Abstract
The primary cilium, a sensory appendage that is present in most mammalian cells, plays critical roles in signaling pathways and cell cycle progression. Mutations that affect the structure or function of primary cilia result in ciliopathies, a group of developmental and degenerative diseases that affect almost all organs and tissues. Our understanding of the constituents, development, and function of primary cilia has advanced considerably in recent years, revealing pathogenic mechanisms that potentially underlie ciliopathies. In the brain, the primary cilia are crucial for early patterning, neurogenesis, neuronal maturation and survival, and tumorigenesis, mostly through regulating cell cycle progression, Hedgehog signaling, and WNT signaling. We review these advances in our knowledge of primary cilia, focusing on brain development, and discuss the mechanisms that may underlie brain abnormalities in ciliopathies.
The primary cilium, a hair-like projection from the surface of a cell, is a sensory organelle present in most mammalian cells. It detects physical and chemical cues from the environment, including light (in photoreceptor cells), mechanical forces, growth factors, and neurotransmitters. In proliferating cells, the assembly and disassembly of the primary cilium are closely associated with cell cycle progression because this organelle grows from the basal body, a modified centriole that organizes the mitotic spindle during cell division. At the nexus of environmental inputs and the cell cycle machinery, primary cilia critically regulate cellular behaviors to ensure proper development and homeostasis. Consequently, structural and functional defects in cilia cause diverse developmental and degenerative diseases, which are collectively termed ciliopathies.1 Despite their structural prominence, these organelles were functionally obscure until relatively recently. At the beginning of this century, genetic studies linked primary cilia with a key developmental signaling pathway and human diseases.2, 3, 4 Subsequent genetic, cellular, biochemical, genomic, and proteomic studies have revealed the detailed constituents, development, and function of primary cilia, generating insights into the mechanisms underlying diseases caused by defective cilia. We will review these advances in our knowledge, with a particular focus on brain development and diseases.
The Structure of Primary Cilia
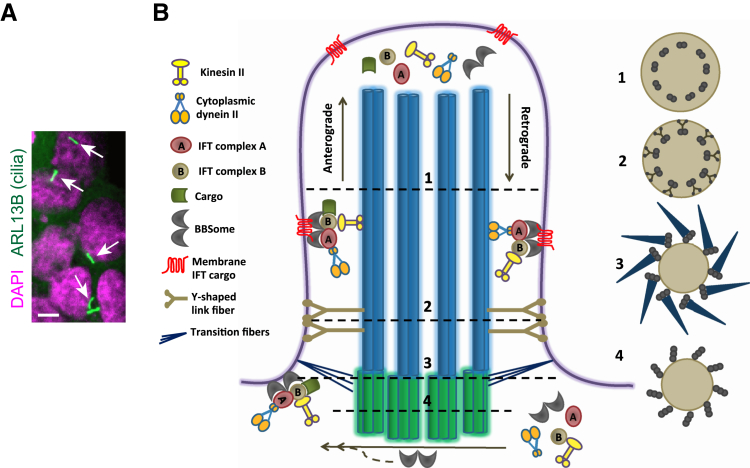
The primary cilium is a slender projection that is typically 1- to 10-μm long and 0.2- to 0.3-μm wide (Figure 1A). At the core of this organelle is the axoneme, a ring of nine microtubule doublets (in a 9+0 arrangement) that runs longitudinally through the organelle. Motile cilia (secondary cilia) contain an additional central pair of microtubules that form the 9+2 axoneme structure. The axoneme grows from the basal body, a barrel-like structure consisting of a ring of nine microtubule triplets (Figure 1B). On completing cell division, the mother centriole converts into the basal body to initiate ciliogenesis. The mother centriole, but not the daughter centriole, possesses distal appendages composed of at least five proteins [centrosomal protein (CEP) 164, CEP89, CEP83, sodium channel and clathrin linker 1, and fas binding factor 1] that are recruited by distal centriole proteins, including oro-facial-digital syndrome 1, C2 calcium dependent domain containing 3, and outer dense fiber of sperm tails 2.5, 6, 7, 8, 9, 10 The distal appendages are essential for anchoring the mother centriole to the ciliary membrane, and they become the transition fibers of the basal body (Figure 1B).7, 8, 10, 11 Transition fibers are essential to the recruitment of components of intraflagellar transport (IFT),10, 11, 12, 13 a bidirectional microtubule-based transport system that operates between the ciliary tip and base.14 IFT builds and maintains the ciliary axoneme by supplying axonemal components to the ciliary tip. Kinesin II motors drive the anterograde transport from the ciliary base to the tip, and cytoplasmic dynein II motors drive the retrograde transport from the ciliary tip to the base. The motors bind to their cargoes through adaptors called IFT particles, which are composed of two interacting complexes (the IFT A and IFT B complexes) that contain at least 22 proteins (Table 1).14 The BBSome, a complex of eight conserved proteins that are mutated in a human ciliopathy called Bardet-Biedl syndrome,15 controls the assembly and recycling of IFT particles at the ciliary base and tip, respectively.16 The BBSome also controls the trafficking of certain membrane proteins in and out of the cilia.17, 18 A proximal region of the cilium above the basal body forms the transition zone. The transition zone is defined by the presence of Y-shaped link fibers, with one end attached to each microtubule doublet of the axoneme and the other two ends attached to the arrangement of membranous particles known as the ciliary necklace. The transition zone is composed of at least two distinct, but interacting, protein complexes, containing at least 16 and 10 proteins, respectively.19, 20, 21, 22
Figure 1.
Structure of primary cilia. A: Immunofluorescence image of primary cilia (arrows) extending from neural stem cells cultured from embryonic mouse brains. Cilia are labeled by anti-ARL13B antibody (green), and nuclei are stained by DAPI (magenta). B: Schematic of the structure of the primary cilium. The basal body, a modified mother centriole that contains a ring of nine microtubule triplets, attaches to the plasma membrane via transition fibers. The transition fibers recruit components of intraflagellar transport (IFT), a bidirectional transport system that operates between the ciliary tip and base. IFT builds and maintains cilia by supplying axonemal components to the tip of the axoneme, a ring of nine microtubule doublets that grow from the basal body. Kinesin II and cytoplasmic dynein II motors drive anterograde IFT (from the base to the tip) and retrograde IFT, respectively. IFT motors bind to cargoes via IFT particles, adaptor complexes composed of IFT A and IFT B. The BBSome, a complex of proteins that are mutated in Bardet-Biedl syndrome, coordinates the assembly of the IFT complex at the ciliary base and its recycling at the tip. The BBSome also functions as an IFT adaptor for some cargoes, including membrane proteins. The proximal end of the cilium above the basal body is the transition zone, which is defined by the presence of Y-shaped link fibers that connect axonemes with the ciliary membrane. The Y-shaped link includes two interacting complexes composed of proteins that are mutated in ciliopathies. The transition zone, together with the transition fibers, forms the ciliary gate that regulates the trafficking of ciliary components. The ciliary gate, IFT, and BBSome, by actively controlling ciliary trafficking, establish and maintain the cilium as a compartment with constituents distinct from the rest of the cell. Mutations affecting ciliary structure and function, including the ciliary gate, IFT, and BBSome, result in ciliopathies, a group of developmental and degenerative diseases that can affect almost all organs and tissues. At right are schematic drawings of cross sections of the cilium at different levels indicated with dashed lines: ciliary axoneme (1), transition zone (2), distal end of the basal body with transition fibers (3), and basal body (4). Scale bar = 5 μm (A).
Table 1.
