Abstract
Humans engage in cooperative childcare, which includes some elements not found in other animals, such as the presence of post-reproductive helpers, extensive food sharing among adults and a pervasive sexual division of labour. In animals, cooperative offspring care has typically been studied in two different contexts. The first mainly involves helpers contributing care in cooperatively breeding family groups; the second context is allomaternal care in species usually not categorized as cooperative breeders (e.g. plural and communal breeders, often without male care). Comparative analyses suggest that cooperative breeding and allomaternal care in plural and communal breeders have distinct evolutionary origins, with humans fitting neither pathway entirely. Nevertheless, some critical proximate mechanisms of helping, including hormonal regulators, are likely to be shared across species. Other mechanisms may vary among species, such as social tolerance, proactive prosociality or conditional mother–infant bonding. These are presumably associated with specific details of the care system, such as whether all group members contribute, or whether mothers can potentially raise offspring alone. Thus, cooperative offspring care is seen in different contexts across animal lineages, but may nonetheless share several important psychological characteristics. We end by discussing how work on humans may play a unifying role in studying cooperative offspring care.
Keywords: cooperative breeding, allomaternal care, comparative analyses, human evolution
1. Introduction
Evolution involves descent with modification, and therefore generally leads to increasing morphological, physiological and behavioural diversification. Sometimes newly evolved, derived traits in one lineage are functionally similar to traits in other lineages, a phenomenon called evolutionary convergence. Examples include the evolution of flight, warning coloration, complex eyes [1] or cooperative breeding [2,3]. However, the underlying genetic basis of these convergently evolved traits will only rarely be identical [1].
Tinbergen [4] famously distinguished between proximate and ultimate aspects of behaviour, implying that selection for a function is actually selection of a mechanism. Thus, despite differences in the genetic foundation, it is not implausible to expect similarity at higher levels of proximate causation. For behavioural traits, we may therefore ask whether the underlying motivations or psychological predispositions (i.e. an individual's attitudes towards sets of objects or behavioural options, typically reflected in explicit decision-making [5]) are similar enough to warrant giving them the same label, at least in members of the same broad lineage.
Here, we examine the case of cooperative offspring care. Although definitions of cooperative breeding have drifted over time [6–8], it is usually defined as conspecifics helping parents raise their young [2,8] (see also below). By this definition, humans are cooperative breeders, but our cooperative breeding almost certainly arose from a different ancestral state [9,10] than it did in birds [3] and other mammals [2], among which it evolved independently multiple times and largely from similar ancestral social systems. Moreover, despite the variability among and between birds and mammals, the human version differs in so many respects from both (elaborated below) that various experts have argued humans should not be given the same label [11,12]. This debate suggests that it is important to recognize the potential heterogeneity of cooperative offspring care in different species or lineages.
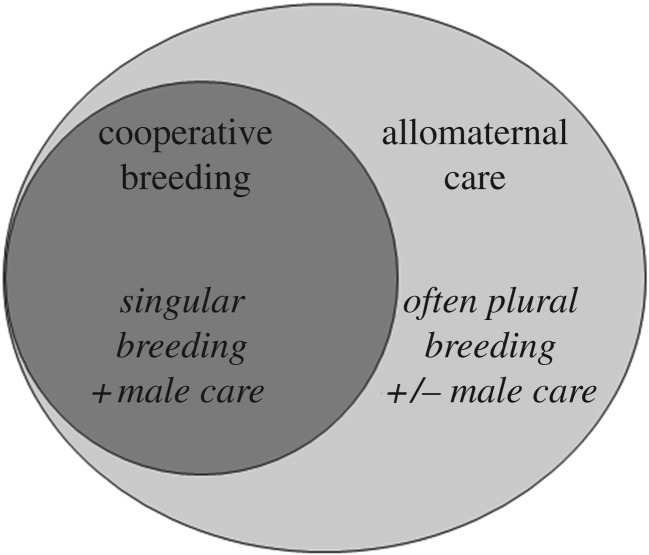
Indeed, one possible reason for confusion about the state of humans versus the other cooperative breeders is that allomaternal care is observed in two contexts (figure 1). The first context is cooperative breeding in species with biparental care (either birds or mammals, including callitrichid primates). Research here often focuses on what makes helping by non-reproducing individuals adaptive [8]. This work considers several forms of helping behaviours, such as incubation, sentinelling, babysitting, provisioning or carrying, but major foci are the role of kin selection [13,14], and cases where helpers contribute to rearing non-kin or distant relatives [15].
Figure 1.
Contexts in which cooperative offspring care in animals is typically studied.
The second context is allomaternal care in group-living mammals with plural breeding, such as elephants, coatis, dolphins, sperm whales and many primates, with quite variable paternal involvement. Researchers ask how much of which kind of help a breeding female receives in the form of provisioning, infant carrying or babysitting and what benefits she derives from this [16–19]. Importantly, this perspective acknowledges that help can be vital to immatures even if the costs to helpers are modest, as when an experienced female elephant helps the calf of another mother to move out of a ditch from which it could not escape without help [20]. This kind of allomaternal care overlaps with communal breeding, where reproductive females help each other [16], but it is perhaps best to separate the non-nursing care considered here from allonursing, which has almost certainly evolved independently [21,22] and only involves adult females that currently have dependent offspring. In sum, allomaternal care is seen in both cooperative and non-cooperative breeders, and the form, function and proximate regulation of the behaviours involved may differ as well.
The aim of this paper is to examine to what extent shared offspring care in humans and other mammals or birds represents overlapping sets of traits, each of which may be homogeneous in terms of function and regulation. Ultimately, we wish to assess the extent to which major psychological predispositions of humans that make us different from the other great apes can be traced back to the evolution of the human-specific form of cooperative breeding, as postulated by the cooperative breeding hypothesis for human cooperation [17,23].
We begin with an overview of cooperative offspring care in birds and mammals, and then present the human case. Then, we discuss the differences in the ancestral states between cooperative breeders and species with allomaternal care that are usually not considered cooperative breeders. We find that cooperative offspring care has multiple independent origins and varies in form and function. We will see that many of the distinct elements of extensive allomaternal care in cooperative and independent breeders share hormonal and psychological mechanisms, despite having different histories and somewhat different functions.
2. Cooperative offspring care: basic description
Most definitions of cooperative breeding describe systems where helpers assist a breeding pair in raising offspring [7,8], and thereby exclude species in which allomaternal care but no male care is found (but see [24,25]). In species where non-parents help, it is most often a family or extended family affair where offspring remain with their parents beyond independence and assist them in raising younger siblings [26]. Nevertheless, it is quite rare for helpers to only direct their care towards offspring of their own parents, but they direct it towards offspring with varying degrees of kinship.
In birds, helpers are usually sexually mature [3]; in mammals they are commonly juvenile [7]. Interestingly, in almost all birds where young remain in the family until the next breeding season, they act as helpers [3]. By contrast, as elaborated below, in most mammals where young remain with their mother after weaning they do not provide allomaternal care [27], showing that delayed dispersal in mammals does not inevitably lead to cooperative breeding. Estimates of the prevalence of cooperative breeding vary because tropical species remain poorly studied [28], but it appears more common in birds than in mammals: about 15–25% of bird species [3,8,28] versus about 2.5–3% or more of mammal species [2,25,29]. There is considerable variation on the theme of cooperative families [8]. For instance, reproductive skew can be moderate or extremely high [14], and in some species, cooperative breeding is facultative, whereas it is obligate in others.
