Abstract
In tropical tree communities, processes occurring during early life stages play a critical role in shaping forest composition and diversity through differences in species' performance. Predicting the future of tropical forests depends on a solid understanding of the drivers of seedling survival. At the same time, factors determining spatial and temporal patterns of seedling survival can play a large role in permitting species coexistence in diverse communities. Using long-term data on the survival of more than 45 000 seedlings of 238 species in a Neotropical forest, we assessed the relative importance of key abiotic and biotic neighbourhood variables thought to influence individual seedling survival and tested whether species vary significantly in their responses to these variables, consistent with niche differences. At the community level, seedling survival was significantly correlated with plant size, topographic habitat, neighbourhood densities of conspecific seedlings, conspecific and heterospecific trees and annual variation in water availability, in descending order of effect size. Additionally, we found significant variation among species in their sensitivity to light and water availability, as well as in their survival within different topographic habitats, indicating the potential for niche differentiation among species that could allow for species coexistence.
Keywords: density dependence, shade, size dependence, resource niche partitioning, habitat association, tropical forest dynamics
1. Background
The sheer number of tree species coexisting in tropical forest communities continues to inspire debate among ecologists over mechanisms that allow for high local diversity [1,2]. Understanding the ecological mechanisms that create and maintain diversity in forests is important because woody plants make up the bulk of biomass in forests. Additionally, tree species diversity can exert strong influences on ecosystem functions [3]. Early survival is one of the strongest filters on plant community composition [4]. Furthermore, many of the mechanisms proposed to explain species coexistence in plant communities are hypothesized to act on early life stages [5–7]. It is vital that we understand the factors that determine the survival of tree species at early life stages to better comprehend, predict, conserve and manage forests.
Both niche and neutral processes can give rise to species coexistence [8]. Neutral processes, such as random colonization and extinction of species, allow for ecologically equivalent species to randomly walk through the landscape with coexistence maintained by these mechanisms over relatively long time scales [9]. Alternatively, niche processes allow for stable coexistence through partitioning of resources by species to avoid competitive exclusion [10,11]. The question of whether there are enough niches for hyper-diverse plant species assemblages to coexist remains controversial [10], because plants require only a few limiting resources to survive: light, water and a small suite of soil micro- and macro-nutrients. Trade-offs in plant performance among species along these axes of variation represents one requirement for niche differentiation and coexistence via niche processes. Spatial and temporal variation in these factors creates potential abiotic niches for specialists, but whether variation is large enough for hundreds of species to coexist remains unresolved [2,12,13]. A critical first step is to assess whether species do in fact vary widely in their responses to abiotic variables and to determine which abiotic factors are likely to contribute most to species coexistence via niche partitioning.
The density, distance and identity of neighbour plants can have a great influence on the probability of survival [6,7,14–21]. This is due not only to inter- and intra-specific competition for resources, but also interactions with host-specific herbivores and pathogens attracted by presence and abundance of species [19,22,23]. Host-specific pests and pathogens attracted to dense patches of a species could limit the successful establishment of dominant species and create a spacing mechanism that enhances diversity [6,7]. Considerable evidence supports the existence of negative conspecific density-dependent seedling recruitment and survival in both tropical and temperate forests [20,24,25]. However, negative effects of conspecific density could be masked by habitat effects (e.g. higher survival in areas of high density because high-density areas correspond to preferred habitats or lower survival in areas of low density because of resource limitations). Thus, considering both the biotic neighbourhood and abiotic conditions simultaneously is critical for revealing their relative influence [26].
Here, we focus on the factors that mediate the success of individual seedlings in a tropical forest community. Our aims are (i) to elucidate the impact of biotic and abiotic factors on the probability of seedling survival of woody species at the community level and (ii) to quantify the variation among species in their response to these factors. While previous studies have analysed individual or subsets of these biotic and abiotic factors in relation to tree seedling survival, our study is unprecedented in scope. We analyse annual to biennial observations of seedling mortality for 45 242 individuals of 238 woody plant species collected over 10 years, combined with spatially and temporally explicit data on relevant abiotic and biotic variables. In combination, our results provide the most complete picture to date of the relative importance of key abiotic and biotic factors driving seedling survival and potentially fostering species coexistence in a diverse tropical forest.
2. Material and methods
We conducted the study in the seasonal lowland moist tropical forest of the 50 ha Forest Dynamics Plot (FDP) on Barro Colorado Island (BCI), Panama (9o9′ N, 79o51′ W) (electronic supplementary material, figure S1). All woody plants ≥1 cm DBH (diameter at 1.3 m above-ground) in the FDP have been identified to species, measured and mapped at 5-year intervals [27,28]. In 2001, we established a permanent array of marked 1 m2 seedling plots, with one plot in the centre of each 5 × 5 m quadrat of the FDP (20 000 plots). All free-standing, woody seedlings and saplings ≥20 cm tall and <1 cm DBH were tagged, measured and identified to species within each plot, with the exception of a small subset of plots that were skipped to avoid damage to ongoing monitoring efforts by other researchers [15,29]. Seedling plots were censused every year between 2001 and 2017, with the exceptions of 2005, 2007, 2010 and 2015. In the analysis presented here, we use the data from 2003 to 2013 due to the availability of data on understorey light availability during that period.
Prior to running all models, we tested for correlations between predictor variables and found they were relatively weak (all r2 < 0.09; electronic supplementary material, table S1). Furthermore, variable inflation factors of predictors in all models were all less than 1.9 (electronic supplementary material, table S2). We report the modification of risk of annual mortality by the independent variables, which represent the increase or decrease of risk of mortality when the variable increased by two standard deviations (s.d.). Using 2 s.d. allows for comparability of continuous and categorical coefficients [30]. All analyses were carried out in R using the package lme4.
(a). Community-wide survival model
We analysed the probability of seedling mortality using a generalized linear mixed-effects model (GLMM) with binomial errors and a complementary log–log link to assess the relative importance of factors determining individual seedling survival (see the electronic supplementary material, methods for model details). We examined mortality of each seedling in the dataset over the first census interval after it recruited into the census, with a log(time) offset to account for differences in the length of the census interval (range: 0.6–2.5 years). Species and sample plot were included as random intercepts to account for variation among species in baseline mortality rate and spatial autocorrelation in mortality. Fixed effects included biotic and abiotic factors likely to be important to seedling mortality.
