Abstract
Target of rapamycin (TOR) is an evolutionarily conserved protein kinase that controls multiple cellular processes upon various intracellular and extracellular stimuli. Since its first discovery, extensive studies have been conducted both in yeast and animal species including humans. Those studies have revealed that TOR forms two structurally and physiologically distinct protein complexes; TOR complex 1 (TORC1) is ubiquitous among eukaryotes including animals, yeast, protozoa, and plants, while TOR complex 2 (TORC2) is conserved in diverse eukaryotic species other than plants. The studies have also identified two crucial regulators of mammalian TORC1 (mTORC1), Ras homolog enriched in brain (RHEB) and RAG GTPases. Of these, RAG regulates TORC1 in yeast as well and is conserved among eukaryotes with the green algae and land plants as apparent exceptions. RHEB is present in various eukaryotes but sporadically missing in multiple taxa. RHEB, in the budding yeast Saccharomyces cerevisiae, appears to be extremely divergent with concomitant loss of its function as a TORC1 regulator. In this review, we summarize the evolutionarily conserved functions of the key regulatory subunits of TORC1 and TORC2, namely RAPTOR, RICTOR, and SIN1. We also delve into the evolutionary conservation of RHEB and RAG and discuss the conserved roles of these GTPases in regulating TORC1.
Keywords: target of rapamycin (TOR), kinase, GTPase, signaling, TORC1, TORC2, RHEB, RAG
1. Introduction
Target of rapamycin (TOR) is a phosphoinositide-3 kinase-related protein kinase that plays pivotal roles in controlling a wide variety of cellular processes in response to a broad spectrum of intracellular and extracellular stimuli [1]. TOR was first identified through a genetic screen for budding yeast mutants that are resistant to the immunosuppresant rapamycin [2]. Subsequent identification of TOR in humans and other species revealed evolutionary conservation of TOR from yeast to humans [3]. Expanding genome data of diverse species have revealed that TOR exists ubiquitously in eukaryotes of all five major clades, Opisthokonta (animals, yeast and fungi), Amoebozoa (protozoa), Excavata (protozoa), SAR (protozoa, brown algae), and Plantae (red algae, green algae, land plants) [4] (Figure 1; Table 1). Among the eukaryotic species, notable exceptions are obligate intracellular parasites, such as Plasmodium falciparum that belongs to the phylum Apicomplexa in the SAR clade and Encephalitozoon intestinalis that belongs to the phylum Microsporidia in the fungal kingdom [5] (Table 1). Thus, it is likely that TOR arose in the last eukaryotic common ancestor (LECA) and has stayed vital in all eukaryotes except those exclusively living in an extremely stable environment such as the inside of host cells.
Figure 1.
A consensus cladogram of the five major eukaryotic clades with selected eukaryotes. The cladogram was constructed based on the proposed phylogenetic relationships in [4]. LECA: last eukaryotic common ancestor.
Table 1.
Appearance of target of rapamycin (TOR) signaling components among eukaryotic species.
| Major Clade | Kingdom | Species | RICTOR | SIN1 | TOR | LST8 | RAPTOR | RHEB | TSC1 | TSC2 | RAG-A/B | RAG-C/D | DEPDC5 | NPRL2 | NPRL3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Opisthokonta | Metazoa | Homo sapiens | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Metazoa | Ciona intestinalis | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Metazoa | Drosophila melanogaster | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Metazoa | Schistosoma mansoni | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| Opisthokonta | Metazoa | Caenorhabditis elegans | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||
| Opisthokonta | Metazoa | Nematostella vectensis | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Metazoa | Trichoplax adhaerens | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Amphimedon queenslandica | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Opisthokonta | Capsaspora owczarzaki | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Opisthokonta | Fungi | Mitosporidium daphniae | ✔ | ✔ | ✔ | ||||||||||
| Opisthokonta | Fungi | Encephalitozoon intestinalis | |||||||||||||
| Opisthokonta | Fungi | Rozella allomycis | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| Opisthokonta | Fungi | Allomyces macrogynus | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| Opisthokonta | Fungi | Batrachochytrium dendrobatidis | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Fungi | Gonapodya prolifera | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Opisthokonta | Fungi | Conidiobolus coronatus | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Fungi | Smittium culicis | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Opisthokonta | Fungi | Rhizophagus irregularis | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Fungi | Lobosporangium transversale | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Fungi | Mortierella elongata | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Fungi | Mucor circinelloides | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Fungi | Neocallimastix californiae | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Fungi | Puccinia sorghi | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Fungi | Schizophyllum commune | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Fungi | Cutaneotrichosporon oleaginosus | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Fungi | Tilletiaria anomala | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Fungi | Ustilago maydis | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Fungi | Dothistroma septosporum | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Fungi | Cladophialophora bantiana | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Opisthokonta | Fungi | Aspergillus fumigatus | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Opisthokonta | Fungi | Trichopyton equinum | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Opisthokonta | Fungi | Botrytis cinerea | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Fungi | Colletotrichum graminicola | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Fungi | Fusarium fujikuroi | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Fungi | Neurospora crassa | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Fungi | Schizosaccharomyces pombe | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Fungi | Saitoella complicata | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Fungi | Yarrowia lipolytica | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Fungi | Candida albicans | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Opisthokonta | Fungi | Ogataea parapolymorpha | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Opisthokonta | Fungi | Eremothecium gossypii | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| Opisthokonta | Fungi | Kluyveromyces lactis | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Opisthokonta | Fungi | Candida glabrata | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| Opisthokonta | Fungi | Saccharomyces cerevisiae | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||
| Amoebozoa | Acanthamoeba castellanii | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| Amoebozoa | Dictyostelium discoideum | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||
| Amoebozoa | Entamoeba histolytica | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||
| Excavata | Naegleria gruberi | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| Excavata | Bodo saltans | ✔ | ✔ | ✔ | ✔ 1 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| Excavata | Angomonas deanei | ✔ | ✔ | ✔ | ✔ 1 | ✔ | ✔ | ✔ | ✔ | ||||||
