Abstract
Under strong pathogen pressure, insects often evolve resistance to infection. Many insects are also protected via immune memory (immune priming), whereby sublethal exposure to a pathogen enhances survival after secondary infection. Theory predicts that immune memory should evolve when the pathogen is highly virulent, or when pathogen exposure is relatively rare. However, there are no empirical tests of these hypotheses, and the adaptive benefits of immune memory relative to direct resistance against a pathogen are poorly understood. To determine the selective pressures and ecological conditions that shape immune evolution, we imposed strong pathogen selection on flour beetle (Tribolium castaneum) populations, infecting them with Bacillus thuringiensis (Bt) for 11 generations. Populations injected first with heat-killed and then live Bt evolved high basal resistance against multiple Bt strains. By contrast, populations injected only with a high dose of live Bt evolved a less effective but strain-specific priming response. Control populations injected with heat-killed Bt did not evolve priming; and in the ancestor, priming was effective only against a low Bt dose. Intriguingly, one replicate population first evolved priming and subsequently evolved basal resistance, suggesting the potential for dynamic evolution of different immune strategies. Our work is the first report showing that pathogens can select for rapid modulation of insect priming ability, allowing hosts to evolve divergent immune strategies (generalized resistance versus specific immune memory) with potentially distinct mechanisms.
Keywords: Bacillus thuringiensis, immune priming, pathogen selection, specific immunity, Tribolium castaneum
1. Introduction
A large body of work shows that strong pathogen pressure drives the evolution of resistance mechanisms in insect hosts, reducing the fitness impact of infection [1–3]. In addition, many insects exhibit a form of immune memory (priming response), gaining increased protection against a pathogen after initial sublethal exposure to the pathogen (reviewed in [4,5]). Immune priming is also observed across multiple natural populations of flour beetles [6]. Thus, in addition to basal resistance, immune priming is probably a significant immune strategy across insects. Mathematical models predict that such immune memory strongly impacts disease prevalence [7,8] and can alter population dynamics [9]. However, we have limited empirical information about the evolutionary benefits of priming relative to the innate immune responses that confer basal resistance against a pathogen (without priming).
We also know very little about the selective pressures and ecological conditions that shape the evolution of insect immune priming versus basal resistance to a pathogen. A general mathematical model examining the impact of various immune strategies on host population growth suggests that the frequency and duration of infection are key determinants of immune function [10]. Although this model does not specifically address the evolution of immune function in insects, it makes the general prediction that adaptive immunity (responsible for immune memory) is more likely to evolve when infection is relatively rare. By contrast, assuming greater maintenance costs of constitutively expressed resistance, innate immunity should be more advantageous under frequent infection. However, it is unclear whether this assumption holds for insects, because we have limited understanding of the molecular mechanisms responsible for insect immune priming. Recent evidence suggests that components of innate immunity—cellular and humoral defences—play a critical role in the priming response of diverse insect orders such as Diptera [11–13], Coleoptera [14,15] and Hymenoptera [16]. Yet the extent of overlap between priming and resistance pathways, and their relative costs, are not well understood [14,16]. Thus, from an evolutionary perspective it is not even clear whether insect immune memory and resistance are distinct phenomena. Hence, we do not know whether priming can evolve rapidly, and whether (and when) it is adaptive in natural populations.
To begin to understand the impact of pathogen pressure on the evolution of alternative immune strategies (priming response versus resistance), we performed experimental evolution with the red flour beetle Tribolium castaneum, a cosmopolitan pest of stored grain products [17]. Tribolium castaneum is an emerging model system for insect immunity and host–pathogen co-evolution [18,19], and several studies have demonstrated the beetles' ability for specific immune memory [5,6,20,21]. We allowed replicate outbred laboratory populations of flour beetles to evolve with their natural insect pathogen Bacillus thuringiensis (strain DSM 2046, henceforth ‘Bt'; described in [20]). The pathogen causes significant mortality in the ancestral beetle population (approx. 65% mortality within 2 days; electronic supplementary material, figure S1), imposing strong selection on their immune function. The Bt toxin is also a widely used insecticide that kills insects that ingest it, and a large body of work has analysed the evolution of resistance to Bt in many insect pests (reviewed in [22]). However, with oral infection, individual differences in feeding rates can cause large variation in the pathogen dose received by the host. To minimize individual variation in pathogen exposure, in our experiments we separately injected each adult beetle with Bt cells.
Importantly, although the ancestral population was capable of mounting a priming response against Bt infection (approx. 8000 cells per beetle [6]), this basal priming ability was ineffective against the higher dose of infection used here (approx. 12 000 cells; electronic supplementary material, figure S1). Thus, at the beginning of the selection experiment, beetle populations had low effective basal resistance and no priming ability against a high dose of Bt. We tested whether populations evolve stronger priming or higher resistance when exposed to a single severe infection each generation (no priming opportunity) versus when given the opportunity for priming (first injected with heat-killed bacteria, then infected with live pathogens). We found that populations showed divergent responses to Bt infection, either evolving immune priming against the specific Bt strain used for selection, or more effective but non-specific basal resistance (i.e. greater survival following the specific infection dose). Note that our measurement of resistance is perhaps confounded by the potential evolution of tolerance (the ability to minimize the cost of infection and resulting immune response) [23], and our current experiments cannot distinguish between resistance and tolerance. Nonetheless, we provide the first empirical evidence for rapid evolution of vertebrate-like specific immune memory in insects, and provide an experimental framework to understand the evolution of divergent immune strategies in response to pathogen selection.
2. Material and methods
We generated an outbred T. castaneum stock population (‘ancestral' population, DA-IK) using adults from 10 wild-caught lines collected from different locations across India [6]. We maintained this line as a large population (greater than 5000 adults) on whole-wheat flour on a 45-day discrete generation cycle at 34°C for approximately 2 years (approx. 16 generations) before starting our experiments.