Biotic factors included the initial (log-transformed) height of the focal seedling, and the density of seedling and tree neighbours. We calculated seedling neighbourhood densities as the numbers of conspecific or heterospecific seedlings within the same 1 m2 plot as the focal seedling at the start of the census interval. Tree neighbourhood densities were calculated for each focal seedling based on the size and distance to focal seedling (i.e. DBH0.25/distance) of conspecific and heterospecific individuals ≥1 cm DBH within 30 m of the centre of the seedling plot (based on comparison of models using various measures of tree neighbourhood density; see the electronic supplementary material, figure S2). We treat all heterospecific species as equivalent in our model because phylogenetic relatedness of heterospecific neighbours had been shown to add little predictive power in neighbourhood analyses [31].
Abiotic variables included measures of light, soil nutrient and water availability. As a measure of light availability, we calculated a canopy shade index based on the method of [32] at a height of 0.5 m above the forest floor using annual canopy census data collected for every 5 m block in the FDP (see the electronic supplementary material, methods). Data on soil nutrient concentrations in the BCI FDP at the 20 × 20 m scale were available [33]. Because many soil nutrients were correlated, we used the first three PC axes, which described greater than 75% of the variation, in our models (electronic supplementary material, figure S3). We included two variables to capture temporal variation in water availability: mean daily rainfall during the census interval and a dry season severity index to capture the maximum degree of drought stress seedlings experienced within the census interval, calculated based on evapotranspiration data from BCI [34]. To capture broad-scale spatial edaphic variation, we assigned seedling plots to one of five previously described topographic habitat types in the BCI FDP [35]. These habitats vary in soil moisture availability and include (from driest to wettest): high plateau, low plateau, slope, stream and swamp. Species' relative abundances vary among the habitats, but there is large overlap in the species present. In our models, we set slope as the baseline category because it probably represents the least stressful habitat type because it retains soil moisture longer into the dry season compared with the plateau habitats [35]. We excluded seedling plots in quadrats that could not be clearly assigned to a topographic habitat type or that fell in an approximately 2 ha area of secondary forest in the plot. In addition, we excluded plots within 27.5 m of the edge of the 50 ha plot because they were lacking sufficient canopy data to calculate the canopy shade index. Our dataset for analysis included 45 242 seedlings in 10 914 seedling plots and 238 species. We present detailed descriptions of methods for each variable in the electronic supplementary material, information.
We first constructed a GLMM to examine community-wide responses to the biotic and abiotic variables, and the effects of these variables were not allowed to vary among species. Abundant species more strongly influence coefficients as a result. However, coefficients from this community-wide model represent the mean response of any given individual seedling in the community, and thus can assess the relative importance of key biotic and abiotic factors in driving community-wide patterns of seedling mortality.
(b). Species random slope mortality models
Species coexistence via resource niche partitioning depends in part on variation among species in response to abiotic factors. In addition, tree species are known to vary in their responses to neighbour densities [15]. Thus, we also constructed a GLMM similar to the model described above, but which included random effects that allowed species to vary in their responses to each of the independent continuous variables (i.e. varying slopes models). This model quantifies the variation among species in response to each continuous predictor variable, as well as the mean response across species (rather than individuals) to the predictor variable. We compared this model with the model with no random slope terms (i.e. the ‘community-wide' model) using a likelihood ratio test [36] to assess whether there was significant among-species variation (consistent with species niche partitioning). To assess the potential for species coexistence via habitat partitioning, in a separate model we tested for variation in species performance within each habitat. This model produced species-specific estimates of responses to each habitat category relative to the consistent baseline of the slope habitat, which we then used to test for correlations or potential trade-offs in performance between species in different habitat pairs (e.g. species with relatively high survival in one habitat have relatively low survival in other habitats, rather than the same species having high survival in all habitats).
3. Results
(a). Relative importance of factors determining individual seedling mortality
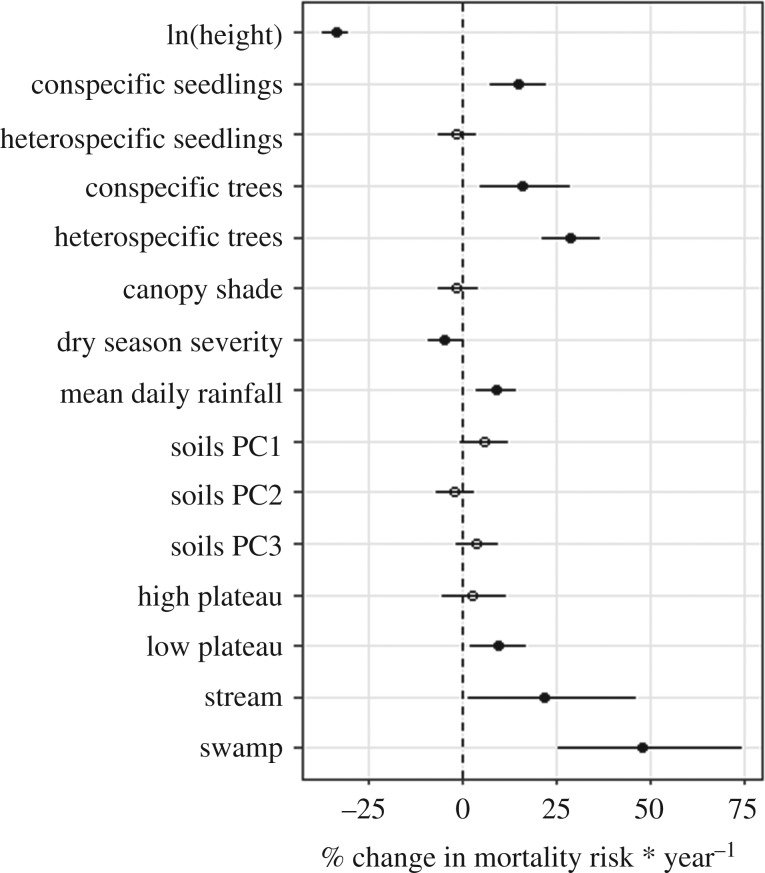
We found that the habitat where a seedling is located had the strongest influence on the likelihood of mortality (figure 1). Seedlings in the swamp and stream habitats had the highest increase in risk of annual mortality over the baseline (slope habitat), with a 49.3% and 23.0% increase, respectively. Risk of mortality was slightly, but not significantly, higher in the high-plateau habitat than the slope habitat. Seedlings in the low plateau had a 9.9% increase in the risk of mortality relative to the slope habitat. Seedling height was the second greatest risk factor for seedling mortality, with a 2 s.d. increase in height resulting in a 33.7% decrease in risk of mortality. After height, neighbour densities were the next strongest predictor variables. Specifically, increasing heterospecific tree density and conspecific tree and seedling densities by 2 s.d. increased mortality risk by 28.8%, 16.0% and 14.6%, respectively (note that 1 s.d. change of heterospecific tree density was 1.8 times greater than conspecific tree density). By contrast, heterospecific seedlings had no significant effect in the model. In terms of remaining abiotic variables, increasing mean daily rainfall increased seedling mortality risk by 8.9%, while increasing dry season severity decreased mortality risk by 4.6%. Changes in soils and canopy shade index had no significant effects on seedling mortality.
