Abstract
Objective:
To assess whether any alteration of B-cell subset distribution and/or the cytokine production capacities of B cells could be associated with any stage of MS and could be predictive of MS evolution.
Methods:
We prospectively enrolled radiologically isolated syndrome (RIS), clinically isolated syndrome (CIS), naive patients with relapsing remitting MS (RRMS) of any disease modifying drug, and healthy controls (HCs). Peripheral blood B-cell subset distributions and the interleukin (IL)-6/IL-10–producing B-cell ratio were assessed by flow cytometry to evaluate their proinflammatory and anti-inflammatory functional properties.
Results:
Twelve RIS, 46 CIS, 31 RRMS patients, and 36 HCs were enrolled. We observed that a high IL-6/IL-10–producing B-cell ratio in patients with RIS/CIS was associated with the evolution of the disease in the short term (6 months). This imbalance in cytokine production was mainly explained by an alteration of the production of IL-10 by B cells, especially for the transitional B-cell subset. In addition, a significant increase in IgD−/CD27− B cells was detected in patients with CIS and RRMS compared with HCs (p = 0.01). Apart from this increase in exhausted B cells, no other variation in B-cell subsets was observed.
Conclusions:
The association between a high IL-6/IL-10–producing B-cell ratio and the evolution of patients with RIS/CIS suggest a skew of B cells toward proinflammatory properties that might be implicated in the early phases of MS disease.
MS is a well-known T cell–dependent disease. Cumulative data revealed the involvement of B cells in the pathophysiology of the disease.1 Although the efficacy of B cell–targeting drugs2 implies a key role for B cells in MS, the exact molecular mechanisms of this role remain to be defined.1 The lack of impact of B-cell depletion on CSF oligoclonal bands suggests that the role of B cells in the lesional processes is not restricted to antibody-dependent mechanisms but could involve their cellular functions.1 Data from experimental autoimmune encephalomyelitis models showed that B cells can balance either a proinflammatory response by producing interleukin (IL)-63 or a regulatory response through IL-10 production.4 Some B-cell subsets have been associated with such pro- and anti-inflammatory profiles. Previous studies working on cytokine production by B cells in MS focused on anti-inflammatory cytokines (IL-10) and proinflammatory cytokines (IL-6, granulocyte-macrophage colony-stimulating factor [GM-CSF], lymphotoxin [LT], or tumor necrosis factor–alpha [TNF]α) in established MS disease including patients with disease modifying drugs. Most of those transversal studies showed a decrease of IL-10 and an increase of proinflammatory cytokines produced by B cells i.e., LT and TNFα,5 IL-6,3 or GM-CSF.6 However, Duddy et al.7 did not find any difference in LT and TNFα secretion by B cells. Michel et al.8 did not observe any alteration in IL-10 production by B cells in MS. Such discrepancy might be explained by the studied population, especially the treatment status of patients with MS, but also by heterogeneous methodological conditions mixing early and late MS, treated and naive patients, precluding any firm conclusion. We therefore conducted this prospective study focusing on the early phases of MS disease in patients naive of any disease modifying drug. We aimed to analyze whether from the initial phase of MS naive of any disease modifying drug, the evolution of the disease may be associated with any imbalance in cytokine production capacities by B cells.
METHODS
Patients and healthy volunteers.
Patients in the MS group were enrolled from the Department of Neurology in the University Hospital of Lille. In that group, patients with radiologically isolated syndrome (RIS) and clinically isolated syndrome (CIS) were included as well as those with relapsing remitting MS (RRMS) who were defined according to the 2010 McDonald criteria.9 RIS was defined according to Okuda criteria.10 Patients with CIS did not fulfill temporal and spatial dissemination for MS at baseline. Exclusion criteria were any history of taking disease modifying drugs; previous corticosteroid use for management of relapses was accepted. However, all blood samples had to be collected at least 1 month after the last steroid intake. Healthy subjects were enrolled as a control.
