Abstract
Increasing evidence indicates that the adult neurogenic niche of the ventricular-subventricular zone (V-SVZ), beyond serving as a potential site of origin, affects the outcome of malignant brain cancers. Glioma contact with this niche predicts worse prognosis, suggesting a supportive role for the V-SVZ environment in tumor initiation or progression. In this review, we describe unique components of the V-SVZ that may permit or promote tumor growth within the region. Cell-cell interactions, soluble factors, and extracellular matrix composition are discussed, and the role of the niche in future therapies is explored. The purpose of this review is to highlight niche intrinsic factors that may promote or support malignant cell growth and maintenance, and point out how we might leverage these features to improve patient outcome.
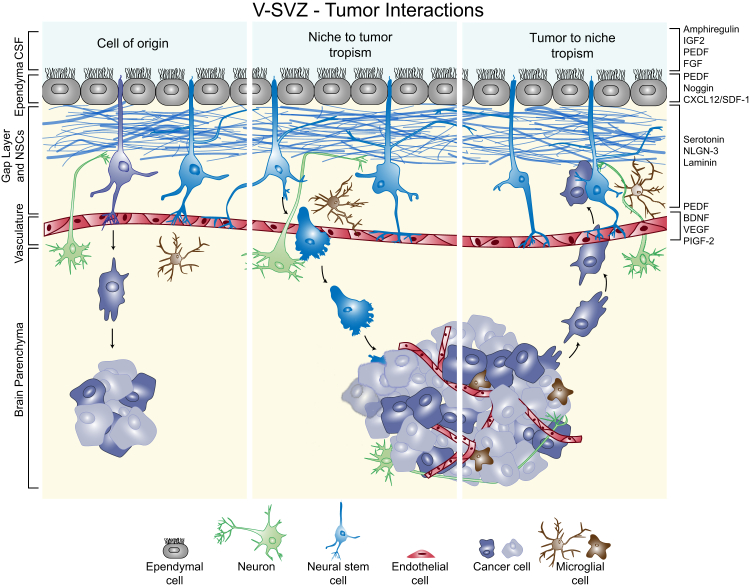
Extensive work during the past decades has demonstrated the existence of two neurogenic niches in the adult mammalian brain: the ventricular-subventricular zone (V-SVZ) and the subgranular zone (SGZ). The cellular constituents, intercellular interactions, and extracellular components of these niches support stem cell maintenance and differentiation.1, 2, 3 The V-SVZ is the larger of the two niches, and recently there has been increased focus on the role of this niche in high-grade (III and IV) gliomas, the most common primary malignant neoplasms of the adult brain. Interest in the V-SVZ heightened with the emergence of the cancer stem cell theory, which posits that a fraction of cancer cells are self-renewing progenitors at the apex of a cancer cell hierarchy, capable of generating all cell types found in a tumor.4 This hypothesis is supported by similarities in gene expression between non-neoplastic stem cells and cancer cells, as well as by their shared capacity for proliferation. In the setting of brain cancer, it has been proposed that neural stem cells of the V-SVZ are cells of origin for brain cancers, although more recent tumor models implicate additional progenitor and mature cells in tumor development (Figure 1).5, 6 The development of neoplasia after genetic ablation of tumor suppressors and exogenous up-regulation of growth factors in the rodent V-SVZ have further supported this hypothesis.7 Cell of origin notwithstanding, the concept of a stem-like niche within tumors is one with significant therapeutic implications.8, 9 In addition to the possibility that gliomas originate within the V-SVZ, some tumors may co-opt this niche, taking advantage of an existing system that promotes proliferation and migration of progenitor cells in early development. In support of this hypothesis, radiographic studies show that contact with the V-SVZ is a negative prognostic factor for grade IV gliomas.10 Given that neural stem cell niche components may enhance glioma initiation, maintenance, and/or recurrence, the interaction between the V-SVZ and tumor cells warrants investigation, and this review will focus on the interplay between adult stem cell niches and neoplastic cells in this context. We will briefly introduce the V-SVZ niche and summarize the unique features that may provide a selective advantage to cancerous cells.
Figure 1.
The ventricular-subventricular zone (V-SVZ) niche contains ependymal cells (gray) that contact the lateral ventricle and cerebrospinal fluid (CSF). Neural stem cells (NSCs; blue) have an apical contact with the CSF and a basal contact with the vasculature (red). In the human, astrocytic processes (blue) lie beneath the ependyma. Neurons (green) from the brain parenchyma innervate the niche. Surveying or resting microglia (brown) surveil the niche microenvironment and can become activated in the presence of tumor cells. Three proposed roles of the niche in malignant brain tumors are depicted. Left panel: Neural stem cells may acquire mutations that lead to cancer (purple). Middle panel: Neural stem cells can home toward tumors and eliminate tumor cells. Right panel: Tumor cells can migrate toward the V-SVZ and take up residence in the niche. A subset of factors demonstrated to be involved in these regions and discussed in the text (Direction from the Top: CSF Factors in Normal and Malignant Biology, Local Associations: Cellular Constituents of the Niche, Basal Foundations: Vascular Contact, The Niche as a Refuge) are listed on the right. BDNF, brain-derived neurotrophic factor; FGF, fibroblast growth factor; IGF2, insulin-like growth factor 2; NLGN-3, neuroligin-3; PEDF, pigment epithelium-derived factor; PIGF-2, placental growth factor 2; SDF-1, stromal-derived factor 1; VEGF, vascular endothelial growth factor.
Distinct Cellular Neighborhoods: The V-SVZ and SGZ
The two regions of adult neurogenesis, the V-SVZ and the SGZ, contain multiple cell types and specialized contacts, including a prominent vascular component. These features cooperate to maintain an environment permissive to ongoing neurogenesis. The V-SVZ (sometimes referred to as the SVZ or the subependymal zone) is the larger of these two niches and is located immediately adjacent to the lateral ventricles in the cerebrum. The rodent V-SVZ primarily generates interneurons destined for the olfactory bulb, and the early postnatal human brain recapitulates this production of olfactory interneurons. The pediatric human V-SVZ also contributes interneurons to the ventromedial prefrontal cortex via a medial migratory stream and a large population of newly born cells to additional forebrain areas through a structure termed the Arc.11, 12 In adult humans, robust migration to the olfactory bulb is absent, and V-SVZ neurogenesis appears to be a rare event, although limited contribution of neurons to the neighboring striatum may occur.13, 14 Adult V-SVZ generation of mature neurons can occur in the setting of brain injury, as has been shown in adult rats after ischemic stroke.15
The astrocyte-like neural stem cells (NSCs) of the V-SVZ have a polarity defined by an apical primary cilium immersed in ventricular cerebrospinal fluid (CSF) and a basal vascular contact, resulting in a cytoarchitecture reminiscent of embryonic radial glia progenitors.16 Ependymal cells within this niche line the lateral walls of the lateral ventricles and also contact the CSF, into which they project motile cilia to aid in CSF flow.17, 18 Ependymal cell bodies are arranged in pinwheels around the apical contacts of the NSCs (a planar organization not seen in nonneurogenic regions of the ventricular system).16, 19 NSCs produce rapidly dividing C cells (alias transit-amplifying cells) that undergo a limited number of divisions to produce neuroblasts, which then migrate out of the niche and eventually generate mature neurons.20 The neural stem cell bodies remain in close contact with neuroblasts, whereas their long, radial glia-esque processes contact the blood vessels that supply nutrients and oxygen to the niche.2, 20 Microglial cells are also found within the niche, as are contacts from neighboring and distant neurons.13, 21, 22, 23, 24
The SGZ is located at the interface of the hilus and dentate gyrus in the hippocampus.2 It, too, contains radial-glia–like neural stem cells that produce neurons via transit-amplifying cells. In this niche, the apical portion of the NSC contacts a blood vessel, the highly branched opposite process contacts neuronal processes and glial cells, and the cell body contacts mature granule neurons.25, 26 In contrast to V-SVZ NSCs, SGZ NSCs do not extend processes into the ventricles to contact CSF, a point of difference between these germinal regions that may be relevant to tumor progression.
