Abstract
AIM
To evaluate the behaviour of two fast-setting polymethylmethacrylate (PMMA) cements CMW® 2G and Palacos® fast R + G, as reference: Standard-setting Palacos® R + G.
METHODS
The fast-setting cements CMW® 2G and Palacos® fast R + G were studied, using standard-setting high viscosity Palacos® R + G as a reference. Eleven units (of two batch numbers) of each cement were tested. All cements were mixed as specified by the manufacturer and analysed on the following parameters: Handling properties (mixing, waiting, working and hardening phase) according to Kuehn, Mechanical properties according to ISO 5833 and DIN 53435, Fatigue strength according to ISO 16402, Benzoyl Peroxide (BPO) - Content by titration, powder/liquid-ratio by weighing, antibiotic elution profile by High Performance Liquid Chromatography. All tests were done in an acclimatised laboratory with temperatures set at 23.5 °C ± 0.5 °C and a humidity of > 40%.
RESULTS
Palacos® fast R + G showed slightly shorter handling properties (doughing, hardening phase, n = 12) than CMW® 2G, allowing to reduce operative time and to optimise cemented cup implantation. Data of the quasistatic properties of ISO 5833 and DIN 53435 of both cements tested was comparable. The ISO compressive strength (MPa) of Palacos® fast R + G was significantly higher than CMW® 2G, resulting in ANOVA (P < 0.01) and two sample t-test (P < 0.01) at 0.05 level of significance (n = 20). Palacos® fast R + G showed a higher fatigue strength of about 18% mean (ISO 16402) of 15.3 MPa instead of 13.0 MPa for CMW® 2G (n = 5 × 106 cycles). Palacos® fast R + G and CMW® 2G differed only by 0.11% (n = 6) with the former having the higher content. The BPO-content of both cements were therefore comparable. CMW® 2G had a powder/liquid ratio of 2:1, Palacos® fast R + G of 2.550:1 due to a higher powder content. Despite its higher gentamicin content, CMW® 2G showed a significantly lower antibiotic elution over time than Palacos® fast R + G (n = 3).
CONCLUSION
Both cements are compliant with international standards and are highly suitable for their specified surgical indications, affording a time-saving measure without detriment to the mechanical properties.
Keywords: Polymethylmethacrylate, Elution, Fast-setting, Viscosity, Antibiotic, Fatigue, Arthroplasty
Core tip: Polymethylmethacrylate (PMMA) cements provide reliable fixation of the implants in joint arthroplasty. Fast-setting high viscosity PMMA cements exist that have altered setting characteristics compared to standard setting cements. These potentially offer benefits to surgeons based upon their handling properties. Such cements have gained popularity in arthroplasty surgery as described in the United Kingdom and Australian National Joint Registries. The use of fast-setting cements has various beneficial as well as economic effects, such as time-saving and better antibiotic elution.
INTRODUCTION
Polymethylmethacrylate (PMMA) cements provide reliable fixation of the implants in joint arthroplasty[1,2]. The chemical composition of each bone cement accounts for its mechanical and handling properties. The polymerization reaction of PMMA is divided in to four phases: Mixing, waiting, working and setting. Bone cements are classified based upon the amount of time they spend in each of these phases. The most popular cements are high viscosity varieties which have the best results in registry figures but also offer the surgeon short waiting and extra-long working phases[3]. However, high viscosity cements can take up to 13 min to set[4].
Fast-setting high viscosity cements exist that have altered setting characteristics compared to standard setting cements. These potentially offer benefits to surgeons based upon their handling properties. Such cements have gained popularity in arthroplasty surgery as described in the United Kingdom and Australian National Joint Registries[2,5].
Fast-setting high viscosity cements are characterised by a short mixing, moderate working and very short hardening phase in comparison to standard-setting high viscosity PMMA cements[6,7]. They potentially offer benefits to both surgeon and patient based on their handling properties. These benefits might include: (1) Reduced operative time. Fast setting cements are homogenised quickly and demonstrate very short mixing and waiting phases, allowing them to be applied rapidly[8,9]. Usually, fast setting cements are setting at the same time that a standard high viscosity cement is reaching the end of their working properties. By reducing the operative time, they may offer an economic advantage[10-12]. Of more importance, as longer operative time is correlated with increased risk of prosthetic joint infection, they may also offer an advantage in reducing infection[13,14]; (2) cemented cup implantation. The short waiting phase of fast setting cements might have the advantage of minimising the risk of bottoming out of the socket during insertion[15]. A high viscosity, fast setting cement flows away less readily during pressurisation and thus, once the correct cup position is achieved, it can be kept under pressure during the working phase with less progressive movement[16]; (3) cemented knee arthroplasty. Some authors have advocated the use of a sequential mixing technique to ensure even cement mantles in total knee arthroplasty[6]. Fast setting cements are central to this technique in order to avoid excessive operative time. In addition, others state that high viscosity fast setting cements tend to penetrate the cancellous bone less deeply. This reduces the peak temperature at the interface and facilitates cement removal at revision arthroplasty[17]; (4) cement spacer and bead production: The handling properties of fast setting cements allows manipulation of the dough more rapidly, facilitating the production of hand-made spacers and beads[18]; and (5) augmentation surgery. The short waiting phase of fast setting high viscosity cements leads to the dough sticking less to surgeon’s gloves. These favourable handling properties will be of benefit when filling bone defects or augmenting the fixation of screws in osteoporotic bone[19].