Figure 1.
Community-wide estimates of relative influence of abiotic and biotic variables on the mortality of seedlings. Categorical habitat classes are estimated relative to performance in the slope habitat. Error bars represent 2 s.e. around the mean estimate. Filled points indicate parameter estimates significantly different from zero at the alpha = 0.05 level.
(b). Variation among species in response to abiotic and biotic variables
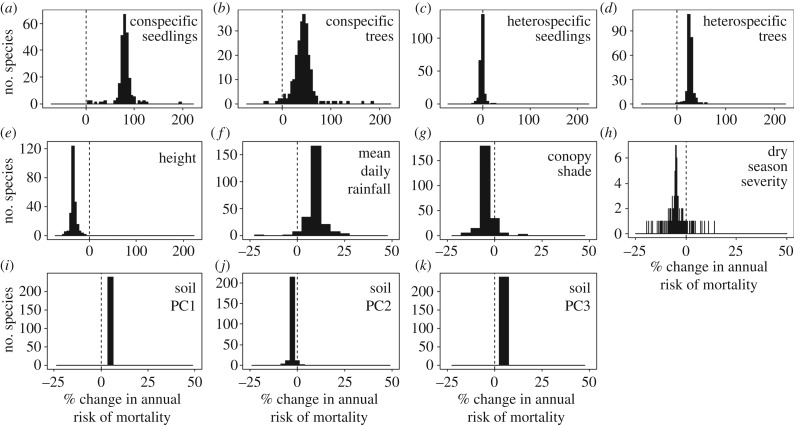
Allowing species to vary in their response to the continuous abiotic and biotic variables resulted in a significantly better model fit compared with the community level model (likelihood ratio test, p < 0.0001; electronic supplementary material, table S3 and figure S4). Species varied the most in their response to conspecific tree density, followed by conspecific seedling density, initial height, heterospecific tree density, dry season severity, heterospecific seedling density, mean daily rainfall and canopy shade in descending order (figure 2). There was very little species level variation in response to soils. Correlations of species’ responses to predictors tended to be non-significant or negative for abiotic variables (electronic supplementary material, table S4).
Figure 2.
Summary of species-specific variable response to 2 s.d. increase of variables in the model allowing species-specific responses. Conspecific seedlings (a; mean ± s.d. = 79.7 ± 16.6), conspecific trees (b; 43.7 ± 25.7), heterospecific seedlings (c; −1.7 ± 4.2), heterospecific trees (d; 27.5 ± 5.7), initial seedling height (e; −33.8 ± 6.0), mean daily rainfall (f; 9.5 ± 4.1), canopy shade (g; −4.2 ± 3.2), dry season severity (h; −4.8 ± 4.1), soils PCA 1–3 (i–k; 4.7 ± 1.1 × 10−6, −2.5 ± 0.8, 3.64 ± 3.1 × 10−5, respectively). Vertical dashed line indicates no change in risk of mortality. Note the horizontal scale differs between (a–e) and (f–k).
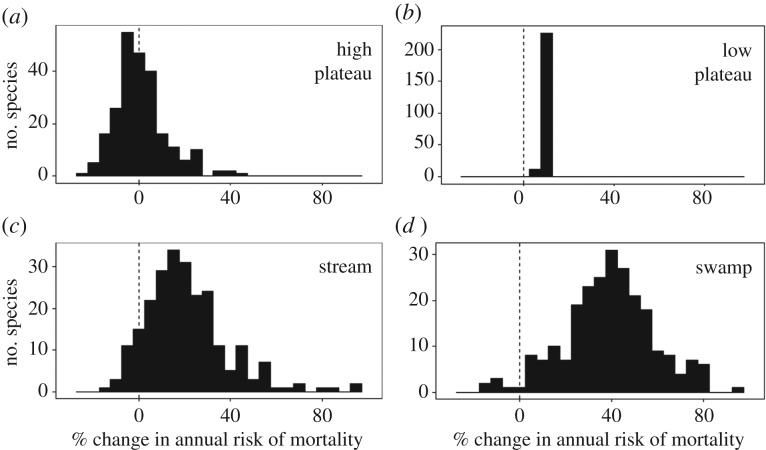
Species also varied significantly in response to habitat categories, indicated by a significantly better model fit for the model with species random effects for each habitat category compared to the community-wide model (likelihood ratio test, p = 0.034; electronic supplementary material, table S3). Species-specific responses to habitat (compared to the baseline slope habitat) indicate that the high plateau, stream and swamp had the greatest variation, while the low plateau had relatively little variation (figure 3). When examining correlations of species performance among habitats (relative to their baseline performance in the slope habitat), we found correlations of species random estimates between the swamp and all other habitats were negative, while correlations between pairs of the other habitats were positive (table 1).
Figure 3.
Summary of varying species mortality response in differing habitats relative to performance in the slope habitat for the high plateau (a; mean ± s.d. = 0.9 ± 11.4), low plateau (b; 8.2 ± 0.5), stream (c; 20.8 ± 18.2) and swamp (d; 39.1 ± 19.2). The horizontal axis represents species-specific response of the estimates for each habitat. Variation indicates species level differences in mortality in each habitat.
Table 1.