| Excavata | Trypanosoma brucei | ✔ | ✔ | ✔ 1 | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||
| Excavata | Leishmania major | ✔ | ✔ | ✔ | ✔ 1 | ✔ | ✔ | ✔ | ✔ | ||||||
| Excavata | Giardia intestinalis | ✔ | ✔ | ✔ | ✔ | ||||||||||
| Excavata | Spironucleus salmonicida | ✔ | ✔ | ✔ | ✔ | ||||||||||
| SAR | Rhizaria | Reticulomyxa filosa | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| SAR | Rhizaria | Plasmodiophora brassicae | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| SAR | Rhizaria | Bigelowiella natans | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| SAR | Alveolata | Stylonychia lemnae | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| SAR | Alveolata | Oxytricha trifallax | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| SAR | Alveolata | Paramecium tetraurelia | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||
| SAR | Alveolata | Tetrahymena thermophila | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| SAR | Alveolata | Vitrella brassicaformis | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| SAR | Alveolata | Plasmodium falciparum | |||||||||||||
| SAR | Alveolata | Hammondia hammondi | ✔ | ✔ | ✔ | ||||||||||
| SAR | Alveolata | Toxoplasma gondii | ✔ | ✔ | ✔ | ||||||||||
| SAR | Alveolata | Cyclospora cayetanensis | |||||||||||||
| SAR | Alveolata | Eimeria maxima | |||||||||||||
| SAR | Alveolata | Cryptosporidium pavrum | |||||||||||||
| SAR | Alveolata | Theileria annulata | |||||||||||||
| SAR | Stramenopiles | Aureococcus anophagefferens | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||
| SAR | Stramenopiles | Saprolegnia diclina | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| SAR | Stramenopiles | Aphanomyces invadans | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| SAR | Stramenopiles | Pythium irregulare | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| SAR | Stramenopiles | Phytophthora infestans | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| SAR | Stramenopiles | Plasmopara halstedii | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| SAR | Stramenopiles | Hyaloperonospora arabidopsis | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| SAR | Stramenopiles | Albugo candida | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| SAR | Stramenopiles | Nannochloropsis gaditana | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| SAR | Stramenopiles | Blastocystis hominis | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||
| SAR | Stramenopiles | Phaeodactylum tricornutum | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| Plantae | Rhodophyta | Cyanidioschyzon melorae | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||
| Plantae | Rhodophyta | Galdieria sulphuraria | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||
| Plantae | Rhodophyta | Chondrus crispus | ✔ | ✔ | ✔ | ||||||||||
| Plantae | Chlorophyta | Ostreococcus lucimarinus | ✔ | ✔ | ✔ | ||||||||||
| Plantae | Streptophyta | Selaginella moellendorffii | ✔ | ✔ | ✔ | ||||||||||
| Plantae | Streptophyta | Amborella trichopoda | ✔ | ✔ | ✔ | ||||||||||
| Plantae | Streptophyta | Oryza sativa | ✔ | ✔ | ✔ | ||||||||||
| Plantae | Streptophyta | Arabidopsis thaliana | ✔ | ✔ | ✔ |
1 A Trypanosoma brucei protein (NCBI: XP_828034) was experimentally determined as a lethal with sec thirteen 8 (LST8) ortholog, although this protein shows only limited similarity to human and yeast LST8 [6]. The LST8 orthologs in Leishmania major, Bodo saltans, and Angomonas deanei were identified by the NCBI BLAST program with Trypanosoma brucei LST8 as a query. DEPDC5: DEP domain containing 5; NPRL2: nitrogen permease regulator 2-like protein; NPRL3: nitrogen permease regulator 3-like protein; RAPTOR: regulatory associated protein of mTOR; RHEB: Ras homolog enriched in brain; RICTOR: rapamycin-insensitive companion of mTOR; SIN1: stress-activated protein kinase interacting protein 1; TOR: target of rapamycin; TSC1, 2: tuberous sclerosis complex 1, 2.
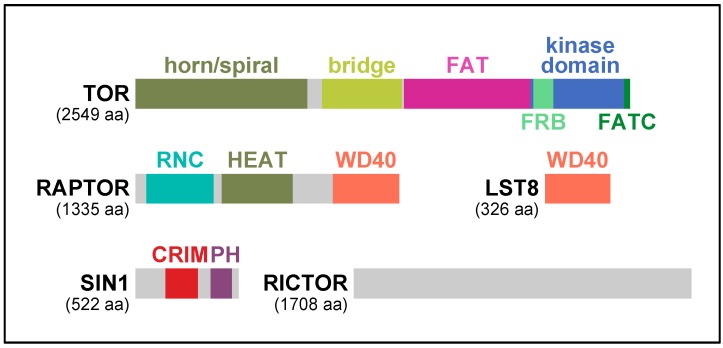
TOR kinase possesses multiple characteristic motifs and domains: α-helical HEAT (Huntington-EF3-PP2A-TOR1) repeats, the FRB (FKBP-Rapamycin Binding) domain, the FAT (FRAP–ATM–TRAPP) domain, the FATC (FAT C-terminus) domain, and the phosphoinositide-3 kinase domain (Figure 2). In the crystal structures of the C-terminal half of human TOR, the kinase domain is in an enzymatically active conformation [7]. However, the active site is located at the bottom of the deep catalytic cleft and sterically hindered by the surrounding structural elements, where multiple hyper-activating mutations of TOR have been identified [7]. Thus, it has been proposed that activity of TOR is controlled primarily by restricting active-site access [7]. Recent cryo-electron microscopy (cryo-EM) analyses of human and fungal TOR also revealed that the N-terminal HEAT repeats of TOR form two solenoid structures: a highly curving structure called “horn” or “spiral” and a less curving structure called “bridge” [8,9,10] (Figure 2). Two molecules of TOR shape a two-fold symmetry ring by physical contact between the “horn/spiral” and the “bridge” [8,9,10]. Considering the significant homologies throughout the N-terminal HEAT repeat region of TOR, it is expected that the characteristic solenoid structures as well as the two-fold symmetric ring formation are very common among TOR orthologs.
Figure 2.
Domain structure of the subunits of the TOR complexes in human. TOR: target of rapamycin; RAPTOR: regulatory associated protein of mTOR; LST8: lethal with sec thirteen 8; SIN1: stress-activated protein kinase interacting protein 1; RICTOR: rapamycin-insensitive companion of mTOR; FAT: FRAP–ATM–TRAPP; FRB: FKBP-Rapamycin Binding; FATC: FAT C-terminus; RNC: RAPTOR N-terminal conserved; HEAT: Huntington-EF3-PP2A-TOR1; CRIM: conserved region in the middle; PH: pleckstrin homology.
TOR forms two functionally and structurally distinct protein complexes TOR complex 1 (TORC1) and 2 (TORC2), of which only TORC1 is sensitive to rapamycin [11] (Figure 3). TORC1 inhibition by rapamycin is mediated by physical binding of rapamycin and the peptidyl-prolyl cis-trans isomerase FKBP12 to the FRB domain of TOR kinase [11]. Among eukaryotic species, TORC1 contains the TORC1-specific regulatory subunit regulatory associated protein of mTOR (RAPTOR) and TORC2 with two TORC2-specific subunits, rapamycin-insensitive companion of mTOR (RICTOR) and stress-activated protein kinase interacting protein 1 (SIN1) (Figure 3). The two TOR complexes share the same catalytic subunit TOR kinase and a regulator subunit called LST8 (Figure 3), and the physiological and biochemical distinction of the two complexes is mainly determined by the complex-specific regulatory subunits. Below, we summarize and discuss the evolutionarily conserved molecular functions of RAPTOR, RICTOR, and SIN1 subunits, with emphasis on their structures. Since the small GTPases Ras homolog enriched in brain (RHEB) and RAG have been emerging as critical regulators of mammalian TORC1, we also review the molecular functions and evolutionary conservation of these small GTPases and their regulators [1,12]. The evolution of nutrient-sensing pathways regulating TORC1 is also discussed in a recent review article [13]. Note that, throughout this review, we utilize human protein names without the prefixes “m” (for mammal) or “h” (for human) to describe each component in the TOR signaling pathways [14].
Figure 3.
Composition and regulation of TORC1 and TORC2 in human. DEPDC5: DEP domain containing 5; GATOR1: GAP activity towards RAGs 1; NPRL2: nitrogen permease regulator 2-like protein; NPRL3: nitrogen permease regulator 3-like protein; RHEB: Ras homolog enriched in brain; TORC1, 2: TOR complex 1, 2; TSC1, 2: tuberous sclerosis complex 1, 2.
