Abstract
With this study we have demonstrated that in vitro transduction of normal human CD4+ T lymphocytes with NPM-ALK results in their malignant transformation. The transformed cells become immortalized and display morphology and immunophenotype characteristic of patient-derived anaplastic large-cell lymphomas. These unique features, which are strictly dependent on NPM-ALK activity and expression, include perpetual cell growth, proliferation, and survival; activation of the key signal transduction pathways STAT3 and mTORC1; and expression of CD30 (the hallmark of anaplastic large-cell lymphoma) and of immunosuppressive cytokine IL-10 and cell-surface protein PD-L1/CD274. Implantation of NPM-ALK–transformed CD4+ T lymphocytes into immunodeficient mice resulted in formation of tumors indistinguishable from patients' anaplastic large-cell lymphomas. Our findings demonstrate that the key aspects of human carcinogenesis closely recapitulating the features of the native tumors can be faithfully reproduced in vitro when an appropriate oncogene is used to transform its natural target cells; this in turn points to the fundamental role in malignant cell transformation of potent oncogenes expressed in the relevant target cells. Such transformed cells should permit study of the early stages of carcinogenesis, and in particular the initial oncogene–host cell interactions. This experimental design could also be useful for studies of the effects of early therapeutic intervention and likely also the mechanisms of malignant progression.
Anaplastic large-cell lymphomas (ALCLs) carrying anaplastic lymphoma kinase (ALK) comprise a distinct clinicopathological entity.1, 2, 3 ALK+ ALCLs are derived from CD4+ T lymphocytes, typically occur in children and young adults, and involve soft tissues and other extranodal sites. As the name implies, ALCLs consist of large, highly atypical cells with prominent nuclei and abundant cytoplasm and thus bear little resemblance to their normal CD4+ T-cell counterparts, either resting or activated. They also display a unique phenotype, with variable loss of CD3 and other T-cell markers and strong expression of CD30 (a cell surface receptor in the TNF-R superfamily).
Although ALK is physiologically expressed only in a subset of immature neuronal cells,1 its aberrant expression has been identified in a subset of ALCL4, 5 and subsequently also in a spectrum of histologically diverse malignancies, including subsets of a large B-cell lymphoma, inflammatory myofibroblastic tumor, and non–small cell lung carcinoma.1, 2, 3 The aberrant expression of ALK typically results in these malignancies from chromosomal translocations involving the ALK gene and various partner genes, with the nucleophosmin gene (NPM1; alias NPM) being by far the most common partner in ALK+ ALCL.1 The NPM-ALK chimeric protein is constitutively activated through autophosphorylation4, 5 and is highly oncogenic, as has been documented mainly in patient-derived cell lines and transgenic mouse models.6, 7, 8, 9, 10 NPM-ALK activates a number of signal transduction pathways, including STAT311, 12 and mTORC1 (along with its downstream target, S6RP).13 Chronic activity of these signaling pathways leads to the persistent modulation of a number of genes, which results in sustained cell proliferation, resistance to cell death, and other oncogenic properties. NPM-ALK is capable of fostering evasion of the antitumor immune response by inducing expression of potent immunosuppressive proteins (namely, the cytokine IL-10 and the cell membrane bound ligand PD-L1/CD274).14, 15
Materials and Methods
Lentiviral Transduction of CD4+ T Lymphocytes
Deidentified purified human CD4+ T cells were obtained from the Human Immunology Core facility at the University of Pennsylvania under an Institutional Review Board–approved protocol. The CD4+ T cells were activated by coculture with anti-CD3/28 antibody-coated beads at a 1:3 cell/bead ratio. The cells were transduced 24 hours later by exposure to lentiviral vectors containing NPM-ALK (wild type or kinase-deficient K210R mutant) either alone or together with GFP as part of T2A fusion construct. Fresh medium was added to the cells on day 3 and twice weekly thereafter. On day 5, the magnetic beads were removed. Transduction efficiency was determined using flow cytometry to examine expression of GFP or NPM-ALK; the latter was accomplished using anti-ALK antibody (J606; BD Biosciences, San Jose, CA) and a Cytofix/Cytoperm fixation and permeabilization kit (BD Biosciences).
Cell Lines
The standard cell lines used in the present study have been described previously.13, 14, 15 In brief, the SUDHL-1 [obtained from Dr. Stephen Morris (St. Jude Hospital, Memphis, TN)] and JB6 cell lines were derived from ALK+ ALCL and the 2A (Mac-2A) cell line was derived from the primary cutaneous ALK-ALCL [both obtained from Dr. Marshall Kadin (Beth Israel Hospital, Boston MA)]. The MyLa2059 and MyLa3675 cell lines were derived from cutaneous T-cell lymphomas and obtained from Dr. Niels Ødum (University of Copenhagen, Copenhagen, Denmark). The Jeko cell line was derived from a mantle B-cell lymphoma and obtained from Dr. Raymond Lai (University of Alberta, Edmonton, AB, Canada). HEK 293 cells are derived from human embryonic kidney and purchased from the ATCC (Manassas, VA).
