Abstract
Antarctic krill form some of the highest concentrations of animal biomass observed in the world's oceans potentially due to their prolific ability to swarm. Determining the movement of Antarctic krill within swarms is important to identify drivers of their behaviour and their biogeochemical impact on their environment. We examined vertical velocity within approximately 2000 krill swarms through the combined use of a shipborne echosounder and an acoustic Doppler current profiler. We revealed a pronounced downward anomaly in vertical velocity within swarms of −0.6 cm s−1 compared with vertical motion outside the swarm. The anomaly changed over the diel cycle, with smaller downward anomalies occurring at night. Swarms in regions of high phytoplankton concentrations (a proxy for food availability) also exhibited significantly smaller downward anomalies. We propose that the anomaly is the result of downward velocities generated by the action of krill beating their swimming appendages. During the night and in high phytoplankton availability, when krill are more likely to feed to the point of satiation, swimming activity is lowered and the anomaly is reduced. Our findings are consistent with laboratory work where krill ceased swimming and adopted a parachute posture when sated. Satiation sinking behaviour can substantially increase the efficiency of carbon transport to depth through depositing faecal pellets at the bottom of swarms, avoiding the reingestion and break-up of pellets by other swarm members.
Keywords: Euphausia superba, acoustic Doppler current profiler, Southern Ocean, faecal pellets, carbon flux
1. Background
Swarming is a common behavioural trait in pelagic marine organisms that can improve fitness through reducing predation and increasing foraging success [1]. Swarms of Antarctic krill form some of the highest concentrations of animal biomass observed in the world's ocean, reaching densities of up to 2 Mt over an area of 100 km2 [2]. Krill swarms have been observed in a wide range of configurations such as small compact aggregations (10–100 m long, 2–20 m thick [3]), extensive layers (41 km long [4]), superswarms [2] and dispersed formations throughout the water column [5]. The prolific ability of Antarctic krill to swarm may be a major factor in these organisms achieving arguably the highest monospecific biomass of any free-living animal on Earth (200–400 Mt [6]).
Advances in remote sensing and rapid data processing of underway cruise data are achieving unprecedented insights into the biomass distribution of krill swarms [7]. However, our understanding of how krill organize themselves and behave within swarms has not progressed to the same degree because of a lack of in situ observations, particularly in more open ocean environments [8]. Our best insights into within-swarm behaviour are presently from laboratory-based methods which have revealed the mechanisms of individual krill swimming. For instance, Murphy et al. [9] showed that krill swim through a metachronal beating of the pleopods (abdominal swimming appendages). Catton et al. [10] visualized the flow fields generated by free-swimming krill, which were generally downward and of the order of −1 to −4 cm s−1 over a distance of around 4 cm below the krill. This pattern was similar whether the krill were measured singularly or within small coordinated groups. Tarling & Johnson [11] showed that krill may not continuously swim, but alter between periods when the pleopods beat continuously and cease beating altogether. During beat cessation, the pleopods are splayed out as if to control descent, which corresponds to the parachute mode captured by U. Kils using underwater photography of krill in situ (http://www.ecoscope.com). This parachute mode was found to occur more frequently in krill with full stomachs compared to those with empty stomachs [11]. Krill with fuller stomachs also beat their pleopods at a lower frequency and with decreased strength [12]. This suggests that krill undergo satiation sinking, where they descend during periods of digestion to reascend to the surface layers to feed when digestion is complete [13].
One potential means of examining behaviour within swarms is through acoustic Doppler current profilers (ADCPs), which measure Doppler shift in particles, principally as a means of measuring water velocity and direction. The instrument makes its calculations based on the assumption that all ensonified particles move passively [14], but this assumption may be violated if particles within any ensonifed layers are dominated by directionally swimming organisms. For instance, Wilson & Firing [15] found that residuals from tidal fits to ADCP data were conspicuously large at sunrise, which they considered to be a bias from coherent horizontal swimming of dominant acoustic targets. Demer et al. [16] and Tarling & Thorpe [17] used this bias to measure the horizontal direction and velocity of fish schools and krill swarms, respectively. ADCPs can also measure currents in the vertical dimension. Vertical currents are generally an order of magnitude lower than horizontal currents and any substantial vertical movements resolved by ADCPs are frequently attributed to the vertical migrations of pelagic organisms [18].
In this study, we measure instantaneous vertical velocities within krill swarms across the Scotia Sea (Southern Ocean) using acoustic information obtained through a combination of a ship-borne ADCP and a multifrequency EK60 echosounder. Our objectives are twofold: first, to discern what identifiable effects krill swarms have on measured vertical flows within the water column; and, second, whether variability in these flows can provide insights into factors affecting the internal organization of krill swarms. In particular, although krill swarms have been documented to undertake diel vertical migration, it is notably variable and even absent in certain instances [19]. Given that some swarms have vertical extents that can span much of the surface mixed layer, there remains the possibility that diel patterns of behaviour exist within the swarms themselves. Furthermore, the substantial thicknesses of many swarms (between 20 and 40 m [20]) means that not all individuals will be within the layer of greatest food concentration at any one time, suggesting that satiation sinking may be an important mechanism of positional turnover within swarms. Obtaining evidence of such behavioural traits will advance our understanding of how krill swarms operate and their sensitivity to prevailing environmental conditions.