Humans spent most of their evolutionary history as nomadic hunter–gatherers or foragers [30]. The rearing system of foragers involves extensive allomaternal care [16]. Fathers or other adult men often make major energetic contributions [31,32], as do grandmothers [33], though generally less than fathers or men generally [34]. Grandmothers and older children provide babysitting services [35], either in the band's camp or during foraging [36], which allows mothers to forage more or more efficiently. The energetic contributions of the immatures, however, are modest [37], largely because the skill-intensive foraging niche is mastered only late during ontogeny. Communal nursing is common, but generally involves close kin [38], and the caloric transfers provided by fathers and grandmothers are far more important. Taken together, mothers receive abundant help by allomothers. This includes significant help from non-breeding helpers, both from pre- and post-reproductive age categories, and all helpers flexibly complement each other's contributions. As the saying goes, it takes a village to raise a child.
Help does not merely flow towards immature offspring, however. There is also systematic adult–adult food sharing. First, within families, men and women forage on distinct sets of food items [39], which they subsequently share or exchange. Second, meat and honey, largely acquired by men, are also shared widely in a camp, reflecting the strong male bonds in a foraging band [40].
Despite cooperative breeding, reproductive skew in forager societies is modest. The social unit of foragers is a multi-level system of bands within a larger macro-band or community [41]. Crucially, each band contains multiple, interdependent families as well as unattached adults. Owing to pair bonds between unrelated individuals, different families are not necessarily all closely related to each other [30,42]. As a result of opportunistic dispersal and pair bonds, relatively few dyads are at the full-sib level [42]. Much support therefore goes to non-kin, because of extensive between-family sharing. However, because the sharing provides temporal stability at various time scales [43], bands would rarely be viable if they contained only a single family.
In sum, in humans the cooperative family element is complemented by male–male sharing of valuable foods, extensive care by post-reproductive women and two-way sharing within the pair bond, plus some sharing and caring by unmarried adults. Thus, in humans the help is not just directed at immatures but also at adults of either sex.
3. Evolutionary origins
Here, we briefly discuss the evolutionary origins of help in rearing offspring, distinguishing cooperative breeding (figure 2a,b), allomaternal care in plural breeders (figure 2c), and the human system (figure 2d).
Figure 2.
The evolutionary pathway towards classic cooperative breeding (CB) in (a) birds and (b) mammals, and towards (c) allomaternal care in plural breeders and (d) cooperative breeding in humans.
(a). Cooperative families in birds and mammals
Cooperative breeding among birds probably arose in two steps [3]. First, in pair-living species, offspring began to stay with their parents beyond nutritional independence, leading to family living. The conditions favouring family living were thought to be high risk of predation and opportunities for skill learning for the immatures [44–46]. Second, where in such family groups young were still around during the next breeding attempt, they typically helped their parents rearing the next brood(s) despite already being of reproductive age. This was presumably facilitated by less predictable food supplies through increasing environmental variability [47], and thus steeper fitness benefits from receiving help.
Cooperative breeding among pair-living mammals also probably evolved in two steps. The transition towards pair living appears less prevalent in mammals than in birds because male mammals can less readily assist their lactating females in raising offspring through provisioning. This transition towards pair living may be facilitated by different evolutionary drivers [48,49]. Among infant-carrying primates, for instance, social units generally contain both sexes, presumably to reduce infanticide risk, so when groups become smaller, associated pairs may remain [50] (see also [51,52] for a summary of the debate regarding the role of infanticide for pair living). In mammals, not all males living in pairs engage in direct infant care, and young often disperse late enough to be able to help rear the next set of young (26.6% in mammals; 11.1% in birds; M.G. 2017, unpublished data), but they usually do not help. The first step towards cooperative breeding in pair-living mammals therefore probably was that males began to provide care [49]. Secondarily, this allowed females to increase their reproductive effort, which made it advantageous for older young to begin helping to rear their younger siblings [2]. This final transition towards help by offspring may have been facilitated, as in birds, by an increase in the variability in food availability [29].
In sum, in birds, where 55% of species are pair living [3], family living appeared to be the critical precondition for the transition to cooperative breeding. In mammals by contrast, where 95% of species show female-only care [49], it was more likely male parental care and the accompanying increased female reproductive investment that was the critical precondition (figure 2a,b). However, in both birds and mammals, high average relatedness in the social units was a precondition and thus kin selection responsible for the evolution of cooperative breeding in most cases [2,3].
(b). Allomaternal care without cooperative breeding
In a range of species, help is provided by pre-reproductive helpers, breeding is plural and male care is often absent. Allomothers may protect and babysit infants, which allows mothers to forage more efficiently. Examples include elephants [20], sperm whales [53] and primates, where non-mothers, including other females that are not close relatives, may show extensive infant carrying and babysitting [16,18,54]. Females make up the most common class of allomothers, especially but not exclusively adolescents [16].
Although the actual helping effort is often modest, it may have a major impact on the survival of the offspring [55] or the mother's rate of infant production [19], as confirmed by comparative studies [56,57]. This explains why effective allomaternal help can evolve despite lower relatedness between helpers and offspring, as in non-monogamous species (figure 2c).
(c). Humans
Palaeo-anthropologists assume that the earlier forms of the genus Homo, around 2 Ma, lived in large mixed-sex groups, just like our closest-living relatives [10]. We can conclude this, for instance, because no terrestrial or semi-terrestrial primate species is socially monogamous [58]. In later forms, we see evidence for communal defence against predators [59] as well as cooperative hunting, as suggested by the hunting of large bovids [60]. It is therefore most likely that our evolutionary history never showed the state of isolated dispersed, territorial male–female pairs that set the scene for cooperative breeding in other mammals and birds. Cooperative breeding in our lineage therefore had probably evolved along another path [9].
Any reconstruction must remain speculative at this stage [10,61], but the elements listed earlier almost certainly evolved partly independently. As suggested in figure 2d, likely crucial early elements were the presence of male–male bonds and non-exclusive male–female friendships, the increasing difficulties of immatures to feed themselves (and thus steeper fitness benefits of provisioning them), and the gradually increasing difficulty of giving birth, which may have led to the evolution of midlife menopause, and thus provisioning and helping by grandmothers [10,59,62]. Critically, the extensive help and sharing seen in humans seems not due to unusually high relatedness between helpers and offspring [9]. Rather, human immatures could not be reared successfully without extensive allomaternal care and interdependent adult human foragers could not survive without the extensive within-band sharing [30,43].