Western Blotting
Western blotting was performed using antibodies against p-ALK, p-STAT3, p-S6RP, total S6RP (all from Cell Signaling Technology, Danvers, MA), total NPM-ALK (BD Pharmingen, San Diego, CA), and total STAT3 and β-actin (both from Santa Cruz Biotechnology, Santa Cruz, CA), according to standard protocols.
Cell Migration
Cells were incubated for 20 hours in fetal bovine serum–free RPMI1640 medium, washed, resuspended in quenching medium (5% bovine serum albumin–RPMI). They were applied at concentration of 2 × 106/mL in 250 μL to the top chamber of the Transwell culture system (Millipore–Chemicon International, Temecula, CA) and 400 μL of RPMI medium with fetal bovine serum was added to the lower chamber. The plates were covered and incubated for 24 hours at 37°C in a 5% CO2–enriched atmosphere. Cells that passed through the membrane were collected from the lower chamber and added to a 96-well plate. Lysis buffer and dye solution containing CyQUANT green dye (Life Technologies) was added to all samples for 15 minutes at room temperature, and the plate was examined with a fluorescence plate reader (Molecular Devices, Sunnyvale, CA) using a 480/520-nm filter set.
Colony Formation
Cells were plated for 21 days in semisolid agar prepared according to the standard protocol. The number of growing colonies was counted using an inverted light microscope.
Flow Cytometry
The cells were analyzed using a FACSCalibur fluorescence-activated cell sorting system (BD Biosciences), and the data acquisition and analysis were performed using CellQuest Pro software version 6.03 (BD Biosciences). For the standard cell-surface staining, 0.5 × 106 to 1.0 × 106 cells were incubated for 20 minutes at 4°C with 10 to 20 μL of fluorescein isothiocyanate–conjugated, phycoerythrin-conjugated, or allophycocyanin-conjugated standard anti–T-cell and anti–B-cell antibodies, PD-L1, or isotype control antibody (BioLegend, San Diego, CA). The intracellular staining was performed by using commercially available fixation and permeabilization reagents from BD Biosciences or from Life Technologies (Carlsbad, CA). In brief, 0.5 × 106 to 1.0 × 106 washed membrane-stained or unstained cells were fixed for 15 minutes at room temperature with 100 μL of fixation and permeabilization solution or fixation medium. After washing the cells were resuspended in 100 μL of PBS or permeabilization medium, and incubated for 15 minutes at room temperature with 10 to 20 μL of phycoerythrin-labeled ALK or isotype control antibody (BD Biosciences). After additional washing, cells were analyzed by flow cytometry using a FACSCalibur system (BD Biosciences); data acquisition and analysis were performed using CellQuest Pro software version 6.03 (BD Biosciences).
Immunohistochemistry
Immunohistochemical staining was performed on formalin-fixed, paraffin-embedded cell blocks or xenotransplant tumor tissues using standard methods. In brief, the slides were heat-treated for antigen retrieval in 10 mmol/L citrate buffer and sections were incubated with diluted primary antibodies to ALK, CD30, CD2, MUM1, Ki-67 (all from Dako, Carpinteria, CA), and CD3 (Novocastra; Leica Microsystems, Wetzlar, Germany). For interpretation, the immunostained slides were evaluated by light microscopy.
Cytogenetics
A metaphase-arresting colcemid solution was added to cell cultures for 2 hours. Cells were exposed to hypotonic solution for 40 minutes at 37°C, followed by three changes of 1:3 glacial acetic acid/methanol solution. The cell suspension was dropped onto water-wetted microscope slides. Dried slides were aged in an oven at 600°C for 14 hours and stained with Wright's stain for G-banding. Metaphase spreads were analyzed under a bright-field microscope at ×100 magnification, images were captured, and karyotypes were prepared using GeneVision (Applied Imaging, Santa Clara, CA).
T-Cell Receptor Gene Rearrangement
DNA was extracted from cultured cells using conventional column-based methods (Qiagen, Valencia, CA). Two separate multiplex PCR amplifications were performed, using primers to relatively well-conserved regions in the V and J gene segments of the T-cell receptor gamma locus (TRG). PCR products were separated by capillary electrophoresis using an ABI 3130xl system (Life Technologies). Peak size and height were determined using GeneMapper software version 3.7 (Life Technologies). PCR product sizes are expected to range between 200 bp and 250 bp for the TRG V gene segments 1 to 8 primer mix, and between 150 bp and 200 bp for the TRG V gene segments 9 to 11 primer mix.
siRNA Assay
A mixture of four siRNAs specific for ALK or control siRNAs (Dharmacon; Thermo Fisher Scientific, Waltham, MA) was introduced into cells for 72 hours using Lipofectamine 2000 transfection reagent (Life Technologies) as described previously15 for SUDHL-1 cells and Nucleofector Solution T (Amaxa; Lonza, Walkersville, MD) for CD4+ T-cell–derived NA1 cells.
IL-10 Assay
IL-10 expression was examined using a human cytokine 10-plex antibody bead kit (Life Technologies) according to the manufacturer's protocol. Sample acquisition was performed using a Luminex FlexMAP-3D system (Life Technologies), and analyses were performed using the associated xPONENT software version 4.0. A nine-point standard curve at threefold dilutions was used, with the range defined by 80% to 120% of expected/observed values. Samples were tested in duplicate, and the coefficient of variation was <10%. Results are expressed as decrease in cells treated with the ALK inhibitor CEP-28122, relative to untreated cells.