V-SVZ Contact and Patient Outcome
High-grade gliomas, most notably grade IV glioblastomas (GBMs), are defined by their invasive presentation, and a subset of these tumors appears to spread specifically within the ventricular-subventricular zone (alias subependymal spread).27, 28, 29 V-SVZ spread is evaluated by magnetic resonance imaging in pediatric and adult gliomas and is interpreted as the presence of contrast enhancement and/or abnormally elevated T2-weighted signal within the subependymal region.30, 31, 32 Although microscopic analysis of this region is uncommon because of the rarity of resections that include the V-SVZ, histologic sections show increased cell density at resection, including cytologically atypical glial cells with enlarged, hyperchromatic, angular nuclei.33 Immunohistochemistry of a cell-cycle–associated antigen, Ki-67, can be used to highlight the atypical population, because it is normally infrequently expressed in adult V-SVZ.11, 13 Preferential spread in the V-SVZ region has been described in high-grade astrocytoma autopsy cases.29 Likewise, in pediatric diffuse intrinsic pontine gliomas, contact with the ventricle or V-SVZ correlates with V-SVZ tumor infiltration and nodule formation along the ventricle.34 In addition, both isocitrate dehydrogenase wild-type and isocitrate dehydrogenase–mutant GBMs as well as brain metastases have demonstrated such spread through the subependyma.27, 28 Although isocitrate dehydrogenase–mutant gliomas occur more frequently in the frontal lobe than isocitrate dehydrogenase wild-type tumors, there appears to be no difference between the two groups in V-SVZ contact.35 However, the literature on the topic is sparse and warrants further investigation.
Recent evaluation of clinical data indicates that glioblastoma patients whose tumors infiltrate or contact the V-SVZ have worse outcomes. A meta-analysis of multiple studies demonstrated that radiographic contact of GBM with the V-SVZ is associated with significantly decreased overall survival,10 independent of extent of tumor resection.36 V-SVZ contacting glioblastomas also display earlier recurrence after treatment compared with V-SVZ noncontacting GBMs,10, 36 and these recurrences are more likely to contact the V-SVZ.37, 38 Some studies further describe a tendency for V-SVZ contacting glioblastomas to be multifocal at diagnosis36, 38, 39 and to recur after treatment at sites distant from the initial tumor site,39, 40, 41 although the latter observation is debated.36, 42 Strikingly, GBM contact with the SGZ has not been found to influence survival,36 suggesting that features unique to the V-SVZ contribute to outcome. The prognostic value of V-SVZ contact may be attributable, in part, to cancer cell access to the ventricles and CSF, a feature unique to the V-SVZ niche. However, the V-SVZ and SGZ differ in several additional ways, including the presence of a gap layer in the V-SVZ that is absent in the SGZ and the closer proximity of the V-SVZ to major white matter tracts. At the molecular level, differences between secreted factors in the V-SVZ and SGZ remain poorly defined. Niche-enriched factors may be derived from different cellular sources, present at different levels, or delivered through specific cell-cell contacts. Each of these variations might affect signaling between niche and tumor cells. Cumulatively, these clinical data suggest a possible distinct, aggressive biology of V-SVZ contacting tumors, although attempts to date have failed to identify transcriptional signatures unique to V-SVZ contacting tumors. Only a limited number of candidate signatures have been found, which may not be cancer cell derived.43 Alternatively, the impact of V-SVZ contact raises the possibility of niche-derived factors that positively affect one or more aspects of tumor biology, including glioma growth, therapy resistance, dissemination, and immunomodulation.
Direction from the Top: CSF Factors in Normal and Malignant Biology
Cerebrospinal fluid is produced by the choroid plexus and fills the ventricles. Ciliated ependymal cells, including those in the V-SVZ, help to maintain CSF flow.44, 45 CSF acts as a protective cushion for the central nervous system and a provider of secreted factors as well as a mechanism for waste removal to maintain homeostasis. The V-SVZ is the only neurogenic niche that directly contacts the ventricles, which contain soluble factors that regulate NSC quiescence and proliferation beyond those traditionally contained in stem cell culture medium (Figure 1).2, 46 The CSF milieu has been explored most thoroughly in the embryo, but the NSC regulatory factors insulin-like growth factor 2, amphiregulin, and pigment epithelium-derived factor (PEDF), which can promote NSC proliferation and self-renewal, have all been detected in adult brain.47, 48, 49 More important, these factors found in the CSF have also been shown to regulate glioma cell growth and may be a source of growth stimuli to malignant cells.50, 51, 52
In addition to providing NSCs and cancer cells with soluble factors and signals, the CSF is a potential reservoir for cancer cells and cancer cell–derived secreted factors. Along with radiographic imaging, analysis of CSF cytology obtained via lumbar puncture, although infrequently performed, is used for detection and diagnosis of malignancies.53 Tumor DNA can be detected in the CSF of cancer patients, especially in patients whose tumors are located at CSF-brain interfaces.54 Glioma cells are also known to release exosomes loaded with RNA species that can be detected in the CSF.55, 56 The CSF can also serve as an avenue for tumor diffusion in gliomas, although this phenomenon is clinically prominent only in a rare subset of GBMs with primitive neuronal components.57 Thus, the CSF may both supply neoplasms with mitogenic cues and harbor tumor cells with the potential to seed additional tumor sites.
Local Associations: Cellular Constituents of the Niche
NSC interactions with surrounding cells directly influence cell proliferation and stemness. V-SVZ ependymal cells preserve the self-renewal capacity of NSCs, in part by producing PEDF, which promotes symmetric division of NSCs and suppression of differentiation genes. PEDF can similarly suppress glioma stem cell differentiation and promote gliomasphere formation and sex determining region Y-box 2 expression.51 Ependymal cells also secrete the bone morphogenic protein inhibitor noggin, which may help maintain glioma cell tumor-initiating capacity,58, 59 and C-X-C motif chemokine (CXCL)12, which can induce glioma cell homing to the neurogenic niche (Figure 1).60, 61
In addition to local cell-derived signals, neuron-derived signals are another component of the V-SVZ niche. Whole-mount electron microscopy of murine ventricles has revealed serotonergic axons derived from soma in the raphe nuclei projecting along the ventricular walls amid ependymal microvilli. These cells release serotonergic vesicles that act on the serotonin receptors on NSCs and the choroid plexus. Activation of these receptors on the NSCs results in increased proliferation, whereas serotonin stimulation of the choroid plexus can result in release of fibroblast growth factor into the CSF, a known proliferation stimulant for normal and neoplastic cells.21, 62 Excitatory cholinergic neurons have been found to increase normal stem cell proliferation in the V-SVZ, and a population of ventral neurons adjacent to the murine V-SVZ directs fate specification through the production of sonic hedgehog.63, 64 Similarly, cortical neurons projecting to patient-derived glioma xenografts induced an increase in cell proliferation through neuroligin-3 stimulation, suggesting that excitatory neuronal stimulation can regulate glioma growth (Figure 1).65
NSCs can also communicate with each other. For example, NSCs in the SGZ generate gap junctions with each other via connexins 43 and 30. Without these gap junctions, there is a marked reduction in NSC proliferation and number of progeny, reminiscent of the critical role of connexin 43 in radial glia during early cortical development.66, 67 Glioma cells can also use connexin 43 to establish gap junctions, sometimes with normal astrocytes, that provide unique advantages by facilitating invasion of brain parenchyma. In this context, gap junctions may enhance glioma growth by permitting the exchange of factors, including ATP from astrocyte to glioma cell.68 Outside the cell, distinct laminin structures in the V-SVZ, called fractones, are morphologically characterized by thin stalk-like projections with intermittent bulbs. These structures are in close physical contact with the ependyma, endothelia, and NSCs and are hypothesized to serve as reservoirs of mitogens for NSCs.69 Glioma cells invading the niche may also contact these stores. Laminin is further observed on the ventricular face of the V-SVZ at the center of ependymal pinwheels, where ependymal cells and NSCs contact each other.70 Laminin signaling occurs via the interaction of dystroglycan on ependymal cells with integrin α6 on NSCs and is required for ependymal pinwheel formation and promotion of NSC interactions with blood vessels.70, 71 Integrin α6 expression is lost as NSCs differentiate.71 Glioma cells with stem cell characteristics (enrichment of CD133, oligodendrocyte transcription factor, and Nestin) also express integrin α6. These cells have increased sphere-forming capacity and tumor-propagating abilities relative to integrin-negative or bulk tumor cells. In addition, these cells are closely associated with the perivascular niche.72 These data suggest that the rich laminin environment of the V-SVZ may readily support a glioma stem cell population while inhibiting differentiation.