2. Material and methods
Data were analysed from a survey carried out by the RRS James Clark Ross between 9 January and 16 February 2003 within the Scotia Sea sector of the Southern Ocean. Survey transects were transited at speeds of 9–18 km h−1 and covered approximately 13 000 km (electronic supplementary material, figure S1). Acoustic data were collected using a combination of a calibrated Simrad split-beam EK60 echosounder with 38 and 120 kHz transducers and an RD Instruments narrow-band 153.6 kHz ship-mounted ADCP (full details on the configurations of the acoustic instruments and data matching procedures are given in electronic supplementary material). Net deployments were made intermittently along the transects from which krill population structure was determined and used for the parametrization of target identification models. Swarms that were detected within 100 km of any coastline were excluded from the analysis to ensure that the study only considered the open-ocean situation, given that krill adopt different behavioural strategies in more inshore regions [21]. A vertical velocity anomaly (wnet, cm s−1) was determined for each swarm where there was a valid estimate of ADCP derived vertical velocity both within the swarm (wobs) and outside the swarm (i.e. above and/or below the swarm, wpre; electronic supplementary material, dataset S1). wobs was taken to be the vertical velocity from the single ADCP bin corresponding to the mid-depth and mid-length point of the swarm. To determine vertical velocity outside the swarm, the closest bin above and below the swarm's vertical extent at the mid-length point of the swarm was chosen because this allowed all sizes of swarms to be measured on a similar basis. In total, wnet was derived for a total of 2043 swarms. The same procedure was followed in a further analysis to identify any artefacts in the wnet calculation method and to derive a baseline level of wnet to which the influence of krill swarms could be compared. This analysis drew ‘fake’ swarms of similar dimensions to observed swarms (electronic supplementary material, table S1) in swarm-devoid regions and determined wnet as above (see electronic supplementary material). The effect of light on swarm behaviour was tested through matching observed swarms with photosynthetically active radiation (PAR), measured by a parlite quantum sensor (Kipp and Zonen), which collected measurements at 5 s intervals, subsequently averaged into 1 min intervals. Phytoplankton availability for each swarm was derived through matching to the relevant spatial 4 × 4 km pixel of 8-day synthesized sea surface chlorophyll a (Chl a) images provided by the MODIS instrument on board the Aqua satellite (operated by NASA).
3. Results
(a). Vertical velocity anomalies within krill swarms
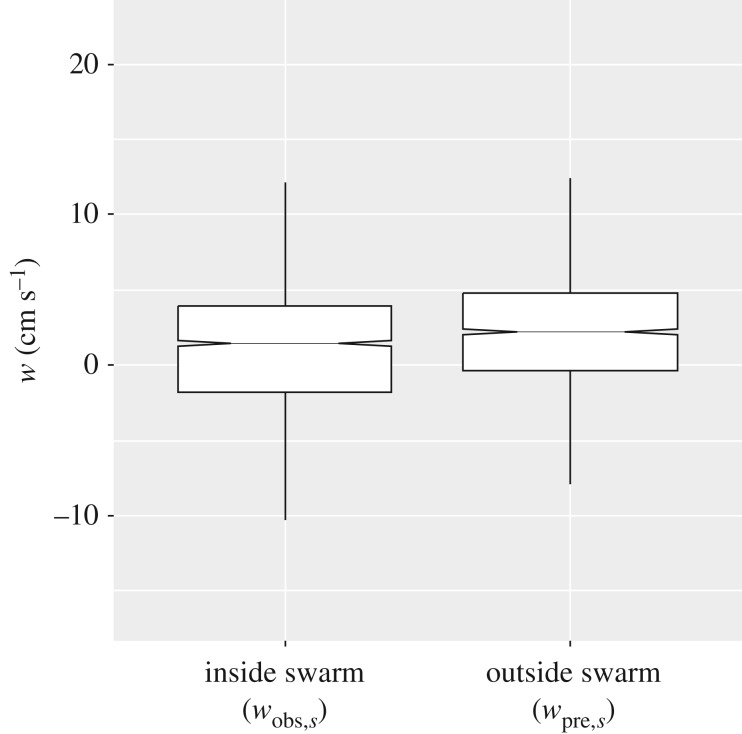
Our method compared the vertical velocities (w, cm s−1) within the swarm with those immediately outside (both above and below the swarm) with derive a vertical velocity anomaly, wnet (figure 1). Vertical velocities inside and outside the swarms were significantly different, with median wnet being downwards at −0.61 cm s−1 (Mann–Whitney (MW) rank sum test, U = 18740070, T = 3928016, n(small) = 2043, n(big) = 2043, p = < 0.001). We verified that the pattern was not an artefact of the processing method through carrying out the same calculations in areas where there were no krill swarms (termed ‘fake’ swarms) for which we found there to be no significant difference between vertical velocities outside of and within fake swarm regions (MW test, U = 4439200, T = 8880890, n(small) = 2980, n(big) = 2980, p = 0.988; figure 2).
