scholar]
- 99.Taylor TN, Hass H, Kerp H. 1997. A cyanolichen from the Lower Devonian Rhynie chert. Am. J. Bot. 84, 992–1004. ( 10.2307/2446290) [DOI] [PubMed] [Google Scholar]
- 100.Karatygin IV, Snigirevskaya NS, Vikulin SV. 2009. The most ancient terrestrial lichen Winfrenatia reticulata: a new find and new interpretation. Paleontol. J. 43, 107–114. ( 10.1134/S0031030109010110) [DOI] [Google Scholar]
- 101.Selden PA, Nudds JR. 2012. Evolution of fossil ecosystems, 2nd edn London, UK: Manson Publishing Ltd. [Google Scholar]
- 102.Krings M, Dotzler N, Galtier J, Taylor TN. 2009. Microfungi from the upper Visean (Mississippian) of central France: Chytridiomycota and chytrid-like remains of uncertain affinity. Rev. Palaeobot. Palynol. 156, 319–328. ( 10.1016/j.revpalbo.2009.03.011) [DOI] [Google Scholar]
- 103.Kusano S. 1936. On the parasitism of Olpidium. Jap. J. Bot. 8, 155–187. [Google Scholar]
- 104.Sahtiyanci S. 1962. Studien über einige wurzelparasitäre Olpidiaceen. Arch. Mikrobiol. 41, 187–228. ( 10.1007/BF00409505) [DOI] [Google Scholar]
- 105.Karling JS. 1981. Predominantly holocarpic and eucarpic simple biflagellate phycomycetes, 2nd edn Vaduz, Liechtenstein: J. Cramer. [Google Scholar]
- 106.Letcher PM, Powell MJ. 2012. A taxonomic summary and revision of Rhizophydium (Rhizophydiales, Chytridiomycota). Tuscaloosa, AL: University Printing, The University of Alabama. [Google Scholar]
- 107.Grosshart HP, Wurzbacher C, James TY, Kagami M. 2016. Discovery of dark matter fungi in aquatic ecosystems demands a reappraisal of the phylogeny and ecology of zoosporic fungi. Fung. Ecol. 19, 28–38. ( 10.1016/j.funeco.2015.06.004) [DOI] [Google Scholar]
- 108.Hanson AM. 1944. Three new saprophytic chytrids. Torreya 44, 30–33. [Google Scholar]
- 109.Pons D, Locquin MV. 1981. Mycokidstonia sphaerialoides Pons & Locquin, gen. et sp. nov., Ascomycètes fossile Dévonien. Cah. Micropaléontol. 1, 101–104. [Google Scholar]
- 110.Stockland JN, Siltonen J, Jonsson BG.. 2012. Biodiversity in dead wood. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 111.Gleason FH, Karpov SA, Lilje O, Macarthur DJ, van Otgen FF, Sime-Ngando T. 2014. Zoosporic parasites of phytoplankton. In Freshwater fungi and fungal-like organisms (eds Jones EBG, Hyde KD, Pang KL), pp. 279–304. Berlin, Germany: Walter de Gruyter GmbH. [Google Scholar]
- 112.Chen JJ, Cui BK, Zhou LW, Korhonen K, Dai YC. 2015. Phylogeny, divergence time estimation and biogeography of the genus Heterobasidion (Basidiomycota, Russulales). Fung. Div. 71, 185–200. ( 10.1007/s13225-014-0317-2) [DOI] [Google Scholar]
- 113.Karlsson M, et al. 2015. Insights on the evolution of mycoparasitism from the genome of Clonostachys rosea. Genome Biol. Evol. 7, 465–480. ( 10.1093/gbe/evu292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tkacz A, Poole P. 2015. Role of root microbiota in plant productivity. J. Exp. Bot. 66, 2167–2175. ( 10.1093/jxb/erv157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.LePage BA, Currah RS, Stockey RA. 1994. The fossil fungi of the Princeton chert. Int. J. Plant Sci. 155, 828–836. ( 10.1086/297221) [DOI] [Google Scholar]
- 116.Stockey RA, Rothwell GW, Addy HD, Currah RS. 2001. Mycorrhizal association of the extinct conifer Metasequoia milleri. Mycol. Res. 105, 202–205. ( 10.1017/S0953756200003221) [DOI] [Google Scholar]
- 117.Strullu-Derrien C, Kenrick P, Rioult JP, Strullu DG. 2011. Evidence of parasitic Oomycetes (Peronosporomycetes) infecting the stem cortex of the Carboniferous seed fern Lyginopteris oldhamia. Proc. R. Soc. B 278, 675–680. ( 10.1098/rspb.2010.1603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Krings M, Taylor TN, Dotzler N, Persichini G. 2012. Fossil fungi with suggested affinities to the Endogonaceae from the Middle Triassic of Antarctica. Mycologia 104, 835–844. ( 10.3852/11-384) [DOI] [PubMed] [Google Scholar]
- 119.Krings M, White JF, Dotzler N, Harper CJ. 2013. A putative zygomycetous fungus with mantled zygosporangia and apposed gametangia from the Lower Coal Measures (Carboniferous) of Great Britain. Int. J. Plant Sci. 174, 269–277. ( 10.1086/668247) [DOI] [Google Scholar]