Correlation coefficients for species mortality response between habitats (lower boxes) and p-values (upper). Performance responses are calculated relative to the baseline habitat (slope; see Material and methods).
| habitat | high plateau | low plateau | stream | swamp |
|---|---|---|---|---|
| high plateau | <0.0001 | <0.0001 | <0.001 | |
| low plateau | 0.851 | <0.001 | <0.0001 | |
| stream | 0.969 | 0.755 | <0.0001 | |
| swamp | −0.979 | −0.858 | −0.984 |
4. Discussion
Using long-term, spatially explicit data on survival of more than 45 000 woody seedlings of 238 tree and shrub species, we assessed the relative importance of key abiotic and biotic variables for mortality at early life stages in a diverse tropical forest. We found strong evidence that species vary in their probability of mortality in different habitats, under different environmental conditions, and in response to differences in neighbour identity and density. The observed species-specific responses to abiotic variables and habitat suggest trade-offs in performance, a necessary condition for coexistence through niche-based processes. Additionally, the significant negative conspecific density effects observed in this forest further support the potential for stabilizing forces to contribute to the maintenance of tropical tree diversity.
(a). Relative importance of factors determining individual seedling mortality
At the individual level, habitat had the strongest influence on probability of mortality. Seedlings in the swamp and stream habitats were the most likely to die during their first census period. The swamp area is seasonally flooded requiring seedlings to be adapted to inundation for prolonged periods. The stream also experiences episodic flooding and potential soil erosion that may make it difficult to thrive. The plateaus tend to have lower soil moisture compared with the slopes, as shown by previous studies in this forest [37], and would seemingly be the most sensitive habitats to drought conditions; however, our results suggest that the high plateau and slope habitats have the same effect on overall mortality while the low-plateau habitat is slightly worse for seedling survival. This may be because the high plateau contains more drought resistance species compared to the slope habitat [38], and as a result seedling survival is equivalent in the two habitats at the community level.
After habitat, seedling mortality risk was most strongly influenced by seedling height and the local biotic neighbourhood. Seedling height is well established as influential on the probability of mortality [39–41], presumably due to advantages in resource acquisition. In terms of biotic variables, seedling mortality increased with increasing density of conspecific seedlings and tree basal area supporting the idea that conspecific negative density-dependent mortality is an important dynamic process in this tropical forest [15] and in forests generally [42]. Previous experimental work at this site suggests host-specific soil-borne pathogens may be at least partially responsible for increased seedling morality in the presence of conspecifics [22]. Seedling mortality also increased with heterospecific tree basal area, probably due to shading, competition for below-ground resources and/or generalist natural enemies. Although the effect of 2 s.d. of heterospecific tree basal area was stronger than the effect of 2 s.d. of conspecific tree basal area on seedling mortality risk, the effect of conspecific trees was stronger than heterospecific trees on a per unit basal area scale (i.e. on the raw, unscaled data) and was thus consistent with the idea that conspecific neighbours more negatively influence focal plant survival relative to heterospecific neighbours. A previous analysis at our study site found a negligible effect of heterospecific neighbourhood on seedling survival [15]. Our results differ here potentially because our model included both biotic and abiotic variables simultaneously (in contrast to [15]). Overall, the biotic neighbourhood a seedling finds itself in has a significant impact on its risk of mortality.
Seedling mortality risk at the community level was reduced by increasing dry season severity and increased by increasing mean rainfall. Drier dry seasons in this forest may correspond with greater light availability in the understorey due to lower cloud cover, which may result in reduced seedling mortality [43]. Furthermore, there are many drought-resistant species in this forest [38], which may mask negative effects of low water availability when looking only at the community level response. Conversely, wetter years may benefit pathogens and herbivores and thus reduce seedling survival [44]. It is important to note that our study did not include any years with El Niño events, which can lead to extremely severe droughts in this region and have been associated with elevated tree mortality at our study site in Panama [45], as well as elsewhere in the tropics [46,47]. Thus, it remains unclear how the seedling community as a whole would respond to drought conditions more extreme than those observed in the decade over which we monitored seedlings in this study.
We found no significant effect of soil nutrients or canopy shade on seedling mortality in the community level model. However, this lack of significance at the community level for canopy shade could be due to the low frequency of extreme high and low light areas in this old growth forest (resulting in under-sampling of these conditions), and potentially also due to the variation among species in response to light, with some species suffering greater mortality and other species less in response to increasing canopy shade, as revealed by the species random slopes model (figure 2). Soil nutrient availability varies with topography in the BCI plot, so it is possible that the lack of significant nutrient effects was because such effects were being captured by the topographic habitat types included in the model. To test this (post hoc), we ran the community-level model excluding topographic habitats and did find a significant effect of soil PC1 (electronic supplementary material, figure S5).
(b). Among-species variation in factors affecting mortality
Variation among species in response to the abiotic factors tested indicates the potential for niche partitioning in this community. The variation in response to mean annual rainfall and dry season severity indicates that species differ in response to water availability and dry season intensity. Past work has shown that drought can play a large role in structuring tropical forest communities [38,48,49]. Seedlings may be at particular risk for mortality from low water availability because they typically have less well developed root systems that cannot access deep soil moisture during times of water stress [50,51] and via asymmetric competition with larger trees for water. The episodic shifts in the El Niño/Southern Oscillation (ENSO) strongly affect the climate in Panama, and in much of the western hemisphere. Strong ENSO events result in greater mortality rates in canopy trees on the FDP and other tropical forests [45,52,53]. Potential changes in ocean temperatures and atmospheric conditions based on climate model predictions associated with climate change could bring about increased variability in rainfall and extreme climatic events in the region [54–56]. Our results suggest that there is wide variation in species level response to dry season severity which would mean shifts in community composition with increasing frequency and intensity of extreme conditions [57]. It is important to note that our sampling period does not include an ENSO event, and our results should therefore be regarded as conservative estimates of species' responses to dry season severity and mean annual rainfall. The species-specific responses to water availability observed here, coupled with temporal variation in rainfall at the site and the long lifetimes of tropical trees, could contribute to coexistence via the storage effect [5,58,59]. Spatio-temporal variation in water availability may also provide the conditions for coexistence of species through hydrological niche segregation in edaphic conditions and trade-offs in gas exchange function among species [60]. Future work could examine the response of species to the interaction between habitats and climatic variables, as well as test for positive covariance between environmental variables and competition, as essential component of the storage effect [61].
Species also varied in response to canopy shade with the majority having lower mortality with increased shade indicating that most species survive well in low light while some survive better in higher light environments (figure 2). Decreased risk of mortality with greater shade may seem counterintuitive at first given the low light levels most understorey plants experience in tropical forests [62,63], but this is consistent with previous research on sapling survival at BCI where most species had greater mortality in higher light conditions [40]. We assume that the increase in survival with increasing canopy shade is due to intense competition in light gaps, which would lead to higher mortality [64]. Additionally, many species are likely to have a low-light compensation point and are well adapted to survival in the shade because in forests where gap creation is random and infrequent, understorey persistence is essential for high fitness, particularly for dispersal limited species [28].