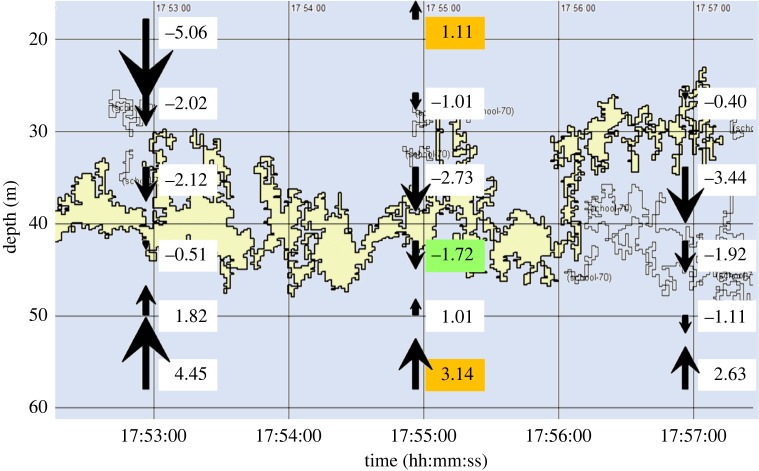
Figure 1.
Example of a krill swarm resolved by an EK60 echosounder. The krill swarm (yellow irregular object) was observed on 27 January 2003 at 58.80° S, 41.69° W. ADCP vertical velocities are superimposed (boxes and arrows; cm s−1). The green box denotes the mid-swarm vertical velocity wobs while the orange boxes are values above and below the limits of the swarm, which are averaged to determine wpre. Vertical velocity anomaly (wnet) represents wobs minus wpre which is −3.85 cm s−1 in the present example, representing a downward anomaly.
Figure 2.
Comparison of vertical velocities (w) measured inside and immediately outside of krill swarms. Refer to figure 1 for illustration of ADCP bin selection. Measurements for outside of swarm represent the average of the closest bins above and below the swarm's vertical extent at the mid-length point of the swarm. Notched horizontal line represents the median, limits of boxes the 25th and 75th percentiles, and vertical lines 1.5 times the interquartile range.
(b). Relationship to the diel cycle
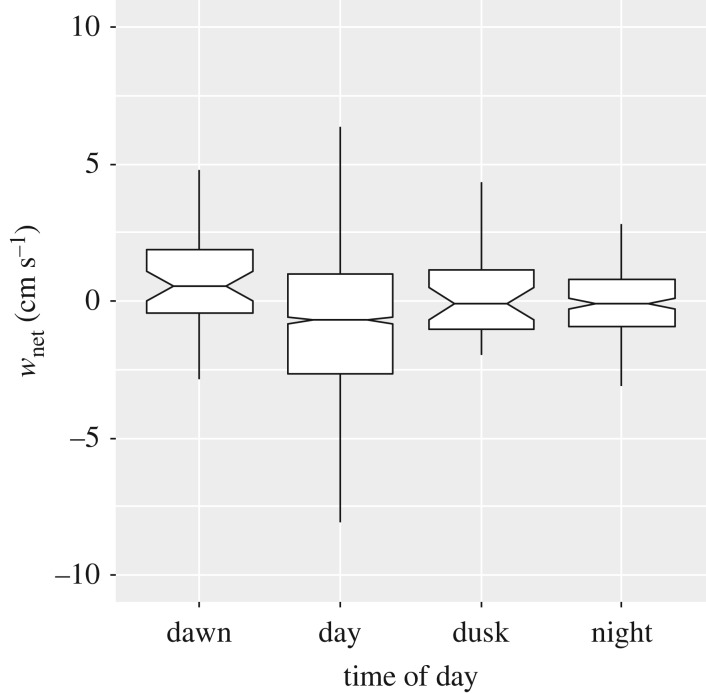
We found there to be a significant difference in wnet between different phases of the diel cycle (Kruskal–Wallis one-way ANOVA, H = 29.98, 3 d.f., p < 0.001; figure 3). Individual significant differences were found between day and night (all pairwise comparison, Dunns Method, difference in ranks 183.1, Q = 3.794), and between dawn and day (difference in ranks 327.4, Q = 3.757). All other comparisons did not show significant differences. Daytime contained the lowest median value for wnet (−0.71 cm s−1) with night-time and dusk also exhibiting negative (downward) median vertical velocity anomalies (both being −0.10 cm s−1). wnet was positive (upward) during dawn (0.50 cm s−1). During the daytime, we found no influence of different levels of daylight on wnet when comparing between low PAR and high PAR situations (MW test, U = 199090, T = 379208, n(small) = 588, n(big) = 689, p = 0.597).
Figure 3.
Downward vertical velocity anomaly (wnet) at different phases of the diel cycle. A positive value for wnet represents an upward anomaly, a negative value a downward anomaly. Notched horizontal line represents the median, limits of boxes the 25th and 75th percentiles, and vertical lines 1.5 times the interquartile range.
