Abstract
Plant life cycles underwent fundamental changes during the initial colonization of the land in the Early Palaeozoic, shaping the direction of evolution. Fossils reveal unanticipated diversity, including new variants of meiotic cell division and leafless gametophytes with mycorrhizal-like symbioses, rhizoids, vascular tissues and stomata. Exceptional fossils from the 407-Ma Rhynie chert (Scotland) play a key role in unlocking this diversity. These fossils are reviewed against progress in our understanding of the plant tree of life and recent advances in developmental genetics. Combining data from different sources sheds light on a switch in life cycle that gave rise to the vascular plants. One crucial step was the establishment of a free-living sporophyte from one that was an obligate matrotroph borne on the gametophyte. It is proposed that this difficult evolutionary transition was achieved through expansion of gene expression primarily from the gametophyte to the sporophyte, establishing a now extinct life cycle variant that was more isomorphic than heteromorphic. These changes also linked for the first time in one developmental system rhizoids, vascular tissues and stomata, putting in place the critical components that regulate transpiration and forming a physiological platform of primary importance to the diversification of vascular plants.
This article is part of a discussion meeting issue ‘The Rhynie cherts: our earliest terrestrial ecosystem revisited’.
Keywords: plant, life cycle, fossil, homeodomain, gametophyte
1. Introduction
Life has existed on land for over 2.7 Gyr in the form of communities of bacteria and archaea inhabiting shallow bodies of freshwater and sediments. Later, these were joined by simple eukaryotes and sometime during the Late Neoproterozoic or Early Palaeozoic the green algal ancestors of plants [1]. The earliest land plants were probably simple filamentous organisms [2,3], and their initial diversification was accompanied by the evolution of fundamental organs and tissue systems, including modes of reproduction and dispersal, axes and stems, vascular system, diverse rooting structures and later leaves and wood [4–6]. From within the background consortium of microorganisms there arose mutualistic associations between plants and fungi [7,8] that were critical to nutrient provision and to soil formation [9]. These innovations were made possible by radical changes in life cycle [10–13]. Land plants evolved a biphasic life cycle in which a haploid gamete producing plant (gametophyte) alternates with a diploid spore producing plant (sporophyte). In bryophytes, the sporophyte is an obligate matrotroph existing as a simple stalked capsule that is always borne on and physiologically tied to the free-living leafy or thalloid gametophyte. This physiological dependence is broken in the basal clades of vascular plants. Here, the two phases of the life cycle lead independent existences, and thus freed the sporophyte was able to undergo massive evolutionary development, becoming the most conspicuous and productive element of terrestrial ecosystems.
The hypothesized shift from obligate matrotrophy was a pivotal event in plant evolution [6,11,12,14], but it is poorly understood in both mechanistic and ecological terms. Furthermore, although the broad outlines of life cycle evolution in plants can be inferred from the tree of life [10–13,15], much still remains unclear or ambiguous. One fundamental issue is that relationships among basal clades of land plants are still unsettled and alternative tree topologies are possible and even plausible [16–21]. Furthermore, the evolutionary transitions from one life cycle state to another are poorly understood. These difficulties are exacerbated by the ancient nature of the clades, which are isolated and divergent remnants of over 400 Myr of evolution [4]. To what extent, therefore, are the life cycles of modern species representative of those common in early floras? Tantalizing evidence from fossils hints at greater diversity in the past. Here, I review this fossil evidence against the background of recent progress in the plant tree of life and advances in developmental genetics. Together, these lines of evidence provide insights into how a major change in life cycle led to the evolution of the vascular plants.
2. Life cycle evolution—the view from the top of the tree
(a). Land plants evolved from charophytes with haplontic life cycles
The ground-breaking early cladistic analysis of green algae and bryophytes published by Mishler & Churchill in 1985 [22] did much to clarify thinking around life cycle evolution in early land plants (e.g. antithetic versus homologous theories) [13], and subsequent molecular phylogenetic treatments have further improved and constrained evolutionary scenarios [10,12,13,15], but many uncertainties remain. Land plants (embryophytes) are a monophyletic group that belongs to the green plant clade Streptophyta [23]. Their closest relatives are the charophycean algae. This is predominantly a freshwater group with some inhabiting moist terrestrial habitats and a few secondarily adapted to brackish or alkaline waters. Phylogenetic studies indicate that the closest relatives of land plants are to be found among the ‘higher charophytes’ (i.e. Coleochaetophyceae, Charophyceae s.str., Zygnematophyceae), and various sister groups have been proposed [3,24–26]. Recently, approaches based on phylotranscriptomics [17] and on whole plastid genomes [18] found robust support for a sister group relationship between land plants and Zygnematophyceae. This distinctive class of algae comprises some 4000 living species of coccoid, filamentous and colonial forms that lack a flagellate stage and that reproduce sexually by conjugation [27]. Molecular studies, therefore, point to a single origin of land plants from freshwater or perhaps terrestrial algae [2,28].
One can infer from the morphology of living charophytes that the last common ancestor (LCA) of land plants and higher charophytes was an alga probably with branched filaments and oogamous reproduction [2]. It shared various distinctive cellular features (e.g. plasmodesmata, phragmoplast, core cell wall polysaccharides), physiological and metabolic systems (e.g. type of photorespiration, phytochrome system), and similarities in spermatogenesis and male gamete ultrastructure with the charophytes [20,22,29–31]. Less certain is that it might already have been archegoniate, possessing multicellular gametangia (antheridia, archegonia) of the type in Charophyceae s.str. [10]. Land plants, therefore, evolved from organisms with a haplontic life cycle, which is one in which mitosis and development happen only in the haploid phase. They inherited key cellular and metabolic features from streptophyte green algae, but they evolved most of their fundamental organs and tissue systems on land.
(b). Phylogenetic uncertainty leads to ambiguity in tracing the early evolution of the land plant life cycle
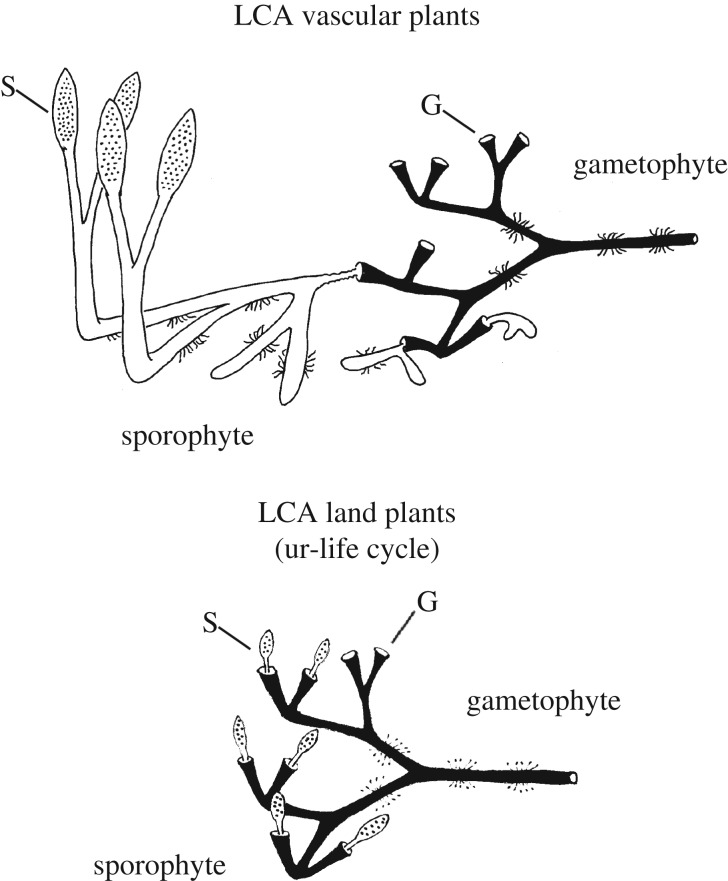
The tree of life clearly implies that the transition to land involved a shift from an ancestral haplontic life cycle to a haploid–diploid one, which was present in the LCA of land plants. In haploid–diploid life cycles mitosis and development happen in both haploid and diploid phases, giving rise to gametophytes and sporophytes respectively (figure 1). The nature of this original or primitive life cycle (ur-life cycle) and how it subsequently evolved is less certain, which is due in part to conflicting phylogenetic evidence. Early phylogenetic studies based on comparative morphology found bryophytes to be a paraphyletic group, with liverworts sister to all other land plants and mosses sister to the vascular plants [22]. Subsequent studies focusing mainly on molecular data recovered almost every possible alternative tree topology, which is not to say that every one is equally plausible. Increasing the amount of molecular data through genomics [18] and transcriptomics [17] and critical evaluation of the methods and systematic biases [16,18] are beginning to clarify some of the strengths and weaknesses of alternative topologies, but they have not yet provided an unambiguous answer. Based on reanalysis of molecular data from two previous studies Cox et al. [16] concluded that the prevailing hypothesis of full bryophyte paraphyly to vascular plants, and especially the sister group relationship between vascular plants and hornworts [18,32], is likely an artefact of convergent base composition induced by synonymous substitutions. A recent phylotranscriptomics analysis [17] recovered three different primary hypotheses: all grouped liverworts with mosses, which is a feature of some morphological datasets [20], but differed in the relationships of hornworts and vascular plants. Analysis of whole plastid genomes also recovered a liverwort–moss clade, once third codons were removed or the nucleotides converted to amino acids [18]. At present there seems to be insufficient grounds to reject with confidence any of these hypotheses [16,17]. Phylogenetic analyses, therefore, do not yet provide an unambiguous framework for tracing life cycle evolution among basal land plants, but they do impose some constraints.