All biological procedures and statistical analyses are described in e-Methods at http://links.lww.com/NXI/A15, figures e-1 to e-9 at http://links.lww.com/NXI/A19. For all subjects, phenotypic and functional studies of B cells were performed at the time of the inclusion, i.e., at baseline, to assess (1) any differences between healthy controls (HCs) and the subgroups of patients with MS and (2) to define a potential prognosis biomarker associated with the evolution of the disease. Functional studies were focused on the analysis of intracellular IL-6 and IL-10 production by B cells.
All patients were routinely clinically visited at baseline before blood sampling and every 6 months. In our clinical practice, usually brain and spinal MRIs were performed at diagnosis time and 3 months afterward for patients with CIS.
Standard protocol approvals, registrations, and patients consents.
This study was approved by the local ethical committee (CPP Nord Ouest IV no. IDRCB: 2014 A00248 39). Informed written consent was obtained from all participants.
RESULTS
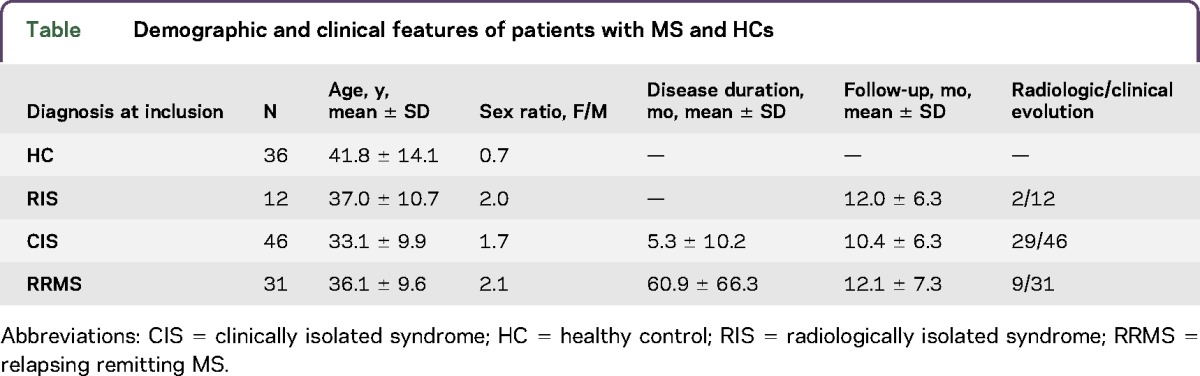
Eighty-nine patients with MS and 36 HCs were enrolled between 2013 and 2016 (table). There was no difference in age and sex between the different groups.
Table.
Demographic and clinical features of patients with MS and HCs
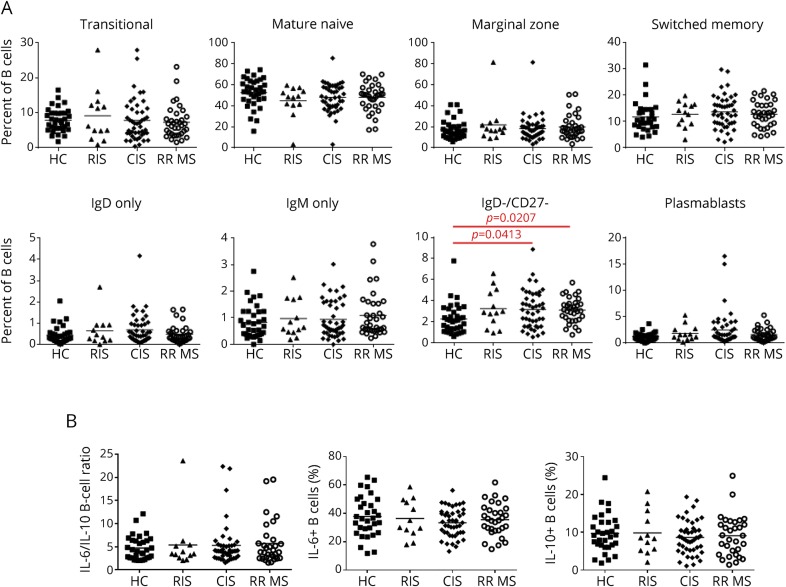
The frequency of IgD−/CD27− B cells is increased in CIS and RRMS.