(c). Relationship to surface Chl a
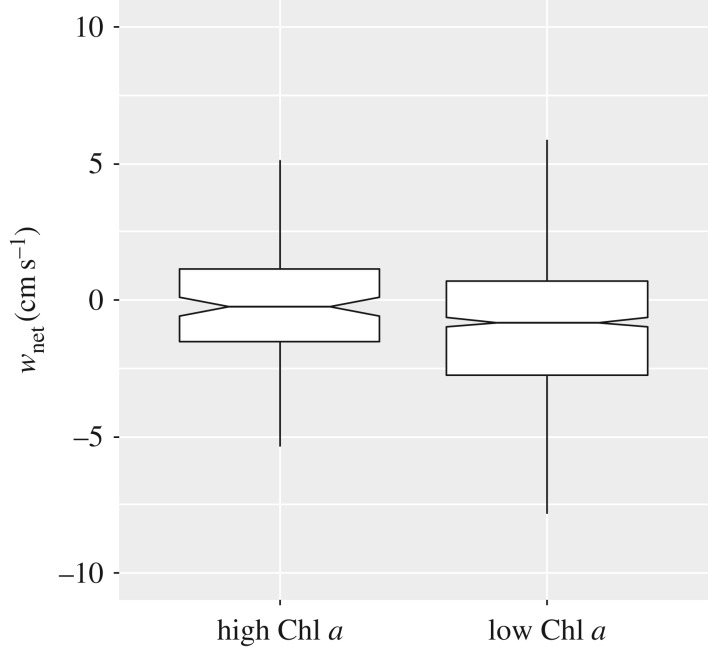
wnet was significantly more negative in regions with low levels of surface Chl a compared with regions where surface Chl a was high, both when including all times of day and night (MW test, U = 58654, T = 88409, n(small) = 149, n(big) = 912, p = 0.007) and when restricting the analysis to daytime only (MW test, U = 39204, T = 55969, n(small) = 103, n(big) = 872, p = 0.035). Across all times of day and night, median wnet was −0.81 cm s−1 in low Chl a conditions compared with −0.25 cm s−1 when Chl a was high (figure 4).
Figure 4.
Downward vertical velocity anomaly (wnet) in high versus low Chl a. High Chl a was defined as being values ≥ 0.5 mg m−3, and low values, <0.5 mg m−3. A positive value for wnet represents an upward anomaly, a negative value, a downward anomaly. Notched horizontal line represents the median, limits of boxes, the 25th and 75th percentiles, and vertical lines, 1.5 times the interquartile range.
4. Discussion
(a). Vertical velocity anomalies within krill swarms
Through comparing vertical velocities within and immediately outside of swarms, we determined there to be a downward velocity anomaly within swarms of −0.6 cm s−1. Such an anomaly did not exist over similar dimensions of the water column where there were no krill swarms. Although it can be deduced that krill within swarms are responsible for the anomaly, it remains unclear how they produce it. One possibility is that it reflects the movement of the krill themselves within the body of the swarm. Alternatively, it may be generated by the movement they impart to the water through the beating of their pleopods, assuming that pleopod beating deflects small particles downwards, so generating a negative Doppler shift detectable by the ship-borne ADCP.
Although we do not have direct evidence on how the anomalies are generated within swarms, we can rule out certain explanations based on other available evidence. For instance, if the anomaly is produced by the movement of individuals within swarms, it implies that the average swarm must always be migrating downwards. Swarms are typically found within the top 100 m of the water column and maintain this relatively narrow vertical distribution over diel cycles [19]. Such a bias towards downward moving swarms would be contrary to our understanding of krill swarm distribution and behaviour. It is further possible that the downward anomaly may reflect an avoidance behaviour in krill with respect to the survey ship, as has been found in fish during trawling [16,22]. However, there were no nets in the water during the acoustic observations included in the present analysis. Furthermore, wnet significantly varied according to time of day which rules out a response to ship's noise, which can be assumed to be relatively constant day and night. Another explanation is that krill may be responding to a shadowing of light by the vessel. The average depth of a swarm was 50 m below the vessel, by which depth light is not fully attenuated. We tested this possibility by comparing wnet between high and low PAR situations, assuming that any shadowing effect would have been more marked when PAR was high, and found there to be no significant difference in wnet between these two light environments. Although some avoidance behaviour cannot be ruled out, it does not offer a consistent explanation for the patterns and cycles we observed in wnet.
In the case of the downward deflection of small particles through pleopod beating, it is necessary first to consider the swimming action of Antarctic krill. When swimming, Antarctic krill rely on a mix of both drag-based and momentum-based swimming [10]. Their body size and density means that they are negatively buoyant and must beat their pleopods continuously in order to maintain their position within the water column [23]. When hovering, the majority of the thrust required to maintain position is directed downwards [23]. This may be less the case when swimming forwards although a large downward component is still produced [10]. These downward velocities will collectively dominate the vertical velocity signal wherever the krill are resident in sufficiently high concentrations. Nevertheless, ADCPs do not resolve water movement directly but rely on detecting Doppler shift in particles that are assumed to represent water movement. Within krill swarms, likely candidates of such particles are the background zooplankton communities and suspended particulate matter, as were also resolved in ‘fake’ swarm regions (areas devoid of swarms that were used as controls). A further matter is that ADCPs average all velocities within respective depth-time bins, which will modulate the influence of specific sources such as the wakes of swimming krill. This, therefore, may explain why our observed anomaly of −0.6 cm s−1 is below the range expected in terms of the downward water movements imparted by swarming Antarctic krill, which are of the order of −1 to −4 cm s−1 [10].