Genes Delineating the Ciliary Compartment and Associated Diseases
| Complex | Official gene symbol | Alias | Diseases |
|---|---|---|---|
| IFT A | WDR19 (IFT144) | ATD5, CED4, NPHP13, SRTD5 | CED4, NPHP13, SLS8, SRTD5 |
| IFT140 | MZSDS, SRTD9 | SRTD9 | |
| IFT122 | CED1, WDR10, WDR10p, WDR140 | CED1 | |
| TTC21B (IFT139) | ATD4, THM1, SRTD4, JBTS11, NPHP12 | JBTS11, NPHP12, SRTD4 | |
| WDR35 (IFT121) | CED2, IFTA1, SRTD7 | CED2, SRTD7 | |
| IFT43 | CED3 | CED3 | |
| IFT B | IFT172 | SLB, Wim, RP71, BBS20, NPHP17, SRTD10 | BBS20, NPHP17, RP71, SRTD10 |
| IFT88 | TG737 | ||
| IFT81 | DV1, CDV1, CDV1R | SRPS | |
| IFT80 | ATD2, SRTD2, WDR56 | SRTD2 | |
| IFT74 | CMG1, BBS20, CCDC2 | BBS20 | |
| IFT57 | HIPPI, MHS4R2, ESRRBL1 | OFD | |
| TTC30B (IFT70) | Fleer, IFT70B | ||
| TTC26 (IFT56) | |||
| TRAF3IP1 (IFT54) | MIPT3, SLSN9 | SLS9 | |
| IFT52 | NGD2, NGD5, CGI-53 | SRTD16 | |
| IFT46 | CFAP32, C11orf2, C11orf60 | ||
| CLUAP1 (IFT38) | LCA | ||
| HSPB11 (IFT25) | |||
| IFT27 | RAYL, BBS19, RABL4 | BBS19 | |
| IFT22 | RABL5 | ||
| IFT20 | |||
| Kinesin II motor | KIF3A | FLA10, KLP-20 | |
| KIF3B | FLA8, HH0048, KLP-11 | ||
| KIFAP3 | FLA3, KAP3, SMAP, KAP-1 | ||
| Cytoplasmic dynein II | WDR34 | DIC5, FAP133, SRTD11, bA216B9.3 | SRTD11 |
| WDR60 | SRPS6, SRTD8, FAP163 | SRPS6, SRTD8 | |
| TCTEX1D2 | SRTD17 | ||
| NUDCD3 | NudCL | ||
| DYNC2LI1 (LIC3) | LIC3, D2LIC | SRTD15 | |
| BBSome | BBS1 | BBS2L2 | BBS1 |
| BBS2 | BBS, RP74 | BBS2, RP74 | |
| BBS4 | BBS4 | ||
| BBS5 | BBS5 | ||
| BBS7 | BBS2L1 | BBS7 | |
| TTC8 (BBS8) | RP51 | BBS8, RP51 | |
| BBS9 | B1, D1, C18, PTHB1 | BBS9 | |
| BBIP1 (BBS18) | BBIP10 | BBS18 | |
| Transition zone MKS module | B9D1 | MKS9, EPPB9, MKSR1, JBTS27 | JBTS27, MKS9 |
| B9D2 | MKS10, MKSR2, ICIS-1 | MKS10 | |
| MKS1 | MES, MKS, BBS13, POC12, JBTS28 | BBS13, JBTS28, MKS1 | |
| AHI1 | JBTS3 | JBTS3 | |
| CC2D2A | MKS6, JBTS9 | COACH syndrome, JBTS9, MKS6 | |
| TCTN1 | JBTS13 | JBTS13 | |
| TCTN2 | MKS8, JBTS24 | JBTS24, MKS8 | |
| TCTN3 | OFD4, JBTS18 | JBTS18, OFD4 | |
| KCTD10 | BTBD28, ULRO61, MSTP028, hBACURD3 | ||
| TMEM237 | JBTS14, ALS2CR4 | JBTS14 | |
| TMEM231 | MKS11, JBTS20, ALYE870, PRO1886 | JBTS20, MKS11 | |
| TMEM218 | |||
| TMEM216 | HSPC244 | JBTS2, MKS2 | |
| TMEM138 | HSPC196 | Mutated in JBTS2 without TMEM216 mutation | |
| TMEM107 | GRVS638, PRO1268 | JBTS, MKS, OFD | |
| TMEM67 | MKS3, JBTS6, NPHP11, MECKELIN | COACH syndrome, JBTS6, NPHP11, MKS3 | |
| TMEM17 | |||
| CEP290 | MKS4, POC3, rd16, BBS14, JBTS5, LCA10, NPHP6, SLSN6 | BBS14, JBTS5, LCA10, MKS4, NPHP6, SLS6 | |
| Transition zone NPHP module | NPHP1 | NPH1, JBTS4, SLSN1 | JBTS4, NPHP1, SLS1 |
| INVS | INV, NPH2, NPHP2 | Heterotaxia, NPHP2 | |
| NPHP3 | MKS7, NPH3, RHPD, RHPD1, CFAP31 | NPHP3, MKS7, RHPD1 | |
| NPHP4 | POC10, SLSN4 | NPHP4, SLS4 | |
| IQCB1 (NPHP5) | PIQ, NPHP5, SLSN5 | NPHP4, SLS5 | |
| RPGRIP1L | FTM, MKS5, CORS3, JBTS7, NPHP8, PPP1R134 | COACH syndrome, JBTS7, MKS5, NPHP8 | |
| NEK8 | JCK, NPHP9, RHPD2, NEK12A | NPHP9, RHPD2 | |
| ANKS3 | Congenital heart disease, heterotaxia | ||
| ANKS6 | PKDR1, SAMD6, NPHP16, ANKRD14 | NPHP16 | |
| ATXN10 | E46L, SCA10, HUMEEP | Spinocerebellar ataxia 10 | |
| Transition fiber | CEP83 | CCDC41, NPHP18, NY-REN-58 | NPHP18 |
| CEP89 | CEP123, CCDC123 | ||
| SCLT1 | CAP1A, CAP-1A | ||
| FBF1 | Alb | ||
| CEP164 | NPHP15 | NPHP15 |
BBS, Bardet-Biedl syndrome; CED, cranioectodermal dysplasia; COACH, cerebellar vermis hypo/aplasia, oligophrenia, ataxia congenital, coloboma, and hepatic fibrosis; IFT, intraflagellar transport; JBTS, Joubert syndrome; LCA, Leber congenital amaurosis; MKS, Meckel-Gruber syndrome; NPHP, nephronophtisis; OFD, oro-facial-digital syndrome; RHPD, renal-hepatic-pancreatic dysplasia; RP, retinitis pigmentosa; SLS, Senior-Løken syndrome; SRPS, short-rib–polydactyly syndrome; SRTD, short-rib thoracic dysplasia; TMEM, transmembrane protein.
Although the ciliary lumen and membrane are contiguous with the rest of the cytosol and cell membrane, the primary cilium is a distinct compartment with unique protein and lipid composition. This distinction is established and maintained by IFT, the BBSome, and the ciliary gate at the base of the cilium, which together regulate trafficking into and out of the cilium. The ciliary gate is formed by the transition fibers and the transition zone.23 The basal body, transition fibers, transition zone, IFT, and BBSome are essential for ciliary assembly, maintenance, and function; therefore, mutations in their respective components disrupt ciliary structure, function, or both, causing ciliopathies.
Ciliopathies: Diseases Caused by Dysfunctional Cilia
Among the initial evidence implicating dysfunctional primary cilia in human diseases were the findings that mutations that disrupt IFT components cause heterotaxia24 and polycystic kidney disease (PKD)4 in mice and that proteins mutated in Bardet-Biedl syndrome may function in the basal body and cilia.2 The ciliopathies, a group of diseases caused by dysfunctional primary cilia, encompass more than a dozen syndromes that have long been considered as distinct entities [namely, acrocallosal syndrome, Alström syndrome, Bardet-Biedl syndrome, cranioectodermal dysplasia (alias Sensenbrenner syndrome), endocrine-cerebro-osteodysplasia, hydrolethalus syndrome, Joubert syndrome (JBTS), Leber congenital amaurosis, McKusick-Kaufman syndrome, Meckel-Gruber syndrome, mental retardation, truncal obesity, retinal dystrophy, and micropenis syndrome, nephronophtisis, oro-facial-digital syndrome, Senior-Løken syndrome, short-rib thoracic dysplasia, and Usher syndrome]. Short-rib thoracic dysplasia includes Ellis–van Creveld syndrome, Jeune asphyxiating thoracic dysplasia, Mainzer-Saldino syndrome, and short-rib–polydactyly syndrome. Ciliopathies are genetically heterogeneous, reflecting the many genes involved in ciliary structure and function. For example, >90 genes have been ascribed to nephronophtisis.1 Ciliopathies are mostly recessive disorders, but heterozygous mutations in more than one gene can result in a disease of this type. Moreover, different mutations in the same gene or even the same mutation can result in clinically distinct ciliopathies, suggesting that ciliary proteins undergo complex interactions and have context-dependent functions.1
Ciliopathies are also phenotypically heterogeneous and can affect almost all organs, reflecting the near ubiquity of primary cilia in human cells and highlighting the important functions of this organelle in diverse cell types. The pleiotropic features of distinct ciliopathies often overlap; they may include brain malformation, neurologic impairment, retinal degeneration, skeletal anomalies, congenital heart diseases, hepatic fibrosis, and cystic-fibrotic kidney. Brain phenotypes are frequently, but not always, manifested and include encephalocele, holoprosencephaly, microcephaly, polymicrogyria, heterotopia, intracerebral cysts, hippocampal dysgenesis, corpus callosum agenesis, hydrocephalus, cerebellar hypoplasia, and cognitive deficits (eg, intellectual disability and autism spectrum disorder).25, 26 Many of these phenotypes are shared across ciliopathies, albeit with varying frequencies, indicating that similar cellular and developmental functions of primary cilia are disrupted in the different ciliopathies.
Cellular Functions of Primary Cilia
Structural Barrier to Cell Cycle Progression
Before undergoing mitosis, a proliferating cell must disassemble its primary cilium to release the basal body and convert it into the centriole in the centrosome that organizes the mitotic spindle.27, 28 Notably, cells also transiently resorb their primary cilia when they enter the S phase.28 These early findings suggested that primary cilia influence cell cycle progression. Three decades after these observations, recent studies have demonstrated that primary cilia function as structural checkpoints for cell cycle progression.
Serum stimulation that triggers the entry of serum-starved cells into the S phase also activates Aurora kinase A (AURKA) at the basal body. In turn, AURKA phosphorylates and activates histone deacetylase 6, thereby stimulating axonemal tubulin deacetylation and ciliary resorption.29 Tricoplein, a protein originally identified as a keratin-interacting protein, also activates AURKA at the basal body.30 Accordingly, a histone deacetylase 6 inhibitor or the depletion of tricoplein, AURKA, or histone deacetylase 6 inhibits ciliary disassembly.29, 30, 31 Remarkably, these manipulations also inhibit serum-triggered S-phase entry in cells that have primary cilia, but not in cells that lack primary cilia.30, 31 These results suggest that the disrupted disassembly and persistence of cilia inhibit cells from entering the S phase.