2. RAPTOR, the Signature Subunit of TORC1
When TORC1 was first discovered in yeast and mammals, RAPTOR was identified as the signature subunit of TORC1 [11,15,16,17]. Except for ciliates, such as Paramecium tetraurelia and Tetrahymena thermophile, RAPTOR is found in every eukaryotic species that possess functional TOR kinase (Table 1) [5]. Therefore, it is presumed that RAPTOR arose with TOR in LECA and has been conserved during the evolution of eukaryotes. In its primary structure, RAPTOR has several characteristic segments. At the N-terminus is a region highly homologous among RAPTOR orthologs, and thus called the RAPTOR N-terminal Conserved (RNC) domain [8,15] (Figure 2). In the ternary structure of human and fungal RAPTOR, RNC forms a caspase-like fold with several extra helices [8,10]. The extra helices are tightly packed and placed between the caspase-like fold and the α-solenoidal HEAT repeat structure lying in the middle of RAPTOR [8,10] (Figure 2). At the C-terminus is a WD40 repeat domain that shapes a seven-bladed β-propeller [8,10,15,16] (Figure 2).
RAPTOR plays multiple essential roles in TORC1, including assembly and stabilization of the complex as well as substrate recognition. According to the recent cryo-EM studies, the extra helices of RNC and the first several helices of the HEAT repeats together form a wedge to stabilize the interaction between the two α-solenoidal structures “horn/spiral” and “bridge” of TOR [8,10]. Since RNC and the HEAT repeats are highly conserved in the primary sequences of the RAPTOR orthologs, those helical regions should retain similar structural characteristics for the assembly and stabilization of TORC1 among species. Mutations in the WD40 domain also compromise the assembly and stabilization of TORC1 [15,18], but ternary structure of TORC1 does not clearly illustrate how the WD40 domain of RAPTOR is involved in the TORC1 architecture [8,10].
In mammals, p70 ribosomal S6 kinase 1 (S6K1) and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1), two of the best-characterized TORC1 substrates, possess a five amino-acid stretch called TOR Signaling (TOS) motif [19,20]. Through their TOS motifs, RAPTOR physically interacts with S6K1 and 4E-BP1, resulting in their phosphorylation by TORC1. In the cryo-EM structure of human TORC1, the caspase fold of RAPTOR is in proximity to the active site of TOR kinase, implying that the caspase-fold region directly binds the TOS motif to bring the substrates toward the catalytic center of the complex [8,10]. The model is further reinforced by the fact that caspase proteases recognize four-residue sequences ending with aspartic acid, as the fourth position of the five-amino acid-long TOS motifs is occupied by aspartic acid. However, it remains to be experimentally determined if the caspase fold of mammalian RAPTOR physically binds the TOS motif. In addition, not all the mammalian TORC1 substrates have apparent TOS motif-like sequences [21]. It should also be noted that the TOS motif has not been reported in TORC1 substrates of non-animal species including fungi, although the caspase-fold region of RAPTOR is highly conserved also in those species.
3. TORC2 and Two Key Regulatory Subunits: RICTOR and SIN1
Rapamycin-insensitive TORC2 is the second complex formed by TOR kinase. TORC2 was first discovered in the budding yeast Saccharomyces cerevisiae with RICTOR and SIN1 as its regulatory subunits [11,17], followed by identification of TORC2 in other organisms including mammals [22,23,24]. Currently, TORC2 has been identified in four of the five eukaryotic clades with Plantae being the only exception (Table 1) [5]. Therefore, it is speculated that TORC2 arose in LECA but was lost at the very beginning of the evolution of algae and plant. It is also conceivable that TORC2 arose after Plantae diverged from LECA, although how the five major clades of eukaryotes diverged during evolution remains unresolved [4].
The TORC2-specific subunit RICTOR plays indispensable roles for the TORC2 function, of which the proper assembly and stabilization of TORC2 appear to be its primary role [25,26]. The amino acid sequence of RICTOR is highly conserved among species [11,24], but unfortunately, no detailed structural information has been available for this essential TORC2 subunit. A chemical crosslinking study of TORC2 in budding yeast demonstrated that the very C-terminus of RICTOR occupies the vicinity of the FRB domain of TOR kinase; indeed, C-terminal truncation of RICTOR is sufficient to make budding yeast TORC2 sensitive to rapamycin [27]. Thus, the C-terminus of RICTOR prevents the rapamycin-FKBP12 complex from binding to the FRB domain of TOR kinase in TORC2, which makes TORC2 insensitive to rapamycin [27].
SIN1 is another conserved regulatory subunit of TORC2 essential for its function. SIN1 was identified in the fission yeast Schizosaccharomyces pombe as a protein that interacts with the stress-activated mitogen-activated protein (MAP) kinase Spc1 (also known as Sty1) [28]. Nonetheless, significance of the interaction remains unclear until today [22]. Subsequent identification of SIN1 as a component of TORC2 opened the door for studying the SIN1 function [11,23]. In its primary structure, SIN1 is divided into several discrete regions [29,30,31]. The N-terminal region is homologous only between closely related species but rather divergent among a wide variety of species. The C-terminal region is composed of a PH (pleckstrin homology) domain, a well-known lipid-binding domain that selectively binds phospho-inositides in cellular membranes [32] (Figure 2). The central region is most highly conserved in SIN1 and hence called conserved region in the middle (CRIM) [30] (Figure 2).
In the absence of SIN1, TORC2 is disassembled in budding yeast and animals, suggesting a critical role of SIN1 in the stabilization of TORC2 [23,26,33]. However, the importance of SIN1 in TORC2 assembly may differ among species, as the remaining TORC2 subunits stay associated in fission yeast cells lacking SIN1 [34]. Chemical crosslinking of budding yeast TORC2 revealed configuration of SIN1 in TORC2; the N-terminus of SIN1 is positioned beside the RICTOR subunit, the region N-terminal to CRIM is located next to LST8, and the C-terminal PH domain is near the kinase domain of TOR [27]. Proximity of the SIN1 PH domain to the TOR kinase domain was observed also by electron microscopy [27].
The CRIM domain is a ubiquitin-like domain with a characteristic acidic protrusion [34]. From yeast to humans, the CRIM domain functions as a substrate-recruiting module in TORC2, by directly binding TORC2 substrates in a manner dependent on the acidic protrusion [34,35,36]. The CRIM domain can distinguish the TORC2 substrates, such as human AKT and protein kinase C (PKC), from the TORC1 substrate S6K1, though these kinases all belong to the same AGC family [34]. It remains to be determined how CRIM specifically recognizes the catalytic domain of the certain AGC kinases [34]. The CRIM domain is dispensable for TORC2 assembly because TORC2 is fully assembled with the CRIM-less mutant SIN1 in fission yeast as well as in mammalian cells [34].
The C-terminal PH domain is highly conserved among SIN1 orthologs in diverse species, but its physiological role and significance seem to be somewhat controversial. In budding yeast, the SIN1 PH domain is essential for the TORC2 function [37]. Because of its ability to bind phospho-inositide and localize to the plasma membrane, the SIN1 PH domain has been proposed to target TORC2 to the cell surface of budding yeast [37]. Further corroboration of the model would be possible by introducing point mutations that disable the PH domain for binding phospho-inositide. In fission yeast, the SIN1 PH domain is dispensable for the TORC2 function [34]. Fission yeast TORC2 is also localized at the plasma membrane, but the membrane localization is observed even in mutant cells lacking the SIN1 subunit [38]. In mammals, TORC2 has been observed at various subcellular locations, including the endoplasmic reticulum, mitochondria, mitochondria-associated endoplasmic reticulum membranes, early and late endosomes, and the plasma membrane [39,40,41]. The SIN1 PH domain appears contributing the plasma membrane localization of TORC2 [39,42], although the physiological significance of the PH domain and the plasma membrane localization remains obscure in mammals.