(a). Immune priming and challenge
A detailed protocol is given in ‘supplementary methods' section (electronic supplementary material). Briefly, we used the insect pathogen Bacillus thuringiensis (DSM 2046) (Bt) isolated from a Mediterranean flour moth [20] to impose pathogen selection. For priming (primary exposure), we pricked adults between the head and thorax, using a 0.1 mm insect pin dipped in heat-killed overnight culture of Bt as described by Khan et al. [6]. Under natural conditions, individuals are more likely to experience live infection rather than killed pathogens. However, using live cells for priming would incur costs of infection and impose mortality, selecting for priming as well as greater basal resistance. To minimize these effects, we used heat-killed bacteria that would prime the beetle immune system by eliciting an immune response without any cost of infection (also see [16,20,21]). We used insect Ringer solution to perform mock priming (see [6]). Six days after priming, we challenged individuals with live bacterial culture (secondary exposure), delivering approximately 12 000 live cells per beetle.
(b). Experimental evolution
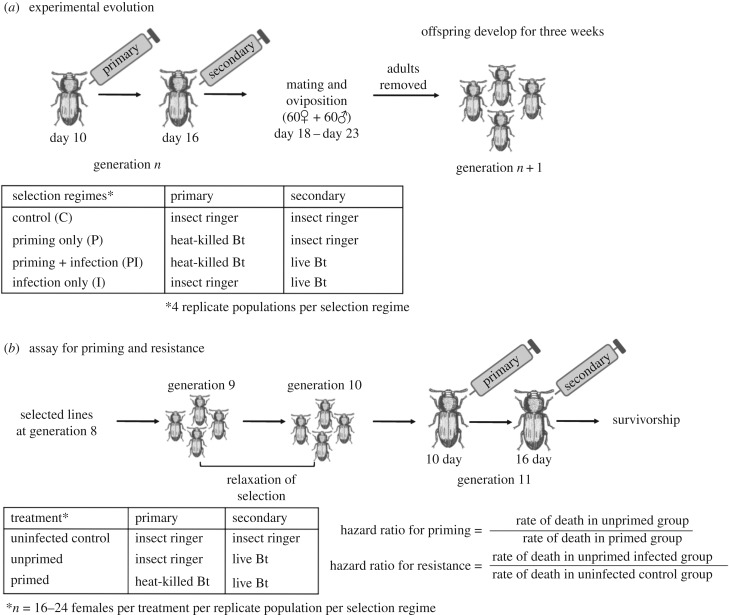
For artificial selection, we used four selection regimes—control (C), primed only (P), primed and infected (PI) and infected only (I)—each with four replicate populations (C1 to C4, P1 to P4, PI1 to PI4 and I1 to I4). The detailed experimental protocol is shown in figure 1a and described in the electronic supplementary material. Each generation, we isolated pupae from each population and allowed eclosed adults to initiate the next generation after the relevant selection treatment. For all injections and while measuring survivorship, we isolated individuals in 96-well microplates containing flour. At generations 8 and 11, we collected an additional set of pupae from each replicate population to generate standardized populations. These populations were maintained under relaxed selection (i.e. no mock injection, priming or pathogen infection) for two generations to minimize non-genetic parental effects [2,3]. For subsequent assays of evolved priming response and basal resistance (described below), we used individuals from these standardized populations.
Figure 1.
(a) Design of experimental evolution and selection regimes. (b) Generating standardized beetles to measure evolved priming and resistance.
(c). Quantifying evolved priming and basal resistance
To measure evolved priming and resistance after eight generations of selection, we randomly assigned 10-day-old virgin females from each standardized population to one of three treatments, as described in figure 1b (see electronic supplementary material for details). After the primary and secondary exposure, we isolated beetles in wells of 96-well microplates with flour. We noted survival of these standardized females every 6 h for 2 days and then every 24 h for the following 50 days (n = 16–24 females per treatment per population). For standardized males and females derived after 11 generations of pathogen selection, we noted survival at the same intervals for the experimental selection window of 7 days (n = 16–26 sex per treatment per population). Simultaneously, we re-evaluated the impact of bacterial infection and priming on females from the unhandled ancestral beetle population as described above (n = 22–24 females per treatment per population).
We used standardized females derived at generation 8 to test whether the evolved priming response and resistance were specific to the pathogen strain used for the selection experiment (i.e. Bt) (see electronic supplementary material for details). We used a different pathogenic strain of B. thuringiensis (MTCC 6905, henceforth ‘Bt1', isolated from silkworm) to prime and challenge 10-day-old standardized virgin females as described above (n = 16–24 females per treatment per population). We could not estimate Bt1 priming in I4 lines for logistical reasons. Similarly, we also tested whether evolved priming ability requires immune activation via priming with the same pathogen strain or whether priming with a different strain could also induce a protective response (experimental details are given in the electronic supplementary material).
For each experiment, we first analysed survival data for all selection regimes using a mixed-effects Cox model implemented in R using the package coxme [24]. We fitted separate models to estimate evolved Bt resistance and priming response, with replicate populations as random effects (see electronic supplementary material for details). For resistance, we used data from the unprimed infected and the uninfected control treatments; for priming, we used data from the unprimed and primed treatments. While this analysis provides an overall estimate of each effect, we could not conduct meaningful comparisons between selection regimes using this framework. Therefore we then separately analysed survival data for each population.