We found little variation among species in response to soils PC axes. The lack of response may be due to soil heterogeneity at scales finer than was sampled. While it would have been ideal to have soil samples at every one of the seedling plots, it was not logistically feasible due to the large number of plots (approx. 20 000). Also, seedling growth may be sensitive to soil nutrients, while seedling mortality is not. Alternatively, woody species may be less sensitive to soil variation than other plant types, such as the herb layer [65].
Species varied widely in their response to conspecific seedling and tree neighbour density and less in response heterospecific tree and seedling density (figure 2). Even with the wide variation among species in effect of conspecific density dependence, all species examined had increased risk of mortality with increasing conspecific seedlings and all but six had increased risk of mortality from increasing conspecific tree density. Species level responses to conspecific tree and seedling densities were positively correlated (electronic supplementary material, table S4), suggesting that conspecific neighbours of any size tend to increase the probability of mortality for seedlings. This finding is suggestive of differences in species-specific enemy pressure or intra-specific competition, with an overall negative effect at the community level. This finding confirms what was shown for seedlings in this forest in a previous analysis that was restricted to neighbourhood variables [15], and is consistent with recent work that found that conspecific negative density dependence is a major structuring mechanism globally [66].
Species mortality risk varied most in response to the swamp, stream and high-plateau habitats indicating potential abiotic filtering in those habitats (figure 3). Species whose seedlings survived well in the swamp tended to survive poorly in other habitats (table 1), suggesting trade-offs in species' ability to tolerate inundated soils versus ability to perform well in well-drained soils. Such a trade-off could permit coexistence via habitat partitioning. For comparisons among the remaining habitats (high plateau, low plateau and stream), species response was positively correlated suggesting that rather than promoting coexistence through trade-offs in habitat-specific seedling survival, variation among species in mortality results in environmental filtering that excludes or lowers the abundance of species that are poorly suited for harsher habitats. Previous work at our study site has shown that, in general, seedlings of drought-resistant species survive better than seedlings of drought sensitive species [57], but the difference is even more pronounced in the drier plateau habitats, leading to decreased relative abundance of drought sensitive species on the plateaus [48], consistent with environmental filtering. Habitat-specific mortality is a likely mechanism for habitat associations observed in studies of species composition at this forest [48,67,68], as well as other tropical and temperate forests where species exhibit affinities for habitats [29,69–72]. However, our results suggest that the relative contribution of habitat partitioning to coexistence is limited. Future work should investigate coexistence within habitats, which may be fostered by the strong biotic interactions observed in the present study and/or by microhabitat preferences at scales finer than our habitats.
5. Conclusion
Our results demonstrate niche partitioning may contribute to coexistence in this forest through differential responses to abiotic factors as well as conspecific negative density-dependent mortality. Although studies in shorter-lived, lower-diversity communities have demonstrated that the type of niche differences observed here can lead to species coexistence [73,74], proving that these differences contribute to coexistence in long-lived, diverse forests remain a challenge. In particular, some of the effects observed here may vary over ontogeny [25,75]. However, our results demonstrate that species experienced differential mortality risks based on abiotic factors allowing for niche partitioning along multiple resource axes. Future work could investigate how functional traits interact with biotic and abiotic factors to cause differential seedling survival among species to gain a mechanistic framework for predictive modelling of community dynamics [76,77]. Assessing the degree to which niche-based processes contribute to species coexistence by overcoming fitness differences is a critical next step for understanding how diverse tropical forests are structured.
Supplementary Material
Acknowledgements
We thank Salomon Aguilar, Rolando Perez and the BCI seedling census field team for data collection and species identifications and Suzanne Lao for data management. Portions of this work benefited greatly from discussions with many people during CTFS-ForestGEO workshops (NSF DEB-1046113): R. Foster as plot founder; S. Lao for data management; S. Dolins for database design; plus hundreds of fieldworkers for the census work; the National Science Foundation, Smithsonian Tropical Research Institute and MacArthur Foundation for the bulk of the financial support. Soils data provided by STRI Soils Initiative and Jim Dalling, Robert John, Kyle Harms, Robert Stallard and Joe Yavitt, and funded by NSF DEB021104, 021115, 0212284, 0212818 and OISE 0314581.
Data accessibility
Seedling data: http://datadryad.org/resource/doi:10.5061/dryad.fm654 [78]; soil data: http://ctfs.si.edu/webatlas/datasets/bci/soilmaps/BCIsoil.html; climate data: http://biogeodb.stri.si.edu/physical_monitoring/research/barrocolorado; canopy data: https://doi.org/10.5479/data.bci20140711; tree data: https://doi.org/10.5479/data.bci.20130603.
Authors' contributions
The study was designed by D.J.J. and L.S.C. Statistical analyses were carried out by D.J.J. Canopy shade data were processed by R.C. BCI tree data are curated and maintained by R.C. and S.P.H. The initial draft of the manuscript was written by D.J.J. and L.S.C. All authors contributed to the critical revision of the manuscript.
Competing interests
We have no competing interests.
Funding
Funding for the collection of seedling data was provided by National Science Foundation LTREB awards (NSF DEB-1464389 to L.S.C. and S.P.H.).