Basal Foundations: Vascular Contact
The endothelial cells and pericytes comprising the vascular elements of the V-SVZ are closely associated with NSCs (Figure 1). The V-SVZ vasculature consists of a planar plexus with large vessels that branch into smaller ones, unlike cortical vessels, which are smaller and branch more frequently.73 Most of the vessels in the brain, including those in the cortex and striatum, are encapsulated by pericytes and astrocytic end feet, which together compose the blood-brain barrier. In the V-SVZ, however, patches of vessels are devoid of this covering. At these sites, NSCs and transit-amplifying cells contact the vasculature, and cell division is observed.71, 74 Vasculature lacking pericytes and astrocytes appears to be more permissive to exchange of factors, as demonstrated by experiments in rodents showing that dye conjugates carried in the bloodstream can access the V-SVZ niche directly.73 These authors also found that soluble growth factors, hormones, nutrients, and oxygen from the blood can infiltrate the CSF via choroid plexus blood vessels, supporting a model whereby the V-SVZ niche receives support from the ventricular and vascular aspects of the niche.73 Not only do vascular elements regulate access to serum factors, but these cells can also directly stimulate niche signaling. Endothelial cell secretions include PEDF, brain-derived neurotrophic factor, placental growth factor 2, and vascular endothelial growth factor. Placental growth factor 2 and vascular endothelial growth factor enhance neuroblast proliferation, whereas PEDF promotes NSC self-renewal and vascular endothelial growth factor improves neuronal survival.75, 76, 77, 78, 79 To this end, NSCs with access to endothelial cell–cultured medium undergo increased symmetric self-renewing divisions relative to those cultured with cortical cells.74 Direct physical contact with endothelial cells prevents stem cell differentiation and promotes expression of stemness genes (such as Nestin and the Notch effector Hes5) by signaling through ephrinB2 and Jagged1.80 Blood vessels from different regions of the brain can also differentially influence NSC growth. Surprisingly, although both cortical and subventricular endothelial cells can result in an increase in NSC cell number in vitro, cortical endothelial cells do so to a greater degree.79
In high-grade gliomas, the blood-brain barrier is often compromised because of the high rate of angiogenesis and tortuous neovasculature. Furthermore, like normal stem cells, cancer stem-like cells tend to cluster near blood vessels.81 Incomplete coverage of vessels by support cells, including pericytes, has been observed in gliomas and raises the possibility that glioma cells may receive serum-derived factors that normally influence NSCs.82 These cues include growth signals as well as nutrients and oxygen. When medulloblastoma stem-like cells (expressing Nestin, CD133, or both) are co-cultured with endothelial cells, they maintain expression of Nestin and CD133 and form larger spheres. In vivo, coinjection of endothelial cells and medulloblastoma stem-like cells accelerates tumor growth.81 Glioma cells have also been shown to be able to assume a pericyte-like phenotype in vivo, including up-regulation of pericyte markers, a close physical association with the vasculature, and a functional role in maintaining tumor growth.83 This may be mediated by direct signaling from endothelial cells via the Notch ligand Jagged80 because Notch signaling in GBM cells has been shown to promote a pericyte cell phenotype and result in increased tumor vasculature.84 In addition, cancer stem cells secrete vascular endothelial growth factor and can promote endothelial cell migration, branching, and tube formation.85 Invasion of the V-SVZ, with its rich vascular support, may provide rapid tumor cell access to factors that support aggressive growth.
The Niche as a Refuge
Features that endow the V-SVZ niche with the ability to support and maintain neural stem cells may also cause it to serve as a refuge for neoplastic cells. In recent years, several factors have been identified that can attract or direct cells to this refuge. Ependymal and endothelial cells in the V-SVZ express the CXCL12 (alias stromal-derived factor 1), which facilitates tumor cell homing to stem cell niches in other cancers.86 GBM patient-derived xenografts were found to co-opt CXCL12/C-X-C chemokine receptor 4 signaling to occupy the V-SVZ and reside in the niche (Figure 1).61 Orthotopic xenografts of human glioma cells injected into the striatum of immunodeficient mice contained a subset of tumor cells that migrated toward the V-SVZ. Upon integration in the niche, the human cells expressed markers of NSCs and even began to migrate toward the olfactory bulb, suggesting that they were co-opting niche cues and adopting NSC behaviors. Dissection of these tumor cells from the host mouse and subsequent investigation of sphere-forming capacity and tumor initiation indicated that these cells were tumor propagating.61, 87 Furthermore, glioma cells expressing C-X-C chemokine receptor 4 found in the V-SVZ were shown to be more radioresistant in vitro and in vivo because of signaling through V-SVZ–derived CXCL12.88 More recently, conditioned medium from murine V-SVZ was shown to increase diffuse intrinsic pontine glioma cell invasiveness by producing a protein complex, including pleiotrophin, a neurite outgrowth promoting factor.89 In vivo interruption of either of these signaling mechanisms, stromal-derived factor 1 or pleiotrophin, reduced the number of identifiable cancer cells that reached the V-SVZ. These findings suggest that glioma recurrence and progression may be driven by tumor cell homing to the V-SVZ with subsequent adoption of niche-based, therapy-protective interactions.
A study of glioblastoma patients undergoing tumor resection guided by 5-aminolevulinic acid provided evidence for glioma cell migration to a neurogenic niche in humans. 5-Aminolevulinic acid is a metabolic precursor of fluorescent porphyrins that can accumulate in cancer cells.90 After peripheral injection of 5-aminolevulinic acid, fluorescent cells were detected in the V-SVZ in 65% of patients.33 Histologic analysis of resected tissue confirmed glioma involvement, and driver mutations matching the primary tumor samples were detected. Importantly, the V-SVZ resident cancer cells were capable of generating spheres in vitro and tumors in vivo, confirming that the cells found in the niche can be tumor propagating.33 Intriguingly, tumor cells found in the niche tended to be of the mesenchymal transcriptional subtype, regardless of the subtype assigned to the bulk tumor.33, 91 This finding suggests that either the V-SVZ niche imposes a similar gene expression profile on the glioma cells or a certain cell phenotype is particularly capable of V-SVZ infiltration.
