Abstract
The honey bee is a major pollinator whose health is of global concern. Declines in bee health are related to multiple factors, including resource quality and pesticide contamination. Intensive agricultural areas with crop monocultures potentially reduce the quality and quantity of available nutrients and expose bee foragers to pesticides. However, there is, to date, no evidence for synergistic effects between pesticides and nutritional stress in animals. The neonicotinoids clothianidin (CLO) and thiamethoxam (TMX) are common systemic pesticides that are used worldwide and found in nectar and pollen. We therefore tested if nutritional stress (limited access to nectar and access to nectar with low-sugar concentrations) and sublethal, field-realistic acute exposures to two neonicotinoids (CLO and TMX at 1/5 and 1/25 of LD50) could alter bee survival, food consumption and haemolymph sugar levels. Bee survival was synergistically reduced by the combination of poor nutrition and pesticide exposure (−50%). Nutritional and pesticide stressors reduced also food consumption (−48%) and haemolymph levels of glucose (−60%) and trehalose (−27%). Our results provide the first demonstration that field-realistic nutritional stress and pesticide exposure can synergistically interact and cause significant harm to animal survival. These findings have implications for current pesticide risk assessment and pollinator protection.
Keywords: bee health, carbohydrates, clothianidin, thiamethoxam, haemolymph, food consumption
1. Introduction
Pollinators provide essential ecosystem services, contributing to wild plant biodiversity [1] and sustaining agricultural productivity [2]. The honey bee is a major pollinator species, and its poor health is related to multiple factors [3,4], including resource quality [5] and pesticide contamination [6]. Concern is therefore growing about honey bee nutrition and the potential for synergistic effects between pesticide exposure and nutrition [7,8].
Intensive agriculture with crop monocultures modifies natural land use, reduces natural habitats and plant diversity [9], and decreases the quality and quantity of nutrients in nectar and pollen [7,10]. Honey bees pollinate multiple crops and can therefore be vulnerable to such reduced food quality. Nutritional stress plays a crucial role in bee losses and poor colony health [7,11]. In fact, nutritional deficits were identified as a major cause of colony losses in the USA between 2007 and 2015 (21–58%) [12].
Agriculture also exposes foragers to pesticides [13]. Attention has focused on the neonicotinoid pesticides [14] because of their adverse impacts on pollinator health [15]. Neonicotinoids are globally used systemic insecticides [16] that can be found in the nectar and pollen collected by foragers [17], and are highly toxic to bees [16]. Bees can be exposed to pesticides that drift from treated fields when they forage on flowering strips, buffer zones, and cover or catch crops [18]. Furthermore, neonicotinoids are highly persistent and are found in environmental reservoirs such as water and soil [17]. Consequently, plants could take up neonicotinoids years after the actual treatment, resulting in prolonged contamination [13,17].
Clothianidin (CLO) and thiamethoxam (TMX) are commonly used neonicotinoids, and CLO is also a degradation product of TMX [16]. These neurotoxic insecticides are agonists of nicotinic acetylcholine receptors (nAChRs) [16] and impair bees in multiple ways [15,19]. Neonicotinoids have additive and synergistic effects on honey bees in combination with health stressors such as nosemosis and Varroa infestation (for review, see [20]). Moreover, the combination of poor nutrition and pesticide exposure may be especially problematic given that some genes can be upregulated by pesticide or pollen stress [21]. To date, there is no evidence for negative synergistic effects between pesticides and nutritional stressors in any animal studies [22]. However, good nutrition can help: bees were typically more resistant to pesticides when fed pollen diets [21,23]. Food quality can influence the effect of toxins on the health of other arthropods, such as Daphnia [24–26] and Diaptornus [27]. A few studies have demonstrated synergies between starvation and contamination from heavy metals, PAHs or PCBs on aquatic animals (fish, amphipods and molluscs) (for review, see [22]). However, these are not pesticides. We therefore decided to study the interactive effects of field-realistic neonicotinoids and nutritional stress on a major pollinator species.
We focused on honey bees because they are an important pollinator and are an indicator of how insect pollinators can respond to environmental stressors [28]. Bee foragers are particularly important because they are the only colony members that spend a significant proportion of their time flying [29] and therefore have significant energy needs. Unlike other insects, in which flight is initially powered by glycogen and subsequently by lipids, honey bee flight is entirely powered by sugars in the honey stomach after the depletion of glycogen reserves [30]. Sugar is therefore essential for foraging because flight has high-energetic demands [31,32]: forager metabolic activity increases 50–100 times during flight [31]. A bee could need up to 12 mg of sugar to sustain itself for each 1 h of flight [33]. To deal with such high energy demands, sugars are quickly absorbed into the bee's haemolymph [34].
Honey bees store only small amounts of glycogen in their flight muscles [35] and thus have high haemolymph sugar levels relative to other insects [36]. Haemolymph sugar content is therefore a good indicator of bee nutritional and physiological status. Trehalose, a disaccharide composed of two d-glucose molecules, is the most abundant sugar in honey bee haemolymph [36,37] and can be rapidly metabolized into d-glucose to release energy [37]. d-glucose is another major component of bee haemolymph [38] and is used to power motor activities directly [39].
We therefore tested the combined effects of sublethal, field-realistic acute exposures (see Material and methods) to two neonicotinoids (CLO and TMX at 1/5 and 1/25 of their LD50) and nutritional stress (limited sugar quantity and quality) on forager survival, food consumption and haemolymph sugar levels. Haemolymph sugar levels were assessed 2 h after treatment to test for potential rapid alterations caused by pesticide administration. Survival and sugar consumption were assessed over a longer period (4 days). We studied foragers because they spend a majority of their time foraging, an energy-intensive task [31] that can also expose bees to neonicotinoid-contaminated nectar.
2. Material and methods
This study was conducted in the summer of 2015 in Bologna, Italy. We used five queen-right honey bee (Apis mellifera ligustica) colonies located in the experimental apiary of the Council for Agricultural Research and Economics, Agriculture and Environment Research Centre (CREA-AA). The colonies were healthy, produced honey and showed no sign of disease throughout the season. They were managed according to an organic production protocol [40], and we used standard inspection techniques [41] to confirm that our colonies did not have detectable disease or parasite infestations. Colonies were inspected at least once per week.