RT-qPCR
For reverse transcription quantitative PCR (RT-qPCR), total RNA was extracted using a Qiagen RNeasy kit and was reverse-transcribed using an ABI high-capacity RNA-to-cDNA kit (Life Technologies). Expression levels of NPM-ALK mRNA were quantified by using an ABI PRISM 7700 sequence detection system with TaqMan gene expression assay kits (NPM-ALK, Hs03024829; β-actin, Hs9999903) (Life Technologies) and SYBR Green assay (Life Technologies), using forward and reverse primers for IL-10 (5′-AAGGCGCATGTGAACTCC-3′ and 5′-AAGGCATTCTTCACCTGCTC-3′) and for GAPDH (5′-TCTCCAGAACATCATCCCTGCCTC-3′ and 5′-TGGGCCATGAGGTCCACCACCCTG-3′). All assays were performed in duplicate. The fold difference in RNA levels was calculated on the basis of the difference between CT values (ΔCT) obtained for control and individual mRNA.
MTT Enzymatic Conversion Assay
Cells suspended at 2 × 104 cells per well were incubated at 37°C in microtiter plates for up to 44 hours, then incubated with MTT (Promega, Madison, WI) for 4 hours. Well contents were solubilized overnight in the medium containing 10% SDS and 0.01 mol/L HCl. Absorbance at 570 nm in each well was measured using a Titertek Multiskan spectrophotometer (Thermo Fisher Scientific).
Bromodeoxyuridine Incorporation Assay
The assay was performed using a cell-proliferation enzyme-linked immunosorbent assay (ELISA; Roche Diagnostics, Indianapolis, IN) according to the manufacturer's protocol. In brief, cells were cultured at a concentration of 2 × 104 cells per well for 44 hours and labeled with bromodeoxyuridine for 4 hours. After centrifugation, supernatant removal, and plate drying, the cells were fixed and the DNA was denatured using FixDenat reagent (Roche Diagnostics). The amount of incorporated bromodeoxyuridine was determined by incubation with a specific antibody conjugated with horseradish peroxidase, followed by colorimetric conversion of the substrate and absorbance evaluation in the ELISA plate reader.
TUNEL Assay for DNA Fragmentation
TUNEL assay was performed using an ApoAlert DNA fragmentation assay kit (BD Biosciences) according to the manufacturer's protocol. In brief, cells were cultured at 0.5 × 104 cells per well for 48 hours and then were collected, washed, fixed, permeabilized with 70% ethanol, washed again, and incubated in terminal deoxynucleotidyl transferase incubation buffer for 1 hour at 37°C. The reaction was stopped by adding 20 mmol/L EDTA. The cells were washed twice, resuspended in 0.5 mL of propidium iodide–RNase–PBS, collected, and analyzed by flow cytometry using a FACSCalibur system (BD Biosciences); data acquisition and analysis were performed using CellQuest Pro software (BD Biosciences).
Annexin V Expression Assay
For annexin V expression assay, cells were treated with the ALK inhibitor CEP-28122 (100 nmol/L) for 48 hours or with ALK siRNA (100 pmol/L) for 72 hours. After treatment, cells were washed with PBS and stained with anti–annexin V antibody and propidium iodide for 10 minutes, according to the manufacturer's instructions (Roche Diagnostics). The stained cells were analyzed by flow cytometry using a FACSCalibur system (BD Biosciences); data acquisition and analysis were performed using CellQuest Pro software (BD Biosciences).
Mouse Xenograft Tumor Formation
For tumor growth studies, NOD/SCID/IL-2Rγcnull (NSG; stock no. 005557; Jackson Laboratory, Bar Harbor, ME) mice were generated at the Stem Cell and Xenograft Core facility (University of Pennsylvania School of Medicine) using stock breeders obtained from the Jackson Laboratory. Mice were housed in sterile conditions using HEPA-filtered microisolator cages and were fed with irradiated food and acidified water. All experiments were performed using mice aged 8 weeks. The protocol was approved by the Institutional Animal Care and Use Committee. The NPM-ALK–transformed CD4+ T-cell lines NA1 and ALK+ ALCL–derived SUDHL-1 line were transduced to express luciferase. On day 0, individual mice were implanted with 3 × 106 cells by intraperitoneal administration. Mice were monitored weekly for tumor growth by visual examination and, starting with week 3, by bioluminescence imaging, which was performed on anesthetized mice using a Xenogen Spectrum system and Living Image software version 3.2 (Caliper–PerkinElmer, Hopkinton, MA). For imaging, 10 mg/kg d-luciferin (Caliper–PerkinElmer) suspended in PBS at a concentration of 15 mg/mL was administered intraperitoneally. Mice were imaged at 12 minutes after luciferin injection, and serial images were collected at various exposures. Data were analyzed with Living Image software version 3.2, using images taken with identical settings for mice in each group at each time point. Imaging data were converted to photons/second, photons/cm2, and photons/steradian to normalize each image for exposure time, f-stop, binning, and mouse size.