Serum stimulation also induces phosphorylation of Tctex-1, a light-chain subunit of cytoplasmic dynein, leading to its dissociation from the dynein complex and its accumulation at the transition zone, where it triggers ciliary resorption and S-phase entry.32 Similar to the depletion of tricoplein, AURKA, or histone deacetylase 6, the depletion of Tctex-1 inhibits ciliary resorption and S-phase entry in ciliated cells but not in cells lacking primary cilia, whereas the expression of a phosphomimic Tctex-1 accelerates ciliary resorption and S-phase entry. Notably, an actin polymerization inhibitor, cytochalasin D, blocks ciliary disassembly that is triggered by serum or phosphomimic Tctex-1, suggesting that Tctex-1 and serum disassemble cilia by a mechanism involving actin polymerization.32 A recent study linked the roles of actin polymerization, AURKA, and ciliary resorption.33 In quiescent cells, the plasma membrane is enriched with phosphatidylinositol 4,5-bisphospate, whereas the ciliary membrane is enriched with phosphatidylinositol 4-phospate, because cilia contain high levels of phosphoinositide-5-phosphatase (INPP5E) that hydrolyzes the 5-phosphate of phosphatidylinositol 4,5-bisphospate.33 INPP5E is mutated in ciliopathies, JBTS, and mental retardation, truncal obesity, retinal dystrophy, and micropenis syndrome.34, 35 Serum stimulation depletes INPP5E from primary cilia via AURKA.33 The depletion of INPP5E increases ciliary phosphatidylinositol 4,5-bisphospate, leading to actin regulator accumulation and actin polymerization in primary cilia, which, in turn, triggers the excision of the ciliary tips, followed by ciliary resorption. Blocking the excision of the ciliary tips inhibits ciliary resorption and cell cycle entry on serum stimulation.33 These results are consistent with those of previous studies, which showed that INPP5E mutation accelerates ciliary disassembly and S-phase entry.34, 35
The length of cilia also affects S-phase entry. NudE neurodevelopment protein 1 and centrosomal-P4.1-associated protein (alias centromere protein J, CENPJ; centrosomal proteins, mutations of which cause microcephaly) negatively regulate the length of cilia.36, 37 The depletion of Nde-1 results in increased ciliary length and delays S-phase entry after serum stimulation; however, there is no delay in S-phase entry in cells that cannot produce cilia.37 Similarly, fibroblasts derived from microcephaly patients and neural progenitor cells carrying a CPAP mutation have long cilia, resulting in retarded ciliary disassembly and cell cycle entry.36 The depletion of cell cycle-related kinase (alias cyclin-dependent kinase 20, CDK20) or intestinal cell kinase, two kinases that negatively regulate ciliary length, also inhibits S-phase entry in a primary cilia–dependent manner.38 Intestinal cell kinase mutations cause endocrine-cerebro-osteodysplasia. Rab8a, a BBSome-interacting small GTPase, is a positive regulator of ciliary length.15 The expression of constitutively active Rab8a lengthens primary cilia and delays S-phase entry. These findings suggest that abnormally long cilia disrupt timely ciliary disassembly and S-phase entry.
At the G2/M transition, a centrosomal serine/threonine kinase, Nek-2, triggers the disassembly of primary cilia.39 Nek-2 phosphorylates Kif24, a microtubule-depolymerizing kinesin, inducing ciliary disassembly. The depletion of Nek-2 or Kif24 in breast cancer cells that express these enzymes at high levels and, thus, lack primary cilia restores cilia and decreases cell proliferation. However, the depletion of Nek-2 or Kif24 fails to decrease the proliferation of breast cancer cells that cannot make cilia. These findings suggest that the persistence of primary cilia inhibits the G2/M transition.
Taken together, these findings demonstrate that primary cilia regulate cell cycle progression as a structural barrier to S- and M-phase entry. Cell cycle kinetics critically regulate the proliferation and differentiation of neural progenitor cells.40 Alterations to the cell cycle progression resulting from defects in the assembly and disassembly of cilia may affect the proliferation and differentiation of neural progenitors in embryonic and adult brains, leading to developmental malformations and diseases.
Signaling Hub
The roles of primary cilia in key developmental signaling pathways were first recognized as a result of a genetic screen for embryonic patterning mutations in mice. The study revealed that mutations that disrupt IFT proteins and, therefore, primary cilia cause patterning defects by disrupting Hedgehog (HH) signaling.3 This and subsequent studies firmly established the essential roles of primary cilia in HH signaling that regulates many aspects of animal development, from early embryonic patterning to tissue homeostasis in adults.41 Defective HH signaling causes various developmental malformations, including neural tube defects, holoprosencephaly, microcephaly, craniofacial defects, skeletal abnormalities, and polydactyly, whereas aberrantly active HH signaling can lead to tumors, including medulloblastomas, basal cell carcinomas, rhabdomyosarcomas, meningiomas, and odontogenic tumors.42 In vertebrates, canonical HH signaling is triggered by one of three HH proteins [Sonic Hedgehog (SHH), Indian Hedgehog, or Desert Hedgehog] and culminates in changes in the transcriptional program brought about by changing the balance of the activator and repressor forms of GLI family zinc finger transcription factors. In the absence of HH ligands, Patched 1, a 12-transmembrane receptor, localizes to the primary cilium and inhibits HH signaling, in part by inhibiting ciliary accumulation and the activation of a G-protein–coupled receptor-like protein called Smoothened (SMO).43 On binding to HH, Patched 1 exits primary cilia, leading to the accumulation and activation of SMO inside them.43, 44 Activated SMO induces ciliary accumulation of GLI2 and GLI3 transcription factors and their binding partner SUFU negative regulator of hedgehog signaling, leading to the formation of GLI transcriptional activators.45 In the absence of primary cilia, SMO cannot activate GLI transcription factors. In the absence of HH ligand, GLI2 and GLI3 are cleaved by proteasomes to become transcriptional repressors that inhibit the expression of HH target genes. The formation of GLI repressors in the absence of HH also requires primary cilia.45, 46, 47, 48 Thus, primary cilia are required to both turn on and turn off HH target-gene expression, and most of the developmental defects in ciliary mutant mice can be attributed to defective HH signaling.
WNT signaling is another key regulator of animal development. The role of primary cilia in WNT signaling is rather controversial.49 WNT binds to Frizzled receptors to trigger either canonical or noncanonical signaling pathways. Canonical WNT signaling turns on target-gene expression via β-catenin, whereas noncanonical signaling is independent of β-catenin and primarily controls cytoskeletons involved in planar cell polarity. Several studies in cell cultures, fish, frogs, and mice have shown that defects in the primary cilium or basal body potentiate canonical WNT signaling activity and disrupt noncanonical WNT signaling.50, 51, 52, 53, 54, 55, 56 However, other studies have shown that defective primary cilia do not affect WNT signaling in cell cultures, fish, or mice.57, 58 Therefore, the roles of primary cilia in WNT signaling remain unclear, but they are likely to be subtle and context dependent. Notably, recent studies have shown that during Drosophila wing development, several, but not all, IFT components are required for WNT signaling.59, 60 Cells in developing fly wings lack primary cilia61; thus, some IFT components and other ciliary proteins may function in WNT signaling independently of primary cilia.
Developmental Functions of Primary Cilia and Disease Mechanisms
Forebrain Patterning, Heterotopia, Intracerebral Cysts, and Agenesis of the Corpus Callosum
The central nervous system develops from a neural tube formed from a single layer of neural progenitor cells. The patterning or formation of distinct regions of the central nervous system is achieved through progressive divisions, along the dorsoventral and rostrocaudal axes, of neural progenitor domains. Morphogens secreted by discrete populations of cells form concentration gradients along those axes, and the gradients specify different fates of neural progenitors, thereby patterning the central nervous system. A hypomorphic mutation in Ift88 (Ift88cbs/cbs), a gene encoding a component of the IFT particle, causes severe disorganization of telencephalic structures, resulting in malformed dorsomedial structures (the cortical hem, hippocampal primordium, and choroid plexus), incomplete divisions between the dorsal and ventral (pallial and subpallial) forebrain and between the telencephalon and diencephalon, and the formation of rosette-shaped heterotopia containing a central lumen.62 These phenotypes are strikingly similar to those observed in Gli3 mutant mice.63, 64, 65 Consistent with the requirement for primary cilia in GLI3 repressor production, Ift88cbs/cbs mutants produce GLI3 repressors inefficiently. Mutations affecting other components of IFT (IFT39) or the transition zone (retinitis pigmentosa GTPase regulator interacting protein 1-like) result in similar phenotypes.66, 67 Early patterning defects in Rpgrip1l mutant mice also cause agenesis of the corpus callosum.68 Notably, transgenic expression of a GLI3 repressor form rescues the patterning and corpus callosum defects in Rpgrip1l mutant mice.66, 68 The neural progenitor cells (neuroepithelial cells and radial glia) lining the ventricle are highly polarized in the apicobasal axis. The loss of Arl13b, a gene mutated in JBTS, at embryonic day (E) 9.5 or earlier, but not later, reverses the apicobasal polarity of neural progenitors, resulting in the inversion of the entire cortical organization.69 This phenotype has not been observed in other ciliary mutants, and the underlying mechanism remains unclear. Taken together, these findings suggest that primary cilia regulate the patterning and morphogenesis of the forebrain, mostly by regulating GLI3 repressor formation, and they provide mechanistic insights into the morphologic defects seen in ciliopathies, including heterotopia, intracerebral cysts, and agenesis of the corpus callosum.
