Abstract
Objective:
Influenza A virus infections are still a major health problem and the choices available for the control and treatment of the disease are limited. This research evaluated in vitro and in vivo antiviral effects of Peganum harmala L. seeds (PHS) extract against influenza A virus.
Materials and Methods:
In this research, in vitro anti-influenza A virus activity of the extract was assessed in Madin-Darby canine kidney (MDCK) cells. In order to evaluate anti-influenza activity of PHS extract in vivo, BALB/c mice were infected with 5LD50 of mouse-adapted influenza virus (H1N1; PR8) and received 200 mg/kg/day of PHS extract or 20 mg/kg/day oseltamivir. Lungs of seven mice per group were removed on day 3 post-infection and lung virus titers were determined by qRT-PCR. Mice survival, body weights and general conditions were observed for up to 14 days post-infection.
Results:
The results demonstrated that, the ethanolic extract of PHS possesses high activity against influenza virus with IC50 value of 15.7 (CI95%:11.7-21) μg/ml in MDCK cells. Our results also showed that, oral administration of PHS extract (200 mg/kg/day) or oseltamivir (20 mg/kg/day) to infected mice, increased the survival rate, reduced body weight loss, and decreased lung virus titer.
Conclusion:
Based on our findings, P. harmala seeds extract can inhibit influenza A virus replication in vitro and in vivo. Therefore, isolation and characterization of the plant’s active compounds and investigation of the underlying mechanisms of its antiviral action are highly suggested.
Key Words: Influenza A virus, Antiviral activity, Peganum harmala L.
Introduction
One of the most common human respiratory tract pathogens with high morbidity and mortality risk, is influenza virus which has been considered as a public health concern (WHO, 2016 ▶). The high rates of antigenic drift and shift lead to emergence of novel human and non-human influenza viruses with the ability to cross the species barriers and become pathogenic in their new hosts (Cannell et al., 2008 ▶; Dawood et al., 2009 ▶).
A group of anti-influenza virus agents including amantadine and rimantadine which are matrix protein (M2) ion-channel inhibitors, interferes with viral uncoating within the host cells. These drugs are only effective against influenza A virus and widespread drug resistance has been observed. The other group, oseltamivir and zanamivir, are neuraminidase (NA) inhibitors (Jackson et al., 2011 ▶) and are widely used in the treatment of both seasonal and pandemic influenza virus infections. However, oseltamivir-resistant H1N1 strains with NA H275Y mutation were found to be circulated since 2007–2008 (Dapat et al., 2013 ▶; van der Vries et al., 2013 ▶). Since no highly immunogenic vaccine with considerable efficiency has been yet developed against this virus and because drug-resistant strains of this virus are emerging (Hayden, 2009 ▶; Jefferson et al., 2009 ▶; Lackenby et al., 2008 ▶), the need for development of new highly effective anti-influenza agents for treatment of both seasonal and pandemic influenza infections, seems to be urgent.
Medicinal plants have been used for many years for the treatment of human diseases (Sewell and Rafieian-Kopaei, 2014 ▶). A large number of natural compounds has been examined to be introduced as new herbal medicines and remedies (Akbari et al., 2013 ▶; Madihi et al., 2013 ▶; Shahrani et al., 2007 ▶). The compounds with natural origin, especially those isolated from herbs, have been shown to be reliable sources for development of new drugs (Karimi et al., 2016 ▶; Moradi et al., 2016 ▶; Moradi et al., 2016 ▶; Moradi et al., 2017 ▶). Some of these herbal medicines have become therapeutic agents with promising results (Sarrafchi et al., 2016 ▶; Shayganni et al., 2016 ▶).
Peganum harmala L. (family Zygophyllaceae), a perennial, glabrous plant which grows spontaneously in semi-arid conditions, steppe areas and sandy soils, is native to eastern Mediterranean region. This plant is known as “Espand” in Iran, “Harmel” in North Africa and “African rue”, “Mexican rue” or “Turkish rue” in the United States (Mahmoudian et al., 2002 ▶). For a long time, seeds, fruits, roots, and bark of P. harmala have been used as folk medicine in Iran, Turkey, and China to treat coughs, rheumatism, hypertension, diabetes and asthma (Mina et al., 2015 ▶; Moloudizargari et al., 2013 ▶; Zhao et al., 2011 ▶).
P. harmala contains alkaloids, flavonoids and anthraquinones (Bukhari et al., 2008 ▶). The pharmacologically active compounds of P. harmala are several alkaloids which are found especially in the seeds and roots. These include β-carbolines such as harmine, harmaline (identical with harmidine), harmalol and harman and quinazoline derivatives like vasicine and vasicinone (Mahmoudian et al., 2002 ▶).
A literature review revealed that P. harmala and its active alkaloids possess a wide range of pharmacological activities like cardiovascular (Aarons et al., 1977 ▶; Berrougui et al., 2006 ▶), neurologic (Berrougui et al., 2006 ▶; Fortunato et al., 2009 ▶; Nasehi et al., 2010 ▶; Splettstoesser et al., 2005 ▶), anticancer (Hamsa and Kuttan, 2010 ▶; 2011; Zaker et al., 2007 ▶), antidiabetic (Mina et al., 2015 ▶; Moloudizargari et al., 2013 ▶), antispasmodic, anticholinergic, antihistaminic and antiadrenergic effects (Aqel and Hadidi, 1991 ▶) as well as angiogenic inhibitory properties (Moloudizargari et al., 2013 ▶). Several studies have reported anti-parasidal (Astulla et al., 2008 ▶; Sathiyamoorthy et al., 1999 ▶), antifungal, antibacterial (Nenaah, 2010 ▶), insecticidal (Jbilou et al., 2008 ▶; Rharrabe et al., 2007), and antiviral (Asgarpanah and Ramezanloo, 2012 ▶; Kiani et al., 2008 ▶) effects for P. harmala alkaloids.
This research evaluated in vitro and in vivo antiviral activity of seeds extract of P. harmala L. against influenza A virus infection.