Given that neurogenic niches may harbor cancer cells with tumor-propagating capabilities and increased radioresistance,88 some groups have retrospectively examined the impact of targeting the V-SVZ and SGZ with radiation therapy. Although a moderate survival benefit from niche irradiation has been reported, even in the absence of radiographically detectable contact, not all retrospective studies of such treatment found a survival benefit.92 In fact, a recent study involving 61 patients reported inferior survival when the ipsilateral neural stem cell compartment (V-SVZ and SGZ) received a high dose of radiation (55.2 Gy).93 The effect of radiation on noncancerous cells of the stem cell niche warrants consideration when interpreting survival effects. In rodents, it has been shown that either whole brain or V-SVZ targeted radiation can result in sustained loss of cell proliferation and neural precursor production. In patients, necrosis of the V-SVZ can occur after radiation treatment, and this complication correlates with worse performance status.94, 95, 96 At this time, there are two ongoing clinical trials to investigate the clinical efficacy of explicitly targeting or sparing the V-SVZ during irradiation (NCT02177578 and NCT01478854, respectively). The results of the only prospective clinical trial investigating neural stem cell niche irradiation in GBM97 are promising, reporting significantly improved overall survival when the V-SVZ was irradiated and a trend toward improved survival when the SGZ was irradiated.
Targeted Strikes: NSCs in Therapy
Although the stem cell niche may support the formation, maintenance, or recurrence of neoplasms, noncancerous NSCs have the potential to serve a therapeutic role. Normal neural stem cells exhibit targeted migration to sites of tumor engraftment and some antitumor effects (Figure 1). Rats receiving xenografts of glioma cells together with NSCs show improved survival in comparison to animals that receive glioma cells only.98 A survival benefit is maintained in a model of established tumors, when NSCs are administered 3 days after tumor engraftment. Furthermore, NSCs injected into brains millimeters outside the engrafted tumor will migrate toward the cancer cells, encapsulate the tumor, and penetrate the mass, even showing migration toward multifocal tumor sites separate from the bulk.99, 100 The NSCs do not infiltrate the surrounding brain parenchyma or contralateral hemisphere, suggesting they are specifically targeting the tumor. Migrating NSCs exert an antitumor effect, observed as a reduction in tumor volume 1 to 2 weeks after injection.98, 100 This effect is observed in multiple brain cancer types, including glioma and medulloblastoma.99
The ability of NSCs to seek out tumor cells has inspired a host of studies in which therapies are transported by NSCs for delivery to cancerous cells. Multiple strategies have been used for both the generation of tumor-homing NSCs and therapy delivery. Human neural stem cells and NSCs transdifferentiated from skin fibroblasts have both been shown to successfully target glioma cells in vitro and in vivo.101, 102 These engineered NSCs induce glioma cell apoptosis via expression of soluble tumor necrosis factor–α-related apoptosis–inducing ligand102, 103 and cytosine deaminase (paired with 5-formylcytosine).104 Therapeutic NSCs can promote glioma cell differentiation in addition to apoptosis.101 When applied to orthotopic xenograft models in rodents, NSC-delivered therapies have been shown to prolong survival. Importantly, the NSCs retain their ability to target tumor cells after steroid therapy or radiotherapy, both of which are routinely administered during patient care. In addition to effectively reducing tumor burden, these therapies may prove to have fewer off-target effects than currently used pharmaceuticals. Compared with soluble tumor necrosis factor–α-related apoptosis–inducing ligand administered intravenously or injected into the tumor, tumor necrosis factor–α-related apoptosis–inducing ligand secreted by NSCs was not detected in organs other than the brain, persisted through 24 hours, and resulted in tumor volume reduction.105 This result suggests that NSC-mediated therapies may show pharmacokinetic features preferable to routinely administered chemotherapies. The preliminary investigation of therapeutic NSCs for brain cancers is the basis of an ongoing clinical trial.104
Conclusions and Persisting Questions
The study of neurogenic niches in the adult brain is a rich and active field of research. Beyond the function of these sites as generators of new neurons, emerging data reveal significant effects of neurogenic niches on the behavior of malignant gliomas. Treatment options for these aggressive neoplasms are limited, and patient survival remains dismal; therefore, the potential role of these niches in tumor initiation, maintenance, or recurrence merits further research. Improvements in tumor therapy may include targeting niche factors and disrupting niche–tumor cell interactions, with radiotherapeutic targeting of the V-SVZ representing a first step along this course. As our knowledge of the normal niche continues to expand, newly revealed features may also drive better understanding of tumor cause and therapy response. Areas of interest include the impact of cell-to-cell heterogeneity and lineage priming within normal NSCs on the disease state and the contribution of microglia, the major innate immune population within the brain, to normal and tumor-bearing V-SVZ. Studies from the mouse brain indicate that neural stem cells are spatially diverse, meaning that stem cells from different regions of the V-SVZ produce different progeny.2, 63, 106, 107, 108, 109, 110 Examination of spatial differences in tumor-forming or tumor-homing capabilities of heterogeneous NSCs may further inform the design of targeted therapies. Detailed investigations of the role of innate and adaptive immune cells in this niche will also be critical to understanding how these tumors may evade immune detection or targeted immunotherapy approaches (eg, anti–programmed cell death protein 1/cytotoxic T-lymphocyte antigen-4 agents). Microglia help to define and maintain the neurogenic niche, and recent studies indicate that the V-SVZ resident population is functionally distinct and temporally dynamic, exhibiting an immature phenotype that changes with organismal age.23, 111 Finally, glioma research currently benefits from the ample available patient tissue from primary tumor resections, as well as a plethora of imaging data collected during routine care. One persistent challenge is the integration of molecular information (bulk and single-cell genomic and proteomic approaches) with spatial information obtained from clinical imaging. Recent advances in both the preparation of single-cell suspensions and the collection of high-dimensional data will enhance our ability to map specific populations of cancer and niche cells, providing a better understanding of the impact of V-SVZ niche diversity on tumor behavior.112, 113
Acknowledgments
We thank Akshitkumar Mistry, Nalin Leelatian, Allison Greenplate, Gabrielle Rushing, and members of the Ihrie and Irish laboratories (Vanderbilt University) for helpful discussion and critical feedback. We apologize to our colleagues whose work could not be discussed in depth within space limitations.
Footnotes
Neural Regeneration and Development Theme Issue
Supported by NIH/National Institute of Neurological Diseases and Stroke grant R01NS096238 (R.A.I.), Department of Defense Idea Development Award W81XWH-16-1-0171 (R.A.I.), Vanderbilt-Ingram Cancer Center (VICC) Ambassadors Award and Discovery Award P30 CA68485 (R.A.I.), a VICC Michael David Greene Brain Cancer Fund gift (R.A.I.), and Ann Faulkenberry Memorial Award SBTA-0001199 from the Southeastern Brain Tumor Foundation (R.A.I.).
Disclosures: None declared.
This article is part of a review series on neural regeneration and developmental biology in health and disease.