The phenotypical analysis of peripheral blood lymphocytes showed both similar counts of lymphocytes and distribution of CD4+, CD8+ and total T cells, NK cells, and total B cells between HCs and patients with RIS, CIS, and RRMS (figure e-3).
We observed a similar distribution of the different B-cell subsets (transitional, mature naive, marginal zone, switched memory B cells, IgM-only, IgD-only B cells, and plasmablasts) (figures e-1 and 1A). Double-negative IgD−/CD27− B cells were the only subset that was significantly increased in patients with CIS and RRMS when compared with HCs (p = 0.01).
Figure 1. Phenotype and functional properties of B cells in MS and HCs at baseline.
(A) Comparison of B-cell subset distribution in MS patient groups and HCs. Comparison of percentages of transitional, mature naive, marginal zone, switched memory, IgM-only, IgD-only B cells, exhausted B cells, and plasmablasts. (B) Comparison of functional properties of B cells in MS patient groups and HCs. Comparison of percentages of IL-6– and IL-10–producing B cells and IL-6/IL-10–producing B-cell ratio. CIS = clinically isolated syndrome; HC = healthy control; IL = interleukin; RIS = radiologically isolated syndrome; RRMS = relapsing remitting MS.
No difference in IL-10 and IL-6 production by B cells in patients with MS compared with controls.
Stimulating B cells with CD40 and CpG reliably induced IL-6 and IL-10 production. After 48 hours of polyclonal stimulation, no differences in IL-6 or IL-10–expressing B cells or in IL-6/IL-10–producing B-cell ratio were observed between the different groups of patients and HCs (figure 1B). Total B cells did not present any alteration in IL-10 and IL-6 production at any phase of MS disease (RIS, CIS, or RRMS stage) compared with HCs.
Comparison of B-cell subset distribution according to the MS evolution over time.
Among cytometric and functional B-cell characteristics obtained at baseline after 48-hour polyclonal stimulation, we searched for parameters with a predictive value of the disease evolution at the maximum follow-up of 11.2 months (±6.7). In the following 2 groups, RIS/CIS and RRMS, we distinguished patients who evolved and patients who did not, at 6 months (short term) and after 6 months (long term). Evolution was defined as (1) conversion from RIS to CIS, (2) conversion from CIS to MS according to 2010 MacDonald criteria, and (3) another relapse for RRMS (table).
We did not find any association between the different lymphocytes including B cells populations, at baseline, and the evolution at short term and long term in patients with RIS/CIS and in patients with RRMS at short term (figures e-4 and e-5). No statistical analysis was performed for patients with RRMS for the long-term evolution (n = 2).
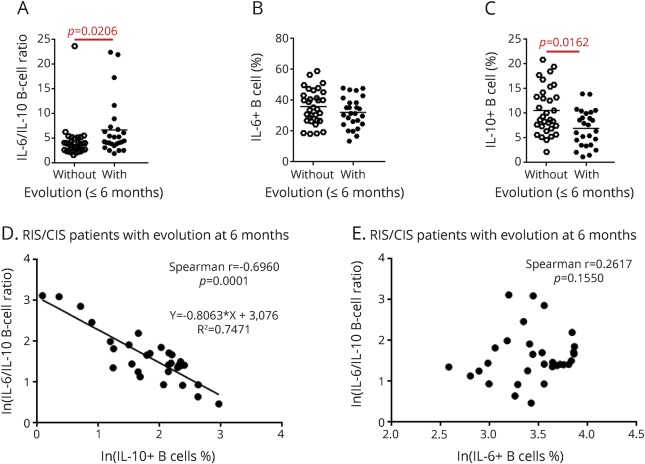
An increased IL-6/IL-10–producing B-cell ratio is associated with the evolution of MS.