Materials and Methods
Plant collection and extraction
Seeds of P. harmala were purchased from a reliable drugstore. Then, in the Herbarium of Medical Plants Research Center of the Shahrekord University of Medical Sciences (Iran), genus and species of the plant were identified and confirmed (herbarium number MPSKUMS-188). The seeds were powdered and then extracted using maceration method. The plant material was dissolved in 96% ethyl alcohol and kept at room temperature for 96 hr. Then, the mixture was filtered and concentrated under nearly vacuum pressure at 40°C using rotary evaporator. The extracts were dissolved at 37°C in 10% dimethylsulphoxide (DMSO) to give a stock solution of 10 mg/mL, filtered and stored at 4°C until analysis. The little percentage of DMSO present in the wells (maximum 0.1%) has no effect on the results of the experiments (Jadhav et al., 2012 ▶).
Cell culture and influenza virus propagation
Madin Darby Canine Kidney (MDCK) cell line, and mouse-adapted influenza virus (A/Puerto Rico/8/34 (H1N1; PR8) and swine influenza virus (A/Iran/12/2014 (H1N1)) were obtained from Influenza Unit, Pasteur Institute of Iran. MDCK cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco, USA), supplemented with 10% Fetal Bovine Serum (FBS) (Gibco, USA) and 1% penicillin-streptomycin (Gibco, USA), at 37°C in humidified incubator with 5% CO2.
Virus titration
A standard 50% tissue culture infectious doses (TCID50) method was applied for virus titration (Kim et al., 2010 ▶). When, 90% confluent MDCK cells were prepared in 96-well plates, the cell culture medium was aspirated and washed twice with phosphate-buffered saline (PBS). Then, 200μl of 10-fold dilutions of virus in DMEM with 0.5μg/mL trypsin TPCK, was added into the wells and incubated for 2 days. Consequently, 50μl of culture medium was taken from each well and transferred to a U-bottomed 96-well plate for hemagglutination assessment (WHO, 2011 ▶). TCID50 was calculated based on the method of Reed and Muench (Reed and Muench, 1938 ▶).
Cytotoxicity assay
The effects of PHS extract on the viability of MDCK were determined using 3-(4, 5-dimethylthiazol-2ol) 2, 5 diphenyl tetrazolium bromide (MTT; Sigma, USA) assay. When the cell monolayer was confluent, the cells were incubated with 100 µL/well of various concentrations of PHS extract (in triplicates) in 96-well microtiter plates for another 2 days. After the incubation period, the supernatants were removed from the wells and 50 μL of an MTT solution (1 mg/mL in PBS) was added to each well. The plates were incubated for 4 hr at 37°C, and 100 μL of DMSO (Samchun, Korea) was added to the wells to dissolve MTT crystals. The absorbance was read using an enzyme-linked immunosorbent assay (ELISA) reader (StataFax 2100, USA) at 570 nm. The percentage of toxicity was calculated using the following formula:
Toxicity (%) = [100– (ODT/ODC) ×100], where ODT and ODC refer to the absorbance of the test substance and the control, respectively (Mosmann, 1983 ▶). The 50% cytotoxic concentration (CC50) was defined as the cytotoxic concentration of the extract by regression analysis.
Anti-influenza virus activity of PHS extract
Confluent monolayers of MDCK cells were prepared in 96 or 12-well plates. swine influenza A virus was inoculated into the cells at 100 TCID50, and the virus-infected cells were incubated in the presence of each compound at various concentrations. Oseltamivir served as the positive control. Virus replication and virus titration were assessed using different methods including cytopathic effect reduction assay, hemagglutination assay, and real-time quantitative RT-PCR.
Cytopathic effect (CPE) reduction assay
When the cell monolayer was 90% confluent, the cell culture medium of cells was aspirated. Next, cells were washed with PBS and infected with 100 TCID50 of influenza A (H1N1) virus for 1 hr. Then, the virus was removed and the cells were treated with 2-fold diluted nontoxic concentrations of PHS extract (6.25, 12.5, 25, and 50 µg/ml) and incubated for 48 hr. DMSO 0.1% and oseltamivir (Sigma, USA) were used as negative and positive controls, respectively. Cell viability was also determined using a previously described MTT assay (Kodama et al., 1996 ▶). The procedure was carried out in triplicate. The 50% inhibitory concentration (IC50) was determined based on a curve of inhibition versus the concentration of the extract. Selectivity index, as a marker of antiviral activity, was determined as the ratio of CC50/IC50.
Hemagglutinin (HA) assay
Confluent MDCK cells in 12-well plates were infected with 100 TCID50 of influenza A (H1N1) virus, and incubated for 1 hr at 37oC. Then, the virus was removed and the cells were treated with 2-fold diluted nontoxic concentration of PHS extract (5, 10, 20, 40 and 80 µg/ml). To evaluate the presence of the virus in cell culture, the cell culture supernatants were harvested at 24 and 48 hr post-infection. Serial dilutions of the cell culture supernatants were mixed with the same volume of 0.5% chicken red blood cells (RBCs) in a U-bottomed 96-well plate for 45 min at room temperature. The hemagglutination activity was determined by measuring the dilution factor of the samples required for complete chicken RBC agglutination (Jang et al., 2014 ▶).
Quantitative reverse transcription-PCR
We used real-time PCR to quantify the presence of virus in the media of MDCK cells after infection with influenza virus. Next, in 12-well plates, 90%-confluent MDCK cells were infected with the virus and treated with 2-fold dilutions of nontoxic concentrations of PHS extract (5, 10, 20, 40 and 80 µg/ml) or control for 48 hr. Viral RNA was extracted from the culture supernatant, using a viral nucleic acid extraction kit (Yekta tajhiz azma Co., Iran), and reverse-transcribed to cDNA using RevertAid First Strand cDNA synthesis kit (Thermo scientific, Lithuania) and an influenza A viral RNA-specific universal Uni12 primer (5’-AGCAAAAGCAGG-3’). Quantitative PCR was performed using influenza M gene primers (5’-GGCAAATGGTACAGGCAATG-3’ and 5’- AGCAACGAGAGGATCACTTG-3’) (Mehrbod et al., 2012 ▶) and 2X SYBER Green Master Mix (Thermo scientific, Lithuania) with a Rotor-Gene Q (Corbett, Qiagen, Germany). The viral RNA level in the virus-infected cells was considered 100%, and the relative viral RNA levels in test samples were calculated.