(b). Diel periodicity in anomalies
We found that the downward vertical velocity anomaly was significantly greater during the daytime than in other phases of the 24 h cycle. The downward anomaly was around −0.7 cm s−1 during the day compared with around −0.1 cm s−1 during dusk and night-time. The diel change in this anomaly implies that the swimming behaviour of individuals within the swarm must also be altering on a diel basis. Assuming that the anomaly is the result of the downward velocity imparted to the water by krill pleopod beating, it follows that either the power or the duration of these beats decreases during the night.
In free-running dark and light : dark incubations, Gaten et al. [24] found that krill have complex diel rhythms in swimming behaviour made of two circadian components, one shorter than 24 h and one longer than 24 h, to which is added a further 12 h rhythmic component. Godlewska [19] also identified a 24 h and 12 h component in the diel vertical migration patterns of Antarctic krill derived from acoustic and net sample analyses. We propose that one manifestation of this behavioural periodicity is phases of stronger and weaker pleopod beating over the course of the day–night cycle.
One interesting observation was the positive anomaly of 0.5 cm s−1 observed at dawn. Krill during this survey were observed to undertake a reverse vertical migration from 80 m during the night to 40 m during the day (G.A.T. 2003, personal observation). The positive anomaly may be the one instance where the upward movement of the krill themselves dominates the ADCP estimate of vertical velocity. Such upward anomalies are consistent with ADCP observations of other swarming euphausiid species during upward migration phases [25].
(c). Satiation sinking in krill swarms
Our further finding was that downward velocities were significantly lower in high-phytoplankton food environments (for which surface Chl a was used as a proxy). This suggests that the process of feeding also has implications on krill swimming behaviour. At an individual level, laboratory-based tethering experiments have shown that satiation in krill can cause a decrease in swimming activity and the adoption of a parachute posture which may facilitate periods of controlled sinking [11,12]. In the natural environment, this implies that swarms within regions of high food availability will be more likely to contain individuals undergoing satiation sinking. In this scenario, a fraction of the krill population stops beating and outsplays their pleopods when their stomachs are full. This means that they will no longer contribute to the generation of downward velocities. The result is a decrease in the overall downward anomaly. It follows that downward anomalies are likely to be smaller in rich feeding environments where satiation is more likely to occur.
Given that sinking individuals must be replaced by other upwardly swimming individuals if the swarm is to remain intact, there will be a continual vertical overturn of individuals within swarms found in food-rich environments. This continual upward and downward movement of individuals will have an impact on swarm organization, particularly inter-individual distances and packing concentrations. In a further analysis taken across all swarms identified during the present survey, we found that packing concentrations were significantly lower both during night-time and in high food environments (MW test: night versus day, U = 101822, T = 115118 n(small) = 163, n(big) = 1802, p = < 0.001; high versus low Chl a, U = 43048, T = 54223, n(small) = 149, n(big) = 912, p = < 0.001). When adopting the parachute posture in satiation sinking mode, krill no longer have the requirement to maintain optimal positions relative to their nearest neighbours, which will lead to swarm structure becoming less organized and more dispersed. Direct demonstrations in controlled conditions would be a logical next step to support this hypothesis.
(d). Influence of swarms on vertical flows and mixing
The vertical velocity anomaly that we observed within krill swarms implies that these swarms have a resolvable and significant impact on the velocities of the bodies of water they occupy. This supports the position of earlier studies considering the influence that krill swarms have on ocean mixing. Huntley & Zhou [26], for instance, calculated that swarms produce turbulent energy at a rate that is three to four orders of magnitude greater than the background average rate of turbulent energy dissipation. Kunze et al. [27] similarly found turbulence that was three to four orders of magnitude larger during the dusk ascent of a dense acoustic-scattering layer of krill compared with background levels during the day and that this elevated the daily averaged mixing in the inlet by a factor of 100. Nevertheless, further studies have not found evidence of increased turbulence within aggregations of marine organisms or during periods of vertical migration [28,29]. Although not universal, the impact of swarms and vertical migration on ocean mixing may be significant in certain situations, particularly in the seasonally stratified layers and in coastal regions during summer, facilitating the upward mixing of limiting nutrients from depth [26,27]. This may indeed be an important process in the continuation of large blooms that are major hotspots for krill [30].
