Abstract
Duchenne muscular dystrophy (DMD) is characterized by striated muscle weakness, cardiomyopathy, and respiratory failure. Since oxidative stress is recognized as a secondary pathology in DMD, the efficacy of antioxidant intervention, using the superoxide scavenger tempol, was examined on functional and biochemical status of dystrophin-deficient diaphragm muscle. Diaphragm muscle function was assessed, ex vivo, in adult male wild-type and dystrophin-deficient mdx mice, with and without a 14-day antioxidant intervention. The enzymatic activities of muscle citrate synthase, phosphofructokinase, and lactate dehydrogenase were assessed using spectrophotometric assays. Dystrophic diaphragm displayed mechanical dysfunction and altered biochemical status. Chronic tempol supplementation in the drinking water increased diaphragm functional capacity and citrate synthase and lactate dehydrogenase enzymatic activities, restoring all values to wild-type levels. Chronic supplementation with tempol recovers force-generating capacity and metabolic enzyme activity in mdx diaphragm. These findings may have relevance in the search for therapeutic strategies in neuromuscular disease.
Keywords: Duchenne muscular dystrophy, mdx, tempol, oxidative stress, diaphragm, antioxidant
1. Introduction
Duchenne muscular dystrophy (DMD) is the most common form of inherited muscle disease in childhood, with an estimated incidence of 1:3500 male births [1]. DMD is caused by a deficiency in the protein dystrophin, which is a component of the dystrophin associated protein complex (DAPC) [2]. The DAPC has a structural role in linking the actin cytoskeleton to the extracellular matrix, thus stabilizing the sarcolemma during muscle contraction and relaxation [3]. Dystrophin deficiency results in destabilization of the DAPC, leading to muscle weakness and fragility, resulting in muscle damage, fibrosis, and necrosis [4]. Inflammation is secondary to muscle damage in DMD, with attendant disruption to Ca2+ homeostasis, oxidative stress, and mitochondrial dysfunction [5]. DMD patients suffer severe limb and respiratory muscle weakness [6]. Patients have compromised lung function due to diaphragm muscle weakness, altered chest wall compliance, and scoliosis [7]. Trans-diaphragmatic pressures are low in DMD boys [8], and disordered breathing can occur, particularly during sleep, leading to obstructive sleep apnea and hypoventilation [9]. Cardio-respiratory failure is the leading cause of death in DMD.
Oxidative stress is the result of increased reactive oxygen species (ROS) and/or decreased antioxidant capacity. ROS, in particular superoxide anions, are highly chemically reactive substances that can react with nucleic acids, lipids, and proteins, thus hindering cellular metabolism, resulting in cell injury [10]. Low levels of ROS are essential for optimal force production during muscle contraction, and act as important physiological signaling molecules that alter enzymatic activity and gene expression [11]. Large amounts of ROS have deleterious effects on muscle force and endurance, such as during strenuous exercise and chronic disease states, such as muscular dystrophy [12,13,14].
The most widely studied preclinical model of DMD is the dystrophin-deficient mdx mouse. Diaphragm muscle from mdx mice displays similar characteristics to DMD patients, including loss of function [15], muscle fibrosis and necrosis [16], inflammation, and oxidative stress [17]. Respiratory deficits are present in the mdx mouse, including hypoventilation [18]. An imbalance between ROS production and antioxidant scavenging capacity in muscle can promote muscle damage and mitochondrial dysfunction, with resultant impaired muscle function. Antioxidant capacity and estimates of ROS turnover have been examined in a number of tissues from mdx mice, including skeletal and cardiac muscle, and the brain. Markers of oxidative stress are increased in hearts from mdx mice [19], as well as in limb and diaphragm muscle [20,21,22]. Increased superoxide dismutase activity is reported in mdx cerebellum and prefrontal cortex, and this increase in antioxidant activity was associated with decreased lipid peroxidation, suggesting a protective role of superoxide dismutase in mdx mouse brain [23].
Disruption of the DAPC in DMD results in nitric oxide synthase (NOS) displacement from the sarcolemma [3]. NOS has a physiological role in the synthesis of nitric oxide (NO), which exerts protective effects in muscle in response to cell injury. Activity of the superoxide generating enzyme complex nicotinamide adenine dinucleotide phosphate-oxidase (NOX) is increased in mdx muscle [21,24]. Pharmacological inhibition of NOX, using apocynin, improved calcium handling and contractility in mdx hearts [24]. Similarly, inhibition of ROS with diapocynin reduced ROS production and prevented force loss induced by eccentric contractions in dystrophic limb muscle [25]. Increased ROS can activate pro-inflammatory pathways, resulting in inflammation and fibrosis, and can promote Ca2+ dysregulation. Recent data suggest a complex interaction between oxidative stress, Ca2+ dysregulation, and inflammation as secondary mediators of pathology in DMD [5,26,27].
Currently no cure exists for DMD, therefore, much work is focused on discovering novel therapies for the treatment of the disease. In the present study, we used the mdx mouse model of DMD to investigate the therapeutic potential of tempol on diaphragm muscle force and oxidative and glycolytic metabolic enzyme activities. Tempol is a membrane permeable superoxide dismutase mimetic, which exerts antioxidant effects by scavenging superoxide anions. Tempol acts by catalyzing the reaction of superoxide into oxygen or hydrogen peroxide. Previous work has shown that tempol exerts a positive inotropic effect on rat pharyngeal dilator muscle function ex vivo [28], and prevents hypoxia-induced muscle weakness [29,30] and oxidative stress [31,32]. It is also established that tempol prevents temperature-dependent force loss in mouse and rat muscle ex vivo [33]. We hypothesized that chronic antioxidant therapy in mdx mice would improve diaphragm muscle function.
2. Materials and Methods
2.1. Ethical Approval
Procedures involving live animals were performed under license in accordance with Irish and European legislation, following prior approval by University College Cork’s animal research ethics committee. The approval number from University College Cork’s animal research ethics committee is AEEC 2013/035.
2.2. Experimental Animals
Male and female wild-type (C57BL/10ScSnJ) and mdx (C57BL/10ScSn-Dmdmdx/J) mice were purchased from the Jackson Laboratory (Jackson Laboratory, Bar Harbor, ME, USA) and bred at University College Cork’s animal housing facility. Animals were housed conventionally in a temperature- and humidity-controlled facility, operating on a 12 h light–12 h dark cycle with food and water available ad libitum. Male mice were studied at 14 weeks of age, and were assigned to four groups: wild-type (26.2 ± 1.6 g; n = 7), mdx (29.2 ± 2.8 g; n = 7), mdx + tempol in vitro (32.2 ± 2.5 g; n = 7) and mdx + tempol in vivo (30.1 ± 2.2g; n = 9); mdx animals were randomly assigned to groups. The mdx + tempol in vivo group received tempol (1 mM 4-hydroxy-TEMPO; Sigma Aldrich, Wicklow, Ireland) in their drinking water for two weeks (from 12 to 14 weeks of age), equivalent to a dose of 20–35 mg/g body weight, taking an estimate of fluid intake and known body mass gain over the intervention period. The dose was informed by previously published work in rat, wherein tempol proved efficacious in preventing respiratory muscle dysfunction in response to redox stress associated with exposure to chronic intermittent hypoxia [29,30].
2.3. Muscle Physiology
2.3.1. Ex Vivo Muscle Preparation
Animals were anaesthetized with 5% isoflurane in air, and euthanized by cervical dislocation. Diaphragm muscle was immediately excised, with central tendon and rib intact for functional studies. Additional diaphragm muscle samples were snap frozen in liquid nitrogen and stored at −80 °C for later analysis. Diaphragm muscle was suspended vertically in a water-jacketed tissue bath at 35 °C, filled with Krebs solution (in mM: 120 NaCl, 5 KCl, 2.5 Ca2+ gluconate, 1.2 MgSO4, 1.2 NaH2PO4, 25 NaHCO3 and 11.5 glucose) and d-tubocurarine (25 μM). Preparations were equilibrated with hyperoxic (95% O2/5% CO2) gas. The rib end of the preparation was attached to an immobile hook at the base of a muscle holder, and the central tendon was attached to a dual mode lever transducer system (Aurora Scientific Inc., Aurora, ON, Canada) with non-elastic string, to allow the assessment of isometric and isotonic contractile properties. For the mdx + tempol in vitro group, diaphragm muscle from mdx mice was studied in Krebs solution containing 1 mM tempol, which has been shown to exert positive inotropic effects on rat respiratory muscle in isolated muscle preparations [28,29]).