In vivo study
Female BALB/c mice (weighing 18–23 g) were obtained from Pasteur institute (Tehran, Iran). Mice were housed at 25oC with controlled 12 hr-12 hr light-dark cycles. Food and water were freely available. All experiments were executed in accordance with the Guide for the Care and Use at Laboratory Animals and approved by research and ethics committee of Shahrekord University of Medical Sciences (approval No. IR. SKUMS.REC.1395.160).
For in vivo toxicity determinations, the dose of PHS extract which was lethal to mice was determined. Mice were randomly divided into 7 groups of 5 mice and treated with 2-fold diluted doses of PHS extract (80, 161, 312.5, 625, 1250, and 2500 mg/kg/day) or control by oral gavage once a day for 5 days. The mice were monitored daily for signs of toxicity and death for 21 days. Lethal dose (LD50) was calculated using GraphPad Prism 6 (GraphPad Software, La Jolla, CA). We also calculated the median infectious dose (LD50) of PR8 in mice using Reed and Muench method (Reed and Muench, 1938 ▶).
Protective efficacy in mice
In order to evaluate anti-influenza activity of PHS extract in vivo, mice were randomly divided into 4 groups of 14. Mice were anesthetized by intraperitoneal injection of 100 mg/kg ketamine hydrochloride (Parke-Davis, Pontypool, UK) and 4 mg/kg xylazine (Bayer, Bury St, Edmunds, UK) and infected with 5LD50 of mouse-adapted Influenza virus (A/Puerto Rico/8/34 (H1N1; PR8) in 50 μL sterile PBS via the intranasal route. Group 1 was infected with virus and did not receive treatment. Groups 2 and 3 were infected with virus and received 200 mg/kg/day of PHS extract or 20 mg/kg/day of oseltamivir (sigma, USA) orally via gavage once a day during days 1–5 post-infection (PI) starting 1 hr post-infection. Group 4 received PHS extract 200 mg/kg/day orally for 5 days without virus inoculation.
For the evaluation of body weight and survival rate, seven mice from each group were observed daily for morbidity and weighed once a day for 14 days. Based on our data, percentage of mortality (M), index of protection (IP) = (Mp -Me)/Mp ×100, where Mp and Me are mortality in virus control group and drug-treated groups, correspondingly) and mean day to death (MDD) were calculated.
In order to determine infectious titer of the virus in lung tissue, lungs of the other seven mice per group, were removed on day 3, and PI (peak of virus titer (Perrone et al., 2008 ▶) and Lung virus titers were determined by real-time qRT-PCR using primers for NS1 gene (Zhang et al., 2013 ▶).
Lung viral titers analysis
Total RNA was extracted from the lung homogenates using Trizol Reagent (1 ml of Trizol for 30 mg tissue), according to the manufacturer’s instruction (Invitrogen, Carlsbad, CA). Real-time polymerase chain reaction (RT-PCR) reaction was performed using a Rotor-Gene 3000 (Corbett, Qiagen, Germany) at a total volume of 10 μl containing 1μl of synthesized cDNA solution, 5μl of 2X SYBER Green Master Mix (Thermo scientific, Lithuania) and 500 nM of each primer. Amplification program included a denaturation at 95°C for 10 min followed by 45 cycles at 95°C for 15 sec, 54 or 58°C for 20 sec, and 72°C for 25 sec. Following the amplification, the specificity of the amplified products was confirmed using melting curve analysis. Viral RNA was normalized against RNA loading for each sample using the β-actin RNA as an internal standard. The primers for PR8 NS1 gene were: forward 5’- CATAATGGATCCAAACACTGTGTC -3’ and reverse 5’- CCTCTTAGGGATTTCTGATCTCGG -3’. The primers for β-actin were: forward 5’- CGGTCAGGTCATCACTATCGG -3’ and reverse 5’- TCTTTACGGATGTCAACGTCACAC -3’.
The levels of viral RNA were expressed as the ratio of viral RNA to β-actin mRNA (viral RNA/β-actin mRNA). Each assay was performed in duplicate for each sample. Relative quantification of viral RNA to β-actin was determined using the 2−ΔCt = 2− (Ct, NS1− Ct, β-actin) equation (Schmittgen and Livak, 2008 ▶).
Statistical analyses
The IC50 and CC50 values were calculated using GraphPad Prism Demo (GraphPad software, San Diego, California, USA). The data were analyzed by Kruskal-Wallis test, and expressed as mean±SD. Survival times were compared using the log-rank test. Differences in values among different groups at p≤0.05 were considered statistically significant.
Results
Cytotoxicity and antiviral activity of PHS extracts in MDCK cells
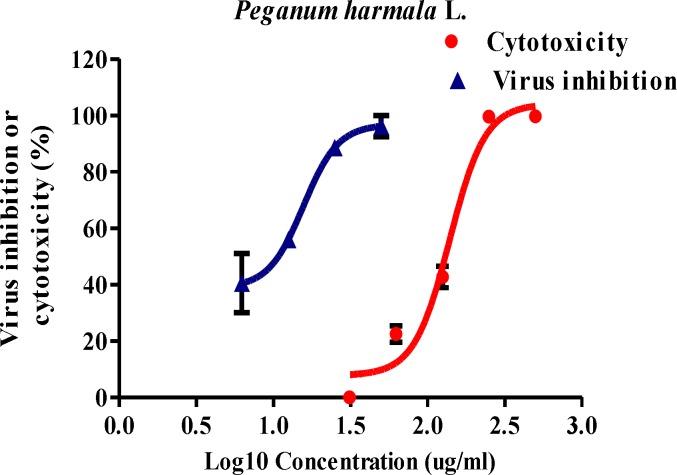
The results showed that the CC50 value of PHS extract in MDCK cells was 139.2 (CI95%:118.2-163.9) μg/ml. The analysis showed a significant relation between the concentration of the extract and cell death (p<0.01). To evaluate the effect of PHS extract on influenza virus replication, virus-infected MDCK cells were treated with non-cytotoxic concentrations of PHS extract for 48 hr to investigate the cytopathic effect and the viability of uninfected and infected cells measured by MTT. Based on nonlinear regression analysis, IC50 of PHS extract on influenza A (H1N1) was 15.7 μg/ml (CI95%:11.7-21) with SI value of 8.87 (Figure 1).