Results
NPM-ALK Induces Malignant Transformation of Normal CD4+ T Lymphocytes
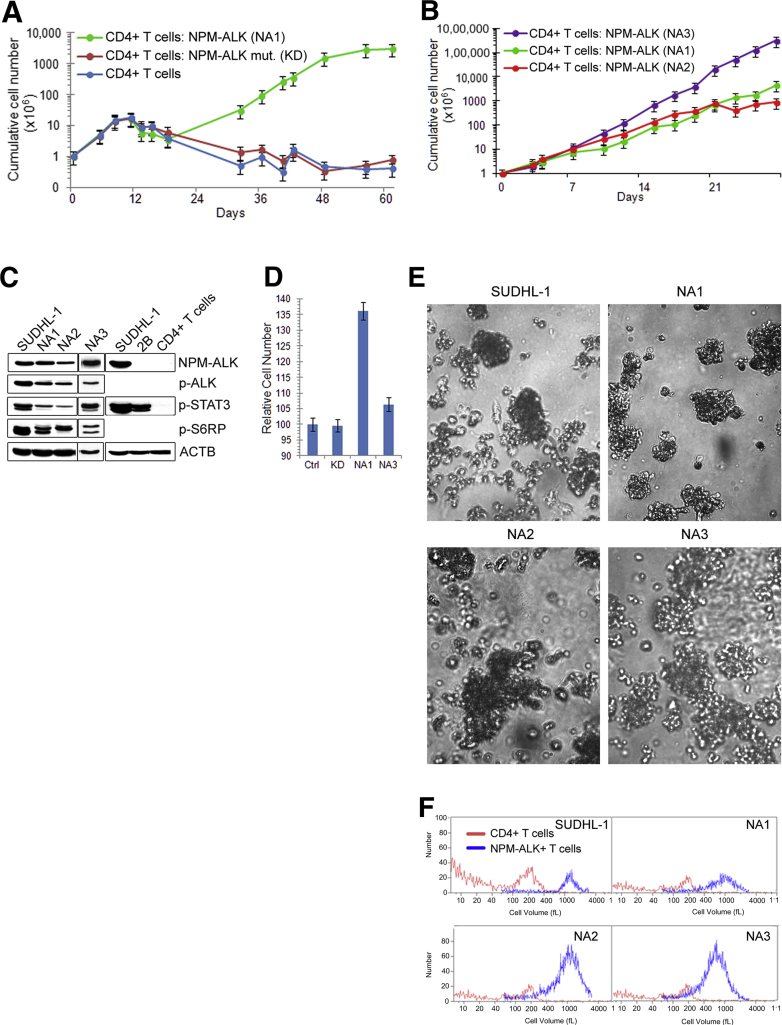
Given the highly oncogenic phenotype of NPM-ALK and the CD4+ T-cell derivation of ALK+ ALCL,1, 2, 3 we transduced purified normal CD4+ T lymphocytes with lentiviral vector expressing the kinase, after having preactivated the cells with anti-CD3 and anti-CD28 antibodies to foster an effective transduction. Separate pools of the preactivated CD4+ T cells were transduced with either an NPM-ALK mutant devoid of enzymatic activity (NPM-ALK-KD) or were left untransduced. Transfection with the native NPM-ALK led to sustained growth of the target cells (Figure 1A). Although they displayed somewhat higher transfection efficiency (Supplemental Figure S1), the cells expressing inactive NPM-ALK had reached the growth plateau by week 2 and began to decline shortly afterward, similar to untransfected cells. The same pattern of cell growth was seen in three independent consecutive experiments, in which the transfection of CD4+ T cells with wild-type NPM-ALK resulted in the establishment of cell lines designated NA1, NA2, and NA3. These cell lines display a steady growth rate (Figure 1B) and remain in continuous culture for at least 8 months, whereas the control cell populations ceased to grow by 3 to 4 weeks of culture (Supplemental Figure S2). The NA1, NA2, and NA3 cell lines display sustained expression of NPM-ALK, as well as phosphorylation of the kinase, similar to the control ALK+ ALCL–derived SUDHL-1 cells (Figure 1C). These cell lines also display phosphorylation of the direct target of NPM-ALK, STAT3, and of its indirect, mTORC1-dependent target S6RP. Cells of the NA1, NA2, and NA3 lines are also very large, matching the size of SUDHL-1 cells and markedly exceeding the size of the control CD3- and CD28-stimulated CD4+ T cells (Figure 1F). Furthermore, these cells can migrate (Figure 1D) and form colonies (Figure 1E) and thus display additional features of transformed cells.
Figure 1.
NPM-ALK–induced malignant transformation of normal human CD4+ T lymphocytes. A: Cell growth curves of NPM-ALK–expressing CD4+ T cells. Purified CD4+ T cells were stimulated with bead-immobilized CD3 and CD28 antibodies and either transduced with wild-type NPM-ALK (NA1) or enzymatically inactive NPM-ALK mutant (KD) or left untransfected. Triplicate cell cultures were counted using a cell counter. B: Cell growth curves of NPM-ALK–transfected CD4+ T cells (NA1, NA2, NA3) from three separate, consecutive experiments. C: Activation of ALK, STAT3, and mTORC1 pathways as determined by phosphorylation status of ALK, STAT3, and S6RP. Expression of total NPM-ALK and β-actin (ACTB) served as controls. ALK+ ALCL–derived cell line SUDHL-1, ALK-ALCL cell line 2B, and normal unstimulated CD4+ T cells were used as additional positive and negative controls. D: Migration of NPM-ALK–transfected cells determined using a Transwell culture system. Cells transfected with the enzymatically inactive NPM-ALK (KD) and untransfected cells (Ctrl) served as controls. P = 0.01 for NA1 and P = 0.04 for NA3 versus combined KD and Ctrl. E: Colony formation by the NPM-ALK–transfected NA1, NA2, NA3, and control ALK+ ALCL–derived SUDHL-1 cells. F: Cell volume of NPM-ALK–transfected and untransfected CD4+ T cells as determined by cell counter analysis (Beckman Coulter, Brea, CA). The ALK+ ALCL–derived cell line SUDHL-1 served as a positive control.