There is extensive published data on the mechanical properties of standard-setting high viscosity cements. However, the varied possible clinical applications, described above, for the use of fast-setting cements demands a similar detailed knowledge of their properties. There is therefore a need to describe the clinically relevant cement properties of these newer cements including, handling behaviour, antibiotic elution, quasistatic and dynamic mechanical properties.
The aim of this research is to study the behaviour of two fast-setting cements and compare these against the “gold-standard” clinically proven standard-setting high viscosity cement, Palacos® R + G. The design was an in vitro non-interventional, experimental and prospective comparative study.
MATERIALS AND METHODS
Materials
The fast-setting cements CMW® 2G and Palacos® fast R + G were studied, using standard-setting high viscosity Palacos® R + G as a reference[20-22]. Eleven units (of two batch numbers) of each cement were tested (Table 1).
Table 1.
Testing material
| Product | Powder (g) | Liquid (mL) | Batch number | Viscosity |
| DePuy CMW® 2G | 40 | 20 | #3620322 | Very high-viscosity |
| #3572255 | Very high-viscosity | |||
| Palacos® fast R + G | 51 | 20 | #7743 | Very high-viscosity |
| FZ52#050214 | Very high-viscosity | |||
| Reference: Palacos® R + G | 40.8 | 20 | #7735 | Standard high viscosity |
| #7753 | Standard high viscosity |
Methods
All cements were mixed manually as specified by the manufacturer, in porcelain crucibles with metal spatulas by our team, consisting of experienced laboratory technicians of Heraeus Holding GmbH and me, after intense training for several weeks with different cements.
All cements were analysed on the following parameters: (1) Handling properties (mixing, waiting, working and hardening phase) according to Kuehn[7]; (2) Mechanical properties according to ISO 5833[23] and DIN 53435[24]; (3) Fatigue strength according to ISO 16402[25]; (4) Benzoyl Peroxide (BPO) - content by titration, powder/liquid-ratio by weighing; and (5) Antibiotic elution profile by High Performance Liquid Chromatography (HPLC)[26] (Table 2). All tests were done in an acclimatised laboratory with temperatures set at 23.5 °C ± 0.5 °C and a humidity of > 40%.
Table 2.
Test parameters
| Parameter | Characteristics | More details | Sample size | Scale of measure - Ratio scale | Descript. + analyt. statistics |
| Handling properties in vitro | Mixing, doughing and waiting phase | Elapsed time | 12 | Duration | Median, quartile, boxplot |
| Working phase | Elapsed time | 12 | Duration | ||
| Hardening phase | Elapsed time | 12 | Duration | ||
| Quasistatic mechanical properties | ISO bending strength | In MPa | 12 | Metric | Median, quartile, boxplot, arithmetic mean, ANOVA, independent two-sample t-test |
| ISO flexural modulus | In MPa | 12 | Metric | ||
| ISO compressive strength | In MPa | 20-24 | Metric | ||
| Dynstat notched impact strength | In kJ/m2 | 16 | Metric | ||
| Dynstat bending strength | In MPa | 16 | Metric | ||
| Dynamic mechanical properties | In MPa | 5 | Metric | Chart, bar graph and standard deviation | |
| BPO-content | In % | 6 | Metric | Arithmetic mean, standard deviation, bar graph | |
| Powder/liquid-ratio | g:mL | Ratio | Bar graph | ||
| Elution profile | Gentamicin release per mould body | In µg/FK | 3 | Metric | Table, S/N-curve |
Fatigue testing: Fatigue was tested according to ISO 16402[25]. The run follows the four-point bending test method described in ISO 5833[24]. The dynamic testing is executed with a pulsating sinusoidal loading under force control. The tests are continued until failure occurs or the specimen reaches a predetermined maximum number of cycles (n = 5 × 106). The specimens had the dimensions 3.3 mm × 10 mm × 70 mm[27].
Gentamicin elution: Cylindrical cement specimens (d = 25 mm, h = 10 mm) with a surface of approximately 3.1 cm2 were used. For the dissolution the PMMA cement samples were stored at 37 °C in dissolution medium (0.1 M phosphate buffer, pH 7.4). Aliquots were taken and the dissolution medium was renewed at day 1, 3, 7, 14, and 21. An appropriate amount of dissolution medium was added to ensure that the samples were completely covered. The dissolution medium samples were stored at -20 °C until analysis. Sample preparation and the determination of concentrations were done at AZB (Analytisches Zentrum Biopharm GmbH Berlin).
Sample preparation: For the preparation of calibration standards 7.646 mg gentamicin sulphate (equivalent to 5.0 mg gentamicin) were dissolved in 25 mL water to achieve a 200 μg/mL gentamicin stock solution. Working solutions were prepared at 100, 250, 500, 750, 1000, 2500, 5000 and 7500 ng/mL by serial dilutions with water. Tobramycin was used as an internal standard. The internal standard working solution was prepared at a concentration of 25 μg/mL in water.
Up to eight calibration standards from 100-7500 ng/mL were prepared by spiking 200 μL of gentamicin working solutions with 18 μL internal standard working solution. The study samples were diluted by a factor of 20 with water and prepared according to the calibration standards by adding internal standard working solution[26].
Determination of concentrations: Concerning the liquid chromatography (LC) mass spectroscopy (MS) conditions, chromatographic separation was performed on a modular HPLC 1200 Series (Agilent Technologies, Waldbronn, Germany) using a Luna C18 (II) column, 150 mm × 2 mm, with two C18, 4 mm × 2 mm, guard columns (Phenomenex, Aschaffenburg, Germany) thermostated at 25 °C (gentamicin), respectively.