We exposed bees to a nutritional stress (limited access to nectar or ad libitum access to nectar with low-sugar concentrations) and a neonicotinoid treatment. These treatments were administered individually and in combination to test for synergistic interactions [42]. After exposure, we measured the effects of the nutritional and neonicotinoid stressors on survival (up to 4 days after treatment), food consumption (up to 4 days after treatment), and glucose and trehalose haemolymph levels (2 h after treatment). We repeated the experiment four times (twice for each pesticide), using a total of 2840 foragers from five different colonies. We report mean ± 1 s.e., and superscript ‘DS’ indicates the statistical tests that passed the Dunn–Sidak correction for multiple pairwise comparisons. Further details are reported in the electronic supplementary material.
(a). Sugar diet treatments
We define nutritional stress as limited access to nectar or access to nectar with low-sugar concentrations. We tested sugar diets with different quantities (amounts) and qualities (concentrations) of sucrose. We provided the bees either ad libitum or limited (10 µl) quantities of sugar solution. The quality of the sugar diet was either rich (50% (w/w) sucrose solution), intermediate (32.5%) or poor (15%).
Our nutritional stresses are field-realistic. Foragers can be exposed to the sugar concentrations that we tested when foraging for nectar or consuming non-ripened honey stored in the nest. Bees collect nectar containing 5–80% (w/v) sugar concentration [43,44], but sugar concentrations can be as low as 2% [43]. Nectar is converted into honey in the hive via ripening, a process that increases sugar concentrations [44]. However, this process starts in the hive only [44]. Counterintuitively, foragers even dilute the sugar concentration in nectar by approximately 1% during nectar collection [45]. Thus, foragers can consume nectar containing less than 5% sugar while foraging and flying outside the nest.
Inside the nest, nectar is ripened gradually over a period taking up to 5 [45] or even 21 days [44]. When nectar is rapidly collected in large quantities, bees do not immediately ripen it; instead they deposit the nectar, largely unconcentrated, into storage cells [45]. Ripening is therefore influenced by multiple factors: weather, honey flow conditions, collection rates, colony strength, amount and concentration of nectar, extent of available storage cells, temperature, humidity and ventilation conditions [45]. Bees can thus be exposed to largely unconcentrated nectar for several days when consuming carbohydrates stored in the hive.
Individual carbohydrate intake can also be limited by non-foraging periods. In fact, lack of sufficient food stores is a common cause of winter colony losses [11] (i.e. involved in 58% of the colonies lost in the USA in 2014–2015 [12]). In our study, we therefore tested this limited carbohydrate scenario in two ways: feeding bees with a limited amount of sucrose solution or, in a separate treatment, feeding bees no nutrients (0% sucrose).
(b). Neonicotinoid treatments
We followed the most recent international guidelines for pesticide tests on bees [46]. We tested sublethal acute oral exposure to field-realistic doses of two neonicotinoid pesticides: CLO and TMX. Our doses were field-realistic because bees can consume higher doses of CLO and TMX while collecting contaminated nectar in the field for a short period (1 h) (see details below). Treatments consisted of a control dose (pesticide-free) or a neonicotinoid dose (dose) that was either 1/25 (lower dose, TMX = 0.2 ng/bee, CLO = 0.16 ng/bee) or 1/5 (higher dose, TMX = 1 ng/bee, CLO = 0.8 ng/bee) of their respective LD50 (TMX = 5 ng/bee, CLO = 4 ng/bee) [47,48]. The no nutrients diet was pesticide-free. The higher doses used for each neonicotinoid reflect field-realistic scenarios with elevated neonicotinoid contamination. Calculations based on European Food Safety Authority (EFSA) [49] data confirm that our sublethal doses were lower than the worst-case scenario in which bees foraged for 1 h on nectar that was contaminated with CLO or TMX after a seed treatment (maximum field-realistic doses: CLO = 1 ng/bee/1 h, TMX = 0.66 ng/bee/1 h) or a transplant-drip application (maximum field-realistic dose of TMX = 1.80 ng/bee/1 h).
For CLO, the EFSA [49] calculated that foragers can consume up to 1 ng/bee in 1 h of nectar foraging. This calculation was based on the field-realistic concentration of CLO in nectar (9 ppb, found in oilseed rape nectar after seed treatment application [49]) and sugar in oilseed rape nectar (10% (w/w) [44,50]). A previous study similarly estimated that a forager can acutely consume up to 1.36 ng of CLO in a foraging trip when collecting nectar on oilseed rape fields grown from seeds treated with CLO [42]. In fact, CLO can occur at even higher field-realistic concentrations in nectar (e.g. 10 ppb [17,51]) and pollen (e.g. 41 ppb [52]) than those used in our study.
Similarly, for TMX, EFSA [49] calculated that foragers can consume up to 0.66 ng/bee in 1 h of foraging for nectar (10% (w/w) sugar, oilseed rape) with 5 ppb of TMX (concentration found in nectar after seed treatment application [49]). However, foragers can consume up to 1.80 ng/bee in 1 h of foraging for nectar with 15 ppb of TMX (concentration found in nectar after transplant-drip application [51]). TMX also is found at higher concentrations in nectar (e.g. 17 ppb [52]; 19 ppb [51]; 20 ppb [53]) and pollen (e.g. 127 ppb [52]) than those used in our study. Further details on our neonicotinoid treatments are provided in the electronic supplementary material.
3. Results
(a). Combined nutritional and neonicotinoid stressors synergistically reduced survival
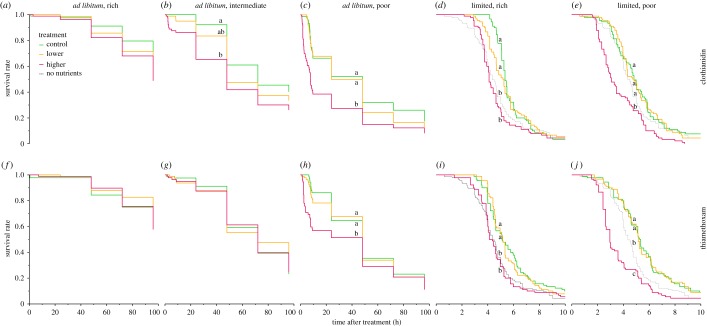
Survival was monitored up to 4 days after exposure to the neonicotinoids. Sublethal and field-realistic doses of neonicotinoids did not significantly reduce survival when foragers were fed ad libitum rich diets (Kaplan–Meier, p > 0.13; electronic supplementary material, table S1; figure 1a,f). However, neonicotinoids significantly reduced the survival of bees fed the ad libitum diets with qualities that were intermediate (CLO; figure 1b) or poor (CLO and TMX, Kaplan–Meier, p < 0.01; figure 1c,h). Bees fed higher pesticide doses had significantly lower survival when compared with control bees (CLO: within poor- and intermediate-quality diets groups; TMX: within the poor-quality diet group) and lower dose (CLO: within the poor-quality diet group) (p < 0.0170, Kaplan–MeierDS).