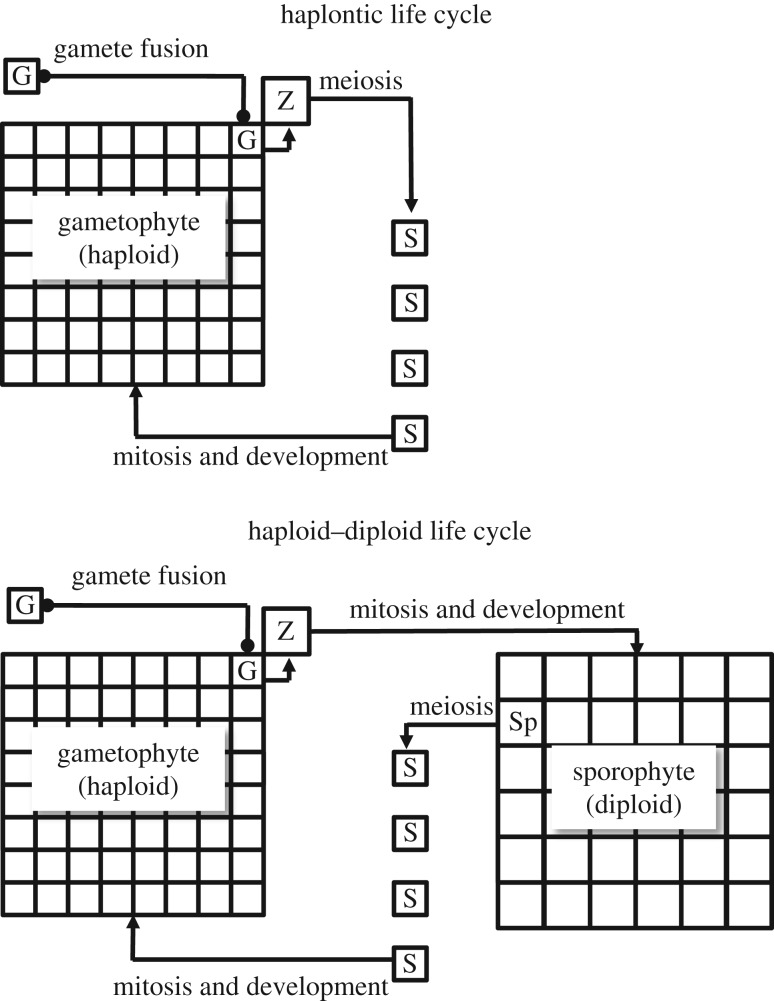
Figure 1.
Diagrammatic representation of haplontic and haploid–diploid life cycles. In these examples gamete (G) fusion takes place on the gametophyte, which retains the ensuing zygote (Z). Haplontic: zygotic meiosis gives rise directly to zoospores (S). Haploid–diploid: zygote undergoes mitosis and development. Spores (S) develop from spore mother cells (Sp) on the sporophyte. In bryophytes the sporophyte is matrotrophic (i.e. retained on and nurtured by the gametophyte). In basal vascular plants, gametophyte and sporophyte are separate, free-living organisms.
(c). Leading hypothesis proposes that the ur-life cycle of land plants was haploid–diploid with obligate matrotrophic sporophytes
In most phylogenetic analyses the bryophytes emerge as a paraphyletic group (figure 2). This led to the development of the prevailing view that the ur-life cycle in the LCA of land plants was bryophyte-like [11,12]. This is consistent with F. O. Bower's antithetic theory of the origin of the alternation of generations, which postulated that the first sporophytes evolved from matrotrophic zygotes through the interpolation of somatic cell divisions prior to meiosis, thus creating a simple matrotrophic sporophyte that was little more than a capsule within which spores developed [13,33]. Within this bryophyte paraphyly paradigm, the relative phylogenetic placement of liverworts, hornworts and mosses also has implications for inferring details of the ur-life cycle and the shift to independent sporophytes in the lineage leading to the vascular plants [22]. Ligrone et al. [12] proposed that the ur-life cycle possessed a free-living gametophyte that had bifurcating, leafless, radially symmetrical axes bearing unicellular rhizoids and mucilage papillae. Vascular tissues if present were simple tubular cells with perforate walls. The sporophytes were obligate matrotrophs of the gametophyte, comprising foot, short seta and sporangium. Alternative phylogenetic models of bryophyte paraphyly might have other implications for specific features of archetypes [3], but they would still all infer that the ur-life cycle had obligate matrotrophic sporophytes and that there was a transition to free-living ones in the vascular plants.
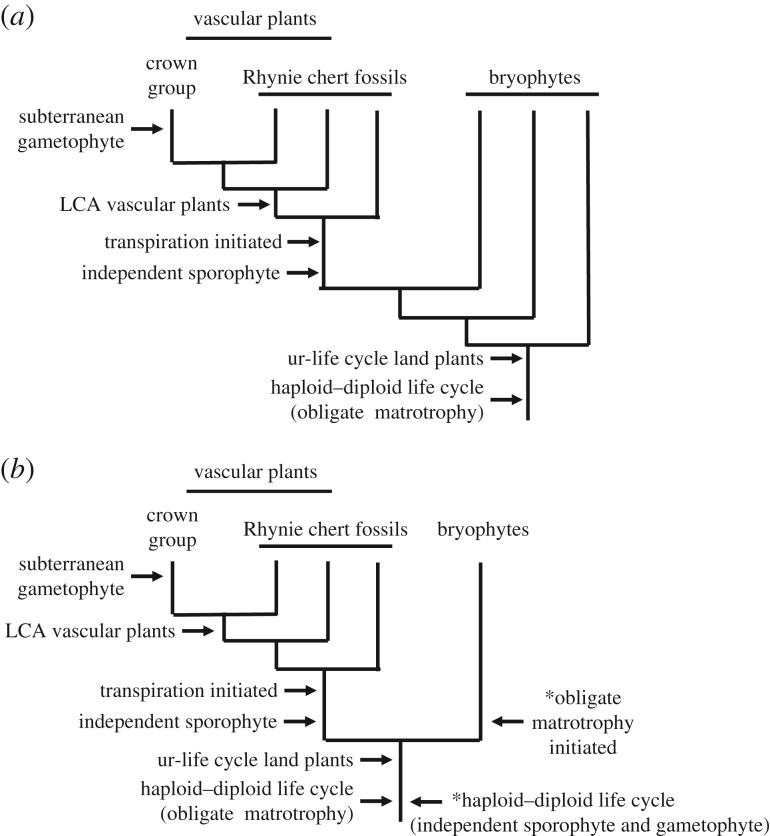
Figure 2.
Outline tree topologies showing aspects of conflict among phylogenies of basal land plants and some key events in life cycle evolution discussed. (a) Full bryophyte paraphyly to vascular plants. (b) Bryophyte monophyly. Key events discussed in text (arrows on left) occur in same sequence and equivalent points on both trees. *Two arrows on right of lower tree indicate key points argued in the Stebbins & Hill hypothesis of life cycle evolution [34].
(d). Alternative phylogenies can imply other modes of life cycle evolution
Arguably the most radical alternative hypothesis of relationships among land plants is the finding of bryophyte monophyly in some analyses (figure 2) [16]. If this is correct, it would necessitate a complete revision of our understanding of early life cycle evolution. It could reopen the door to hypotheses of the type proposed by Stebbins & Hill [34], which postulated the form of the ancestral life cycle, the mechanism by which it evolved, and the means by which the modern vascular plant and bryophyte life cycles diverged. In the Stebbins & Hill model the ur-life cycle was envisaged as isomorphic, with independent gametophyte and sporophyte phases (figure 2). Plant bodies were simple, possibly a little more complex than the charophycean alga Coleochaete. An attractive aspect of this hypothesis is that it proposed a mechanism for the evolution of the sporophyte from an ancestral haplontic life cycle and an ecological argument for how it became established and how the bryophyte and vascular plant life cycles subsequently diverged. Adaptations to life on land evolved first in the gametophyte and were later expressed in the sporophyte through insertion of a somatic phase between zygote formation and meiosis. Bryophyte and vascular plant life cycles were thought to have diverged as different ecological strategies for coping with life on land, opening the door to exploiting seasonal variation or differences in microhabitat at a local scale. Stebbins & Hill's is not the only plausible model of life cycle evolution compatible with bryophyte monophyly (figure 2). Nevertheless, this hypothesis has very different evolutionary implications. Because independent gametophyte and sporophyte phases already existed in the hypothetical ur-life cycle, under this model obligate matrotrophy becomes a derived characteristic of bryophytes.
3. Life cycles preserved in the rocks
The potential of the fossil record to shed light on early life cycle evolution has long been acknowledged, but until recently it was rightly judged to be of quite limited value [34]. First, there are preservation and collector biases. The first emergent land plants derived from charophycean algae were small, simple organisms lacking the more robust tissues of their modern relatives [2]. Therefore, they are both difficult to recognize as fossils and vulnerable during the fossilization process. Second, there are biases in the rock record meaning that the freshwater terrestrial sediments in which these organisms lived are rare during the critical Ordovician and Silurian Periods [4,34]. However, recent research demonstrates that discovering such environments although challenging is possible [1,35–37]. Third, the nature and affinities of some common Silurian and Devonian fossils that are potentially relevant remain enigmatic (e.g. Spongiophyton, Orestovia, Protosalvinia, Parka) [38,39]. Despite these difficulties, fossil evidence is now providing insights into life cycle variants in the earliest land-colonizing plants [40].
(a). The packaging of the products of meiosis was more diverse in early plants
The early record of dispersed spores includes forms with conspicuous trilete marks that first appeared in the upper part of the Ordovician Period (figure 3g–i) [41]. These are typical of the vascular plants and some bryophytes, where the trilete mark is indicative of meiosis. Preceding and overlapping with these is an extinct class of spores known as cryptospores (Ordovician–Devonian) (figure 3a–f) [42–45]. These were much more diverse, taking the form of alete monads and other types that were dispersed as tetrads and dyads, some of which were enveloped in a second wall layer [40]. Even though the affinities of most cryptospores are obscure, some are now known to be produced by minute land plants that possessed a combination of features not found together in living species [46–50]. Their fossilized remains are highly fragmentary [40], so many aspects of their overall morphology, biology and affinity still remain unclear. The cryptospore producers (termed cryptophytes) are thought to belong to a grade of organization that encompassed elements possibly of the land plant stem group or stem group members of the bryophytes and the vascular plants [5,40]. Today, obligate tetrads are found only in a handful of living hepatics, including Riccia, Sphaerocarpos and Cryptothallus; dyads (figure 3b,e) are not a product of normal meiosis. The fossil record of cryptospores, therefore, testifies to a far greater versatility in the ways that the products of meiosis were packaged among ancient embryophytes, but the forces driving this diversity and ultimately curtailing it remain obscure. At a mechanistic level these differences can be attributed to changes in the timing of sporopollenin deposition during meiotic cell division [40,51]. The abundance of spore dyads in the early fossil record raises the possibility that these rather than tetrads were the principal products of meiosis in the archetypical land plants [40].
Figure 3.