For each standardized replicate population, we performed Cox proportional hazard survival analysis using the software JMP 10. We calculated resistance to infection as the estimated hazard ratio of unprimed infected versus uninfected control groups (rate of death in unprimed infected group/rate of death in the uninfected control group). A hazard ratio significantly greater than one indicates an enhanced risk of mortality in the infected group (i.e. lower resistance). We calculated the survival benefit of priming as the hazard ratio of unprimed versus primed groups. A hazard ratio significantly greater than one indicates increased risk of mortality in the unprimed group compared to primed individuals (i.e. a significant survival benefit of priming). With four replicate populations per regime, we did not have sufficient statistical power to compare mean hazard ratios across the four regimes. Therefore, we focus our analysis on the number of replicate populations that showed significant priming response or increased resistance to infection within each selection regime.
3. Results
(a). Experimental evolution with Bt causes rapid decline in Bt-induced mortality
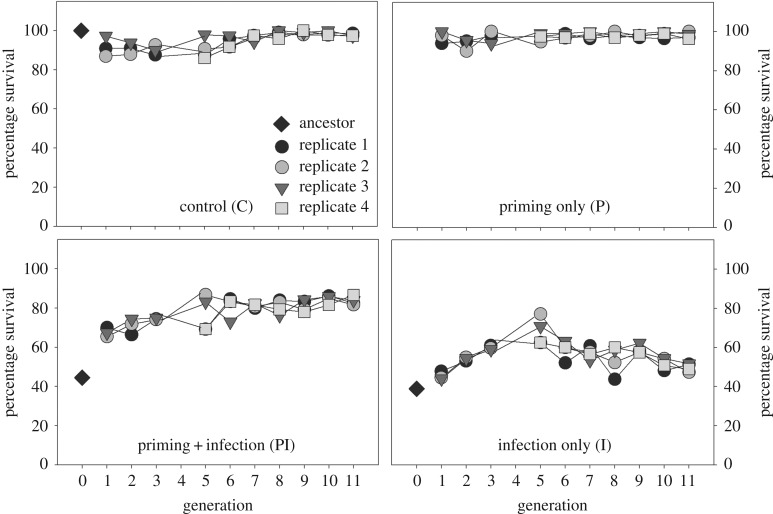
We allowed replicate beetle populations to evolve under strong pathogen selection (infection with live Bt cells each generation), either with or without an opportunity for priming (pricked with buffer or heat-killed Bt cells before live Bt infection; figure 1a). As expected, the high infection dose in each generation imposed substantial mortality (and therefore strong selection) on populations in I (infection only) and PI (priming + infection) regimes. However, within a single generation of selection, post-infection survival (after 2 days) increased from approximately 40% in the ancestor to approximately 45% in I populations, and as high as approximately 68% in PI populations (figure 2). By the 11th generation of selection, adult survival had increased further: approximately 50% adults in I populations and approximately 80% adults from PI populations survived infection. As expected, adults in the C (control) and P (priming only) populations that were never exposed to Bt maintained very high survival (approx. 90–100%) throughout the experiment (figure 2). Note that regardless of variation in post-infection survival, we used 60 mating pairs to initiate each successive generation for all populations (accounting for expected mortality, we infected a larger number of beetles; see Material and methods). This design allowed us to ensure strong selection on survival every generation, without imposing a genetic bottleneck.
Figure 2.
Adult survival during the first 48 h after infection with live Bt cells (day 16–18; figure 1a), during the course of experimental evolution. Only PI and I beetles were infected, and C and P beetles were injected with buffer.
(b). Evolved priming and basal resistance are mutually exclusive immune strategies
After eight generations of selection, we tested whether females from standardized populations (after relaxing selection) had evolved stronger priming or higher resistance (for logistical reasons, we could not test males). Mixed-effects models fitted to survival data revealed a significant impact of the selection regime on the difference between uninfected control versus infection treatments (i.e. resistance; p < 0.001), as well as the difference between unprimed versus primed treatments (i.e. priming; p = 0.007) (electronic supplementary material, table S1). Thus, during experimental evolution, replicate populations across regimes evolved different degrees of resistance and priming. To understand these differences in detail, we separately analysed survival data for each population with Cox proportional hazard analysis.
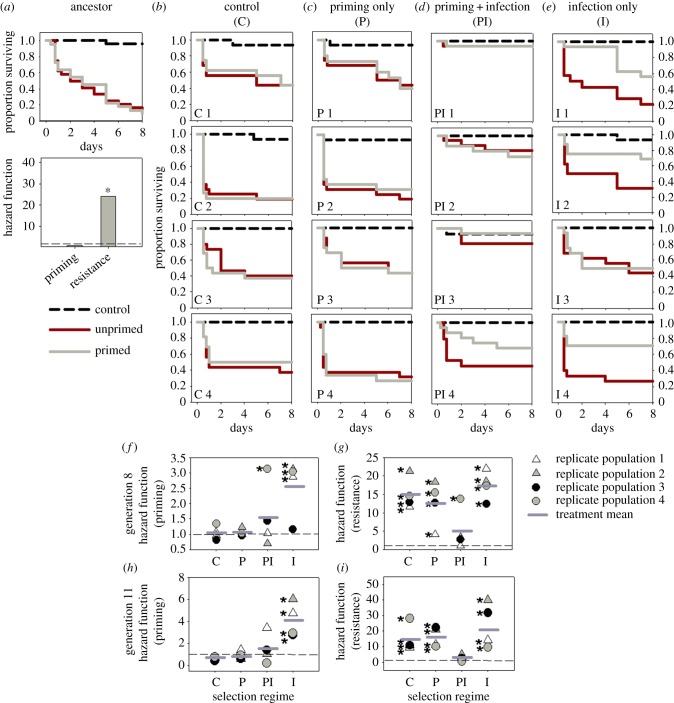
We calculated resistance to infection as the estimated hazard ratio of unprimed infected versus uninfected control groups, and the survival benefit of priming as the hazard ratio of unprimed versus primed groups (see Material and methods for details). As expected, females from populations that were not exposed to live Bt (unhandled ancestral population, and populations from C and P regimes) showed high mortality after infection and no survival benefit of priming (figure 3a–c,f,g). Thus, neither priming nor resistance had evolved in these populations. We found that highly effective basal resistance to Bt had evolved in three of four PI populations, conferring survival rates nearly as high as uninfected control beetles (figure 3d,g). However, in the fourth population (population PI4), we observed significant priming ability that conferred a threefold survival advantage (figure 3f). Note that all PI populations were first injected with heat-killed and then live Bt each generation, allowing an opportunity for the evolution of priming-induced survival benefits. Despite this opportunity for priming, only one population showed evidence of priming ability after eight generations of selection. In contrast with PI populations, three of four populations in the I regime (infected with a single high dose of Bt) evolved priming ability rather than resistance. Although unprimed I beetles remained highly susceptible to infection, priming improved survival significantly (approx. threefold increase in survival; figure 3e–g). Interestingly, the fourth population (I3) evolved neither priming nor improved basal resistance (figure 3e–g). We found comparable results when we analysed female survival beyond the experimental selection window (until day 50; electronic supplementary material, figure S2C–F). The only population (PI4) that did not evolve higher resistance showed priming instead (twofold survival benefit; electronic supplementary material, figure S2C,E,F). Thus, the survival benefit of evolved basal resistance or priming lasts beyond the selection window. We did not find any difference in survival of uninfected control beetles from selected populations until day 50 (cf. survival curves in electronic supplementary material, figure S2; p > 0.05), suggesting the absence of long-term costs of selection. However, we cannot exclude the possibility of survival costs arising at a later stage.