To assess the predictive value for MS outcome of IL-6 and IL-10 production by B cells at baseline, we compared IL-6/IL-10–producing B-cell ratio at baseline between evolving and not-evolving patients with MS. We observed that the IL-6/IL-10–producing B-cell ratio was significantly increased in patients with RIS/CIS who evolved at 6 months (p = 0.021, figure 2A). Next, we compared the IL-6– and IL-10–producing B cells separately and did not observe any variation in IL-6–producing B cells according to the outcome of patients with RIS/CIS. Conversely, we observed a significant decrease of IL-10–producing B cells (p = 0.0016) at baseline in patients with RIS/CIS who evolved at 6 months (figure 2, B and C). We did not observe any association between the IL-6/IL-10 ratio and the evolution of the RIS/CIS group in the long term and of the patients with RRMS in the short term (figure e-6, A–F).
Figure 2. Functional properties of B cells according to disease evolution in patients with RIS/CIS.
(A–C) Comparison of IL-6/IL-10–producing B-cell ratios (A), percentages of IL-6–producing B cells (B), and percentages of IL-10–producing B cells (C) between patients who had evolved and patients who had not at 6 months. (D and E) Correlation between IL-6/IL-10–producing B-cell ratio and percentages of IL-10 (D) or IL-6–producing B cells (E) in evolving patients with RIS/CIS MS at 6 months. CIS = clinically isolated syndrome; IL = interleukin; RIS = radiologically isolated syndrome.
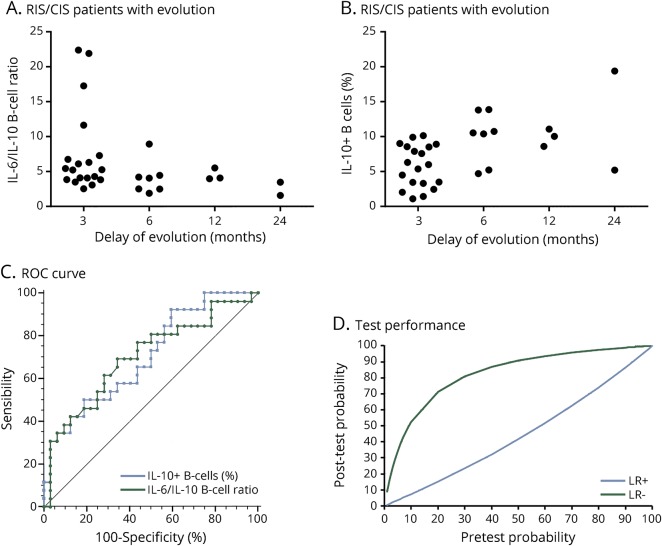
IL-6/IL-10 ratio was significantly correlated with IL-10 but not with IL-6 B-cell production (figure 2, D and E). The IL-6/IL-10–producing B-cell ratio was also inversely correlated with the delay of evolution of the disease (rspearman = −0.42; p = 2.10−2). Even not significant, the IL-6/IL-10–producing B-cell ratio is higher in patients with early evolution of disease (figure 3A). In addition, the percentage of IL-10–producing B cells was also correlated with the delay of evolution of the disease (rspearman = 0.54; p = 2.10−3). Even not significant, the percentage of IL-10–producing B-cell ratio is lower in patients with early evolution of disease (figure 3B).
Figure 3. Analysis of the prognostic value of IL-10–producing B cells or IL-6/IL-10–B-cell ratio measurements in patients with RIS/CIS MS.