(e). Biogeochemical impact of satiation sinking
One of the major consequences of the vertical movement of pelagic organisms is that they contribute to the transport of carbon and nitrogen from the food rich layers at the surface to the ocean interior, a process otherwise referred to as the ‘biological pump’ [31]. In the case of synchronised vertical migration, this would occur at dawn, when organisms that had just fed at the surface migrate downwards and defecate, respire and excrete in the ocean interior [32]. This active transport of materials downwards avoids interception and break-up en route which otherwise limits the efficiency of the passive process of dead matter and faeces sinking through gravity alone from the surface layers to depth. However, the fact that active transport from synchronized vertical migration occurs during just a short time window around dawn limits the overall contribution of this process to the biological pump. Under a scenario of satiation sinking within krill swarms, active transport will occur whenever there is sufficient food available for individuals to become sated and sink [13]. As well as short-circuiting the community of organisms that feed on detritus in the upper water column, this behaviour also ensures that a large fraction of faeces are egested towards the bottom of the krill swarm, so avoiding refiltering and interference by other swarm members.
Our proposal that satiation sinking is common within krill swarms is supported by observations showing that krill faecal pellets can dominate the material collected by deep sediment traps at many localities within the Southern Ocean [33–36]. In the present study region, Manno et al. [37] found that faecal pellets can make up 91% of total sedimentary particulate carbon, with around a fifth being derived from krill. Krill faecal pellets have also been found to dominate sinking material further up the water column, in the region just below the surface mixed layer [38,39]. Indeed, Belcher et al. [39] found krill faecal pellets were sometimes just as abundant below the surface mixed layer as within it, even though the majority of krill swarms themselves did not extend below the surface mixed layer, showing that faecal pellets can be exported from swarms very efficiently. This would not be the case if the majority of faecal pellets were generated randomly within swarms and had to pass through much of the swarm before being exported rather than being produced mostly towards the bottom of swarms as a result of satiation sinking. The contribution to the biological pump of satiation sinking within krill swarms can be substantial, potentially sequestering 23 Mt of carbon to the ocean interior each year within the Southern Ocean [11].
5. Conclusion
Our evidence shows that the presence of krill swarms produces a downward anomaly in the background level of vertical movement in the water column of −0.6 cm s−1. Rather than being the result of the movement of individual krill within the swarm, we interpret this anomaly to be the product of the downward velocities generated by krill beating their pleopods continuously and so allowing them to overcome their negative buoyancy and remain pelagic. The downward anomaly was found to be significantly smaller during night-time and in regions of high phytoplankton food availability (high Chl a) when feeding levels are likely to be high. The latter result is congruent with the findings of laboratory experiments in which krill with full stomachs were more likely to cease beating and outsplay their pleopods in a phase of controlled sinking. The consistency between in situ observations and laboratory results indicates that satiation sinking is likely to be a common feature within krill swarms. Satiation sinking can increase the probability of faecal pellets remaining intact and sinking to depth. This may help to explain the high concentrations of krill faecal pellets found below the surface mixed layer and at bathypelagic depths within the Southern Ocean.
Supplementary Material
Acknowledgements
We thank the crew and scientists aboard the RRS James Clark Ross during the cruise JR82, D. Bone for assembling and maintaining the net gear and N. Cunningham for organizing and retrieving data. S. Fielding and T. Klevjer assisted in the acoustic identification and visualization of krill swarms, as reported in previously published works. A. Atkinson, D. Pond and R. Shreeve helped with the morphometric measurement and maturity staging of Antarctic krill captured by nets during the cruise, and H. Venables gave advice on the ADCP data. MODIS chlorophyll data were provided by the NASA Ocean Biology Processing Group.
Ethics
All work was undertaken with relevant ethics approval.
Data accessibility
Antarctic krill swarm dataset available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.p46k6 [40].
Authors' contributions
G.A.T. participated in the krill sampling expedition, carried out the statistical analysis on the acoustic data, developed the hypotheses and drafted the manuscript. S.E.T. participated in the krill sampling expedition, processed and matched the ADCP data to the krill acoustic data and contributed to manuscript writing. Both authors give final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by the Natural Environment Research Council, UK through its support of the Ecosystems program at the British Antarctic Survey.