Cortical Neural Progenitor Expansion and Microcephaly
There are three modes of neural progenitor cell division: self-amplifying division to produce two progenitors, self-renewing division to produce one progenitor and one differentiating cell, and self-consuming division to produce two differentiating cells. The cell cycle kinetics control the division mode of neural progenitors, thereby affecting the number of neural progenitors, the number of neural cells, and the overall brain size.40 In the developing cerebral cortex, a shortened G1 phase increases self-renewing and amplifying division of neural progenitors at the expense of differentiating division, whereas a lengthened G1 induces cell cycle exit and differentiation.32, 70, 71, 72, 73 Consistent with these observations, delayed ciliary disassembly and G1/S transition attributable to depletion of Tctex-1 and mutations in CPAP cause premature differentiation of neural progenitors in the mouse embryonic cortex and microcephaly patient–derived neural progenitors in culture, respectively.32, 36 In contrast, conditionally ablating cilia by using conditional alleles of IFT components (kinesin family member 3A, IFT88, and IFT139) in neural progenitors in the early stage mouse embryo (E9 or earlier) results in the expansion of neural progenitors at the expense of neuron production.74, 75 The increased proliferation and expansion of neural progenitors in a Kif3a mutant are largely attributable to defects in GLI3 repressor production in the absence of cilia; the expression of a mutant GLI3 that cannot be processed to a repressor form or the conditional loss of GLI3 causes a similar increase in the proliferation of neural progenitors.75 Notably, the extent of neural progenitor expansion in these Gli3 mutant cortices is smaller than that in Kif3a mutants, suggesting additional roles for cilia in early cortical neural progenitors. The absence of a structural barrier to cell cycle progression (ie, primary cilia) may contribute to the increased proliferation. Increased canonical WNT signaling in cortical neural progenitors attributable to defective cilia may also contribute to the expansion of neural progenitors62, 76, 77; canonical WNT signaling promotes self-amplifying division of neural progenitors. In contrast to the loss of cilia at an early stage of corticogenesis, ablating cilia in cortical progenitors by using conditional Kif3a or Ift88 alleles (Kif3aloxP/loxP or Ift88loxP/loxP) in mice at midgestation (later than E10) has little, if any, effect on cerebral cortex development.78 At this stage, the loss of cilia may be insufficient by itself to affect the cell cycle kinetics of neural progenitors and may result in the concomitant loss of GLI2/3 activators that counterbalances the loss of GLI3 repressors. Taken together, these findings suggest that mutations that disrupt ciliary disassembly may contribute to microcephaly by delaying the cell cycle entry of neural progenitors, whereas the loss of cilia increases neural progenitors at the expense of neuron production in the early stages but not the mid-late stages of corticogenesis.
The primary neural progenitors in mammalian brains are radial glia (RGs), whose cell bodies reside in the ventricular zone (VZ) at the apical side of the developing brain and are, hence, called ventricular RGs (vRGs) or apical RGs. vRGs have a radial process that extends to the pial surface and serves as a scaffold for migrating neurons. vRGs produce neurons directly or indirectly via outer RGs (oRGs; alias basal RGs) and/or intermediate progenitors (IPs) that occupy the subventricular zone (SVZ). oRGs are disconnected from the ventricle but maintain certain characteristics of vRGs, including radial processes that contribute to the tangential dispersion of neurons.79, 80, 81 The expansion of oRGs and IPs is critical for the expansion and folding of the neocortex.79, 80, 81, 82, 83, 84, 85 In particular, oRGs are rare in species with small/smooth brains but are greatly expanded in species with large/folded brains, especially humans, and their expansion is thought to underlie the complexity of the human brain.79, 80, 81, 86 A recent study showed that HH signaling is necessary and sufficient to expand both oRGs and IPs.85 Remarkably, elevating HH signaling in vRGs and their progenies in E13.5 mouse embryos expands oRGs and IPs, resulting in neocortical expansion and folding with normal cytoarchitecture in the otherwise smooth mouse neocortex. Removing cilia completely blocked the expansion of oRGs, IPs, and the neocortex.85 HH signaling also expands oRGs in human cerebral organoids, suggesting that HH signaling is a conserved mechanism to expand oRGs and the neocortex.85 HH signaling activity is higher in the human fetal cortex than in the mouse embryonic cortex.85 Thus, in humans, defective cilia may result in fewer oRGs and a smaller cortex, whereas in mice, in which HH signaling is weak and oRGs are rare, defective cilia or the loss of cilia at midcorticogenesis may have little effect on cortical size.
Adult Neurogenesis in the Hippocampus and Learning Disabilities
The adult mammalian brain maintains neural stem cells that, throughout the life of the organism, produce neurons in the hippocampal dentate gyrus, a structure essential for learning and memory. The continuous production and incorporation of new neurons in the dentate gyrus is important for circuit plasticity, learning, and memory. HH signaling, acting through primary cilia, is essential for the formation of adult neural stem cells in the dentate gyrus. Conditionally ablating SMO or cilia by using SmoloxP/loxP, Kif3aloxP/loxP, or StumpyloxP/loxP (a gene encoding a basal body protein) in embryonic precursors to hippocampal adult neural stem cells blocks the expansion of those precursors, resulting in a severe reduction in the number of adult neural stem cells.87, 88 Rpgrip1l mutant mice and hypomorphic Ift88 mutant mice show similar phenotypes.88
In contrast to the critical roles of primary cilia in the expansion and establishment of adult neural stem cells during perinatal development, postnatal ablation of cilia by using Ift20loxP/loxP in adult neural stem cells and their progenies does not affect the proliferation or self-renewal of adult neural stem cells but decreases the proliferation of their progeny (ie, intermediate progenitors).89 Thus, HH signaling may be dispensable for adult neural stem cells but is required for intermediate progenitors, although HH signaling is active in both cell types.90 A subsequent reduction in neuron production in the mutant dentate gyrus impairs cognitive functions, including spatial learning and special novelty recognition.89 Primary cilia are also essential for the synaptic integration of newborn neurons in the dentate gyrus.91 Disrupting cilia by expressing a dominant-negative Kif3a or shRNA against Ift88 shortens the dendrites of newborn neurons by increasing canonical WNT signaling. Defective cilia increased canonical WNT signaling activity in newborn neurons. Removing β-catenin rescues dendritic shortening in neurons that have defective cilia, whereas the expression of constitutively active β-catenin shortens dendrites. Expressing a dominant-negative Kif3a also impairs cilia and dendritic growth in developing neocortical neurons.92
Canonical WNT signaling increases neurogenesis in the adult dentate gyrus.93 Although the loss of cilia increases canonical WNT signaling in newborn neurons, it appears not to increase WNT signaling in adult neural stem cells and intermediate progenitors, because the loss of cilia decreased, rather than increased, proliferation and neurogenesis.89 These observations exemplify the cell type–specific function of primary cilia in WNT signaling. In summary, primary cilia play critical roles in adult neurogenesis in the dentate gyrus, being involved in the formation of adult neural stem cells, the proliferation of intermediate progenitors, and the synaptic integration of newborn neurons. The disruption of any of these processes is likely to contribute to cognitive impairments, including the learning disabilities seen in ciliopathy patients.
Adult Neurogenesis in the Ventricular-Subventricular Zone and Hydrocephalus
In contrast to the dentate gyrus, primary cilia are required for only a subset of neural stem cells in a different adult neurogenic area [namely, the ventricular-SVZ (V-SVZ) of the lateral ventricle].78, 88 Conditionally ablating cilia in the V-SVZ decreased proliferation only in the anterior ventral V-SVZ, where HH signaling is high. Although primary cilia per se are required only in restricted stem cells, cilia are essential for the establishment and maintenance of the neurogenic niche in the V-SVZ. The primary cilia of vRGs, the precursors of ependymal cells, are crucial for establishing ependymal planar cell polarity (PCP),94, 95 which is essential for the proper circulation of cerebrospinal fluid (CSF). Defective CSF circulation can cause hydrocephalus and abnormal migration of neurons born in the V-SVZ.96 The continuous beating of planar-polarized ependymal cilia generates unidirectional CSF flow. Ependymal cells have two forms of PCP: translational polarity (the asymmetric localization of cilia and basal bodies within the apical side) and rotational polarity of individual cilia. Translational polarity of the basal body is present in vRGs as early as E16, long before the asymmetric localization of PCP proteins that occurs at postnatal day 0.94, 97 Early ablation of primary cilia in vRGs by E14.5 disrupts the translational polarity of vRGs and, subsequently, ependymal PCP, whereas later ablation of cilia by postnatal day 2 affects only the rotational polarity and not the translational polarity in ependymal cells.94 The asymmetrically localized basal bodies in vRGs may provide a positional cue for the asymmetric distribution of newly synthesized basal bodies that will nucleate motile cilia. Notably, the mechanosensory proteins PKD1 and PKD2, mutations of which cause PKDs, localize to vRG cilia. In mice whose vRGs lack primary cilia, PKD1, or PKD2, the translational polarity of vRGs is abnormal, as are the asymmetric distribution of a core PCP protein (Vangl2) essential for establishing PCP in ependymal cells, the ependymal PCP, the CSF flow, and the ventricular size.95 It has been suggested that hydrodynamic force instructs PCP in ependymal cells.98 The PKD1 and PKD2 in vRG cilia may sense passive CSF flow in the embryonic brain ventricle and establish PCP in vRGs. Thus, mutations affecting cilia may lead to hydrocephalus by disrupting the PCP in ependymal cells, the structure of ependymal cilia, or both.