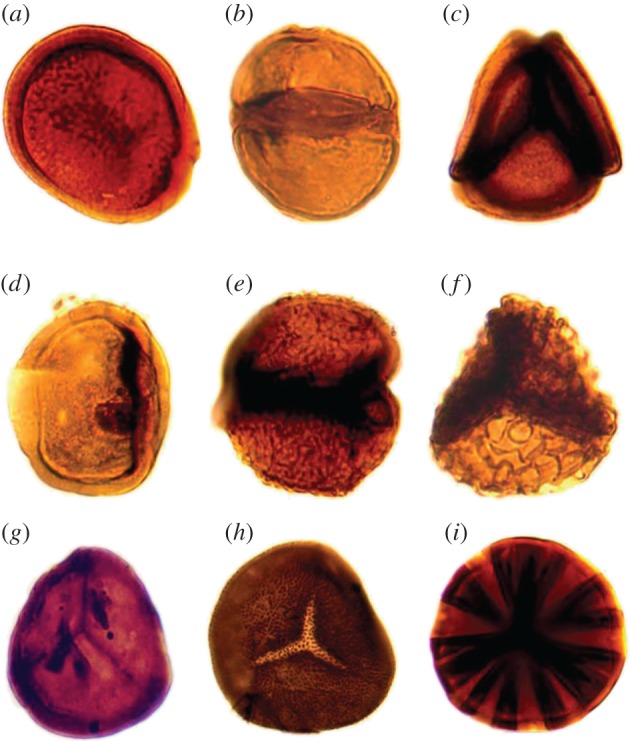
Fossil spores showing the diversity of the products of meiosis in early land floras. (a–f) Cryptospores from the Upper Ordovician (ca 450 Ma) of Oman. (a) Monad. (b) Dyad. (c) Tetrad. (d) Monad enclosed in an envelope. (e) Dyad enclosed in an envelope. (f) Tetrad enclosed in an envelope. (g) Unornamented trilete from the Lower Devonian (ca 415 Ma) of the Anglo-Welsh Basin. (h–i) Ornamented trilete from the Lower Devonian Rhynie chert (ca 407 Ma). Spores ca 20–30 µm diameter. First published in [4] (courtesy Charles Wellman). (Online version in colour.)
(b). New life cycle variants in the 407-Ma Rhynie chert
Whereas fossil spores provide insights into the diversity of meiosis in early land floras, and where found inside sporangia tantalizing glimpses of the sporophyte phase of the life cycle, the earliest evidence for whole-life cycles comes from the Rhynie chert locality near Aberdeen (Scotland). The Rhynie chert was discovered in 1912 and key elements of the flora were described in a ground-breaking series of papers published between 1917 and 1921 [52,53]. The plant bearing cherts formed in a geothermal wetland in which plants grew on or close to sinter surfaces where they were fossilized in silica by outwash from a hot spring system [54–58]. The site is exceptional because of the quality of the preservation, the faithful capturing of intimate associations among elements of the biota, and all in close proximity to the actual habitats in which they flourished. The vascular plants originally documented were all interpreted as sporophytes. These were small with simple bifurcating axes and most were leafless and rootless (figure 4). Axes in contact with the ground were rhizomatous or bulbous bearing rhizoids. Direct evidence for gametophytes was lacking. Reflecting on this, Kidston & Lang [59] and later Bower [33] concluded that the absence of evidence of gametophytes implied that they must have been even simpler and less robust than the sporophytes. Others disagreed. One influential alternative interpretation held that the rhizomes were in fact gametophytes from which upright aerial sporophytes developed [60]; however, key evidence for the gametophytic status was disputed [61,62]. Although this interpretation did not receive universal acceptance, it drew attention to the possibility that the two phases of the life cycle might have strongly resembled one another.
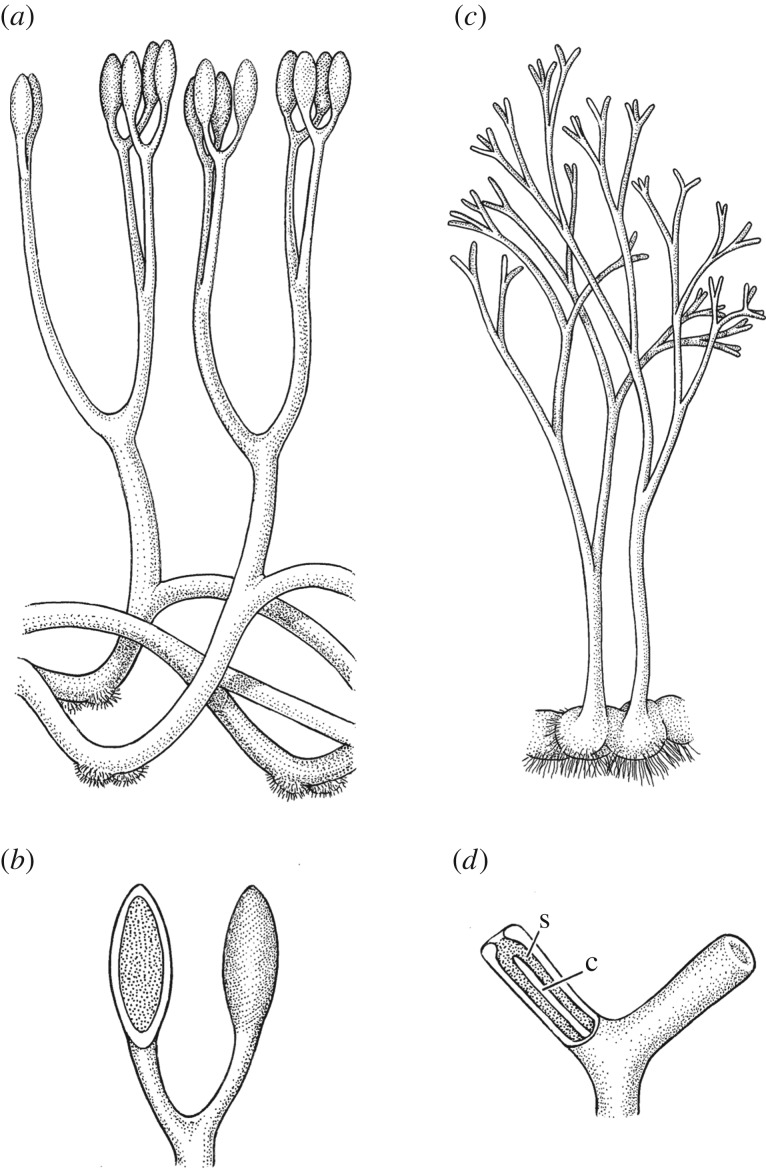
Figure 4.
Two Rhynie chert sporophytes reconstructed. (a,b) Aglaophyton majus: (a) habit (ca 15 cm tall) and (b) detail of sporangia with one on left cut away to reveal inner spore cavity. (c,d) Horneophyton lignieri: (c) habit (ca 10 cm tall) and (d) detail of sporangia with one on left cut away to reveal inner spore cavity (s) and columella (c). Adapted from drawings by Pollyanna Lidmark first published in [19].
This idea was later borne out in a series of works by Remy et al. [63] (reviewed in [64]), which provided compelling evidence for gametophytes, including the key observation of well-preserved archegonia and antheridia. The essential stages of the life cycles are now known for four of the six species of land plant [62,65]. The simple axes bearing the gametangia were upright and cylindrical with a central strand of vascular tissues, a cortex and an epidermis with stomata and rhizoids at the base [62,63]. The gametophytes were, therefore, free-living. Two distinct size categories are known. Remyophyton and Lyonophyton were tiny plants, significantly smaller than their corresponding sporophytes (Rhynia and Aglaophyton). Remyophyton had upright cylindrical axes (10–20 mm long) that typically were unbranched, and that bore embedded archegonia (figure 5a). In larger specimens, antheridia were borne on peltate to bowl-shaped apices. Remyophyton grew in dense stands a few centimetres in diameter containing hundreds of gametangiophores arising from globular rhizoid-bearing bases. Plants of Lyonophyton developed fleshy protocorms, from which several gametangiophores arose. Archegoniophores bifurcated, whereas antheridiophores were unbranched. The tips were slightly expanded with shallow central depressions (figures 5b, 6). Plants were small, growing to a height of about 2 cm. The second category of gametophytes, Langiophyton and Kidstonophyton, were larger plants. Although their overall growth form is still incompletely known they are thought to be comparable in size but still somewhat smaller than their corresponding sporophytes (Horneophyton and Nothia). Their upright axes were also cylindrical, and they contained massive conducting tissues. Antheridia and archegonia were borne on well-developed peltate to bowl-shaped apices [62,63]. In general, the gametangia were larger than those of modern vascular plants [63]. Gametophytes were seemingly dioecious [63,65], but one cannot rule out the possibility of gender diphasy. It is unclear whether the sporophytes were cosexual or dioecious [65]. These fossils, therefore, demonstrate the existence of a completely new life cycle variant in land plants. Its unique and key defining feature is that the gametophyte and sporophyte are indistinguishable histologically, except for the presence of either gametangia or sporangia. The two parts of the life cycle bore a much greater degree of similarity than is found among living species.
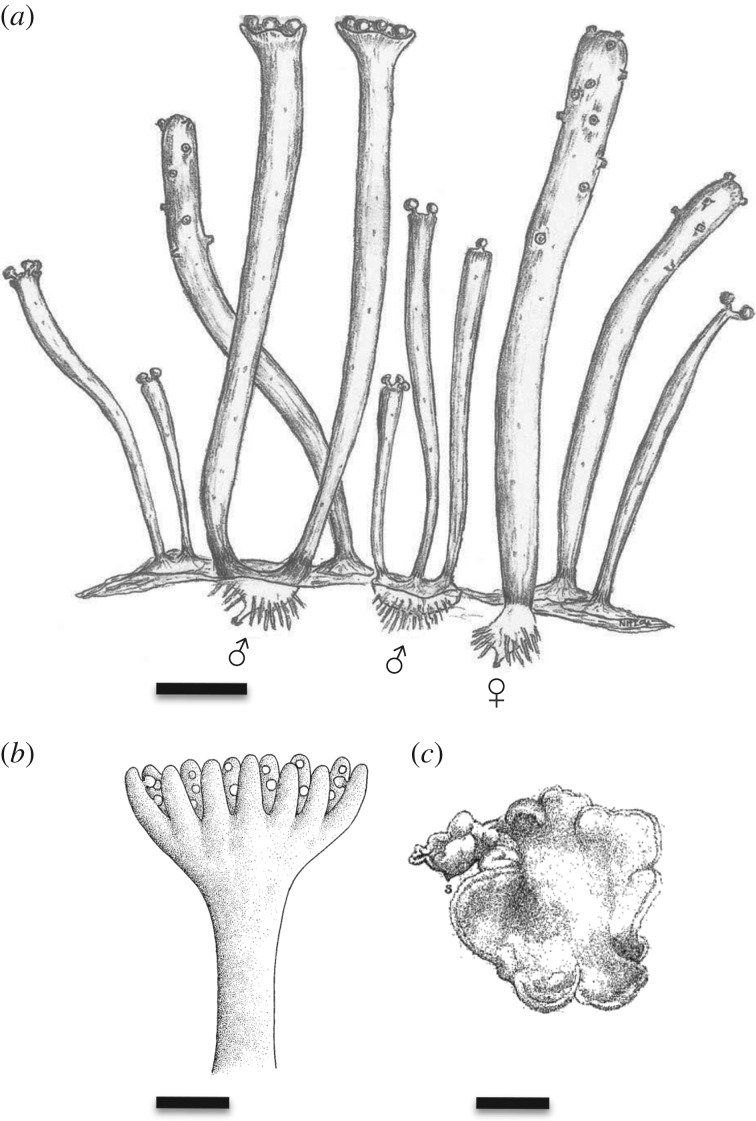
Figure 5.
