Abstract
Zoosporic fungi are key saprotrophs and parasites of plants, animals and other fungi, playing important roles in ecosystems. They comprise at least three phyla, of which two, Chytridiomycota and Blastocladiomycota, developed a range of thallus morphologies including branching hyphae. Here we describe Retesporangicus lyonii gen. et sp. nov., an exceptionally well preserved fossil, which is the earliest known to produce multiple sporangia on an expanded hyphal network. To better characterize the fungus we develop a new method to render surfaces from image stacks generated by confocal laser scanning microscopy. Here, the method helps to reveal thallus structure. Comparisons with cultures of living species and character state reconstructions analysed against recent molecular phylogenies of 24 modern zoosporic fungi indicate an affinity with Blastocladiomycota. We argue that in zoosporic fungi, kinds of filaments such as hyphae, rhizoids and rhizomycelium are developmentally similar structures adapted for varied functions including nutrient absorption and anchorage. The fossil is the earliest known type to develop hyphae which likely served as a saprotrophic adaptation to patchy resource availability. Evidence from the Rhynie chert provides our earliest insights into the biology of fungi and their roles in the environment. It demonstrates that zoosporic fungi were already diverse in 407 million-year-old terrestrial ecosystems.
This article is part of a discussion meeting issue ‘The Rhynie cherts: our earliest terrestrial ecosystem revisited’.
Keywords: fossil fungus, hyphae, Blastocladiomycota, Chytridiomycota, phylogeny, confocal laser scanning microscopy
1. Introduction
Fungi were evolving at the dawn of terrestrial life. However, in contrast with plants, the fossil record of early Fungi is meagre. One site of exceptional preservation is the Rhynie chert (Aberdeenshire, Scotland, UK), dating to 407 Ma [1], which is providing critical insights into an early terrestrial ecosystem [2]. Fossilization occurred through episodic inundation of the biota with fluids from a nearby hot-spring system, and the resulting silicification preserved organisms to the cellular level [3,4]. Fungi were preserved in situ along with their hosts or other interacting organisms (e.g. [5–8]) and the most common fungi in this ecosystem seem to be zoosporic members of Chytridiomycota and Blastocladiomycota [9–20], but attributing affinity can be problematic owing to fairly limited characters and difficulties reconstructing such minute organisms. Molecular phylogenies show that following the separation of Fungi from the animal clade in the Proterozoic Aeon, first the Cryptomycota and then Chytridiomycota and Blastocladiomycota were the earliest fungi to diverge [21]. As saprotrophs or parasites of plants, animals or other fungi, early diverging fungi fulfil important ecological roles in modern ecosystems. Neocallimastigomycetes is a clade of anaerobic fungi derived from aerobic ancestors that inhabit vertebrate digestive tracts. As the sister group to the remaining Chytridiomycota [22], they are variously recognized at the phylum level (Neocallimastigomycota) [23] or as a class within Chytridiomycota [24]. Cryptomycota are endoparasites [25] that have yet to be recognized in the fossil record and could be difficult to distinguish from the cellular debris of their hosts. Chytridiomycota and Blastocladiomycota, on the other hand, produce recognizable, walled thalli (bodies) of varying complexity. These latter two phyla are separated on the basis of zoospore ultrastructure, life cycle and phylogenetic position based on rDNA analyses [26,27]. In both phyla, body or ‘thallus’ type varies by genus and species. Some genera in each phylum have monocentric thalli with determinate growth that is complete when all nuclei are packaged into zoospores in the single sporangium. Monocentric thalli often have rhizoids, which are narrow, anucleate filaments that serve in anchorage and in nutrient uptake. Other genera are rhizomycelial, with indeterminate growth that continues as nuclei squeeze through narrow rhizoidal segments to populate rhizoidal swellings which may expand into new sporangia. A few genera are hyphal with nuclei that are distributed more or less regularly within cylindrical filaments of protoplasm. A hyphal individual can usually produce multiple sporangia. Among these different thallus types, intermediates exist, as might be expected given that phylogenies suggest that polycentric forms evolved repeatedly from monocentric ancestors [28]. Establishment of the systematic affinity of fossils is challenging because extant phyla share parallel diversity of thallus types [26].
Here we describe Retesporangicus lyonii gen. et sp. nov., as a fossil zoosporic fungus from the Rhynie chert. In order to address the difficulties of reconstructing thallus morphology of minute organisms with potentially complex networks of intertwining connections, we use light microscopy and confocal laser scanning microscopy (CLSM) and we develop a method to extract isosurfaces and create three-dimensional models from the confocal image stacks. Harvey et al. [29] first noted this fossil, providing a photograph and an initial description but without naming the fungus. Our sophisticated microscopic analyses allow us to refute Harvey et al.'s suggestion. To better determine the affinities of the fossil, we compare its structures with those observed of living cultures of putative related species. Character state reconstructions are then analysed for 24 modern species together with the new fossil all constrained against the topologies of recent molecular phylogenetic trees. The development of such combined multidisciplinary approaches is necessary to enable better characterization of the diversity and interactions of fungi in early terrestrial ecosystems.
2. Methods
(a). Fossil Fungi
We examined and photographed specimens in thin section 149/CT/B from the collection of the University of Aberdeen with a Leica 250C stereomicroscope and a Nikon Eclipse LV100ND compound microscope. Depth of field was enhanced through z-stack montage. We acquired confocal images with a Nikon A1-Si confocal laser scanning microscope. We used a 40× oil-immersion objective and selected an area-of-interest around the object for digital enlargement. Autofluorescence signal was collected with four photomultiplier detectors (425–475 nm for the 405 nm laser, 500–550 nm for the 488 nm laser, 570–620 nm for the 561 nm laser and 675–725 nm for the 640 nm laser). Samples were visualized with a 30 µm confocal pinhole, and 100 to 400 z-stacks were acquired for each detector. The fluorescence signal from each z-stack was then projected onto a maximum projection image and used to generate a three-dimensional model of the sample (Nikon NIS-Elements software, http://www.nis-elements.com) (see [19] for additional information). A second series of three-dimensional reconstructions was produced from the confocal datasets. Extraction of the compressed photomultiplier channel z-stacks to individual images (.bmp/.tiff) was performed in ImageJ [30] (using the Nikon ND2 plugin [31]). Channels devoid of information were discarded, and remaining channels merged into a single dataset after application of brightness/contrast corrections. Subsequent manual segmentation of the dataset in SPIERSedit (see [32] for additional information) allowed the production of isosurface-based models in SPIERSview [33]. These three-dimensional models were used to produce ray-traced animations and figures in Blender™ [34].