(A) Comparison of baseline IL-6/IL-10–producing B-cell ratios between patients with RIS/CIS in whom disease has evolved at 3, 6, 12, and 24 months. (B) Comparison of baseline percentages of IL-10–producing B cells between patients with RIS/CIS in whom disease has evolved at 3, 6, 12, and 24 months. (C) ROC curve representation of IL-10–producing B-cell percentage and IL-6/IL-10–producing B-cell ratio according to the capacity to predict the evolution of the disease. (D) Representative graph of the posttest probability values according to pretest probability values, positive and negative likelihood ratios of IL-6/IL-10–producing B-cell ratio. CIS = clinically isolated syndrome; IL = interleukin; LR+ = positive likelihood ratio; LR− = negative likelihood ratio; RIS = radiologically isolated syndrome; ROC = receiver operating characteristic.
We then performed receiver operating characteristic (ROC) curve analysis to find a cutoff value for the IL-6/IL-10 ratio defining a higher risk of evolution. Areas under the curve were at 0.7 for the IL-6/IL-10–producing B-cell ratio and for the percentage of IL-10–producing B cells (figure 3C). Seven of 8 patients with RIS/CIS (87.5%) having a value above a ratio cutoff of 6.3 defined by an ROC curve analysis evolved at 6 months, with a good specificity of 97%. With a ratio below 6.3, 19 of 50 patients with RIS/CIS (38%) evolved giving a sensitivity of 31%. The ROC curve analyses performed for the percentage of IL-10–producing B cells show the same performances with a cutoff at 4.5% (figure 3D). For the IL-10–producing B-cell test, the graph representing its performance is similar (data not shown).
Even if no specific lymphocyte distributions at baseline seem to predict any disease evolution, an alteration of IL-10–expressing B cells and the IL-6/IL-10–producing B-cell ratio, at the very early phase of the disease, is significantly associated with an evolution in the short term.
Potential B-cell subsets and mechanisms involved in the alteration of IL-10 production in early-phase MS.
Using short 5-hour stimulation instead of long 48-hour stimulation in patients in the RIS/CIS group, we did not observe any further difference in IL-10–producing B cells in evolving patients compared with nonevolving patients (figure e-7). We hypothesized that according to different stimulation methods, different B-cell subsets were involved in IL-10 production. We tested both stimulation methods in the different B-cell subsets from HCs. We observed that IL-10 was mainly produced by memory B cells with the short 5-hour stimulation, whereas IL-10 was mainly produced by marginal zone and transitional B cells with the long 48-hour one (figure e-8).
To decipher the mechanisms of IL-10 production by B cells after long stimulation, we explored the type I interferon pathway. Using peripheral blood mononuclear cells (PBMC) from HCs, we analyzed IL-10 production by B cells after long stimulation with or without interferon-blocking antibodies (figure e-9). Blocking Ab had no effect on the IL-10 production by B cells.
DISCUSSION
The main results of this study are as follows: (1) an increase of the IL-6/lL-10 ratio in patients with RIS/CIS at baseline is significantly associated with an activity of the disease at 6 months; (2) this proinflammatory imbalance is linked to a decrease of the IL-10–producing B cells, without alteration of the IL-6–producing B cells; (3) this alteration of IL-10 production in patients with RIS/CIS who evolved concerns mainly transitional B cells and is independent of type I interferon secretion; (4) this feature was not observed when considering baseline samples from patients with RRMS, suggesting that impaired regulatory functions of B cells affect the early phase of MS disease.