Cerebellar Development, Cerebellar Hypoplasia, and Medulloblastoma
Cerebellar granule neurons constitute more than half of the neurons in the human brain. SHH signaling drives the massive proliferation of cerebellar granule neuron precursor cells (GNPs) during perinatal development. Conditionally ablating primary cilia in GNPs by using Kif3aloxP/loxP, Ift88loxP/loxP, or StumpyloxP/loxP disrupts SHH signaling in GNPs, the proliferation of GNPs, and the growth and foliation of the cerebellum.87, 99, 100 Similarly, SHH signaling and GNP proliferation are severely impaired in the cerebellum of human fetuses with hydrolethalus syndrome, Jeune asphyxiating thoracic dysplasia, JBTS, or Meckel-Gruber syndrome.101 In JBTS, the cerebellar vermis is generally much more severely disrupted than are the hemispheres. However, the extent of GNP proliferation defects in the vermis and hemispheres was similar in five of six JBTS specimens examined, suggesting that there are additional mechanisms by which severe vermal defects are induced in JBTS. Notably, both humans with JBTS and mutant mice that lack either Ahi1 or Cep290, two genes that are mutated in JBTS, show midline fusion defects in their cerebella.102 In Ahi1-null mutants, canonical WNT signaling and the proliferation of cerebellar cells surrounding the midline are decreased. Remarkably, activating canonical WNT signaling with lithium chloride rescued defects in proliferation and midline fusion and partially rescued size and foliation defects in the Ahi-null mutant cerebellum. Taken together, these results suggest that defective canonical WNT signaling in the cerebellar midline and defective SHH signaling in GNPs contribute to the defects in cerebellar growth and foliation seen in ciliopathies.
Although SHH signaling is essential for GNP proliferation, deregulated SHH signaling causes unrestrained GNP proliferation, leading to medulloblastoma, which is the most common pediatric brain cancer. Primary cilia play dual and opposing roles in medulloblastoma development.103 Medulloblastomas resulting from constitutively active SMO, an upstream activator of SHH signaling, require intact primary cilia to grow. Surprisingly, however, primary cilia must be removed for medulloblastomas to form when constitutively active GLI2, a transcription factor downstream of the primary cilia, drives the tumor. These findings suggest that, depending on the oncogenic mutation, primary cilia are either required or suppressive for tumor growth. Medulloblastoma comprises four subgroups: SHH, WNT, group 3, and group 4. These subgroups are characterized by their gene-expression signatures and signaling pathways that promote cancer. Notably, primary cilia are present almost exclusively in the SHH and WNT groups and absent in the other two groups, suggesting that primary cilia promote SHH and WNT medulloblastoma but suppress the other groups.103 The loss of GLI3 repressor, caused by the loss of cilia, was hypothesized to mediate the tumor-suppressive function of primary cilia. However, this hypothesis remains to be substantiated and may not be applied to group 3 or 4 medulloblastomas that are not driven by HH signaling.
Conclusion and Perspective
Substantial progress has been made in revealing the cellular and developmental functions of primary cilia and the molecular functions of numerous ciliary proteins, including those that are mutated in ciliopathies. In the brain, primary cilia play critical roles at various stages of neurogenesis, from early patterning, through the proliferation and differentiation of neural stem/progenitors in fetuses and adults, to the maturation of neurons and tumorigenesis. So far, the evidence indicates that primary cilia act mainly as regulators of the cell cycle, HH signaling, and WNT signaling. These discoveries raise more questions: i) How do primary cilia play distinct roles in different cell types or even in the same cell type at different stages of neurogenesis? For example, ablating cilia in cortical progenitors just 1 day apart (E8.5 versus E9.5) results in strikingly different phenotypes.74 Understanding cell-type specificity will also be critical to elucidating the role of primary cilia in WNT signaling. The molecular composition and structure of cilia may vary between cell types, resulting in and reflecting distinct functions of the cilia in diverse cell types. ii) What are the molecular functions of ciliary proteins? The loss of some ciliary proteins, such as Ahi1 and CEP290, affects cerebellar development without affecting ciliogenesis.102 Similarly, a hypomorphic Ift88cbs/cbs mutation severely disrupted brain patterning without disturbing ciliogenesis.62 Ciliary proteins may directly participate in cellular functions of primary cilia in addition to fulfilling their structural roles. iii) How do mutations identified from ciliopathies affect the function of encoded proteins and primary cilia? Our knowledge of ciliopathy genes has been mostly obtained from loss-of-function studies and is limited with regard to the effects of mutations identified in human subjects. Moreover, mouse models do not fully reflect human diseases. Mice engineered to have mutations corresponding to those identified in ciliopathy and organoid models generated from patient-derived pluripotent stem cells will provide further insights into the function of ciliopathy proteins and the related disease mechanisms. iv) What are the functions of cilia in mature neurons? Some neurotransmitter receptors localize to neuronal cilia, suggesting their role in mature neurons.104 Indeed, the loss of cilia in hypothalamic neurons causes hyperphagia and obesity.105 Neuronal cilia may be important for the health and survival of some neurons. ATXN10 and TTBK2, mutations of which cause spinocerebellar ataxia, a neurodegenerative disease characterized by progressive ataxia and atrophy of the cerebellum and brainstem, constitute the transition zone and initiate ciliogenesis, respectively.22, 106 HTT, a gene mutated in another neurodegenerative disease, Huntington disease, regulates ciliogenesis.107 Notably, IFT is disrupted in the photoreceptors of a Huntington disease mouse model.108
Answering these questions will help us to better understand the details of ciliary function and disease mechanisms, which will be crucial to prevent and treat ciliopathies. Given the importance of primary cilia in brain development, from neural progenitor proliferation to neuronal maturation and survival, mechanistic understanding of ciliary function will have broad implications in repair and replacement of damaged neural cells.
Acknowledgment
We thank Dr. Keith A. Laycock for scientific editing of the manuscript.
Footnotes
Neural Regeneration and Development Theme Issue
Supported by The Sontag Foundation Distinguished Scientist Award (Y.-G.H.), a Whitehall Foundation Research grant (Y.-G.H.), American Lebanese Syrian Associated Charities (Y.-G.H.), and NIH/NCI Cancer Center Core Support grant CA021765 (St. Jude Children's Research Hospital).
Disclosures: None declared.
This article is part of a review series on neural regeneration and developmental biology in health and disease.