For gentamicin the mobile phase A was 0.11 mol/L trifluoroacetic acid/methanol (50:50) and mobile phase B was acetonitrile. An isocratic separation was achieved with an A:B ratio of 95:5 at a flow rate of 0.25 mL/min. The run time was 2.5 min and the total cycle time was less than 3 min. Injection volume was 2 µL. Under the described conditions the four gentamicin components C1, C2, C2a and C1a co-eluted. The HPLC method was previously used by Heller et al[26] to determine gentamicin in biopsy samples. The detection of the co-eluted gentamicin components was carried out using an API 4000 QTrap (Applied Biosystems, Darmstadt, Germany). Ionisation was carried out with an electrospray interface (positive polarity) using the mass selective detector in the multiple reaction monitoring mode (MRM). The extracted ion chromatograms of the following ion transitions were stored and calculated: 478.4 → 322.3 m/z (gentamicin C1), 464.4 → 322.3 m/z (gentamicin C2 and C2a), 450.3 → 322.3 m/z (gentamicin C1a.) and 468.4 → 163.1 m/z (internal standard). The three ion transitions of gentamicin components were summed with Analyst (Applied Biosystems, Darmstadt, Germany) and concentrations were calculated with Excel (Microsoft, Unterschleißheim, Germany).
Statistical analysis
The differences of the middle level (= mean) were analyzed by univariate ANOVA (analysis of variance) with repeated measures for more than two paired samples compared with post-hoc Tukey test. The method P values, adjusted according to the Tukey-method were compared with the significance level α = 0.05, and a comparison was considered statistically significant if P < α. In addition, the average group differences and the associated 95%CIs were estimated from this model.
The metric ISO variable mechanism and handling properties were tested descriptively by median, quartile, box plot and span and presented summarized in tabular form for all ISO and DIN mechanical results and as a bar chart for all tested handling results.
By calculating the mean values including the standard deviation and a bar chart, the metric parameters of the (di) benzoyl peroxide content (BPO-content) was descriptively displayed three times for each batch of fast-setting cements. The ratio of polymer powder to monomer liquid was presented with metric parameters as a bar graph. The metric data for all antibiotic elution data were tabulated after the 1st, 3rd and 5th day and presented with its standard deviation as a curve diagram.
Metric data of all fatigue strength data were shown as follows: The quasi-static ISO flexible strength in MPa were indicated by mean and its standard deviation, the fatigue strength in MPa at 5 × 106 cycles and the consequent percentage share of the quasi-static bending strength as a table. It was prepared as SN curve (= S/N curve) in a 95%CI[27].
RESULTS
Handling properties under in vitro condition
For CMW® 2G the polymer powder is filled into the vessel before the monomer liquid. This is contrary to the Palacos® fast R + G and Palacos® R + G process, which both require the filling of the monomer liquid first followed by the polymer powder. The latter technique seems to allow better initial mixing of the liquid and powder moieties. The handling properties of both tested fast setting cements are similar (n = 12), but the differences are as follows. CMW® 2G reached the end of the doughing phase according to ISO 5833 at 50 s, approximately 20 s later than Palacos® fast R + G (Figure 1). Palacos® fast R + G was workable at
Figure 1.
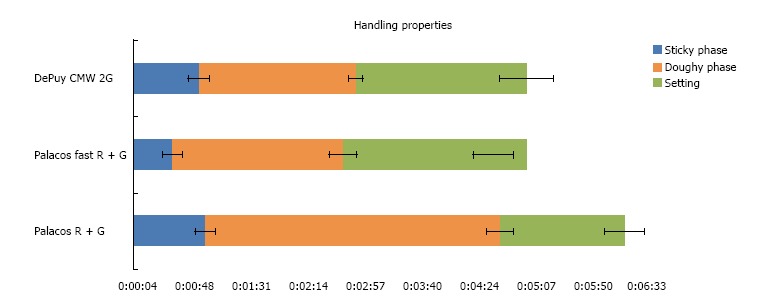
Graphical presentation of handling properties (sticky phase, doughy phase and setting) of tested fast setting cements and the reference material. Data is presented as mean ± SD.
35 s, immediately after mixing because the dough was no longer sticky and an additional waiting phase is not necessary. CMW® 2G showed a small waiting phase (when still sticky to touch) of approximately 15-20 s after the end of mixing and thus could not be applied until approximately 60 s. Both fast setting cements tested had a similar end of working phase at 3 min after start of mixing. Palacos® fast R + G showed a slightly shorter setting time than CMW® 2G.
Quasistatic mechanical properties
Both fast setting cements tested fulfilled the quasistatic properties of ISO 5833 and DIN 53435[23,25]. Data of ISO bending strength (MPa) of both cements were similar with no statistical difference due to the one-way analysis of variance (ANOVA) (P = 0.06) and independent two sample t-test (P = 0.058) at 0.05 level of significance (n = 12). ISO flexural modulus (MPa) of both cements was also similar, resulting in: ANOVA (P = 0.869) and independent two sample t-test (P = 0.868) (for both α = 0.05, n = 12). The ISO compressive strength (MPa) of Palacos® fast R + G was significantly higher than CMW® 2G, ANOVA (P < 0.01) and t-test (P < 0.01) at 0.05 level of significance (n = 20). Dynstat bending strength (MPa) was comparable [ANOVA (P = 0.15) and t-test (P = 0.15) (n = 16)], as was the Dynstat notched impact strength (kJ/m2) [ANOVA (P = 0.196) and t-test (P = 0.200) (n = 16)] of both fast-setting cements (Tables 3 and 4).