Figure 1.
Survival of bees exposed to combined nutritional and pesticide stressors. We exposed bees to a control (green lines), lower (orange) or higher (red) field-realistic sublethal dose of CLO (a–e) or TMX (f– j). We fed bees ad libitum diets of rich (a,f), intermediate (b,g) or poor (c,h) quality, and limited diets of rich (d,i) or poor (e,j) quality. Because of the low survival rate and to facilitate graphical display, we show the survival of bees fed limited diets until 10 h after treatment only. We also fed bees a no nutrients diet (0% sucrose concentration, dotted dark grey lines), and their survival was compared to bees fed limited diets. Different letters indicate significant differences (Kaplan–MeierDS test). Main effects and sample sizes are shown in electronic supplementary material, table S1.
CLO and TMX also reduced the survival of bees fed limited-quantity diets with either rich (figure 1d,i) or poor (figure 1e,j) sugar qualities (Kaplan–Meier, p < 0.0001; electronic supplementary material, table S1). Specifically, higher doses of both neonicotinoids significantly reduced survival when compared with control and lower doses, at all diet qualities (p < 0.0170, Kaplan–MeierDS). Increased death of bees fed neonicotinoids and poor-quality diets occurred 2–3 h after treatment (up to 0%, 6% and 19% mortality, respectively, 1 h, 2 h and 3 h after treatment; electronic supplementary material, table S2).
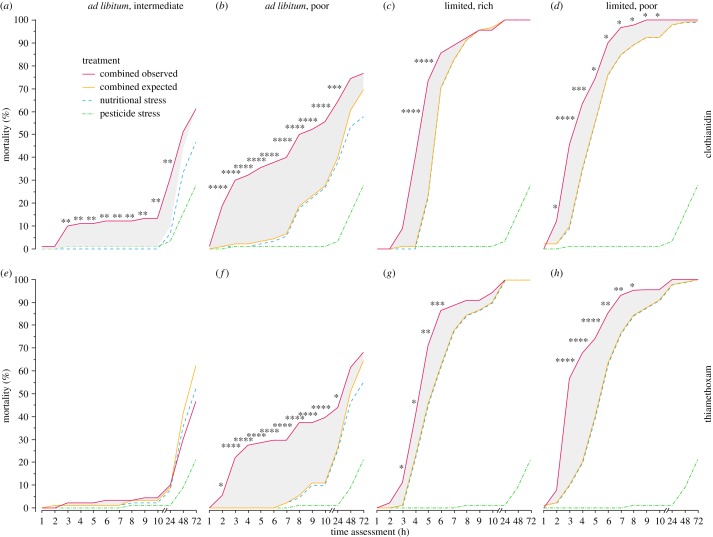
There was a significant synergistic reduction in survival elicited by all combinations of nutritional stresses (ad libitum intermediate, ad libitum poor, limited high and limited poor) and the higher pesticide dose (binomial proportion test, Holm correction; figure 2; electronic supplementary material, table S3). Ad libitum poor diets synergistically reduced survival between 2–24 h (CLO and TMX, SESrange = 5–33%; figure 2b,f), and ad libitum intermediate diets synergistically reduced survival between 3–24 h (CLO, SESrange = 9–21%; figure 2a). There was no significant synergistic effect on the survival of bees exposed to the ad libitum intermediate diet and TMX. Limited poor diets synergistically reduced survival between 2–10 h (CLO, SESrange = 8–36%; figure 2d) and 3–8 h (TMX, SESrange = 11–48%; figure 2h), and limited rich diets synergistically reduced survival between 4–5 h (CLO, SESrange = 39–50%; figure 2c) and 3–6 h (TMX, SESrange = 10–24%; figure 2g).
Figure 2.
Synergistic effects of nutritional and neonicotinoid stressors on bee survival across time. We tested the individual and combined effects of each nutritional stress (treatment A, blue, dashed lines) and the higher neonicotinoid sublethal field-realistic dose of either CLO (a–d) or TMX (e–h) (treatment B, green, dashed and dotted lines), and compared their expected (orange, full lines) and observed (red, full lines) combined effects (treatment AB). The size of the synergistic effects is highlighted by the grey-shaded area between expected and observed mortality. Asterisks indicate significant synergistic effects (i.e. significant difference between mortality of expected and observed combined treatment) at specific time assessments (binomial proportion tests, Holm corrected, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). Synergistic effect sizes for each time assessment are shown in electronic supplementary material, table S3.
Receiving no nutrients (i.e. starvation) was better than receiving some nutrients with pesticides. Within the limited-quantity diet trial, we tested an additional diet containing no nutrients (10 µl of pure water). Bees fed the no nutrients diet had significantly higher survival than those fed the limited-quantity diet of poor quality (10 µl of 15% sucrose solution) containing the higher pesticide dose of either CLO or TMX (electronic supplementary material, table S1 and figure 1e,j). The survival of bees fed the no nutrients diet was significantly lower than that of bees fed limited poor diets containing the control and lower dose (TMX: at 15–50%; CLO: at 50%; figure 1).
(b). Combined nutritional and neonicotinoid stressors reduced sugar consumption
We assessed the sucrose consumption of bees fed the ad libitum diet only because bees that received a limited-quantity diet only had access to a fixed amount of food (10 µl). We calculated the actual mass of pure sucrose consumed per bee per day. There was no significant effect of CLO on sugar consumption of foragers fed rich- and intermediate-quality diets (GLMs, p > 1.40; electronic supplementary material, table S4 and figure S1A). However, there was a significant effect of CLO on consumption of bees fed a poor-quality diet (GLMs, p < 0.0001; electronic supplementary material, figure S1A). Specifically, control bees consumed significantly more sucrose than lower (−31%) and higher (−48%) dose bees, and lower dose bees consumed more than bees treated with higher doses (−25%, contrast testDS). There was no significant effect of TMX on sucrose consumption at any diet quality (GLM, p > 0.3; electronic supplementary material, table S4 and figure S1B).