Figure 3.
Survival curves and estimated hazard ratios for priming and resistance to Bt (a) in the ancestral population (n = 22–24 females per treatment) (b–g) after eight generations of selection (n = 16–24 females per treatment population) and (h,i) after 11 generations of selection (n = 16–26 females per treatment per population). (f,g) Hazard ratios calculated from survival curves shown in (b–e). Survival curves for panels (h) and (i) are given in electronic supplementary material, figure S3. (f,h) The survival benefit of priming (a greater hazard ratio indicates higher benefit of priming); (g,i) the resistance to infection (a greater hazard ratio indicates higher susceptibility to infection, or lower resistance). (f–i) Horizontal dark grey lines denote group mean hazard ratios and dashed lines indicate a hazard ratio of 1. Asterisks denote hazard ratios significantly different from 1 (p ≤ 0.05). (Online version in colour.)
Analysing standardized females derived after 11 generations of selection, we again found a significant impact of selection regime on evolved resistance as well as priming (p < 0.001 in each case; electronic supplementary material, table S1). Specifically, females from all I populations now showed priming (two to sixfold survival benefit) within the selection window (figure 3h; electronic supplementary material, figure S3), and all PI populations showed higher resistance (figure 3i; electronic supplementary material, figure S3). Thus, females from population PI4 showed priming after eight generations of selection, but subsequently evolved resistance within three additional generations (cf. figure 3f–g and figure 3h–i). For three replicate populations from each selection regime, we also measured priming and resistance in standardized males after 11 generations of selection. As with females, males from three PI populations had evolved resistance, and two of three I populations evolved significant priming response (approx. fourfold survival benefit; electronic supplementary material, figure S4). The third I population showed a similar trend (approx. 2.5-fold survival benefit of priming), but the response was marginally non-significant (p = 0.061; electronic supplementary material, figure S4D–E). Thus, both sexes evolved similar immune strategies in response to Bt infection (cf. electronic supplementary material, figures S3 and S4).
The high basal resistance in evolved PI populations may prevent the detection of a weak but significant priming response, especially given our sample size of 16–24 beetles per treatment per population. Therefore, we infected PI beetles with a higher bacterial dose (approx. 16 500 cells per beetle) that imposed greater mortality (approx. 85% within 48 h) than the dose used during experimental evolution (approx. 50% within 48 h). However, we still did not observe significant priming (electronic supplementary material, figure S5). Together, our results suggest that under pathogen selection, a population can evolve either improved resistance or priming ability, but not both.
(c). Evolved priming is strain-specific, but evolved resistance is generalized
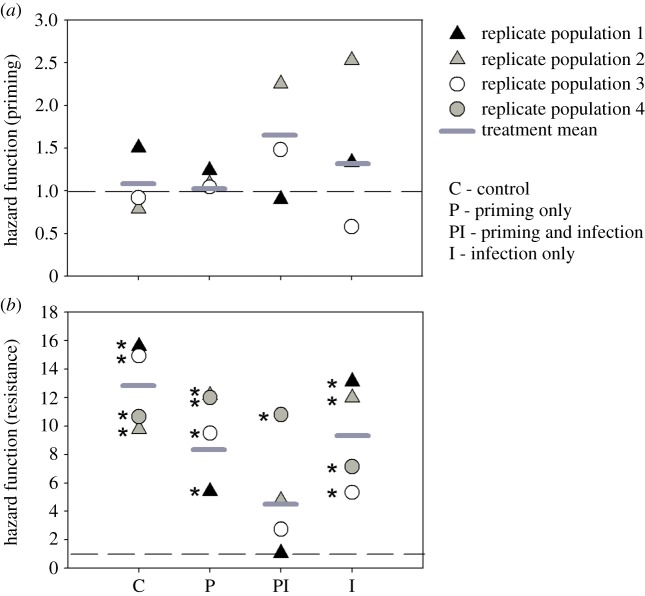
To test whether the evolved priming response and resistance were specific to the Bt strain used to impose selection, we used another highly virulent B. thuringiensis strain (Bt1; see Material and methods), against which the ancestral population could mount a priming response at a low dose (approx. 8000 cells; electronic supplementary material, figure S6). None of the populations showed priming ability against a higher dose of Bt1 (approx. 12 000 cells per beetle; figure 4a; electronic supplementary material, figure S7A–D). Thus, the evolved priming response may be specific to the pathogen strain imposing selection. By contrast, evolved resistance was non-specific: PI populations that evolved resistance against Bt were also more resistant to Bt1 (three of four populations, except PI4; figure 4b; electronic supplementary material, figures S7C). We also tested whether the evolved survival benefit against Bt infection requires specific priming with the same strain (homologous priming). Only I beetles receiving homologous priming showed a survival benefit (2.5- to threefold survival benefit; electronic supplementary material, figure S8B,C), whereas heterologous priming (priming with Bt1) failed to protect against Bt infection (electronic supplementary material, figure S8B,D). Thus, the evolved priming in I populations requires strain-specific immune activation by Bt, rather than a general non-specific immune induction.