References
- 1.Silva-Vargas V., Crouch E.E., Doetsch F. Adult neural stem cells and their niche: a dynamic duo during homeostasis, regeneration, and aging. Curr Opin Neurobiol. 2013;23:935–942. doi: 10.1016/j.conb.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Fuentealba L.C., Obernier K., Alvarez-Buylla A. Adult neural stem cells bridge their niche. Cell Stem Cell. 2012;10:698–708. doi: 10.1016/j.stem.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonaguidi M.A., Song J., Ming G.L., Song H. A unifying hypothesis on mammalian neural stem cell properties in the adult hippocampus. Curr Opin Neurobiol. 2012;22:754–761. doi: 10.1016/j.conb.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reya T., Morrison S.J., Clarke M.F., Weissman I.L. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 5.Sanai N., Alvarez-Buylla A., Berger M.S. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353:811–822. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 6.Zong H., Parada L.F., Baker S.J. Cell of origin for malignant gliomas and its implication in therapeutic development. Cold Spring Harb Perspect Biol. 2015;7 doi: 10.1101/cshperspect.a020610. a020610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J., McKay R.M., Parada L.F. Malignant glioma: lessons from genomics, mouse models, and stem cells. Cell. 2012;149:36–47. doi: 10.1016/j.cell.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plaks V., Kong N., Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbertson R.J., Rich J.N. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 10.Mistry A.M., Hale A.T., Chambless L.B., Weaver K.D., Thompson R.C., Ihrie R.A. Influence of glioblastoma contact with the lateral ventricle on survival: a meta-analysis. J Neurooncol. 2017;131:125–133. doi: 10.1007/s11060-016-2278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanai N., Nguyen T., Ihrie R.A., Mirzadeh Z., Tsai H.H., Wong M., Gupta N., Berger M.S., Huang E., Garcia-Verdugo J.M., Rowitch D.H., Alvarez-Buylla A. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paredes M.F., James D., Gil-Perotin S., Kim H., Cotter J.A., Ng C., Sandoval K., Rowitch D.H., Xu D., McQuillen P.S., Garcia-Verdugo J.M., Huang E.J., Alvarez-Buylla A. Extensive migration of young neurons into the infant human frontal lobe. Science. 2016;354 doi: 10.1126/science.aaf7073. aaf7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanai N., Tramontin A.D., Quinones-Hinojosa A., Barbaro N.M., Gupta N., Kunwar S., Lawton M.T., McDermott M.W., Parsa A.T., Manuel-Garcia Verdugo J., Berger M.S., Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 14.Ernst A., Alkass K., Bernard S., Salehpour M., Perl S., Tisdale J., Possnert G., Druid H., Frisen J. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 15.Kernie S.G., Parent J.M. Forebrain neurogenesis after focal ischemic and traumatic brain injury. Neurobiol Dis. 2010;37:267–274. doi: 10.1016/j.nbd.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mirzadeh Z., Merkle F.T., Soriano-Navarro M., Garcia-Verdugo J.M., Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Bigio M.R. The ependyma: a protective barrier between brain and cerebrospinal fluid. Glia. 1995;14:1–13. doi: 10.1002/glia.440140102. [DOI] [PubMed] [Google Scholar]
- 18.Sawamoto K., Wichterle H., Gonzalez-Perez O., Cholfin J.A., Yamada M., Spassky N., Murcia N.S., Garcia-Verdugo J.M., Marin O., Rubenstein J.L., Tessier-Lavigne M., Okano H., Alvarez-Buylla A. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- 19.Kokovay E., Wang Y., Kusek G., Wurster R., Lederman P., Lowry N., Shen Q., Temple S. VCAM1 is essential to maintain the structure of the SVZ niche and acts as an environmental sensor to regulate SVZ lineage progression. Cell Stem Cell. 2012;11:220–230. doi: 10.1016/j.stem.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Doetsch F., Garcia-Verdugo J.M., Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong C.K., Chen J., Cebrian-Silla A., Mirzadeh Z., Obernier K., Guinto C.D., Tecott L.H., Garcia-Verdugo J.M., Kriegstein A., Alvarez-Buylla A. Axonal control of the adult neural stem cell niche. Cell Stem Cell. 2014;14:500–511. doi: 10.1016/j.stem.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoglinger G.U., Arias-Carrion O., Ipach B., Oertel W.H. Origin of the dopaminergic innervation of adult neurogenic areas. J Comp Neurol. 2014;522:2336–2348. doi: 10.1002/cne.23537. [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro Xavier A.L., Kress B.T., Goldman S.A., Lacerda de Menezes J.R., Nedergaard M. A distinct population of microglia supports adult neurogenesis in the subventricular zone. J Neurosci. 2015;35:11848–11861. doi: 10.1523/JNEUROSCI.1217-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dennis C.V., Suh L.S., Rodriguez M.L., Kril J.J., Sutherland G.T. Human adult neurogenesis across the ages: an immunohistochemical study. Neuropathol Appl Neurobiol. 2016;42:621–638. doi: 10.1111/nan.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seri B., Garcia-Verdugo J.M., Collado-Morente L., McEwen B.S., Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478:359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- 26.Palmer T.D., Willhoite A.R., Gage F.H. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Iacoangeli M., Di Rienzo A., Colasanti R., Zizzi A., Gladi M., Alvaro L., Nocchi N., Di Somma L.G., Scarpelli M., Scerrati M. Endoscopy-verified occult subependymal dissemination of glioblastoma and brain metastasis undetected by MRI: prognostic significance. Onco Targets Ther. 2012;5:449–456. doi: 10.2147/OTT.S39429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willard N., Kleinschmidt-DeMasters B.K. Massive dissemination of adult glioblastomas. Clin Neuropathol. 2015;34:330–342. doi: 10.5414/NP300882. [DOI] [PubMed] [Google Scholar]
- 29.Tamura M., Ohye C., Nakazato Y. Pathological anatomy of autopsy brain with malignant glioma. Neurol Med Chir (Tokyo) 1993;33:77–80. doi: 10.2176/nmc.33.77. [DOI] [PubMed] [Google Scholar]
- 30.Lim D.A., Cha S., Mayo M.C., Chen M.H., Keles E., VandenBerg S., Berger M.S. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol. 2007;9:424–429. doi: 10.1215/15228517-2007-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radbruch A., Lutz K., Wiestler B., Baumer P., Heiland S., Wick W., Bendszus M. Relevance of T2 signal changes in the assessment of progression of glioblastoma according to the Response Assessment in Neurooncology criteria. Neuro Oncol. 2012;14:222–229. doi: 10.1093/neuonc/nor200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu S., Wang Y., Fan X., Ma J., Ma W., Wang R., Jiang T. Anatomical involvement of the subventricular zone predicts poor survival outcome in low-grade astrocytomas. PLoS One. 2016;11:e0154539. doi: 10.1371/journal.pone.0154539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piccirillo S.G., Spiteri I., Sottoriva A., Touloumis A., Ber S., Price S.J., Heywood R., Francis N.J., Howarth K.D., Collins V.P., Venkitaraman A.R., Curtis C., Marioni J.C., Tavare S., Watts C. Contributions to drug resistance in glioblastoma derived from malignant cells in the sub-ependymal zone. Cancer Res. 2015;75:194–202. doi: 10.1158/0008-5472.CAN-13-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caretti V., Bugiani M., Freret M., Schellen P., Jansen M., van Vuurden D., Kaspers G., Fisher P.G., Hulleman E., Wesseling P., Vogel H., Monje M. Subventricular spread of diffuse intrinsic pontine glioma. Acta Neuropathol. 