References
- 1.Braun D.A., Hildebrandt F. Ciliopathies. Cold Spring Harb Perspect Biol. 2017;9 doi: 10.1101/cshperspect.a028191. a028191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansley S.J., Badano J.L., Blacque O.E., Hill J., Hoskins B.E., Leitch C.C., Kim J.C., Ross A.J., Eichers E.R., Teslovich T.M., Mah A.K., Johnsen R.C., Cavender J.C., Lewis R.A., Leroux M.R., Beales P.L., Katsanis N. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425:628–633. doi: 10.1038/nature02030. [DOI] [PubMed] [Google Scholar]
- 3.Huangfu D., Liu A., Rakeman A.S., Murcia N.S., Niswander L., Anderson K.V. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 4.Pazour G.J., Dickert B.L., Vucica Y., Seeley E.S., Rosenbaum J.L., Witman G.B., Cole D.G. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graser S., Stierhof Y.D., Lavoie S.B., Gassner O.S., Lamla S., Le Clech M., Nigg E.A. Cep164, a novel centriole appendage protein required for primary cilium formation. J Cell Biol. 2007;179:321–330. doi: 10.1083/jcb.200707181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singla V., Romaguera-Ros M., Garcia-Verdugo J.M., Reiter J.F. Ofd1, a human disease gene, regulates the length and distal structure of centrioles. Dev Cell. 2010;18:410–424. doi: 10.1016/j.devcel.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joo K., Kim C.G., Lee M.S., Moon H.Y., Lee S.H., Kim M.J., Kweon H.S., Park W.Y., Kim C.H., Gleeson J.G., Kim J. CCDC41 is required for ciliary vesicle docking to the mother centriole. Proc Natl Acad Sci U S A. 2013;110:5987–5992. doi: 10.1073/pnas.1220927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanos B.E., Yang H.J., Soni R., Wang W.J., Macaluso F.P., Asara J.M., Tsou M.F. Centriole distal appendages promote membrane docking, leading to cilia initiation. Genes Dev. 2013;27:163–168. doi: 10.1101/gad.207043.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thauvin-Robinet C., Lee J.S., Lopez E., Herranz-Perez V., Shida T., Franco B., Jego L., Ye F., Pasquier L., Loget P., Gigot N., Aral B., Lopes C.A., St-Onge J., Bruel A.L., Thevenon J., Gonzalez-Granero S., Alby C., Munnich A., Vekemans M., Huet F., Fry A.M., Saunier S., Riviere J.B., Attie-Bitach T., Garcia-Verdugo J.M., Faivre L., Megarbane A., Nachury M.V. The oral-facial-digital syndrome gene C2CD3 encodes a positive regulator of centriole elongation. Nat Genet. 2014;46:905–911. doi: 10.1038/ng.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye X., Zeng H., Ning G., Reiter J.F., Liu A. C2cd3 is critical for centriolar distal appendage assembly and ciliary vesicle docking in mammals. Proc Natl Acad Sci U S A. 2014;111:2164–2169. doi: 10.1073/pnas.1318737111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt K.N., Kuhns S., Neuner A., Hub B., Zentgraf H., Pereira G. Cep164 mediates vesicular docking to the mother centriole during early steps of ciliogenesis. J Cell Biol. 2012;199:1083–1101. doi: 10.1083/jcb.201202126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deane J.A., Cole D.G., Seeley E.S., Diener D.R., Rosenbaum J.L. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr Biol. 2001;11:1586–1590. doi: 10.1016/s0960-9822(01)00484-5. [DOI] [PubMed] [Google Scholar]
- 13.Wei Q., Xu Q., Zhang Y., Li Y., Zhang Q., Hu Z., Harris P.C., Torres V.E., Ling K., Hu J. Transition fibre protein FBF1 is required for the ciliary entry of assembled intraflagellar transport complexes. Nat Commun. 2013;4:2750. doi: 10.1038/ncomms3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taschner M., Lorentzen E. The intraflagellar transport machinery. Cold Spring Harb Perspect Biol. 2016;8: doi: 10.1101/cshperspect.a028092. a028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nachury M.V., Loktev A.V., Zhang Q., Westlake C.J., Peranen J., Merdes A., Slusarski D.C., Scheller R.H., Bazan J.F., Sheffield V.C., Jackson P.K. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 16.Wei Q., Zhang Y., Li Y., Zhang Q., Ling K., Hu J. The BBSome controls IFT assembly and turnaround in cilia. Nat Cell Biol. 2012;14:950–957. doi: 10.1038/ncb2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin H., White S.R., Shida T., Schulz S., Aguiar M., Gygi S.P., Bazan J.F., Nachury M.V. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 2010;141:1208–1219. doi: 10.1016/j.cell.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lechtreck K.F., Johnson E.C., Sakai T., Cochran D., Ballif B.A., Rush J., Pazour G.J., Ikebe M., Witman G.B. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J Cell Biol. 2009;187:1117–1132. doi: 10.1083/jcb.200909183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chih B., Liu P., Chinn Y., Chalouni C., Komuves L.G., Hass P.E., Sandoval W., Peterson A.S. A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nat Cell Biol. 2011;14:61–72. doi: 10.1038/ncb2410. [DOI] [PubMed] [Google Scholar]
- 20.Dowdle W.E., Robinson J.F., Kneist A., Sirerol-Piquer M.S., Frints S.G., Corbit K.C., Zaghloul N.A., van Lijnschoten G., Mulders L., Verver D.E., Zerres K., Reed R.R., Attie-Bitach T., Johnson C.A., Garcia-Verdugo J.M., Katsanis N., Bergmann C., Reiter J.F. Disruption of a ciliary B9 protein complex causes Meckel syndrome. Am J Hum Genet. 2011;89:94–110. doi: 10.1016/j.ajhg.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Gonzalo F.R., Corbit K.C., Sirerol-Piquer M.S., Ramaswami G., Otto E.A., Noriega T.R., Seol A.D., Robinson J.F., Bennett C.L., Josifova D.J., Garcia-Verdugo J.M., Katsanis N., Hildebrandt F., Reiter J.F. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet. 2011;43:776–784. doi: 10.1038/ng.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sang L., Miller J.J., Corbit K.C., Giles R.H., Brauer M.J., Otto E.A., Baye L.M., Wen X., Scales S.J., Kwong M., Huntzicker E.G., Sfakianos M.K., Sandoval W., Bazan J.F., Kulkarni P., Garcia-Gonzalo F.R., Seol A.D., O'Toole J.F., Held S., Reutter H.M., Lane W.S., Rafiq M.A., Noor A., Ansar M., Devi A.R., Sheffield V.C., Slusarski D.C., Vincent J.B., Doherty D.A., Hildebrandt F., Reiter J.F., Jackson P.K. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell. 2011;145:513–528. doi: 10.1016/j.cell.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Gonzalo F.R., Reiter J.F. Open sesame: how transition fibers and the transition zone control ciliary composition. Cold Spring Harb Perspect Biol. 2017;9 doi: 10.1101/cshperspect.a028134. a028134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nonaka S., Tanaka Y., Okada Y., Takeda S., Harada A., Kanai Y., Kido M., Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 25.Han Y.G., Alvarez-Buylla A. Role of primary cilia in brain development and cancer. Curr Opin Neurobiol. 2010;20:58–67. doi: 10.1016/j.conb.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waters A.M., Beales P.L. Ciliopathies: an expanding disease spectrum. Pediatr Nephrol. 2011;26:1039–1056. doi: 10.1007/s00467-010-1731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rieder C.L., Jensen C.G., Jensen L.C. The resorption of primary cilia during mitosis in a vertebrate (PtK1) cell line. J Ultrastruct Res. 1979;68:173–185. doi: 10.1016/s0022-5320(79)90152-7. [DOI] [PubMed] [Google Scholar]
- 28.Tucker R.W., Pardee A.B., Fujiwara K. Centriole ciliation is related to quiescence and DNA synthesis in 3T3 cells. Cell. 1979;17:527–535. doi: 10.1016/0092-8674(79)90261-7. [DOI] [PubMed] [Google Scholar]
- 29.Pugacheva E.N., Jablonski S.A., Hartman T.R., Henske E.P., Golemis E.A. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoko A., Matsuyama M., Goto H., Ohmuro-Matsuyama Y., Hayashi Y., Enomoto M., Ibi M., Urano T., Yonemura S., Kiyono T., Izawa I., Inagaki M. Trichoplein and Aurora A block aberrant primary cilia assembly in proliferating cells. J Cell Biol. 2012;197:391–405. doi: 10.1083/jcb.201106101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gradilone S.A., Radtke B.N., Bogert P.S., Huang B.Q., Gajdos G.B., LaRusso N.F. HDAC6 inhibition restores ciliary expression and decreases tumor growth. Cancer Res. 2013;73:2259–2270. doi: 10.1158/0008-5472.CAN-12-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li A., Saito M., Chuang J.Z., Tseng Y.Y., Dedesma C., Tomizawa K., Kaitsuka T., Sung C.H. Ciliary transition zone activation of phosphorylated Tctex-1 controls ciliary resorption, S-phase entry and fate of neural progenitors. Nat Cell Biol. 2011;13:402–411. doi: 10.1038/ncb2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phua S.C., Chiba S., Suzuki M., Su E., Roberson E.C., Pusapati G.V., Setou M., Rohatgi R., Reiter J.F., Ikegami K., Inoue T. Dynamic remodeling of membrane composition drives cell cycle through primary cilia excision. Cell. 2017;168:264–279.e15. doi: 10.1016/j.cell.2016.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bielas S.L., Silhavy J.L., Brancati F., Kisseleva M.V., Al-Gazali L., Sztriha L., Bayoumi R.A., Zaki M.S., Abdel-Aleem A., Rosti R.O., Kayserili H., Swistun D., Scott L.C., Bertini E., Boltshauser E., Fazzi E., Travaglini L., Field S.J., Gayral S., Jacoby M., Schurmans S., Dallapiccola B., Majerus P.W., Valente E.M., Gleeson J.G. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet. 2009;41:1032–1036. doi: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacoby M., Cox J.J., Gayral S., Hampshire D.J., Ayub M., Blockmans M., Pernot E., Kisseleva M.V., Compere P., Schiffmann S.N., Gergely F., Riley J.H., Perez-Morga D., Woods C.G., Schurmans S. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat Genet. 2009;41:1027–1031. doi: 10.1038/ng.427. [DOI] [PubMed] [Google Scholar]