4. The major elements of cooperative offspring care
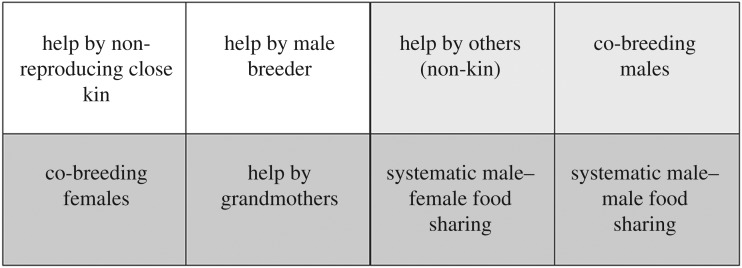
The unexpected heterogeneity in cooperative offspring care [7,8] suggests it might be useful to examine the broad phenomenon as a bundle of potentially independent elements. In figure 3, the white block contains the two core components of cooperative breeding in birds and mammals: help by breeding males and help by non-reproducing close kin. As noted above, in mammals, we can find cases where the breeding males do not help, but others provide care [18,21]. In addition, help may also be provided by others than older siblings or the sire (light grey) [15]. While this is common in humans, it is also common in other species, and may reflect the need for individuals to be members of a group rather than solitary floaters, which can have substantially lower survival [63]. In this case, helpers are not always close kin of those they help, and we may expect a pay-to-stay system [64]. Alternatively, helping relatives rearing offspring may be the best option to maximize inclusive fitness in a given situation. For instance, when their own nest fails, white-fronted bee-eaters and long-tailed tits provision the nestlings of relatives [65,66]. Helping by unrelated individuals may be even more common than expected when the focus is not exclusively on provisioning [21].
Figure 3.
The main elements of cooperative offspring care systems. Entries in the first row are the major elements of classic cooperative breeding (cooperative families); those in the second row are not.
Another additional element of cooperative breeding can be co-breeding by males. Especially in mammalian carnivores, some birds and callitrichid monkeys, paternity is not always monopolized by a single male [15,67–69]. This may reflect concessions to maintain critical collective action, as in social hunters that require more than two hunters to kill or defend prey [70], or the need for multiple helpers, as in obligate cooperative breeders, which means that a single breeding pair cannot establish a new group [71]. Some tamarin species, for instance, may fail to breed as pairs and are therefore routinely polyandrous [69,72]. Humans are quite different, because all men in forager groups are pair bonded, at least in principle. Yet this may merely be on one endpoint of a continuum because in areas of low productivity marriage arrangements may become polyandrous [73].
The elements in the lower row of figure 3 are not traditionally considered part of cooperative breeding (see also §2). In many group-living mammals, but especially primates [21], breeding females receive some allomaternal help (i.e. infant carrying, babysitting and sometimes allonursing), usually by kin that are selective in their help (e.g. [74]). Thus, female co-breeding is frequently found in allomaternal care systems, but the co-breeding found in humans is unusual due to the intensity of allomaternal care.
Premature menopause, and thus the presence of non-reproducing older females (grandmothers), is found not only in humans but has also been reported for some whales (e.g. in orcas). However, because menopause in orcas should probably not be considered allomaternal care [75], it may well be that grandmothering with menopause is uniquely human.
At least two elements, finally, appear uniquely elaborated to humans. The first is systematic male–female food sharing, a reflection of the sexual division of labour. Although provisioning of the female by the male is also seen in various cooperative breeders, especially during incubation in birds [76], this provisioning is not reciprocal. The second element is systematic, reciprocal sharing of valuable foods by a local band's men [77], which is an expression of the male-bonding component of human social organization. Male bonding is also seen in chimpanzees, lions or raptors, but they show opportunistic sharing around a kill [78–80] rather than the transport of the quarry to a central home base and thus proactive sharing, as seen in humans. Thus, again, this element may be uniquely human, although the provisioning of adult African wild dogs that remain in the den to guard the young while the rest were out to hunt may be an intermediate case [67].
Because the patchy distribution of these elements suggests independent origins, it may be worth asking whether they are also regulated by distinct processes. However, due to the great similarity in the actual actions involved, they may actually have come to share proximate mechanisms. We now turn to this question.
5. Proximate mechanisms of cooperative offspring care
As implied by Tinbergen's framework [4], selection for allomaternal care behaviours requires selection on a proximate mechanism, such as a genetically based hormonal regulatory system, which can bring about changes in psychological preferences and predispositions. Here, we ask whether these mechanisms are characteristic for cooperative breeders in the commonly accepted sense or extensive allomaternal care per se, and to what extent variation in the elements of cooperative breeding discussed above (figure 3) might influence which proximate mechanisms are selected for in a given species.
(a). Hormonal mechanisms
At the hormonal level, the regulatory system involved in maternal behaviour seems also involved in allomaternal behaviour by male breeders, kin helpers and other allomaternal care providers [81–84]. For instance, in meerkats, peripheral administration of oxytocin increases provisioning and affiliation with pups [85]. In marmosets, oxytocin increases not only in mothers after the birth of infants but in all group members. It is also associated with infant licking and food sharing [86], and reflects social bonds among adults [87]. The oxytocin system also seems involved in human grandmothering [88]. Likewise, increased levels of prolactin are associated with a higher helper effort in Florida scrub jays [89], meerkats [90] and marmosets [82,91]. Importantly, the same pattern also holds for allomaternal care in plural, non-cooperative breeders [92].
Hormonal systems regulating maternal behaviours often also have other sex-specific reproductive functions, which might interfere with the reproductive functions of the opposite sex. Some differences in the hormonal regulation of male versus female allomaternal care must therefore be expected [93–95]. Furthermore, the necessity to be physiologically ready to reproduce independently in case a breeding opportunity arises will impose further constraints [96,97]. In fact, trade-offs between infant care and independent reproduction may predict species differences in helping. For instance, these trade-offs may bias against helping in species with year-round nesting but not in seasonal species [98]. In the latter, individuals who did not manage to breed will anyway have to wait until the next year to mate, and therefore the reduced testosterone levels associated with helping [96,99] will not compromise mating success in the following year.
Neuro-endocrine mechanisms often regulate behaviour by modulating psychological predispositions and motivations, which we will discuss now. In the case of helping behaviour, these may include social tolerance, spontaneous or proactive prosocial predispositions, as well as the readiness of mothers to share their offspring and to bond with a newborn infant.
(b). Psychological adaptations in helpers
The most common helping behaviours are provisioning, protection and vigilance, and in primates, infant carrying [8,21]. All require high social tolerance towards the offspring. Whenever these behaviours have to be coordinated in close proximity to other group members, high tolerance towards adult group members is also required, such as around the nest in birds or around and inside the den in burrowing mammals. In species where infants are carried and thus transferred from one caregiver to another, all potential carriers must be highly tolerant. Both observations from the wild [69,100,101] and empirical evidence in captivity [102–104] suggest the amount of allomaternal care is indeed correlated with group-level social tolerance in primates. Important in the context of this paper, what is decisive is the extent of allomaternal care, rather than whether or not a species qualifies as cooperative breeder. We are not aware of similar comparative analyses for a link between social tolerance and cooperative breeding in other lineages, although in birds, high social tolerance near the nest probably determines whether species breed cooperatively or not [105].
Living in larger groups with high reproductive skew increases competition between group members for breeding slots, which may lead to high degrees of context specificity of social tolerance. For instance, in callitrichid monkeys [97,106], the high social tolerance during everyday activities is punctuated by episodes of intense competition when breeding vacancies become available, a pattern also found in cooperatively breeding apostlebirds [107] (M.G. 2017, unpublished data) and acorn woodpeckers [108]. In very large groups with extreme reproductive skew, one possible way of dealing with increased competition is to restrict tolerance mostly to offspring. This, however, requires that helping can be organized in a way that minimizes the need for close behavioural coordination between adults.