2014;128:605–607. doi: 10.1007/s00401-014-1307-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai A., Kharbanda S., Pope W.B., Tran A., Solis O.E., Peale F., Forrest W.F., Pujara K., Carrillo J.A., Pandita A., Ellingson B.M., Bowers C.W., Soriano R.H., Schmidt N.O., Mohan S., Yong W.H., Seshagiri S., Modrusan Z., Jiang Z., Aldape K.D., Mischel P.S., Liau L.M., Escovedo C.J., Chen W., Nghiemphu P.L., James C.D., Prados M.D., Westphal M., Lamszus K., Cloughesy T., Phillips H.S. Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J Clin Oncol. 2011;29:4482–4490. doi: 10.1200/JCO.2010.33.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mistry A.M., Dewan M.C., White-Dzuro G.A., Brinson P.R., Weaver K.D., Thompson R.C., Ihrie R.A., Chambless L.B. Decreased survival in glioblastomas is specific to contact with the ventricular-subventricular zone, not subgranular zone or corpus callosum. J Neurooncol. 2017;132:341–349. doi: 10.1007/s11060-017-2374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen L., Chaichana K.L., Kleinberg L., Ye X., Quinones-Hinojosa A., Redmond K. Glioblastoma recurrence patterns near neural stem cell regions. Radiother Oncol. 2015;116:294–300. doi: 10.1016/j.radonc.2015.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jafri N.F., Clarke J.L., Weinberg V., Barani I.J., Cha S. Relationship of glioblastoma multiforme to the subventricular zone is associated with survival. Neuro Oncol. 2013;15:91–96. doi: 10.1093/neuonc/nos268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adeberg S., Konig L., Bostel T., Harrabi S., Welzel T., Debus J., Combs S.E. Glioblastoma recurrence patterns after radiation therapy with regard to the subventricular zone. Int J Radiat Oncol Biol Phys. 2014;90:886–893. doi: 10.1016/j.ijrobp.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 40.Nestler U., Lutz K., Pichlmeier U., Stummer W., Franz K., Reulen H.J., Bink A., Group ALA Glioma Study Anatomic features of glioblastoma and their potential impact on survival. Acta Neurochir (Wien) 2015;157:179–186. doi: 10.1007/s00701-014-2271-x. [DOI] [PubMed] [Google Scholar]
- 41.Sonoda Y., Saito R., Kanamori M., Kumabe T., Uenohara H., Tominaga T. The association of subventricular zone involvement at recurrence with survival after repeat surgery in patients with recurrent glioblastoma. Neurol Med Chir (Tokyo) 2014;54:302–309. doi: 10.2176/nmc.oa.2013-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimura M., Lee Y., Miller R., Castillo M. Glioblastoma multiforme: relationship to subventricular zone and recurrence. Neuroradiol J. 2013;26:542–547. doi: 10.1177/197140091302600507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jungk C., Mock A., Exner J., Geisenberger C., Warta R., Capper D., Abdollahi A., Friauf S., Lahrmann B., Grabe N., Beckhove P., von Deimling A., Unterberg A., Herold-Mende C. Spatial transcriptome analysis reveals Notch pathway-associated prognostic markers in IDH1 wild-type glioblastoma involving the subventricular zone. BMC Med. 2016;14:170. doi: 10.1186/s12916-016-0710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siyahhan B., Knobloch V., de Zelicourt D., Asgari M., Schmid Daners M., Poulikakos D., Kurtcuoglu V. Flow induced by ependymal cilia dominates near-wall cerebrospinal fluid dynamics in the lateral ventricles. J R Soc Interface. 2014;11:20131189. doi: 10.1098/rsif.2013.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohata S., Alvarez-Buylla A. Planar organization of multiciliated ependymal (E1) cells in the brain ventricular epithelium. Trends Neurosci. 2016;39:543–551. doi: 10.1016/j.tins.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silva-Vargas V., Maldonado-Soto A.R., Mizrak D., Codega P., Doetsch F. Age-dependent niche signals from the choroid plexus regulate adult neural stem cells. Cell Stem Cell. 2016;19:643–652. doi: 10.1016/j.stem.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 47.Lehtinen M.K., Zappaterra M.W., Chen X., Yang Y.J., Hill A.D., Lun M., Maynard T., Gonzalez D., Kim S., Ye P., D'Ercole A.J., Wong E.T., LaMantia A.S., Walsh C.A. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69:893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thouvenot E., Urbach S., Dantec C., Poncet J., Seveno M., Demettre E., Jouin P., Touchon J., Bockaert J., Marin P. Enhanced detection of CNS cell secretome in plasma protein-depleted cerebrospinal fluid. J Proteome Res. 2008;7:4409–4421. doi: 10.1021/pr8003858. [DOI] [PubMed] [Google Scholar]
- 49.Falk A., Frisen J. Amphiregulin is a mitogen for adult neural stem cells. J Neurosci Res. 2002;69:757–762. doi: 10.1002/jnr.10410. [DOI] [PubMed] [Google Scholar]
- 50.Soroceanu L., Kharbanda S., Chen R., Soriano R.H., Aldape K., Misra A., Zha J., Forrest W.F., Nigro J.M., Modrusan Z., Feuerstein B.G., Phillips H.S. Identification of IGF2 signaling through phosphoinositide-3-kinase regulatory subunit 3 as a growth-promoting axis in glioblastoma. Proc Natl Acad Sci U S A. 2007;104:3466–3471. doi: 10.1073/pnas.0611271104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yin J., Park G., Kim T.H., Hong J.H., Kim Y.J., Jin X., Kang S., Jung J.E., Kim J.Y., Yun H., Lee J.E., Kim M., Chung J., Kim H., Nakano I., Gwak H.S., Yoo H., Yoo B.C., Kim J.H., Hur E.M., Lee J., Lee S.H., Park M.J., Park J.B. Pigment epithelium-derived factor (PEDF) expression induced by EGFRvIII promotes self-renewal and tumor progression of glioma stem cells. PLoS Biol. 2015;13:e1002152. doi: 10.1371/journal.pbio.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lorente M., Carracedo A., Torres S., Natali F., Egia A., Hernandez-Tiedra S., Salazar M., Blazquez C., Guzman M., Velasco G. Amphiregulin is a factor for resistance of glioma cells to cannabinoid-induced apoptosis. Glia. 2009;57:1374–1385. doi: 10.1002/glia.20856. [DOI] [PubMed] [Google Scholar]
- 53.Chhieng D.C., Elgert P., Cohen J.M., Jhala N.C., Cangiarella J.F. Cytology of primary central nervous system neoplasms in cerebrospinal fluid specimens. Diagn Cytopathol. 2002;26:209–212. doi: 10.1002/dc.10013. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y., Springer S., Zhang M., McMahon K.W., Kinde I., Dobbyn L., Ptak J., Brem H., Chaichana K., Gallia G.L., Gokaslan Z.L., Groves M.L., Jallo G.I., Lim M., Olivi A., Quinones-Hinojosa A., Rigamonti D., Riggins G.J., Sciubba D.M., Weingart J.D., Wolinsky J.P., Ye X., Oba-Shinjo S.M., Marie S.K., Holdhoff M., Agrawal N., Diaz L.A., Jr., Papadopoulos N., Kinzler K.W., Vogelstein B., Bettegowda C. Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc Natl Acad Sci U S A. 2015;112:9704–9709. doi: 10.1073/pnas.1511694112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akers J.C., Ramakrishnan V., Kim R., Skog J., Nakano I., Pingle S., Kalinina J., Hua W., Kesari S., Mao Y., Breakefield X.O., Hochberg F.H., Van Meir E.G., Carter B.S., Chen C.C. MiR-21 in the extracellular vesicles (EVs) of cerebrospinal fluid (CSF): a platform for glioblastoma biomarker development. PLoS One. 2013;8:e78115. doi: 10.1371/journal.pone.0078115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skog J., Wurdinger T., van Rijn S., Meijer D.H., Gainche L., Sena-Esteves M., Curry W.T., Jr., Carter B.S., Krichevsky A.M., Breakefield X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perry A., Miller C.R., Gujrati M., Scheithauer B.W., Zambrano S.C., Jost S.C., Raghavan R., Qian J., Cochran E.J., Huse J.T., Holland E.C., Burger P.C., Rosenblum M.K. Malignant gliomas with primitive neuroectodermal tumor-like components: a clinicopathologic and genetic study of 53 cases. Brain Pathol. 2009;19:81–90. doi: 10.1111/j.1750-3639.2008.