Table 3.
ISO mechanical properties of all tested cements
|
ISO 5833:2002 |
||||||
| Bending strength (MPa) | Flexural modulus (MPa) | Compressive strength (MPa) | ||||
| Limit | > 50 | > 1800 | > 70 | |||
| DePuy CMW® 2G | 75.39 | 67.3 (-8.09) | 3002.75 | 2775 (-227.75) | 91.53 | 80.94 (-10.59) |
| 81.8 (+6.41) | 3159 (+156.25) | 99.11 (+7.58) | ||||
| Palacos® R + G | 65.79 | 61.7 (-4.09) | 2552 | 2383 (-169) | 87.46 | 81.92 (-5.54) |
| 68.7 (+2.91) | 2659 (+107) | 93.13 (+5.67) | ||||
| Palacos® fast R + G | 72.17 | 69.3 (-2.87) | 2995.18 | 2784 (-211.18) | 106.25 | 100.26 (-5.99) |
| 76.2 (+4.03) | 3118 (+122.82) | 110.51 (+4.26) | ||||
Table 4.
DIN mechanical properties of all tested cements
|
DIN 53435 |
||||
| Bending strength (MPa) | Notched impact strength (kJ/m2) | |||
| Limit | ||||
| DePuy CMW® 2G | 73.1 | 63.88 (-9.22) | 2.94 | 2.01 (-0.93) |
| 82.81 (+9.71) | 3.98 (+1.04) | |||
| Palacos® R + G | 71.21 | 65.06 (-7.15) | 3.2 | 2.39 (-0.81) |
| 79.09 (+7.85) | 4.50 (+1.3) | |||
| Palacos® fast R + G | 76.35 | 65.91 (-10.44) | 3.19 | 2.39 (-0.8) |
| 88.3 (+11.95) | 3.98 (+0.79) | |||
Dynamic mechanical strength (fatigue)
CMW® 2G had a higher initial quasistatic ISO bending strength (62.3 ± 7.2 MPa) for fatigue according to ISO 16402 than Palacos® fast R + G (55.3 ± 1.1 MPa). Subsequently Palacos® fast R + G showed a higher fatigue strength of about 18% mean (ISO 16402) of 15.3 MPa instead of 13.0 MPa for CMW® 2G (n = 5 × 106 cycles). All dynamic mechanical testing results showed no statistical significance (Figure 2).
Figure 2.
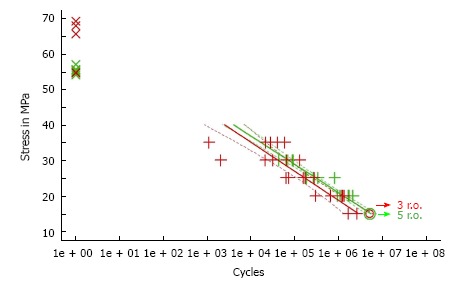
Fatigue strength (n = 5 × 106 cycles) of both tested fast setting cements, CMW® 2G (in red) and Palacos® fast R + G (in green). Data is presented in a S/N curve. Lines: Result of the linear regression and the “narrow” confidence band at the 95% level; x: Quasi-static values; +: Failed under fatigue load; o: Run-outs, regression with + and o.
BPO-content
Palacos® fast R + G and CMW® 2G differed only by 0.11% with the former having the higher content (Figure 3, mean values of all batches tested). The BPO-content of both cements were therefore comparable.
Figure 3.
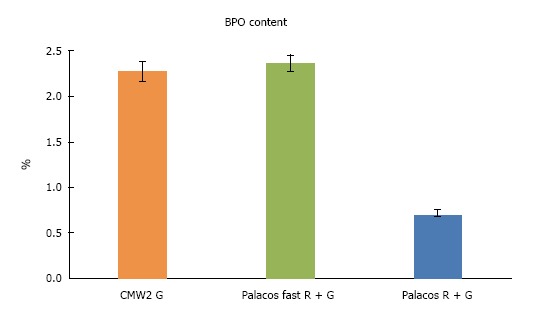
Graphical presentation of Benzoyl Peroxide-content of all tested cements. Data is presented as mean ± SD. BPO: Benzoyl peroxide.
Powder/liquid-ratio
CMW® 2G had a powder/liquid ratio of 2:1, Palacos® fast R + G of 2.550:1 due to a higher powder content (Figure 4).
Figure 4.
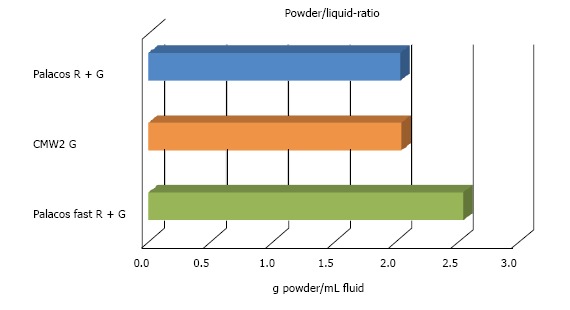
Graphical presentation of powder/liquid ratio of all tested cements. Data is presented as absolute.