Social tolerance is a necessary precondition for helping, but not sufficient: additional psychological mechanisms are necessary. One possibility is that each of the specific helping behaviours of a given species is the result of automatic triggering by specific cues (e.g. feeding triggered by begging cues, or caring for larvae by olfactory cues). This type of regulation can be non-flexible and prone to misdirected offspring care [24]. Alternatively, the helping behaviours may be regulated more generally by a psychological prosocial helping disposition. The latter seems to be the case in at least some mammals. For instance, oxytocin is involved in the regulation of multiple infant care behaviours (e.g. food sharing and infant licking in marmosets [86]), a variety of other cooperative behaviours such as sentinel behaviour and digging (meerkats [85]), as well as in experimentally assessed proactive prosociality between adults (marmosets: [109]).
For primates, experimental comparative evidence over a large number of species supports a link between proactive prosociality and cooperative offspring care [103]. Again, interspecific variation in proactive prosociality was better explained by the extent of allomaternal care rather than qualifying as a cooperative breeder or not as, as was the case for tolerance. Such a link has been questioned, in particular for non-primate species (see [110,111], but also [104]), but recent evidence from dolphins [112] and corvids [113] suggests that it may not be limited to primates. More comparative data will help to further narrow down in which lineages and under which conditions allomaternal care is linked with social tolerance and proactive prosociality.
Both in the wild and in naturalistic situations in captivity, proactive food sharing in callitrichids is predominantly directed at immatures [114], which may appear conflicting, with experimental evidence suggesting proactive prosociality between adults as well. However, even food sharing with immatures is not indiscriminate, but increases when food is difficult to obtain for the immatures (e.g. Leontopithecus chrysomelas [115], Saguinus oedipus [116], Callithrix jacchus [117]). In experimental prosociality tasks, food cannot be obtained independently at all by potential recipients, and it is in exactly this situation where proactive prosociality between adults is measured. Such situations may be rare in naturalistic conditions, where proactive prosociality between adults is therefore more likely involved in facilitating cooperation, as in collective action and cooperative behaviour in various contexts, including cooperative food harvesting, vigilance and other forms of predator protection, territorial and resource defence [100,118], but also cooperative communication [119]. In fact, cooperation tasks reveal that callitrichids are more likely than capuchin monkeys, chimpanzees or orangutans to maintain high levels of cooperation even if for some time they do not obtain a reward for it [120]. This corresponds to the situation in humans, where prosocial tendencies assessed in experimental tasks [121] likewise do not imply that all resources are shared with others all the time, but that this predisposition is context dependent and involved in facilitating a broad range of cooperative behaviours. In fact, due to more complex cognitive abilities in humans, and perhaps supported by uniquely human evolutionary processes such as cultural group selection, this may well have given rise to some of the uniquely derived human features such as large-scale cooperation and language [17].
(c). Psychological adaptations in mothers: tolerance and conditional mother–offspring bonding
Psychological adaptations may not only be required in helpers, but also in mothers. First, mothers have to tolerate others around their offspring, which is not obvious. Young primate females, for instance, often show a high motivation to interact with immatures, but may also leave the infants behind or even abuse them when their interest wanes. These cases are best described as kidnapping and do not benefit the infant [16,122]. Mothers may therefore only allow kin to handle infants [123], and maternal tolerance towards others is thus kin biased [16]. However, in classic cooperative breeders, such as callitrichids, selective intolerance is also found towards related female helpers when they potentially start breeding [86,97].
Second, in specialized cooperative breeders where raising infants without help is not possible, a mother's readiness to invest in the offspring may be contingent on the perceived availability of allomaternal care. When females perceive a lack of help, they may choose not to invest in the current offspring. At the proximate level, this may be mirrored in conditional postpartum responsiveness of mothers to infants, as proposed for humans and other cooperatively breeding primates, which can prevent mothers from immediately bonding with the new-born and thus enables her to eventually reject it [124].
Since more systematic reliance on helpers can only evolve once mothers have acquired some levels of tolerance towards potential allomothers, together this supports the crucial role of kin selection early in the evolutionary trajectories towards allomaternal care in all contexts, including cooperative breeding. By contrast, the conditional investment by mothers in their new-born offspring, depending on the availability of help, must be a more derived adaptation, only expected in obligate cooperative breeders.
6. Discussion
This special issue addresses the question of how studying humans can help identifying biological fundamentals. The obvious answer must be that no one species is more important than another one, because fundamental biological processes can only be established based on broad patterns observed across species. Nonetheless, humans often appear unique among animals in many ways, which can lead to a new perspective on related phenomena in animals. We have focused on human cooperative child care, and doing so indirectly shed new light on the variability of shared offspring care in animals.
Consideration of the human form of cooperative breeding led to the realization that allomaternal care is seen in two distinct contexts: cooperative breeding, where the modal pattern is that offspring help their parents raise younger siblings, and allomaternal care in independent breeders, where help tends to be less spectacular and pervasive. In humans, we see both elements, plus others, such as male bonding, which led to a male predisposition to share food with allies, and a pronounced sexual division of labour in the pair bond.
The evolutionary origins of cooperative breeding and allomaternal care appear clearly distinct, as do the evolutionary trajectories that led to cooperative breeding in birds and mammals [2,3]. In birds, family living is a critical precondition, whereas in mammals it is male care. In the human case, we find both elements, but also midlife menopause and male bonding, although it is difficult to assess in which order these derived traits evolved. However, despite these distinct evolutionary histories, strong convergences nonetheless appear to exist at the proximate (hormonal) level.
As to psychological predispositions, comparative primate data arguably support a direct link between allomaternal care and social tolerance and proactive prosociality. For researchers interested in the evolution of prosociality and tolerance in humans, primates are most relevant, given the importance of path dependence in evolution. Whether such a link also exists in other lineages remains an empirical question, but its pursuit is one example in which research on humans can give rise to efforts to establish biological fundamentals. The same comparative work on primates also revealed that the overall extent of allomaternal care, rather than being classified as a cooperative breeder or not, is more important for the prevalence of these psychological predispositions. These results show that it is fruitful to combine the perspectives of cooperative breeding and allomaternal care, which became necessary because humans show a combination of both.
In sum, studying the role of cooperative breeding in human cooperation has not only led to new hypotheses and insights regarding human evolution, but can also impact research on biological fundamentals in other animals. In particular, it can lead to a better integration of the rather separate research traditions of cooperative breeding and allomaternal care, raises the issue of lineage specificity of biological processes and offers a test case for how deeply shared proximate convergence can be found in functionally convergent traits.
Data accessibility
This article has no additional data.
Authors' contributions
J.B., M.G. and C.v.S. wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
This work has been funded by SNF project 31003A-12979 to J.M.B.; by SNF project 310030B_160363 and the A.H. Schultz Foundation to C.v.S.; and by the National Science Centre, Poland, through the European Union's Horizon 2020 research and innovation programme (Marie Sklodowska-Curie grant no. 665778) to M.G.