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lim D.A., Tramontin A.D., Trevejo J.M., Herrera D.G., Garcia-Verdugo J.M., Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 59.Piccirillo S.G., Reynolds B.A., Zanetti N., Lamorte G., Binda E., Broggi G., Brem H., Olivi A., Dimeco F., Vescovi A.L. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 60.Kokovay E., Goderie S., Wang Y., Lotz S., Lin G., Sun Y., Roysam B., Shen Q., Temple S. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. 2010;7:163–173. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goffart N., Kroonen J., Di Valentin E., Dedobbeleer M., Denne A., Martinive P., Rogister B. Adult mouse subventricular zones stimulate glioblastoma stem cells specific invasion through CXCL12/CXCR4 signaling. Neuro Oncol. 2015;17:81–94. doi: 10.1093/neuonc/nou144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soumier A., Banasr M., Kerkerian-Le Goff L., Daszuta A. Region- and phase-dependent effects of 5-HT(1A) and 5-HT(2C) receptor activation on adult neurogenesis. Eur Neuropsychopharmacol. 2010;20:336–345. doi: 10.1016/j.euroneuro.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 63.Ihrie R.A., Shah J.K., Harwell C.C., Levine J.H., Guinto C.D., Lezameta M., Kriegstein A.R., Alvarez-Buylla A. Persistent sonic hedgehog signaling in adult brain determines neural stem cell positional identity. Neuron. 2011;71:250–262. doi: 10.1016/j.neuron.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paez-Gonzalez P., Asrican B., Rodriguez E., Kuo C.T. Identification of distinct ChAT(+) neurons and activity-dependent control of postnatal SVZ neurogenesis. Nat Neurosci. 2014;17:934–942. doi: 10.1038/nn.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Venkatesh H.S., Johung T.B., Caretti V., Noll A., Tang Y., Nagaraja S., Gibson E.M., Mount C.W., Polepalli J., Mitra S.S., Woo P.J., Malenka R.C., Vogel H., Bredel M., Mallick P., Monje M. Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell. 2015;161:803–816. doi: 10.1016/j.cell.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elias L.A., Wang D.D., Kriegstein A.R. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- 67.Kunze A., Congreso M.R., Hartmann C., Wallraff-Beck A., Huttmann K., Bedner P., Requardt R., Seifert G., Redecker C., Willecke K., Hofmann A., Pfeifer A., Theis M., Steinhauser C. Connexin expression by radial glia-like cells is required for neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2009;106:11336–11341. doi: 10.1073/pnas.0813160106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin J.H., Takano T., Cotrina M.L., Arcuino G., Kang J., Liu S., Gao Q., Jiang L., Li F., Lichtenberg-Frate H., Haubrich S., Willecke K., Goldman S.A., Nedergaard M. Connexin 43 enhances the adhesivity and mediates the invasion of malignant glioma cells. J Neurosci. 2002;22:4302–4311. doi: 10.1523/JNEUROSCI.22-11-04302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mercier F., Kitasako J.T., Hatton G.I. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451:170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- 70.McClenahan F.K., Sharma H., Shan X., Eyermann C., Colognato H. Dystroglycan suppresses notch to regulate stem cell niche structure and function in the developing postnatal subventricular zone. Dev Cell. 2016;38:548–566. doi: 10.1016/j.devcel.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shen Q., Wang Y., Kokovay E., Lin G., Chuang S.M., Goderie S.K., Roysam B., Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lathia J.D., Gallagher J., Heddleston J.M., Wang J., Eyler C.E., Macswords J., Wu Q., Vasanji A., McLendon R.E., Hjelmeland A.B., Rich J.N. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6:421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tavazoie M., Van der Veken L., Silva-Vargas V., Louissaint M., Colonna L., Zaidi B., Garcia-Verdugo J.M., Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shen Q., Goderie S.K., Jin L., Karanth N., Sun Y., Abramova N., Vincent P., Pumiglia K., Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 75.Andreu-Agullo C., Morante-Redolat J.M., Delgado A.C., Farinas I. Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nat Neurosci. 2009;12:1514–1523. doi: 10.1038/nn.2437. [DOI] [PubMed] [Google Scholar]
- 76.Ramirez-Castillejo C., Sanchez-Sanchez F., Andreu-Agullo C., Ferron S.R., Aroca-Aguilar J.D., Sanchez P., Mira H., Escribano J., Farinas I. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9:331–339. doi: 10.1038/nn1657. [DOI] [PubMed] [Google Scholar]
- 77.Leventhal C., Rafii S., Rafii D., Shahar A., Goldman S.A. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- 78.Jin K., Zhu Y., Sun Y., Mao X.O., Xie L., Greenberg D.A. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crouch E.E., Liu C., Silva-Vargas V., Doetsch F. Regional and stage-specific effects of prospectively purified vascular cells on the adult V-SVZ neural stem cell lineage. J Neurosci. 2015;35:4528–4539. doi: 10.1523/JNEUROSCI.1188-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ottone C., Krusche B., Whitby A., Clements M., Quadrato G., Pitulescu M.E., Adams R.H., Parrinello S. Direct cell-cell contact with the vascular niche maintains quiescent neural stem cells. Nat Cell Biol. 2014;16:1045–1056. doi: 10.1038/ncb3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Calabrese C., Poppleton H., Kocak M., Hogg T.L., Fuller C., Hamner B., Oh E.Y., Gaber M.W., Finklestein D., Allen M., Frank A., Bayazitov I.T., Zakharenko S.S., Gajjar A., Davidoff A., Gilbertson R.J. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 82.Watkins S., Robel S., Kimbrough I.F., Robert S.M., Ellis-Davies G., Sontheimer H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun. 2014;5:4196. doi: 10.1038/ncomms5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheng L., Huang Z., Zhou W., Wu Q., Donnola S., Liu J.K., Fang X., Sloan A.E., Mao Y., Lathia J.D., Min W., McLendon R.E., Rich J.N., Bao S. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153:139–152. doi: 10.1016/j.cell.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guichet P.O., Guelfi S., Teigell M., Hoppe L., Bakalara N., Bauchet L., Duffau H., Lamszus K., Rothhut B., Hugnot J.P. Notch1 stimulation induces a vascularization switch with pericyte-like cell differentiation of glioblastoma stem cells. Stem Cells. 2015;33:21–34. doi: 10.1002/stem.1767. [DOI] [PubMed] [Google Scholar]
- 85.Bao S., Wu Q., Sathornsumetee S., Hao Y., Li Z., Hjelmeland A.B., Shi Q., McLendon R.E., Bigner D.D., Rich J.N. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 86.Shiozawa Y., Pedersen E.A., Havens A.M., Jung Y., Mishra A., Joseph J., Kim J.K., Patel L.R., Ying C., Ziegler A.M., Pienta M.J., Song J., Wang J., Loberg R.D., Krebsbach P.H., Pienta K.J., Taichman R.S. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121:1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kroonen J., Nassen J., Boulanger Y.G., Provenzano F., Capraro V., Bours V., Martin D., Deprez M., Robe P., Rogister B. Human glioblastoma-initiating cells invade specifically the subventricular zones and olfactory bulbs of mice after striatal injection. Int J Cancer. 2011;129:574–585. doi: 10.1002/ijc.25709. [DOI] [PubMed] [Google Scholar]