Understanding the impact of the B-cell role in MS pathophysiology involves the analysis of peripheral blood B-cell subset distribution in patients, trying to associate any phenotypic characteristics with functional properties. Conflicting results are found in the literature. Immature B-cell phenotype (such as transitional B cells) has been associated with an anti-inflammatory profile,11 whereas memory B-cell phenotype has been associated with a more proinflammatory profile.7
Studies usually focused on the distribution of the following subsets: transitional, mature naive, and memory B cells. Although Haas et al.12 described an increase of transitional B cells in CIS and RRMS patients with active disease, Lee-Chang et al.13 found a decrease of those same cells in the same patient groups. Memory B-cell decrease have also been described in RRMS,12,14 whereas Michel et al.8 did not find any modification in the distribution of B-cell subsets in MS compared with HCs. In our study, we did not find any alteration in the distribution of those B-cell subsets at any phase of the disease. The only exception was that the double-negative (IgD−/CD27−) B-cell subset is overrepresented in CIS and RRMS groups compared with HCs. Such double-negative B cells have been reported as increased in other autoimmune conditions (systemic lupus and Sjögren syndrome)15,16 but have not been explored in MS except by Haas et al.12 who also described their overrepresentation in CIS and RRMS. These cells are described as exhausted B cells in a chronic activation status, but their functional properties remain elusive.17,18 We observed that the distribution of some B-cell subsets like transitional B cells was more heterogeneous in patients with MS.12,13 This could mean that, despite an apparent homogeneity through the clinical clustering of patients in defined groups (RIS, CIS, and RRMS), every individual patient presents a peculiar heterogeneous disease history. Indeed Carr et al.19 recently reported how peripheral blood immune cell distribution could be heterogeneous and how that distribution was shaped by environmental conditions. Besides the lack of association between B-cell subsets and the stage of MS at baseline in our study, we did not find any association with MS evolution either. These data suggest that the phenotypic analysis of peripheral blood B cells could be insufficiently informative. We therefore assessed a more functional analysis of B cells through their cytokine production capacities.
We hypothesized that initial phases of the disease could reflect a more homogenous pathologic process before any additional modifications brought by treatments and cumulative damage occurrence. In addition to patients with RRMS, we did select early MS disease (RIS and CIS) patients who are naive of any immunomodulating or immunosuppressive drugs. We did not see any difference in IL-6 and IL-10 production by B cells between HCs and RIS, CIS, or RRMS nor between the different subgroups of patients. Such discrepant results with literature might be linked to the different methodological approaches: cells studied and stimulation methods used. We chose to use stimulated fresh PBMCs to analyze specific cytokine production by B cells using a cytometric approach. Iwata et al.20 demonstrated that this technique using PBMC is as convenient as purified B cells for the specific cytokine production analysis. Moreover, the stimulation pathway we used in our study has been demonstrated as optimal for the analysis of cytokine production by B cells.21
In the longitudinal phase of our study, we concluded that, in the very early phases of MS (for RIS and CIS), the higher the IL-6/IL-10 B-cell ratio, the poorer the prognosis at 6 months.
The increase of this ratio was mainly explained by a decrease of IL-10 production. Indeed, although IL-6 production did not significantly vary, the decrease of the capacity of B cells to produce IL-10 could confer a higher proinflammatory property on those B cells. Moreover, in the group of patients who evolved at 6 months, we observed an inverse correlation between time to progression and IL-6/IL-10 ratio. A lower ratio was associated with a longer time to evolution.
Using an ROC curve approach, we delineated a cutoff to define a test with a high specificity. More than 87% of patients with RIS/CIS who presented less than 4.5% of B-cell–producing IL-10 have evolved at 6 months, corresponding to a specificity of 97%, but a low sensitivity of 31%. This approach points out that, under this percentage of IL-10–producing B cells, the patients with RIS/CIS are at substantial risk of developing an active disease.
Those results were obtained with a long stimulation while they were not observed after short stimulation of B cells. We could hypothesize that the decrease in the level of IL-10 B-cell production may be restricted to transitional B cells. Moreover, previous studies have shown that IL-10 production by transitional B cells after long TLR9 stimulation could depend on type I interferon produced by PBMC.19 However, we did show that IL-10 B-cell production was independent of type I interferon synthesis in our population.
In the RRMS group, we did not observe that IL-6/IL-10 ratios and IL-10 B cells were associated with any further disease activity during the follow-up. These data suggest that at the RRMS stage, peripheral blood no longer reflected the initial pathologic processes but, perhaps, a more heterogeneous process linked to accumulation, in a single patient, of lesions at various stages.