- 120.Locatelli ER. 2014. The exceptional preservation of plant fossils: a review of taphonomic pathways and biases in the fossil record. In Reading and writing of the fossil record: preservational pathways to exceptional fossilization, vol. 20 (eds Laflamme M, Schiffbauer JD, Darroch SAF), pp. 237–258. Bolder, CO, USA: Paleontological Society Papers. [Google Scholar]
- 121.Taylor TN, Taylor EL. 2000. The Rhynie chert ecosystem: a model for understanding fungal interactions. In Microbial endophytes (eds Bacon CW, White JF), pp. 31–47. New York, NY: Marcel Dekker. [Google Scholar]
- 122.Berbee ML, Taylor JW. 2007. Rhynie chert: a window into a lost world of complex plant-fungus interactions. New Phytol. 174, 475-479. ( 10.1111/j.1469-8137.2007.02080.x) [DOI] [PubMed] [Google Scholar]
- 123.Bonfante P, Genre A. 2008. Plants and arbuscular mycorrhizal fungi: an evolutionary-developmental perspective. Trends Plant Sci. 13, 492–498. ( 10.1016/j.tplants.2008.07.001) [DOI] [PubMed] [Google Scholar]
- 124.Field KJ, Pressel S, Duckett JG, Rimington WR, Bidartondo MI.. 2015. Symbiotic options for the conquest of land. Trends Ecol. Evol. 30, 477–486. ( 10.1016/j.tree.2015.05.007) [DOI] [PubMed] [Google Scholar]
- 125.Li DW, Castañeda-Ruiz RF, LaMondia J. 2016. Evolution of fungi and update on ethnomycology. In Biology of microfungi (ed. Li DW.), pp. 237–266. New York: NY: Springer. [Google Scholar]
- 126.Krings M, Taylor TN, Harper CJ.. 2017. Early fungi: Evidence from the fossil record. In The fungal community, its organization and role in the ecosystem (eds Dighton J, White JF), pp. 37–46, 4th edn Boca Raton, FL: CRC Taylor and Francis. [Google Scholar]
- 127.Redman RS, Litvintseva A, Sheehan KB, Henson JM, Rodriguez RJ. 1999. Fungi from geothermal soils in Yellowstone National Park. Appl. Environ. Microbiol. 65, 5193–5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Henson J, Redman R, Rodriguez R, Stout R. 2005. Fungi in Yellowstone's geothermal soils and plants. Yellowstone Sci. 13, 25–30. [Google Scholar]
- 129.Pan WZ, Huang XW, Wei KB, Zhang CM, Yang DM, Ding JM, Zhang KQ. 2010. Diversity of thermophilic fungi in Tengchong Rehai National Park revealed by ITS nucleotide sequence analyses. J. Microbiol. 48, 146–152. ( 10.1007/s12275-010-9157-2) [DOI] [PubMed] [Google Scholar]
- 130.Zhou WN, White JF Jr, Soares MA, Torres MS, Zhou ZP, Li HY. 2015. Diversity of fungi associated with plants growing in geothermal ecosystems and evaluation of their capacities to enhance thermotolerance of host plants. J. Plant Interact. 10, 305–314. ( 10.1080/17429145.2015.1101495) [DOI] [Google Scholar]
- 131.Green DG, Sadedin S. 2005. Interactions matter—complexity in landscapes and ecosystems. Ecol. Complex. 2, 117–130. ( 10.1016/j.ecocom.2004.11.006) [DOI] [Google Scholar]
- 132.Bairey E, Kelsic ED, Kishony R. 2016. High-order species interactions shape ecosystem diversity. Nat. Commun. 7, 12285 ( 10.1038/ncomms12285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jordano P. 2016. Chasing ecological interactions. PLoS Biol. 14, e1002559 ( 10.1371/journal.pbio.1002559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Beimforde C, et al. 2014. Estimating the Phanerozoic history of the Ascomycota lineages: Combining fossil and molecular data. Mol. Phylogen. Evol. 78, 386–398. ( 10.1016/j.ympev.2014.04.024) [DOI] [PubMed] [Google Scholar]
- 135.Hibbett D, Blanchette R, Kenrick P, Mills B. 2016. Climate, decay, and the death of the coal forests. Curr. Biol. 26, R543–R576. ( 10.1016/j.cub.2016.01.014) [DOI] [PubMed] [Google Scholar]
- 136.Spatafora JW, et al. 2016. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 108, 1028–1046. ( 10.3852/16-042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Powell CL, Trewin NH, Edwards D. 2000. Palaeoecology and plant succession in a borehole through the Rhynie cherts, Lower Old Red Sandstone, Scotland. Geol. Soc. London Spec. Pub. 180, 439–457. ( 10.1144/GSL.SP.2000.180.01.23) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All thin sections and original digital images of P. tayloriana are deposited in the Bayerische Staatssammlung für Paläontologie und Geologie (SNSB-BSPG), Munich, Germany. Additional material is housed in the Abteilung Paläobotanik, Geologisch-Paläontologisches Institut, Westfälische Wilhelms-Universität, Münster, Germany (prefix P).