References
- 1.Foster EG, Ritz DA, Osborn JE, Swadling KM. 2001. Schooling affects the feeding success of Australian salmon (Arripis trutta) when preying on mysid swarms (Paramesopodopsis rufa). J. Exp. Mar. Biol. Ecol. 261, 93–106. ( 10.1016/s0022-0981(01)00265-9) [DOI] [PubMed] [Google Scholar]
- 2.Nowacek DP, Friedlaender AS, Halpin PN, Hazen EL, Johnston DW, Read AJ, Espinasse B, Zhou M, Zhu YW. 2011. Super-aggregations of krill and Humpback whales in Wilhelmina Bay, Antarctic Peninsula. PLoS ONE 6, e19173 ( 10.1371/journal.pone.0019173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalinowski J, Witek Z. 1985. Scheme for classifying Antarctic krill. BIOMASS Handbook Ser. 27, 1–12. [Google Scholar]
- 4.Watkins JL, Murray AWA. 1998. Layers of Antarctic krill, Euphausia superba: are they just long krill swarms? Mar. Biol. 131, 237–247. ( 10.1007/s002270050316) [DOI] [Google Scholar]
- 5.Everson I. 1982. Diurnal variations in mean volume backscattering strength of an Antarctic krill (Euphausia superba) patch. J. Plankt. Res. 4, 155–162. ( 10.1093/plankt/4.1.155) [DOI] [Google Scholar]
- 6.Atkinson A, et al. 2008. Oceanic circumpolar habitats of Antarctic krill. Mar. Ecol. Prog. Ser. 362, 1–23. ( 10.3354/meps07498) [DOI] [Google Scholar]
- 7.Siegel V, Watkins JL. 2016. Distribution, biomass and demography of Antarctic krill, Euphausia superba. In Biology and ecology of Antarctic krill (ed. Siegel V.), pp. 21–100. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 8.Hamner WM, Hamner PP. 2000. Behavior of Antarctic krill (Euphausia superba): schooling, foraging, and antipredatory behavior. Can. J. Fish. Aquat. Sci. 57, 192–202. ( 10.1139/cjfas-57-S3-192) [DOI] [Google Scholar]
- 9.Murphy DW, Webster DR, Kawaguchi S, King R, Yen J. 2009. Locomotory biomechanics of Antarctic krill. Integr. Compar. Biol. 49, E121. [Google Scholar]
- 10.Catton KB, Webster DR, Kawaguchi S, Yen J. 2011. The hydrodynamic disturbances of two species of krill: implications for aggregation structure. J. Exp. Biol. 214, 1845–1856. ( 10.1242/jeb.050997) [DOI] [PubMed] [Google Scholar]
- 11.Tarling GA, Johnson ML. 2006. Satiation gives krill that sinking feeling. Curr. Biol. 16, R83–R84. ( 10.1016/j.cub.2006.01.044) [DOI] [PubMed] [Google Scholar]
- 12.Johnson ML, Tarling GA. 2008. Influence of individual state on swimming capacity and behaviour of Antarctic krill. Mar. Ecol. Prog. Ser. 366, 99–110. ( 10.3354/meps07533) [DOI] [Google Scholar]
- 13.Pearre S. 2003. Eat and run? The hunger/satiation hypothesis in vertical migration: history, evidence and consequences. Biol. Rev. 78, 1–79. ( 10.1017/S146479310200595X) [DOI] [PubMed] [Google Scholar]
- 14.Woodward WE, Appell GF. 1986. Current velocity measurements using acoustic Doppler backscatter: a review. IEEE J. ocean. Engng. 11, 3–6. ( 10.1109/JOE.1986.1145147) [DOI] [Google Scholar]
- 15.Wilson CD, Firing E. 1992. Sunrise swimmers bias acoustic Doppler current profiles. Deep-Sea Res. 39, 885–892. ( 10.1016/0198-0149(92)90127-F) [DOI] [Google Scholar]
- 16.Demer DA, Barange M, Boyd AJ. 2000. Measurements of three-dimensional fish school velocities with an acoustic Doppler current profiler. Fish. Res. 47, 201–214. ( 10.1016/s0165-7836(00)00170-3) [DOI] [Google Scholar]
- 17.Tarling GA, Thorpe SE. 2014. Instantaneous movement of krill swarms in the Antarctic circumpolar current. Limnol. Oceanogr. 59, 872–886. ( 10.4319/lo.2014.59.3.0872) [DOI] [Google Scholar]
- 18.Pleuddemann AJ, Pinkel R. 1989. Characterization of the patterns of diel migration using a Doppler sonar. Deep-Sea Res. 36, 509–530. ( 10.1016/0198-0149(89)90003-4) [DOI] [Google Scholar]
- 19.Godlewska M. 1996. Vertical migrations of krill (Euphausia superba Dana). Pol. Arch. Hydrobiol. 14, 9–63. ( 10.1007/BF00297159) [DOI] [Google Scholar]
- 20.Tarling GA, Klevjer T, Fielding S, Watkins J, Atkinson A, Murphy E, Korb R, Whitehouse M, Leaper R. 2009. Variability and predictability of Antarctic krill swarm structure. Deep-Sea Res. Part II 56, 1994–2012. ( 10.1016/j.dsr.2009.07.004) [DOI] [Google Scholar]
- 21.Klevjer TA, Tarling GA, Fielding S. 2010. Swarm characteristics of Antarctic krill Euphausia superba relative to the proximity of land during summer in the Scotia Sea. Mar. Ecol. Prog. Ser. 409, 157–170. ( 10.3354/meps08602) [DOI] [Google Scholar]
- 22.Olsen K, Lovik A. 1982. Observed fish reactions to a surveying vessel with special reference to herring, cod, capelin and polar cod. Symp. Fish. Acoust. 48, 21–24. [Google Scholar]