(b). Modern Fungi
Photographs are from cultures of Physocladia obscura JEL 513, Catenomyces persicinus JEL 342, Nowakowskiella sp. JEL 335, Catenaria sp. JEL 194, Catenophlyctis variabilis JEL 575, Lacustromyces hiemalis JEL 31 and Polychytrium aggregatum JEL 109. Cultures were maintained on mPmTG agar (2.0 g l−1 glucose, 0.4 g l−1 peptonized milk, 0.4 g l−1 tryptone, 10.0 g l−1 agar), with transfers every one to two weeks or every three months in some cases. JEL = Joyce E. Longcore U. Maine Chytrid Collection.
Morphological analysis to incorporate the fossil into a phylogeny of extant taxa: to infer the distribution of character states among living taxa, we drew a 24-taxon tree that combined the branching order from published, DNA-based phylogenies of zoosporic fungi, from James et al. [26] for Chytridiomycota and Porter et al. [35] for Blastocladiomycota. We illustrated variation in thallus type with drawings from original photographs or published work. For the fossil and the 24 taxa of zoosporic fungi, we coded character states of six characters: thallus type (monocentric, rhizomycelial/polycentric, hyphal); presence or absence of rhizoids; presence or absence of any kind of operculum (lid) blocking the exit until zoospore release from the zoosporangia; presence or absence of elongate zoospore discharge tubes in at least some zoosporangia; ornamentation of resting sporangium wall; and germination of resting sporangium (unknown or via a crack, circumscissile opening, or pore). Character states for most of the extant taxa were from Karling [36]. Barr [37] was the source of character states for Spizellomyces punctatus. Character states for Neocallimastigomycetes were based on Neocallimastix frontalis [38] and Longcore et al. [39] was the source for Batrachochytrium dendrobatidis.
We next searched for the most parsimonious position of the fossil, given the character matrix while applying the phylogeny of the 24 extant taxa as a topological constraint. We assumed character state changes were unordered and unweighted. Branch and bound searches in PAUP* [40] then incorporated the fossil taxon into the pre-existing 24 taxon phylogeny. We evaluated the effects of alternative assumptions about character states on inferences about the fossil's relationships. Using Mesquite 3.10 [41], again assuming that character state changes were equally weighted and unordered, we reconstructed ancestral character states using parsimony for a phylogeny that incorporated the fossil into the phylogeny of 24 extant taxa.
(c). Systematics
Kingdom: Fungi
Phylum: Blastocladiomycota
Class: incertae sedis
Genus: Retesporangicus Strullu-Derrien, gen. nov.
Diagnosis: extensively branched hyphal thallus with intercalary or terminal thin-walled zoosporangia and thick-walled resting sporangia. Zoosporangia and resting sporangia varying in size and shape. Endo-operculate.
Species: R. lyonii Strullu-Derrien, sp. nov.
Diagnosis: as in generic diagnosis. Hyphae isodiametric, ca 3.5 µm in diameter. Zoosporangia either oval, pyriform, elongate, elongate with a long discharge tube, triangular, triangular with a long discharge tube or spherical (from 18–34 × 44–75 µm up to 30 µm in diameter). Resting sporangia globose to ovoid (42 µm in diameter to 36–62 × 75–85 µm).
Etymology: genus name refers to the hyphal network on which sporangia develop. The specific epithet honours A. G. Lyon from Cardiff University who prepared this original material.
Holotype hic designatus: specimens in petrographic thin section no. 149-CT-B from the collections at the School of Geosciences, King's College, University of Aberdeen in Scotland (figure 1).
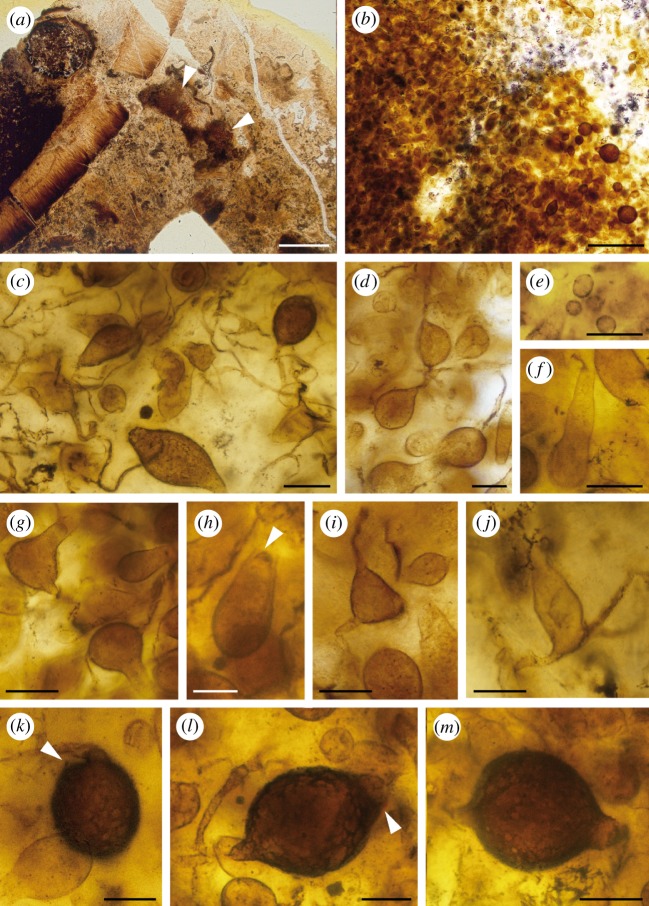
Figure 1.
Retesporangicus lyonii. Holotype (slide no. 149-CT-B from the collections of the University of Aberdeen, Scotland, UK). (a) Global view showing two brown areas containing the fungal thalli. (b) Thalli from (a) in higher magnification. (c,d) Hyphae and swellings. (e) Zoospores released from the reproductive structures. (f–j) Zoosporangia of various shapes and sizes; arrowhead in (h), endo-operculum. (k–m) Resting sporangia; arrowheads in (k) and (l), indicate cracks in wall, possibly sites of germination. Scale bars: a, 0.25 cm; b, 200 µm; d,j,k,l,m, 20 µm; c,e,f,g,h,i, 30 µm.
Locality: Rhynie, northwest of Aberdeen (Scotland, UK). Age: Lower Devonian (ca 407 Ma [1])
MycoBank [42–44] nos: MB 820924 (genus), MB 820925 (species).