2.3.2. Isometric Protocol
The muscle strips were stimulated via field stimulation with platinum electrodes at supramaximal voltage. Optimal length (Lo), the length producing maximum twitch force, was obtained by repeated isometric twitch stimulation (supramaximal voltage; 1 ms duration) at varying muscle lengths, achieved by adjustment of a micro-positioner. The Lo was recorded, and the muscle remained at this length for the remainder of the experiment. Following a 10 min equilibration period, contractile properties were examined. First, a single twitch was elicited, and twitch force, contraction time (CT; time to peak force), and half-relaxation time (½ RT; time for force to decay by 50%) were determined. Next, an isometric tetanic contraction was elicited by stimulating muscle strips with supramaximal voltage at 100 Hz for 300 ms duration. Peak isometric tetanic force (Fmax) was determined [34,35].
2.3.3. Isotonic Protocol
Concentric contractions were evoked at varying loads in an incremental step-test (0%, 5%, 10%, 20%, 30%, 40%, 50%, 60%, 80%, 100%; % of Fmax). Each contraction was interspaced by 1 min, and muscle length returned to Lo following each contraction. Total shortening was considered the maximum distance shortened during contraction; shortening velocity was measured during the initial 30 ms of shortening [34,36]. Mechanical work (force x total shortening) and power (force x shortening velocity) were measured at each % load [34,37].
2.4. Muscle Biochemistry
2.4.1. Tissue Preparation
Diaphragm samples stored at −80 °C were removed and allowed to defrost at 4 °C for 5 min. All procedures were performed at 4 °C to prevent protein degradation. Samples were homogenized in a lysis buffer (RIPA) made up from 10× RIPA, deionized water, 200 mM sodium fluoride, 100 mM phenylmethylsulfonyl fluoride, protease cocktail inhibitor 1, and phosphatase cocktail inhibitor 2. Following the homogenization process, the reactant mixtures were centrifuged (15,339× g) and the supernatants were harvested. Total amount of protein for each tissue sample was determined using Pierce® Bicinchoninic Acid Assay (BCA assay, Thermo Scientific, Fisher, Dublin, Ireland). Supernatants were aliquoted and stored at −80 °C for future use.
2.4.2. Metabolic Enzyme Assays
Tissue homogenates were used for enzymatic activity assays. The experimental procedures for citrate synthase, phosphofructokinase, and lactate dehydrogenase activity assays were performed in accordance with the technical bulletins of a citrate synthase assay kit (CS0720; Sigma-Aldrich, Wicklow, Ireland), phosphofructokinase activity colorimetric assay kit (MAK093; Sigma-Aldrich), and lactate dehydrogenase activity assay kit (MAK066; Sigma-Aldrich), respectively. Results are presented as enzymatic activity per 1 mg of protein in tissue homogenate.
2.5. Data Analysis
Specific force was normalized for muscle tissue cross-sectional area (CSA), and calculated in N/cm2. Muscle CSA was estimated for each muscle strip by dividing the muscle mass (weight in grams) by the product of muscle Lo (cm) and muscle density (assumed to be 1.06 g/cm3). For isotonic load relationships, data were expressed as the measured parameter versus % load. Total muscle shortening was normalized to Lo and expressed in Lo/s. Maximal total shortening (Smax) and maximum shortening velocity (Vmax) occurred at 0% load. Maximum mechanical work (Wmax) and power (Pmax) occurred at ~30–40% load.
2.6. Statistical Analysis
Values are expressed as mean ± SD or are represented graphically as box and whisker plots (median, 25th–75th centile, and minimum and maximum). Data were statistically compared by unpaired Student’s t tests with Welch’s correction where appropriate, and two-way analysis of variance (ANOVA); for data from incremental load tests). p < 0.05 was deemed to be statistically significant.
3. Results
3.1. Isometric Force and Twitch Contractile Kinetics
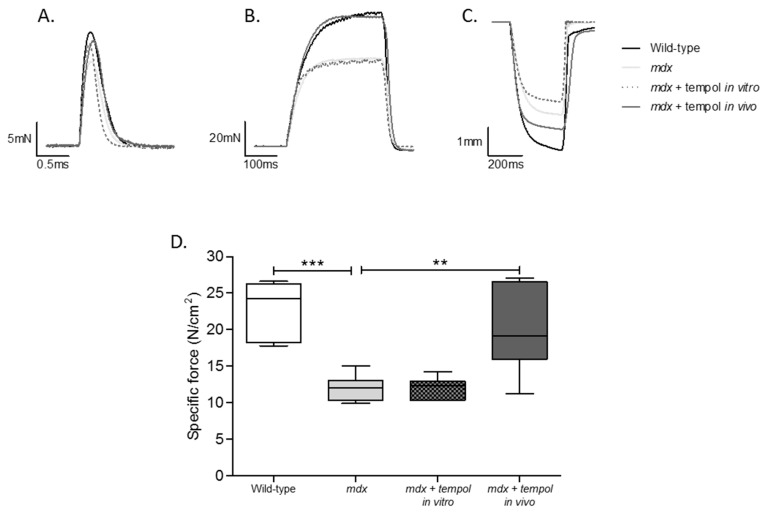
Representative original traces for diaphragm muscle twitch (A) and tetanic (B) contractions and maximum unloaded shortening (C) are shown in Figure 1 for wild-type, mdx, mdx + tempol in vitro and mdx + tempol in vivo. Table 1 shows data for diaphragm muscle twitch force and contractile kinetics from all four groups. Twitch force was significantly lower in mdx compared with wild-type diaphragm (p = 0.0066, Student’s t test). There was a significant decrease in diaphragm ½ RT following tempol administration in vivo in mdx mice (p = 0.0046). Twitch force was significantly higher in mdx + tempol in vivo compared with mdx (p = 0.0111). Fmax was significantly lower in mdx diaphragm compared with wild-type (p < 0.0001; Figure 1D). Fmax was significantly higher in mdx + tempol, in vivo, compared with mdx (p = 0.0069), such that values were equivalent to wild-type values for peak force generation. Bath application of tempol to mdx diaphragm had no effect on force-generating capacity or contractile kinetics compared with mdx (Figure 1 and Table 1).
Figure 1.
Peak Isometric Tetanic Force. Representative traces for muscle twitch (A) and tetanic (B) contractions and maximum unloaded shortening (C) for diaphragm muscle from wild-type, mdx, mdx + tempol in vitro and mdx + tempol in vivo. Group data for diaphragm muscle peak tetanic force (D) from wild-type (n = 7), mdx (n = 7), mdx + tempol in vitro (n = 7) and mdx + tempol in vivo (n = 8). For the mdx + tempol in vitro group, diaphragm muscle preparations were studied in Krebs solution containing 1 mM tempol in vitro. The mdx + tempol in vivo group received 1 mM tempol in their drinking water for two weeks. Values are expressed as box and whisker plots (median, 25–75% centiles and minimum and maximum values) and data were statistically compared by Student’s t tests. *** p < 0.0001; ** p = 0.0069.
Table 1.
Diaphragm Muscle Contractile Properties.
| Wild-Type (n = 7) | Mdx (n = 7) | mdx + Tempol In Vitro (n = 7) | mdx + Tempol In Vivo (n = 8) | Student’s t Test | |
|---|---|---|---|---|---|
| CT (ms) | 18.0 ± 1.8 | 20.5 ± 4.5 | 17.9 ± 1.5 | 20.2 ± 3.1 | $: p = 0.2196; †: p = 0.1796; £: p = 0.8777 |
| ½ RT (ms) | 23.5 ± 0.6 | 23.5 ± 0.5 | 23.2 ± 0.2 | 17.8 ± 3.9 | $: p = 0.9803; †: p = 0.1197; £: p = 0.0046 |
| Pt (N/cm2) | 5.1 ± 1.7 | 2.5 ± 0.7 | 2.7 ± 0.7 | 4.0 ± 1.2 | $: p = 0.0066; †: p = 0.4821; £: p = 0.0111 |
| Wmax (J/cm2) | 1.3 ± 0.5 | 0.7 ± 0.2 | 0.7 ± 0.4 | 1.5 ± 0.7 | $: p = 0.0276; †: p = 0.6852; £: p = 0.0085 |
| Pmax (W/cm2) | 9.0 ± 3.8 | 5.8 ± 0.9 | 4.7 ± 1.6 | 11.0 ± 4.9 | $: p = 0.0709; †: p = 0.1329; £: p = 0.0217 |
| Smax (L/Lo) | 0.32 ± 0.06 | 0.28 ± 0.07 | 0.24 ± 0.03 | 0.34 ± 0.10 | $: p = 0.3457; †: p = 0.1532; £: p = 0.1936 |
| Vmax (Lo/s) | 3.5 ± 1.3 | 3.8 ± 0.8 | 2.7 ± 0.9 | 4.5 ± 2.0 | $: p = 0.6766; †: p = 0.0353; £: p = 0.3433 |
Values (mean ± SD) for twitch contraction time (CT), twitch half-relaxation time (½ RT), peak twitch force (Pt), maximum mechanical work (Wmax), maximum mechanical power (Pmax), peak shortening (Smax), and peak shortening velocity (Vmax) of diaphragm muscle from the following groups: wild-type (n = 7), mdx (n = 7), mdx + tempol in vitro (n = 7) and mdx + tempol in vivo (n = 8). For the mdx + tempol in vitro group, diaphragm muscle preparations were studied in Krebs solution containing 1 mM tempol in vitro. The mdx + tempol in vivo group received 1 mM tempol in their drinking water for two weeks. Data were statistically compared by unpaired Student’s t tests with Welch’s correction where appropriate. $: Wild-Type vs. mdx; †: mdx vs. mdx Tempol in vitro; £: mdx vs. mdx Tempol in vivo.