Gametophytes and gametangiophores ancient and modern. (a) A reconstruction of mature male and female Remyophyton delicatum gametophytes. The antheridia are generally borne on shorter axes and the archegonia on longer ones. The substrate has been omitted to reveal the protocorms and rhizoids. Scale bar, 2 mm. Reproduced by permission of the Royal Society of Edinburgh from [62]. (b) Reconstruction of Lyonophyton rhyniense gametangiophore and short part of subtending axis. Antheridia borne on upper surface of cup. First published in [64]. Scale bar, 2 mm. (c) Illustration of subterranean gametophyte of living Lycopodium annotinum. Gametangia (not shown) borne on upper surface of convoluted disc. Scale bar, ca 2 mm. First published in [66].
Figure 6.
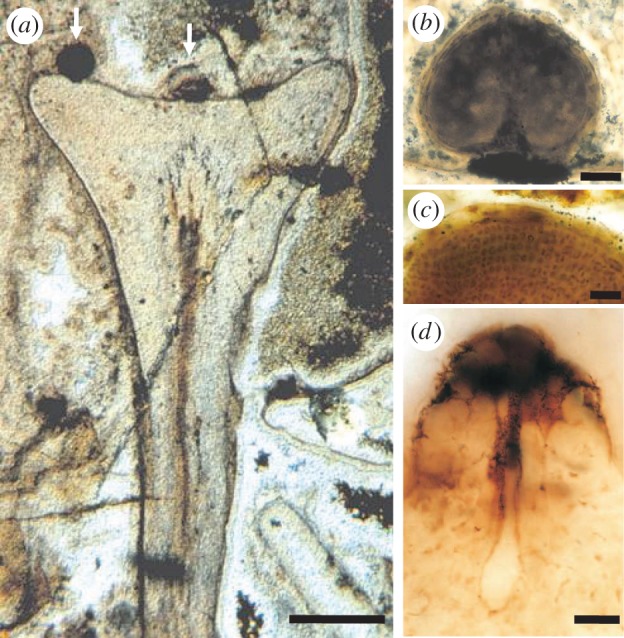
Fossilized gametangiophore of Lyonophyton rhyniense from the Rhynie chert. (a) Longitudinal section through a cup-shaped gametangiophore bearing two visible antheridia on the upper surface (arrows). The subtending axis contains a central strand of vascular tissues. Scale bar, 1 mm. (b) Longitudinal section through a spherical antheridium. Scale bar, 100 µm. (c) Sperm cells inside antheridium. Scale bar, 30 µm. (d) Archegonium in longitudinal section showing neck, neck canal and egg chamber. Scale bar, 30 µm. Reproduced by permission of the Royal Society of Edinburgh from [62].
(c). The gametophyte phase is under-recorded in early fossil floras
Exceptional cellular preservation in the Rhynie chert enabled the identification of gametangia, leading finally to the recognition of the gametophyte generation. However, in most Late Silurian and Early Devonian sites fossils are less well preserved, typically taking the form of thin coalified films (figure 7) [38]. Frequently, epidermal features are preserved as cuticles and the more robust internal tissues in minerals, but cellular level detail of gametangia is only likely to survive under the most exceptional conditions [62]. Other cues are required to recognize gametophytes, and evidence from the Rhynie chert suggests several possible lines of enquiry. First, in several species the most distinctive feature is the gametangiophore itself. This developed through expansion of the apex into a rimmed disc or cup-like structure up to 1 cm in diameter (figures 5b, 6a) [63]. Such features could be preserved as compressions. Second, although gametangia are small and their cellular structure unlikely to survive fossilization, their outlines and position might also be conserved as compression features. Moreover, we would anticipate observing such features on the putative gametangiophores [63,64]. Third, overall size might also provide clues. In several species the gametophyte is significantly smaller than the sporophyte, and in one it is known to grow in dense stands [62]. Even the larger gametophytes are thought to be somewhat smaller than corresponding sporophytes, but rigorous comparative study is lacking. Finally, differences in ploidy between sporophyte and gametophyte should manifest itself as differences in cell volume [70]. Guard cell length of stomata, a widely used proxy in plants for genome size [71], might, therefore, provide an additional means of distinguishing the two phases of the life cycle. Palaeobotanical works overwhelmingly document sporophytes because of their often distinctive sporangia, but at most sites there are abundant additional remains that are less readily attributable. These represent a pool of potentially gametophytic or sporophytic materials. Gametophytes have gone unrecognized partly because they resemble sporophytes and partly owing to their diagnostic features being more subtle. The gametophyte was, therefore, probably much more prominent in these early environments than the written record suggests.
Figure 7.
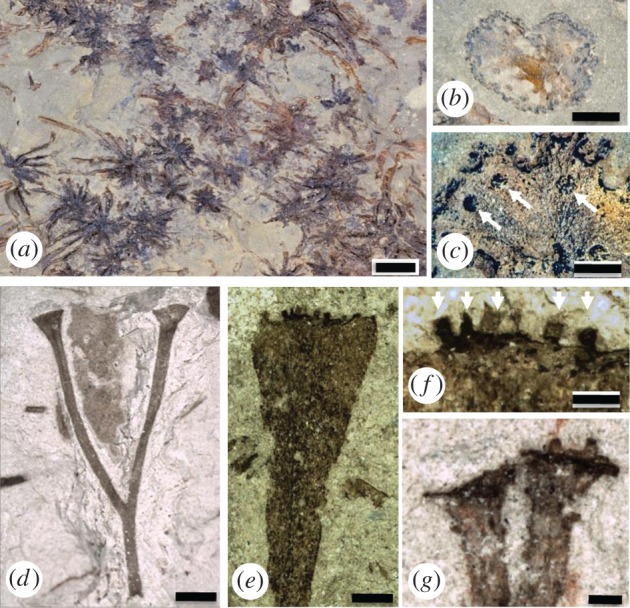
Putative fossil gametophytes and sporophytes preserved as thin coalified films (Early Devonian). (a–c) Sciadophyton (Germany). (a) Basal regions of several plants with leafless axes radiating from a central point. Scale bar, 1 cm. (b) Upper surface of disc-shaped terminal gametangiophore. Scale bar, 0.5 cm. (c) Details of upper surface of gametangiophore with small circular mounds (arrows) interpreted as gametangia. Scale bar, 1 mm. (d) Cooksonia paranensis (Brazil) sporophyte with trumpet-shaped apices interpreted as sporangia. Scale bar, ca 2 mm. First published in [67]. (e,f) Pisa37 (Brazil). First published in [68]. (e) Gametangiophore with flared apex. Scale bar, 2 mm. (f) Details of apex with small rounded to elongate bodies (arrows) interpreted as gametangia. Scale bar, 1 mm. (g) Pertonella sp. (Brazil) with flared apices (interpreted as sporangia) bearing small projections. Scale bar, 0.5 mm. First published in [69]. Images d, e, f, g courtesy of Philippe Gerrienne.
(d). Gametophytes are known from other geological sites
Unequivocal fossil evidence of gametophytes at sites other than the Rhynie chert is sparse. The most compelling examples are named Sciadophyton, and occur widely in sediments of the Early Devonian [72,73]. These take the form of distinctive coalified compressions of narrow, leafless axes (approx. 2.0–2.6 mm wide; greater than 9 cm long) that diverge from a central point like the spokes of a wheel (figure 7a). Axes bifurcate infrequently. In specimens that are partly mineralized, a vascular strand of simple helically thickened tracheids (S-type cells) was observed [74]. Each branch terminates in a shallow cup (approx. 3.5–15.0 mm wide; figure 7b) with a weakly lobed margin bearing oval to circular bodies (approx. 0.20–0.55 mm wide; figure 7c) on the upper surface. The oval bodies are typically smaller and denser at the margins and larger and less dense towards the middle. Fossils of Sciadophyton can exceed 9 cm in length. Other fossils, named Calyculiphyton, from the Early Devonian of Germany, possess a different mode of branching [63,75]. The axes are similarly leafless (1.0–2.0 mm wide) but with strong main leader and subordinate laterals in helical arrangement. Each branch terminates in a shallow cup (1.9–8.4 mm wide) with lobed or entire margins bearing centrally located, superficial, clavate to circular bodies (ca 0.6 mm diameter) on the upper surface. Plants of Calyculiphyton would have exceeded 20 cm in length. These fossils are remarkably similar to some of the larger gametophytes from the Rhynie chert. In particular, the cup-shaped gametangiophores are comparable in shape, size, and position. Furthermore, the circular or clavate bodies borne on the upper surface are similar to antheridia. So, even though the histology of the putative gametangia of Sciadophyton and Calyculiphyton is not preserved, the combination of other features makes a compelling case for gametophytes.
(e). Life cycle phases can be misattributed
The gametangiophores of Sciadophyton are quite distinctive, making them easily recognizable in compression floras at many sites. More recently documented fossils from the Rhynie chert show that the gametangiophores of another species were not nearly so distinctive, and they were a lot smaller (Remyophyton; figure 5a) [62]. Could such structures be observed in coalified materials? One recent example from the Lower Devonian of Brazil indicates that they can (figure 7e,f) [68]. This is a fragment of putative gametangiophore with a truncate end measuring 6 mm in width. Minute peg-like to clavate protrusions emerging from the end are plausible gametangia. Careful observations of small coalified fossils could yield further information on the gametophyte generation in early floras.
Some forms of gametangiophore converge on sporangia in shape and size, meaning that they can be difficult to distinguish, opening up the possibility of mistaking gametophyte for sporophyte and vice versa. Examples of fossils of ambiguous nature include some attributed to Cooksonia from the Lower Devonian of Eifel, Germany [76]. These particular specimens possess numerous 4 cm long bifurcating axes arising from a central point. Each terminates in an expanded head interpreted as a sporangium, implying that it was a sporophyte. Alternatively, this fossil could equally plausibly be reinterpreted as a gametophyte [77]. The putative sporangia are highly variable in shape, with the better developed ones resembling cup-shaped gametangiophores. Furthermore, spores—a diagnostic feature of sporangia—were not observed. Here, features originally interpreted as sporangia might in fact be gametangiophores fossilized at different developmental stages.