(d). Description of the fungus
The thin section contains several detached fragments of the hymenial layer of Prototaxites taiti [45] (figure 1a). Among debris and in close vicinity to these fragments are two brown areas, which contain hundreds of tiny fungal structures varying from small and spherical (7.5 µm in diameter) to large and ovoid (62 × 85 µm) (figure 1b).
Different elements interpreted as belonging to the same fungus are described below.
— Filamentous structures. Connections occur between swellings (figure 1c,d); however, they are not easy to distinguish among the debris. Some filaments may emerge from multiple sites on a swelling (figure 1g,i,j).
— More than 100 small spherical structures 7.5–11.5 µm in diameter (figure 1e).
— Thin-walled swellings of different shapes and sizes (figure 1f–j). They range from oval (20–38 × 50–69 µm), through pyriform (19–25 × 39–56 µm), elongate (18–23 × 44–75 µm), elongate with an elongate neck (discharge tube) (26–30 × 48–71 µm), triangular (21 × 30 µm), triangular with an elongate neck (34 × 54 µm) to spherical (20–30 in diameter) (figure 1f–j). Some of these swellings appear to be endo-operculate (figure 1h, arrowhead). This type of operculation consists of a thickened membrane-like structure under the outer wall.
— Thicker-walled and dark swellings of different shape and size (figure 1k–m), varying from globose to ovoid (36–62 × 75–85 µm). The wall does not appear to be perfectly smooth, but the type of ornamentation cannot be defined. The outer wall of these swellings seems to crack open (figure 1k,l, arrowheads).
Additional data obtained from CSLM and further analysis of the confocal datasets.
— The three-dimensional reconstructions (figure 2e; electronic supplementary material, movie S2e) allow observation of details of the filaments. They are ca 3.5 µm in diameter, highly branched and connect the swellings.
— Reconstructions of the two types of swellings allowed comparison of the thickness of the wall (electronic supplementary material, figure S1), the content of the swellings (figure 2a–d) and the connection with the filaments (figure 2e). Some sporangia with thick walls still contain spores but these seem to be highly degraded (electronic supplementary material, figure S1c). The darker thick-walled swellings (electronic supplementary material, figure S1d) were difficult to image in confocal microscopy as the beam penetration was weak through the wall; this implies that their recorded thickness is likely underestimated. Some of the swellings are cut in half and appear to be empty (electronic supplementary material, figure S1).
— Swellings are separated from the filaments by septa (figure 2b,e, arrowheads; electronic supplementary material, figure S1b and movies S2b,e).
— Several of the swellings contain spherical tiny structures ca 6–7 µm in diameter (figure 2a–d; electronic supplementary material, movies S2a–d and figure S1a–c).
Figure 2.
Three-dimensional reconstructions of zoosporangia (a–d) and thallus (e) of Retesporangicus lyonii obtained by our new imaging method. Zoosporangium in light brown, spores in yellow–green inside the zoosporangium, hyphae in dark brown. In images a–d the wall of the sporangium appears in transparency (on the right) to enable observation of its content. Black arrowheads = septa. Scale bars: a,b,d = 30 µm; c,e = 20 µm.
(e). Interpretation of the structures
We interpret the filaments as hyphae rather than rhizoids based on their diameter, which is on the wider side within the realm of possibility for chytrid rhizoids, and also because these filaments appear equal in diameter throughout. In modern fungi, rhizoids lack nuclei whereas nuclei are regularly distributed throughout the hyphae. Nuclear distribution is a character that we have been unable to observe in the fossils as these are unlikely to be preserved.
We interpret the thin-walled swellings as zoosporangia-containing zoospores 6–7 µm in diameter (figures 1f and 2a–d; electronic supplementary material, figure S1a,b), which is the range for many modern species. Some zoospores have been released from the reproductive structures (figure 1e). The empty dark thicker-walled swellings are considered to be resting sporangia (figure 1k–m; electronic supplementary material, figure S1d).
In summary, we interpret the thallus as hyphal and extensively branched with intercalary or terminal thin-walled and thick-walled swellings of various shapes and sizes. Spores are preserved inside the zoosporangia. Taking into account the restricted area and close proximity in which the fungal structures developed, the similarities in hyphal connection, structure content and size range of the reproductive structures, we consider that all elements belong to the same fungus.
(f). Comparison with other fossils
Harvey et al. [29] recorded the fossil we now describe as a mycelial fungus bearing vesicles and spores. They illustrated the fossil with one photograph and pointed out that its vesicles were of two types with a pyriform–ovate type being the most frequently observed. The vesicle shape suggested to Harvey et al. that the fossil was not unlike Apodachlya pyrifera Zopf, a modern oomycete. However, the match to the modern species was not perfect and they noted that hyphal constrictions were not discernible in the fossil and that the oogonia and sporangia of A. pyrifera were smaller than the vesicles of the fossil. Nowadays the oomycetes and hyphochytrids, together with some marine flagellates, are included in the Heterokonta, along with the photosynthetic chromistan (golden-brown) algae, brown algae and diatoms [46,47]. We describe the fossil as a true zoosporic fungus. We dismiss a possible oomycete affinity because thick-walled resting sporangia rather than oogonia with antheridia are evident in the material. Arguing against a relationship with Apodachlya, our three-dimensional images show clearly that hyphae are not constricted at their point of attachment to sporangia.
Among the true fungi, none of the Chytridiomycota earlier reported from the Rhynie chert resembles closely Retesporangicus lyonii, and the differences, presented below, justify erecting a new genus and species. Globicultrix nugax [16]) was a microfungus that showed rhizomycelial morphology. It developed within fungal spores and consisted of slender branched filaments and terminal, usually apophysate sporangia. Krispiromyces discoides, which associated with the freshwater alga Palaeonitella cranii [10], Cultoraquaticus trewinii, which developed on a structure of unknown affinity [20], and Illmanomyces corniger, found associated with a fungal spore, had relatively complete epibiotic zoosporangia with an endobiotic rhizoidal system [17]. Cultoraquaticus trewinii and I. corniger have been compared with modern Chytridiomycota orders Spizellomycetales [48] and Rhizophydiales [49]. These fungi differ from R. lyonii, which comprised intercalary or terminal sporangia and resting sporangia connected by an extensive, highly branched hyphal network with connections that might emerge from multiple sites on a sporangium. In contrast to those of the four previously described fossil Chytridiomycota, sporangia and resting sporangia in R. lyonii varied in shape and size. Another fossil, Trewinomyces annulifer, with mixed similarities with Chytridiomycota and Blastocladiomycota [18] comprised a multibranched intramatrical rhizoidal system, but unlike R. lyonii, it produced clusters of elongate, unbranched extramatrical hyphae, each hypha bearing a single terminal sporangium.