3.2. Isotonic Contractile Parameters and Kinetics
Table 1 shows data for diaphragm muscle isotonic contractile parameters. Wmax was significantly lower in mdx compared with wild type diaphragm (p = 0.0276; Student t test). Wmax was significantly elevated in mdx + tempol in vivo compared with mdx (p = 0.0085). Pmax was lower in mdx diaphragm compared with wild-type, but this did not reach statistical significance (p = 0.0709). Pmax was significantly elevated in mdx + tempol in vivo compared with mdx (p = 0.0217), completely restoring Pmax to wild-type values. No significant differences were noted for Smax between groups. Bath application of tempol to mdx diaphragm significantly decreased Vmax compared with mdx (p = 0.0353), but had no effect on Wmax or Pmax.
3.3. Isotonic Load Relationships
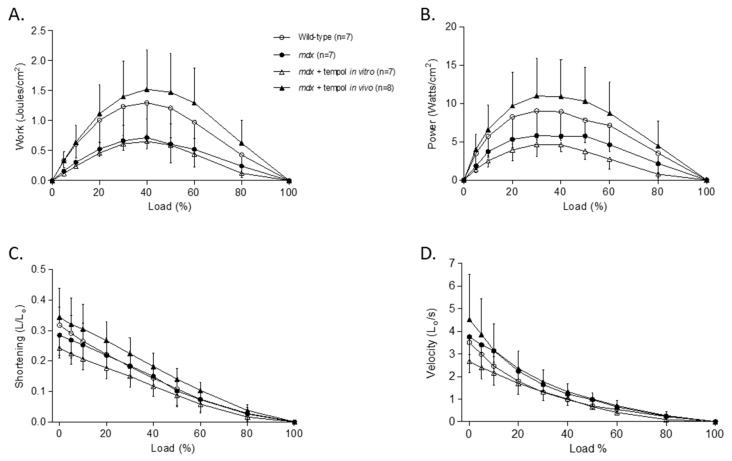
Figure 2A–D shows data for diaphragm muscle isotonic load relationships. Loading had a significant effect on work (p < 0.0001; two-way ANOVA; Figure 2A), power (p < 0.0001; Figure 2B), shortening (p < 0.0001; Figure 2C) and shortening velocity (p < 0.0001; Figure 2D) for diaphragm muscle from all four groups. Work (p = 0.0071) and power production (p = 0.0115) were significantly reduced in mdx diaphragm compared with wild-type. Tempol supplementation in vivo in mdx mice significantly increased work (p = 0.0063) and power (p = 0.0177) production compared with mdx. Bath application of tempol significantly reduced mdx diaphragm power production (p = 0.0037) and shortening velocity (p = 0.0159) compared with mdx.
Figure 2.
Diaphragm Muscle Isotonic Contractile Properties. Group data (mean ± SD) for work—(A), power—(B), shortening—(C) and shortening velocity—(D) load relationships for diaphragm muscle from wild-type (n = 7), mdx (n = 7), mdx + tempol in vitro (n = 7) and mdx + tempol in vivo (n = 8). For the mdx + tempol in vitro group, diaphragm muscle preparations were studied in Krebs solution containing 1 mM tempol in vitro. The mdx + tempol in vivo group received 1 mM tempol in their drinking water for two weeks. Data were statistically compared by two-way analysis of variance (ANOVA). Work: Load: p < 0.0001; Gene: p = 0.0071; tempol in vitro: p = 0.5020; tempol in vivo: p = 0.0063; Power: Load: p < 0.0001; Gene: p = 0.0115; tempol in vitro: p = 0.0037; tempol in vivo: p = 0.0177; Shortening: Load: p < 0.0001; Gene: p = 0.7068; tempol in vitro: p = 0.0995; tempol in vivo: p = 0.1117; Velocity: Load: p < 0.0001; Gene: p = 0.1756; tempol in vitro: p = 0.0159; tempol in vivo: p = 0.5427.
3.4. Metabolic Enzyme Activity
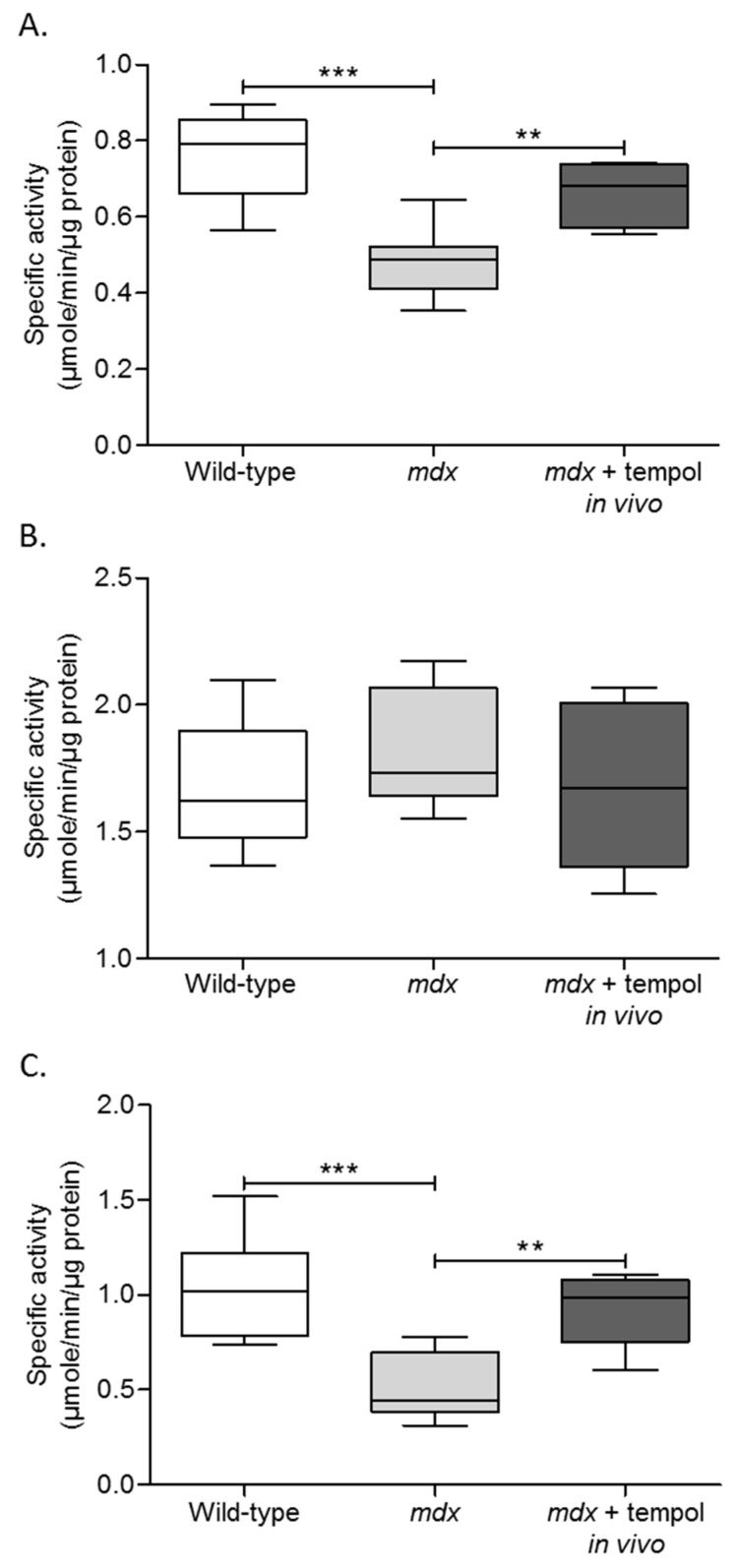
Figure 3A–C shows data for diaphragm muscle metabolic enzyme activities. Citrate synthase activity was significantly lower in mdx diaphragm compared with wild-type (p = 0.0003; unpaired Student’s t test; Figure 3A). Chronic tempol supplementation in mdx significantly increased diaphragm citrate synthase activity compared with mdx (p = 0.005). No significant differences were noted between groups for diaphragm phosphofructokinase activity (Figure 3B).
Figure 3.
Metabolic Enzyme Activities. Group data for citrate synthase (A), phosphofructokinase (B), and lactate dehydrogenase (C) enzyme activities in diaphragm muscle from wild-type, mdx and mdx + tempol in vivo. mdx + tempol in vivo received 1 mM tempol in their drinking water for two weeks. Values are expressed as box and whisker plots (median, 25–75% centiles and minimum and maximum values), and data were statistically compared by unpaired Student’s t tests. (A) *** p = 0.0003; ** p = 0.005. (C) *** p = 0.001; ** p = 0.0018.