Characteristics of the NPM-ALK–Transformed Cells
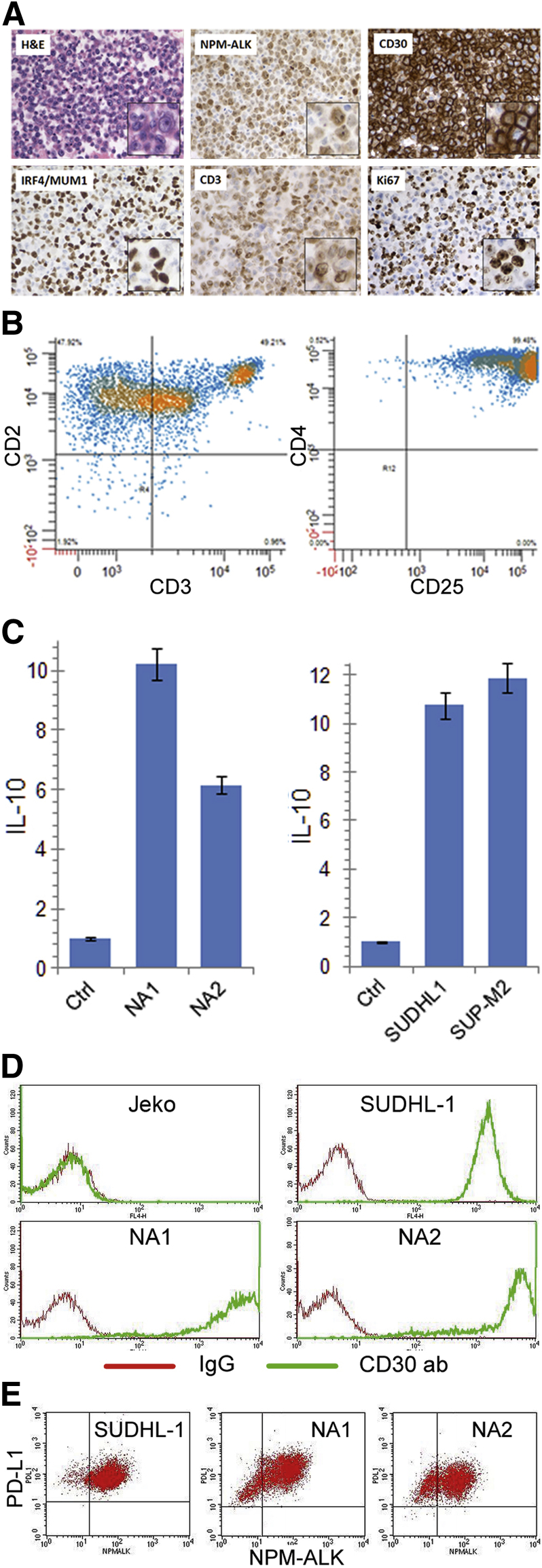
We next examined the morphology and immunophenotype of the NPM-ALK–transformed CD4+ T cells. These cells displayed predominantly large nuclei with prominent nucleoli and a moderate to abundant amount of the eosinophilic cytoplasm (Figure 2A). In addition to expressing NPM-ALK, these cells weakly expressed T-cell–related CD3 antigen and strongly expressed proliferation-related Ki-67 antigen, as determined by immunohistochemistry. Of note, the cells universally and strongly expressed CD30 and IRF4 (alias MUM1) antigens. Marked loss of CD3 expression was confirmed by flow cytometry, and diminished expression of CD5 and strong expression of CD4 and CD25 were also noted (Figure 2B). Strong CD30 expression was also confirmed by this method (Figure 2D). The cells further mimicked ALK+ ALCL cells by variably expressing the T-cell markers CD2 and CD7 (Supplemental Table S1).
Figure 2.
Morphological and immunophenotypic features of NPM-ALK–transformed CD4+ T cells. A: H&E staining and immunohistochemical analysis for NPM-ALK, CD30, IRF4 (alias MUM1), CD3, and Ki-67 in NA1 cells. B: Multiparameter flow cytometry analysis of NA1 cells for expression of T-cell markers CD2 and CD3, and CD4 and CD25. C: Expression of IL-10 mRNA determined by RT-qPCR in NA1 and NA2 cells, with CD3 and CD28–stimulated, NPM-ALK–untransfected CD4+ T cells (Ctrl) serving as negative control. ALK+ ALCL cell lines SUDH-L1 and SUP-M2 served as positive controls. ∗P = 0.01 for the experimental versus control cells. D: Flow cytometry analysis of the NA1 and NA2 for CD30 expression. ALK+ ALCL–derived SUDHL-1 and mantle cell lymphoma–derived Jeko cell lines served as positive and negative control, respectively. E: Expression of the immunosuppressive PD-L1/CD274 protein by NA1, NA2, and control SUDHL-1 cells. Original magnification: ×200; ×400 (insets).