(c). Sublethal doses of neonicotinoids reduced glucose and trehalose haemolymph levels
Glucose and trehalose haemolymph levels were only assessed on bees fed the ad libitum-quantity diet, because insufficient haemolymph was extractable from bees that were only fed the limited-quantity diet (10 µl). The haemolymph was extracted 2 h after the neonicotinoid exposure. There was a significant effect of CLO on glucose (p = 0.0092) and trehalose (p = 0.0021) haemolymph levels when foragers were fed a diet of rich quality (50% sucrose) (GLM; electronic supplementary material, table S5 and figure S2A). Specifically, the haemolymph of control bees contained higher levels of glucose than bees fed the higher (+26%) and lower (+27%) CLO doses. Control bee haemolymph also contained higher levels of trehalose than the haemolymph of bees fed the CLO higher dose (+26%, contrast testDS).
Likewise, there was a significant effect of TMX on glucose (p = 0.0122) haemolymph levels when foragers were fed diets of rich quality (GLM; electronic supplementary material, table S5 and figure S2B). Specifically, control bee haemolymph contained higher levels of glucose than that of bees exposed to lower (+55%) and higher (+60%) TMX doses (contrast testDS).
(d). Effects of nutritional deficits on pesticide-free bees
(i). Nutritional deficits decreased the survival of pesticide-free bees
As expected, the survival of pesticide-free bees fed the limited-quantity diet was significantly lower than the that of pesticide-free bees fed the ad libitum diet (Kaplan–Meier, χ2 = 762.32, d.f. = 1, p < 0.0001).
There was a significant effect of diet quality on the survival of pesticide-free foragers fed ad libitum (Kaplan–Meier, p < 0.0001; electronic supplementary material, figure S3A and table S6). Specifically, foragers fed lower-quality diets had a significantly shorter survival (Kaplan–MeierDS, d.f. = 1, p < 0.0001; poor versus intermediate: χ2 = 35.62; poor versus rich: χ2 = 100.16; intermediate versus rich: χ2 = 41.43; electronic supplementary material, figure S3A).
There was a significant effect of diet quality on the survival of pesticide-free foragers fed limited-quantity diets (Kaplan–Meier, p < 0.0001; electronic supplementary material, figure S3B and table S6). Specifically, bees fed lower-quality diets had significantly reduced survival (Kaplan–Meier, d.f. = 1; poor versus rich: χ2 = 5.45, p = 0.0196; no nutrients versus rich: χ2 = 37.30, p < 0.0001; no nutrients versus poor: χ2 = 9.02, p = 0.0027; electronic supplementary material, figure S3B).
(ii). Lower-quality diets reduced glucose and trehalose levels in the haemolymph
In pesticide-free foragers, there was a significant effect of diet quality on glucose (GLM, χ7,2 = 22.42, p < 0.0001) and trehalose (GLM, χ7,2 = 37.30, p < 0.0001) levels (electronic supplementary material, figure S3E,F). As expected, forager haemolymph of bees fed rich diets contained significantly higher levels of both glucose and trehalose than those fed intermediate (+49% and+23%, respectively) and poor (+68% and +48%) diets (contrast testDS).
(iii). Diet quality influenced sucrose consumption
There was a significant effect of diet quality on sucrose consumption of pesticide-free foragers (GLMs, χ7,2 = 171.09, p < 0.0001; electronic supplementary material, figure S3C). Foragers consumed significantly less sucrose when they were fed lower-quality diets (rich versus poor: −72%; rich versus intermediate: −33%; intermediate versus poor: −58%, contrast testDS; electronic supplementary material, figure S3C). There was no significant effect of diet quality on the volume of the sucrose solutions consumed daily by the foragers (GLMs, χ7,2 = 1.43, p = 0.488; electronic supplementary material, figure S3D).
4. Discussion
One of the most common routes of honey bee pesticide exposure is via foragers collecting nectar and pollen. We demonstrate, for the first time, that nutritional stresses can act synergistically with a sublethal, field-realistic pesticide exposure and reduce honey bee survival. We also show that the exposure to nutritional and pesticide stressors impairs bee haemolymph energy levels and food consumption. Although prior research demonstrated that a good pollen diet can increase bee resistance to pesticides [21,23], and that food quality influences the effect of toxins on arthropod health [24–27], this is the first study to demonstrate the negative synergistic effects of sugar caloric restriction and pesticides in animals.
Bees that did not undergo nutritional stress were not significantly impaired by TMX or CLO. Forager survival was not significantly altered by any field-realistic doses of these neonicotinoids when they were fed optimal-quality and -quantity sugar diets (electronic supplementary material, table S1; figure 1a,f). This result also confirms that our doses were sublethal. However, bees fed a poor nutritional diet experienced detrimental synergistic effects, up to a 50% mortality increase when compared with the expected non-synergistic (additive) effects. Each neonicotinoid synergistically reduced survival of bees fed diets of low quality (32.5% and 15% sugar concentration) or quantity (limited 10 µl of sugar solution) (electronic supplementary material, tables S1 and S3; figures 1 and 2). This adverse synergistic effect of neonicotinoids and poor nutrition appeared rapidly after treatment (2 h; electronic supplementary material, table S2) and lasted up to 1 day (figure 2). Interestingly, starvation was less severe than pesticide exposure: bees survived longer when fed a pesticide-free diet containing no nutrients (pure water), when compared with bees that consumed a sugar diet of poor nutritional value, but containing a sublethal dose of pesticide (electronic supplementary material, table S1; figure 1e,j).
The combination of nutritional and neonicotinoid stressors also reduced food consumption (electronic supplementary material, figure S1). In all of our consumption experiments, bees were only fed pure sucrose solutions. Neonicotinoids were administered separately, prior to measuring consumption. Consumption was therefore not influenced by the presence of neonicotinoids in the sucrose solutions [54]. When foragers were fed the richest-quality diets, their consumption was not significantly altered by any prior neonicotinoid exposure. However, all acute doses of CLO significantly reduced subsequent food consumption when bees were exposed to the poorest quality diet, suggesting that neonicotinoids alter foragers' energy metabolism or feeding behaviour.
What accounts for this change in feeding? TMX reduced forager motor functioning (acute exposure, 1.34 ng/bee; 2-day chronic exposure, rangeTMX daily doses = 1.42–3.48 ng/bee d−1) and food consumption (1 day of chronic exposure) [55]. The reduced motor functioning of neonicotinoid-treated bees may lead to decreased energy consumption and food intake [55]. Similarly, Kessler et al. [54] showed that chronic exposure to CLO (0.1–1 µM, 25–250 ppb) and TMX (0.1–1 µM, 29–292 ppb) reduced honey bee food consumption.