Within the current circumscription of the genus Cooksonia [67], some fossil are undoubtedly sporophytes because spores have been observed in situ. Others with flared, trumpet-shaped ends might plausibly represent a more or less isomorphic gametophyte phase. One of these is Cooksonia paranensis from the Early Devonian of Brazil [69]. This was interpreted as a sporophyte that developed from a minute thalloid gametophyte [77]. Bifurcating axes (0.3 mm–1.1 mm wide) with flared ends (0.58 mm–3.84 mm wide) (sporophyte; figure 7d) developed from a small carbonized layer at the base of the plant (thalloid gametophytes). No plausible gametangia were identified and no spores or dehiscence feature were observed [67]. A fossil such as this could be reinterpreted as a gametophyte bearing trumpet-shaped gametangiophores similar to those of the Rhynie chert gametophytes (e.g. Lyonophyton; figure 6a).
Other fossils present similar difficulties of interpretation. Specimens attributed to Pertonella from the same site as C. paranensis are fragments (less than 8.0 mm long) of minute bifurcating axis (0.8–1.2 mm wide) with flared, trumpet-shaped ends (figure 7g) [69]. These bear minute projections (approx. 0.25 mm width/length) with rounded or truncated tops. The fossil was interpreted as a sporophyte, but no spores were recovered. It could plausibly be reinterpreted as a gametophyte resembling the Rhynie chert gametophyte Remyophyton (figure 5a) [62]. The projections might be the compressed remains of archegonia, which in Remyophyton are distributed on and below the slightly flared apices. None of the above reinterpretations of fossils originally described as sporophytes is certain. Attention is drawn to these examples simply to make the point that other plausible interpretations are open. There is a tendency in the literature always to presume sporophytic status, which becomes less tenable with our developing understanding of the Rhynie chert life cycles.
4. Origin of the vascular plant life cycle
(a). The problem of the matrotrophic sporophyte
The significance of these discoveries for our understanding of the early evolution of life cycles in land plants hinges on determining the relative position of the fossils in the plant tree of life. Phylogenetic analyses place Rhynie chert plants including Rhynia, Aglaophyton and Horneophyton in the vascular plant stem group [19,21,77,78] (figure 2). Although the sporophyte generation of fossils attributed to Sciadophyton still remains speculative [64], some of the gametophytes are known to possess distinctive tracheids similar to those of Rhynia (S-type), also strongly indicating vascular plant stem group. Other fossils are still too poorly characterized to place with assurance into the plant tree of life. Calyculiphyton is potentially highly significant because its overall habit is more indicative of the extinct zosterophylls, hinting at possible affinities within the vascular plant crown group. The new life cycle variants that are known in sufficient detail are, therefore, more closely related to the vascular plants than to the bryophytes. Furthermore, it is likely that this relationship holds under either scenario of monophyletic or paraphyletic bryophytes (figure 2). The life cycle of the LCA of the vascular plants, therefore, possessed free-living, leafless gametophyte and sporophyte. Where known, the fossils show that the gametophyte is smaller than its associated sporophyte [68], but both phases possessed similar tissue systems and general growth architecture. The key evolutionary step in the origin of the vascular plants was the transition of the sporophyte from obligate matrotrophy in the ur-life cycle to free-living status [6,11,12,14] (figure 2). Intuitively, this seems to be the most difficult of evolutionary steps. How does a typically tiny, poorly equipped, parasitic phase of the life cycle manage to complete the transition to self-supporting autotroph?
(b). Shifts in genome expression between life cycle phases release the sporophyte and initiate the process of transpiration
An answer to the problem was foreseen in 1980 by Stebbins & Hill in a different context [34]. They faced a similar challenge explaining the origin of the sporophyte generation in developing their hypothesis of the land plant ur-life cycle. To put it in modern terms, the answer is likely to lie in the recruitment of ancient genes and gene regulatory networks (GRNs) from the more developed and already autotrophic gametophyte, and now there is a growing body of corroborating evidence from the molecular developmental genetics of moss (Physcomitrella patens) and angiosperm (Arabidopsis thaliana) model organisms [79,80]. Significantly, two recent well-documented examples involve tissue systems related to rooting and water conduction, which are key functions in a free-living plant. Rhizoid and protonemal development in moss gametophytes and root hair development in vascular plant sporophytes share an ancient GRN kernel employing common ROOT HAIR DEFECTIVE SIX-LIKE (RSL) Class I and Class II transcription factors and LOTUS JAPONICUS ROOTHAIRLESS1-LIKE (LRL) genes [80–82]. Thus, cells involved in anchorage and nutrient acquisition use the same basic genetic programme in both gametophyte and sporophyte. Hydroid development in the moss gametophyte and xylem development in the vascular plant sporophyte are regulated by the same group of transcription factors, indicating that these systems too are homologous developmentally [83]. Furthermore, polar auxin transport—a key regulator of sporophyte development in flowering plants—is now known to be essential to the patterning of development in both phases of the moss life cycle [84,85]. These discoveries in plant developmental genetics are consistent with the life cycle variants in early fossils, which imply that similar gene expression profiles and auxin-mediated regulation of tissue differentiation operated in both haploid and diploid phases of their life cycles.
The problem of the transition from obligate matrotrophic sporophyte to free-living one might, therefore, have been solved by the gametophyte. Key modes of development and functionality that enabled independent autotrophic existence (e.g. apical meristem, axial growth form, water transport, anchoring and absorption) evolved first in the gametophyte and were later expressed in the sporophyte enabling it to become free-living too. In the fossils similar histology in both phases of the life cycle indicates that this did not happen in a piecemeal fashion. More likely, the sporophyte of the vascular plants evolved by wholesale co-option of those aspects of the gametophyte developmental programme that enabled it to live and grow as an independent organism. In other words, during the evolution of the vascular plant life cycle there was a transitional phase in which the gametophyte body plan was substantially expressed in the sporophyte (figure 8). This need not have resulted in a precisely isomorphic phenotype, but it furnished the sporophyte with a sufficient phenotype to live freely.
Figure 8.
Origin of the vascular plant life cycle. The sporophyte in the last common ancestor (LCA) of land plants is an obligate matrotroph. The free-living sporophyte of the LCA of the vascular plants evolved by an expansion of gene expression such that the gametophyte body plan, already capable of free-living autotrophic existence, was substantially expressed in the sporophyte. S, sporangium; G, gametangiophore.
Substantial changes in life cycle of the type envisaged here would also have major structural and physiological consequences. Whereas rhizoids and primitive vascular system probably evolved in the gametophyte [12], stomata are thought to have originated in the matrotrophic sporophyte to facilitate spore discharge through capsule desiccation, only later acquiring a role in the regulation of gaseous exchange [86,87]. The hypothesized expansion of genome expression in the two phases of the life cycle brought together new combinations of cell types and organs. Thus, rhizoids, vascular system and stomata were linked developmentally for the first time. This change in life cycle, therefore, also put in place the key components that regulate transpiration, which formed a physiological platform of primary importance to the establishment and subsequent diversification of the vascular plants.
(c). Reduction and simplification of gametophyte linked to subterranean mycotrophic phase
The life cycles of the fossil plants discussed here are at odds with the widely held view that the gametophyte generation of the earliest vascular plants was a simple thalloid plant akin to the modern hornworts or the surficial photosynthetic gametophytes of ferns [12,15,22]. Their axial growth and the presence of a vascular system and stomata are notable differences. How far did this new life cycle variant persist into the vascular plant crown group and are there any vestiges in species living today? One hypothesis holds that gametophyte reduction happened early in the stem group, resulting in a simple thalloid form akin to the autotrophic gametophytes of some modern pteridophytes [77]. A second proposes that highly differentiated gametophytes persisted into the vascular plant crown group in early members of the euphyllophytes and lycophytes [64,88]. Neither yet has clear support. Discovery of the gametophyte of the Rhynie chert lycopod Asteroxylon mackiei (vascular plant crown group) could, therefore, prove decisive. A later reduction of the gametophyte from complex axial ancestral forms would be more consistent with the view that axial gametophytes are plesiomorphic in living vascular plants [13]. The subterranean gametophytes of Ophioglossaceae, Psilotaceae and Lycopodiaceae typically take an axial, branched form with rhizoids and mycorrhizal fungi [89]. Vascular systems are generally absent, but tracheids, phloem and endodermis were observed in the gametophytes of one polyploid race of Psilotum [89]. Certain forms of Lycopodiaceae gametophytes are especially interesting because apical growth ceases with the apex differentiating into a terminal trumpet-shaped or cup-shaped structure on which the sporophyte develops. Other non-axial forms develop into distinctive disc-shaped gametangiophores with a convoluted margin (figure 5c). Both types resemble known or suspected fossil gametangiophores. The loss of vasculature, stomata and the reduced axial growth form could be explained by the intriguing idea that the gametophyte generation of vascular plants went through a persistent subterranean mycotrophic phase [13].
5. Fossil phenotypes are relevant to plant developmental genetics
In addition to providing insights into the origins of the life cycles of vascular plants, the Rhynie chert fossils present an additional set of phenotypes against which to evaluate our growing understanding of the molecular developmental basis of life cycle regulation. There has been much progress recently in our understanding of the genetic basis of the alternation of generations [90,91]. In green plants two families of homeodomain proteins play key roles: KNOTTED-LIKE HOMEOBOX (KNOX) and BELL-LIKE HOMEOBOX (BELL). Their interactions are known to be deep rooted in gamete gender identity and zygote development of the chlorophyte alga Chlamydomonas reinhardtii [92]. KNOX and BELL genes underwent numerous duplications within the streptophyte clade giving rise to multiple paralogues [93,94]. The KNOX family split into two classes within the charophytes (Class I, Class II) before the evolution of the multicellular diploid sporophyte [94,95]. In the moss Physcomitrella patens, both are crucial for sporophyte development. Neither class of KNOX genes is functional during vegetative growth of the gametophyte, but both are activated in the egg cell and the surrounding archegonial cells. Developmental genetics further indicates that in P. patens one BELL paralogue is involved in the haploid to diploid transition [96] and that KNOX2 activity in the developing sporophyte represses the gametophyte genetic programme [97]. In the gametophyte, sporophyte-specific developmental programmes are repressed via epigenetic control of sporophyte-specific gene expression exerted by POLYCOMB REPRESSIVE COMPLEX 2 (PRC2) genes [98]. This works by silencing transcription factor genes via histone methylation and known targets of PRC2 include KNOX and BELL. Thus, some of the genetic machinery controlling the haploid-to-diploid transition in green algae is conserved in land plants and this has been extended and developed via expansion and neofunctionalization of the relevant gene families and via the evolution of novel epigenetic mechanisms.