Figure 4.
Estimated hazard ratios for (a) immune priming response and (b) resistance to Bt1 infection across replicate populations of each regime (n = 16–24 females per treatment per population). Horizontal grey lines denote group mean hazard ratios and dashed lines indicate a hazard ratio of 1. Asterisks denote hazard ratios significantly different from 1 (p ≤ 0.05).
4. Discussion
Empirical evidence for immune memory in insects has grown rapidly in recent years, but the selective pressures and ecological conditions that shape its adaptive evolution remain largely unexplored. Here, we used experimental evolution to reveal a causal role for pathogen-imposed selection in the rapid, adaptive evolution of immune memory (priming ability) in an insect. We found that priming ability evolved repeatedly in I populations, where beetles were directly exposed to a single high dose of infection with Bt each generation. Importantly, beetles from these populations were as susceptible to the pathogen as control populations (C and P regimes), and showed no increase in basal resistance. Thus, the evolved priming ability did not confer substantial survival benefits during experimentally imposed selection. Surprisingly, we found that despite an opportunity for priming, PI populations consistently evolved increased basal resistance rather than significant immune priming. Thus, one of the most striking outcomes of our experiment is the highly parallel yet mutually exclusive evolution of priming in I populations, and increased resistance in PI populations. Intriguingly, one population (PI4) first evolved priming ability and then gained resistance within three generations, suggesting that immune priming may be selectively favoured as an intermediate step preceding increased resistance. Although this single observation should be interpreted cautiously, it suggests the potential for rapid and complex dynamics in the evolution of alternate immune strategies.
What prevented the evolution and maintenance of priming in PI populations? Conversely, why did not a resistance allele(s) sweep through I populations, despite the large potential selective advantage? One way to approach these questions is to determine the relative costs and benefits of hypothetical ‘priming' versus ‘resistance’ alleles, and ask whether their net benefit may vary as a function of I and PI treatments. It is clear that the survival benefit of evolved resistance was greater than the benefit of evolved priming in standardized populations, suggesting that a resistance allele should always outcompete a priming allele. Furthermore, the net benefit of evolved priming in I lines during experimental evolution was lower than that of evolved resistance in PI lines—after 11 generations, survival had increased marginally from 40% to 50% in I lines, but was as high as 80% in PI lines. Therefore, weak selection for a resistance allele in I lines cannot explain the observed lack of resistance.
What about the relative physiological costs of priming and resistance alleles? Generalized resistance in insects is associated with overexpression of fast acting non-specific immune effectors such as phenoloxidase, and the production of reactive oxygen species (ROS) [25]. Such immune effectors may facilitate rapid clearance of pathogens, but can also impose large physiological costs of inflammation via damage to vital host organs such as Malpighian tubules [26]. Resistance to ingested Bt toxin also involves multiple different mechanisms across various insects (reviewed in [27]). For instance, a recent study with the wax moth Galleria melonella found that evolved resistance to ingested Bt toxin was associated with upregulation of genes relevant for inflammation (e.g. ROS) and tissue repair [28]; it is not clear whether direct injection of Bt cells in the haemolymph selected for similar responses in our beetles. By contrast, specific priming may require blood cell differentiation or Toll pathway activation [11,12], which is potentially less toxic to host tissues [29–31]. Thus, generalized resistance may be physiologically more costly than a specific priming response. McDade and colleagues have proposed a similar hypothesis for human immune function, drawing upon data from vertebrates to suggest that the cost of specific adaptive immune responses is lower than that of non-specific innate immune responses [32]. Could a greater cost of basal resistance explain the pattern of evolution that we observe? A general mathematical model to explore the emergence of various forms of immune defence offers some clues [10]. Assuming that more effective defence incurs a greater maintenance cost, this model predicts that under frequent pathogen attacks, a population's growth rate is maximized by constitutively expressed resistance via innate immune responses. On the other hand, at a lower pathogen frequency, lower maintenance costs of inducible adaptive immunity make it more favourable. Our I and PI selection regimes broadly resemble these conditions of low versus high frequency of infection: I populations received a single infection each generation, whereas PI populations were exposed to Bt antigens twice (primary exposure introduced heat-killed cells directly into the beetle haemolymph to activate the immune responses, whereas secondary exposure introduced live Bt). Thus, a large maintenance cost of generalized resistance combined with low frequency of pathogen exposure in I populations may have prevented the evolution of resistance and favoured the evolution of priming ability.