Elution profile
CMW® 2G contains 2.5% gentamicin sulphate in the powder, Palacos® fast R + G only 1.25% gentamicin. Both cements showed a typical biphasic antibiotic elution profile with high initial release of gentamicin within the first 24 h. Despite its higher gentamicin content, CMW® 2G however released only approximately half the amount gentamicin as compared to Palacos® fast R + G after the first 24 h. Further CMW® 2G showed a much lower antibiotic elution over time than Palacos® fast R + G. Additionally, after day 3, CMW® 2G had a significantly lower gentamicin release than Palacos® fast R + G (Figure 5).
Figure 5.
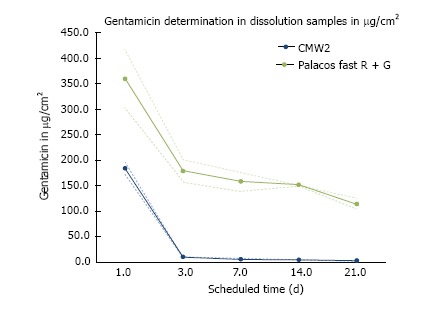
Graphical presentation of elution profile of CMW® 2G (in blue) and Palacos® fast R + G (in green) (specimen surface 3.1 cm2). Data is presented as mean ± SD.
DISCUSSION
Fast-setting high viscosity cements have gained popularity in clinical applications owing to their advantageous handling properties and possible associated cost saving potential. Economically the rapid use immediately after mixing and the quick setting is of importance. In the United Kingdom and Australia such fast-setting cements are now widely used[2,5].
Palacos® fast R + G and CMW® 2G were characterised by different mechanical and handling properties when compared to standard-setting high viscosity PMMA cements. The key question, is whether such fast-setting behaviour lends itself to use in all cement clinical applications for bone cement? The relatively short mixing, moderate working and setting phases when compared to standard-setting high viscosity may be less favourable in some scenarios such as femoral stem insertion when the downside of incomplete insertion would be detrimental to the outcome of the surgical procedure. None the less the fact that these cements will have set whilst standard versions are still in their working phase does offer some potential benefits to the surgeon, as detailed in the introduction.
The altered handling characteristics of CMW® 2G are the clinically corollary of the special PMMA bead formulation together with an increased content of BPO[28]. In contrast, Palacos® fast R + G utilises an increased powder-liquid (polymer to monomer) ratio in combination with an increased BPO content to achieve the different handling properties.
Both tested fast setting cements are characterised by a short doughy/waiting phase with Palacos® fast R + G being ready to handle immediately after mixing. During THA these properties might be advantageous such as allowing better component position control during cup insertion with less extrusion and movement of the cement. The viscosity within the working or application phase is ideal for good cement penetration into the cancellous bone. Surgical technique must be altered to allow the use of fast-setting cements because the viscosity increases quickly and results in an earlier setting.
With regards to the handling properties of the two cements tested, Palacos® fast R + G may offer some benefits to surgeon over CMW® 2G. The latter is workable from about 60 s until 4 min after the start of mixing. The material sets at approx 5 min[20]. The viscosity at the end of the working phase is high and the cement becomes warmer quickly. Palacos® fast R + G is workable immediately after mixing at 35-40 s until 4 min after the start of mixing. This results in a slightly longer working phase at 23 °C. It is expected that these differences in the handling behaviour of the tested fast setting cements will be significantly higher at lower ambient temperatures.
Fast-setting cements can be used immediately after mixing during TKA in a sequential cementing technique. Before touching the cement users should be aware that CMW® 2G is sticky for slightly longer than Palacos® fast R + G. High viscosity fast-setting cements do not penetrate the cancellous bone as deeply as their standard-setting versions. The higher the monomer (liquid) content in a PMMA cement, the higher the temperature reached during setting[29]. The higher powder-liquid ratio of Palacos® fast R + G results in a lower peak temperature in comparison to CMW® 2G. This might protect against thermal damage of the bone with use of the Palacos® fast R + G cement.
To our knowledge, only two fast-setting high viscosity cements are marketed today. Another cement is marketed as a fast setting cement - Simplex®P SpeedSet® (Stryker®). This is not a high viscosity fast-setting cement as studied in this paper, but rather represents a slightly faster-setting version of their medium viscosity Simplex P[30].
The altered setting behavior of Simplex®P Speed Set® is achieved by using smaller copolymer particles which leads to an increased surface area of the powder beads. Such a change of the polymeric composition produces a more viscous material. Further, a higher BPO content is used to speed up the polymerisation reaction, leading to a faster setting time in comparison with the original Simplex®P cement[31]. The handling properties of Simplex®P Speed
Set® are markedly different from those of standard viscosity cements, such as Palacos® R + G. Simplex®P SpeedSet® is characterised by a doughing time of 2.53 min, a working time of approximately 4.8 min and a setting time of 8.2 min according to FDA510 (k) K 053198[28]. The reference, the standard viscous Palacos® R + G showed a working time of approximately 4 min, a doughing of approximately 55 s and a setting of 6 to 7 min. Due to this, Simplex®P SpeedSet® is not a high viscosity fast-setting cement.
The clinical success of cemented arthroplasty relies upon the strength of the interfaces between the bone, the cement and the implants. Registry figures have confirmed the efficacy of combining implants and cements with optimal mechanical properties and design (NJR 2015). When assessing a new type of cement one must ensure that they will be able to withstand the varying loads they will encounter in vivo. The minimum requirements for mechanical properties on an acrylic cement to be used in human applications are described in ISO 5833 and DIN 53435[23,24]. Both the fast-setting cements we tested in this study fulfilled these requirements. In addition, it was noted that Palacos® fast R + G had a statistically significant higher ISO compressive strength (MPa) than CMW® 2G. Nevertheless, quasistatic tests on PMMA cements usually conveys similar strength values between brands.