References
- 1.Futuyma DJ. 1998. Evolutionary biology. Sunderland, MA: Sinauer. [Google Scholar]
- 2.Lukas D, Clutton-Brock T. 2012. Life histories and the evolution of cooperative breeding in mammals. Proc. R. Soc. B 279, 4065–4070. ( 10.1098/rspb.2012.1433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griesser M, Drobniak SM, Nakagawa S, Botero CA, Morlon H. 2017. Family living sets the stage for cooperative breeding and ecological resilience in birds. PLoS Biol. 15, e2000483 ( 10.1371/journal.pbio.2000483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tinbergen N. 1963. On aims and methods of ethology. Ethology 20, 410–433. [Google Scholar]
- 5.Lichtenstein S, Slovic P.. 2006. The construction of preference. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 6.Skutch AF. 1961. Helpers among birds. Condor 63, 198–226. ( 10.2307/1365683) [DOI] [Google Scholar]
- 7.Solomon NG, French JA. 1997. Cooperative breeding in mammals. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 8.Koenig WD, Dickinson J. 2016. Cooperative breeding in vertebrates: studies of ecology, evolution, and behavior. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 9.Kramer KL, Russell AF. 2015. Was monogamy a key step on the Hominin road? Reevaluating the monogamy hypothesis in the evolution of cooperative breeding. Evol. Anthropol. 24, 73–83. ( 10.1002/evan.21445) [DOI] [PubMed] [Google Scholar]
- 10.Van Schaik CP. 2016. The primate origins of human nature. New York, NY: John Wiley & Sons. [Google Scholar]
- 11.Silk JB, House BR.. 2016. The evolution of altruistic social preferences in human groups. Phil. Trans. R. Soc. B 371, 20150097 ( 10.1098/rstb.2015.0097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogin B, Bragg J, Kuzawa C. 2014. Humans are not cooperative breeders but practice biocultural reproduction. Ann. Hum. Biol. 41, 368–380. ( 10.3109/03014460.2014.923938) [DOI] [PubMed] [Google Scholar]
- 13.Boomsma JJ. 2009. Lifetime monogamy and the evolution of eusociality. Phil. Trans. R. Soc. B 364, 3191–3207. ( 10.1098/rstb.2009.0101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koenig WD, Dickinson JL. 2004. Ecology and evolution of cooperative breeding in birds. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 15.Riehl C. 2013. Evolutionary routes to non-kin cooperative breeding in birds. Proc. R. Soc. B 280, 20132245 ( 10.1098/rspb.2013.2245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hrdy SB. 1999. Mother nature: maternal instincts and how they shape the human species. New York, NY: Ballantine Books.
- 17.Burkart JM, Hrdy SB, Van Schaik CP. 2009. Cooperative breeding and human cognitive evolution. Evol. Anthropol. 18, 175–186. ( 10.1002/evan.20222) [DOI] [Google Scholar]
- 18.Tecot SR, Baden AL.. 2015. Primate allomaternal care. In Emerging trends in the social and behavioral sciences: an interdisciplinary, searchable, and linkable resource (eds Scott R, Kosslyn S), pp. 1–16. Hoboken, NJ: Wiley & Sons. [Google Scholar]
- 19.Lahdenperä M, Mar KU, Lummaa V.. 2016. Nearby grandmother enhances calf survival and reproduction in Asian elephants. Sci. Rep. 6, 446 ( 10.1038/srep27213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee PC. 1987. Allomothering among African elephants. Anim. Behav. 35, 278–291. ( 10.1016/S0003-3472(87)80234-8) [DOI] [Google Scholar]
- 21.Isler K, van Schaik CP. 2012. Allomaternal care, life history and brain size evolution in mammals. J. Hum. Evol. 63, 52–63. ( 10.1016/j.jhevol.2012.03.009) [DOI] [PubMed] [Google Scholar]
- 22.Lewis SE, Pusey AE.. 1997. Factors influencing the occurrence of communal care in plural breeding mammals. In Cooperative breeding in mammals (eds Solomon NG, French JA), pp. 335–363. New York: NY: Cambridge University Press. [Google Scholar]
- 23.Hrdy SB. 2009. Mothers and others: the evolutionary origins of mutual understanding. Cambridge, MA: Harvard University Press. [Google Scholar]
- 24.Griesser M, Suzuki TN. 2016. Occasional cooperative breeding in birds and the robustness of comparative analyses concerning the evolution of cooperative breeding. Zool. Lett. 2, 1 ( 10.1186/s40851-016-0041-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell AF. 2004. Mammals: comparisons and contrasts. In Ecology and evolution of cooperative breeding in birds (eds Koenig WD, Dickinson J), pp. 210–227. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 26.Drobniak SM, Wagner G, Mourocq E, Griesser M. 2015. Family living: an overlooked but pivotal social system to understand the evolution of cooperative breeding. Behav. Ecol. 26, 805–811. ( 10.1093/beheco/arv015) [DOI] [Google Scholar]
- 27.Jones KE, et al. 2009. PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals: ecological archives E090-184. Ecology 90, 2648 ( 10.1890/08-1494.1) [DOI] [Google Scholar]
- 28.Ke D, Griesser M, Huang Z. 2016. Cooperative breeding among Chinese passerines: inference from social behavior, diet, and migratory status. J. Field Ornithol. 87, 204–212. ( 10.1111/jofo.12152) [DOI] [Google Scholar]
- 29.Lukas D, Clutton-Brock T. 2017. Climate and the distribution of cooperative breeding in mammals. Open sci. 4, 160897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill K, et al. 2011. Co-residence patterns in hunter-gatherer societies show unique human social structure. Science 331, 1286–1289. ( 10.1126/science.1199071) [DOI] [PubMed] [Google Scholar]
- 31.Marlowe FW. 2003. The mating system of foragers in the standard cross-cultural sample. Cross Cult. Res. 37, 282–306. ( 10.1177/1069397103254008) [DOI] [Google Scholar]
- 32.Hill K, Hurtado AM. 2009. Cooperative breeding in South American hunter–gatherers. Proc. R. Soc. B 276, 3863–3870. ( 10.1098/rspb.2009.1061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hawkes K, O'Connell F, Blurton Jones N.. 1989. Hardworking Hadza grandmothers. In Comparative socioecology: the behavioural ecology of humans and other mammals (eds Standen V, Foley RA), pp. 341–366. Oxford, UK: Blackwell Scientific Publications. [Google Scholar]
- 34.Gurven M, Hill K. 2009. Why do men hunt? Curr. Anthropol. 50, 51–74. ( 10.1086/595620) [DOI] [PubMed] [Google Scholar]