4. RHEB and TSC
RHEB is a Ras-like small GTPase essential for TORC1 activity in mammals [43]. Although the precise molecular mechanism is unknown, GTP-bound active RHEB physically binds and stimulates TORC1 activity [44,45] (Figure 3). RHEB is inactivated by its own GTPase activity, which is promoted by GTPase activating protein (GAP) activity of the tuberous sclerosis complex (TSC) protein complex [43] (Figure 3). In mammals, the TSC complex is composed of three subunits TSC1, TBC1D7, and TSC2 [46]. Of these, TSC2 alone is sufficient for the GAP activity toward RHEB in vitro [47], while both TSC1 and TSC2 are indispensable for the function of the TSC complex in vivo [43]. Multiple physiological stimuli, such as cellular energy status and extracellular growth factors, converge on the TSC complex to regulate the guanine-nucleotide binding state of RHEB [1].
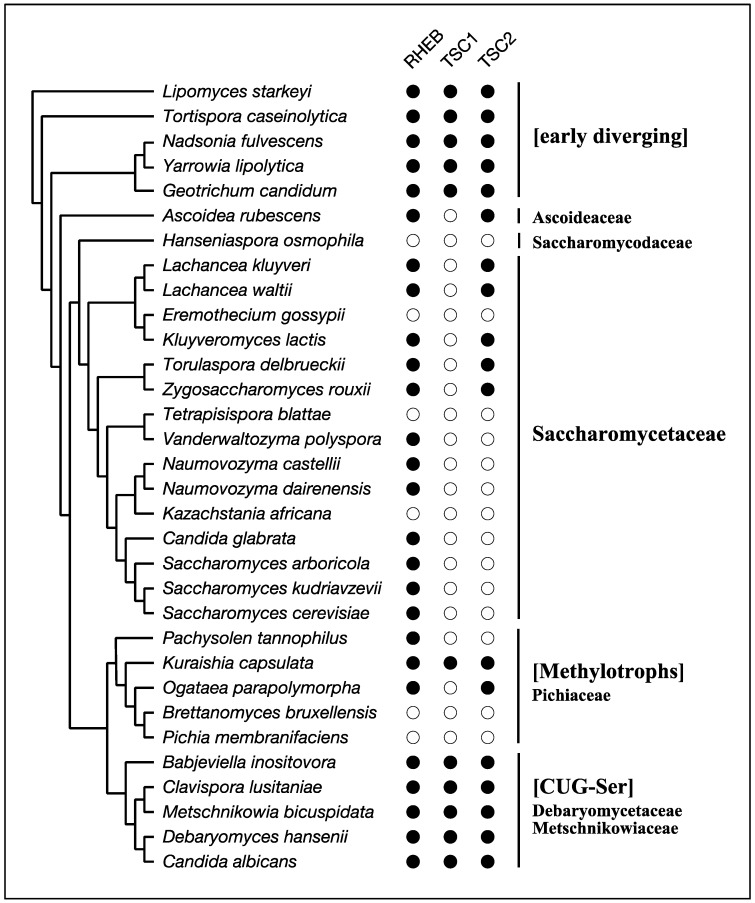
While RHEB is absolutely essential for TORC1 activity in mammals, its requirement appears to substantially vary among Opisthokonta that include mammals as well as insects, worms, filamentous fungi and yeast. RHEB is an essential activator of TORC1 in the fly Drosophila melanogaster [48,49]. While TBC1D7 is a vertebrate specific protein, TSC1 and TSC2 co-exist in the fly and function together as GAP for RHEB [50]. RHEB also acts as a positive regulator of TORC1 in Caenorhabditis elegans [51]. Both TSC1 and TSC2, however, are absent in the genome of the Caenorhabditis species C. brenneri, C. briggasae, C. elegans, C. japonica, and C. remanei (Table 1; data not shown). Moreover, neither RHEB nor the TSC subunits can be found in the genomes of the worm species Hymenolepis microstoma, Echinococcus granulosus, Echinococcus multilocularis, Opisthorchis viverrini, Schistosoma haematobium, and Schistosoma mansoni (Table 1; data not shown). Therefore, a certain animal species may partially or completely lose RHEB-dependent regulation of TORC1, although the absence of RHEB and the TSC subunits described above is contingent on accurate genome annotations of those species. In the fungal kingdom, RHEB is indispensable for TORC1 activity and cellular viability in the fission yeast Schizosaccharomyces pombe [52,53,54], while it appears to play only a limited role in the viability and virulence of Aspergillus fumigatus [55]. Interestingly, this pathogenic fungus as well as Cladophialophora bantiana and Trichopyton equinum is a member of the class Eurotiomycetes (Table 1), in which all the nineteen species we examined lack TSC1 (Table 1; data not shown), implying unique function and regulation of RHEB in this class of fungi.
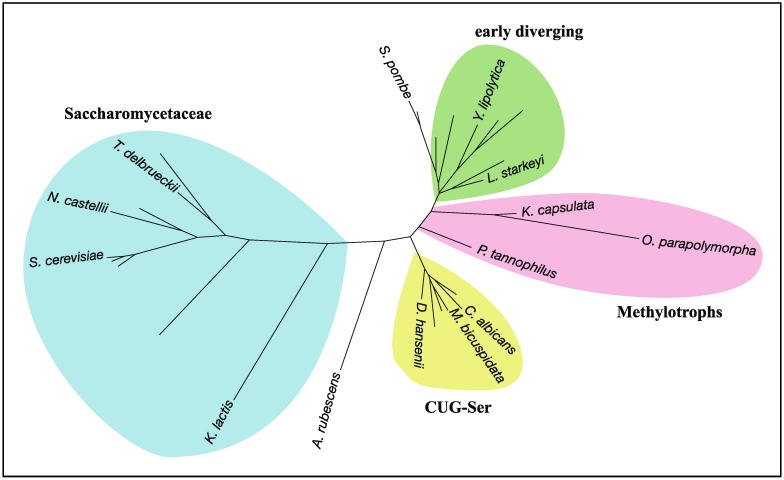
The best-known species that lacks RHEB-dependent regulation of TORC1 among Opisthokonta is the budding yeast Saccharomyces cerevisiae. Its genome carries a gene encoding a RHEB-like small GTPase, but the gene product does not function as an activator of TORC1 [56,57,58]. This yeast species also lacks genes for TSC1 and TSC2 (Table 1; Figure 4). S. cerevisiae belongs to the subphylum Saccharomycotina, which is composed mainly of three major clades, CUG-Ser, Methylotrophs, and Saccharomycetaceae as well as several early diverging members (Figure 4) [59]. RHEB, TSC1, and TSC2 are conserved among the early diverging members, such as Lipomyces starkeyi and Yarrowia lipolytica (Table 1; Figure 4). Members of the CUG-Ser clade, where the CUG codon is translated to Ser instead of Leu due to genetic changes of the tRNACAG, also possess RHEB, TSC1, and TSC2 (Table 1; Figure 4). In Candida albicans, a member of the CUG-Ser clade, RHEB is involved in nitrogen starvation-induced filamentation but dispensable for cellular viability, implying a diminished contribution of RHEB to the regulation of TORC1 in this clade [60]. On the other hand, all members in the Saccharomycetaceae clade partially or completely lack RHEB and the TSC subunits. Moreover, RHEB identified in this clade is substantially divergent from those in other members of Saccharomycotina (Figure 5). Such divergence in Saccharomycetaceae implies that RHEB had lost its role in the regulation of TORC1 during early evolution of Saccharomycetaceae and carries out different cellular functions. The Methylotrophs clade also exhibits sporadic loss of RHEB, TSC1, or TSC2 (Figure 4), and how TORC1 is regulated in the absence of RHEB, TSC1, or TSC2 in the Methylotrophs species remains unknown.