The early fossil life cycle variants discussed here differ significantly from contemporary plant life cycles in the much greater similarity between sporophyte and gametophyte. Although similar, it has been pointed out rightly that they are not strictly isomorphic [10,68]. Where known, gametophytes are generally smaller than sporophytes, but overall size and growth architecture of the larger Rhynie chert gametophytes is still unknown. Also, we should bear in mind that differences in growth form between phases of the life cycle do not necessarily imply underlying differences in genetic regulation. In the brown seaweed Ectocarpus, life cycle phases that are near isomorphic under laboratory culture can exhibit marked heteromorphism in the field [99]. Nevertheless, from a developmental perspective the significant point about the fossils is that with the exception of the phase defining organs (i.e. gametangia, sporangia) all tissue types and organ systems are expressed in both gametophyte and sporophyte. One might, therefore, conclude that broadly the same genetic programme is being expressed in the two phases of the life cycle. Thus, if the expression and function of KNOX/BELL in the sporophyte (repression of gametophyte development) and PRC2 in the gametophyte (repression of sporophyte development) are conserved in Viridiplantae the downstream gene regulatory networks that they control must differ in the Rhynie chert plants from those in modern vascular plants and the balance of these controls has also shifted between life cycle phases.
6. Future directions
Fossils are beginning to reveal new and diverse life cycle variants among the earliest land plants. The nature of these life cycles is still comparatively poorly understood and further surprises may be in store. Studies of spore development in fossil and living plants could shed further light on the mysterious early meiotic variants [100,101]. Further basic information is needed on overall growth habit of the gametophyte phase of most Rhynie chert plants as well as comparisons with meristem structure in living groups (e.g. Lycopodiaceae). The Rhynie chert still holds information on the life cycle of the earliest crown group vascular plants (e.g. Asteroxylon mackiei), which is currently unknown, but is crucial to understanding how and perhaps why the gametophyte phase of vascular plants became reduced and simplified. The Rhynie chert is a unique site, but compression fossil floras are much more abundant. The ecological roles of the gametophyte and its prevalence in these early marginal lake and river environments may have been greatly underestimated and needs reappraisal.
Recent research on model organisms in green plants highlights the central importance of homeodomain transcription factors and epigenetics in the regulation of life cycles. There is still much to learn about how KNOX/BELL expression and chromatin modification by PRC2 influence the development of gametophytes and sporophytes in groups that bridge the phylogenetic gap between model moss (P. patens) and model angiosperms (e.g. A. thaliana) [90,98]. Particularly interesting taxa include vascular plants with well-developed axial gametophytes, including Lycopodiaceae, Psilotaceae and Ophioglossaceae. Gametangiophores in some Lycopodiaceae possess a meristematic feature similar to that in some of the earliest known fossil gametophytes, and diploid gametophytes of Psilotaceae have been known to differentiate vascular tissues. The shift in life cycle from matrotrophic sporophyte to free-living one was caused by a significant redeployment of genetic regulatory mechanisms. These changes also linked for the first time in one developmental system rhizoids, vascular tissues and stomata, putting in place the critical components that regulate transpiration in the vascular plants.
Broadening comparisons to life cycles in other eukaryotes could deepen our understanding of the early evolution of novel plant life cycle variants and the ecological conditions under which they flourished [90]. Many red, brown and green seaweeds have haploid and diploid phases that encompass a full spectrum of forms from extreme heteromorphy to isomorphy [15]. Moreover, recent molecular phylogenetic analyses reveal considerable switching among life-history strategies [102,103]. During the crown group radiation of brown algae (Cretaceous–Paleogene) isomorphic life cycles evolved repeatedly from heteromorphic antecedents [102]. The most widely evoked ecological explanation for the maintenance and diversity of haploid–diploid life cycles postulates that they have a capacity to exploit a broader range of environmental conditions because the two ploidy phases occupy different ecological niches [99]. So, as originally envisaged by Stebbins & Hill [34], the evolution of haploid–diploid life cycles in land plants and the subsequent divergence of the two phases might be explicable in ecological terms. In many seaweeds the life cycle alternates between crustose and foliose phases, which has been interpreted as an adaptation to seasonal variation [99]. Crustose forms growing as filaments in crevices in the substratum can withstand harsh winter conditions, whereas foliose forms are able to take advantage of more benign spring conditions to grow rapidly and to reproduce. Similar forces might have been at play driving the early evolution of morphological differences between sporophyte and gametophyte in vascular plants. Perhaps the gametophyte generation went through a persistent subterranean mycotrophic phase (crustose form) whereas the sporophytes flourished on a more seasonal basis above ground (foliose form). Increasingly detailed phylogenies of algae together with an understanding of the genetic regulatory basis of their life cycles will help in furthering our understanding of the early evolution of life cycles in plants.
Acknowledgements
I thank Dianne Edwards, Liam Dolan, Hans Kerp, Gar Rothwell and John L. Bowman for their many very stimulating papers and their astute discussion and the latter two additionally for their reviews of the manuscript. I am indebted to the European Union funded PLANTORIGINS-ITN for financial support and to my colleagues in this programme for their insights, constructive criticisms and for their collaborative interdisciplinary perspectives. Last, but not least, I thank the Hooke Committee of the Royal Society of London for financial support and for hosting and publishing this discussion meeting.
Data accessibility
This article has no additional data.
Competing interests
I have no competing interests.
Funding
This study was supported by PLANT developmental biology: discovering the ORIGINS of form (FP7-PEOPLE-INT-2008). I am grateful to EMBO for financial support to attend the workshop on new model systems for early land plant evolution at the Gregor Mendel Institute of Molecular Plant Biology, Vienna in June 2016.
References
- 1.Wellman CH, Strother PK. 2015. The terrestrial biota prior to the origin of land plants (embryophytes): a review of the evidence. Palaeontology 58, 601–627. ( 10.1111/pala.12172) [DOI] [Google Scholar]
- 2.Delwiche CF, Cooper ED. 2015. The evolutionary origin of a terrestrial flora. Curr. Biol. 25, R899–R910. ( 10.1016/j.cub.2015.08.029) [DOI] [PubMed] [Google Scholar]
- 3.Bowman JL. 2013. Walkabout on the long branches of plant evolution. Curr. Opin. Plant Biol. 16, 70–77. ( 10.1016/j.pbi.2012.10.001) [DOI] [PubMed] [Google Scholar]
- 4.Kenrick P, Wellman CH, Schneider H, Edgecombe GD. 2012. A timeline for terrestrialization: consequences for the carbon cycle in the Palaeozoic. Phil. Trans. R. Soc. B 367, 519–536. ( 10.1098/rstb.2011.0271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards D, Kenrick P. 2015. The early evolution of land plants, from fossils to genomics: a commentary on Lang (1937) ‘On the plant-remains from the Downtonian of England and Wales’. Phil. Trans. R. Soc. B 370, 20140343 ( 10.1098/rstb.2014.0343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomescu AMF, Wyatt SE, Hasebe M, Rothwell GW. 2014. Early evolution of the vascular plant body plan—the missing mechanisms. Curr. Opin. Plant Biol. 17, 126–136. ( 10.1016/j.pbi.2013.11.016) [DOI] [PubMed] [Google Scholar]
- 7.Strullu-Derrien C, Kenrick P, Pressel S, Duckett JG, Rioult JP, Strullu DG. 2014. Fungal associations in Horneophyton ligneri from the Rhynie Chert (c. 407 million year old) closely resemble those in extant lower land plants: novel insights into ancestral plant–fungus symbioses. New Phytol. 203, 964–979. ( 10.1111/nph.12805) [DOI] [PubMed] [Google Scholar]
- 8.Taylor TN, Krings M, Taylor EL. 2015. Fossil fungi. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 9.Mitchell RL, Cuadros J, Duckett JG, Pressel S, Mavris C, Sykes D, Najorka J, Edgecombe GD, Kenrick P. 2016. Mineral weathering and soil development in the earliest land plant ecosystems. Geology 44, 1007–1010. ( 10.1130/G38449.1) [DOI] [Google Scholar]
- 10.Niklas KJ, Kutschera U. 2010. The evolution of the land plant life cycle. New Phytol. 185, 27–41. ( 10.1111/j.1469-8137.2009.03054.x) [DOI] [PubMed] [Google Scholar]
- 11.Qiu YL, Taylor AB, McManus HA. 2012. Evolution of the life cycle in land plants. J. Syst. Evol. 50, 171–194. ( 10.1111/j.1759-6831.2012.00188.x) [DOI] [Google Scholar]
- 12.Ligrone R, Duckett JG, Renzaglia KS. 2012. Major transitions in the evolution of early land plants: a bryological perspective. Ann. Bot. 109, 851–871. ( 10.1093/aob/mcs017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haig D. 2008. Homologous versus antithetic alternation of generations and the origin of sporophytes. Bot. Rev. 74, 395–418. ( 10.1007/s12229-008-9012-x) [DOI] [Google Scholar]