Fossils with suggested affinity with Blastocladiomycota also differ from R. lyonii. Paleoblastocladia milleri developed two nearly identical morphological types of thalli, which consisted of hyphae bearing branched rhizoids [15]. The sporothallus bore terminal zoosporangia and resting sporangia. The gametothallus was formed of barrel-shaped gametangia organized in pairs. Palaeozoosporites renaultii [19] had thalli made of branches dividing more or less isotomously to produce zoosporangia in small clusters and then sympodially to produce resting sporangia.
(g). Comparison with extant zoosporic fungi in a phylogenetic context
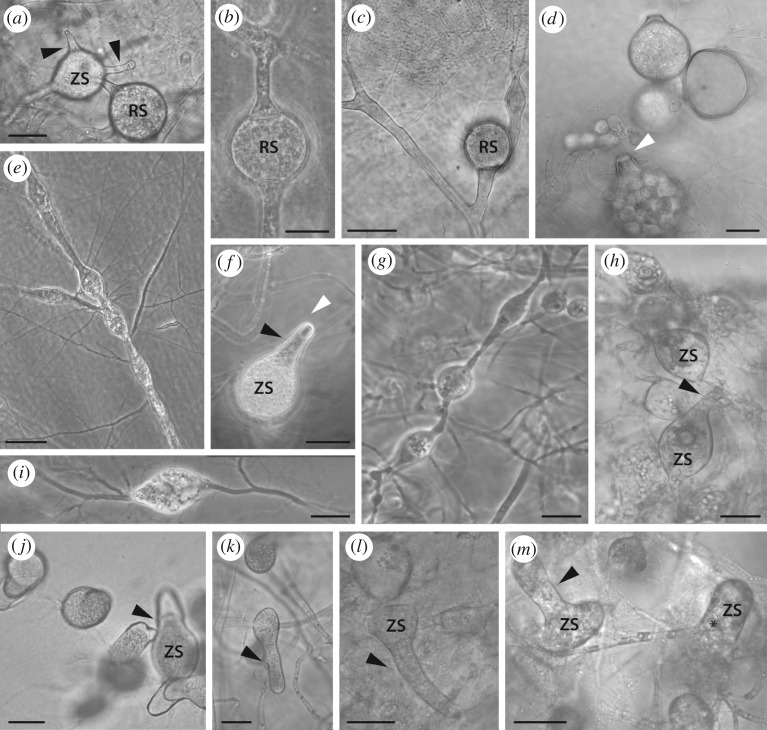
Like the fossil, polycentric species in modern Chytridiomycota and Blastocladiomycota produce multiple sporangia per individual thallus (figure 3). Sporangia, as in Retesporangicus, are produced by highly branched filaments in Lacustromyces haemalis (figure 3a,b), Polychytrium aggregatum (figure 3c,d), Nowakowskiella sp. (figure 3e,f), Catenomyces persicinus (figure 3g,h) and Physocladia obscura (figure 3i). Polycentric Blastocladiomycota include Catenaria sp. (figure 3j,k) and Catenophlyctis variabilis (figure 3l,m). In both Chytridiomycota and Blastocladiomycota, sporangia may be intercalary (figure 3b,g–m) and attached to two (figure 3b,l,m), three, or more filaments (not illustrated), or they may be terminal (figure 3c). The fossil appears to have intercalary sporangia attached to two or three hyphae; they can also be terminal. As in Retesporangicus, sporangia can develop long discharge tubes for zoospore release in varied taxa from Blastocladiomycota and Chytridiomycota including Catenaria, sp. (figure 3j,k), Catenophlyctis (figure 3l,m), Catenomyces persicinus (figure 3h) and, as diagrammed in Karling [36], Nowakowskiella spp. Endo-operculation seems to occur in the fossil as in Catenomyces while exo-operculation characterizes Nowakowskiella (figure 3f). An operculum is a characteristic that appears in various members of Chytridiomycota but type of operculum can vary even within a species so the shared character may not provide reliable evidence about relationships.
Figure 3.
Diversity of thallus and sporangium morphology in modern Chytridiomycota (a–i) and Blastocladiomycota (j–m). (a,b) Lacustromyces hiemalis (Polychytriales). (a) Thick-walled resting sporangium connected to an intercalary, thin-walled sporangia with two discharge tubes. (b) Intercalary thick-walled resting sporangium. (c,d) Polychytrium aggregatum (Polychytriales). (c) Resting sporangium on hypha-like filaments. (d) Zoospore discharge through an exit papilla (white arrowhead). (e,f) Nowakowskiella (JEL78) (Cladochytriales). (e) Rhizomycelium with swellings and abundant rhizoids. (f) Sporangium with operculum (white arrowhead). (g,h) Catenomyces persicinus JEL 342 (Rhizophlyctidales). (g) Rhizomycelium, (h) zoosporangia. (i) Physocladia obscura JEL 513 (Chytridiales). Highly branched rhizoids emerge from multiple sites on a swelling. (j,k) Catenaria sp. JEL 194 (Blastocladiales). Pyriform sporangia develop long discharge tubes. (l,m) Catenophlyctis variabilis JEL 575 (Blastocladiales). Variation in shapes of sporangia with long discharge tubes. Hyphal thalli (c,j,k). Black arrowheads, discharge tube; asterisk, zoospore; RS, resting sporangium; ZS, zoosporangium. Scale bars: a,b = 30 µm; d,f,h,j,m = 10 µm; c,e,g,i = 20 µm; k,l = 40 µm.
The tree in figure 4 shows a strict consensus of the four equally parsimonious positions for R. lyonii relative to a pre-existing phylogeny of zoosporic fungi. In all four trees, R. lyonii appeared to be nested in phylum Blastocladiomycota. Trees (length, 29 steps) had a consistency index (CI) of 0.31 and a retention index (RI) of 0.51. In three of the trees, R. lyonii formed a clade with Coelomycidium sp. and Coelomomyces punctatus. The three trees differed only in branching order within this clade. The fourth tree showed R. lyonii as sister taxon to Catenaria anguillulae.
Figure 4.