Because ALK+ ALCL cells universally express the immunosuppressive molecules IL-1014 and PD-L1,15 we next examined the NPM-ALK–transduced CD4+ cells for expression of these two immunosuppressive molecules. Indeed, both IL-10 and PD-L1 were expressed by the transduced cells, similar to the ALK+ ALCL–derived cell lines (Figure 2, C and E).
To further characterize the NPM-ALK–transformed CD4+ T cells, we evaluated their karyotype and clonality. Both NA1 and NA2 cells displayed essentially normal cytogenetics, with only occasional random changes identified (Supplemental Figure S3). However, these two cell lines, as well as NA3, displayed from two to four distinct peaks in the T-cell receptor gamma chain rearrangement PCR study that used two separate primer pairs (Supplemental Table S2), indicating the monoclonal to oligoclonal nature of the transformed CD4+ T lymphocytes.
Transformed Cells are Strictly NPM-ALK–Dependent
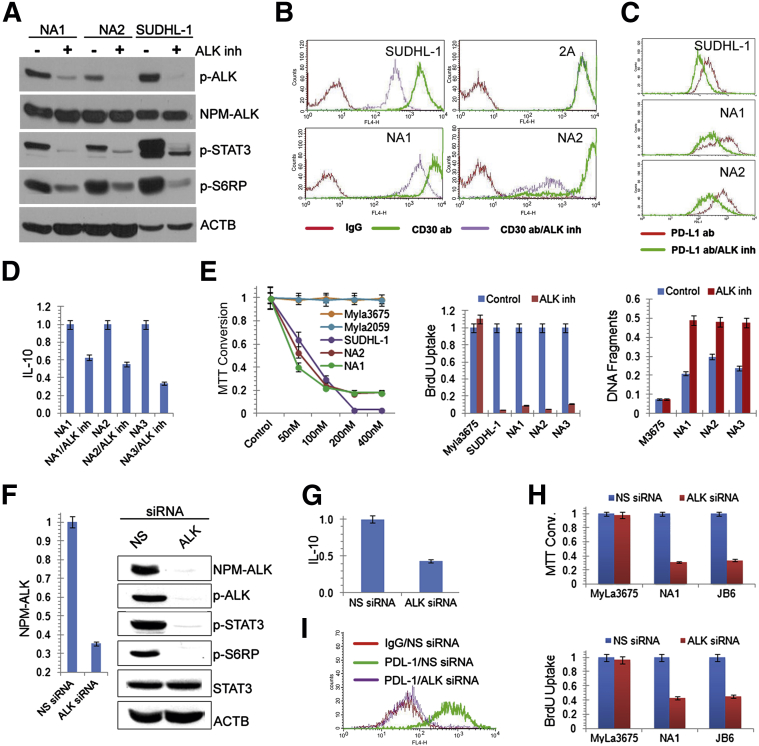
In the next series of experiments, we determined the effects of NPM-ALK suppression on these cells, using first a highly selective ALK inhibitor16 and then ALK-targeting siRNA. Cell signaling was ALK-dependent in the transformed cells, as documented by the ALK inhibitor–induced suppression of phosphorylation of the key proteins (ie, ALK itself, STAT3, and S6RP) (Figure 3A). A similar result was obtained in the NPM-ALK–transfected epithelial HEK 293 cells, although a higher dose of the inhibitor had to be used to suppress ALK, STAT3, and S6RP phosphorylation, because of the high concentration of NPM-ALK expressed by these easily transfectable cells (Supplemental Figure S4). HEK 293 cells transfected with the inactive NPM-ALK-KD mutant failed to phosphorylate ALK, STAT3, and S6RP, further supporting the key role of NPM-ALK in their activation.
Figure 3.
NPM-ALK–dependence of the transformed CD4+ T cells. A–D: Suppressive effect of the ALK inhibitor CEP-28122 (100 nmol/L) on phosphorylation of the cell-signaling proteins ALK, STAT3, and S6RP in NA1, NA2, and control SUDHL-1 cells (A); expression of CD30, with CD30-ALK− T-cell line 2A serving as a negative control (B); expression of PD-L1 (C); and synthesis of IL-10 (D). E: Effects of ALK inactivation by CEP-28122 on cell growth (left panel), cell proliferation (middle panel), and cell apoptotic rate (right panel). ALK− T-cell lines MyLa2059 and MyLa3675 and ALK+ ALCL cell line SUDHL-1 served as controls. F–I: Depletion of NPM-ALK mediated by ALK siRNA and its effect on phosphorylation of the cell-signaling proteins STAT3 and S6RP (F), IL-10 expression (G), cell growth (H), and PD-L1 expression (I). Nonspecific (NS) siRNA was used as a negative control (F–I). BrdU, bromodeoxyuridine.