- 36.Gabriel E., Wason A., Ramani A., Gooi L.M., Keller P., Pozniakovsky A., Poser I., Noack F., Telugu N.S., Calegari F., Saric T., Hescheler J., Hyman A.A., Gottardo M., Callaini G., Alkuraya F.S., Gopalakrishnan J. CPAP promotes timely cilium disassembly to maintain neural progenitor pool. EMBO J. 2016;35:803–819. doi: 10.15252/embj.201593679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim S., Zaghloul N.A., Bubenshchikova E., Oh E.C., Rankin S., Katsanis N., Obara T., Tsiokas L. Nde1-mediated inhibition of ciliogenesis affects cell cycle re-entry. Nat Cell Biol. 2011;13:351–360. doi: 10.1038/ncb2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Y., Roine N., Makela T.P. CCRK depletion inhibits glioblastoma cell proliferation in a cilium-dependent manner. EMBO Rep. 2013;14:741–747. doi: 10.1038/embor.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim S., Lee K., Choi J.H., Ringstad N., Dynlacht B.D. Nek2 activation of Kif24 ensures cilium disassembly during the cell cycle. Nat Commun. 2015;6:8087. doi: 10.1038/ncomms9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dehay C., Kennedy H. Cell-cycle control and cortical development. Nat Rev. 2007;8:438–450. doi: 10.1038/nrn2097. [DOI] [PubMed] [Google Scholar]
- 41.Bangs F., Anderson K.V. Primary cilia and mammalian hedgehog signaling. Cold Spring Harb Perspect Biol. 2017;9 doi: 10.1101/cshperspect.a028175. a028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Briscoe J., Therond P.P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 43.Rohatgi R., Milenkovic L., Scott M.P. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 44.Corbit K.C., Aanstad P., Singla V., Norman A.R., Stainier D.Y., Reiter J.F. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 45.Haycraft C.J., Banizs B., Aydin-Son Y., Zhang Q., Michaud E.J., Yoder B.K. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huangfu D., Anderson K.V. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu A., Wang B., Niswander L.A. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–3111. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- 48.May S.R., Ashique A.M., Karlen M., Wang B., Shen Y., Zarbalis K., Reiter J., Ericson J., Peterson A.S. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol. 2005;287:378–389. doi: 10.1016/j.ydbio.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 49.Oh E.C., Katsanis N. Context-dependent regulation of Wnt signaling through the primary cilium. J Am Soc Nephrol. 2013;24:10–18. doi: 10.1681/ASN.2012050526. [DOI] [PubMed] [Google Scholar]
- 50.Cano D.A., Murcia N.S., Pazour G.J., Hebrok M. Orpk mouse model of polycystic kidney disease reveals essential role of primary cilia in pancreatic tissue organization. Development. 2004;131:3457–3467. doi: 10.1242/dev.01189. [DOI] [PubMed] [Google Scholar]
- 51.Corbit K.C., Shyer A.E., Dowdle W.E., Gaulden J., Singla V., Chen M.H., Chuang P.T., Reiter J.F. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- 52.Gerdes J.M., Liu Y., Zaghloul N.A., Leitch C.C., Lawson S.S., Kato M., Beachy P.A., Beales P.L., Demartino G.N., Fisher S., Badano J.L., Katsanis N. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet. 2007;39:1350–1360. doi: 10.1038/ng.2007.12. [DOI] [PubMed] [Google Scholar]
- 53.Jonassen J.A., San Agustin J., Follit J.A., Pazour G.J. Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J Cell Biol. 2008;183:377–384. doi: 10.1083/jcb.200808137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones C., Roper V.C., Foucher I., Qian D., Banizs B., Petit C., Yoder B.K., Chen P. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet. 2008;40:69–77. doi: 10.1038/ng.2007.54. [DOI] [PubMed] [Google Scholar]
- 55.Ross A.J., May-Simera H., Eichers E.R., Kai M., Hill J., Jagger D.J., Leitch C.C., Chapple J.P., Munro P.M., Fisher S., Tan P.L., Phillips H.M., Leroux M.R., Henderson D.J., Murdoch J.N., Copp A.J., Eliot M.M., Lupski J.R., Kemp D.T., Dollfus H., Tada M., Katsanis N., Forge A., Beales P.L. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 56.Simons M., Gloy J., Ganner A., Bullerkotte A., Bashkurov M., Kronig C., Schermer B., Benzing T., Cabello O.A., Jenny A., Mlodzik M., Polok B., Driever W., Obara T., Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang P., Schier A.F. Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development. 2009;136:3089–3098. doi: 10.1242/dev.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ocbina P.J., Tuson M., Anderson K.V. Primary cilia are not required for normal canonical Wnt signaling in the mouse embryo. PLoS One. 2009;4:e6839. doi: 10.1371/journal.pone.0006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balmer S., Dussert A., Collu G.M., Benitez E., Iomini C., Mlodzik M. Components of intraflagellar transport complex A function independently of the cilium to regulate canonical Wnt signaling in drosophila. Dev Cell. 2015;34:705–718. doi: 10.1016/j.devcel.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vuong L.T., Mukhopadhyay B., Choi K.W. Kinesin-II recruits Armadillo and Dishevelled for Wingless signaling in Drosophila. Development. 2014;141:3222–3232. doi: 10.1242/dev.106229. [DOI] [PubMed] [Google Scholar]
- 61.Han Y.G., Kwok B.H., Kernan M.J. Intraflagellar transport is required in Drosophila to differentiate sensory cilia but not sperm. Curr Biol. 2003;13:1679–1686. doi: 10.1016/j.cub.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 62.Willaredt M.A., Hasenpusch-Theil K., Gardner H.A., Kitanovic I., Hirschfeld-Warneken V.C., Gojak C.P., Gorgas K., Bradford C.L., Spatz J., Wolfl S., Theil T., Tucker K.L. A crucial role for primary cilia in cortical morphogenesis. J Neurosci. 2008;28:12887–12900. doi: 10.1523/JNEUROSCI.2084-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fotaki V., Yu T., Zaki P.A., Mason J.O., Price D.J. Abnormal positioning of diencephalic cell types in neocortical tissue in the dorsal telencephalon of mice lacking functional Gli3. J Neurosci. 2006;26:9282–9292. doi: 10.1523/JNEUROSCI.2673-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Theil T., Alvarez-Bolado G., Walter A., Ruther U. Gli3 is required for Emx gene expression during dorsal telencephalon development. Development. 1999;126:3561–3571. doi: 10.1242/dev.126.16.3561. [DOI] [PubMed] [Google Scholar]
- 65.Tole S., Ragsdale C.W., Grove E.A. Dorsoventral patterning of the telencephalon is disrupted in the mouse mutant extra-toes(J) Dev Biol. 2000;217:254–265. doi: 10.1006/dbio.1999.9509. [DOI] [PubMed] [Google Scholar]
- 66.Besse L., Neti M., Anselme I., Gerhardt C., Ruther U., Laclef C., Schneider-Maunoury S. Primary cilia control telencephalic patterning and morphogenesis via Gli3 proteolytic processing. Development. 2011;138:2079–2088. doi: 10.1242/dev.059808. [DOI] [PubMed] [Google Scholar]
- 67.Stottmann R.W., Tran P.V., Turbe-Doan A., Beier D.R. Ttc21b is required to restrict sonic hedgehog activity in the developing mouse forebrain. Dev Biol. 2009;335:166–178. doi: 10.1016/j.ydbio.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Laclef C., Anselme I., Besse L., Catala M., Palmyre A., Baas D., Paschaki M., Pedraza M., Metin C., Durand B., Schneider-Maunoury S. The role of primary cilia in corpus callosum formation is mediated by production of the Gli3 repressor. Hum Mol Genet. 2015;24:4997–5014. doi: 10.1093/hmg/ddv221. [DOI] [PubMed] [Google Scholar]
- 69.Higginbotham H., Guo J., Yokota Y., Umberger N.L., Su C.Y., Li J., Verma N., Hirt J., Ghukasyan V., Caspary T., Anton E.S. Arl13b-regulated cilia activities are essential for polarized radial glial scaffold formation. Nat Neurosci. 2013;16:1000–1007. doi: 10.1038/nn.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Glickstein S.B., Monaghan J.A., Koeller H.B., Jones T.K., Ross M.E. Cyclin D2 is critical for intermediate progenitor cell proliferation in the embryonic cortex. J Neurosci. 2009;29:9614–9624. doi: 10.1523/JNEUROSCI.2284-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lange C., Huttner W.B., Calegari F. Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell. 2009;5:320–331. doi: 10.1016/j.stem.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 72.Mairet-Coello G., Tury A., Van Buskirk E., Robinson K., Genestine M., DiCicco-Bloom E. p57(KIP2) regulates radial glia and intermediate precursor cell cycle dynamics and lower layer neurogenesis in developing cerebral cortex. Development. 2012;139:475–487. doi: 10.1242/dev.067314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pilaz L.J., Patti D., Marcy G., Ollier E., Pfister S., Douglas R.J., Betizeau M., Gautier E., Cortay V., Doerflinger N., Kennedy H., Dehay C. Forced G1-phase reduction alters mode of division, neuron number, and laminar phenotype in the cerebral cortex. Proc Natl Acad Sci U S A. 2009;106:21924–21929. doi: 10.1073/pnas.0909894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Snedeker J., Schock E.N., Struve J.N., Chang C.F., Cionni M., Tran P.V., Brugmann S.A., Stottmann R.W. Unique spatiotemporal requirements for intraflagellar transport genes during forebrain development. PLoS One. 2017;12:e0173258. doi: 10.1371/journal.pone.0173258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilson S.L., Wilson J.P., Wang C., Wang B., McConnell S.K. Primary cilia and Gli3 activity regulate cerebral cortical size. Dev Neurobiol. 