Dynamic mechanical testing of cements probably offers more clinically relevant information relevant to the long-term survival of cemented implants. Such properties include visco-elastic properties and fatigue testing[9]. Fatigue behaviour of PMMA cement was tested in this study because it is more sensitive to acrylic variation and can frequently distinguish between material differences in composition or preparation[32]. Palacos® fast R + G showed higher fatigue strength according to ISO 16402. This may depend upon the different sterilisation techniques used in the preparation of the two cements tested explains these differences. CMW® 2G powder is sterilised by gamma irradiation, whereas Palacos® cements are sterilised by Ethylene oxide (ETO). Irradiation has been shown by other authors to reduce the molecular weight of the polymer beads by half, which results in lower fatigue strengths of the resultant cement[33,34].
In revision surgery for prosthetic joint infection, local antibiotic delivery is a proven component of treatment in the form of hand-made antibiotic-loaded cement spacers and beads[35,36]. Fast-setting cements may be preferable for this indication if they appropriate handling and elution characteristics? Firstly, the highly viscous dough of fast setting cements after mixing allows an easy manual application without the cement sticking to the gloves. Secondly, both cements tested showed standard bi-phasic elution of the antibiotic in vitro. Palacos® fast R + G showed superior gentamicin release at all stages, despite a lower antibiotic content in comparison to CMW® 2G. Palacos® fast R + G contains the same hydrophilic co-polymers that are present in Palacos® R + G. It is these co-polymers in combination with the special polymer/monomer ratios of Palacos® cements that accounts for the increased antibiotic release compared with other cement brands[37]. This phenomenon has been described by numerous authors for the standard-setting Palacos® R + G and it would appear to hold true for the fast-setting version as well[7,38].
The use of eligible, fast-setting high viscosity PMMA cements are already described in national joint registries for both knee and hip arthroplasty[5,6]. Palacos® fast R + G and CMW® 2G are both highly suitable for their specified surgical indications as they afford a time-saving measure without detriment to the mechanical properties. Both are compliant with international standards and we have described in this study their relative handling and mechanical properties in order to inform surgeons so that they might apply them to their practice.
Due to Palacos® fast R + G’s shorter doughy/waiting phase compared to CMW® 2G, it is ready to apply immediately after mixing. During surgeries Palacos® fast R + G allows better component position control during cup insertion with less extrusion and movement of the cement compared to CMW® 2G.
A higher powder-liquid ratio of Palacos® fast R + G results in a lower peak temperature compared to CMW® 2G, which might protect against thermal damage of the bone with use of the Palacos® fast R + G cement.
Palacos® fast R + G had a statistically significant higher ISO compressive strength (MPa) than CMW® 2G, it also showed higher fatigue strength according to ISO 16402, likely due to different sterilisation techniques. Palacos® fast R + G also showed a much higher gentamicin release profile at all stages, despite a lower antibiotic content compared to CMW® 2G.
COMMENTS
Background
Polymethylmethacrylate (PMMA) cements provide reliable fixation of the implants in joint arthroplasty. Fast-setting high viscosity cements exist that have altered setting characteristics compared to standard setting cements.
Research frontiers
PMMA is widely used for implant fixation in orthopaedic and trauma surgery. Bone cements also act as space-filler and elastic buffer, distributing forces evenly between prosthesis and bone.
Innovations and breakthroughs
Fast-setting PMMA cements offer benefits to both surgeon and patient based on their handling properties. These benefits might include reduced operative time and therefore economic advantage and decreasing risk of infection, also due to better antibiotic release because of special polymer/monomer ratio.
Application
Fast-setting PMMA bone cements have gained popularity in knee arthroplasty and hip replacement surgery as described in the United Kingdom and Australian National Joint Registries over the last years.
Terminology
The chemical composition of PMMA cements accounts for its mechanical and handling properties. The polymerization reaction of PMMA is divided in to four phases: Mixing, waiting, working and setting. Bone cements are classified based upon the amount of time they spend in each of these phases. Fast-setting cements are characterised by a short mixing, moderate working and very short hardening phase.
Peer-review
The study is very well executed and presented.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Analytisches Zentrum Berlin (AZB), Germany for their assistance with the elution tests and the Fraunhofer Institut Freiburg, Germany for their assistance with the fatigue testing. Neil Ayron Caraan is a medical student at the Medical University of Vienna and the tests were carried out as part of his thesis. The thesis was supervised by Windhager R, Kuehn KD and Zentgraf N. The material was provided by Heraeus Medical GmbH. This article contains no studies on humans or animals.
Footnotes
Institutional review board statement: This manuscript does not involve human or human subjects.
Institutional animal care and use committee statement: This manuscript does not involve animal or animal subjects.
Conflict-of-interest statement: The authors have no conflicts of interest to disclose.
Data sharing statement: Technical appendix, statistical code and dataset available from the corresponding author at neil.caraan@gmx.at.
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country of origin: Austria
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: December 30, 2016
First decision: March 27, 2017
Article in press: November 8, 2017
P- Reviewer: Cui Q, Drampalos E, Drosos GI, Fenichel I, Malik H, Nikolaou VS S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
Contributor Information
Neil Ayron Caraan, Department of Orthopaedics and Orthopaedic Surgery, Medical University of Vienna, Vienna 1090, Austria.