- 88.Goffart N., Lombard A., Lallemand F., Kroonen J., Nassen J., Di Valentin E., Berendsen S., Dedobbeleer M., Willems E., Robe P., Bours V., Martin D., Martinive P., Maquet P., Rogister B. CXCL12 mediates glioblastoma resistance to radiotherapy in the subventricular zone. Neuro Oncol. 2017;19:66–77. doi: 10.1093/neuonc/now136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qin E.Y., Cooper D.D., Abbott K.L., Lennon J., Nagaraja S., Mackay A., Jones C., Vogel H., Jackson P.K., Monje M. Neural precursor-derived pleiotrophin mediates subventricular zone invasion by glioma. Cell. 2017;170:845–859.e19. doi: 10.1016/j.cell.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stummer W., Stocker S., Wagner S., Stepp H., Fritsch C., Goetz C., Goetz A.E., Kiefmann R., Reulen H.J. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery. 1998;42:518–525. doi: 10.1097/00006123-199803000-00017. discussion 525–526. [DOI] [PubMed] [Google Scholar]
- 91.Verhaak R.G., Hoadley K.A., Purdom E., Wang V., Qi Y., Wilkerson M.D., Miller C.R., Ding L., Golub T., Mesirov J.P., Alexe G., Lawrence M., O'Kelly M., Tamayo P., Weir B.A., Gabriel S., Winckler W., Gupta S., Jakkula L., Feiler H.S., Hodgson J.G., James C.D., Sarkaria J.N., Brennan C., Kahn A., Spellman P.T., Wilson R.K., Speed T.P., Gray J.W., Meyerson M., Getz G., Perou C.M., Hayes D.N., Cancer Genome Atlas Research Network Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nourallah B., Digpal R., Jena R., Watts C. Irradiating the subventricular zone in glioblastoma patients: is there a case for a clinical trial? Clin Oncol (R Coll Radiol) 2017;29:26–33. doi: 10.1016/j.clon.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 93.Achari R., Arunsingh M., Badgami R.K., Saha A., Chatterjee S., Shrimali R.K., Mallick I., Arun B. High-dose neural stem cell radiation may not improve survival in glioblastoma. Clin Oncol (R Coll Radiol) 2017;29:335–343. doi: 10.1016/j.clon.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 94.Panagiotakos G., Alshamy G., Chan B., Abrams R., Greenberg E., Saxena A., Bradbury M., Edgar M., Gutin P., Tabar V. Long-term impact of radiation on the stem cell and oligodendrocyte precursors in the brain. PLoS One. 2007;2:e588. doi: 10.1371/journal.pone.0000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Achanta P., Capilla-Gonzalez V., Purger D., Reyes J., Sailor K., Song H., Garcia-Verdugo J.M., Gonzalez-Perez O., Ford E., Quinones-Hinojosa A. Subventricular zone localized irradiation affects the generation of proliferating neural precursor cells and the migration of neuroblasts. Stem Cells. 2012;30:2548–2560. doi: 10.1002/stem.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iuchi T., Hatano K., Kodama T., Sakaida T., Yokoi S., Kawasaki K., Hasegawa Y., Hara R. Phase 2 trial of hypofractionated high-dose intensity modulated radiation therapy with concurrent and adjuvant temozolomide for newly diagnosed glioblastoma. Int J Radiat Oncol Biol Phys. 2014;88:793–800. doi: 10.1016/j.ijrobp.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 97.Malik M., Akram K.S., Joseph D., Valiyaveettil D., Ahmed S.F. Prospective study of irradiation of potential stem cell niches in glioblastoma. Int J Radiat Oncol Biol Phys. 2015;93:S111. [Google Scholar]
- 98.Jeon J.Y., An J.H., Kim S.U., Park H.G., Lee M.A. Migration of human neural stem cells toward an intracranial glioma. Exp Mol Med. 2008;40:84–91. doi: 10.3858/emm.2008.40.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim S.K., Kim S.U., Park I.H., Bang J.H., Aboody K.S., Wang K.C., Cho B.K., Kim M., Menon L.G., Black P.M., Carroll R.S. Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin Cancer Res. 2006;12:5550–5556. doi: 10.1158/1078-0432.CCR-05-2508. [DOI] [PubMed] [Google Scholar]
- 100.Staflin K., Lindvall M., Zuchner T., Lundberg C. Instructive cross-talk between neural progenitor cells and gliomas. J Neurosci Res. 2007;85:2147–2159. doi: 10.1002/jnr.21344. [DOI] [PubMed] [Google Scholar]
- 101.Liu S., Yin F., Zhao M., Zhou C., Ren J., Huang Q., Zhao Z., Mitra R., Fan W., Fan M. The homing and inhibiting effects of hNSCs-BMP4 on human glioma stem cells. Oncotarget. 2016;7:17920–17931. doi: 10.18632/oncotarget.7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bago J.R., Okolie O., Dumitru R., Ewend M.G., Parker J.S., Werff R.V., Underhill T.M., Schmid R.S., Miller C.R., Hingtgen S.D. Tumor-homing cytotoxic human induced neural stem cells for cancer therapy. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aah6510. eaah6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shah K., Bureau E., Kim D.E., Yang K., Tang Y., Weissleder R., Breakefield X.O. Glioma therapy and real-time imaging of neural precursor cell migration and tumor regression. Ann Neurol. 2005;57:34–41. doi: 10.1002/ana.20306. [DOI] [PubMed] [Google Scholar]
- 104.Aboody K.S., Najbauer J., Metz M.Z., D'Apuzzo M., Gutova M., Annala A.J., Synold T.W., Couture L.A., Blanchard S., Moats R.A., Garcia E., Aramburo S., Valenzuela V.V., Frank R.T., Barish M.E., Brown C.E., Kim S.U., Badie B., Portnow J. Neural stem cell-mediated enzyme/prodrug therapy for glioma: preclinical studies. Sci Transl Med. 2013;5:184ra59. doi: 10.1126/scitranslmed.3005365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hingtgen S.D., Kasmieh R., van de Water J., Weissleder R., Shah K. A novel molecule integrating therapeutic and diagnostic activities reveals multiple aspects of stem cell-based therapy. Stem Cells. 2010;28:832–841. doi: 10.1002/stem.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fuentealba L.C., Rompani S.B., Parraguez J.I., Obernier K., Romero R., Cepko C.L., Alvarez-Buylla A. Embryonic origin of postnatal neural stem cells. Cell. 2015;161:1644–1655. doi: 10.1016/j.cell.2015.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Llorens-Bobadilla E., Zhao S., Baser A., Saiz-Castro G., Zwadlo K., Martin-Villalba A. Single-cell transcriptomics reveals a population of dormant neural stem cells that become activated upon brain injury. Cell Stem Cell. 2015;17:329–340. doi: 10.1016/j.stem.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 108.Merkle F.T., Mirzadeh Z., Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- 109.Kelsch W., Mosley C.P., Lin C.W., Lois C. Distinct mammalian precursors are committed to generate neurons with defined dendritic projection patterns. PLoS Biol. 2007;5:e300. doi: 10.1371/journal.pbio.0050300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Young K.M., Fogarty M., Kessaris N., Richardson W.D. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci. 2007;27:8286–8296. doi: 10.1523/JNEUROSCI.0476-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Solano Fonseca R., Mahesula S., Apple D.M., Raghunathan R., Dugan A., Cardona A., O'Connor J., Kokovay E. Neurogenic niche microglia undergo positional remodeling and progressive activation contributing to age-associated reductions in neurogenesis. Stem Cells Dev. 2016;25:542–555. doi: 10.1089/scd.2015.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leelatian N., Doxie D.B., Greenplate A.R., Mobley B.C., Lehman J.M., Sinnaeve J., Kauffmann R.M., Werkhaven J.A., Mistry A.M., Weaver K.D., Thompson R.C., Massion P.P., Hooks M.A., Kelley M.C., Chambless L.B., Ihrie R.A., Irish J.M. Single cell analysis of human tissues and solid tumors with mass cytometry. Cytometry B Clin Cytom. 2017;92:68–78. doi: 10.1002/cyto.b.21481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wei W., Shin Y.S., Xue M., Matsutani T., Masui K., Yang H., Ikegami S., Gu Y., Herrmann K., Johnson D., Ding X., Hwang K., Kim J., Zhou J., Su Y., Li X., Bonetti B., Chopra R., James C.D., Cavenee W.K., Cloughesy T.F., Mischel P.S., Heath J.R., Gini B. Single-cell phosphoproteomics resolves adaptive signaling dynamics and informs targeted combination therapy in glioblastoma. Cancer Cell. 2016;29:563–573. doi: 10.1016/j.ccell.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]