References
- 1.Leigh EG, Davidar P, Dick CW, Puyravaud JP, Terborgh J, ter Steege H, Wright SJ. 2004. Why do some tropical forests have so many species of trees? Biotropica 36, 447–473. [Google Scholar]
- 2.Wright SJ. 2002. Plant diversity in tropical forests: a review of mechanisms of species coexistence. Oecologia 130, 1–14. ( 10.1007/s004420100809) [DOI] [PubMed] [Google Scholar]
- 3.Hooper DU, et al. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35. ( 10.1890/04-0922) [DOI] [Google Scholar]
- 4.Harcombe PA. 1987. Tree life-table. Bioscience 37, 557–568. ( 10.2307/1310666) [DOI] [Google Scholar]
- 5.Chesson P. 2000. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366. ( 10.1146/annurev.ecolsys.31.1.343) [DOI] [Google Scholar]
- 6.Connell JH. 1971. On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forests. In Dynamics of populations (eds PJD Boer, G Gradwell), pp. 298–310. Wageningen, the Netherlands: Center for Agricultural Publishing and Documentation. [Google Scholar]
- 7.Janzen DH. 1970. Herbivores and number of tree species in tropical forests. Am. Nat. 104, 501–528. ( 10.1086/282687) [DOI] [Google Scholar]
- 8.Gravel D, Canham CD, Beaudet M, Messier C. 2006. Reconciling niche and neutrality: the continuum hypothesis. Ecol. Lett. 9, 399–409. ( 10.1111/j.1461-0248.2006.00884.x) [DOI] [PubMed] [Google Scholar]
- 9.Hubbell SP. 2001. The unified neutral theory of biodiversity and biogeography. Princeton, NJ: Princeton University Press. [Google Scholar]
- 10.Silvertown J. 2004. Plant coexistence and the niche. Trends Ecol. Evol. 19, 605–611. ( 10.1016/j.tree.2004.09.003) [DOI] [Google Scholar]
- 11.Tilman D. 1994. Competition and biodiversity in spatially structured habitats. Ecology 75, 2–16. ( 10.2307/1939377) [DOI] [Google Scholar]
- 12.Chesson P. 2000. General theory of competitive coexistence in spatially-varying environments. Theor. Popul. Biol. 58, 211–237. ( 10.1006/tpbi.2000.1486) [DOI] [PubMed] [Google Scholar]
- 13.Gravel D, Guichard F, Hochberg ME. 2011. Species coexistence in a variable world. Ecol. Lett. 14, 828–839. ( 10.1111/j.1461-0248.2011.01643.x). [DOI] [PubMed] [Google Scholar]
- 14.Kobe RK, Vriesendorp CF. 2011. Conspecific density dependence in seedlings varies with species shade tolerance in a wet tropical forest. Ecol. Lett. 14, 503–510. ( 10.1111/j.1461-0248.2011.01612.x) [DOI] [PubMed] [Google Scholar]
- 15.Comita LS, Muller-Landau HC, Aguilar S, Hubbell SP. 2010. Asymmetric density dependence shapes species abundances in a tropical tree community. Science 329, 330–332. ( 10.1126/science.1190772) [DOI] [PubMed] [Google Scholar]
- 16.Swamy V, Terborgh JW. 2010. Distance-responsive natural enemies strongly influence seedling establishment patterns of multiple species in an Amazonian rain forest. J. Ecol. 98, 1096–1107. ( 10.1111/j.1365-2745.2010.01686.x) [DOI] [Google Scholar]
- 17.Bagchi R, et al. 2011. Spatial patterns reveal negative density dependence and habitat associations in tropical trees. Ecology 92, 1723–1729. ( 10.1890/11-0335.1) [DOI] [PubMed] [Google Scholar]
- 18.Bai XJ, et al. 2012. Effects of local biotic neighbors and habitat heterogeneity on tree and shrub seedling survival in an old-growth temperate forest. Oecologia 170, 755–765. ( 10.1007/s00442-012-2348-2) [DOI] [PubMed] [Google Scholar]
- 19.Augspurger CK. 1984. Pathogen mortality of tropical tree seedlings: experimental studies of the effects of dispersal distance, seedling density, and light conditions. Oecologia 61, 211–217. ( 10.1007/BF00396763) [DOI] [PubMed] [Google Scholar]
- 20.Comita LS, Queenborough SA, Murphy SJ, Eck JL, Xu K, Krishnadas M, Beckman N, Zhu Y. 2014. Testing predictions of the Janzen–Connell hypothesis: a meta-analysis of experimental evidence for distance- and density-dependent seed and seedling survival. J. Ecol. 102, 845–856. ( 10.1111/1365-2745.12232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilbert GS, Harms KE, Hamill DN, Hubbell SP. 2001. Effects of seedling size, El Nino drought, seedling density, and distance to nearest conspecific adult on 6-year survival of Ocotea whitei seedlings in Panama. Oecologia 127, 509–516. ( 10.1007/s004420000616) [DOI] [PubMed] [Google Scholar]
- 22.Mangan SA, Schnitzer SA, Herre EA, Mack KML, Valencia MC, Sanchez EI, Bever JD. 2010. Negative plant-soil feedback predicts tree-species relative abundance in a tropical forest. Nature 466, 752–755. ( 10.1038/nature09273) [DOI] [PubMed] [Google Scholar]
- 23.Terborgh J. 2012. Enemies maintain hyperdiverse tropical forests. Am. Nat. 179, 303–314. ( 10.1086/664183) [DOI] [PubMed] [Google Scholar]
- 24.Carson WP, Anderson JT, Leigh EGJ, Schnitzer SA. 2008. Challenges associated with testing and falsifying the Janzen–Connell hypothesis: a review and critique. In Tropical forest community ecology (eds Carson WP, Schnitzer SA), p. 536 Hoboken, NJ: Wiley-Blackwell. [Google Scholar]
- 25.Piao T, Comita L, Jin G, Kim J. 2013. Density dependence across multiple life stages in a temperate old-growth forest of northeast China. Oecologia 172, 207–217. ( 10.1007/s00442-012-2481-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, Mi XC, Comita LS, Zhang LW, Ren HB, Ma KP. 2010. Community-level consequences of density dependence and habitat association in a subtropical broad-leaved forest. Ecol. Lett. 13, 695–704. ( 10.1111/j.1461-0248.2010.01468.x) [DOI] [PubMed] [Google Scholar]
- 27.Condit R. 1998. Tropical forest census plots: methods and results from barro Colorado island, Panama and a comparison with other plots. New York, NY: Berlin, Germany: Springer. [Google Scholar]
- 28.Hubbell SP, Foster RB, O'Brien ST, Harms KE, Condit R, Wechsler B, Wright SJ, de Lao SL. 1999. Light-gap disturbances, recruitment limitation, and tree diversity in a neotropical forest. Science 283, 554–557. ( 10.1126/science.283.5401.554) [DOI] [PubMed] [Google Scholar]