Figure 1.
Anti-influenza virus activity and cytotoxicity of Peganum harmala L. extracts in MDCK cell lines. Confluent MDCK cells without virus or after influenza A virus infection, were exposed to different concentrations of the extract for 48 hr. Cell viability was measured using MTT assay. Results are expressed as means ± SD from three experiments
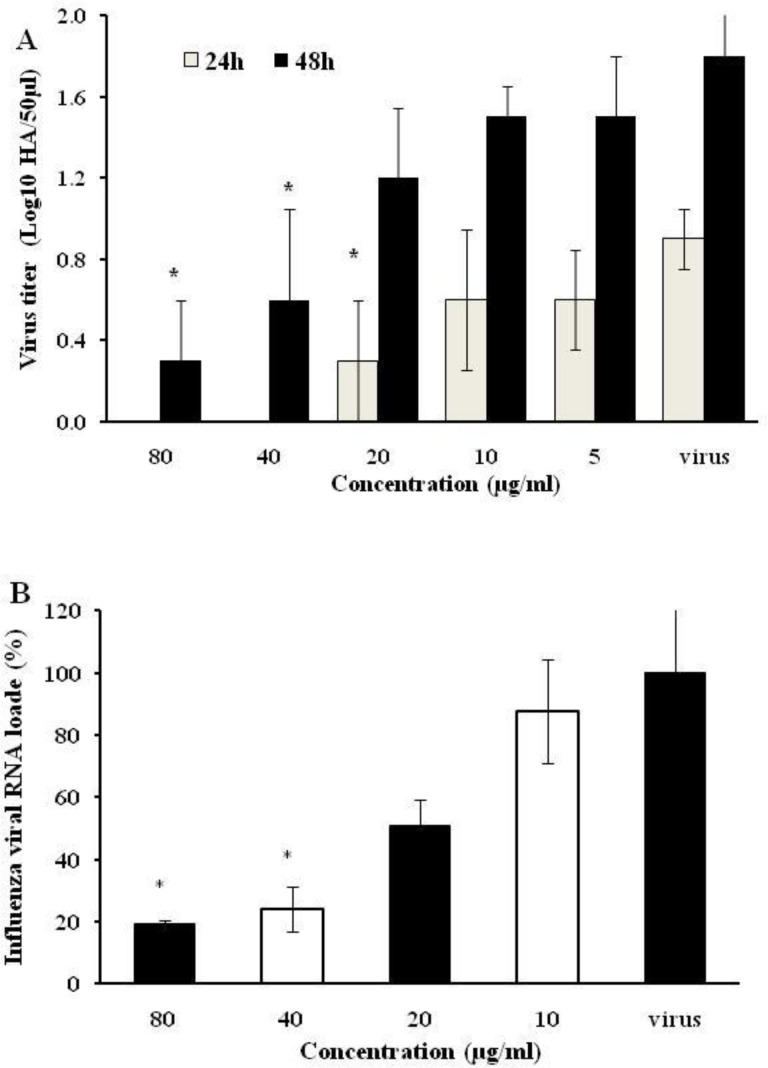
Antiviral activity of PHS extract against influenza virus was assessed by hemagglutination endpoint test. PHS inhibitory effect resulted in a significant decrease in log 10 virus titer as tested by hemagglutination assay (Figure 2A). In addition, PHS extract effects on viral genome load levels were reflected as decrements in log 10 copy number in treatments which were calculated through absolute quantification. Quantitative analysis of the M gene of influenza A virus PCR products exposed to PHS extract, showed statistically significant decrements in viral load compared to the virus sample (Figure 2B).
Figure 2.
Reduction in influenza viral titers in the culture supernatants treated with the Peganum harmala seeds extract. The influenza A H1N1-infected MDCK cells were incubated with different concentrations of the extract for 24 and 48 hr. The supernatants were used for hemagglutination assay (A) or for viral RNA quantification by quantitative RT-PCR using influenza M gene (B). * p<0.05 from values obtained for treated samples compared to virus control (untreated sample). The data were analyzed by SPSS using Kruskal-Wallis test. The data are expressed as mean±SD of three independent experiments
In vivo therapeutic efficacy of PHS extract against PR8 (H1N1) influenza virus
Oral treatment with PHS extract (once a day for 5 days) indicated an approximate LD50 of 519 mg/kg/day for the extract.
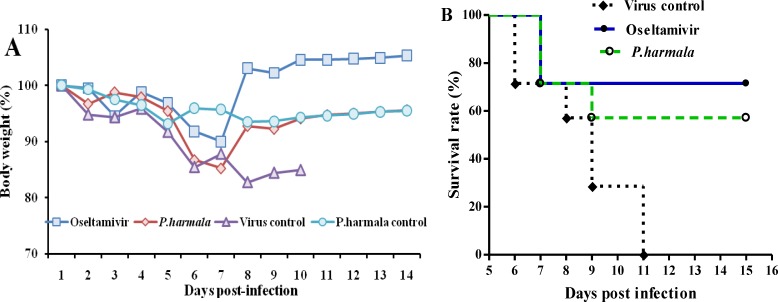
The effect of oral treatment with PHS extract on the survival rate of influenza-infected mice was evaluated. We observed that treatment of mice with 200mg/kg PHS extract and 20 mg/kg oseltamivir decreased body weight loss (Figure 3A) and increased survival rates (Figure 3B) of infected mice. While the MDD of virus control mice was 8.57±2.07 days, the mice administered with 200 mg/kg PHS extract and 20 mg/kg oseltamivir survived for 11.85±3.97 days and 12.7±3.9 days, respectively (p<0.05; Table 1).