In a consensus of four equally parsimonious trees, the fossil Retesporangicus lyonii appears within Blastocladiomycota. The tree was reconstructed by using morphological characters to integrate R. lyonii into published phylogenies of Blastocladiomycota [35] and Chytridiomycota [26]. Drawings illustrate the diversity of thallus types. In both phyla, hyphae with approximately cylindrical walls, rhizomycelia (indicated by icons showing swellings linked by filaments) and monocentric thalli (indicated by a single circle, usually with attached filamentous rhizoids) all occur. Sources of the photographs that were traced for drawings were: (a) [50, fig. 22]; (b) K. Wells, teaching collection the University of California; (c) [51, fig. 15]; (d) J. Longcore; (e) [52, figs 51 and 57], reprinted with permission from Mycologia, ©The Mycological Society of America; (f,g,i,j) J. Dee; (h) [53, fig. 1], with permission from the American Journal of Botany.
3. Discussion and conclusion
Several chytrid morphotypes suggestive of Chytridiomycota or Blastocladiomycota have been identified in the Rhynie chert, including epibiotic and endobiotic holocarpic forms (holocarpic = whole thallus differentiates into a sporangium) and eucarpic forms (eucarpic = some part of the thallus, e.g. rhizoids or hyphae as in R. lyonii do not become part of the sporangium) [9–20]. Monocentric chytrid-like globular forms connected with filaments (rhizoids) are associated with aquatic algae, land plants, and land plant and fungal spores ([54] and references therein; [18,20]). Relatively complete epibiotic zoosporangia and endobiotic rhizoidal systems are evident in Krispiromyces discoides [10], Cultoraquaticus trewinii [20] and Illmanomyces corniger [17]. On the other hand, Globicultrix nugax [16], Paleoblastocladia milleri [15] and Palaeozoosporites renaultii [19] as well as R. lyonii developed polycentric thalli.
Phylogenetic comparison placed R. lyonii in Blastocladiomycota, albeit without strong support from multiple congruent characters. Considering the individual characters in turn, monocentric thalli appeared ancestral overall and in Chytridiomycota, but within Blastocladiomycota, hyphae were reconstructed as ancestral for the majority of taxa following the basal split in the phylum (electronic supplementary material, figure S2). Rhizoids were lost from Synchytrium macrosporum and in Monoblepharidomycetes (Chytridiomycota) and from the shared ancestor of a Blastocladiomycota clade that included Coelomycidium sp., Coelomomyces punctatus and, in some reconstructions, R. lyonii (electronic supplementary material, figure S3). Opercula in zoosporangia appear to have originated convergently in three of the sampled lineages of Chytridiomycota as well as in the fossil (electronic supplementary material, figure S4). All of the operculate taxa had endo-opercula with the exception of exo-operculate Chytriomyces hyalinus. Elongate discharge tubes for zoospore release (figure 3a,f,h,m) evolved independently in several lineages of the Chytridiomycota and Blastocladiomycota (electronic supplementary material, figure S5). Consistent with their frequent appearance among modern Chytridiomycota (figure 3a–c) and Blastocladiomycota, smooth-walled resting sporangia were reconstructed as ancestral in both phyla (electronic supplementary material, figure S6). Unlike the other five individual characters which were homoplasious (CIs 0.20–0.33), resting sporangium germination had a CI of 1.00 (electronic supplementary material, figure S7). This was because, in Blastocladiomycota, germination consistently involved dehiscence with a crack in the sporangium wall while, in the outgroup Rozella allomycis and in Chytridiomycota (where known), resting sporangia germinated with a pore (electronic supplementary material, figure S7). The high CI may reflect a large amount of missing data rather than absence of convergence. Helpful though germination type appears to be for classification (electronic supplementary material, figure S7), it has yet to be observed in many taxa, sometimes because resting sporangia are unknown and sometimes because resting sporangia remain dormant for months or even years.
Four characters, germination of the resting sporangium, type of thallus, development of an elongate zoospore discharge tube, and absence of rhizoids, were largely responsible for R. lyonii's phylogenetic position, based on analyses that involved alternative character state coding and re-analysis in PAUP*, or manual modification of the position of R. lyonii in trees using Mesquite. Just changing the coding for germination in R. lyonii's resting sporangial germination to ‘unknown’ rather than ‘crack’ increased the number of equally parsimonious positions for R. lyonii to 8. In addition to nested positions in Blastocladiomycota, without the information about germination, the fossil could equally parsimoniously group with any of the three species in Chytridiomycota that had zoosporangia with opercula and long discharge tubes. Coding both germination and thallus type as ‘unknown’ removed the information that had supported R. lyonii's position in Blastocladiomycota so that the fossil fell into Chytridiomycota, always together with operculate taxa with long discharge tubes. Assuming germination involved a ‘crack’ and that thallus type was ‘hyphal’ (as appeared to be correct) and then manually forcing the fossil to appear within a clade in Chytridiomycota with other taxa with operculate, long-necked sporangia only increased the tree length from 29 to 30.
Within Blastocladiomycota, parsimony places R. lyonii with Catenaria or with Coelomycidium and Coelomomyces. Of these possibilities, R. lyonii most resembles Catenaria. Catenaria species, like R. lyonii, can grow as saprotrophs. Catenaria species variously associate with nematodes, insect eggs or other invertebrates and some will grow on boiled grass [52]. Unlike Catenaria, both Coelomycidium and Coelomomyces are obligate internal parasites of insects and other invertebrates and nutrient absorption in both involves a wall-less, plasmodial phase [35]. Although the phase is termed ‘hyphal’ in Coelomomyces owing to general shape, it is difficult to know whether its ancestry was from a walled hypha. Catenaria is not closely related to Coelomycidium and Coelomomyces within Blastocladiomycota [34]. This explains why resolution of internal branching in Blastocladiomycota is lost in a consensus of equally parsimonious positions of R. lyonii (as in figure 4). In our analyses, within Blastocladiomycota, Physoderma maydis usually appeared to diverge before R. lyonii split from its closest relatives. However, Physoderma spp. are derived parasites of plants with distinctive autoapomorphic traits that provide minimal signal about phylogenetic relationships. We tentatively placed the fossil in Blastocladiomycota incertae sedis as its position relative to known genera is uncertain and it may even represent a member of the stem group.