Lactate dehydrogenase activity was significantly lower in mdx diaphragm compared with wild-type (p = 0.001; Figure 3C). Chronic tempol supplementation in mdx significantly increased diaphragm lactate dehydrogenase activity compared with mdx (p = 0.0018).
4. Discussion
The main findings of this study are (1) diaphragm muscle weakness in mdx mice is evidenced by reduced specific force, work and power output; (2) dystrophin-deficiency in mdx diaphragm is associated with reduced citrate synthase and lactate dehydrogenase enzyme activities, whereas phosphofructokinase activity is equivalent to wild-type; (3) chronic tempol supplementation in mdx mice completely restored diaphragm force- and power-generating capacity; (4) chronic tempol supplementation significantly increased mdx diaphragm citrate synthase and lactate dehydrogenase enzyme activities to wild-type levels; (5) acute bath application of tempol had limited effects on dystrophic diaphragm function ex vivo.
Diaphragm muscle thickening and fat accumulation are described in DMD patients, associated with diaphragm muscle weakness [38,39]. Ventilatory capacity decreases with age, since DMD is a progressive disease [8,40]. Respiratory failure is a leading cause of mortality in DMD. Although DMD is primarily caused by a genetic abnormality resulting in the absence of the dystrophin protein, many secondary pathologies have been identified as contributing to the muscle pathology observed in DMD, including inflammation, Ca2+ dysregulation, and redox stress [5,26].
Diaphragm muscle degeneration, fibrosis, and dysfunction are reported in mdx mice, which display an overt pathology similar to human DMD [16]. In the present study, we report diaphragm weakness in mdx mice consistent with other reports [15,16,41]. Diaphragm weakness in mdx was characterized by reduced twitch and tetanic muscle force and reduced Wmax. Diaphragm muscle mechanical work and power generation were reduced across the load continuum, revealing severe intrinsic mechanical dysfunction in dystrophic diaphragm muscle. These functional deficits were associated with altered metabolic enzyme activities, characterized by reduced citrate synthase and lactate dehydrogenase activity. Citrate synthase catalyzes the first step within the Krebs cycle, the condensation of acetyl-coenzyme A with oxaloacetate to form citrate, and serves as a marker of the mitochondrial matrix. Reduced citrate synthase activity suggests reduced mitochondrial activity, and hence, aerobic capacity in mdx diaphragm. This reduction in mitochondrial activity may relate to a loss of mitochondrial content, resulting in reduced oxidative capacity, or may be due to oxidative stress-induced mitochondrial dysfunction or mitophagy. Phosphofructokinase catalyzes the phosphorylation of fructose-6-phosphate to fructose-1,6-bisphosphate, thus functioning as a key regulatory step in the glycolytic pathway. Phosphofructokinase activity was unchanged in mdx diaphragm compared with wild-type. Lactate dehydrogenase catalyzes the forward and backward conversion of pyruvate to lactate, with the skeletal muscle isoform kinetically favoring conversion from pyruvate to lactate. Since glycolysis, which diverts pyruvate to lactate, serves to diminish redox stress [42], reductions in lactate dehydrogenase activity can cause oxidative stress associated with increased cellular oxygen consumption [43]. Metabolic reprogramming in mdx and DMD muscle is complex, and likely temporally regulated in dynamic fashion. Indeed, contrary to our observation, a recent report revealed elevated activity in oxidative enzymes of the Krebs cycle in mdx diaphragm [44].
Indicators of oxidative stress, such as 4-hydroxynonenal (4-HNE) and dihydroethdium (DHE), are increased in mdx diaphragm, revealing increased oxidative stress which likely has functional implications [17]. Antioxidant enzymes, such as superoxide dismutase, catalase and glutathione peroxidase, are elevated in limb muscle from mdx mice [20], which may contribute to the mild limb muscle phenotype which is observed in mdx mice compared with the diaphragm muscle, which more closely represents the human diaphragm pathology. Studies have shown that NOX subunits and superoxide production are increased in skeletal muscle of mdx mice [21,45]. Oxidative stress contributes to cardiomyocyte dysfunction in dystrophic hearts, and NOX2 inhibition resulted in restoration of calcium handling and contractility, and reduced collagen expression in cardiomyocytes [19,24]. The NOX subunits gp91phox, p67phox and Rac1 are increased in the tibialis anterior of mdx mice, associated with increased superoxide production [21].
Tempol has previously been shown to have positive inotropic effects on rat pharyngeal dilator (respiratory) muscle performance ex vivo [28,29]. Interestingly, tempol was shown to ameliorate upper airway muscle weakness in a rat model of chronic intermittent hypoxia [29]. In a similar model of chronic intermittent hypoxia, tempol exerted modest effects on diaphragm muscle force, but reversed chronic intermittent hypoxia-induced diaphragm fatigue [30]. Chronic tempol supplementation has also been shown to prevent sustained hypoxia induced pharyngeal dilator muscle weakness [31], but not diaphragm muscle dysfunction [37]. Collectively, these studies illustrate that tempol can exert inotropic effects on rodent respiratory muscle and ameliorate muscle weakness in models of hypoxic stress via superoxide scavenging, although this capacity appears muscle specific and may be dependent on the mode of hypoxia (sustained versus intermittent). This may have relevance to putative hypoxia-dependent remodeling in mdx muscle. DMD patients [9,46] and mdx mice [47,48] hypoventilate. DMD patients exhibit nocturnal hypoxemia and sleep-disordered breathing [49,50], and exposure to chronic intermittent hypoxia has been shown to further weaken mdx diaphragm [51]. Therefore, hypoxic stress may be implicated in mdx and DMD pathology, such that antioxidant strategies (such as tempol), proven to prevent hypoxia-dependent respiratory muscle weakness and fatigue, may be especially useful.
Since elevated superoxide production is reported in the diaphragm muscle of mdx mice, we sought to examine the efficacy of the superoxide scavenger tempol on mdx diaphragm muscle function. To our knowledge, this is the first report on the effects of tempol on muscle function and metabolic enzyme activity in the mdx mouse. Chronic tempol supplementation resulted in increased diaphragm force generation, revealed both in twitch and tetanic contractions, impressively restoring these functional indices to values equivalent to wild-type values. Work- and power-generating capacity expressed as a function of load were significantly increased in diaphragm from tempol supplemented mice compared with mdx, resulting in a complete restoration of work- and power-generating capacity. Wmax and Pmax were similarly significantly increased. Interestingly, tempol decreased ½ RT for mdx diaphragm, suggesting an increased rate of calcium reuptake into the sarcoplasmic reticulum during muscle relaxation. These functional improvements in mdx diaphragm are likely attributable to scavenging of excessive ROS in mdx diaphragm [17,21,45], and improvements in calcium handling.
In the current study, the finding that acute bath application of tempol significantly reduced mdx diaphragm Vmax, demonstrates the functional significance of ROS signaling, most likely superoxide, within mdx diaphragm muscle preparations. Basal ROS are known to have physiological roles in cross-bridge cycling and muscle force generation [11]. However, elevated levels of ROS orchestrate a pro-oxidant environment, which can serve to impair cross-bridge cycling, and thus, reduce force production. A tonic inhibitory effect of ROS on respiratory muscle function, suppressing force-generating capacity, was revealed by the observation of positive inotropic effects of acute bath application of the superoxide scavengers, tempol, and tiron [28]. Moreover, H2O2 has been shown to cause concentration-dependent inotropic effects on rat respiratory muscle function, with high concentrations of ROS resulting in muscle weakness and fatigue, an effect blocked by catalase [52]. Notwithstanding the capacity for antioxidant scavenging to acutely affect muscle performance, the general lack of effect of bath application of tempol in mdx diaphragm is not surprising. We posit that chronically elevated levels of ROS in mdx diaphragm induce structural abnormalities that cannot be reversed by acute tempol application. Rather, it is evident that chronic antioxidant supplementation is required to re-establish redox homeostasis, returning ROS to physiological levels, limiting redox stress, allowing restoration of muscle function. The functional improvements in mdx diaphragm extended to restoration of citrate synthase and lactate dehydrogenase enzyme activities. Improved metabolic enzyme activity has obvious consequences for improved mdx diaphragm performance. We used enzyme activity as a surrogate marker for oxidative stress, since these proteins are often targets of redox stress, and can in turn be drivers of redox stress owing to alterations in bioenergetics. We interpret the restoration of aerobic and glycolytic enzyme activities as indirect evidence of the efficacy of tempol in ameliorating oxidative stress in mdx diaphragm, with the important added benefit of restoring energy homeostasis and ATP-generating capacity.