2012;72:1196–1212. doi: 10.1002/dneu.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abdelhamed Z.A., Wheway G., Szymanska K., Natarajan S., Toomes C., Inglehearn C., Johnson C.A. Variable expressivity of ciliopathy neurological phenotypes that encompass Meckel-Gruber syndrome and Joubert syndrome is caused by complex de-regulated ciliogenesis, Shh and Wnt signalling defects. Hum Mol Genet. 2013;22:1358–1372. doi: 10.1093/hmg/dds546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wheway G., Abdelhamed Z., Natarajan S., Toomes C., Inglehearn C., Johnson C.A. Aberrant Wnt signalling and cellular over-proliferation in a novel mouse model of Meckel-Gruber syndrome. Dev Biol. 2013;377:55–66. doi: 10.1016/j.ydbio.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 78.Tong C.K., Han Y.G., Shah J.K., Obernier K., Guinto C.D., Alvarez-Buylla A. Primary cilia are required in a unique subpopulation of neural progenitors. Proc Natl Acad Sci U S A. 2014;111:12438–12443. doi: 10.1073/pnas.1321425111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fietz S.A., Kelava I., Vogt J., Wilsch-Brauninger M., Stenzel D., Fish J.L., Corbeil D., Riehn A., Distler W., Nitsch R., Huttner W.B. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat Neurosci. 2010;13:690–699. doi: 10.1038/nn.2553. [DOI] [PubMed] [Google Scholar]
- 80.Hansen D.V., Lui J.H., Parker P.R., Kriegstein A.R. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- 81.Reillo I., de Juan Romero C., Garcia-Cabezas M.A., Borrell V. A role for intermediate radial glia in the tangential expansion of the mammalian cerebral cortex. Cereb Cortex. 2011;21:1674–1694. doi: 10.1093/cercor/bhq238. [DOI] [PubMed] [Google Scholar]
- 82.Florio M., Albert M., Taverna E., Namba T., Brandl H., Lewitus E., Haffner C., Sykes A., Wong F.K., Peters J., Guhr E., Klemroth S., Prufer K., Kelso J., Naumann R., Nusslein I., Dahl A., Lachmann R., Paabo S., Huttner W.B. Human-specific gene ARHGAP11B promotes basal progenitor amplification and neocortex expansion. Science. 2015;347:1465–1470. doi: 10.1126/science.aaa1975. [DOI] [PubMed] [Google Scholar]
- 83.Nonaka-Kinoshita M., Reillo I., Artegiani B., Martinez-Martinez M.A., Nelson M., Borrell V., Calegari F. Regulation of cerebral cortex size and folding by expansion of basal progenitors. EMBO J. 2013;32:1817–1828. doi: 10.1038/emboj.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stahl R., Walcher T., De Juan Romero C., Pilz G.A., Cappello S., Irmler M., Sanz-Aquela J.M., Beckers J., Blum R., Borrell V., Gotz M. Trnp1 regulates expansion and folding of the mammalian cerebral cortex by control of radial glial fate. Cell. 2013;153:535–549. doi: 10.1016/j.cell.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 85.Wang L., Hou S., Han Y.G. Hedgehog signaling promotes basal progenitor expansion and the growth and folding of the neocortex. Nat Neurosci. 2016;19:888–896. doi: 10.1038/nn.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martinez-Cerdeno V., Cunningham C.L., Camacho J., Antczak J.L., Prakash A.N., Cziep M.E., Walker A.I., Noctor S.C. Comparative analysis of the subventricular zone in rat, ferret and macaque: evidence for an outer subventricular zone in rodents. PLoS One. 2012;7:e30178. doi: 10.1371/journal.pone.0030178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Breunig J.J., Sarkisian M.R., Arellano J.I., Morozov Y.M., Ayoub A.E., Sojitra S., Wang B., Flavell R.A., Rakic P., Town T. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci U S A. 2008;105:13127–13132. doi: 10.1073/pnas.0804558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Han Y.G., Spassky N., Romaguera-Ros M., Garcia-Verdugo J.M., Aguilar A., Schneider-Maunoury S., Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- 89.Amador-Arjona A., Elliott J., Miller A., Ginbey A., Pazour G.J., Enikolopov G., Roberts A.J., Terskikh A.V. Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: implications for learning and memory. J Neurosci. 2011;31:9933–9944. doi: 10.1523/JNEUROSCI.1062-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ahn S., Joyner A.L. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- 91.Kumamoto N., Gu Y., Wang J., Janoschka S., Takemaru K., Levine J., Ge S. A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nat Neurosci. 2012;15:399–405. doi: 10.1038/nn.3042. S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guadiana S.M., Semple-Rowland S., Daroszewski D., Madorsky I., Breunig J.J., Mykytyn K., Sarkisian M.R. Arborization of dendrites by developing neocortical neurons is dependent on primary cilia and type 3 adenylyl cyclase. J Neurosci. 2013;33:2626–2638. doi: 10.1523/JNEUROSCI.2906-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lie D.C., Colamarino S.A., Song H.J., Desire L., Mira H., Consiglio A., Lein E.S., Jessberger S., Lansford H., Dearie A.R., Gage F.H. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 94.Mirzadeh Z., Han Y.G., Soriano-Navarro M., Garcia-Verdugo J.M., Alvarez-Buylla A. Cilia organize ependymal planar polarity. J Neurosci. 2010;30:2600–2610. doi: 10.1523/JNEUROSCI.3744-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ohata S., Herranz-Perez V., Nakatani J., Boletta A., Garcia-Verdugo J.M., Alvarez-Buylla A. Mechanosensory genes Pkd1 and Pkd2 contribute to the planar polarization of brain ventricular epithelium. J Neurosci. 2015;35:11153–11168. doi: 10.1523/JNEUROSCI.0686-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sawamoto K., Wichterle H., Gonzalez-Perez O., Cholfin J.A., Yamada M., Spassky N., Murcia N.S., Garcia-Verdugo J.M., Marin O., Rubenstein J.L., Tessier-Lavigne M., Okano H., Alvarez-Buylla A. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- 97.Boutin C., Labedan P., Dimidschstein J., Richard F., Cremer H., Andre P., Yang Y., Montcouquiol M., Goffinet A.M., Tissir F. A dual role for planar cell polarity genes in ciliated cells. Proc Natl Acad Sci U S A. 2014;111:E3129–E3138. doi: 10.1073/pnas.1404988111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guirao B., Meunier A., Mortaud S., Aguilar A., Corsi J.M., Strehl L., Hirota Y., Desoeuvre A., Boutin C., Han Y.G., Mirzadeh Z., Cremer H., Montcouquiol M., Sawamoto K., Spassky N. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat Cell Biol. 2010;12:341–350. doi: 10.1038/ncb2040. [DOI] [PubMed] [Google Scholar]
- 99.Chizhikov V.V., Davenport J., Zhang Q., Shih E.K., Cabello O.A., Fuchs J.L., Yoder B.K., Millen K.J. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. J Neurosci. 2007;27:9780–9789. doi: 10.1523/JNEUROSCI.5586-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spassky N., Han Y.G., Aguilar A., Strehl L., Besse L., Laclef C., Ros M.R., Garcia-Verdugo J.M., Alvarez-Buylla A. Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev Biol. 2008;317:246–259. doi: 10.1016/j.ydbio.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aguilar A., Meunier A., Strehl L., Martinovic J., Bonniere M., Attie-Bitach T., Encha-Razavi F., Spassky N. Analysis of human samples reveals impaired SHH-dependent cerebellar development in Joubert syndrome/Meckel syndrome. Proc Natl Acad Sci U S A. 2012;109:16951–16956. doi: 10.1073/pnas.1201408109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lancaster M.A., Gopal D.J., Kim J., Saleem S.N., Silhavy J.L., Louie C.M., Thacker B.E., Williams Y., Zaki M.S., Gleeson J.G. Defective Wnt-dependent cerebellar midline fusion in a mouse model of Joubert syndrome. Nat Med. 2011;17:726–731. doi: 10.1038/nm.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Han Y.G., Kim H.J., Dlugosz A.A., Ellison D.W., Gilbertson R.J., Alvarez-Buylla A. Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med. 2009;15:1062–1065. doi: 10.1038/nm.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Green J.A., Mykytyn K. Neuronal ciliary signaling in homeostasis and disease. Cell Mol Life Sci. 2010;67:3287–3297. doi: 10.1007/s00018-010-0425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davenport J.R., Watts A.J., Roper V.C., Croyle M.J., van Groen T., Wyss J.M., Nagy T.R., Kesterson R.A., Yoder B.K. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol. 2007;17:1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goetz S.C., Liem K.F., Jr., Anderson K.V. The spinocerebellar ataxia-associated gene Tau tubulin kinase 2 controls the initiation of ciliogenesis. Cell. 2012;151:847–858. doi: 10.1016/j.cell.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Keryer G., Pineda J.R., Liot G., Kim J., Dietrich P., Benstaali C., Smith K., Cordelieres F.P., Spassky N., Ferrante R.J., Dragatsis I., Saudou F. Ciliogenesis is regulated by a huntingtin-HAP1-PCM1 pathway and is altered in Huntington disease. J Clin Invest. 2011;121:4372–4382. doi: 10.1172/JCI57552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karam A., Tebbe L., Weber C., Messaddeq N., Morle L., Kessler P., Wolfrum U., Trottier Y. A novel function of Huntingtin in the cilium and retinal ciliopathy in Huntington's disease mice. Neurobiol Dis. 2015;80:15–28. doi: 10.1016/j.nbd.2015.05.008. [DOI] [PubMed] [Google Scholar]