The idea of extinction of whole taxa or organization types is familiar to plant and animal palaeontologists, but may be overlooked by palaeomycologists [55]. When Retesporangicus lyonii grew 407 Ma ago, fossil evidence [54] and molecular dating [56,57] agree that all known fungal phyla were also present and so it is reasonable to evaluate R. lyonii's fit with Chytridiomycota and Blastocladiomycota. It is also possible that R. lyonii represents an extinct lineage from outside these phyla that happened to share a convergent morphology. Complicating classification, hyphal or rhizomycelial species evolved frequently and recently from monocentric ancestors. As filaments of protoplasm surrounded by a cell wall, rhizoids, rhizomycelium and hyphae are probably homologous and they share functions of anchorage and nutrient uptake. The convergent transitions from small, determinate, monocentric, rhizoidal species to more expansive rhizomycelial or hyphal forms may have been favoured when food sources became large enough to support extended growth and multiple centres of reproduction [28]. Whatever its affiliations, R. lyonii represents the earliest known zoosporic fungus that could produce multiple sporangia by using an expanded hyphal network to take advantage of dispersed nutrients.
Of the Rhynie chert zoosporic fungi described to date, several life strategies have been observed. Some like G. nugax grew within a single fragment of a substrate [16]. Several, like C. trewinii [20], T. annulifer [18] and P. milleri [15], had rhizoids that formed within a single substrate fragment for nutrient acquisition and anchorage, while sporangia formed externally. Because the hyphal structures in T. annulifer and P. milleri formed outside of the substrate and bore sporangia, they presumably functioned primarily in reproduction rather than in locating or absorbing nutrients. In contrast, R. lyonii had no evident rhizoids and so its hyphae were the only structures that appeared positioned to assimilate nutrients. Retesporangicus lyonii was observed among plant and fungal detritus, in close vicinity with fragments of Prototaxites [45]. It likely developed as a saprotroph in this palaeoecosystem (figure 1a), the detritus being mainly amorphous elements and parts of Prototaxites tubes (hyphae?). Hyphae of R. lyonii span several patches of debris rather than attaching to a particular piece of a single substrate. We interpret R. lyonii as the first known zoosporic lineage that could deploy hyphae to locate and then digest food which may have been patchy in its distribution.
Retesporangicus lyonii increases our knowledge of the polycentric thallus morphology and shows that a higher diversity of zoosporic fungi than previously known occurred 407 Ma ago. Saprotrophy and parasitism have been suggested for several species in freshwater and soil environments but surprisingly, only indirect associations with arthropods have been found to date [20]. It has been proposed that through mineral weathering, saprotrophic and mutualistic fungi must have contributed to global carbon cycles in early environments [58–60]. Retesporangicus lyonii with its extensive hyphal network might have been an efficient contributor.
Supplementary Material
Acknowledgements
The authors thank Professor Nigel Trewin (Aberdeen University) and Professor Dianne Edwards (Cardiff University) for providing specimens for investigation. They acknowledge Mycologia and the American Journal of Botany for permission to reuse images for making the drawings. M.L.B. thanks the National Science and Engineering Research Council of Canada for Discovery grant no. RGPIN-2016-03746. C.S.-D. thanks the European Commission under the Marie Curie Intra-European Fellowship Programme FP7-People-2011 (SYMBIONTS 298735) and the Palaeontological Association, UK (grant no. PA-RG201602).
Data accessibility
Supplementary three-dimensional model data are available at the Zenodo repository (doi:10.5281/zenodo.801505).
Authors' contributions
C.S.-D. and R.H. recognized the fungus. C.S.-D. did the light microscopy. M.L.B. did the phylogenetic analysis. C.S.-D. and T.G. did the confocal microscopy. A.R.T.S. did the three-dimensional reconstructions and helped C.S.-D. interpret the fossil structures. J.E.L., M.L.B. and J.D. collected the data on modern fungi. C.S.-D. and M.L.B. wrote the first draft of the manuscript. C.S.-D., M.L.B., J.E.L. and P.K. wrote the final draft of the manuscript with the contributions of the other co-authors. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
C.S.-D. received grants from the European Commission, Programme FP7-People-2011(SYMBIONTS 298735) and from the Palaeontological Association, UK (grant no. PARG201602). M.L.B. received a grant from the National Science and Engineering Research Council of Canada (Discovery grant no. RGPIN-2016-03746).
References
- 1.Mark DF, Rice CM, Trewin NH. 2013. Discussion on a high-precision U–Pb age constraint on the Rhynie Chert Konservat-Lagerstätte: time scale and other implications. J. Geol. Soc. 170, 701–703. ( 10.1144/jgs2011-110) [DOI] [Google Scholar]
- 2.Trewin NH, Rice CM. 2004. The Rhynie hot-spring system. Geology, biota and mineralisation. Proceedings of the Conference held in 2003. Trans. R. Soc. Edinb. Earth Sci. 94, 283–521. ( 10.1017/S0263593300000687) [DOI] [Google Scholar]
- 3.Trewin NH, Fayers SR, Kelman R. 2003. Subaqueous silicification of the contents of small ponds in an Early Devonian hot spring complex, Rhynie, Scotland. Can. J. Earth Sci. 40, 1697–1712. ( 10.1139/e03-065) [DOI] [Google Scholar]
- 4.Channing A, Edwards D. 2013. Wetland megabias: ecological and ecophysiological filtering dominates the fossil record of hot spring flora. Palaeontology 56, 523–526. ( 10.1111/pala.12043) [DOI] [Google Scholar]