Figure 3.
In vivo antiviral activity of Peganum harmala seeds extract against PR8 (H1N1) influenza virus in mice. Female BALB/c mice were intranasally infected with a mouse-adapted PR8 (H1N1) influenza virus (5LD50). One hour later, seven mice per group received placebo, P. harmal seeds extract (200 mg/kg/day) or oseltamivir (20 mg/kg/day) once daily for 5 days, orally. (A) Body weights and (B) survival were monitored daily
Table 1.
Effect of oral treatment with Peganum harmala seeds extract on lung virus yield and mice survival in PR8 influenza virus-infected mice
| Group | Dead/total (M, %) | IP, % | MDD a , b | Viral RNA titer / β-actin gene expression b , c |
|---|---|---|---|---|
| PHS +virus | 3/7 (42.85) | 57.15 | 11.85±3.97 | 1.07×10-5± 7.87×10-6* |
| oseltamivir + virus | 2/7 (28.57) | 71.43 | 12.7±3.9* | 6.08×10-7± 5.03×10-7* |
| Virus control | 7/7 (100) | N/A | 8.57±2.07 | 8.43×10-5± 2.96×10-5 |
| PHS control | 0/7 (0) | - | >14 | N/A |
PHS: Peganum harmala seeds extract (200 mg/kg/day), oseltamivir (20 mg/kg/day), M: Mortality, IP: Index of protection,
MDD: Mean day to death of mice dying prior to day 14;
Data is represented as mean ± SD;
the levels of viral RNA were expressed as the ratio of viral RNA to β-actin mRNA (viral RNA/β-actin mRNA). Relative quantification of viral RNA to β-actin was determined using the 2−ΔCt = 2− (Ct, NS1− Ct, β-actin) equation.
p<0.05 versus virus control.
The effect of the extract on viral loads in lungs of seven mice per group was examined on day 3 PI. We observed that treatment of mice with oseltamivir reduced viral titers. Similarly, viral load in virus-infected lungs was reduced in PHS extract (200mg/kg/day)-treated group, compared to the infected mice without therapy (Table 1).
Discussion
Infection with influenza A virus is still a major health issue, and the available choices for the control and treatment of the disease are limited. Natural products and their derivatives have, historically, been considered as invaluable therapeutic agents (Mirhosseini et al., 2014 ▶; Rouhi-Boroujeni et al., 2016 ▶). Recent technological advances have allowed the researches for the development of anti-viral and also anti-influenza drugs from natural products (Ge et al., 2010 ▶).
In this study, we used MTT cytotoxicity method, hemagglutination assay and qPCR method to evaluate anti-influenza A virus activity of PHS extract in vitro using MDCK cell line. Based on our results, the CC50 (on MDCK cell) and the IC50 value (on influenza A virus) of PHS extract were 139.2 (CI95%:118.2-163.9) μg/ml and 15.7 (CI95%:11.7-21) μg/ml, respectively. The recommended IC50 value, characteristic of herbal extract against infectious diseases is less than 100 μg/mL (Cos et al., 2006 ▶). The PHS extract used in this study had an IC50 value of 15.7 μg/mL which is far below the recommended cut-off and this may indicate that this extract is a potent anti-influenza A virus in vitro. Since PHS extract showed antiviral effects in vitro, BALB/c mice were used to determine in vivo effects of this extract against mouse-adapted influenza A virus. Orally administered PHS 200 mg/kg/day (after infection with influenza virus) for 5 days significantly increased the survival rate, prolonged the mean survival time and reduced the viral titers in the lung. Many plant extracts and compounds have been shown to possess anti-influenza A virus activity, while only a few of which exhibited a protective effect against influenza infection, in vivo (Kim et al., 2010 ▶; Yu et al., 2016 ▶; Zarubaev et al., 2015 ▶).
PHS contains some alkaloids compounds and has been considered from long time ago as a herbal medicine. The pharmacochemical studies of the PHS extract has shown that this herb contains flavonoids, saponins, tannins, compounds reducers, volatile oils, anthraquinones, triterpenes, sterols, and alkaloids. These studies also showed that pharmacologically active compounds of P. harmala include several alkaloids, β-carbolines (such as harmine, harmaline, harman and harmalol) and the quinazoline derivatives (vasicine and vasicinone) (Mina et al., 2015 ▶).
It has been reported that some of the β-carboline alkaloids also have antimicrobial (Shahverdi et al., 2008 ▶), antiviral (Hudson et al., 1986 ▶; Ishida et al., 2001 ▶) and antiplasmodial (Astulla et al., 2008 ▶) activities. It has been also shown that two alkaloid compounds of the β-carbolines (Zhang et al., 2013 ▶) and the quinazoline (Dang et al., 2014 ▶; Pan et al., 2015 ▶) have anti-influenza A virus activity (Chiou et al., 2011 ▶; He et al., 2013 ▶; Zeng et al., 2006 ▶). Therefore, the anti-influenza A virus activity of PHS extract used in this study could be attributed to its alkaloid components. The spectrum of anti-influenza activities and the underlying mechanisms as well as more preclinical evaluations of PHS extract will be done in our future works.
In conclusion, the results presented here suggest that PHS extract can inhibit influenza A virus replication in vitro and in vivo. Therefore, further characterization of PHS active compounds and investigations of the underlying mechanisms of its antiviral activity need to be carried out to enable us to introduce PHS as an anti- influenza A virus agent.