Previously, EUK-134, a novel catalytic mimetic of superoxide dismutase and catalase, was used to examine the role of oxidative stress in mdx diaphragm pathology. EUK-134 reduced markers of oxidative stress, inflammation, and indicators of muscle damage in mdx diaphragm, although only a partial rescue of diaphragm force was observed [53]. Treatment of mdx mice with ascorbic acid, an antioxidant and free radical scavenger, was shown to reduce creatine kinase levels, myonecrosis, inflammation, and the levels of 4-HNE [54]. These studies further highlight the close relationship between ROS and inflammation in dystrophic diaphragm. Beyond antioxidant effects, targeting ROS using antioxidant interventions in mdx mice may also have non-specific anti-inflammatory actions, further supporting their use as a potential therapeutic strategy in DMD. In respect of interventional studies in DMD, our data suggest that superoxide scavengers warrant attention. Tempol is not approved for use in humans, but dietary and/or pharmacotherapies mimicking its actions could prove beneficial in DMD. Many studies have revealed beneficial effects of antioxidants on muscle integrity (Table 2), but few studies have assessed the effects of antioxidants on diaphragm force generating capacity. It will be interesting to determine how tempol compares to previous studies in respect of improvements in diaphragm structure and quality.
Table 2.
Overview of studies assessing antioxidant intervention in mdx mice.
| Antioxidant | Author | Classification | Model | Age | Dose/Method of Delivery | Tissue Examined | Results |
|---|---|---|---|---|---|---|---|
| α-lipoic acid/L-carnitine | Hnia K. et al., 2007 [55] | Free radical scavenger | mdx mouse | 5 weeks old | 250 mg/kg α-lipoic acid/L-carnitine i.p injection for 14 days | Diaphragm | α-lipoic acid/L-carnitine decreased plasma CK levels and decreased muscle fibre central nucleation and fibre variance, antioxidant activity, lipid peroxidation, NF-kB and matrix metalloproteinase activity in mdx diaphragm. Β-dystroglycan expression was increased in mdx diaphragm following α-lipoic acid/L-carnitine. |
| Apocynin | Gonzalez D.R. et al., 2014 [24] | NADPH oxidase inhibitor | mdx cardiac myocytes | - | 100 µM apocynin in vitro | Isolated cardiac myocytes | Apocynin restored contractility in mdx cardiac myocytes and normalised the amplitude of evoked intracellular Ca2+ concentration transients and total SR Ca2+ content. The production of spontaneous diastolic Ca2+ release events was decreased and SR Ca2+ leakage was decreased, thus apocynin improved SR Ca2+ handling and contractility in mdx cardiac myocytes. |
| Ascorbic acid (vitamin C) | Tonon E. et al., 2012 [54] | Antioxidant | mdx mouse | 14 days old | Ascorbic acid 200 mg/kg via oral gavage daily for 14 days | Diaphragm | Ascorbic acid decreased plasma CK levels and diaphragm myonecrosis, inflammation, TNF-α and 4-HNE levels and Evans blue dye staining in mdx mice. |
| Cilostazol | Hermes Tde A.E. et al., 2016 [56] | PDE3 inhibitor | mdx mouse | 14 days old | Cilostazol 100 mg/kg/day for 14 days | Diaphragm | Cilostazol reduced plasma CK and diaphragm myonecrosis, inflammatory cell area and macrophage infiltration, NF-kB and TNF-α content, ROS production and oxidative stress in mdx mice. |
| Diacerhein | Mâncio R.D. et al., 2017 [57] | IL-1β inhibitor | mdx mouse | 14 days old | 20 mg/kg/day diacerhein via oral gavage for 14 days | Diaphragm | Diacerhin reduced plasma CK levels, diaphragm muscle fibre damage and central nucleation, inflammatory mediators, oxidative stress and lipid peroxidation in mdx mice. |
| EUK-134 | Kim J.H. and Lawler J.M. 2012 [53] | Superoxide dismutase mimetic | mdx mouse | 20 days old | 30 mg/kg/day EUK-134 i.p. injection for 8 days | Diaphragm | EUK-134 reduced 4-HNE, total hydroperoxides, positive staining of macrophages and T-cells, activation of NF-κB, p65 protein abundance and the number of centralised myonuclei and variability of fibre size in diaphragm muscle from mdx mice. Diaphragm contractile force was partially rescued following EUK-134 and increased citrate synthase activity in mdx mice. |
| Epigallocatechin-3-gallate (EGCG) | Nakae Y. et al., 2008 [58] | Green tea extract/antioxidant/Polyphenol | mdx mouse | From birth | 5 mg/kg EGCG s.c. injection 4 times per week for 8 weeks | Diaphragm | EGCG had no effect on body weight and no observable toxic effects in the liver and kidney. EGCG decreased plasma CK and decreased the number of lipofuscin granules, necrotic muscle fibres and connective tissue in mdx diaphragm and increased utrophin expression. EGCG did not affect diaphragm isometric force. |
| SNT-NC17/Idebenone | Buyse G.M. et al., 2009 [59] | Antioxidant | mdx mouse | 4 weeks old | 200 mg/kg SNT-MC17/idebenone for 9 months | Heart | SNT-NC17/Idebenone corrected cardiac diastolic dysfunction, improved contractile reserve and voluntary running and decreased cardiac inflammation and fibrosis in mdx mice. |
| L-arginine | Marques M.J. et al., 2010 [60] | Amino acid | mdx mouse | 6 months old | L-arginine in drinking water for 6 months | Heart | L-arginine had no effect on myocardial fibrosis but reduced the density of inflammatory cells in the mdx heart. |
| N-acetylcysteine (NAC) | Williams I.A. and Allen D.G. 2007 [19] | Glutathione precursor | mdx mouse | 3 weeks old | 1% NAC in drinking water for 6 weeks | Heart | NAC reduced DHE levels in mdx hearts, reduced abnormalities in mdx cardiomyocyte Ca2+ handling, returned mdx fractional shortening to WT values but did not affect Ca2+ sensitivity. NAC returned collagen type III and CD68 expression in mdx hearts to WT values. |
| N-acetylcysteine (NAC) | de Senzi Moraes Pinto R. et al., 2013 [61] | Glutathione precursor | mdx mouse | 14 days old | 150 mg/kg NAC i.p. daily for 14 days | Diaphragm | NAC reduced plasma CK levels and reduced TNF-α and 4-HNE protein adduct levels, inflammation, Evans blue dye staining and myonecrosis in mdx diaphragm muscle. |
| Resveratrol | Kuno A. et al., 2013 [62] | SIRT1 activator | mdx mouse | 9 weeks old | 4 g/kg resveratrol enriched diet for 32 weeks | Heart | Resveratrol downregulated the pro-hypertrophic co-activator p300 protein level in the mdx heart thus inhibiting fibre hypertrophy. Resveratrol also suppressed cardiac fibrosis and preserved cardiac diastolic function in mdx hearts. |
| Pentoxifylline | Gosselin L.E. and Williams J.E. 2006 [63] | PDE inhibitor | mdx mouse | 4 weeks old | 16 mg/kg/day pentoxyifylline for 4 weeks | Diaphragm | Pentoxyifylline had no effect on mdx diaphragm force, hydroxyproline concentration, type I and III procollagen mRNA and TGF-β mRNA. |
| Pentoxifylline | Burdi R. et al., 2009 [64] | PDE inhibitor | mdx mouse | 4–5 weeks old | 50 mg/kg/day pentoxyifylline i.p. injection for 4–8 weeks | Diaphragm | Pentoxifylline modestly increased mdx diaphragm isometric tetanic force. |
| Pyrrolidine dithiocarbamate (PDTC) or ursodeoxycholic acid(UDCA) | Graham K.M. et al., 2010 [65] | NF-κB inhibitors | mdx mouse | 30 days old | 50 mg/kg/day PDTC i.p. injection for one month 40 mg/kg/day UDCA i.p. injection for one month |
Diaphragm | Neither PDTC or UDCA influenced collagen deposition or TGF-β1 expression in mdx diaphragm. |
| Quercetin | Hollinger K. et al., 2015 [66] | PGC-1α pathway activator | mdx mouse | 3 months old | 0.2% quercetin-enriched diet for 6 months | Diaphragm | Quercetin preserved diaphragm muscle fibres and reduced centralised nuclei, infiltrating immune cells, TNF-α gene expression and muscle fibrosis in mdx mice. Genes associated with oxidative metabolism were increased following quercetin. |
| Quercetin | Selsby J.T. et al., 2016 [67] | PGC-1α pathway activator | mdx mouse | 2 months old | 0.2% quercetin-enriched diet for 12 months | Diaphragm | Quercetin protected respiratory function in mdx mice during the first 4–6 months and declined thereafter. Mdx diaphragm muscle function and histology were not preserved following 12 months of quercetin treatment. |
| Quercetin | Ballmann C. et al., 2017 [68] | PGC-1α pathway activator | mdx mouse | 2 months old | 0.2% quercetin-enriched diet for 12 months | Heart | Quercetin decreased fibronectin, inflammation and indices of tissue damage while mitochondrial biogenesis and antioxidant enzymes were improved, and quercetin facilitated the assembly of the DAPC in mdx hearts. |
| Quercetin | Ballmann C. et al., 2015 [69] | PGC-1α pathway activator | mdx mouse | 3 weeks old 3 months old |
0.2% quercetin-enriched diet for 6 months | Heart | 3 weeks old: Quercetin increased cytochrome-c and superoxide dismutase 2 protein expression, increased utrophin and decreased matrix metalloproteinase 9 abundance in mdx heart. 3 months old: Quercetin decreased relative and absolute heart weights, damage indicators and TGFβ-1 in mdx heart. |
| Sildenafil | Percival J.M. et al., 2012 [70] | PDE-5 inhibitor | mdx mouse | 3 weeks old | 400 mg/L sildenafil citrate in drinking water for 14 weeks | Diaphragm | Sildenafil modestly increased diaphragm force generating capacity and reduced fibronectin, TNF-α, matrix metalloproteinase 13 and Evans blue dye staining in the mdx diaphragm. Fatigue resistance and TGF-β were unaffected. |
| Vitamin E | Mancio R.D. et al., 2017 [71] | Peroxyl radical scavenger | mdx mouse | 14 day old | 40 mg vitamin E/kg daily via oral gavage for 14 days | Diaphragm | Vitamin E reduced muscle fibre damage, oxidative stress and inflammation processes in mdx diaphragm. |
List of abbreviations: 4-HNE, 4-Hydroxynonenal; Ca2+, calcium; CD68, cluster of differentiation 68; CK, creatine kinase; DHE, dihydroethidium; EGCG, epigallocatechin-3-gallate; IL-1β, interleukin-1 beta; i.p., intra-peritoneal; MMP, matrix metalloproteinase; NAC, N-acetylcysteine; NADPH, nicotinamide adenine dinucleotide phosphate-oxidase; NFκB, nuclear factor lappa-light-chain-enhancer of activated B cells; PDE, phosphodiesterase; PDTC, pyrrolidine dithiocarbamate; PGC-1α, peroxisome proliferator activated receptor gamma co-activator 1 alpha; ROS, reactive oxygen species; SR, sarcoplasmic reticulum; s.c., sub-cutaneous; SIRT-1, sirtuin-1;TGF-β1, tumour growth factor beta 1; TNF-α, tumour necrosis factor alpha; UDCA, ursodeoxycholic acid.