- 5.Remy W, Taylor TN, Hass H, Kerp H. 1994. Four hundred-million-year-old vesicular arbuscular mycorrhizae. Proc. Natl Acad. Sci. USA 91, 11 841–11 843. ( 10.1073/pnas.91.25.11841) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor TN, Kerp H, Hass H. 2005. Life history biology of early land plants: deciphering the gametophyte phase. Proc. Natl Acad. Sci. USA 102, 5892–5897. ( 10.1073/pnas.0501985102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krings M, Taylor TN, Hass H, Kerp H, Dotzler N, Hermsen EJ. 2007. Fungal endophytes in a 400-million-yr-old land plant: infection pathways, spatial distribution, and host responses. New Phytol. 174, 648–657. ( 10.1111/j.1469-8137.2007.02008.x) [DOI] [PubMed] [Google Scholar]
- 8.Strullu-Derrien C, Kenrick P, Pressel S, Duckett JG, Rioult JP, Strullu DG. 2014. Fungal associations in Horneophyton ligneri from the Rhynie Chert (c. 407 Ma) closely resemble those in extant lower land plants: novel insights into ancestral plant–fungus symbioses. New Phytol. 203, 964–979. ( 10.1111/nph.12805) [DOI] [PubMed] [Google Scholar]
- 9.Illman WI. 1984. Zoosporic fungal bodies in the spores of the Devonian fossil vascular plant Horneophyton. Mycologia 76, 545–547. ( 10.2307/3793338) [DOI] [Google Scholar]
- 10.Taylor TN, Hass H, Remy W. 1992. Devonian fungi: interactions with the green alga Palaeonitella. Mycologia 84, 901–910. ( 10.2307/3760288) [DOI] [Google Scholar]
- 11.Taylor TN, Remy W, Hass H. 1992. Parasitism in a 400-million-year-old green alga. Nature 357, 493–494. ( 10.1038/357493a0) [DOI] [Google Scholar]
- 12.Taylor TN, Remy W, Hass H. 1992. Fungi from the Lower Devonian Rhynie chert: Chytridiomycetes. Am. J. Bot. 79, 1233–1241. ( 10.2307/2445050) [DOI] [PubMed] [Google Scholar]
- 13.Taylor TN, Remy W, Hass H. 1994. Allomyces in the Devonian. Nature 367, 601 ( 10.1038/367601a0) [DOI] [Google Scholar]
- 14.Hass H, Taylor TN, Remy W. 1994. Fungi from the Lower Devonian Rhynie chert: mycoparasitism. Am. J. Bot. 81, 29–37. ( 10.2307/2445559) [DOI] [PubMed] [Google Scholar]
- 15.Remy W, Taylor TN, Hass H. 1994. Early Devonian fungi: a blastocladalean fungus with sexual reproduction. Am. J. Bot. 81, 690–702. ( 10.2307/2445647) [DOI] [Google Scholar]
- 16.Krings M, Dotzler N, Taylor TN. 2009. Globicultrix nugax nov. gen. et nov. spec. (Chytridiomycota), an intrusive micro-fungus in fungal spores from the Rhynie chert. Zitteliana A 48/49, 165–170. [Google Scholar]
- 17.Krings M, Taylor TN. 2014. An unusual fossil microfungus with suggested affinities to the Chytridiomycota from the Lower Devonian Rhynie chert. Nova Hedwigia 99, 403–412. ( 10.1127/0029-5035/2014/0205) [DOI] [Google Scholar]
- 18.Krings M, Taylor TN, Martin H. 2016. An enigmatic fossil fungus from the 410 Ma Rhynie chert that resembles Macrochytrium (Chytridiomycota) and Blastocladiella (Blastocladiomycota). Mycologia 108, 303–312. ( 10.3852/15-224) [DOI] [PubMed] [Google Scholar]
- 19.Strullu-Derrien C, Wawrzyniak Z, Goral T, Kenrick P. 2015. Fungal colonization of the rooting system of the early land plant Asteroxylon mackiei from the 407-Myr-old Rhynie Chert (Scotland, UK). Bot. J. Linnean Soc. 179, 201–213. ( 10.1111/boj.12307) [DOI] [Google Scholar]
- 20.Strullu-Derrien C, Goral T, Longcore JE, Olesen J, Kenrick P, Edgecombe G. 2016. A new chytridiomycete fungus intermixed with crustacean resting eggs in a 407-million-year-old continental freshwater environment. PLoS ONE 11, e0167301 ( 10.1371/journal.pone.0167301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berbee ML, James TY, Strullu-Derrien C. 2017. Early diverging fungi: diversity and impact at the dawn of terrestrial life. Annu. Rev. Microbiol. 71, 41–59. ( 10.1146/annurev-micro-030117-020324) [DOI] [PubMed] [Google Scholar]
- 22.James TY, et al. 2006. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 443, 818–822. ( 10.1038/nature05110) [DOI] [PubMed] [Google Scholar]
- 23.Hibbett DS, et al. 2007. A higher-level phylogenetic classification of the Fungi. Mycol. Res. 111, 509–547. ( 10.1016/j.mycres.2007.03.004) [DOI] [PubMed] [Google Scholar]
- 24.Spatafora JW, et al. 2016. A phylum-level classifiation of zygomycete fungi based on genome-scale data. Mycologia 108, 1028–1046. ( 10.3852/16-042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones MDM, Richards TA, Hawksworth DL, Bass D. 2011. Validation and justification of the phylum name Cryptomycota phyl. nov. IMA Fungus 2, 173–175. ( 10.5598/imafungus.2011.02.02.08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.James TY, Letcher PM, Longcore JE, Mozley-Standridge SE, Porter D, Powell MJ, Griffith GW, Vilgalys R. 2006. A molecular phylogeny of the flagellated fungi (Chytridiomycota) and description of a new phylum (Blastocladiomycota). Mycologia 98, 860–871. ( 10.1080/15572536.2006.11832616) [DOI] [PubMed] [Google Scholar]
- 27.James TY, Porter TM, Martin WW. 2014. Blastocladiomycota. In The Mycota, vol. VII (eds McLaughlin DJ, Spatafora JW), pp. 177–207. Berlin, Germany: Springer. [Google Scholar]
- 28.Dee JM, Mollicone M, Longcore JE, Roberson RW, Berbee ML. 2015. Cytology and molecular phylogenetics of Monoblepharidomycetes provide evidence for multiple independent origins of the hyphal habit in the Fungi. Mycologia 107, 710–728. ( 10.3852/14-275) [DOI] [PubMed] [Google Scholar]
- 29.Harvey R, Lyon AG, Lewis PN. 1969. A fossil fungus from the Rhynie Chert. Trans. Br. Mycol. Soc. 53, 155–156. ( 10.1016/S0007-1536(69)80025-2) [DOI] [Google Scholar]
- 30.Abràmoff MD, Magalhães PJ, Ram SJ. 2004. Image processing with ImageJ. Biophotonics Int. 11, 36–42. [Google Scholar]
- 31.Kiriyama S, Nikon Instruments. 2014. Nikon ND2 Reader. Melville, NY: Nikon Instruments.