Acknowledgment
This work was a part of a PhD thesis supported by Shahrekord University of Medical Science, Shahrekord, Iran (Grant No.:2313). Authors are thankful to the Director of Medical Plants Research Center and to the Deputy of Research and Technology of Shahrekord University of Medical Sciences, Shahrekord, Iran.
Conflict of interest
The authors of this research declare no conflict of interest.
References
- Aarons DH, Rossi GV, Orzechowski RF. Cardiovascular actions of three harmala alkaloids: harmine, harmaline, and harmalol. J Pharm Sci. 1977;66:1244–1248. doi: 10.1002/jps.2600660910. [DOI] [PubMed] [Google Scholar]
- Akbari F, Ansari-Samani R, Karimi A, Mortazaei S, Shahinfard N, Rafieian-Kopaei M. Effect of turnip on glucose and lipid profiles of alloxan-induced diabetic rats. Iran J Endocrin Metabol. 2013;14:492–497. [Google Scholar]
- Aqel M, Hadidi M. Direct Relaxant Effect of Peganum Harmala Seed Extract on Smooth Muscles of Rabbit and Guinea Pig. Int J Pharmacogn. 1991;29:176–182. [Google Scholar]
- Asgarpanah J, Ramezanloo F. Chemistry, pharmacology and medicinal properties of Peganum harmala L. Afr J pharm pharmacol. 2012;6:1573–1580. [Google Scholar]
- Astulla A, Zaima K, Matsuno Y, Hirasawa Y, Ekasari W, Widyawaruyanti A, Zaini NC, Morita H. Alkaloids from the seeds of Peganum harmala showing antiplasmodial and vasorelaxant activities. J Nat Med. 2008;62:470–472. doi: 10.1007/s11418-008-0259-7. [DOI] [PubMed] [Google Scholar]
- Berrougui H, Martin-Cordero C, Khalil A, Hmamouchi M, Ettaib A, Marhuenda E, Herrera MD. Vasorelaxant effects of harmine and harmaline extracted from Peganum harmala L seeds in isolated rat aorta. Pharmacol Res. 2006;54:150–157. doi: 10.1016/j.phrs.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Bukhari N, Choi J, Jeon C, Park H, Kim W, Khan MA, Leet S. Phytochemical studies of the alkaloids from Peganum harmala. Applied Cham. 2008;12:101–104. [Google Scholar]
- Cannell JJ, Zasloff M, Garland CF, Scragg R, Giovannucci E. On the epidemiology of influenza. Virol J. 2008;5:422X–425. doi: 10.1186/1743-422X-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou WF, Chen CC, Wei BL. 8-Prenylkaempferol Suppresses Influenza A Virus-Induced RANTES Production in A549 Cells via Blocking PI3K-Mediated Transcriptional Activation of NF-kappaB and IRF3. Evid Based Complement Alternat Med. 2011;2011:920828. doi: 10.1093/ecam/nep066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cos P, Vlietinck AJ, Berghe DV, Maes L. Anti-infective potential of natural products: how to develop a stronger in vitro 'proof-of-concept'. J Ethnopharmacol. 2006;106:290–302. doi: 10.1016/j.jep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Dang Z, Jung K, Zhu L, Lai W, Xie H, Lee KH, Huang L, Chen CH. Identification and Synthesis of Quinolizidines with Anti-Influenza A Virus Activity. ACS Med Chem Lett. 2014;5:942–946. doi: 10.1021/ml500236n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapat C, Kondo H, Dapat IC, Baranovich T, Suzuki Y, Shobugawa Y, Saito K, Saito R, Suzuki H. Neuraminidase inhibitor susceptibility profile of pandemic and seasonal influenza viruses during the 2009-2010 and 2010-2011 influenza seasons in Japan. Antiviral Res. 2013;99:261–269. doi: 10.1016/j.antiviral.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- Fortunato JJ, Reus GZ, Kirsch TR, Stringari RB, Stertz L, Kapczinski F, Pinto JP, Hallak JE, Zuardi AW, Crippa JA, Quevedo J. Acute harmine administration induces antidepressive-like effects and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1425–1430. doi: 10.1016/j.pnpbp.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Ge H, Wang YF, Xu J, Gu Q, Liu HB, Xiao PG, Zhou J, Liu Y, Yang Z, Su H. Anti-influenza agents from Traditional Chinese Medicine. Nat Prod Rep. 2010;27:1758–1780. doi: 10.1039/c0np00005a. [DOI] [PubMed] [Google Scholar]
- Hamsa TP, Kuttan G. Harmine inhibits tumour specific neo-vessel formation by regulating VEGF, MMP, TIMP and pro-inflammatory mediators both in vivo and in vitro. Eur J Pharmacol. 2010;649:64–73. doi: 10.1016/j.ejphar.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Hamsa TP, Kuttan G. Harmine activates intrinsic and extrinsic pathways of apoptosis in B16F-10 melanoma. Chin Med. 2011;6:11. doi: 10.1186/1749-8546-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden F. Developing new antiviral agents for influenza treatment: what does the future hold? Clin Infect Dis. 2009;48 (Suppl 1):S3–13. doi: 10.1086/591851. [DOI] [PubMed] [Google Scholar]
- He J, Qi WB, Wang L, Tian J, Jiao PR, Liu GQ, Ye WC, Liao M. Amaryllidaceae alkaloids inhibit nuclear-to-cytoplasmic export of ribonucleoprotein (RNP) complex of highly pathogenic avian influenza virus H5N1. Influenza Other Respir Viruses. 2013;7:922–931. doi: 10.1111/irv.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JB, Graham EA, Fong R, Hudson LL, Towers GH. Further studies on the antiviral activity of harmine, a photoactive beta-carboline alkaloid. Photochem Photobiol. 1986;44:483–487. doi: 10.1111/j.1751-1097.1986.tb04696.x. [DOI] [PubMed] [Google Scholar]