Apart from the potential role of relative costs of priming versus resistance, we note alternative explanations for the evolution of divergent immune function across regimes. First, wounding during mock priming in I populations could trigger subsequent immune responses [33], leading to generalized priming before actual infection. However, as discussed below, such general upregulation of immunity is unlikely to explain the pathogen-specific priming response that we observed. Second, infected parents may have transferred and exposed their offspring to live Bt before experimental priming or infection (see [34]), increasing offspring immune responses. However, PI beetles would still encounter Bt more frequently than I beetles, and should therefore experience selection for constitutively expressed resistance (rather than priming). Finally, the observed difference in resistance may arise due to variation in the temporal dynamics of immune responses across selection regimes. For example, Bt may kill beetles before innate immune responses (requiring blood-cell proliferation or Toll pathway activation) are activated, reducing the adaptive benefit of a resistance allele in the I regime. On the other hand, if heat-killed Bt sensitized immune pathways before live infection, beetles from PI lines may rapidly produce more haemocytes and AMPs after live infection, resisting the infection more effectively. Therefore, prior priming may buy the host some time during the acute phase of infection, until more efficient resistance mechanisms can be expressed. We suggest further experiments to test these hypotheses.
Strain-specific priming is generally thought to be an exclusive feature of vertebrate adaptive immunity. Here, we found that the evolved priming response in I beetle populations was restricted to the specific, high dose of Bt used for selection. Priming with another Bt strain (Bt1) did not confer a survival advantage after a subsequent infection by live Bt1 (even though the ancestral population showed basal priming response against a lower infection dose of Bt1). Similarly, beetles primed with Bt1 did not gain a survival benefit against Bt. Previous work with flour beetles [6,20] has also revealed a similar degree of specificity of immune priming, allowing differentiation between strains of the same pathogen. What molecular mechanisms underlie the evolved, likely strain-specific immune priming? Previous work suggests a role for phagocytosis [11,13] or blood cell differentiation [12] in mediating pathogen-specific immune priming in fruit flies and mosquitoes. However, the mechanism underlying evolved pathogen strain-specific immune priming in our experimental populations remains unclear. Insects can also produce receptor diversity via alternative splicing of Down syndrome cell adhesion molecule to discriminate between different pathogens [35,36], although its direct role in priming is not yet established. Recent work with vertebrates suggests that cytolytic innate immune effectors such as natural killer cells also possess attributes of adaptive immune responses [37], producing long-lasting, antigen-specific immune memory independent of B cells and T cells [37,38]. Hence, an additional possibility is that the insect equivalent of natural killer cells mediated strain-specific priming in our evolved lines. We also note that ancestral beetles already showed priming against lower doses of Bt infection [6], but not against the high dose of Bt that we used for selection. As the cellular and molecular machinery for mounting a priming response was already present in the beetles, it was perhaps simply modified during experimental evolution to efficiently counter a higher pathogen dose. We thus speculate that the evolved priming response involved a quantitative (rather than qualitative) change—for example, existing immune responses in the ancestral population (transcriptional, translational or functional shifts) after a relatively low dose of Bt infection may be activated further or faster in the evolved lines. In contrast with the apparent specificity of immune priming in I populations, we found that three of four PI populations showed generalized resistance against multiple strains of BT, suggesting that the divergent immune responses are driven by different mechanisms (also see [15]). Clearly, more work is needed to understand how specific immune memory and general resistance are achieved separately in insect immunity despite the lack of cellular mechanisms responsible for adaptive immunity in vertebrates. We speculate that while vertebrate immune memory is mechanistically distinct, functionally it may not be as unique as traditionally believed.
Finally, we note some important limitations of our results and their interpretation. First, our work does not address dose-dependent immune responses, and our conclusions are thus limited to the specific infection dose that we used in our experiments. Second, the two Bt strains we used to test specificity may have inherently different growth or virulence dynamics within the host, such that the priming response against them cannot be directly compared. Third, our estimates of basal resistance are confounded by tolerance to infection. Post-infection survival may increase either via improved ability to kill pathogens or via increased tolerance, where beetles do not kill pathogens more efficiently but reduce the cost of infection, immune response or both [23]. As we estimated priming response and resistance using post-infection survival, it remains unclear whether improved survival via broadly termed ‘resistance' involved direct pathogen clearance or improved tolerance. Fourth, increased post-infection survival may reflect a shift in the evolutionary trade-off between reproduction and survival [39] instead of direct selection for improved pathogen resistance. For instance, reduced reproductive investment due to infection may indirectly lead to improved immune responses. Finally, we could not determine the role of trans-generational immune priming in our experiments, whereby parental exposure to pathogen can prime the immune system of their offspring [6,40]. Hence, it is possible that priming did evolve in PI populations, but it was trans-generational priming (i.e. the survival benefit was transferred to offspring) rather than priming within the same generation. The stark increase in survival of PI beetles within the first generation of selection (from 40% to 68%) may have arisen from trans-generational immune priming during the selection protocol. The observed priming response in I beetles may have also evolved as a correlated response to selection for trans-generational priming. However, given that all experimental lines were standardized under relaxed selection for two generations, it is unlikely that trans-generational mechanisms could explain the difference in survival between PI and I populations. We thus speculate that the observed immune phenotypes in our experiments are genetically heritable.
In summary, we have documented the first experimental demonstration of rapid evolution of insect immune priming. Our work also represents a rare example where selection imposed by the same pathogen led to divergent outcomes—either priming ability or basal resistance, but not both. We hope that our results will motivate further experiments to understand the dynamics and mechanistic basis of evolved priming versus resistance. It is likely that an alternative vertebrate-like immune memory can evolve in invertebrates, but with different underlying molecules [35,41]. We also note that the general view of adaptive immunity as an exclusive mediator of immune memory in vertebrates has been recently challenged by the observation that resistance to reinfection can be achieved even without a functional adaptive immune system [42,43]. In fact, several studies are now exploring the potential of the innate immune memory to aid novel therapeutic strategies for immunodeficiency and autoimmune disorders in vertebrates, including humans (reviewed in [43]). We suggest that insects can also be a useful model system where the evolution and mechanistic basis of innate immune memory can be jointly studied.