Figure 4.
An occurrence chart of RHEB, TSC1 and TSC2 and the consensus cladogram of the subphylum Saccharomycotina. The consensus cladogram was constructed according to the literature [59,61,62]. Occurrence was determined by homology searches with the NCBI BLAST program and by domain searches with the HMMER3 suite (http://hmmer.org) and the Pfam database [63]. Filled circles indicate presence; open circles indicate absence.
Figure 5.
A radial phylogram of RHEB in the subphylum Saccharomycotina. Two species of the subphylum Taphrinomycotina, Schizosaccharomyces pombe and Saitoella complicata, were also included to clarify the root of Saccharomycotina in the phylogram. The phylogenic tree was constructed by the ETE3 toolkit [64] with the workflow option being “standard_fasttree”, followed by tree drawing by Dendroscope 3 [65]. Labeling nodes and coloring taxa were done manually. A. rubescens: Ascoidea rubescens; C. albicans: Candida albicans; D. hansenii: Debaryomyces hansenii; K. capsulata: Kuraishia capsulate; K. lactis: Kluyveromyces lactis; L. starkeyi: Lipomyces starkeyi; M. bicuspidata: Metschnikowia bicuspidate; N. castellii: Naumovozyma castellii; O. parapolymorpha: Ogataea parapolymorpha; P. tannophilus: Pachysolen tannophilus; S. cerevisiae: Saccharomyces cerevisiae; S. pombe: Schizosaccharomyces pombe; T. delbrueckii: Torulaspora delbrueckii; Y. lipolytica: Yarrowia lipolytica.
Extensive surveys of genome databases demonstrate that RHEB and TSC2 are present in multiple taxa outside the Opisthokonta clade [5] (Table 1). In contrast, TSC1 is not readily identifiable due to its limited sequence conservation among distantly related species [5] (Table 1). In Amoebozoa, while only RHEB and TSC2 can be found in Dictyostelium species, Acanthamoeba castellanii has all of RHEB, TSC1, and TSC2 (Table 1), suggesting that certain Amoebozoa species possess the intact RHEB-TSC system. None of the Excavata species, such as Trypanosoma and Giardia, exhibit unequivocal presence of RHEB or the TSC subunits (Table 1). The SAR clade is a huge taxon that includes extremely diverse species [4]. In this clade, the water mold Aphanomyces invadans has all of RHEB, TSC1, and TSC2, and multiple other water molds, such as Pythium irregulare and Phytophthora infestans, possess at least RHEB and TSC2 (Table 1), suggesting that water mold species have the functional RHEB-TSC system. Among Plantae, green algae and land plants have completely lost both RHEB and the TSC subunits, while red algae Rhodophyta appears to retain at least a part of the RHEB-TSC system (Table 1). Appearance of RHEB and TSC2 in such a wide variety of eukaryotes suggests that the RHEB-TSC system arose with TORC1 in LECA but was lost during evolution in multiple taxa, resulting in sporadic occurrence among eukaryotic taxa (Table 1). Obligate, intracellular parasitic species without TOR kinase, such as Plasmodium falciparum and Encephalitozoon intestinalis, also lack both RHEB and the TSC subunits, suggesting that the primary function of RHEB and the TSC complex is to regulate TORC1 [5].
Assuming that RHEB and the TSC complex played a key role in controlling TORC1 activity in LECA, how have those key regulators been lost in certain species like S. cerevisiae where TORC1 activity remains physiologically crucial? In the fission yeast S. pombe, RHEB becomes dispensable when TOR kinase carries an activating mutation [53]. It is, therefore, conceivable that mutation(s) activating TOR kinase arose in the common ancestor of the Saccharomycetaceae clade and hence RHEB became less and less important for TORC1 activity in the descendants. However, it is unclear if TOR kinase is intrinsically more active in those RHEB-less Saccharomyces species. In mammals and certain other species, it is widely accepted that RHEB and the TSC complex are the key regulatory factors funneling a variety of stimuli to strictly control TORC1 activity. Considering the vital roles of RHEB and the TSC complex in modulating TORC1 activity, it is of great interest how TORC1 remains highly responsive to stimuli in species that lack the RHEB-dependent regulation, such as S. cerevisiae.
5. The RAG GTPases and Their GAP Complex GATOR1
As mentioned above, mammalian TORC1 is activated by multiple stimuli such as growth factors, cellular energy levels, and nutrients [1,12]. While most input signals modulate the function of the TSC complex and the activity of RHEB to control TORC1, amino acids stimulate TORC1 activity even in the absence of the TSC complex [57,66]. Amino acid stimuli first induce translocation of cytosolic TORC1 to lysosomes, where GTP-bound, active RHEB resides and interacts physically with TORC1 for its activation. The lysosomal translocation of TORC1 is mediated by physical interaction with the RAG small GTPases, which are members of the RAS super-family [67] (Figure 3). Humans have four RAG genes encoding RAG-A, RAG-B, RAG-C, and RAG-D, of which RAG-A and RAG-B form a heterodimer with either RAG-C or RAG-D. The RAG heterodimer is always at the lysosomal surface, but its guanine-nucleotide binding state is responsive to amino acid stimuli [67]. Upon the stimuli, the RAG heterodimer physically binds TORC1 for its recruitment, most efficiently with RAG-A or RAG-B being in the GTP-bound form and RAG-C or RAG-D in the GDP-bound form [67]. The guanine-nucleotide binding state of RAG-A and RAG-B is modulated by a GAP complex called GAP activity towards RAGs 1 (GATOR1), a trimeric protein complex composed of the catalytic DEP domain containing 5 (DEPDC5) subunit and the two regulatory subunits nitrogen permease regulator 2-like protein (NPRL2) and nitrogen permease regulator 3-like protein (NPRL3) [68] (Figure 3). More details about how the RAG GTPase heterodimer and its regulators control TORC1 activity in response to amino acid stimuli are described in other articles in this issue of Biomolecules [57,66,69].
The budding yeast S. cerevisiae also possesses both the RAG GTPases and the trimeric GATOR1 complex (Table 1). Moreover, it has been reported that, in response to nutritional stimuli, budding yeast RAG and GATOR1 promote TORC1 activity even without RHEB-dependent activation of TORC1 [70,71,72,73,74]. As has been found in mammals, the RAG heterodimer exhibits higher affinity to TORC1 when RAG-A is bound to GTP [71,74]. Both RAG GTPases and TORC1 always reside at the surface of vacuoles (yeast equivalent of mammalian lysosomes) in budding yeast, but nutritional stimuli affect the nucleotide binding state of the RAG heterodimer, altering the pattern of TORC1 distribution on the vacuolar surface [70,74]. It is likely that such a change in TORC1 localization is a part of the mechanism of how the RAG heterodimer activates TORC1 independently of RHEB in budding yeast. Details of the mechanism, however, have to wait for future studies.