- 14.Boyce CK. 2008. How green was Cooksonia? The importance of size in understanding the early evolution of physiology in the vascular plant lineage. Paleobiology 34, 179–194. ( 10.1666/0094-8373(2008)034%5B0179:HGWCTI%5D2.0.CO;2) [DOI] [Google Scholar]
- 15.Mable BK, Otto SP. 1998. The evolution of life cycles with haploid and diploid phases. Bioessays 20, 453–462. ( 10.1002/(SICI)1521-1878(199806)20:6%3C453::AID-BIES3%3E3.0.CO;2-N) [DOI] [Google Scholar]
- 16.Cox CJ, Li B, Foster PG, Embley TM, Civáň P. 2014. Conflicting phylogenies for early land plants are caused by composition biases among synonymous substitutions. Syst. Biol. 63, 272–279. ( 10.1093/sysbio/syt109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wickett NJ, et al. 2014. Phylotranscriptomic analysis of the origin and early diversification of land plants. Proc. Natl Acad. Sci. USA 111, E4859–E4868. ( 10.1073/pnas.1323926111). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruhfel B, Gitzendanner M, Soltis P, Soltis D, Burleigh J. 2014. From algae to angiosperms–inferring the phylogeny of green plants (Viridiplantae) from 360 plastid genomes. BMC Evol. Biol. 14, 23 ( 10.1186/1471-2148-14-23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenrick P, Crane PR. 1997. The origin and early diversification of land plants: a cladistic study. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 20.Renzaglia KS, Duff RJ, Nickrent DL, Garbary DJ. 2000. Vegetative and reproductive innovations of early land plants: implications for a unified phylogeny. Phil. Trans. R. Soc. Lond. B 355, 769–793. ( 10.1098/rstb.2000.0615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao S, Xue J. 2013. The Early Devonian Posongchong flora of Yunnan. Beijing, PR China: Science Press. [Google Scholar]
- 22.Mishler BD, Churchill SP. 1985. Transition to a land flora: phylogenetic relationships of the green algae and bryophytes. Cladistics 1, 305–328. ( 10.1111/j.1096-0031.1985.tb00431.x) [DOI] [PubMed] [Google Scholar]
- 23.Bremer K. 1985. Summary of green plant phylogeny and classification. Cladistics 1, 369–385. ( 10.1111/j.1096-0031.1985.tb00434.x) [DOI] [PubMed] [Google Scholar]
- 24.Zhong B, Xi Z, Goremykin VV, Fong R, Mclenachan PA, Novis PM, Davis CC, Penny D. 2014. Streptophyte algae and the origin of land plants revisited using heterogeneous models with three new algal chloroplast genomes. Mol. Biol. Evol. 31, 177–183. ( 10.1093/molbev/mst200) [DOI] [PubMed] [Google Scholar]
- 25.Finet C, Timme RE, Delwiche CF, Marlétaz F. 2012. Multigene phylogeny of the green lineage reveals the origin and diversification of land plants. Curr. Biol. 22, 1456–1457. ( 10.1016/j.cub.2012.07.021) [DOI] [PubMed] [Google Scholar]
- 26.Laurin-Lemay S, Brinkmann H, Philippe H. 2012. Origin of land plants revisited in the light of sequence contamination and missing data. Curr. Biol. 22, R593–R594. ( 10.1016/j.cub.2012.06.013) [DOI] [PubMed] [Google Scholar]
- 27.Gontcharov AA. 2008. Phylogeny and classification of Zygnematophyceae (Streptophyta): current state of affairs. Fottea 8, 87–104. ( 10.5507/fot.2008.004) [DOI] [Google Scholar]
- 28.Harholt J, Moestrup Ø, Ulvskov P. 2016. Why plants were terrestrial from the beginning. Trends Plant Sci. 21, 96–101. ( 10.1016/j.tplants.2015.11.010) [DOI] [PubMed] [Google Scholar]
- 29.Becker B. 2013. Snow ball Earth and the split of Streptophyta and Chlorophyta. Trends Plant Sci. 18, 180–183. ( 10.1016/j.tplants.2012.09.010) [DOI] [PubMed] [Google Scholar]
- 30.Mikkelsen MD, Harholt J, Ulvskov P, Johansen IE, Fangel JU, Doblin MS, Bacic A, Willats WGT. 2014. Evidence for land plant cell wall biosynthetic mechanisms in charophyte green algae. Ann. Bot. 114, 1217–1236. ( 10.1093/aob/mcu171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu YL. 2008. Phylogeny and evolution of charophytic algae and land plants. J. Syst. Evol. 46, 287–306. ( 10.3724/SP.J.1002.2008.08035) [DOI] [Google Scholar]
- 32.Qiu YL, et al. 2006. The deepest divergences in land plants inferred from phylogenomic evidence. Proc. Natl Acad. Sci. USA 103, 15 511–15 516. ( 10.1073/pnas.0603335103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bower FO. 1935. Primitive land plants. London, UK: Macmillan. [Google Scholar]
- 34.Stebbins GL, Hill GJC. 1980. Did multicellular plants invade the land? Am. Nat. 115, 342–353. ( 10.1086/283565) [DOI] [Google Scholar]
- 35.Strother PK, Battison L, Brasier MD, Wellman CH. 2011. Earth's earliest non-marine eukaryotes. Nature 473, 505–509. ( 10.1038/nature09943) [DOI] [PubMed] [Google Scholar]
- 36.Tomescu AMF, Honegger R, Rothwell GW. 2008. Earliest fossil record of bacterial–cyanobacterial mat consortia: the early Silurian Passage Creek biota (440 Ma, Virginia, USA). Geobiology 6, 120–124. ( 10.1111/j.1472-4669.2007.00143.x) [DOI] [PubMed] [Google Scholar]
- 37.Tomescu AMF, Rothwell GW, Honegger R. 2006. Cyanobacterial macrophytes in an Early Silurian (Llandovery) continental biota: Passage Creek, lower Massanutten Sandstone, Virginia, USA. Lethaia 39, 329–338. ( 10.1080/00241160600876719) [DOI] [Google Scholar]
- 38.Taylor TN, Taylor EL, Krings M. 2009. Paleobotany: the biology and evolution of fossil plants, 2nd edn Amsterdam, The Netherlands: Academic Press. [Google Scholar]
- 39.Fletcher BJ, Beerling DJ, Chaloner WG. 2004. Stable carbon isotopes and the metabolism of the terrestrial Devonian organism Spongiophyton. Geobiology 2, 107–119. ( 10.1111/j.1472-4677.2004.00026.x) [DOI] [Google Scholar]
- 40.Edwards D, Morris JL, Richardson JB, Kenrick P. 2014. Cryptospores and cryptophytes reveal hidden diversity in early land floras. New Phytol. 202, 50–78. ( 10.1111/nph.12645) [DOI] [PubMed] [Google Scholar]
- 41.Steemans P, Le Hérissé A, Melvin J, Miller MA, Paris F, Verniers J, Wellman CH. 2009. Origin and radiation of the earliest vascular land plants. Science 324, 353 ( 10.1126/science.1169659) [DOI] [PubMed] [Google Scholar]
- 42.Richardson JB, Ford JH, Parker F. 1984. Miospores, correlation and age of some Scottish Lower Old Red Sandstone sediments from the Strathmore region (Fife and Angus). J. Geol. Soc. 3, 109–124. ( 10.1144/jm.3.2.109) [DOI] [Google Scholar]
- 43.Strother PK, Beck JH. 2000. Spore-like microfossils from Middle Cambrian strata: expanding the meaning of the term cryptospores. In Pollen and spores: morphology and biology (eds Harley MM, Morton CM, Blackmore S), pp. 413–424. Kew, London, UK: Royal Botanic Gardens. [Google Scholar]
- 44.Steemans P. 2000. Miospore evolution from the Ordovician to the Silurian. Rev. Palaeobot. Palynol. 113, 189–196. ( 10.1016/S0034-6667(00)00059-2) [DOI] [PubMed] [Google Scholar]
- 45.Strother PK. 1991. A classification schema for the cryptospores. Palynology 15, 219–236. ( 10.1080/01916122.1991.9989397) [DOI] [Google Scholar]
- 46.Habgood KS. 2000. Two cryptospore-bearing land plants from the Lower Devonian (Lochkovian) of the Welsh Borderland. Bot. J. Linn. Soc. 133, 203–227. ( 10.1111/j.1095-8339.2000.tb01543.x) [DOI] [Google Scholar]
- 47.Edwards D, Richardson JB, Axe L, Davies KL. 2012. A new group of Early Devonian plants with valvate sporangia containing sculptured permanent dyads. Bot. J. Linn. Soc. 168, 229–257. ( 10.1111/j.1095-8339.2011.01207.x) [DOI] [Google Scholar]
- 48.Edwards D. 2000. The role of Mid-Palaeozoic mesofossils in the detection of early bryophytes. Phil. Trans. R. Soc. Lond. B 355, 733–755. ( 10.1098/rstb.2000.0613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edwards D, Wellman CH, Axe L. 1999. Tetrads in sporangia and spore masses from the Upper Silurian and Lower Devonian of the Welsh Borderland. Bot. J. Linn. Soc. 130, 111–156. ( 10.1111/j.1095-8339.1999.tb00515.x) [DOI] [Google Scholar]
- 50.Edwards D, Duckett JG, Richardson JB. 1995. Hepatic characters in the earliest land plants. Nature 374, 635–636. ( 10.1038/374635a0) [DOI] [Google Scholar]
- 51.Hemsley AR. 1994. The origin of the land plant sporophyte: an interpolational scenario. Biol. Rev. 69, 263–274. ( 10.1111/j.1469-185X.1994.tb01270.x) [DOI] [Google Scholar]
- 52.Trewin NH. 2004. History of research on the geology and palaeontology of the Rhynie area Aberdeenshire, Scotland. Trans. R. Soc. Edinb. Earth Sci. 94, 285–297. ( 10.1017/S0263593300000699) [DOI] [Google Scholar]
- 53.Edwards D, Kenrick P, Dolan L. 2017. History and contemporary significance of the Rhynie cherts—our earliest preserved terrestrial ecosystem. Phil. Trans. R. Soc. B 373, 20160489 ( 10.1098/rstb.2016.0489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rice CM, et al. 1995. A Devonian auriferous hot spring system, Rhynie, Scotland. J. Geol. Soc. 152, 229–250. ( 10.1144/gsjgs.152.2.0229) [DOI] [Google Scholar]
- 55.Rice CM, Ashcroft WA. 2004. The geology of the northern half of the Rhynie Basin, Aberdeenshire, Scotland. Trans. R. Soc. Edinb. Earth Sci. 94, 299–308. ( 10.1017/S0263593300000705) [DOI] [Google Scholar]
- 56.Trewin NH, Rice CM. 2004. The Rhynie Chert hot-spring system: geology, biota and mineralization. Trans. R. Soc. Edinb. Earth Sci. 94, 283–521. [Google Scholar]
- 57.Channing A, Edwards D. 2009. Yellowstone hot spring environments and the palaeo-ecophysiology of Rhynie chert plants: towards a synthesis. Plant Ecol. Divers. 2, 111–143. ( 10.1080/17550870903349359) [DOI] [Google Scholar]
- 58.Channing A, Edwards D. 2009. Silicification of higher plants in geothermally influenced wetlands: Yellowstone as a Lower Devonian Rhynie analog. Palaios 24, 505–521. ( 10.2110/palo.2008.p08-131r) [DOI] [Google Scholar]
- 59.Kidston R, Lang WH. 1921. On Old Red Sandstone plants showing structure, from the Rhynie Chert Bed, Aberdeenshire. Part IV. Restorations of the vascular cryptogams, and discussion on their bearing on the general morphology of the Pteridophyta and the origin of the organization of land-plants. Trans. R. Soc. Edinb. 52, 831–854. ( 10.1017/S0080456800016033) [DOI] [Google Scholar]