- 29.Comita LS, Condit R, Hubbell SP. 2007. Developmental changes in habitat associations of tropical trees. J. Ecol. 95, 482–492. ( 10.1111/j.1365-2745.2007.01229.x) [DOI] [Google Scholar]
- 30.Gelman A, Hill J. 2007. Data analysis using regression and multilevel hierarchical models. New York, NY: Cambridge University Press. [Google Scholar]
- 31.Chen L, et al. In press. Forest tree neighborhoods are structured more by negative conspecific density dependence than by interactions among closely related species. Ecography. [Google Scholar]
- 32.Rueger N, Huth A, Hubbell SP, Condit R. 2009. Response of recruitment to light availability across a tropical lowland rain forest community. J. Ecol. 97, 1360–1368. ( 10.1111/j.1365-2745.2009.01552.x) [DOI] [Google Scholar]
- 33.John R, et al. 2007. Soil nutrients influence spatial distributions of tropical tree species. Proc. Natl Acad. Sci. USA 104, 864–869. ( 10.1073/pnas.0604666104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Condit R, Engelbrecht BMJ, Pino D, Perez R, Turner BL. 2013. Species distributions in response to individual soil nutrients and seasonal drought across a community of tropical trees. Proc. Natl Acad. Sci. USA 110, 5064–5068. ( 10.1073/pnas.1218042110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harms KE, Condit R, Hubbell SP, Foster RB. 2001. Habitat associations of trees and shrubs in a 50-ha neotropical forest plot. J. Ecol. 89, 947–959. ( 10.1111/j.1365-2745.2001.00615.x) [DOI] [Google Scholar]
- 36.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J.-S.S. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. ( 10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 37.Daws M, Mullins C, Burslem DRP, Paton S, Dalling J. 2002. Topographic position affects the water regime in a semideciduous tropical forest in Panamá. Plant Soil 238, 79–89. ( 10.1023/A:1014289930621) [DOI] [Google Scholar]
- 38.Engelbrecht BMJ, Comita LS, Condit R, Kursar TA, Tyree MT, Turner BL, Hubbell SP. 2007. Drought sensitivity shapes species distribution patterns in tropical forests. Nature 447, 80–82. ( 10.1038/nature05747) [DOI] [PubMed] [Google Scholar]
- 39.Davies SJ. 2001. Tree mortality and growth in 11 sympatric Macaranga species in Borneo. Ecology 82, 920–932. ( 10.1890/0012-9658(2001)082%5B0920:TMAGIS%5D2.0.CO;2) [DOI] [Google Scholar]
- 40.Rueger N, Huth A, Hubbell SP, Condit R. 2011. Determinants of mortality across a tropical lowland rainforest community. Oikos 120, 1047–1056. ( 10.1111/j.1600-0706.2010.19021.x) [DOI] [Google Scholar]
- 41.Clark DA, Clark DB. 2001. Getting to the canopy: tree height growth in a neotropical rain forest. Ecology 82, 1460–1472. ( 10.1890/0012-9658(2001)082%5B1460:GTTCTH%5D2.0.CO;2) [DOI] [Google Scholar]
- 42.Johnson DJ, Beaulieu WT, Bever JD, Clay K. 2012. Conspecific negative density dependence and forest diversity. Science 336, 904–907. ( 10.1126/science.1220269) [DOI] [PubMed] [Google Scholar]
- 43.van Schaik CP, Terborgh JW, Wright SJ. 1993. The phenology of tropical forests: adaptive significance and consequences for primary consumers. Annu. Rev. Ecol. Syst. 24, 353–377. ( 10.1146/annurev.es.24.110193.002033) [DOI] [Google Scholar]
- 44.Swinfield T, Lewis OT, Bagchi R, Freckleton RP. 2012. Consequences of changing rainfall for fungal pathogen-induced mortality in tropical tree seedlings. Ecol. Evol. 2, 1408–1413. ( 10.1002/ece3.252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Condit R, Hubbell SP, Foster RB. 1995. Mortality rates of 205 neotropical tree and shrub species and the impact of a severe drought. Ecol. Monogr. 65, 419–439. ( 10.2307/2963497) [DOI] [Google Scholar]
- 46.Williamson GB, Laurance WF, Oliveira AA, Delamônica P, Gascon C, Lovejoy TE, Pohl L. 2000. Amazonian tree mortality during the 1997 El Niño drought. Conserv. Biol. 14, 1538–1542. ( 10.1046/j.1523-1739.2000.99298.x) [DOI] [Google Scholar]
- 47.Williamson GB, Ickes K. 2002. Mast fruiting and ENSO cycles—does the cue betray a cause? Oikos 97, 459–461. ( 10.1034/j.1600-0706.2002.970317.x) [DOI] [Google Scholar]
- 48.Comita LS, Engelbrecht BMJ. 2009. Seasonal and spatial variation in water availability drive habitat associations in a tropical forest. Ecology 90, 2755–2765. ( 10.1890/08-1482.1) [DOI] [PubMed] [Google Scholar]
- 49.Brenes-Arguedas T, Coley PD, Kursar TA. 2009. Pests vs. drought as determinants of plant distribution along a tropical rainfall gradient. Ecology 90, 1751–1761. ( 10.1890/08-1271.1) [DOI] [PubMed] [Google Scholar]
- 50.Cao K-F. 2000. Water relations and gas exchange of tropical saplings during a prolonged drought in a Bornean heath forest, with reference to root architecture. J. Trop. Ecol. 16, 101–116. ( 10.1017/S0266467400001292) [DOI] [Google Scholar]
- 51.Engelbrecht BJ, Kursar T. 2003. Comparative drought-resistance of seedlings of 28 species of co-occurring tropical woody plants. Oecologia 136, 383–393. ( 10.1007/s00442-003-1290-8) [DOI] [PubMed] [Google Scholar]
- 52.Nakagawa M, et al. 2000. Impact of severe drought associated with the 1997 & 1998 El Nino in a tropical forest in Sarawak. J. Trop. Ecol. 16, 355–367. ( 10.1017/S0266467400001450) [DOI] [Google Scholar]
- 53.Phillips OL, et al. 2009. Drought sensitivity of the Amazon rainforest. Science 323, 1344–1347. ( 10.1126/science.1164033) [DOI] [PubMed] [Google Scholar]
- 54.Timmermann A, Oberhuber J, Bacher A, Esch M, Latif M, Roeckner E. 1999. Increased El Nino frequency in a climate model forced by future greenhouse warming. Nature 398, 694–697. ( 10.1038/19505) [DOI] [Google Scholar]
- 55.Goodess CM. 2013. How is the frequency, location and severity of extreme events likely to change up to 2060? Environ . Sci. Policy 27, S4–S14. ( 10.1016/j.envsci.2012.04.001) [DOI] [Google Scholar]