Reinhard Windhager, Department of Orthopaedics and Orthopaedic Surgery, Medical University of Vienna, Vienna 1090, Austria.
Jason Webb, Avon Orthopaedic Centre, North Bristol NHS Trust, Southmead Hospital, Bristol BS10 5NB, United Kingdom.
Nadine Zentgraf, Heraeus Medical GmbH, Wehrheim 61273, Germany; Medical University of Vienna, Vienna 1090, Austria.
Klaus-Dieter Kuehn, Heraeus Medical GmbH, Wehrheim 61273, Germany; Medical University of Vienna, Vienna 1090, Austria.
References
- 1.Charnley J. Acrylic cement in orthopaedic surgery. Edinburgh and London: E S Livingstone; 1970. [Google Scholar]
- 2.NJR. National Joint Registry for England, Wales and Northern Ireland: 12th Annual Report - Prostheses used in hip, knee, ankle, elbow and shoulder replacement procedures 2014. Available form: URL: http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/12th%20annual%20report/NJR%20Online%20Annual%20Report%202015.pdf. [Google Scholar]
- 3.Malchau H, Herberts P. Prognosis of Total Hip Replacement. Revision and re-revision rate in THR. A revision-risk study of 148,359 primary operations. USA: Proceedings of AAOS; 1998. [Google Scholar]
- 4.Kuehn KD, Ege W, Gopp U. Acrylic bone cements: composition and properties. Orthop Clin North Am. 2005;36:17–28, v. doi: 10.1016/j.ocl.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Australian Orthopaedic Association NJRR. National Joint Replacement Registry: Supplementary Report 2015 - Cement in Hip Knee Arthroplasty, 2015. Available from: https://aoanjrr.sahmri.com/documents/10180/217745/Hip%20and%20Knee%20Arthroplasty.
- 6.Wroblewski BM, Siney PD, Fleming PA. Triple taper polished cemented stem in total hip arthroplasty: rationale for the design, surgical technique, and 7 years of clinical experience. J Arthroplasty. 2001;16:37–41. doi: 10.1054/arth.2001.28374. [DOI] [PubMed] [Google Scholar]
- 7.Kühn KD. Properties of PMMA Cement Dough. In: Kühn KD, editor. PMMA cements. Berlin Heidelberg: Springer-Verlag; 2013. pp. 93–112. [Google Scholar]
- 8.Breusch SJ, Kühn KD. [Bone cements based on polymethylmethacrylate] Orthopade. 2003;32:41–50. doi: 10.1007/s00132-002-0411-0. [DOI] [PubMed] [Google Scholar]
- 9.Webb JC, Spencer RF. The role of polymethylmethacrylate bone cement in modern orthopaedic surgery. J Bone Joint Surg Br. 2007;89:851–857. doi: 10.1302/0301-620X.89B7.19148. [DOI] [PubMed] [Google Scholar]
- 10.Yates P, Serjeant S, Rushforth G, Middleton R. The relative cost of cemented and uncemented total hip arthroplasties. J Arthroplasty. 2006;21:102–105. doi: 10.1016/j.arth.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Griffiths EJ, Stevenson D, Porteous MJ. Cost savings of using a cemented total hip replacement: an analysis of the National Joint Registry data. J Bone Joint Surg Br. 2012;94:1032–1035. doi: 10.1302/0301-620X.94B8.28717. [DOI] [PubMed] [Google Scholar]
- 12.Kallala R, Anderson P, Morris S, Haddad FS. The cost analysis of cemented versus cementless total hip replacement operations on the NHS. Bone Joint J. 2013;95-B:874–876. doi: 10.1302/0301-620X.95B7.26931. [DOI] [PubMed] [Google Scholar]
- 13.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710–1715. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedersen AB, Svendsson JE, Johnsen SP, Riis A, Overgaard S. Risk factors for revision due to infection after primary total hip arthroplasty. A population-based study of 80,756 primary procedures in the Danish Hip Arthroplasty Registry. Acta Orthop. 2010;81:542–547. doi: 10.3109/17453674.2010.519908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsch D, Breusch SJ. Flanged or unflanged sockets? In: Breusch SJ, Malchau H, editors. The Well-Cemented Total Hip Arthroplasty. Berlin Heidelberg: Springer Verlag; 2005. pp. 206–208. [Google Scholar]
- 16.Kuehn KD. What is bone cement? In: Breusch SJ, Malchau H, editors. The Well-Cemented Total Hip Arthroplasty. Berlin Heidelberg: Springer Verlag; 2005. pp. 52–59. [Google Scholar]
- 17.Janssen D, Srinivasan P, Scheerlinck T, Verdonschot N. Effect of cementing technique and cement type on thermal necrosis in hip resurfacing arthroplasty--a numerical study. J Orthop Res. 2012;30:364–370. doi: 10.1002/jor.21512. [DOI] [PubMed] [Google Scholar]
- 18.Villanueva-Martínez M, Ríos-Luna A, Chana-Rodriguez F, De Pedro JA, Pérez-Caballer A. Articulating Spacers in Infection of Total Knee Arthroplasty - State of the Art. In: Kinov P, editor. Arthroplasty - Update. Rijeka: InTech; 2013. pp. 555–576. [Google Scholar]
- 19.Kühn KD, Höntzsch D. [Augmentation with PMMA cement] Unfallchirurg. 2015;118:737–748. doi: 10.1007/s00113-015-0059-y. [DOI] [PubMed] [Google Scholar]
- 20.DePuy Johnson and Johnson company. “DePuy CMW Orthopaedic Gentamicin Bone Cements”, instruction for use. USA: DePuy Johnson and Johnson company; 2008. [Google Scholar]
- 21.Heraeus-Medical GmbH. Palacos fast R + G, instruction for use, version 06605. Germany: Heraeus-Medical GmbH; 2015. [Google Scholar]
- 22.Heraeus-Medical GmbH. Palacos R+G, instruction for use, version 06527, 2015. Available from: https://www.heraeus.com/media/media/hme/doc_hme/products_hme/palacos_bone_cement/r_rg_mv_mvg_lv_lvg/ifu/PALACOS_RG_IFU.pdf.