Neonicotinoid consumption also reduced sugar levels in the haemolymph of bees, measured 2 h after pesticide exposure (electronic supplementary material, figure S2). CLO exposure significantly decreased both trehalose and glucose titres. TMX significantly reduced glucose levels, although TMX did not alter sucrose consumption at any diet quality. TMX may have altered sugar metabolism. These alterations were only significant when bees were fed ad libitum diets of the richest quality. Bees fed ad libitum diets of poorer quality had very low haemolymph sugar levels (2 h after treatment) across all pesticide treatments. A likely explanation is that the poorer-quality diets could not fulfil bee nutritional requirements.
The food consumption and haemolymph sugar-level alterations caused by neonicotinoids can disrupt forager energy metabolism, which is important for honey bee colony health [56]. Specifically, the neonicotinoid, imidacloprid, inhibits mitochondria respiration and ATP synthesis [57], and increases brain oxidative metabolism [58]. Similarly, another pesticide (a triazole fungicide, myclobutanil) disrupts energy production through reduced mitochondrial regeneration and ATP production [59]. These energetic changes may have broader behavioural effects, interfering with thermoregulation [60], locomotion [55] and flight [61]. Flight is one of the most energy-intensive tasks [31], is fuelled by sugar oxidation [32], requires flight muscle thermoregulation [62] and is impaired by acute and chronic sublethal TMX exposures [61].
Although CLO and TMX elicited similar results, CLO exerted consistently stronger effects, which also appeared earlier after exposure, when compared with TMX. This may have occurred because TMX targets different nAChR subtypes with a lower affinity than CLO [16]. In fact, CLO (LD50 = 4 ng/bee [47]) is more toxic than TMX (LD50 = 5 ng/bee [48]). Because approximately 36% of TMX degrades to its main metabolic by-product, CLO [16,63], the toxicity of TMX may be enhanced, to a degree, by its degradation to CLO. In cockroaches, the impairing effect of TMX on locomotion is correlated with its degradation to CLO [64].
As expected, richer sugar diets significantly increased survival (electronic supplementary material, figure S3A,B) and haemolymph energy levels (electronic supplementary material, figure S3E,F) in pesticide-free bees. Foragers consumed roughly the same maximum amounts of sucrose solution by volume because they consumed similar volumes of food across diet treatments (64 ± 1 µl/bee d−1, mean of all pesticide-free diets; electronic supplementary material, figure S3D). Bees are evidently unable to compensate for a diet with low-sugar concentration by simply consuming a higher volume of sugar solution. In fact, although the mean sugar levels in the haemolymph of our bees were within the typical concentrations of glucose (2–20 µg µl−1) and trehalose (2–40 µg µl−1) [36,65–68], pesticide-free bees fed lower-quality diets had also lower haemolymph energy levels (electronic supplementary material, figure S3E,F).
Prior insect studies showed that nutritional deprivation impairs the immune functions of the mealworm beetle (Tenebrio molitor L.) [69] and decreases the longevity of the housefly (Musca domestica L.) [70]. Sugar scarcity affects the survival [71] and behaviour [72] of organisms with complex sociality, such as ants. Our results show that nutrient deprivation reduces the lifespan of honey bees, and also compromises their resistance and resilience (i.e. ability to recover from the acute sublethal exposure) to pesticides. These data highlight the fundamental importance of high-quality carbohydrate food for bees.
The behavioural and physiological impairments showed in our study probably compromise bee health, contributing to a broader variety of sublethal side effects (for reviews see [15,19]). Nutrition and pesticide stressors could trigger synergistic effects on other bee species. When compared with honey bees, bumblebees consume more food, while storing a lower quantity of it. They are, therefore, more dependent on available nectar sources than honey bees, while being similarly exposed to pesticides. In addition, bumblebee food consumption can be widely altered by chronic exposures to neonicotinoids, such as CLO (0.1–1 µM and 10 µg l−1), TMX (1, 4, 39 and 98 µg kg−1) and imidacloprid (0.001–1 µM and 0.8–125 µg l−1) [54,73–75].
Current risk-assessment (RA) procedures used for testing chemicals do not fully take into account our current understanding of bee toxicology and health [22,26,76–78]. Our results raise further concerns by suggesting that the sugar diet regime typically used for RA toxicity tests may strongly influence pesticide toxicity. For example, the standard RA guideline for LD50 toxicity tests requires feeding bees with 50% (w/v) sucrose solutions ad libitum [46]. The results of these toxicity tests, obtained by feeding bees with an optimal nutritional diet, may underestimate the toxic effect that chemicals elicit on bees in the field, where foragers can be exposed to a combined nutritional stress (i.e. low-sugar nectar) [7,10,17,19]. Thus, the consequences of low-sugar nectar and neonicotinoid (TMX and CLO) exposure should be considered in assessing risks on insect pollinators. We suggest that RA procedures should test pesticide effects at various nutritional quality levels. More broadly, combined animal exposure to xenobiotic and nutritional stressors is a highly relevant ecological scenario that deserves greater attention.
Supplementary Material
Acknowledgement
We are grateful to Luciano Cavani for the critical technical support for the haemolymph analysis. We thank Harmen Hendriksma for the suggestions provided on the manuscript. We thank the many students involved, especially Andrea Resconi, Michela Taccioli and Marco Gobbetti for their contribution in field and laboratory data collection.
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.kc680 [79].
Author contributions
S.T. conceived the experiments. S.T., J.C.N. and F.S. designed the experiments. S.T. and R.C. collected the data. S.T. and J.C.N. analysed the data. P.M. provided materials and reagents. S.T., J.C.N., F.S., R.C. and P.M. wrote and reviewed the manuscript.
Competing interests
We have no competing interests.
Funding
This study was partially funded by Council for Agricultural Research and Economics, Agriculture and Environment Research Centre and Alma Mater Studiorum, University of Bologna.