In conclusion, we suggested that B cells had a reduced capacity for producing the immunoregulatory cytokine IL-10 that may confer a proinflammatory profile to those cells. This phenomenon is objectively shown only at the early initial phases of the disease and could help at that step to identify patients who will need active treatment. A replication cohort would be helpful to substantiate that this test IL-6/IL-10 ratio in B cells is predictive of early disease activity in patients with RIS/CIS.
ACKNOWLEDGMENT
The authors acknowledge the University of Lille 2 and the Fondation pour l'aide à la recherche sur la sclérose en plaques (ARSEP), which funded the study and Carine Hauspie for technical support.
GLOSSARY
- CIS
clinically isolated syndrome
- EAE
experimental autoimmune encephalomyelitis
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HC
healthy control
- IL
interleukin
- LT
lymphotoxin
- PBMC
peripheral blood mononuclear cell
- RIS
radiologically isolated syndrome
- ROC
receiver operating characteristic
- RRMS
relapsing remitting MS
- TNFα
tumor necrosis factor–alpha
AUTHOR CONTRIBUTIONS
Thomas Guerrier: study concept and design and acquisition, analysis, and interpretation of data. Myriam Labalette: study concept and critical revision of the manuscript for intellectual content. Caty Lee Chang: critical revision of the manuscript for intellectual content. Olivier Outteryck: acquisition of data and critical revision of the manuscript for intellectual content. Guillaume Lefèvre and David Launay: critical revision of the manuscript for intellectual content. Patrick Vermersch: acquisition of data and critical revision of the manuscript for intellectual content. Dubucquoi Sylvain: study concept and design, study supervision, analysis and interpretation of data, and critical revision of the manuscript for intellectual content. Hélène Zéphir: study concept and design, study supervision, acquisition of data, analysis and interpretation of data, and critical revision of the manuscript for intellectual content.
STUDY FUNDING
This study was funded by Université de Lille 2 and the Fondation pour l'aide à la recherche sur la sclérose en plaques (ARSEP).
DISCLOSURE
T. Guerrier received research fellowships from Fondation pour la Recherche, Université Lille 2, and ARSEP; received research grants from Université Lille 2 and ARSEP; and received research support from GENZYME. M. Labalette reports no disclosures. D. Launany received travel funding and/or speaker honoraria from Shire, GSK, CSL Behring, and Pfizer and is an associate editor of La Revue de Medecine Interne. C. Lee-Chang received lecture fees from Merck and research grants from Novartis Pharma AG. O. Outteryck received consulting fees and invitations for national and international congresses from Biogen, Merck, Teva, Sanofi Genzyme, Novartis, Bayer, Sanofi-Aventis, and Roche; received research support from Biogen, Bayer, and Novartis; and received academic research grants from the VISIO Foundation. G. Lefèvre received consulting fees and invitations for national and international congresses from LFB, Octapharma, and Shire, as well as received research supports from LFB, CSL Behring, Air Liquide, Octapharma, GRIFOLS, and GSK. P. Vermersch received honoraria and consulting fees from Biogen Idec, Sanofi Genzyme, Bayer, Novartis, Teva, Merck, Roche, Almirall, and Celgene; received research support from Biogen, Bayer, Novartis, Sanofi Genzyme, Roche, and Novartis-Merck; and served on the scientific advisory board of Sanofi, Biogen, Merck, Teva, Novartis, Roche, Serrvier, and Celgene. S. Dubucquoi: received consulting fees and invitations for national and international congresses from Biogen and Teva; received research support from Genzyme, Octapharma, Roche, CSL Behring, and Novartis; and received academic research grants from Lille University and the ARSEP Foundation. H. Zéphir received fees for consulting or lectures and invitations for national and international congresses from Biogen, Merck, Teva, Sanofi Genzyme, Novartis, and Bayer; received research support from Teva and Roche; and received academic research grants from Académie de Médecine, LFSEP, and ARSEP Foundation. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Cross AH, Stark JL, Lauber J, et al. . Rituximab reduces B cells and T cells in cerebrospinal fluid of multiple sclerosis patients. J Neuroimmunol 2006;180:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kappos L, Li D, Calabresi PA, et al. . Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet 2011;378:1779–1787. [DOI] [PubMed] [Google Scholar]