The current study has relevance to therapeutic interventions in DMD, particularly those aimed at alleviating respiratory deficits in DMD boys. Since diaphragmatic weakness is observed in DMD boys, and likely contributes to breathing disturbances and the development of respiratory failure, strategies aimed at improving diaphragm muscle functional capacity in DMD are attractive. In respect of the current data set, it would be interesting in future studies to examine the effects of tempol supplementation on respiratory and cardiac function in mdx mice.
Limitations
We acknowledge the current study employed a single dose of tempol at one time point in a progressive disease. However, notably, our intervention resulted in full recovery of diaphragm force. Future research should examine dose-dependent effects of tempol in mdx mice on respiratory and non-respiratory muscle structure and function. Mitochondrial function and direct measures of oxidative stress should be examined to determine the mechanism of force recovery in mdx diaphragm following tempol supplementation.
5. Conclusions
In conclusion, our findings show that tempol supplementation in mdx mice is efficacious, serving to restore diaphragm force- and power-generating capacity to levels equivalent to wild-type values. Functional improvements were accompanied by restoration of citrate synthase and lactate dehydrogenase enzyme activities in mdx diaphragm. Our study implicates superoxide anions and downstream ROS as pivotal mediators of dystrophin-deficient respiratory muscle pathophysiology. Recovery of diaphragm muscle contractile function was impressive in our study highlighting the potential utility of tempol and other superoxide scavengers in the treatment of DMD.
Acknowledgments
David P. Burns and Izza Ali were supported by funding from the Department of Physiology, University College Cork. Clement Rieux received Erasmus funding from the European Union. We are grateful to staff of the Biological Services Unit, University College Cork for their support in the breeding and maintenance of the murine colonies.
Author Contributions
David P. Burns and Ken D. O’Halloran conceived and designed the experiments; David P. Burns, Izza Ali, Clement Rieux, James Healy and Greg Jasionek performed the experiments; David P. Burns, Izza Ali, Clement Rieux, and Greg Jasionek analyzed the data; David P. Burns and Ken D. O’Halloran performed statistical analysis and interpretation of the data, and wrote the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Emery A.E. Population frequencies of inherited neuromuscular diseases—A world survey. Neuromuscul. Disord. 1991;1:19–29. doi: 10.1016/0960-8966(91)90039-U. [DOI] [PubMed] [Google Scholar]
- 2.Blake D.J., Weir A., Newey S.E., Davies K.E. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol. Rev. 2002;82:291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- 3.Ehmsen J., Poon E., Davies K. The dystrophin-associated protein complex. J. Cell Sci. 2002;115:2801–2803. doi: 10.1242/jcs.115.14.2801. [DOI] [PubMed] [Google Scholar]
- 4.Kanagawa M., Toda T. The genetic and molecular basis of muscular dystrophy: Roles of cell-matrix linkage in the pathogenesis. J. Hum. Genet. 2006;51:915–926. doi: 10.1007/s10038-006-0056-7. [DOI] [PubMed] [Google Scholar]
- 5.Whitehead N.P., Yeung E.W., Allen D.G. Muscle damage in mdx (dystrophic) mice: Role of calcium and reactive oxygen species. Clin. Exp. Pharmacol. Physiol. 2006;33:657–662. doi: 10.1111/j.1440-1681.2006.04394.x. [DOI] [PubMed] [Google Scholar]
- 6.Yiu E.M., Kornberg A.J. Duchenne muscular dystrophy. Neurol. India. 2008;56:236–247. doi: 10.1111/jpc.12868. [DOI] [PubMed] [Google Scholar]
- 7.Hahn A., Bach J.R., Delaubier A., Renardel-Irani A., Guillou C., Rideau Y. Clinical implications of maximal respiratory pressure determinations for individuals with duchenne muscular dystrophy. Arch. Phys. Med. Rehabil. 1997;78:1–6. doi: 10.1016/S0003-9993(97)90001-0. [DOI] [PubMed] [Google Scholar]
- 8.Khirani S., Ramirez A., Aubertin G., Boulé M., Chemouny C., Forin V., Fauroux B. Respiratory muscle decline in duchenne muscular dystrophy. Pediatr. Pulmonol. 2014;49:473–481. doi: 10.1002/ppul.22847. [DOI] [PubMed] [Google Scholar]
- 9.Smith P.E., Edwards R.H., Calverley P.M. Ventilation and breathing pattern during sleep in duchenne muscular dystrophy. Chest. 1989;96:1346–1351. doi: 10.1378/chest.96.6.1346. [DOI] [PubMed] [Google Scholar]
- 10.Barbieri E., Sestili P. Reactive oxygen species in skeletal muscle signaling. J. Signal Transduct. 2012;2012:982794. doi: 10.1155/2012/982794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reid M.B. Invited review: Redox modulation of skeletal muscle contraction: What we know and what we don’t. J. Appl. Physiol. 2001;90:724–731. doi: 10.1152/jappl.2001.90.2.724. [DOI] [PubMed] [Google Scholar]
- 12.Allen D.G., Whitehead N.P. Duchenne muscular dystrophy—What causes the increased membrane permeability in skeletal muscle? Int. J. Biochem. Cell Biol. 2011;43:290–294. doi: 10.1016/j.biocel.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Jackson M.J. Control of reactive oxygen species production in contracting skeletal muscle. Antioxid. Redox Signal. 2011;15:2477–2486. doi: 10.1089/ars.2011.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen D.G., Gervasio O.L., Yeung E.W., Whitehead N.P. Calcium and the damage pathways in muscular dystrophy. Can. J. Physiol. Pharmacol. 2010;88:83–91. doi: 10.1139/Y09-058. [DOI] [PubMed] [Google Scholar]
- 15.Coirault C., Pignol B., Cooper R.N., Butler-Browne G., Chabrier P.E., Lecarpentier Y. Severe muscle dysfunction precedes collagen tissue proliferation in mdx mouse diaphragm. J. Appl. Physiol. (1985) 2003;94:1744–1750. doi: 10.1152/japplphysiol.00989.2002. [DOI] [PubMed] [Google Scholar]
- 16.Stedman H.H., Sweeney H.L., Shrager J.B., Maguire H.C., Panettieri R.A., Petrof B., Narusawa M., Leferovich J.M., Sladky J.T., Kelly A.M. The mdx mouse diaphragm reproduces the degenerative changes of duchenne muscular dystrophy. Nature. 1991;352:536–539. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- 17.Moraes L.H., de Burgos R.R., Macedo A.B., de Almeida Hermes T., de Faria F.M., Minatel E. Reduction of oxidative damage and inflammatory response in the diaphragm muscle of mdx mice using iron chelator deferoxamine. Biol. Trace Elem. Res. 2015;167:115–120. doi: 10.1007/s12011-015-0290-y. [DOI] [PubMed] [Google Scholar]
- 18.Burns D.P., Roy A., Lucking E.F., McDonald F.B., Gray S., Wilson R.J., Edge D., O’Halloran K.D. Sensorimotor control of breathing in the mdx mouse model of duchenne muscular dystrophy. J. Physiol. 2017;595:6653–6672. doi: 10.1113/JP274792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams I.A., Allen D.G. The role of reactive oxygen species in the hearts of dystrophin-deficient mdx mice. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H1969–H1977. doi: 10.1152/ajpheart.00489.2007. [DOI] [PubMed] [Google Scholar]