References
- 1.Biesmeijer JC, et al. 2006. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313, 351–354. ( 10.1126/science.1127863) [DOI] [PubMed] [Google Scholar]
- 2.Klein A-M, Vaissiere BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T. 2007. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc B 274, 303–313. ( 10.1098/rspb.2006.3721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sánchez-Bayo F, Goulson D, Pennacchio F, Nazzi F, Goka K, Desneux N. 2016. Are bee diseases linked to pesticides? A brief review. Environ. Int. 89–90, 7–11. ( 10.1016/j.envint.2016.01.009) [DOI] [PubMed] [Google Scholar]
- 4.Klein S, Cabirol A, Devaud JM, Barron AB, Lihoreau M. 2017. Why bees are so vulnerable to environmental stressors. Trends Ecol. Evol. 32, 268–278. ( 10.1016/j.tree.2016.12.009) [DOI] [PubMed] [Google Scholar]
- 5.Di Pasquale G, Salignon M, Le Conte Y, Belzunces LP, Decourtye A, Kretzschmar A, Suchail S, Brunet JL, Alaux C. 2013. Influence of pollen nutrition on honey bee health: do pollen quality and diversity matter? PLoS ONE 8, e72016 ( 10.1371/journal.pone.0072016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Traynor KS, Pettis JS, Tarpy DR, Mullin CA, Frazier JL, Frazier M, VanEngelsdorp D. 2016. In-hive pesticide exposome: assessing risks to migratory honey bees from in-hive pesticide contamination in the Eastern United States. Sci. Rep. 6, 1–16. ( 10.1038/srep33207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naug D. 2009. Nutritional stress due to habitat loss may explain recent honeybee colony collapses. Biol. Conserv. 142, 2369–2372. ( 10.1016/j.biocon.2009.04.007) [DOI] [Google Scholar]
- 8.Gonzalez-Varo JP, et al. 2013. Combined effects of global change pressures on animal-mediated pollination. Trends Ecol. Evol. 28, 524–530. ( 10.1016/j.tree.2013.05.008) [DOI] [PubMed] [Google Scholar]
- 9.Kluser S, Peduzzi P. 2007. Global pollinator decline: a literature review. Geneva, Switzerland: UNEP/DEWA/GRID-Europe. [Google Scholar]
- 10.Donkersley P, Rhodes G, Pickup RW, Jones KC, Wilson K. 2014. Honeybee nutrition is linked to landscape composition. Ecol. Evol. 4, 4195–4206. ( 10.1002/ece3.1293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brodschneider R, Crailsheim K. 2010. Nutrition and health in honey bees. Apidologie 41, 278–294. ( 10.1051/apido/2010012) [DOI] [Google Scholar]
- 12.Seitz N, et al. 2016. A national survey of managed honey bee 2014–2015 annual colony losses in the USA. J. Apic. Res. 54, 1–12. ( 10.1080/00218839.2016.1153294) [DOI] [Google Scholar]
- 13.Tosi S, Costa C, Vesco U, Quaglia G, Guido G. 2018. A 3-year survey of Italian honey bee-collected pollen reveals widespread contamination by agricultural pesticides. Sci. Total Environ. 615, 208–218. ( 10.1016/j.scitotenv.2017.09.226) [DOI] [PubMed] [Google Scholar]
- 14.Stokstad E. 2012. Field research on bees raises concern about low-dose pesticides. Science 335, 1555 ( 10.1126/science.335.6076.1555) [DOI] [PubMed] [Google Scholar]
- 15.Pisa L, et al. 2017. An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 2: impacts on organisms and ecosystems. Environ. Sci. Pollut. Res. ( 10.1007/s11356-017-0341-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon-Delso N, et al. 2014. Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 22, 5–34. ( 10.1007/s11356-014-3470-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonmatin J-M, et al. 2014. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. Int. 22, 35–67. ( 10.1007/s11356-014-3332-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.David A, Botías C, Abdul-Sada A, Nicholls E, Rotheray EL, Hill EM, Goulson D. 2016. Widespread contamination of wildflower and bee-collected pollen with complex mixtures of neonicotinoids and fungicides commonly applied to crops. Environ. Int. 88, 169–178. ( 10.1016/j.envint.2015.12.011) [DOI] [PubMed] [Google Scholar]
- 19.Godfray HCJ, Blacquiere T, Field LM, Hails RS, Potts SG, Raine NE, Vanbergen AJ, McLean AR. 2015. A restatement of recent advances in the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc. R. Soc. B 282, 20151821 ( 10.1098/rspb.2014.0558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collison E, Hird H, Cresswell J, Tyler C. 2016. Interactive effects of pesticide exposure and pathogen infection on bee health—a critical analysis. Biol. Rev. 91, 1006–1019. ( 10.1111/brv.12206) [DOI] [PubMed] [Google Scholar]
- 21.Schmehl DR, Teal PEA, Frazier JL, Grozinger CM. 2014. Genomic analysis of the interaction between pesticide exposure and nutrition in honey bees (Apis mellifera). J. Insect Physiol. 71, 177–190. ( 10.1016/j.jinsphys.2014.10.002) [DOI] [PubMed] [Google Scholar]
- 22.Holmstrup M, et al. 2010. Interactions between effects of environmental chemicals and natural stressors: a review. Sci. Total Environ. 408, 3746–3762. ( 10.1016/j.scitotenv.2009.10.067) [DOI] [PubMed] [Google Scholar]
- 23.Wahl O, Ulm K. 1983. Influence pollen feeding and physiological condition on pesticide sensitivity of the honey bee Apis mellifera carnica. Oecologia 59, 106–128. [DOI] [PubMed] [Google Scholar]
- 24.Folt C, Chen C. 1999. Synergism and antagonism among multiple stressors. Limnol. Oceanogr. 44, 864–877. ( 10.4319/lo.1999.44.3) [DOI] [Google Scholar]
- 25.Reyes CA, Ramos-Jiliberto R, González-Barrientos J. 2015. Temporal variability of food determines the outcome of pesticide exposure in Daphnia. Ecol. Res. 30, 451–460. ( 10.1007/s11284-014-1240-4) [DOI] [Google Scholar]
- 26.Antunes SC, Castro BB, Gonçalves F. 2004. Effect of food level on the acute and chronic responses of daphnids to lindane. Environ. Pollut. 127, 367–375. ( 10.1016/j.envpol.2003.08.015) [DOI] [PubMed] [Google Scholar]
- 27.Cooney JD, Beauchamp J, Gehrs CW. 1983. Effects of temperature and nutritional state on the acute toxicity of acridine to the calanoid copepod, Diaptomus clavipes schacht. Environ. Toxicol. Chem. 2, 431–439. ( 10.1002/etc.5620020408) [DOI] [Google Scholar]