Supplementary Material
Acknowledgements
We thank Aparna Agarwal, Rittik Deb, Saurabh Mahajan, Apurva Sarin, L. S. Shashidhara and Ann Tate for critical comments on the manuscript, and N. G. Prasad for B. thuringiensis strains.
Data accessibility
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.rk3gn [44].
Authors' contributions
I.K. conceived of experiments. I.K. and D.A. designed the experiments. A.P. and I.K. carried out the experiments. A.P., I.K. and D.A. analysed data. I.K. and D.A. wrote the manuscript with input from A.P. All the authors gave their final approval for publication.
Competing interests
We have no competing interests.
Funding
We acknowledge funding and support from a SERB-DST Young Investigator Grant to I.K., a DST INSPIRE Faculty award to D.A. and the National Centre for Biological Sciences, India.
References
- 1.Kraaijeveld A, Godfray H. 2008. Selection for resistance to a fungal pathogen in Drosophila melanogaster. Heredity 100, 400–406. ( 10.1038/sj.hdy.6801092) [DOI] [PubMed] [Google Scholar]
- 2.Faria VG, Martins NE, Paulo T, Teixeira L, Sucena É, Magalhães S. 2015. Evolution of Drosophila resistance against different pathogens and infection routes. Evolution 69, 2799–2809. ( 10.1111/evo.12782) [DOI] [PubMed] [Google Scholar]
- 3.Gupta V, Venkatesan S, Chatterjee M, Syed ZA, Nivsarkar V, Prasad NG. 2016. No apparent cost of evolved immune response in Drosophila melanogaster. Evolution 70, 934–943. ( 10.1111/evo.12896) [DOI] [PubMed] [Google Scholar]
- 4.Contreras-Garduño JO, Lanz-Mendoza HU, Franco B, Nava A, Pedraza-Reyes MA, Canales-Lazcano JO. 2016. Insect immune priming: ecology and experimental evidences. Ecol. Entomol. 41, 351–366. ( 10.1111/een.12300) [DOI] [Google Scholar]
- 5.Milutinović B, Peuß R, Ferro K, Kurtz J. 2016. Immune priming in arthropods: an update focusing on the red flour beetle. Zoology 119, 254–261. ( 10.1016/j.zool.2016.03.006) [DOI] [PubMed] [Google Scholar]
- 6.Khan I, Prakash A, Agashe D. 2016. Divergent immune priming responses across flour beetle life stages and populations. Ecol. Evol. 6, 7847–7855. ( 10.1002/ece3.2532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tate AT, Rudolf VHW. 2012. Impact of life stage specific immune priming on invertebrate disease dynamics. Oikos 121, 1083–1092. ( 10.1111/j.1600-0706.2011.19725.x) [DOI] [Google Scholar]
- 8.Tate AT. 2016. A general model for the influence of immune priming on disease prevalence. Oikos 126, 350–360. ( 10.1111/oik.03274) [DOI] [Google Scholar]
- 9.Tidbury HJ, Best A, Boots M. 2012. The epidemiological consequences of immune priming. Proc. R. Soc. B 279, 4505–4512. ( 10.1098/rspb.2012.1841) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayer A, Mora T, Rivoire O, Walczak AM. 2016. Diversity of immune strategies explained by adaptation to pathogen statistics. Proc. Natl Acad. Sci. USA 113, 8630–8635. ( 10.1073/pnas.1600663113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pham LN, Dionne MS, Shirasu-Hiza M, Schneider DS. 2007. A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog. 3, e26 ( 10.1371/journal.ppat.0030026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigues J, Brayner FA, Alves LC, Dixit R, Barillas-mury C. 2012. Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science 329, 1353–1355. ( 10.1126/science.1190689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weavers H, Evans IR, Martin P, Wood W. 2016. Corpse engulfment generates a molecular memory that primes the macrophage inflammatory response. Cell 165, 1658–1671. ( 10.1016/j.cell.2016.04.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenwood JM, Milutinović B, Peuß R, Behrens S, Esser D, Rosenstiel P, Schulenburg H, Kurtz J. 2017. Oral immune priming with Bacillus thuringiensis induces a shift in the gene expression of Tribolium castaneum larvae. BMC Genomics 18, 329 ( 10.1186/s12864-017-3705-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tate AT, Andolfatto P, Demuth JP, Graham AL. 2017. The within-host dynamics of infection in trans-generationally primed flour beetles. Mol. Ecol. 26, 3794–3807. ( 10.1111/mec.14088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barribeau SM, Schmid-Hempel P, Sadd BM. 2016. Royal decree: gene expression in trans-generationally immune primed bumblebee workers mimics a primary immune response. PLoS ONE 11, e0159635 ( 10.1371/journal.pone.0159635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sokoloff A. 1974. The biology of Tribolium with special emphasis on genetic aspects. Oxford, UK: Clarendon. [Google Scholar]
- 18.Milutinović B, Stolpe C, Peuβ R, Armitage SAO, Kurtz J. 2013. The red flour beetle as a model for bacterial oral infections. PLoS ONE 8, e64638 ( 10.1371/journal.pone.0064638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joop G, Roth O, Schmid-Hempel P, Kurtz J. 2014. Experimental evolution of external immune defences in the red flour beetle. J. Evol. Biol. 27, 1562–1571. ( 10.5061/dryad.pf013) [DOI] [PubMed] [Google Scholar]
- 20.Roth O, Sadd BM, Schmid-Hempel P, Kurtz J. 2009. Strain-specific priming of resistance in the red flour beetle, Tribolium castaneum. Proc. R. Soc. B 276, 145–151. ( 10.1098/rspb.2008.1157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roth O, Joop G, Eggert H, Hilbert J, Daniel J, Schmid-Hempel P, Kurtz J. 2010. Paternally derived immune priming for offspring in the red flour beetle Tribolium castaneum. J. Anim. Ecol. 79, 403–413. ( 10.1111/j.1365-2656.2009.01617.x) [DOI] [PubMed] [Google Scholar]
- 22.Tabashnik BE, Brévault T, Carrière Y. 2013. Insect resistance to Bt crops: lessons from the first billion acres. Nat. Biotechnol. 31, 510–521. ( 10.1038/nbt.2597) [DOI] [PubMed] [Google Scholar]
- 23.Ayres JS, Schneider DS. 2012. Tolerance of infections. Annu. Rev. Immunol. 30, 271–294. ( 10.1146/annurev-immunol-020711-075030) [DOI] [PubMed] [Google Scholar]
- 24.Therneau TM.2015. coxme: mixed effects Cox models. R package version 2.2-5. See https://CRAN.R-project.org/package=coxme .