- 20.Kaczor J.J., Hall J.E., Payne E., Tarnopolsky M.A. Low intensity training decreases markers of oxidative stress in skeletal muscle of mdx mice. Free Radic. Biol. Med. 2007;43:145–154. doi: 10.1016/j.freeradbiomed.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Whitehead N.P., Yeung E.W., Froehner S.C., Allen D.G. Skeletal muscle NADPH oxidase is increased and triggers stretch-induced damage in the mdx mouse. PLoS ONE. 2010;5:e15354. doi: 10.1371/journal.pone.0015354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon B.S., Delgado-Diaz D.C., Carson J., Fayad R., Wilson L.B., Kostek M.C. Resveratrol improves muscle function but not oxidative capacity in young mdx mice. Can. J. Physiol. Pharmacol. 2014;92:243–251. doi: 10.1139/cjpp-2013-0350. [DOI] [PubMed] [Google Scholar]
- 23.Comim C.M., Cassol O.J., Jr., Constantino L.C., Constantino L.S., Petronilho F., Tuon L., Vainzof M., Dal-Pizzol F., Quevedo J. Oxidative variables and antioxidant enzymes activities in the mdx mouse brain. Neurochem. Int. 2009;55:802–805. doi: 10.1016/j.neuint.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez D.R., Treuer A.V., Lamirault G., Mayo V., Cao Y., Dulce R.A., Hare J.M. NADPH oxidase-2 inhibition restores contractility and intracellular calcium handling and reduces arrhythmogenicity in dystrophic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2014;307:H710–H721. doi: 10.1152/ajpheart.00890.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ismail H.M., Scapozza L., Ruegg U.T., Dorchies O.M. Diapocynin, a dimer of the NADPH oxidase inhibitor apocynin, reduces ROS production and prevents force loss in eccentrically contracting dystrophic muscle. PLoS ONE. 2014;9:e110708. doi: 10.1371/journal.pone.0110708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawler J.M. Exacerbation of pathology by oxidative stress in respiratory and locomotor muscles with duchenne muscular dystrophy. J. Physiol. 2011;589:2161–2170. doi: 10.1113/jphysiol.2011.207456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capogrosso R.F., Cozzoli A., Mantuano P., Camerino G.M., Massari A.M., Sblendorio V.T., De Bellis M., Tamma R., Giustino A., Nico B., et al. Assessment of resveratrol, apocynin and taurine on mechanical-metabolic uncoupling and oxidative stress in a mouse model of duchenne muscular dystrophy: A comparison with the gold standard, α-methyl prednisolone. Pharmacol. Res. 2016;106:101–113. doi: 10.1016/j.phrs.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Skelly J.R., Bradford A., Jones J.F., O’Halloran K.D. Superoxide scavengers improve rat pharyngeal dilator muscle performance. Am. J. Respir. Cell Mol. Biol. 2010;42:725–731. doi: 10.1165/rcmb.2009-0160OC. [DOI] [PubMed] [Google Scholar]
- 29.Skelly J.R., Edge D., Shortt C.M., Jones J.F., Bradford A., O’Halloran K.D. Tempol ameliorates pharyngeal dilator muscle dysfunction in a rodent model of chronic intermittent hypoxia. Am. J. Respir. Cell Mol. Biol. 2012;46:139–148. doi: 10.1165/rcmb.2011-0084OC. [DOI] [PubMed] [Google Scholar]
- 30.Shortt C.M., Fredsted A., Chow H.B., Williams R., Skelly J.R., Edge D., Bradford A., O’Halloran K.D. Reactive oxygen species mediated diaphragm fatigue in a rat model of chronic intermittent hypoxia. Exp. Physiol. 2014;99:688–700. doi: 10.1113/expphysiol.2013.076828. [DOI] [PubMed] [Google Scholar]
- 31.Lewis P., Sheehan D., Soares R., Varela Coelho A., O’Halloran K.D. Chronic sustained hypoxia-induced redox remodeling causes contractile dysfunction in mouse sternohyoid muscle. Front. Physiol. 2015;6:122. doi: 10.3389/fphys.2015.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis P., O’Halloran K.D. Diaphragm muscle adaptation to sustained hypoxia: Lessons from animal models with relevance to high altitude and chronic respiratory diseases. Front. Physiol. 2016;7:623. doi: 10.3389/fphys.2016.00623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards J.N., Macdonald W.A., van der Poel C., Stephenson D.G. O2•− production at 37 °C plays a critical role in depressing tetanic force of isolated rat and mouse skeletal muscle. Am. J. Physiol. Cell Physiol. 2007;293:C650–C660. doi: 10.1152/ajpcell.00037.2007. [DOI] [PubMed] [Google Scholar]
- 34.Burns D.P., O’Halloran K.D. Evidence of hypoxic tolerance in weak upper airway muscle from young mdx mice. Respir. Physiol. Neurobiol. 2016;226:68–75. doi: 10.1016/j.resp.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 35.O’Halloran K.D. Effects of nicotine on rat sternohyoid muscle contractile properties. Respir. Physiol. Neurobiol. 2006;150:200–210. doi: 10.1016/j.resp.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 36.Burns D.P., Rowland J., Canavan L., Murphy K.H., Brannock M., O’Malley D., O’Halloran K.D., Edge D. Restoration of pharyngeal dilator muscle force in dystrophin-deficient (mdx) mice following co-treatment with neutralizing interleukin-6 receptor antibodies and urocortin 2. Exp. Physiol. 2017;102:1177–1193. doi: 10.1113/EP086232. [DOI] [PubMed] [Google Scholar]
- 37.Lewis P., Sheehan D., Soares R., Coelho A.V., O’Halloran K.D. Redox remodeling is pivotal in murine diaphragm muscle adaptation to chronic sustained hypoxia. Am. J. Respir. Cell Mol. Biol. 2016;55:12–23. doi: 10.1165/rcmb.2015-0272OC. [DOI] [PubMed] [Google Scholar]
- 38.Beck J., Weinberg J., Hamnegård C.H., Spahija J., Olofson J., Grimby G., Sinderby C. Diaphragmatic function in advanced duchenne muscular dystrophy. Neuromuscul. Disord. 2006;16:161–167. doi: 10.1016/j.nmd.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 39.De Bruin P.F., Ueki J., Bush A., Khan Y., Watson A., Pride N.B. Diaphragm thickness and inspiratory strength in patients with duchenne muscular dystrophy. Thorax. 1997;52:472–475. doi: 10.1136/thx.52.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baydur A., Gilgoff I., Prentice W., Carlson M., Fischer D.A. Decline in respiratory function and experience with long-term assisted ventilation in advanced Duchenne’s muscular dystrophy. Chest. 1990;97:884–889. doi: 10.1378/chest.97.4.884. [DOI] [PubMed] [Google Scholar]
- 41.Manning J., Buckley M.M., O’Halloran K.D., O’Malley D. Combined XIL-6R and urocortin-2 treatment restores MDX diaphragm muscle force. Muscle Nerve. 2017;56:E134–E140. doi: 10.1002/mus.25644. [DOI] [PubMed] [Google Scholar]
- 42.Brand K.A., Hermfisse U. Aerobic glycolysis by proliferating cells: A protective strategy against reactive oxygen species. FASEB J. 1997;11:388–395. doi: 10.1096/fasebj.11.5.9141507. [DOI] [PubMed] [Google Scholar]
- 43.Le A., Cooper C.R., Gouw A.M., Dinavahi R., Maitra A., Deck L.M., Royer R.E., Vander Jagt D.L., Semenza G.L., Dang C.V. Inhibition of lactate dehydrogenase a induces oxidative stress and inhibits tumor progression. Proc. Natl. Acad. Sci. USA. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Comim C.M., Hoepers A., Ventura L., Freiberger V., Dominguini D., Mina F., Mendonça B.P., Scaini G., Vainzof M., Streck E.L., et al. Activity of krebs cycle enzymes in mdx mice. Muscle Nerve. 2016;53:91–95. doi: 10.1002/mus.24704. [DOI] [PubMed] [Google Scholar]
- 45.Whitehead N.P., Kim M.J., Bible K.L., Adams M.E., Froehner S.C. A new therapeutic effect of simvastatin revealed by functional improvement in muscular dystrophy. Proc. Natl. Acad. Sci. USA. 2015;112:12864–12869. doi: 10.1073/pnas.1509536112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hukins C.A., Hillman D.R. Daytime predictors of sleep hypoventilation in duchenne muscular dystrophy. Am. J. Respir. Crit. Care Med. 2000;161:166–170. doi: 10.1164/ajrccm.161.1.9901057. [DOI] [PubMed] [Google Scholar]