- Ishida J, Wang HK, Oyama M, Cosentino ML, Hu CQ, Lee KH. Anti-AIDS agents 46 Anti-HIV activity of harman, an anti-HIV principle from Symplocos setchuensis, and its derivatives. J Nat Prod. 2001;64:958–960. doi: 10.1021/np0101189. [DOI] [PubMed] [Google Scholar]
- Jackson RJ, Cooper KL, Tappenden P, Rees A, Simpson EL, Read RC, Nicholson KG. Oseltamivir, zanamivir and amantadine in the prevention of influenza: a systematic review. J Infect. 2011;62:14–25. doi: 10.1016/j.jinf.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Jadhav P, Kapoor N, Thomas B, Lal H, Kshirsagar N. Antiviral potential of selected Indian medicinal (ayurvedic) plants against herpes simplex virus 1 and 2. N Am J Med Sci. 2012;4:641–647. doi: 10.4103/1947-2714.104316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YJ, Achary R, Lee HW, Lee HJ, Lee CK, Han SB, Jung YS, Kang NS, Kim P, Kim M. Synthesis and anti-influenza virus activity of 4-oxo- or thioxo-4,5-dihydrofuro[3,4-c]pyridin-3(1H)-ones. Antiviral Res. 2014;107:66–75. doi: 10.1016/j.antiviral.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jbilou R, Amri H, Bouayad N, Ghailani N, Ennabili A, Sayah F. Insecticidal effects of extracts of seven plant species on larval development, alpha-amylase activity and offspring production of Tribolium castaneum (Herbst) (Insecta: Coleoptera: Tenebrionidae) Bioresour Technol. 2008;99:959–964. doi: 10.1016/j.biortech.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Jefferson T, Di Pietrantonj C, Debalini MG, Rivetti A, Demicheli V. Inactivated influenza vaccines: methods, policies, and politics. J Clin Epidemiol. 2009;62:677–686. doi: 10.1016/j.jclinepi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Karimi A, Moradi MT, Alidadi S, Hashemi L. Anti-adenovirus activity, antioxidant potential, and phenolic content of black tea (Camellia sinensis Kuntze) extract. J Complement Integr Med. 2016;13:357–363. doi: 10.1515/jcim-2016-0050. [DOI] [PubMed] [Google Scholar]
- Kiani S, Shamsi Shahrabadi M, Ataei A, Sajjadi N. Peganum harmala seed extract can prevent HSV-1 replication in vitro. Iran J Virol. 2008;4:11–16. [Google Scholar]
- Kim Y, Narayanan S, Chang KO. Inhibition of influenza virus replication by plant-derived isoquercetin. Antiviral Res. 2010;88:227–235. doi: 10.1016/j.antiviral.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Kodama E, Shigeta S, Suzuki T, De Clercq E. Application of a gastric cancer cell line (MKN-28) for anti-adenovirus screening using the MTT method. Antiviral Res. 1996;31:159–164. doi: 10.1016/0166-3542(96)06966-5. [DOI] [PubMed] [Google Scholar]
- Lackenby A, Thompson CI, Democratis J. The potential impact of neuraminidase inhibitor resistant influenza. Curr Opin Infect Dis. 2008;21:626–638. doi: 10.1097/QCO.0b013e3283199797. [DOI] [PubMed] [Google Scholar]
- Madihi Y, Merrikhi A, Baradaran A, Ghobadi S, Shahinfard N, Ansari R, Karimi A, Mesripour A, Rafieian-Kopaei M. Bioactive components and the effect of hydroalcoholic extract of Vaccinium myrtillus on postprandial atherosclerosis risk factors in rabbits. Pak J Med Sci. 2013;29:384–389. [Google Scholar]
- Mahmoudian M, Jalipour H, Salehian Dardashti P. Toxicity of Peganum harmala: Review and a Case Report. Iran J Pharmacol Therapeut. 2002;1:1–4. [Google Scholar]
- Mehrbod P, Ideris A, Omar AR, Hair-Bejo M, Tan SW, Kheiri MT, Tabatabaian M. Attenuation of influenza virus infectivity with herbal-marine compound (HESA-A): an in vitro study in MDCK cells. Virol J. 2012;9:44. doi: 10.1186/1743-422X-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mina CN, Farzaei MH, Gholamreza A. Medicinal properties of Peganum harmala L in traditional Iranian medicine and modern phytotherapy: a review. J Tradit Chin Med. 2015;35:104–109. doi: 10.1016/s0254-6272(15)30016-9. [DOI] [PubMed] [Google Scholar]
- Mirhosseini M, Baradaran A, Rafieian-Kopaei M. Anethum graveolens and hyperlipidemia: A randomized clinical trial. J Res Med Sci. 2014;19:758–761. [PMC free article] [PubMed] [Google Scholar]
- Moloudizargari M, Mikaili P, Aghajanshakeri S, Asghari MH, Shayegh J. Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacogn Rev. 2013;7:199–212. doi: 10.4103/0973-7847.120524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi B, Heidari-Soureshjani S, Asadi-Samani M, Yang Q. A systematic review of phytochemical and phytotherapeutic characteristics of bitter almond. Int J Pharm Phytopharmacol Res. 2017;7(2):1–9. [Google Scholar]
- Moradi MT, Karimi A, Alidadi S. In vitro antiproliferative and apoptosis-inducing activities of crude ethyle alcohole extract of Quercus brantii L acorn and subsequent fractions. Chin J Nat Med. 2016;14:196–202. doi: 10.1016/S1875-5364(16)30016-4. [DOI] [PubMed] [Google Scholar]
- Moradi MT, Rafieian-Kopaei M, Karimi A. A review study on the effect of Iranian herbal medicines against in vitro replication of herpes simplex virus. Avicenna J Phytomed. 2016;6:506–515. [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nasehi M, Piri M, Nouri M, Farzin D, Nayer-Nouri T, Zarrindast MR. Involvement of dopamine D1/D2 receptors on harmane-induced amnesia in the step-down passive avoidance test. Eur J Pharmacol. 2010;634:77–83. doi: 10.1016/j.ejphar.2010.02.027. [DOI] [PubMed] [Google Scholar]