Thorough database searches have revealed that the RAG heterodimer and the trimeric GATOR1 complex distribute much more ubiquitously than RHEB and the TSC subunits among the eukaryotic taxa (Table 1). In fact, no taxon that apparently lacks RAG and GATOR1 possesses RHEB and the TSC subunits. Our searches also show that intracellular parasitic species, such as Plasmodium falciparum and Encephalitozoon intestinalis, have lost RAG and GATOR1 as well as TORC1 (Table 1). Collectively, it is surmised that RAG and GATOR1 arose with TORC1 in LECA and have been functioning for TORC1 regulation since then. Except for species that extremely diverged from other eukaryotes, such as Giardia intestinalis [75], the only taxon that evidently lacks RAG and GATOR1 is the green algae and land plant clade. Thus, it is very likely that RAG and GATOR1 was lost in this clade during early evolution. Since the green algae and land plants also lack RHEB and the TSC subunits, they have probably evolved TORC1 regulatory mechanisms that are completely different from those of other species [76]. Like certain animals and fungi, there are taxa that retain RAG and GATOR1 as well as RHEB and the TSC subunits. Such species include Acanthamoeba castellanii and Dictyostelium discoideum in Amoebozoa, Plasmodiophora brassicae in Rhizaria, multiple water mold species such as Aphanomyces invadans in Stramenopiles, and red algae Cyanidioschyzon melorae and Galdieria sulphuraria in Rhodophyta (Table 1). There are, however, also multiple taxa that possess the RAG heterodimer and GATOR1 but lack RHEB or the TSC subunits (Table 1), including Trypanosoma species in Excavata, the foram Reticulomyxa filosa and the oceanic unicellular algae Bigelowiella natans in Rhizaria, and free-living ciliates such as Paramecium tetraurelia and Tetrahumena thermophila in Alveolata. Frequent appearance of the RAG GTPases without RHEB implies that the RAG GTPases have an evolutionarily conserved function in TORC1 regulation independent of RHEB. Possibly, such a regulatory mechanism is cryptic in mammals where RHEB is absolutely required for TORC1 activation. The budding yeast S. cerevisiae and other species that lack the RHEB-dependent TORC1 activation mechanism may provide a useful platform to explore the evolutionarily conserved molecular function of the RAG GTPases.
Acknowledgments
This work was supported by a grant from Japan Society for the Promotion of Science (JSPS) Grants-in-Aid to K.S. (26291024).
Author Contributions
H.T. prepared all the figures and table; H.T. wrote the draft; H.T. and K.S. completed the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsor had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Shimobayashi M., Hall M.N. Making new contacts: The mTOR network in metabolism and signalling crosstalk. Nat. Rev. Mol. Cell Biol. 2014;15:155–162. doi: 10.1038/nrm3757. [DOI] [PubMed] [Google Scholar]
- 2.Heitman J., Movva N.R., Hall M.N. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 3.Soulard A., Cohen A., Hall M.N. TOR signaling in invertebrates. Curr. Opin. Cell Biol. 2009;21:825–836. doi: 10.1016/j.ceb.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Katz L.A. Origin and diversification of eukaryotes. Annu. Rev. Microbiol. 2012;66:411–427. doi: 10.1146/annurev-micro-090110-102808. [DOI] [PubMed] [Google Scholar]
- 5.Van Dam T.J.P., Zwartkruis F.J.T., Bos J.L., Snel B. Evolution of the TOR pathway. J. Mol. Evol. 2011;73:209–220. doi: 10.1007/s00239-011-9469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barquilla A., Saldivia M., Diaz R., Bart J.-M., Vidal I., Calvo E., Hall M.N., Navarro M. Third target of rapamycin complex negatively regulates development of quiescence in Trypanosoma brucei. Proc. Natl. Acad. Sci. USA. 2012;109:14399–14404. doi: 10.1073/pnas.1210465109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H., Rudge D.G., Koos J.D., Vaidialingam B., Yang H.J., Pavletich N.P. mTOR kinase structure, mechanism and regulation. Nature. 2013;497:217–223. doi: 10.1038/nature12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aylett C.H.S., Sauer E., Imseng S., Boehringer D., Hall M.N., Ban N., Maier T. Architecture of human mTOR complex 1. Science. 2016;351:48–52. doi: 10.1126/science.aaa3870. [DOI] [PubMed] [Google Scholar]
- 9.Baretić D., Berndt A., Ohashi Y., Johnson C.M., Williams R.L. Tor forms a dimer through an N-terminal helical solenoid with a complex topology. Nat. Commun. 2016;7:11016. doi: 10.1038/ncomms11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang H., Wang J., Liu M., Chen X., Huang M., Tan D., Dong M.-Q., Wong C.C.L., Wang J., Xu Y., et al. 4.4 Å Resolution Cryo-EM structure of human mTOR Complex 1. Protein Cell. 2016;7:878–887. doi: 10.1007/s13238-016-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loewith R., Jacinto E., Wullschleger S., Lorberg A., Crespo J.L., Bonenfant D., Oppliger W., Jenoe P., Hall M.N. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell. 2002;10:457–468. doi: 10.1016/S1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 12.Cornu M., Albert V., Hall M.N. mTOR in aging, metabolism, and cancer. Curr. Opin. Genet. Dev. 2013;23:53–62. doi: 10.1016/j.gde.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Wolfson R.L., Sabatini D.M. The Dawn of the Age of Amino Acid Sensors for the mTORC1 Pathway. Cell Metab. 2017;26:301–309. doi: 10.1016/j.cmet.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall M.N. Talks about TORCs: Recent advances in target of rapamycin signalling. On mTOR nomenclature. Biochem. Soc. Trans. 2013;41:887–888. doi: 10.1042/BST20130092. [DOI] [PubMed] [Google Scholar]
- 15.Kim D.-H., Sarbassov D.D., Ali S.M., King J.E., Latek R.R., Erdjument-Bromage H., Tempst P., Sabatini D.M. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/S0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 16.Hara K., Maruki Y., Long X., Yoshino K., Oshiro N., Hidayat S., Tokunaga C., Avruch J., Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/S0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 17.Wedaman K.P., Reinke A., Anderson S., Yates J., McCaffery J.M., Powers T. Tor kinases are in distinct membrane-associated protein complexes in Saccharomyces cerevisiae. Mol. Biol. Cell. 2003;14:1204–1220. doi: 10.1091/mbc.E02-09-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunlop E.A., Dodd K.M., Seymour L.A., Tee A.R. Mammalian target of rapamycin complex 1-mediated phosphorylation of eukaryotic initiation factor 4E-binding protein 1 requires multiple protein-protein interactions for substrate recognition. Cell Signal. 2009;21:1073–1084. doi: 10.1016/j.cellsig.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 19.Schalm S.S., Blenis J. Identification of a conserved motif required for mTOR signaling. Curr. Biol. 2002;12:632–639. doi: 10.1016/S0960-9822(02)00762-5. [DOI] [PubMed] [Google Scholar]