Because expression of CD30, the hallmark of ALK+ ALCL, has been reported to be NPM-ALK–dependent,17 we examined whether this is the case in the NPM-ALK–transformed CD4+ T cells. Indeed, ALK inhibition diminished CD30 expression not only in the ALK+ ALCL–derived cells but also in the transformed CD4+ T cells, whereas it had no effect on CD30 expression in cells from an ALK− T-cell lymphoma (Figure 3B). Similar to CD30, expression of the immunosuppressive proteins PD-L1 and IL-10 was ALK-dependent in the NPM-ALK–transformed CD4+ T cells (Figure 3, C and D). ALK was also critical for the transformed CD4+ T cells on the functional level, because ALK inhibition suppressed their growth (Figure 3E and Supplemental Figure S5A). Depletion of NPM-ALK by siRNA yielded results similar to its inhibition, including loss of ALK, STAT3, and S6RP phosphorylation (Figure 3F), loss of IL-10 and PD-L1 expression (Figure 3, G and I), and impairment of cell growth (Figure 3H and Supplemental Figure S5B).
In Vivo Tumor Formation
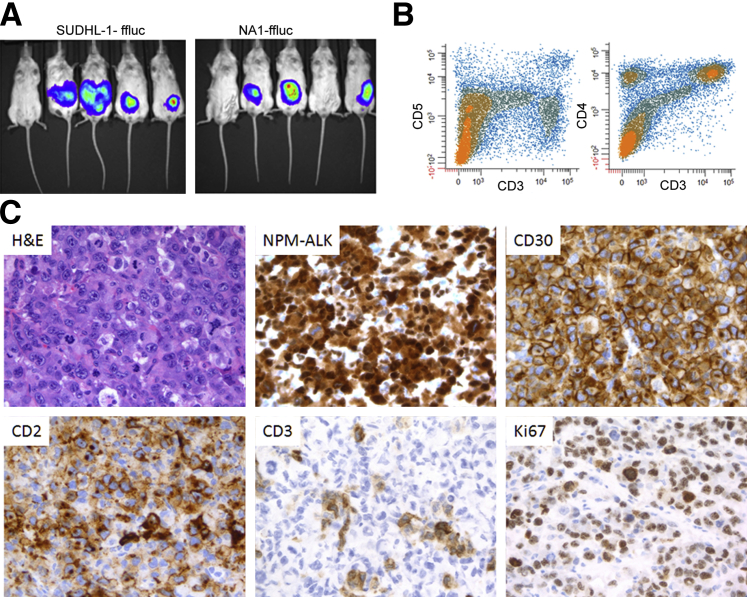
The NPM-ALK–transformed cells are capable of forming tumors in vivo as xenotransplants in the immunodeficient NSG mice (Figure 4). Within 5 weeks after intraperitoneal injection of the transformed cells, three of the five mice developed large tumors infiltrating the peritoneal wall, compared with four of the five mice injected with ALK+ ALCL cells (Figure 4A). These tumors were indistinguishable from native ALK+ ALCL, as determined by the immunophenotype, including variable loss of T-cell antigens, expression of NPM-ALK, CD30, and high proliferative rate and anaplastic large-cell morphology (Figure 4, B and C, and Supplemental Table S3). Taken together, these findings indicate that NPM-ALK induces malignant transformation of normal human CD4+ T lymphocytes.
Figure 4.
Growth of the transformed CD4+ T cells in vivo. A: Ability of NPM-ALK–transfected CD4+ T lymphocytes to form tumors in immunodeficient mice. NA1 and control SUDHL-1 cells were transfected with a vector containing the luciferase gene, injected intraperitoneally at 3 × 106 cells per mouse. The mice were examined for the presence of bioluminescence 5 weeks later. B: Flow cytometry detected expression of T-cell markers CD5 and CD3, and CD4 and CD3, by isolated tumor cells. C: Representative images of H&E staining and immunohistochemical staining for expression of NPM-ALK, CD30, CD2, CD3, and Ki-67 by the tumor tissues. Original magnification, ×400.
Discussion
Our understanding of carcinogenesis has been facilitated by development of various experimental models, including tumor-derived cell lines and oncogene-expressing transgenic mice, but these models have significant limitations. Only a handful of cell lines exist for any given malignancy, and they originate almost exclusively from aggressive, clinically advanced tumors, which precludes study of the early stages of carcinogenesis and the mechanisms of progression. Essentially all transgenic mouse models recapitulate only some features of the human malignancies; this certainly is the case with NPM-ALK transgenic mice. Efforts of several research groups using different gene promoters have resulted in the development of NPM-ALK–driven lymphomas, but all of these lymphomas were of either diverse B-cell or immature T-cell origin.7, 8 None of the transgenic mouse models truly recapitulate features of ALK+ ALCL, a malignancy of mature CD4+ T lymphocytes with highly distinct morphology and phenotype.