- 47.Burns D., Edge D., O’Malley D., O’Halloran K. Respiratory control in the mdx mouse model of duchenne muscular dystrophy. Adv. Exp. Med. Biol. 2015;860:239–244. doi: 10.1007/978-3-319-18440-1_27. [DOI] [PubMed] [Google Scholar]
- 48.Mosqueira M., Baby S.M., Lahiri S., Khurana T.S. Ventilatory chemosensory drive is blunted in the mdx mouse model of Duchenne Muscular Dystrophy (DMD) PLoS ONE. 2013;8:e69567. doi: 10.1371/journal.pone.0069567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barbé F., Quera-Salva M.A., McCann C., Gajdos P., Raphael J.C., de Lattre J., Agustí A.G. Sleep-related respiratory disturbances in patients with Duchenne muscular dystrophy. Eur. Respir. J. 1994;7:1403–1408. doi: 10.1183/09031936.94.07081403. [DOI] [PubMed] [Google Scholar]
- 50.Smith P.E., Edwards R.H., Calverley P.M. Oxygen treatment of sleep hypoxaemia in Duchenne muscular dystrophy. Thorax. 1989;44:997–1001. doi: 10.1136/thx.44.12.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farkas G.A., McCormick K.M., Gosselin L.E. Episodic hypoxia exacerbates respiratory muscle dysfunction in DMD(mdx) mice. Muscle Nerve. 2007;36:708–710. doi: 10.1002/mus.20858. [DOI] [PubMed] [Google Scholar]
- 52.Shortt C.M., O’Halloran K.D. Hydrogen peroxide alters sternohyoid muscle function. Oral. Dis. 2014;20:162–170. doi: 10.1111/odi.12084. [DOI] [PubMed] [Google Scholar]
- 53.Kim J.H., Lawler J.M. Amplification of proinflammatory phenotype, damage, and weakness by oxidative stress in the diaphragm muscle of mdx mice. Free Radic. Biol. Med. 2012;52:1597–1606. doi: 10.1016/j.freeradbiomed.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 54.Tonon E., Ferretti R., Shiratori J.H., Santo Neto H., Marques M.J., Minatel E. Ascorbic acid protects the diaphragm muscle against myonecrosis in mdx mice. Nutrition. 2012;28:686–690. doi: 10.1016/j.nut.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 55.Hnia K., Hugon G., Rivier F., Masmoudi A., Mercier J., Mornet D. Modulation of p38 mitogen-activated protein kinase cascade and metalloproteinase activity in diaphragm muscle in response to free radical scavenger administration in dystrophin-deficient mdx mice. Am. J. Pathol. 2007;170:633–643. doi: 10.2353/ajpath.2007.060344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hermes T.e.A., Macedo A.B., Fogaça A.R., Moraes L.H., de Faria F.M., Kido L.A., Cagnon V.H., Minatel E. Beneficial cilostazol therapeutic effects in mdx dystrophic skeletal muscle. Clin. Exp. Pharmacol. Physiol. 2016;43:259–267. doi: 10.1111/1440-1681.12521. [DOI] [PubMed] [Google Scholar]
- 57.Mâncio R.D., Hermes T.A., Macedo A.B., Mizobuti D.S., Rupcic I.F., Minatel E. Dystrophic phenotype improvement in the diaphragm muscle of mdx mice by diacerhein. PLoS ONE. 2017;12:e0182449. doi: 10.1371/journal.pone.0182449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakae Y., Hirasaka K., Goto J., Nikawa T., Shono M., Yoshida M., Stoward P.J. Subcutaneous injection, from birth, of epigallocatechin-3-gallate, a component of green tea, limits the onset of muscular dystrophy in mdx mice: A quantitative histological, immunohistochemical and electrophysiological study. Histochem. Cell Biol. 2008;129:489–501. doi: 10.1007/s00418-008-0390-2. [DOI] [PubMed] [Google Scholar]
- 59.Buyse G.M., Van der Mieren G., Erb M., D’hooge J., Herijgers P., Verbeken E., Jara A., Van Den Bergh A., Mertens L., Courdier-Fruh I., et al. Long-term blinded placebo-controlled study of SNT-MC17/idebenone in the dystrophin deficient mdx mouse: Cardiac protection and improved exercise performance. Eur. Heart J. 2009;30:116–124. doi: 10.1093/eurheartj/ehn406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marques M.J., Barbin I.C., Taniguti A.P., Oggian D.S., Ferretti R., Santo Neto H. Myocardial fibrosis is unaltered by long-term administration of L-arginine in dystrophin deficient mdx mice: A histomorphometric analysis. Acta Biol. Hung. 2010;61:168–174. doi: 10.1556/ABiol.61.2010.2.5. [DOI] [PubMed] [Google Scholar]
- 61.De Senzi Moraes Pinto R., Ferretti R., Moraes L.H., Neto H.S., Marques M.J., Minatel E. N-acetylcysteine treatment reduces TNF-α levels and myonecrosis in diaphragm muscle of mdx mice. Clin. Nutr. 2013;32:472–475. doi: 10.1016/j.clnu.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 62.Kuno A., Hori Y.S., Hosoda R., Tanno M., Miura T., Shimamoto K., Horio Y. Resveratrol improves cardiomyopathy in dystrophin-deficient mice through Sirt1 protein-mediated modulation of p300 protein. J. Biol. Chem. 2013;288:5963–5972. doi: 10.1074/jbc.M112.392050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gosselin L.E., Williams J.E. Pentoxifylline fails to attenuate fibrosis in dystrophic (mdx) diaphragm muscle. Muscle Nerve. 2006;33:820–823. doi: 10.1002/mus.20523. [DOI] [PubMed] [Google Scholar]
- 64.Burdi R., Rolland J.F., Fraysse B., Litvinova K., Cozzoli A., Giannuzzi V., Liantonio A., Camerino G.M., Sblendorio V., Capogrosso R.F., et al. Multiple pathological events in exercised dystrophic mdx mice are targeted by pentoxifylline: Outcome of a large array of in vivo and ex vivo tests. J. Appl. Physiol. 2009;106:1311–1324. doi: 10.1152/japplphysiol.90985.2008. [DOI] [PubMed] [Google Scholar]
- 65.Graham K.M., Singh R., Millman G., Malnassy G., Gatti F., Bruemmer K., Stefanski C., Curtis H., Sesti J., Carlson C.G. Excessive collagen accumulation in dystrophic (mdx) respiratory musculature is independent of enhanced activation of the NF-kappaB pathway. J. Neurol. Sci. 2010;294:43–50. doi: 10.1016/j.jns.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hollinger K., Shanely R.A., Quindry J.C., Selsby J.T. Long-term quercetin dietary enrichment decreases muscle injury in mdx mice. Clin. Nutr. 2015;34:515–522. doi: 10.1016/j.clnu.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 67.Selsby J.T., Ballmann C.G., Spaulding H.R., Ross J.W., Quindry J.C. Oral quercetin administration transiently protects respiratory function in dystrophin-deficient mice. J. Physiol. 2016;594:6037–6053. doi: 10.1113/JP272057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ballmann C., Denney T., Beyers R.J., Quindry T., Romero M., Selsby J.T., Quindry J.C. Long-term dietary quercetin enrichment as a cardioprotective countermeasure in mdx mice. Exp. Physiol. 2017;102:635–649. doi: 10.1113/EP086091. [DOI] [PubMed] [Google Scholar]
- 69.Ballmann C., Hollinger K., Selsby J.T., Amin R., Quindry J.C. Histological and biochemical outcomes of cardiac pathology in mdx mice with dietary quercetin enrichment. Exp. Physiol. 2015;100:12–22. doi: 10.1113/expphysiol.2014.083360. [DOI] [PubMed] [Google Scholar]
- 70.Percival J.M., Whitehead N.P., Adams M.E., Adamo C.M., Beavo J.A., Froehner S.C. Sildenafil reduces respiratory muscle weakness and fibrosis in the mdx mouse model of Duchenne muscular dystrophy. J. Pathol. 2012;228:77–87. doi: 10.1002/path.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mâncio R.D., Hermes T.A., Macedo A.B., Mizobuti D.S., Valduga A.H., Rupcic I.F., Minatel E. Vitamin E treatment decreases muscle injury in mdx mice. Nutrition. 2017;43–44:39–46. doi: 10.1016/j.nut.2017.07.003. [DOI] [PubMed] [Google Scholar]