- 20.Schalm S.S., Fingar D.C., Sabatini D.M., Blenis J. TOS motif-mediated raptor binding regulates 4E-BP1 multisite phosphorylation and function. Curr. Biol. 2003;13:797–806. doi: 10.1016/S0960-9822(03)00329-4. [DOI] [PubMed] [Google Scholar]
- 21.Dunlop E.A., Tee A.R. The kinase triad, AMPK, mTORC1 and ULK1, maintains energy and nutrient homoeostasis. Biochem. Soc. Trans. 2013;41:939–943. doi: 10.1042/BST20130030. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda K., Morigasaki S., Tatebe H., Tamanoi F., Shiozaki K. Fission yeast TOR complex 2 activates the AGC-family Gad8 kinase essential for stress resistance and cell cycle control. Cell Cycle. 2008;7:358–364. doi: 10.4161/cc.7.3.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacinto E., Facchinetti V., Liu D., Soto N., Wei S., Jung S.Y., Huang Q., Qin J., Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 24.Sarbassov D.D., Ali S.M., Kim D.-H., Guertin D.A., Latek R.R., Erdjument-Bromage H., Tempst P., Sabatini D.M. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 25.Gaubitz C., Prouteau M., Kusmider B., Loewith R. TORC2 Structure and Function. Trends Biochem. Sci. 2016;41:532–545. doi: 10.1016/j.tibs.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Wullschleger S., Loewith R., Oppliger W., Hall M.N. Molecular organization of target of rapamycin complex 2. J. Biol. Chem. 2005;280:30697–30704. doi: 10.1074/jbc.M505553200. [DOI] [PubMed] [Google Scholar]
- 27.Gaubitz C., Oliveira T.M., Prouteau M., Leitner A., Karuppasamy M., Konstantinidou G., Rispal D., Eltschinger S., Robinson G.C., Thore S., et al. Molecular Basis of the Rapamycin Insensitivity of Target of Rapamycin Complex 2. Mol. Cell. 2015;58:977–988. doi: 10.1016/j.molcel.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson M.G., Pino T.S., Tournier S., Buck V., Martin H., Christiansen J., Wilkinson D.G., Millar J.B. Sin1: An evolutionarily conserved component of the eukaryotic SAPK pathway. EMBO J. 1999;18:4210–4221. doi: 10.1093/emboj/18.15.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schroder W.A., Buck M., Cloonan N., Hancock J.F., Suhrbier A., Sculley T., Bushell G. Human Sin1 contains Ras-binding and pleckstrin homology domains and suppresses Ras signalling. Cell Signal. 2007;19:1279–1289. doi: 10.1016/j.cellsig.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Schroder W., Cloonan N., Bushell G., Sculley T. Alternative polyadenylation and splicing of mRNAs transcribed from the human Sin1 gene. Gene. 2004;339:17–23. doi: 10.1016/j.gene.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Wang S.-Z., Roberts R.M. The evolution of the Sin1 gene product, a little known protein implicated in stress responses and type I interferon signaling in vertebrates. BMC Evol. Biol. 2005;5:13. doi: 10.1186/1471-2148-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rebecchi M.J., Scarlata S. Pleckstrin homology domains: A common fold with diverse functions. Annu. Rev. Biophys. Biomol. Struct. 1998;27:503–528. doi: 10.1146/annurev.biophys.27.1.503. [DOI] [PubMed] [Google Scholar]
- 33.Yang Q., Inoki K., Ikenoue T., Guan K.-L. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006;20:2820–2832. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tatebe H., Murayama S., Yonekura T., Hatano T., Richter D., Furuya T., Kataoka S., Furuita K., Kojima C., Shiozaki K. Substrate specificity of TOR complex 2 is determined by a ubiquitin-fold domain of the Sin1 subunit. eLife. 2017;6 doi: 10.7554/eLife.19594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cameron A.J.M., Linch M.D., Saurin A.T., Escribano C., Parker P.J. mTORC2 targets AGC kinases through Sin1-dependent recruitment. Biochem. J. 2011;439:287–297. doi: 10.1042/BJ20110678. [DOI] [PubMed] [Google Scholar]
- 36.Liao H.-C., Chen M.-Y. Target of rapamycin complex 2 signals to downstream effector yeast protein kinase 2 (Ypk2) through adheres-voraciously-to-target-of-rapamycin-2 protein 1 (Avo1) in Saccharomyces cerevisiae. J. Biol. Chem. 2012;287:6089–6099. doi: 10.1074/jbc.M111.303701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berchtold D., Walther T.C. TORC2 plasma membrane localization is essential for cell viability and restricted to a distinct domain. Mol. Biol. Cell. 2009;20:1565–1575. doi: 10.1091/mbc.E08-10-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tatebe H., Morigasaki S., Murayama S., Zeng C.T., Shiozaki K. Rab-family GTPase regulates TOR complex 2 signaling in fission yeast. Curr. Biol. 2010;20:1975–1982. doi: 10.1016/j.cub.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ebner M., Sinkovics B., Szczygieł M., Ribeiro D.W., Yudushkin I. Localization of mTORC2 activity inside cells. J. Cell Biol. 2017;216:343–353. doi: 10.1083/jcb.201610060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Betz C., Stracka D., Prescianotto-Baschong C., Frieden M., Demaurex N., Hall M.N. Feature Article: MTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology. Proc. Natl. Acad. Sci. USA. 2013;110:12526–12534. doi: 10.1073/pnas.1302455110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Betz C., Hall M.N. Where is mTOR and what is it doing there? J. Cell Biol. 2013;203:563–574. doi: 10.1083/jcb.201306041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu P., Gan W., Chin Y.R., Ogura K., Guo J., Zhang J., Wang B., Blenis J., Cantley L.C., Toker A., et al. PtdIns(3,4,5)P3-Dependent Activation of the mTORC2 Kinase Complex. Cancer Discov. 2015;5:1194–1209. doi: 10.1158/2159-8290.CD-15-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heard J.J., Fong V., Bathaie S.Z., Tamanoi F. Recent progress in the study of the Rheb family GTPases. Cell Signal. 2014;26:1950–1957. doi: 10.1016/j.cellsig.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sancak Y., Thoreen C.C., Peterson T.R., Lindquist R.A., Kang S.A., Spooner E., Carr S.A., Sabatini D.M. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol. Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Sato T., Nakashima A., Guo L., Tamanoi F. Specific activation of mTORC1 by Rheb G-protein in vitro involves enhanced recruitment of its substrate protein. J. Biol. Chem. 2009;284:12783–12791. doi: 10.1074/jbc.M809207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dibble C.C., Elis W., Menon S., Qin W., Klekota J., Asara J.M., Finan P.M., Kwiatkowski D.J., Murphy L.O., Manning B.D. TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol. Cell. 2012;47:535–546. doi: 10.1016/j.molcel.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mazhab-Jafari M.T., Marshall C.B., Ishiyama N., Ho J., Di Palma V., Stambolic V., Ikura M. An autoinhibited noncanonical mechanism of GTP hydrolysis by Rheb maintains mTORC1 homeostasis. Structure (Lond. Engl. 1993) 2012;20:1528–1539. doi: 10.1016/j.str.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 48.Patel P.H., Thapar N., Guo L., Martinez M., Maris J., Gau C.-L., Lengyel J.A., Tamanoi F. Drosophila Rheb GTPase is required for cell cycle progression and cell growth. J. Cell Sci. 2003;116:3601–3610. doi: 10.1242/jcs.00661. [DOI] [PubMed] [Google Scholar]