- 28.USEPA 2012. White paper in support of the proposed risk assessment process for bees Washington, DC: USEPA. [Google Scholar]
- 29.Seeley TD. 1995. The wisdom of the hive: the social physiology of honey bee Cambridge, MA: Harvard University Press. [Google Scholar]
- 30.Arrese EL, Soulages JL. 2010. Insect fat body: energy, metabolism, and regulation. Annu. Rev. Entomol. 55, 207–225. ( 10.1146/annurev-ento-112408-085356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beenakkers AMT, Van der Horst DJ, Van Marrewijk WJA. 1984. Insect flight muscle metabolism. Insect Biochem. 14, 243–260. ( 10.1016/0020-1790(84)90057-X) [DOI] [Google Scholar]
- 32.Suarez RK, Lighton JR, Joos B, Roberts SP, Harrison JF. 1996. Energy metabolism, enzymatic flux capacities, and metabolic flux rates in flying honeybees. Proc. Natl Acad. Sci. USA 93, 12 616–12 620. ( 10.1073/pnas.93.22.12616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.EFSA. 2013. EFSA guidance document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA J. 11, 268 ( 10.2903/j.efsa.2013.3295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crailsheim K. 1988. Intestinal transport of sugars in the honeybee (Apis mellifera L.). J. Insect Physiol. 34, 839–845. ( 10.1016/0022-1910(88)90117-5) [DOI] [Google Scholar]
- 35.Neukirch A. 1982. Dependence of the life span of the honeybee (Apis mellifica) upon flight performance and energy consumption. J. Comp. Physiol. B 146, 35–40. ( 10.1007/BF00688714) [DOI] [Google Scholar]
- 36.Fell RD. 1990. The qualitative and quantitative analysis of insect hemolymph sugars by high performance thin-layer chromatography. Camp. Biochem. Physiol. 95, 539–544. ( 10.1016/0300-9629(90)90735-B) [DOI] [Google Scholar]
- 37.Thompson SN. 2003. Trehalose — the insect ‘blood’ sugar. Adv. Insect. Phys. 31, 205–285. ( 10.1016/S0065-2806(03)31004-5) [DOI] [Google Scholar]
- 38.Woodring J, Boulden M, Das S, Gäde G. 1993. Studies on blood sugar homeostasis in the honeybee (Apis mellifera, L.). J. Insect Physiol. 39, 89–97. ( 10.1016/0022-1910(93)90022-J) [DOI] [Google Scholar]
- 39.Gmeinbauer R, Crailsheim K. 1993. Glucose utilization during flight of honeybee (Apis mellifera) workers, drones and queens. J. Insect Physiol. 39, 959–967. ( 10.1016/0022-1910(93)90005-C) [DOI] [Google Scholar]
- 40.Council E. 2007. Council regulation (EC) No 834/2007 of 28 June 2007 on organic production and labelling of organic products and repealing regulation (EEC) No 2092/91. Off. J. Eur. Union. 189, 1–23. [Google Scholar]
- 41.Dietemann V, Ellis JD, Neumann P. 2013. The COLOSS BEEBOOK volume II, standard methods for Apis mellifera pest and pathogen research: introduction. J. Apic. Res. 52, 1–4. ( 10.3896/IBRA.1.52.4.16) [DOI] [Google Scholar]
- 42.Sgolastra F, et al. 2017. Synergistic mortality between a neonicotinoid insecticide and an ergosterol-biosynthesis-inhibiting fungicide in three bee species. Pest Manag. Sci. 73, 1236–1243. ( 10.1002/ps.4449) [DOI] [PubMed] [Google Scholar]
- 43.Abrol DP. 2012. Pollination biology: biodiversity conservation and agricultural production. Berlin, Germany: Springer. [Google Scholar]
- 44.Crane E. 1975. Honey: a comprehensive survey. London, UK: Heinemann. [Google Scholar]
- 45.Atkins EL, Banker R, Butler CG, Cale GH, Cale GH jr, Crane E, Dadant CC. 1975. Gathering, storing and ripening nectar. In The hive and the honey bee (ed. Graham JM.), p. 1324 Hamilton, IL: Dadant & Sons. [Google Scholar]
- 46.OECD/OCDE. 1998. OECD Guideline 213 for the Testing of Chemicals on Honeybee, Acute Oral Toxicity Test, adopted on 21st September 1998. ( ) [DOI]
- 47.EFSA. 2013. Conclusion on the peer review of the pesticide risk assessment for bees for the active substance clothianidin. EFSA J. 11, 3066 ( 10.2903/j.efsa.2013.3066) [DOI] [Google Scholar]
- 48.EFSA. 2013. Conclusion on the peer review of the pesticide risk assessment for bees for the active substance thiamethoxam. EFSA J. 11, 3067 ( 10.2903/j.efsa.2013.3067) [DOI] [Google Scholar]
- 49.EFSA. 2012. Statement on the findings in recent studies investigating sub-lethal effects in bees of some neonicotinoids in consideration of the uses currently authorised in Europe—Version 2012. EFSA J. 10, 2725 ( 10.2903/j.efsa.2012.2752) [DOI] [Google Scholar]
- 50.Pierre J, Mesquida J, Marilleau R, Pham-Delègue MH, Renard M. 1999. Nectar secretion in winter oilseed rape, Brassica napus: quantitative and qualitative variability among 71 genotypes. Plant Breed. 118, 471–476. ( 10.1046/j.1439-0523.1999.00421.x) [DOI] [Google Scholar]
- 51.Dively GP, Kamel A. 2012. Insecticide residues in pollen and nectar of a cucurbit crop and their potential exposure to pollinators. J. Agric. Food Chem. 60, 4449–4456. ( 10.1021/jf205393x) [DOI] [PubMed] [Google Scholar]
- 52.Sanchez-Bayo F, Goka K. 2014. Pesticide residues and bees: a risk assessment. PLoS ONE 9, e94482 ( 10.1371/journal.pone.0094482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stoner KA, Eitzer BD. 2012. Movement of soil-applied imidacloprid and thiamethoxam into nectar and pollen of squash (Cucurbita pepo). PLoS ONE 7, e39114 ( 10.1371/journal.pone.0039114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kessler SC, Tiedeken EJ, Simcock KL, Derveau S, Mitchell J, Softley S, Stout JC, Wright GA. 2015. Bees prefer foods containing neonicotinoid pesticides. Nature 521, 74–76. ( 10.1038/nature14414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tosi S, Nieh JC. 2017. A common neonicotinoid pesticide, thiamethoxam, alters honey bee activity, motor functions, and movement to light. Sci. Rep. 7, 15132 ( 10.1038/s41598-017-15308-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmickl T, Crailsheim K. 2004. Inner nest homeostasis in a changing environment with special emphasis on honey bee brood nursing and pollen supply. Apidologie 35, 249–263. ( 10.1051/apido:2004019) [DOI] [Google Scholar]