- 25.Binggeli O, Neyen C, Poidevin M, Lemaitre B. 2014. Prophenoloxidase activation is required for survival to microbial infections in Drosophila. PLoS Pathog. 10, e1004067 ( 10.1371/journal.ppat.1004067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan I, Agashe D, Rolff J. 2017. Early-life inflammation, immune response and ageing. Proc. R .Soc. B 284, 20170125 ( 10.1098/rspb.2017.0125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferré J, Van Rie J. 2002. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 47, 501–533. ( 10.1146/annurev.ento.47.091201.145234) [DOI] [PubMed] [Google Scholar]
- 28.Dubovskiy IM, et al. 2016. Immuno-physiological adaptations confer wax moth Galleria mellonella resistance to Bacillus thuringiensis. Virulence 7, 860–870. ( 10.1080/21505594.2016.1164367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann JA, Reichhart JM, Hetru C. 1996. Innate immunity in higher insects. Curr. Opin Immunol. 8, 8–13. ( 10.1016/S0952-7915(96)80098-7) [DOI] [PubMed] [Google Scholar]
- 30.Moret Y. 2003. Explaining variable costs of the immune response: selection for specific versus non-specific immunity and facultative life history change. Oikos 102, 213–216. ( 10.1034/j.1600-0706.2003.12496.x) [DOI] [Google Scholar]
- 31.Rowley AF, Powell A. 2007. Invertebrate immune systems-specific, quasi-specific, or nonspecific? J. Immunol. 179, 7209–7214. ( 10.4049/jimmunol.179.11.7209) [DOI] [PubMed] [Google Scholar]
- 32.McDade TW, Georgiev AV, Kuzawa CW. 2016. Trade-offs between acquired and innate immune defenses in humans. Evol. Med. Public Health 1, 1–16. ( 10.1093/emph/eov033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Márkus R, Kurucz É, Rus F, Andó I. 2005. Sterile wounding is a minimal and sufficient trigger for a cellular immune response in Drosophila melanogaster. Immunol. Lett. 101, 108–111. ( 10.1016/j.imlet.2005.03.021) [DOI] [PubMed] [Google Scholar]
- 34.Knorr E, Schmidtberg H, Derya A, Bingsohn L, Vilcinskas A. 2015. Translocation of bacteria from the gut to the eggs triggers maternal transgenerational immune priming in Tribolium castaneum. Biol. Lett. 11, 20150885 ( 10.1098/rsbl.2015.0885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurtz J, Armitage SAO. 2006. Alternative adaptive immunity in invertebrates. Trends Immunol. 27, 9–12. ( 10.1016/j.it.2006.09.001) [DOI] [PubMed] [Google Scholar]
- 36.Armitage SA, Peuß R, Kurtz J. 2015. Dscam and pancrustacean immune memory—a review of the evidence. Dev. Comp. Immunol. 48, 315–323. ( 10.1016/j.dci.2014.03.004) [DOI] [PubMed] [Google Scholar]
- 37.Vivier E, Raulet D, Moretta A, Caligiuri M. 2011. Innate or adaptive immunity? The example of natural killer cells. Science 331, 44–49. ( 10.1126/science.1198687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. 2006. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 7, 507–516. ( 10.1038/ni1332) [DOI] [PubMed] [Google Scholar]
- 39.Stearns SC. 1989. Trade-offs in life-history evolution. Funct. Ecol. 3, 259–268. ( 10.2307/2389364) [DOI] [Google Scholar]
- 40.Zanchi C, Troussard J-P, Moreau J, Moret Y. 2012. Relationship between maternal transfer of immunity and mother fecundity in an insect. Proc. R. Soc. B 279, 3223–3230. ( 10.1098/rspb.2012.0493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agaisse H. 2007. An adaptive immune response in Drosophila? Cell Host Microbe. 1, 91–93. ( 10.1016/j.chom.2007.04.003) [DOI] [PubMed] [Google Scholar]
- 42.Sun JC, Beilke JN, Lanier LL, Francisco S. 2009. Adaptive immune features of natural killer cells. Nature 457, 557–561. ( 10.1038/nature07665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, O'Neill LA, Xavier RJ. 2016. Trained immunity: a program of innate immune memory in health and disease. Science 352, aaf1098 ( 10.1126/science.aaf1098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan I, Prakash A, Agashe D. 2017. Data from: Experimental evolution of insect immune memory versus pathogen resistance Dryad Digital Repository. ( 10.5061/dryad.rk3gn) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Khan I, Prakash A, Agashe D. 2017. Data from: Experimental evolution of insect immune memory versus pathogen resistance Dryad Digital Repository. ( 10.5061/dryad.rk3gn) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.rk3gn [44].