- 35.Hawkes K, O'Connell JF, Blurton Jones NG, Alvarez H, Charnov EL. 1998. Grandmothering, menopause, and the evolution of human life histories. Proc. Natl Acad. Sci. USA 95, 1336–1339. ( 10.1073/pnas.95.3.1336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marlowe FW. 2006. Central place provisioning, the Hadza as an example. In Feeding ecology in apes and other primates (eds Hohmann G, Robbins M, Boesch C), pp. 359–377. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 37.Ivey Henry P, Morelli GA, Tronick EZ. 2005. Child caretakers among Efe foragers of the Itruri Forest. In Hunter–gatherer childhoods: evolutionary, developmental, and cultural perspectives (eds Hewlett BS, Lamb ME), pp. 347–388. Piscataway, NJ: Transaction Publishers. [Google Scholar]
- 38.Hewlett BS, Winn S. 2014. Allomaternal nursing in humans. Curr. Anthropol. 55, 200–229. ( 10.1086/675657) [DOI] [PubMed] [Google Scholar]
- 39.Marlowe FW. 2007. Hunting and gathering: the human sexual division of foraging labor. Cross Cult. Res. 41, 170–195. ( 10.1177/1069397106297529) [DOI] [Google Scholar]
- 40.Foley R, Gamble C. 2009. The ecology of social transitions in human evolution. Phil. Trans. R. Soc. B 364, 3267–3279. ( 10.1098/rstb.2009.0136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Layton R, O'Hara S, Bilsborough A.. 2012. Antiquity and social functions of multilevel social organization among human hunter–gatherers. Int. J. Primatol. 33, 1215–1245. ( 10.1007/s10764-012-9634-z) [DOI] [Google Scholar]
- 42.Apicella CL, Marlowe FW, Fowler JH, Christakis NA. 2012. Social networks and cooperation in hunter–gatherers. Nature 481, 497–501. ( 10.1038/nature10736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaplan HS, Hooper PL, Gurven M. 2009. The evolutionary and ecological roots of human social organization. Phil. Trans. R. Soc. B 364, 3289–3299. ( 10.1098/rstb.2009.0115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griesser M, Suzuki TN. 2017. Naïve juveniles are more likely to become breeders after witnessing predator mobbing. Am. Nat. 189, 58–66. ( 10.1086/689477) [DOI] [PubMed] [Google Scholar]
- 45.Griesser M, Suzuki TN. 2016. Kinship modulates the attention of naïve individuals to the mobbing behaviour of role models. Anim. Behav. 112, 83–91. ( 10.1016/j.anbehav.2015.11.020) [DOI] [Google Scholar]
- 46.Schuppli C, Isler K, van Schaik CP. 2012. How to explain the unusually late age at skill competence among humans. J. Hum. Evol. 63, 843–850. ( 10.1016/j.jhevol.2012.08.009) [DOI] [PubMed] [Google Scholar]
- 47.Rubenstein DR, Lovette IJ. 2007. Temporal environmental variability drives the evolution of cooperative breeding in birds. Curr. Biol. 17, 1414–1419. ( 10.1016/j.cub.2007.07.032) [DOI] [PubMed] [Google Scholar]
- 48.Reichard UH. 2003. Monogamy: past and present. In Monogamy: mating strategies and partnerships in birds, humans and other mammals (eds Reichard UH, Boesch C), pp. 3–25. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 49.Lukas D, Clutton-Brock T. 2013. The evolution of social monogamy in mammals. Science 341, 526–530. ( 10.1126/science.1238677) [DOI] [PubMed] [Google Scholar]
- 50.van Schaik CP, Kappeler PM. 2003. The evolution of social monogamy in primates. In Monogamy: mating strategies and partnerships in birds, humans and other mammals (eds Reichard UH, Boesch C), pp. 59–80. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 51.Opie C, Atkinson QD, Dunbar RIM, Shultz S. 2013. Male infanticide leads to social monogamy in primates. Proc. Natl Acad. Sci. USA 110, 13 328–13 332. ( 10.1073/pnas.1307903110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Opie C, Atkinson QD, Dunbar RIM, Shultz S. 2014. Reply to Lukas and Clutton-Brock: infanticide still drives primate monogamy. Proc. Natl Acad. Sci. USA 111, E1675 ( 10.1073/pnas.1403165111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gero S, Engelhaupt D, Rendell L, Whitehead H. 2009. Who cares? Between-group variation in alloparental caregiving in sperm whales. Behav. Ecol. 20, 838–843. ( 10.1093/beheco/arp068) [DOI] [Google Scholar]
- 54.Fairbanks LA. 1990. Reciprocal benefits of allomothering for female vervet monkeys. Anim. Behav. 40, 553–562. ( 10.1016/S0003-3472(05)80536-6) [DOI] [Google Scholar]
- 55.Tecot SR, Baden AL, Romine N, Kamilar JM. 2013. Reproductive strategies and infant care in the Malagasy primates. In Building babies (ed. Koenig WD.), pp. 321–359. New York: NY: Springer. [Google Scholar]
- 56.Mitani JC, Watts D. 1997. The evolution of non-maternal caretaking among anthropoid primates: do helpers help? Behav. Ecol. Sociobiol. 40, 213–220. ( 10.1007/s002650050335) [DOI] [Google Scholar]
- 57.Ross C, MacLarnon A. 2000. The evolution of non-maternal care in anthropoid primates: a test of the hypotheses. Folia Primatol. 71, 93–113. ( 10.1159/000021733) [DOI] [PubMed] [Google Scholar]
- 58.Schülke O, Ostner J. 2012. Ecological and social influences on sociality. In The evolution of primate societies (eds Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB), pp. 195–219. Chicago, IL: University of Chicago Press. [Google Scholar]
- 59.Willems EP, van Schaik CP. 2017. The social organization of Homo ergaster: inferences from anti-predator responses in extant primates. J. Hum. Evol. 109, 11–21. ( 10.1016/j.jhevol.2017.05.003) [DOI] [PubMed] [Google Scholar]
- 60.Ferraro JV, et al. 2013. Earliest archaeological evidence of persistent hominin carnivory. PLoS ONE 8, e62174 ( 10.1371/journal.pone.0062174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chapais B. 2013. Monogamy, strongly bonded groups, and the evolution of human social structure. Evol. Anthropol. 22, 52–65. ( 10.1002/evan.21345) [DOI] [PubMed] [Google Scholar]
- 62.Hawkes K. 2003. Grandmothers and the evolution of human longevity. Am. J. Hum. Biol. 15, 380–400. ( 10.1002/ajhb.10156) [DOI] [PubMed] [Google Scholar]
- 63.Ridley AR, Raihani NJ, Nelson-Flower MJ. 2008. The cost of being alone: the fate of floaters in a population of cooperatively breeding pied babblers Turdoides bicolor. J. Avian Biol. 39, 389–392. [Google Scholar]
- 64.Kokko H, Johnstone RA, Wright J. 2002. The evolution of parental and alloparental effort in cooperatively breeding groups: when should helpers pay to stay? Behav. Ecol. 13, 291–300. ( 10.1093/beheco/13.3.291) [DOI] [Google Scholar]
- 65.Emlen ST, Wrege PH. 1988. The role of kinship in helping decisions among white-fronted bee-eaters. Behav. Ecol. Sociobiol. 23, 305–315. ( 10.1007/BF00300577) [DOI] [Google Scholar]