- 32.Spencer ART, Hilton J, Sutton MD. 2013. Combined methodologies for three-dimensional reconstruction of fossil plants preserved in siderite nodules: Stephanospermum braidwoodensis nov. sp. (Medullosales) from the Mazon Creek lagerstätte. Rev. Palaeobot. Palynol. 188, 1–17. ( 10.1016/j.revpalbo.2012.09.001) [DOI] [Google Scholar]
- 33.Sutton MD, Garwood RJ, Siveter David J, Siveter Derek J. 2012. SPIERS and VAXML: a software toolkit for tomographic visualisation and a format for virtual specimen interchange. Palaeontol. Electron. 15.2.4T. [Google Scholar]
- 34.Garwood RJ, Dunlop JA. 2014. The walking dead: Blender as a tool for palaeontologists with a case study on extinct arachnids. J. Paleontol. 88, 735–746. ( 10.1666/13-088) [DOI] [Google Scholar]
- 35.Porter TM, Martin W, James TY, Longcore JE, Gleason FH, Adler PH, Letcher PM, Vilgalys R. 2011. Molecular phylogeny of the Blastocladiomycota (Fungi) based on nuclear ribosomal DNA. Fungal Biol. 115, 381–392. ( 10.1016/j.funbio.2011.02.004) [DOI] [PubMed] [Google Scholar]
- 36.Karling JS. 1977. Chytridiomycetarum iconographia. Monticello, NY: Lubrecht & Cramer. [Google Scholar]
- 37.Barr DJS. 1980. An outline for the reclassification of the Chytridiales, and for a new order, the Spizellomycetales. Can. J. Bot. 58, 2380–2394. ( 10.1139/b80-276) [DOI] [Google Scholar]
- 38.Heath IB, Bauchop T, Skipp RA. 1983. Assignment of the rumen anaerobe Neocallimastix frontalis to the Spizellomycetales (Chytridiomycetes) on the basis of its polyflagellate zoospore ultrastructure. Can. J. Bot. 61, 295–307. ( 10.1139/b83-033) [DOI] [Google Scholar]
- 39.Longcore JE, Pessier AP, Nichols DK. 1999. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91, 219–227. ( 10.2307/3761366) [DOI] [Google Scholar]
- 40.Swofford DL. 2002. PAUP*. Phylogenetic analysis using parsimony (*and other methods), version 4. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 41.Maddison WP, Maddison DR. 2016. Mesquite: a modular system for evolutionary analysis, version 3.10. Austin, TX: Mesquite Software; See http://mesquiteproject.org. [Google Scholar]
- 42.Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. 2004. MycoBank: an online initiative to launch mycology into the 21st century. Stud. Mycol. 50, 19–22. [Google Scholar]
- 43.Robert V, Stegehuis G, Stalpers J.2005. The MycoBank engine and related databases. See http://www.mycobank.org .
- 44.Robert V, et al. 2013. MycoBank gearing up for new horizons. IMA Fungus 4, 371–379. ( 10.5598/imafungus.2013.04.02.16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Honegger R, Edwards D, Axe L, Strullu-Derrien C. 2017. Fertile Prototaxites taiti: a basal ascomycete with inoperculate, polysporous asci lacking croziers. Phil. Trans. R. Soc. B 373, 20170146 ( 10.1098/rstb.2017.0146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beakes GW, Thines M, Honda D. 2015. Straminipile “fungi” – taxonomy. eLS. ( 10.1002/9780470015902.a0001984) [DOI] [Google Scholar]
- 47.Strullu-Derrien C. 2016. Aquatic–terrestrial transitions in fungal evolution. In Encyclopedia of evolutionary biology, 1st edn, vol. 2 (ed. Kliman R.), pp. 97–103. Oxford, UK: Academic Press. [Google Scholar]
- 48.Barr DJS. 1984. The classification of Spizellomyces, Gaertneriomyces, Triparticalcar, and Kochiomyces (Spizellomycetales, Chytridiomycetes). Can. J. Bot. 2, 1171–1201. ( 10.1139/b84-161) [DOI] [Google Scholar]
- 49.Letcher PM, Powell MJ, Barr DJS, Churchill PF, Wakefield WS, Picard KT. 2008. Rhizophlyctidales, a new order in Chytridiomycota. Mycol. Res. 112, 1031–1048. ( 10.1016/j.mycres.2008.03.007) [DOI] [PubMed] [Google Scholar]
- 50.Martin WW. 1969. A morphological and cytological study of the development of Coelomomyces punctatus parasitic in Anopheles quadrimaculatus. J. Elisha Mitchell Sci. Soc. 85, 59–72. [Google Scholar]
- 51.Thaxter R. 1896. New or peculiar aquatic fungi. 3. Blastocladia. Bot. Gaz. 21, 45–52. ( 10.1086/327299) [DOI] [Google Scholar]
- 52.Couch JN. 1945. Observations on the genus Catenaria. Mycologia 37, 163–191. ( 10.2307/3754916) [DOI] [Google Scholar]
- 53.Hanson AM. 1945. A morphological, developmental, and cytological study of four saprophytic chytrids. I. Catenomyces persicinus Hanson. Am. J. Bot. 32, 431–438. ( 10.2307/2437362) [DOI] [Google Scholar]
- 54.Taylor T, Krings M, Taylor E. 2015. Fossil fungi. Burlington, MA: Elsevier/Academic Press. [Google Scholar]
- 55.Selosse MA, Strullu-Derrien C. 2015. Origins of the terrestrial flora: a symbiosis with fungi? BIO Web of Conferences 4, 1–12. ( 10.1051/bioconf/20150400009) [DOI] [Google Scholar]
- 56.Chang Y, et al. 2015. Phylogenomic analyses indicate that early fungi evolved digesting cell walls of algal ancestors of land plants. Genome Biol. Evol. 7, 1590–1601. ( 10.1093/gbe/evv090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parfrey LW, Lahr DJG, Knoll AH, Katz LA. 2011. Estimating the timing of early eukaryotic diversification with multigene molecular clocks. Proc. Natl Acad. Sci. USA 108, 13 624–13 629. ( 10.1073/pnas.1110633108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edwards D, Cherns L, Raven JA. 2015. Could land-based early photosynthesizing ecosystems have bioengineered the planet in mid-Palaeozoic times? Palaeontology 58, 803–837. ( 10.1111/pala.12187) [DOI] [Google Scholar]
- 59.Porada P, Lenton TM, Pohl A, Weber B, Mander L, Donnadieu Y, Beer C, Poschi U, Kleidon A. 2016. High potential for weathering and climate effects of non-vascular vegetation in the Late Ordovician. Nat. Commun. 7, 12113 ( 10.1038/ncomms12113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quirk J, Leake JR, Johnson DA, Taylor LL, Saccone L, Beerling DJ. 2015. Constraining the role of early land plants in Palaeozoic weathering and global cooling. Proc. R. Soc. Lond. B 282, 20151115 ( 10.1098/rspb.2015.1115) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supplementary three-dimensional model data are available at the Zenodo repository (doi:10.5281/zenodo.801505).