- 56.IPCC. 2007. Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II, and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change Geneva, Switzerland: IPCC. [Google Scholar]
- 57.Comita LS, Engelbrecht BMJ. 2014. Drought as a driver of tropical tree species regeneration dynamics and distribution patterns. In Forests and global change (eds Coomes DA, Burslem DFRP, Simonson WD), pp. 261–308. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 58.Usinowicz J, Wright SJ, Ives AR. 2012. Coexistence in tropical forests through asynchronous variation in annual seed production. Ecology 93, 2073–2084. ( 10.1890/11-1935.1) [DOI] [PubMed] [Google Scholar]
- 59.Adler PB, HilleRisLambers J, Levine JM. 2009. Weak effect of climate variability on coexistence in a sagebrush steppe community. Ecology 90, 3303–3312. ( 10.1890/08-2241.1) [DOI] [PubMed] [Google Scholar]
- 60.Silvertown J, Araya Y, Gowing D. 2015. Hydrological niches in terrestrial plant communities: a review. J. Ecol. 103, 93–108. ( 10.1111/1365-2745.12332) [DOI] [Google Scholar]
- 61.Ellner SP, Snyder RE, Adler PB. 2016. How to quantify the temporal storage effect using simulations instead of math. Ecol. Lett. 19, 1333–1342. ( 10.1111/ele.12672) [DOI] [PubMed] [Google Scholar]
- 62.Clark DB, Clark DA, Rich PM, Weiss S, Oberbauer SF. 1996. Landscape-scale evaluation of understory light and canopy structures: methods and application in a neotropical lowland rain forest. Can. J. For. Res. 26, 747–757. ( 10.1139/x26-084) [DOI] [Google Scholar]
- 63.Chazdon RL, Pearcy RW. 1991. The importance of sunflecks for forest understory plants—photosynthetic machinery appears adapted to brief, unpredictable periods of radiation. Bioscience 41, 760–766. ( 10.2307/1311725) [DOI] [Google Scholar]
- 64.Welden CW, Hewett SW, Hubbell SP, Foster RB. 1991. Sapling survival, growth, and recruitment: relationship to canopy height in a Neotropical forest. Ecology 72, 35–50. ( 10.2307/1938900) [DOI] [Google Scholar]
- 65.Murphy SJ, Salpeter K, Comita LS. 2016. Higher β-diversity observed for herbs over woody plants is driven by stronger habitat filtering in a tropical understory. Ecology 97, 2074–2084. ( 10.1890/15-1801.1) [DOI] [PubMed] [Google Scholar]
- 66.LaManna JA, et al. 2017. Plant diversity increases with the strength of negative density dependence at the global scale. Science 356, 1389–1392. ( 10.1126/science.aam5678) [DOI] [PubMed] [Google Scholar]
- 67.Kanagaraj R, Wiegand T, Comita LS, Huth A. 2011. Tropical tree species assemblages in topographical habitats change in time and with life stage. J. Ecol. 99, 1441–1452. ( 10.1111/j.1365-2745.2011.01878.x) [DOI] [Google Scholar]
- 68.Baldeck CA, et al. 2013. Habitat filtering across tree life stages in tropical forest communities. Proc. R. Soc. B 280, 20130548 ( 10.1098/rspb.2013.0548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nagamatsu D, Seiwa K, Sakai A. 2002. Seedling establishment of deciduous trees in various topographic positions. J. Veg. Sci. 13, 35–44. ( 10.1111/j.1654-1103.2002.tb02021.x) [DOI] [Google Scholar]
- 70.Webb CO, Peart DR. 2000. Habitat associations of trees and seedlings in a Bornean rain forest. J. Ecol. 88, 464–478. ( 10.1046/j.1365-2745.2000.00462.x) [DOI] [Google Scholar]
- 71.Palmiotto PA, Davies SJ, Vogt KA, Ashton MS, Vogt DJ, Ashton PS. 2004. Soil-related habitat specialization in dipterocarp rain forest tree species in Borneo. J. Ecol. 92, 609–623. ( 10.1111/j.0022-0477.2004.00894.x) [DOI] [Google Scholar]
- 72.Queenborough SA, Burslem D, Garwood NC, Valencia R. 2007. Habitat niche partitioning by 16 species of Myristicaceae in Amazonian Ecuador. Plant Ecol. 192, 193–207. ( 10.1007/s11258-007-9328-3) [DOI] [Google Scholar]
- 73.Levine JM, HilleRisLambers J. 2009. The importance of niches for the maintenance of species diversity. Nature 461, 254–257. ( 10.1038/nature08251) [DOI] [PubMed] [Google Scholar]
- 74.Chu C, Adler PB. 2015. Large niche differences emerge at the recruitment stage to stabilize grassland coexistence. Ecol. Monogr. 85, 373–392. ( 10.1890/14-1741.1) [DOI] [Google Scholar]
- 75.Zhu Y, Comita LS, Hubbell SP, Ma K. 2015. Conspecific and phylogenetic density-dependent survival differs across life stages in a tropical forest. J. Ecol. 103, 957–966. ( 10.1111/1365-2745.12414) [DOI] [Google Scholar]
- 76.Lebrija-Trejos E, Reich PB, Hernández A, Wright SJ. 2016. Species with greater seed mass are more tolerant of conspecific neighbours: a key driver of early survival and future abundances in a tropical forest. Ecol. Lett. 19, 1071–1080. ( 10.1111/ele.12643) [DOI] [PubMed] [Google Scholar]
- 77.McMahon SM, Metcalf CJE, Woodall CW. 2011. High-dimensional coexistence of temperate tree species: functional traits, demographic rates, life-history stages, and their physical context. PLoS ONE 6, 11 ( 10.1371/journal.pone.0016253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Visser MD, Bruijning M, Wright SJ, Muller-Landau HC, Jongejans E, Comita LS, de Kroon H. 2016. Data from: Functional traits as predictors of vital rates across the life cycle of tropical trees. Dryad Digital Repository ( 10.5061/dryad.fm654) [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Visser MD, Bruijning M, Wright SJ, Muller-Landau HC, Jongejans E, Comita LS, de Kroon H. 2016. Data from: Functional traits as predictors of vital rates across the life cycle of tropical trees. Dryad Digital Repository ( 10.5061/dryad.fm654) [DOI]
Supplementary Materials
Data Availability Statement
Seedling data: http://datadryad.org/resource/doi:10.5061/dryad.fm654 [78]; soil data: http://ctfs.si.edu/webatlas/datasets/bci/soilmaps/BCIsoil.html; climate data: http://biogeodb.stri.si.edu/physical_monitoring/research/barrocolorado; canopy data: https://doi.org/10.5479/data.bci20140711; tree data: https://doi.org/10.5479/data.bci.20130603.