- 60.Lemoigne Y. 1970. [New diagnoses of the genus Rhynia and of the species Rhynia gwynne-vaughanii]. Bull. Soc. Bot. Fr. 117, 307–320 (in French with English abstract). ( 10.1080/00378941.1970.10838772) [DOI] [Google Scholar]
- 61.Edwards DS. 1980. Evidence for the sporophytic status of the Lower Devonian plant Rhynia gwynne-vaughanii Kidston and Lang. Rev. Palaeobot. Palynol. 29, 177–188. ( 10.1016/0034-6667(80)90057-3) [DOI] [Google Scholar]
- 62.Kerp H, Trewin NH, Hass H. 2004. New gametophytes from the Early Devonian Rhynie Chert. Trans. R. Soc. Edinb. Earth Sci. 94, 411–428. ( 10.1017/S026359330000078X) [DOI] [Google Scholar]
- 63.Remy W, Gensel PG, Hass H. 1993. The gametophyte generation of some early Devonian land plants. Int. J. Plant Sci. 154, 35–58. ( 10.1086/297089) [DOI] [Google Scholar]
- 64.Kenrick P. 1994. Alternation of generations in land plants: new phylogenetic and palaeobotanical evidence. Biol. Rev. 69, 293–330. ( 10.1111/j.1469-185X.1994.tb01273.x) [DOI] [Google Scholar]
- 65.Taylor TN, Kerp H, Hass H. 2005. Life history biology of early land plants: deciphering the gametophyte phase. Proc. Natl Acad. Sci. USA 102, 5892–5897. ( 10.1073/pnas.0501985102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bruchmann H. Über die Prothallien und Keimpflanzen mehrerer Europäischer Lycopodien. Gotha, Germany: Perthes. (In German.): 1898. [On the prothallus and gametophytes of several European Lycopodiaceae] [Google Scholar]
- 67.Gonez P, Gerrienne P. 2010. A new definition and a lectotypification of the genus Cooksonia Lang 1937. Int. J. Plant Sci. 171, 199–215. ( 10.1086/648988) [DOI] [Google Scholar]
- 68.Gerrienne P, Gonez P. 2011. Early evolution of life cycles in embryophytes: a focus on the fossil evidence of gametophyte/sporophyte size and morphological complexity. J. Syst. Evol. 49, 1–16. ( 10.1111/j.1759-6831.2010.00096.x) [DOI] [Google Scholar]
- 69.Gerrienne P, Bergamaschi S, Pereira E, Rodrigues MAC, Steemans P. 2001. An Early Devonian flora, including Cooksonia, from the Parana Basin (Brazil). Rev. Palaeobot. Palynol. 116, 19–38. ( 10.1016/S0034-6667(01)00060-4) [DOI] [Google Scholar]
- 70.Leitch IJ. 2007. Genome sizes through the ages. Heredity 99, 121–122. ( 10.1038/sj.hdy.6800981) [DOI] [PubMed] [Google Scholar]
- 71.Lomax BH, Hilton J, Bateman RM, Upchurch GR, Lake JA, Leitch IJ, Cromwell A, Knight CA. 2014. Reconstructing relative genome size of vascular plants through geological time. New Phytol. 201, 636–644. ( 10.1111/nph.12523) [DOI] [PubMed] [Google Scholar]
- 72.Remy W, Remy R, Hass H, Schultka S, Franzmeyer F. 1980. [Sciadophyton Steinmann—a gametophyte from the Siegenian]. Argumenta Palaeobotanica 6, 73–94 (in German with English abstract). [Google Scholar]
- 73.Remy W, Hass H, Schultka S. 1992. [Sciadophyton Steinmann emend. Kräusel et Weyland (1930)—the only representative of a Lower Devonian body plan?] Cour. Forschungsinst. Senckenb. 147, 87–91 (in German with English abstract). [Google Scholar]
- 74.Kenrick P, Remy W, Crane PR. 1991. The structure of water-conducting cells in the enigmatic early land plants Stockmansella langii Fairon-Demaret, Huvenia kleui Hass et Remy and Sciadophyton sp. Remy et al. 1980. Argumenta Palaeobotanica 8, 179–191. [Google Scholar]
- 75.Remy W, Schultka S, Hass H. 1991. [Calyculiphyton blanai nov. gen., nov. spec., a gametophyte from the Emsian]. Argumenta Palaeobotanica 8, 119–140 (in German with English abstract). [Google Scholar]
- 76.Schultka S. 2003. Cooksonia—[On the morphology of an early land plant from the Lower Devonian of Eifel]. Courier Forschungs-Institut Senckenberg 241, 7–17 (in German with English abstract). [Google Scholar]
- 77.Gerrienne P, Dilcher DL, Bergamaschi S, Milagres I, Pereira E, Rodrigues MAC. 2006. An exceptional specimen of the early land plant Cooksonia paranensis, and a hypothesis on the life cycle of the earliest eutracheophytes. Rev. Palaeobot. Palynol. 142, 123–130. ( 10.1016/j.revpalbo.2006.05.005) [DOI] [Google Scholar]
- 78.Kenrick P, Crane PR. 1997. The origin and early evolution of plants on land. Nature 389, 33–39. ( 10.1038/37918) [DOI] [Google Scholar]
- 79.Pires ND, Dolan L. 2012. Morphological evolution in land plants: new designs with old genes. Phil. Trans. R. Soc. B 367, 508–518. ( 10.1098/rstb.2011.0252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pires ND, Yi K, Breuninger H, Catarino B, Menand B, Dolan L. 2013. Recruitment and remodeling of an ancient gene regulatory network during land plant evolution. Proc. Natl Acad. Sci. USA 110, 9571–9576. ( 10.1073/pnas.1305457110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Menand B, Yi K, Jouannic S, Hoffmann L, Ryan E, Linstead P, Schaefer DG, Dolan L. 2007. An ancient mechanism controls the development of cells with a rooting function in land plants. Science 316, 1477–1480. ( 10.1126/science.1142618) [DOI] [PubMed] [Google Scholar]
- 82.Tam THY, Catarino B, Dolan L. 2015. Conserved regulatory mechanism controls the development of cells with rooting functions in land plants. Proc. Natl Acad. Sci. USA 112, E3959–E3968. ( 10.1073/pnas.1416324112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu B, et al. 2014. Contribution of NAC transcription factors to plant adaptation to land. Science 343, 1505–1508. ( 10.1126/science.1248417). [DOI] [PubMed] [Google Scholar]
- 84.Bennett TA, et al. 2014. Plasma membrane-targeted PIN proteins drive shoot development in a moss. Curr. Biol. 24, 2776–2785. ( 10.1016/j.cub.2014.09.054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Viaene T, et al. 2014. Directional auxin transport mechanisms in early diverging land plants. Curr. Biol. 24, 2786–2791. ( 10.1016/j.cub.2014.09.056) [DOI] [PubMed] [Google Scholar]
- 86.Duckett JG, Pressel S, P'ng KMY, Renzaglia KS. 2009. Exploding a myth: the capsule dehiscence mechanism and the function of pseudostomata in Sphagnum. New Phytol. 183, 1053–1063. ( 10.1111/j.1469-8137.2009.02905.x) [DOI] [PubMed] [Google Scholar]
- 87.Pressel S, Goral T, Duckett JG. 2014. Stomatal differentiation and abnormal stomata in hornworts. J. Bryol. 36, 87–103. ( 10.1179/1743282014Y.0000000103) [DOI] [Google Scholar]
- 88.Kenrick P. 2000. The relationships of vascular plants. Phil. Trans. R. Soc. Lond. B 355, 847–855. ( 10.1098/rstb.2000.0619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bierhorst DW. 1971. Morphology of vascular plants. New York, NY: Macmillan. [Google Scholar]
- 90.Bowman JL, Sakakibara K, Furumizu C, Dierschke T. 2016. Evolution in the cycles of life. Annu. Rev. Genet. 50, 133–154. ( 10.1146/annurev-genet-120215-035227) [DOI] [PubMed] [Google Scholar]
- 91.Horst NA, Reski R. 2016. Alternation of generations – unravelling the underlying molecular mechanism of a 165-year-old botanical observation. Plant Biol. 18, 549–551. ( 10.1111/plb.12468) [DOI] [PubMed] [Google Scholar]
- 92.Lee J-H, Lin H, Joo S, Goodenough U. 2008. Early sexual origins of homeoprotein heterodimerization and evolution of the plant KNOX/BELL family. Cell 133, 829–840. ( 10.1016/j.cell.2008.04.028) [DOI] [PubMed] [Google Scholar]
- 93.Gao J, Yang X, Zhao W, Lang T, Samuelsson T. 2015. Evolution, diversification, and expression of KNOX proteins in plants. Front. Plant Sci. 6, 882 ( 10.3389/fpls.2015.00882) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Frangedakis E, Saint-Marcoux D, Moody LA, Rabbinowitsch E, Langdale JA. 2016. Nonreciprocal complementation of KNOX gene function in land plants. New Phytol. 216, 591–604. ( 10.1111/nph.14318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sakakibara K. 2016. Technological innovations give rise to a new era of plant evolutionary developmental biology. Adv. Bot. Res. 78, 3–35. ( 10.1016/bs.abr.2016.01.001) [DOI] [Google Scholar]
- 96.Horst NA, Katz A, Pereman I, Decker EL, Ohad N, Reski R. 2016. A single homeobox gene triggers phase transition, embryogenesis and asexual reproduction. Nat. Plants 2, 15209 ( 10.1038/nplants.2015.209) [DOI] [PubMed] [Google Scholar]
- 97.Sakakibara K, Ando S, Yip HK, Tamada Y, Hiwatashi Y, Murata T, Deguchi H, Hasebe M, Bowman JL. 2013. KNOX2 genes regulate the haploid-to-diploid morphological transition in land plants. Science 339, 1067–1070. ( 10.1126/science.1230082) [DOI] [PubMed] [Google Scholar]
- 98.Okano Y, Aono N, Hiwatashi Y, Murata T, Nishiyama T, Ishikawa T, Kubo M, Hasebe M. 2009. A polycomb repressive complex 2 gene regulates apogamy and gives evolutionary insights into early land plant evolution. Proc. Natl Acad. Sci. USA 106, 16 321–16 326. ( 10.1073/pnas.0906997106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Couceiro L, et al. 2015. Evolution and maintenance of haploid–diploid life cycles in natural populations: the case of the marine brown alga Ectocarpus. Evolution 69, 1808–1822. ( 10.1111/evo.12702) [DOI] [PubMed] [Google Scholar]
- 100.Brown RC, Lemmon BE. 2011. Spores before sporophytes: hypothesizing the origin of sporogenesis at the algal–plant transition. New Phytol. 190, 875–881. ( 10.1111/j.1469-8137.2011.03709.x) [DOI] [PubMed] [Google Scholar]
- 101.Taylor WA, Strother PK. 2009. Ultrastructure, morphology, and topology of Cambrian palynomorphs from the Lone Rock Formation, Wisconsin, USA. Rev. Palaeobot. Palynol. 153, 296–309. ( 10.1016/j.revpalbo.2008.09.001) [DOI] [Google Scholar]
- 102.Silberfeld T, Leigh JW, Verbruggen H, Cruaud C, de Reviers B, Rousseau F. 2010. A multi-locus time-calibrated phylogeny of the brown algae (Heterokonta, Ochrophyta, Phaeophyceae): investigating the evolutionary nature of the ‘brown algal crown radiation’. Mol. Phylogenet. Evol. 56, 659–674. ( 10.1016/j.ympev.2010.04.020) [DOI] [PubMed] [Google Scholar]
- 103.Yang EC, Boo SM, Bhattacharya D, Saunders GW, Knoll AH, Fredericq S, Graf L, Yoon HS. 2016. Divergence time estimates and the evolution of major lineages in the florideophyte red algae. Sci. Rep. 6, 21361 ( 10.1038/srep21361) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.