- 66.Russell AF, Hatchwell BJ. 2001. Experimental evidence for kin-biased helping in a cooperatively breeding vertebrate. Proc. R. Soc. B 268, 2169–2174. ( 10.1098/rspb.2001.1790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Creel S, Creel NM. 2002. The african wild dog: behavior, ecology, and conservation. Princeton, NJ: Princeton University Press. [Google Scholar]
- 68.Owens DD, Owens MJ. 1984. Helping behaviour in brown hyenas. Nature 308, 843–845. ( 10.1038/308843a0) [DOI] [PubMed] [Google Scholar]
- 69.Garber PA, Porter LM, Spross J, Di Fiore A. 2016. Tamarins: insights into monogamous and non-monogamous single female social and breeding systems. Am. J. Primatol. 78, 298–314. ( 10.1002/ajp.22370) [DOI] [PubMed] [Google Scholar]
- 70.Smith JE, Swanson EM, Reed D, Holekamp KE. 2012. Evolution of cooperation among mammalian carnivores and its relevance to hominin evolution. Curr. Anthropol. 53, s436–ss52. ( 10.1086/667653) [DOI] [Google Scholar]
- 71.Rubenstein DR. 2016. Superb starlings: cooperation and conflict in an unpredictable environment. In Cooperative breeding in vertebrates: studies of ecology, evolution, and behavior (eds Koenig WD, Dickinson J), pp. 181–196. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 72.Goldizen AW. 1988. Tamarin and marmoset mating systems: unusual flexibility. Trends Ecol. Evol. 3, 36–40. ( 10.1016/0169-5347(88)90045-6) [DOI] [PubMed] [Google Scholar]
- 73.Starkweather KE, Hames R. 2012. A survey of non-classical polyandry. Human Nature 23, 149–172. ( 10.1007/s12110-012-9144-x) [DOI] [PubMed] [Google Scholar]
- 74.O'Brien TG, Robinson J. 1991. Allomaternal care by female wedge-capped capuchin monkeys: effects of age, rank and relatedness. Behaviour 119, 30–50. ( 10.1163/156853991X00355) [DOI] [Google Scholar]
- 75.Croft DP, et al. 2017. Reproductive conflict and the evolution of menopause in killer whales. Curr. Biol. 27, 298–304. ( 10.1016/j.cub.2016.12.015) [DOI] [PubMed] [Google Scholar]
- 76.Silver R, Andrews H, Ball GF. 1985. Parental care in an ecological perspective: a quantitative analysis of avian subfamilies. Am. Zool. 25, 823–840. ( 10.1093/icb/25.3.823) [DOI] [Google Scholar]
- 77.Jaeggi AV, Gurven M. 2013. Reciprocity explains food sharing in humans and other primates independent of kin selection and tolerated scrounging: a phylogenetic meta-analysis. Proc. R. Soc. B 280, 20131615 ( 10.1098/rspb.2013.1615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Silk JB, Brosnan SF, Henrich J, Lambeth SP, Shapiro S. 2013. Chimpanzees share food for many reasons: the role of kinship, reciprocity, social bonds and harassment on food transfers. Anim. Behav. 85, 941–947. ( 10.1016/j.anbehav.2013.02.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schaller GB. 2009. The serengeti lion: a study of predator-prey relations. Chicago, IL: University of Chicago Press. [Google Scholar]
- 80.Cockburn A. 2004. Mating systems and sexual conflict. In Ecology and evolution of cooperative breeding in birds (ed. JL Dickinson), pp. 81–101. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 81.Feldman RA, Bakermans-Kranenburg MJ. 2017. Oxytocin: a parenting hormone. Curr. Opin. Psychol. 15, 13–18. ( 10.1016/j.copsyc.2017.02.011) [DOI] [PubMed] [Google Scholar]
- 82.Storey AE, Ziegler TE. 2016. Primate paternal care: interactions between biology and social experience. Horm. Behav. 77, 260–271. ( 10.1016/j.yhbeh.2015.07.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. 2008. Oxytocin, vasopressin and sociality. Prog. Brain Res. 170, 331–336. ( 10.1016/S0079-6123(08)00427-5) [DOI] [PubMed] [Google Scholar]
- 84.Bales KL, Kim AJ, Lewis-Reese AD, Carter CS. 2004. Both oxytocin and vasopressin may influence alloparental behavior in male prairie voles. Horm. Behav. 45, 354–361. ( 10.1016/j.yhbeh.2004.01.004) [DOI] [PubMed] [Google Scholar]
- 85.Madden JR, Clutton-Brock TH. 2011. Experimental peripheral administration of oxytocin elevates a suite of cooperative behaviours in a wild social mammal. Proc. R. Soc. B 278, 1189–1194. ( 10.1098/rspb.2010.1675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Finkenwirth C, Guerreiro Martins EM, Deschner T, Burkart JM. 2016. Oxytocin is associated with infant-care behavior and motivation in cooperatively breeding marmoset monkeys. Horm. Behav. 80, 10–18. ( 10.1016/j.yhbeh.2016.01.008) [DOI] [PubMed] [Google Scholar]
- 87.Ziegler TE, Crockford C. 2017. Neuroendocrine control in social relationships in non-human primates: field based evidence. Horm. Behav. 91, 107–121. ( 10.1016/j.yhbeh.2017.03.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gray P, Samms-Vaughan M.. 2009. Investigating potential hormonal associations of grandmaternal care in Jamaica. Int. J. Biol. Anthropol. 4. [Google Scholar]
- 89.Schoech SJ, Mumme RL, Wingfield JC. 1996. Prolactin and helping behaviour in the cooperatively breeding Florida scrub-jay, Aphelocoma coerulescens. Anim. Behav. 52, 445–456. ( 10.1006/anbe.1996.0189) [DOI] [Google Scholar]
- 90.Carlson AA, Russell AF, Young AJ, Jordan NR, McNeilly AS, Parlow AF, Brock TC. 2006. Elevated prolactin levels immediately precede decisions to babysit by male meerkat helpers. Horm. Behav. 50, 94–100. ( 10.1016/j.yhbeh.2006.01.009) [DOI] [PubMed] [Google Scholar]
- 91.Mota MT, Sousa MBC. 2000. Prolactin levels of fathers and helpers related to alloparental care in common marmosets, Callithrix jacchus. Folia Primatol. 71, 22–26. ( 10.1159/000021727) [DOI] [PubMed] [Google Scholar]
- 92.Soltis J, Wegner FH, Newman JD. 2005. Urinary prolactin is correlated with mothering and allo-mothering in squirrel monkeys. Physiol. Behav. 84, 295–301. ( 10.1016/j.physbeh.2004.12.006) [DOI] [PubMed] [Google Scholar]
- 93.Gordon IJ, Zagoory-Sharon O, Leckman JF, Feldman RA. 2010. Prolactin, oxytocin, and the development of paternal behavior across the first six months of fatherhood. Horm. Behav. 58, 513–518. ( 10.1016/j.yhbeh.2010.04.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cavanaugh J, French JA. 2013. Post-partum variation in the expression of paternal care is unrelated to urinary steroid metabolites in marmoset fathers. Horm. Behav. 63, 551–558. ( 10.1016/j.yhbeh.2013.02.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gray PB, McHale TS, Carré JM. 2017. A review of human male field studies of hormones and behavioral reproductive effort. Horm. Behav. 91, 52–67. ( 10.1016/j.yhbeh.2016.07.004) [DOI] [PubMed] [Google Scholar]