- 57.Nicodemo D, Maioli MA, Medeiros HCD, Guelfi M, Balieira KVB, De Jong D, Mingatto FE. 2014. Fipronil and imidacloprid reduce honeybee mitochondrial activity. Environ. Toxicol. Chem. 33, 2070–2075. ( 10.1002/etc.2655) [DOI] [PubMed] [Google Scholar]
- 58.Decourtye A, Armengaud C, Renou M, Devillers J, Cluzeau S, Gauthier M, Pham-Delègue MH. 2004. Imidacloprid impairs memory and brain metabolism in the honeybee (Apis mellifera L.). Pestic. Biochem. Physiol. 78, 83–92. ( 10.1016/j.pestbp.2003.10.001) [DOI] [Google Scholar]
- 59.Mao W, Schuler MA, Berenbaum MR. 2017. Disruption of quercetin metabolism by fungicide affects energy production in honey bees (Apis mellifera). Proc. Natl Acad. Sci. USA 114, 201614864 ( 10.1073/pnas.1614864114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tosi S, Démares FJ, Nicolson SW, Medrzycki P, Pirk CWW, Human H. 2016. Effects of a neonicotinoid pesticide on thermoregulation of African honey bees (Apis mellifera scutellata). J. Insect Physiol. 93–94, 56–63. ( 10.1016/j.jinsphys.2016.08.010) [DOI] [PubMed] [Google Scholar]
- 61.Tosi S, Burgio G, Nieh JC. 2017. A common neonicotinoid pesticide, thiamethoxam, impairs honey bee flight ability. Sci. Rep. 7, 1201 ( 10.1038/s41598-017-01361-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heinrich B, Esch H. 1994. Thermoregulation in bees. Am. Sci. 82, 164–170. [Google Scholar]
- 63.Lewis KA, Tzilivakis J, Warner DJ, Green A. 2016. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. 22, 1050–1064. ( 10.1080/10807039.2015.1133242) [DOI] [Google Scholar]
- 64.Benzidane Y, Touinsi S, Motte E, Jadas-Hécart A, Communal P-Y, Leduc L, Thany SH. 2010. Effect of thiamethoxam on cockroach locomotor activity is associated with its metabolite clothianidin. Pest Manag. Sci. 66, 1351–1359. ( 10.1002/ps.2022) [DOI] [PubMed] [Google Scholar]
- 65.Abou-Seif MAM, Maier V, Fuchs J, Mezger M, Pfeiffer E, Bounias M. 1993. Fluctuations of carbohydrates in haemolymph of honeybee (Apis mellifica) after fasting, feeding and stress. Horm. Metab. Res. 25, 4–8. ( 10.1055/s-2007-1002034) [DOI] [PubMed] [Google Scholar]
- 66.Blatt J, Roces F. 2001. Haemolymph sugar levels in foraging honeybees (Apis mellifera carnica): dependence on metabolic rate and in vivo measurement of maximal rates of trehalose synthesis. J. Exp. Biol. 204, 2709–2716. [DOI] [PubMed] [Google Scholar]
- 67.Bounias M, Morgan MRJ. 1984. Induction of honeybee haemolymph sucrase activity by high levels of dietary sucrose. Comp. Biochem. Physiol. B 79, 75–80. ( 10.1016/0305-0491(84)90080-4) [DOI] [Google Scholar]
- 68.Wang Y, Brent CS, Fennern E, Amdam GV. 2012. Gustatory perception and fat body energy metabolism are jointly affected by vitellogenin and juvenile hormone in honey bees. PLoS Genet. 8, e1002779 ( 10.1371/journal.pgen.1002779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siva-Jothy MT, Thompson JJW. 2002. Short-term nutrient deprivation affects immune function. Physiol. Entomol. 27, 206–212. ( 10.1046/j.1365-3032.2002.00286.x) [DOI] [Google Scholar]
- 70.Cooper TM, Mockett RJ, Sohal BH, Sohal RS, Orr WC. 2004. Effect of caloric restriction on life span of the housefly, Musca domestica. FASEB J. 18, 1591–1593. ( 10.1096/fj.03-1464fje) [DOI] [PubMed] [Google Scholar]
- 71.Dussutour A, Simpson SJ. 2012. Ant workers die young and colonies collapse when fed a high-protein diet. Proc. R. Soc. B 279, 2402–2408. ( 10.1098/rspb.2012.0051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grover CD, Kay AD, Monson JA, Marsh TC, Holway DA. 2007. Linking nutrition and behavioural dominance: carbohydrate scarcity limits aggression and activity in Argentine ants. Proc. R. Soc B 274, 2951–2957. ( 10.1098/rspb.2007.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thompson HM, Wilkins S, Harkin S, Milner S, Walters KFA. 2014. Neonicotinoids and bumble bees (Bombus terrestris): effects on nectar consumption in individual workers. Pest Manag. Sci. 71, 946–950. ( 10.1002/ps.3868) [DOI] [PubMed] [Google Scholar]
- 74.Laycock I, Lenthall KM, Barratt AT, Cresswell JE. 2012. Effects of imidacloprid, a neonicotinoid pesticide, on reproduction in worker bumble bees (Bombus terrestris). Ecotoxicology 21, 1937–1945. ( 10.1007/s10646-012-0927-y) [DOI] [PubMed] [Google Scholar]
- 75.Baron GL, Raine NE, Brown MJF. 2017. General and species-specific impacts of a neonicotinoid insecticide on the ovary development and feeding of wild bumblebee queens. Proc. R. Soc. B 284, 20170123 ( 10.1098/rspb.2017.0123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Decourtye A, Henry M, Desneux N. 2013. Environment: overhaul pesticide testing on bees. Nature 497, 188 ( 10.1038/497188a) [DOI] [PubMed] [Google Scholar]
- 77.Stoner K. 2016. Current pesticide risk assessment protocols do not adequately address differences between honey bees (Apis mellifera) and bumble bees (Bombus spp.). Front. Environ. Sci. 4, 79 ( 10.3389/FENVS.2016.00079) [DOI] [Google Scholar]
- 78.Laskowski R, Bednarska AJ, Kramarz PE, Loureiro S, Scheil V, Kudłek J, Holmstrup M. 2010. Interactions between toxic chemicals and natural environmental factors: a meta-analysis and case studies. Sci. Total Environ. 408, 3763–3774. ( 10.1016/j.scitotenv.2010.01.043) [DOI] [PubMed] [Google Scholar]
- 79.Tosi S, Nieh JC, Sgolastra F, Cabbri R, Medrzycki P.2017. Data from: Neonicotinoid pesticides and nutritional stress synergistically reduce survival in honeybees. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Tosi S, Nieh JC, Sgolastra F, Cabbri R, Medrzycki P.2017. Data from: Neonicotinoid pesticides and nutritional stress synergistically reduce survival in honeybees. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.kc680 [79].