- 3.Barr TA, Shen P, Brown S, et al. . B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6–producing B cells. J Exp Med 2012;209:1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol 2002;3:944–950. [DOI] [PubMed] [Google Scholar]
- 5.Bar-Or A, Fawaz L, Fan B, et al. . Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Ann Neurol 2010;67:452–461. [DOI] [PubMed] [Google Scholar]
- 6.Li R, Rezk A, Miyazaki Y, et al. . Proinflammatory GM-CSF–producing B cells in multiple sclerosis and B cell depletion therapy. Sci Transl Med 2015;7:310ra166. [DOI] [PubMed] [Google Scholar]
- 7.Duddy M, Niino M, Adatia F, et al. . Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol 2007;178:6092–6099. [DOI] [PubMed] [Google Scholar]
- 8.Michel L, Chesneau M, Manceau P, et al. . Unaltered regulatory B-cell frequency and function in patients with multiple sclerosis. Clin Immunol 2014;155:198–208. [DOI] [PubMed] [Google Scholar]
- 9.Polman CH, Reingold SC, Banwell B, et al. . Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okuda DT, Siva A, Kantarci O, et al. ; Radiologically Isolated Syndrome Consortium (RISC); Club Francophone de la Sclérose en Plaques (CFSEP). Radiologically isolated syndrome: 5-year risk for an initial clinical event. PLoS One 2014;9:e90509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blair PA, Noreña LY, Flores-Borja F, et al. . CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 2010;32:129–140. [DOI] [PubMed] [Google Scholar]
- 12.Haas J, Bekeredjian-Ding I, Milkova M, et al. . B cells undergo unique compartmentalized redistribution in multiple sclerosis. J Autoimmun 2011;37:289–299. [DOI] [PubMed] [Google Scholar]
- 13.Lee-Chang C, Top I, Zéphir H, et al. . Primed status of transitional B cells associated with their presence in the cerebrospinal fluid in early phases of multiple sclerosis. Clin Immunol 2011;139:12–20. [DOI] [PubMed] [Google Scholar]
- 14.Niino M, Hirotani M, Miyazaki Y, Sasaki H. Memory and naive B-cell subsets in patients with multiple sclerosis. Neurosci Lett 2009;464:74–78. [DOI] [PubMed] [Google Scholar]
- 15.Wei C, Anolik J, Cappione A, et al. . A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J Immunol 2007;178:6624–6633. [DOI] [PubMed] [Google Scholar]
- 16.Saadoun D, Terrier B, Bannock J, et al. . Expansion of autoreactive unresponsive CD21(-/low) B cells in Sjögren's syndrome associated lymphoproliferation. Arthritis Rheum 2013;65:1085–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seifert M, Küppers R. Human memory B cells. Leukemia 2016;30:2283–2292. [DOI] [PubMed] [Google Scholar]
- 18.Wu YCB, Kipling D, Dunn-Walters DK. The relationship between CD27 negative and positive B cell populations in human peripheral blood. Front Immunol 2011;2:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carr EJ, Dooley J, Garcia-Perez JE, et al. . The cellular composition of the human immune system is shaped by age and cohabitation. Nat Immunol 2016;17:461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwata Y, Matsushita T, Horikawa M, et al. . Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 2011;117:530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menon M, Blair PA, Isenberg DA, Mauri C. A regulatory feedback between plasmacytoid dendritic cells and regulatory B cells is aberrant in systemic lupus erythematosus. Immunity 2016;44:683–697. [DOI] [PMC free article] [PubMed] [Google Scholar]