- 96.Muller MN. 2016. Testosterone and reproductive effort in male primates. Horm. Behav. 91, 36–51. ( 10.1016/j.yhbeh.2016.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yamamoto ME, Araujo A, MdF Arruda, Lima AKM, JdO Siqueira, Hattori WT. 2014. Male and female breeding strategies in a cooperative primate. Behav. Process. 109, 27–33. ( 10.1016/j.beproc.2014.06.009) [DOI] [PubMed] [Google Scholar]
- 98.Russell AF. 2001. Dispersal costs set the scene for helping in an atypical avian cooperative breeder. Proc. R. Soc. Lond. B 268, 95–99. ( 10.1098/rspb.2000.1335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.De La Cruz C, Solís E, Valencia J, Chastel O, Sorci G. 2003. Testosterone and helping behavior in the azure-winged magpie (Cyanopica cyanus): natural covariation and an experimental test. Behav. Ecol. Sociobiol. 55, 103–111. ( 10.1007/s00265-003-0674-4) [DOI] [Google Scholar]
- 100.Garber PA. 1997. One for all and breeding for one: cooperation and competition as a tamarin reproductive strategy. Evol. Anthropol. 5, 187–199. ( 10.1002/(SICI)1520-6505(1997)5:6%3C187::AID-EVAN1%3E3.0.CO;2-A) [DOI] [Google Scholar]
- 101.Schaffner CM, Caine NG. 2000. The peacefulness of cooperatively breeding primates. In Natural conflict resolution (eds Aureli F, de Waal FBM), pp. 155–169. Berkeley, CA: University of California Press. [Google Scholar]
- 102.Burkart JM. 2015. Opposite effects of male and female helpers on social tolerance and proactive prosociality in callitrichid family groups. Scientific Reports. 5, 9622 ( 10.1038/srep09622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Burkart JM, et al. 2014. The evolutionary origin of human hyper-cooperation. Nat. Commun. 5, 4747 ( 10.1038/ncomms5747) [DOI] [PubMed] [Google Scholar]
- 104.Burkart JM, van Schaik CP. 2016. Revisiting the consequences of cooperative breeding. J. Zool. 299, 77–83. ( 10.1111/jzo.12322) [DOI] [Google Scholar]
- 105.Ekman J, Griesser M. 2016. Siberian jays: delayed dispersal in absence of cooperative breeding. In Cooperative breeding in vertebrates: studies of ecology, evolution, and behavior (eds Koenig WD, Dickinson J), pp. 6–18. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 106.Digby LJ, Ferrari SF, Saltzman W. 2007. Callitrichines: The role of competition in cooperatively breeding species. In Primates in perspective (eds Campbell CJ, Fuentes A, MacKinnon KC, Panger MA, Bearder SK), pp. 85–105. New York: NY: Oxford University Press. [Google Scholar]
- 107.Griesser M, et al. 2009. Influence of winter ranging behaviour on the social organization of a cooperatively breeding bird species, the apostlebird. Ethology 115, 888–896. ( 10.1111/j.1439-0310.2009.01678.x) [DOI] [Google Scholar]
- 108.Mumme RL, Koenig WD, Pitelka FA. 1983. Reproductive competition in the communal acorn woodpecker: sisters destroy each other's eggs. Nature 306, 583–584. ( 10.1038/306583a0) [DOI] [Google Scholar]
- 109.Finkenwirth C, Burkart JM. 2017. Long-term-stability of relationship structure in family groups of common marmosets, and its link to proactive prosociality. Physiol. Behav. 173, 79–86. ( 10.1016/j.physbeh.2017.01.032) [DOI] [PubMed] [Google Scholar]
- 110.Thornton A, McAuliffe K. 2015. Cognitive consequences of cooperative breeding? A critical appraisal. J. Zool. 295, 12–22. ( 10.1111/jzo.12198) [DOI] [Google Scholar]
- 111.Thornton A, McAuliffe K, Dall SRX, Fernandez-Duque E, Garber PA, Young AJ. 2016. Fundamental problems with the cooperative breeding hypothesis. J. Zool. 299, 84–88. ( 10.1111/jzo.12351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nakahara F, Komaba M, Sato R, Ikeda H, Komaba K, Kawakubo A. 2017. Spontaneous prosocial choice by captive bottlenose dolphins, Tursiops truncatus. Behav. Process. 135, 8–11. ( 10.1016/j.beproc.2016.11.009) [DOI] [PubMed] [Google Scholar]
- 113.Horn L, Scheer C, Bugnyar T, Massen JJM. 2016. Proactive prosociality in a cooperatively breeding corvid, the azure-winged magpie (Cyanopica cyana). Biol. Lett. 12, 20160649 ( 10.1098/rsbl.2016.0649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jaeggi AV, Burkart JM, van Schaik CP. 2010. On the psychology of cooperation in humans and other primates: the natural history of food sharing and experimental evidence of prosociality. Phil. Trans. R. Soc. B 12, 2723–2735. ( 10.1098/rstb.2010.0118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moura AC, Nunes HG, Langguth A. 2010. Food sharing in lion Tamarins (Leontopithecus chrysomelas): does foraging difficulty affect investment in young by breeders and helpers? Int. J. Primatol. 31, 848–862. ( 10.1007/s10764-010-9432-4) [DOI] [Google Scholar]
- 116.Humle T, Snowdon CT. 2008. Socially biased learning in the acquisition of a complex foraging task in juvenile cottontop tamarins (Saguinus oedipus). Anim. Behav. 27, 267–277. ( 10.1016/j.anbehav.2007.05.021) [DOI] [Google Scholar]
- 117.Martins EM G, Burkart JM. 2013. Common marmosets preferentially share difficult to obtain food items. Folia Primatol. 84, 281–282. [Google Scholar]
- 118.Willems EP, van Schaik CP. 2015. Collective action and the intensity of between-group competition in nonhuman primates. Behav. Ecol. 26, 625–631. ( 10.1093/beheco/arv001) [DOI] [Google Scholar]
- 119.Borjon JI, Ghazanfar AA. 2014. Convergent evolution of vocal cooperation without convergent evolution of brain size. Brain Behav. Evol. 84, 93–102. ( 10.1159/000365346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Snowdon CT, Cronin KA. 2007. Cooperative breeders do cooperate. Behav. Process. 76, 138–141. ( 10.1016/j.beproc.2007.01.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fehr E, Fischbacher U. 2004. Social norms and human cooperation. Trends Cogn. Sci. 8, 185–190. ( 10.1016/j.tics.2004.02.007) [DOI] [PubMed] [Google Scholar]
- 122.Hrdy SB. 1976. Care and exploitation of nonhuman primate infants by conspecifics other than the mother. In Advances in the study of behavior, vol. 6 (eds Rosenblatt JS, Hinde RA, Shaw E, Beer C), pp. 101–158. New York: NY: Academic Press. [Google Scholar]
- 123.Pavelka MSM, Fedigan LM, Zohar S. 2002. Availability and adaptive value of reproductive and postreproductive Japanese macaque mothers and grandmothers. Anim. Behav. 64, 407–414. ( 10.1006/anbe.2002.3085) [DOI] [Google Scholar]
- 124.Hrdy SB. 2016. Variable postpartum responsiveness among humans and other primates with ‘cooperative breeding’: a comparative and evolutionary perspective. Horm. Behav. 77, 272–283. ( 10.1016/j.yhbeh.2015.10.016) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.