- 23.ISO. Internationals Standards, ISO 5833:2002 - Implants for surgery - Acrylic resin cements. Orthopaedic Application. Available from: http://www.iso.org/iso/catalogue_detail.htm?csnumber=30980.
- 24.DIN 53435. Testing of plastics; bending test and impact test on dynstat test pieces. Beuth Verlag. 1983. Available from: https://www.beuth.de/de/norm/din-53435/1046244. [Google Scholar]
- 25.ISO International Organization for Standardization. ISO 16402:2008 - Implants for surgery - Acrylic resin cement - Flexural fatigue testing of acrylic resin cements used in orthopaedics 2008. Available from: http://www.iso.org/iso/catalogue_detail.htm?csnumber=32212.
- 26.Heller DN, Peggins JO, Nochetto CB, Smith ML, Chiesa OA, Moulton K. LC/MS/MS measurement of gentamicin in bovine plasma, urine, milk, and biopsy samples taken from kidneys of standing animals. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;821:22–30. doi: 10.1016/j.jchromb.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Soltész U, Schäfer R, Jaeger R, Gopp U, Kühn KD. Fatigue testing of bone cements - comparison of testing arrangements. J Astm Int. 2005;2:1–11. [Google Scholar]
- 28.Smith DC. The genesis and evolution of acrylic bone cement. Orthop Clin North Am. 2005;36:1–10. doi: 10.1016/j.ocl.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Lai PL, Chen LH, Chen WJ, Chu IM. Chemical and physical properties of bone cement for vertebroplasty. Biomed J. 2013;36:162–167. doi: 10.4103/2319-4170.112750. [DOI] [PubMed] [Google Scholar]
- 30.Howmedica Osteonics Corp. Simplex P Speedset Bone Cement. FDA 510(k) K 053198 (2006) Available from: https:// www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm? ID=K053198.
- 31.Popham GJ, Mangino P, Seligson D, Henry SL. Antibiotic-impregnated beads. Part II: Factors in antibiotic selection. Orthop Rev. 1991;20:331–337. [PubMed] [Google Scholar]
- 32.Callister WD. Fatigue. In: Callister WD, editor. Materials Science and Engineering: An Introduction. 7th ed. New York: John Wiley Sons, Inc; 2007. pp. 227–238. Available from: http://user.ceng.metu.edu.tr/~e1630912/Callister%20-%20 Materials% 20Science% 20and%20Engineering%20-%20An%20 Introduction%207e%20(Wiley,%202007).pdf. [Google Scholar]
- 33.Tepic S, Soltesz U. Influence of gamma sterilization on the fatigue strength of bone cement. In: Trans 42nd Ann Mtg, OrthopRes Soc, Atlanta, GA;; 1996. p. 445. Available from: https://books.google.at/books/about/Transactions_of_the_42nd_Annual_Meeting.html?id=rh--twAACAAJredir_esc=y. [Google Scholar]
- 34.Lewis G, Mladsi S. Effect of sterilization method on properties of Palacos R acrylic bone cement. Biomaterials. 1998;19:117–124. doi: 10.1016/s0142-9612(97)00165-8. [DOI] [PubMed] [Google Scholar]
- 35.Witos E, Engesaeter LB. Revision of Infected Total Hip Prostheses in Norway and Sweden. In: Walenkamp GHIM, editor. Local Antibiotics in Arthroplasty. Stuttgart, Germany: Thieme Verlag; 2007. pp. 145–146. Available from: https://books.google.at/books?id=OG51dGWOPogCpg=PR4lpg=PR4dq=Walenkamp+GHIM.+Local+Antibiotics+in+Arthroplasty.+Stuttgart,+Germany:+Thieme+Verlag,+2007source=blots=yPY9IJ0cCMsig=TFI7lmPwMfI0NnA85zc3gxtGiO4hl=desa=Xved=0ahUKEwjnoNu-1fzVAhXLcRQKHfwfDWgQ6AEIMDAA#v=onepageqf=false. [Google Scholar]
- 36.Arora M, Chan EK, Gupta S, Diwan AD. Polymethylmethacrylate bone cements and additives: A review of the literature. World J Orthop. 2013;4:67–74. doi: 10.5312/wjo.v4.i2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webb JCJ. The effects of antibiotic combinations on bone cement properties. Thesis: University of Bristol; 2010. [Google Scholar]
- 38.Perry AC, Rouse MS, Khaliq Y, Piper KE, Hanssen AD, Osmon DR, Steckelberg JM, Patel R. Antimicrobial release kinetics from polymethylmethacrylate in a novel continuous flow chamber. Clin Orthop Relat Res. 2002;(403):49–53. doi: 10.1097/00003086-200210000-00009. [DOI] [PubMed] [Google Scholar]