- 49.Stocker H., Radimerski T., Schindelholz B., Wittwer F., Belawat P., Daram P., Breuer S., Thomas G., Hafen E. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat. Cell Biol. 2003;5:559–565. doi: 10.1038/ncb995. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y., Gao X., Saucedo L.J., Ru B., Edgar B.A., Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat. Cell Biol. 2003;5:578–581. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- 51.Honjoh S., Yamamoto T., Uno M., Nishida E. Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature. 2009;457:726–730. doi: 10.1038/nature07583. [DOI] [PubMed] [Google Scholar]
- 52.Otsubo Y., Yamamato M. TOR signaling in fission yeast. Crit. Rev. Biochem. Mol. Biol. 2008;43:277–283. doi: 10.1080/10409230802254911. [DOI] [PubMed] [Google Scholar]
- 53.Urano J., Sato T., Matsuo T., Otsubo Y., Yamamoto M., Tamanoi F. Point mutations in TOR confer Rheb-independent growth in fission yeast and nutrient-independent mammalian TOR signaling in mammalian cells. Proc. Natl. Acad. Sci. USA. 2007;104:3514–3519. doi: 10.1073/pnas.0608510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang W., Tabancay A.P., Urano J., Tamanoi F. Failure to farnesylate Rheb protein contributes to the enrichment of G0/G1 phase cells in the Schizosaccharomyces pombe farnesyltransferase mutant. Mol. Microbiol. 2001;41:1339–1347. doi: 10.1046/j.1365-2958.2001.02599.x. [DOI] [PubMed] [Google Scholar]
- 55.Panepinto J.C., Oliver B.G., Fortwendel J.R., Smith D.L.H., Askew D.S., Rhodes J.C. Deletion of the Aspergillus fumigatus gene encoding the Ras-related protein RhbA reduces virulence in a model of Invasive pulmonary aspergillosis. Infect. Immun. 2003;71:2819–2826. doi: 10.1128/IAI.71.5.2819-2826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.González A., Hall M.N. Nutrient sensing and TOR signaling in yeast and mammals. EMBO J. 2017;36:397–408. doi: 10.15252/embj.201696010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nicastro R., Sardu A., Panchaud N., De Virgilio C. The Architecture of the Rag GTPase Signaling Network. Biomolecules. 2017;7 doi: 10.3390/biom7030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Urano J., Tabancay A.P., Yang W., Tamanoi F. The Saccharomyces cerevisiae Rheb G-protein is involved in regulating canavanine resistance and arginine uptake. J. Biol. Chem. 2000;275:11198–11206. doi: 10.1074/jbc.275.15.11198. [DOI] [PubMed] [Google Scholar]
- 59.Riley R., Haridas S., Wolfe K.H., Lopes M.R., Hittinger C.T., Göker M., Salamov A.A., Wisecaver J.H., Long T.M., Calvey C.H., et al. Comparative genomics of biotechnologically important yeasts. Proc. Natl. Acad. Sci. USA. 2016;113:9882–9887. doi: 10.1073/pnas.1603941113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsao C.-C., Chen Y.-T., Lan C.-Y. A small G protein Rhb1 and a GTPase-activating protein Tsc2 involved in nitrogen starvation-induced morphogenesis and cell wall integrity of Candida albicans. Fungal Genet. Biol. FGB. 2009;46:126–136. doi: 10.1016/j.fgb.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 61.Shen X.-X., Zhou X., Kominek J., Kurtzman C.P., Hittinger C.T., Rokas A. Reconstructing the Backbone of the Saccharomycotina Yeast Phylogeny Using Genome-Scale Data. G3 (Bethesda Md.) 2016;6:3927–3939. doi: 10.1534/g3.116.034744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kurtzman C.P., Robnett C.J. Relationships among genera of the Saccharomycotina (Ascomycota) from multigene phylogenetic analysis of type species. FEMS Yeast Res. 2013;13:23–33. doi: 10.1111/1567-1364.12006. [DOI] [PubMed] [Google Scholar]
- 63.Finn R.D., Coggill P., Eberhardt R.Y., Eddy S.R., Mistry J., Mitchell A.L., Potter S.C., Punta M., Qureshi M., Sangrador-Vegas A., et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016;44:D279–D285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huerta-Cepas J., Serra F., Bork P. ETE 3: Reconstruction, Analysis, and Visualization of Phylogenomic Data. Mol. Biol. Evol. 2016;33:1635–1638. doi: 10.1093/molbev/msw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huson D.H., Scornavacca C. Dendroscope 3: An interactive tool for rooted phylogenetic trees and networks. Syst. Biol. 2012;61:1061–1067. doi: 10.1093/sysbio/sys062. [DOI] [PubMed] [Google Scholar]
- 66.Yao Y., Jones E., Inoki K. Lysosomal Regulation of mTORC1 by Amino Acids in Mammalian Cells. Biomolecules. 2017;7 doi: 10.3390/biom7030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sancak Y., Peterson T.R., Shaul Y.D., Lindquist R.A., Thoreen C.C., Bar-Peled L., Sabatini D.M. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bar-Peled L., Chantranupong L., Cherniack A.D., Chen W.W., Ottina K.A., Grabiner B.C., Spear E.D., Carter S.L., Meyerson M., Sabatini D.M. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science. 2013;340:1100–1106. doi: 10.1126/science.1232044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Noda T. Regulation of Autophagy through TORC1 and mTORC1. Biomolecules. 2017;7 doi: 10.3390/biom7030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kira S., Kumano Y., Ukai H., Takeda E., Matsuura A., Noda T. Dynamic relocation of the TORC1-Gtr1/2-Ego1/2/3 complex is regulated by Gtr1 and Gtr2. Mol. Biol. Cell. 2016;27:382–396. doi: 10.1091/mbc.E15-07-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sekiguchi T., Kamada Y., Furuno N., Funakoshi M., Kobayashi H. Amino acid residues required for Gtr1p-Gtr2p complex formation and its interactions with the Ego1p-Ego3p complex and TORC1 components in yeast. Genes Cells Devoted Mol. Cell. Mech. 2014;19:449–463. doi: 10.1111/gtc.12145. [DOI] [PubMed] [Google Scholar]
- 72.Stracka D., Jozefczuk S., Rudroff F., Sauer U., Hall M.N. Nitrogen source activates TOR (target of rapamycin) complex 1 via glutamine and independently of Gtr/Rag proteins. J. Biol. Chem. 2014;289:25010–25020. doi: 10.1074/jbc.M114.574335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Panchaud N., Péli-Gulli M.-P., De Virgilio C. Amino acid deprivation inhibits TORC1 through a GTPase-activating protein complex for the Rag family GTPase Gtr1. Sci. Signal. 2013;6:ra42. doi: 10.1126/scisignal.2004112. [DOI] [PubMed] [Google Scholar]
- 74.Binda M., Péli-Gulli M.-P., Bonfils G., Panchaud N., Urban J., Sturgill T.W., Loewith R., De Virgilio C. The Vam6 GEF controls TORC1 by activating the EGO complex. Mol. Cell. 2009;35:563–573. doi: 10.1016/j.molcel.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 75.Morrison H.G., McArthur A.G., Gillin F.D., Aley S.B., Adam R.D., Olsen G.J., Best A.A., Cande W.Z., Chen F., Cipriano M.J., et al. Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science. 2007;317:1921–1926. doi: 10.1126/science.1143837. [DOI] [PubMed] [Google Scholar]
- 76.Pérez-Pérez M.E., Couso I., Crespo J.L. The TOR Signaling Network in the Model Unicellular Green Alga Chlamydomonas reinhardtii. Biomolecules. 2017;7 doi: 10.3390/biom7030054. [DOI] [PMC free article] [PubMed] [Google Scholar]