- 23.Kils U. 1981. Swimming behaviour, swimming performance and energy balance of Antarctic krill Euphausia superba. BIOMASS Sci. Ser. 3, 1–121. [Google Scholar]
- 24.Gaten E, Tarling G, Dowse H, Kyriacou C, Rosato E. 2008. Is vertical migration in Antarctic krill (Euphausia superba) influenced by an underlying circadian rhythm? J. Genet. 87, 473–483. ( 10.1007/s12041-008-0070-y) [DOI] [PubMed] [Google Scholar]
- 25.Tarling GA, Matthews JBL, David P, Guerin O, Buchholz F. 2001. The swarm dynamics of Northern krill (Meganyctiphanes norvegica) and pteropods (Cavolinia inflexa) during vertical migration in the Ligurian Sea observed by an Acoustic doppler current profiler. Deep-Sea Res. Part I 48, 1671–1686. ( 10.1016/S0967-0637(00)00105-9) [DOI] [Google Scholar]
- 26.Huntley ME, Zhou M. 2004. Influence of animals on turbulence in the sea. Mar. Ecol. Prog. Ser. 273, 65–79. ( 10.3354/meps273065) [DOI] [Google Scholar]
- 27.Kunze E, Dower JF, Beveridge I, Dewey R, Bartlett KP. 2006. Observations of biologically generated turbulence in a coastal inlet. Science 313, 1768–1770. ( 10.1126/science.1129378) [DOI] [PubMed] [Google Scholar]
- 28.Gregg MC, Horne JK. 2009. Turbulence, acoustic backscatter, and pelagic nekton in Monterey Bay. J. Phys. Oceanogr. 39, 1097–1114. ( 10.1175/2008JPO4033.1) [DOI] [Google Scholar]
- 29.Rippeth TJ, Gascoigne J, Inall M, Palmer M, Simpson J, Wiles P. 2007. Turbulent dissipation of coastal seas. Science E-letter. See http://www.sciencemag.org/cgi/eletters/313/5794/1768#10043. [Google Scholar]
- 30.Schmidt K, Schlosser C, Atkinson A, Fielding S, Venables HJ, Waluda CM, Achterberg EP. 2016. Zooplankton gut passage mobilizes lithogenic iron for ocean productivity. Curr. Biol. 26, 2667–2673. ( 10.1016/j.cub.2016.07.058) [DOI] [PubMed] [Google Scholar]
- 31.Ducklow HW, Steinberg DK, Buesseler KO. 2001. Upper ocean carbon export and the biological pump. Oceanography 14, 50–58. ( 10.5670/oceanog.2001.06) [DOI] [Google Scholar]
- 32.Wallace MI, Cottier FR, Brierley AS, Tarling GA. 2013. Modelling the influence of copepod behaviour on faecal pellet export at high latitudes. Polar Biol. 36, 579–592. ( 10.1007/s00300-013-1287-7) [DOI] [Google Scholar]
- 33.Dunbar R. 1984. Sediment trap experiments on the Antarctic continental margin. Antarct. J. 19, 70–71. [Google Scholar]
- 34.von Bodungen B, Fischer G, Nöthig E-M, Wefer G. 1987. Sedimentation of krill faeces during spring development of phytoplankton in Bransfield Strait, Antarctica. Mitt. Geol. Paläont. Inst. Univ. Hamburg SCOPE/UNEP Sonderbd. 62, 243–257. [Google Scholar]
- 35.Bathmann U, Fischer G, Müller P, Gerdes D. 1991. Short-term variations in particulate matter sedimentation off Kapp Norvegia, Weddell Sea, Antarctica: relation to water mass advection, ice cover, plankton biomass and feeding activity. Polar Biol. 11, 185–195. ( 10.1007/BF00240207) [DOI] [Google Scholar]
- 36.Accornero A, Manno C, Esposito F, Gambi M. 2003. The vertical flux of particulate matter in the polynya of Terra Nova Bay. Part II. Biological components. Antarct. Sci. 15, 175–188. ( 10.1017/S0954102003001214) [DOI] [Google Scholar]
- 37.Manno C, Stowasser G, Enderlein P, Fielding S, Tarling GA. 2015. The contribution of zooplankton faecal pellets to deep-carbon transport in the Scotia Sea (Southern Ocean). Biogeosciences 12, 1955–2015. ( 10.5194/bg-12-1955-2015) [DOI] [Google Scholar]
- 38.Cadée G, González H, Schnack-Schiel S. 1992. Krill diet affects faecal string settling. Polar Biol. 12, 75–80. ( 10.1007/BF00238267) [DOI] [Google Scholar]
- 39.Belcher A, Tarling G, Manno C, Atkinson A, Ward P, Skaret G, Fielding S, Henson S, Sanders R. 2017. The potential role of Antarctic krill faecal pellets in efficient carbon export at the marginal ice zone of the South Orkney Islands in spring. Polar Biol. 40, 2001–2013. ( 10.1007/s00300-017-2118-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tarling GA, Thorpe SE. 2017. Data from: Oceanic swarms of Antarctic krill perform satiation sinking Dryad Digital Repository. ( 10.5061/dryad.p46k6) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Tarling GA, Thorpe SE. 2017. Data from: Oceanic swarms of Antarctic krill perform satiation sinking Dryad Digital Repository. ( 10.5061/dryad.p46k6) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Antarctic krill swarm dataset available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.p46k6 [40].