- Nenaah G. Antibacterial and antifungal activities of (beta)-carboline alkaloids of Peganum harmala (L) seeds and their combination effects. Fitoterapia. 2010;81:779–782. doi: 10.1016/j.fitote.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Pan QM, Li YH, Hua J, Huang FP, Wang HS, Liang D. Antiviral Matrine-Type Alkaloids from the Rhizomes of Sophora tonkinensis. J Nat Prod. 2015;78:1683–1688. doi: 10.1021/acs.jnatprod.5b00325. [DOI] [PubMed] [Google Scholar]
- Perrone LA, Plowden JK, Garcia-Sastre A, Katz JM, Tumpey TM. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 2008;4:e1000115. doi: 10.1371/journal.ppat.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epid. 1938;27:493–497. [Google Scholar]
- Rharrabe K, Bakrim A, Ghailani N, Sayah F. Bioinsecticidal effect of harmaline on Plodia interpunctella development (Lepidoptera: Pyralidae) Pestic Biochem Physiol. 2007;89:137–145. [Google Scholar]
- Rouhi-Boroujeni H, Heidarian E, Rouhi-Boroujeni H, Deris F, Rafieian-Kopaei M. Medicinal plants with multiple effects on cardiovascular diseases: A systematic review. Curr Pharm Des. 2017;23:999–1015. doi: 10.2174/1381612822666161021160524. [DOI] [PubMed] [Google Scholar]
- Sarrafchi A, Bahmani M, Shirzad H, Rafieian-Kopaei M. Oxidative stress and Parkinson's disease: New hopes in treatment with herbal antioxidants. Curr Pharm Des. 2016;22:238–246. doi: 10.2174/1381612822666151112151653. [DOI] [PubMed] [Google Scholar]
- Sathiyamoorthy P, Lugasi-Evgi H, Schlesinger P, Kedar I, Gopas J, Pollack Y, Golan-Goldhirsh A. Screening for cytotoxic and antimalarial activities in desert plants of the negev and bedouin market plant products. Pharmaceut Biol. 1999;37:188–195. [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Sewell RDE, Rafieian-Kopaei M. The history and ups and downs of herbal medicines usage. J Herbmed Pharmacol. 2014;3:1–3. [Google Scholar]
- Shahrani M, Rafieian M, Shirzad H, Hashemzadeh M, Yousefi H, Khadivi R, Amini SA, Dehghan M, Khayri S, Moradi M, Rahimian G, Gheitasi I. Effect of Allium sativum L extract on acid and pepsin secretion in basal condition and stimulated with vag stimulate in rat. J Med Plant. 2007;6:28–37. [Google Scholar]
- Shahverdi AR, Ostad SN, Khodaee S, Bitarafan L, Monsef-Esfahani HR, Jamalifar H, Nikavar B, Mohseni M. PHCOG MAG: Research Article Antimicrobial and cytotoxicity potential of Peganum harmala smoke. Phcog Mag. 2008;4:236. [Google Scholar]
- Shayganni E, Bahmani M, Asgary S, Rafieian-Kopaei M. Inflammaging and cardiovascular disease: Management by medicinal plants. Phytomedicine. 2016;23:1119–1126. doi: 10.1016/j.phymed.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Splettstoesser F, Bonnet U, Wiemann M, Bingmann D, Busselberg D. Modulation of voltage-gated channel currents by harmaline and harmane. Br J Pharmacol. 2005;144:52–58. doi: 10.1038/sj.bjp.0706024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vries E, Schutten M, Fraaij P, Boucher C, Osterhaus A. Influenza virus resistance to antiviral therapy. Adv Pharmacol. 2013;67:217–246. doi: 10.1016/B978-0-12-405880-4.00006-8. [DOI] [PubMed] [Google Scholar]
- WHO. Avian and other zoonotic influenza: fact sheet. 2016. http://www.who.int/mediacentre/factsheets/avian_influenza/en/
- WHO. Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza. Global Influenza Surveillance and Response System (GISRS) 2011. 151 pp. [Google Scholar]
- Yu J, Wang D, Jin J, Xu J, Li M, Wang H, Dou J, Zhou C. Antiviral activity of SA-2 against influenza A virus in vitro/vivo and its inhibition of RNA polymerase. Antiviral Res. 2016;127:68–78. doi: 10.1016/j.antiviral.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Zaker F, Oody A, Arjmand A. A study on the antitumoral and differentiation effects of peganum harmala derivatives in combination with ATRA on leukaemic cells. Arch Pharm Res. 2007;30:844–849. doi: 10.1007/BF02978835. [DOI] [PubMed] [Google Scholar]
- Zarubaev VV, Garshinina AV, Tretiak TS, Fedorova VA, Shtro AA, Sokolova AS, Yarovaya OI, Salakhutdinov NF. Broad range of inhibiting action of novel camphor-based compound with anti-hemagglutinin activity against influenza viruses in vitro and in vivo. Antiviral Res. 2015;120:126–133. doi: 10.1016/j.antiviral.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Zeng X, Dong Y, Sheng G, Dong X, Sun X, Fu J. Isolation and structure determination of anti-influenza component from Mahonia bealei. J Ethnopharmacol. 2006;108:317–319. doi: 10.1016/j.jep.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Zhang G, Zhang B, Zhang X, Bing F. Homonojirimycin, an alkaloid from dayflower inhibits the growth of influenza A virus in vitro. Acta Virol. 2013;57:85–86. doi: 10.4149/av_2013_01_85. [DOI] [PubMed] [Google Scholar]
- Zhao T, Wang ZT, Branford-White CJ, Xu H, Wang CH. Classification and differentiation of the genus Peganum indigenous to China based on chloroplast trnL-F and psbA-trnH sequences and seed coat morphology. Plant Biol (Stuttg) 2011;13:940–947. doi: 10.1111/j.1438-8677.2011.00455.x. [DOI] [PubMed] [Google Scholar]