In vitro malignant transformation of normal human cells has long been a goal of cancer research. The efforts to recreate carcinogenesis in this manner have met with some success, most notably in immortalizing B lymphocytes using an oncogenic virus and epithelial cells using a combination of oncogenes. Although normal B lymphocytes can routinely be immortalized by human herpesvirus 4 (HHV-4; alias Epstein–Barr virus, or EBV),18 the transformed cells most closely resemble lymphoproliferative disorders seen in transplant patients and other immunodeficient individuals, rather than bona fide lymphomas occurring in the population at large. Furthermore, the EBV genome contains almost 100 genes, with expression of at least nine members from the EBNA and PSMB (alias LMP) gene families being seemingly critical to achieving immortalization. Weinberg and colleagues19, 20, 21 succeeded in neoplastic transformation of normal human fibroblasts and primary breast epithelial cells by simultaneously using three separate retroviral vectors containing genes encoding the hTERT unit of telomerase, the HRAS oncogene, and the simian virus 40 (SV40) early response region coding for large and small viral T antigens. Thus, three and perhaps four distinct genes were required to accomplish the transformation. Perhaps more importantly, these gene combinations, and specifically the SV40 T antigen or antigens, have not been unequivocally implicated in pathogenesis of the naturally occurring human malignancies.
In this context, it is remarkable that we succeeded in transforming normal human CD4+ T lymphocytes using a single oncogene NPM-ALK, and that the transformed cells were morphologically and immunophenotypically virtually indistinguishable from the patient-derived ALK+ ALCL. Previous studies have demonstrated that NPM-ALK tyrosine kinase is a very powerful oncogene, one that is capable of activating several key cell-signaling pathways, including STAT3 and mTORC1.1, 2, 3, 11, 12, 13 Activation of these multiple pathways modulates expression of many diverse genes that regulate the key oncogenic cell functions, including sustained cell growth and evasion of immune response. This pluripotency of ALK, a cell-surface receptor in its native form, is the most likely cause of its ability to transform the normal CD4+ T cells. In principle, other cell-surface tyrosine kinase receptors (such as the EGF-R family or even the IL-3R-mimicking cytoplasmic BCR-ABL (encoded by BCR-ABL1), which is very powerfully oncogenic in its own right and shares a number of characteristics with NPM-ALK22), as well as the other oncogenic forms of ALK, including ELM4-ALK,1, 2, 3 should also be able to effectively transform in vitro the normal cells they target in vivo.
It is interesting that the NPM-ALK immortalized cells are clonal, given the high transduction rate of CD4+ T cells (Supplemental Figure S1). A similar phenomenon has also been noted in the multigene, SV40 T antigen–based cell transformation system,21 and it is a norm in the transgenic mouse models, even though the transgene is expressed in entire tissues, or even the whole organism. At least two scenarios (not mutually exclusive) may be considered for explaining this general phenomenon. In the first scenario, additional genetic changes are always required to achieve malignant cell transformation. In the present study, the high success rate of immortalization achieved in several separate experiments, the normal karyotype of the NPM-ALK–transformed clones, and the young age of the ALK+ ALCL patients argue to some degree against this possibility in our system. The finding that highly malignant rhabdoid cancers in children display very few genetic changes, with a loss of a SMARCB1 gene being the sole common genomic lesion,23 also supports this conclusion. However, the ability of NPM-ALK to impair DNA repair, resulting in an increased mutation rate,24 suggests that secondary genetic changes can occur and may be required.
In the second scenario, only a small subset of the CD4+ T lymphocytes undergoes the effective NPM-ALK–induced malignant transformation. CD4+ T cells are phenotypically and functionally diverse, so it is possible that only a minor subset becomes transformed by NPM-ALK. In accord, a recent study indicates a resemblance of ALK+ ALCL cells to the Th17 subset of the CD4+ T lymphocytes.25 Furthermore, ALK+ ALCL may contain a very minor stem cell population critical for their development, as postulated recently for mucosa-associated lymphoid tissue (MALT) B-cell lymphoma.26 Our model should permit answering these fundamental questions with great precision, and the results may well be applicable to other models of carcinogenesis, including mice transgenic for NPM-ALK (and likely also for other tyrosine kinases and possibly for oncogenes in general).
Recently, Newrzela et al27 described an interesting model in which highly purified murine mature T cells were transduced with NPM-ALK or with another highly potent oncogene, ΔTrkA, and then were injected into Rag-1–deficient mice. Although mice inoculated with the oncogene-transduced T cells obtained from immunocompetent mice with a full T-cell receptor (TCR) repertoire typically failed to establish tumors, mice injected with such T cells obtained from immune-impaired mice transgenic for TCR developed clonal T-cell tumors with high frequency. Of note, coinjection of T cells with the full TCR repertoire and not transduced with the oncogene abrogated the ability of TCR transgenic, oncogene-transduced T cells to establish tumors. This finding clearly points to the key role of T-cell–based immunosurveillance in inhibiting T-cell lymphomagenesis. It is interesting in this context that we succeeded in transforming T cells from healthy individuals. There are at least two major differences between our experimental system and that of Newrzela et al.27 In our system, we dealt with human cells, and the outgrowth of the transformed cells occurred in vitro. The former difference may not have been critical, considering that tumors did develop in the recipient mice as long as the TCR transgenic T cells were used for transduction with the NPM-ALK or ΔTrkA oncogene. However, the in vivo milieu, specifically the antigen-presenting cells, is likely required to mount an effective inhibition of the oncogene-expressing cells by normal immune T cells.
In summary, the oncogenic tyrosine kinase NPM-ALK is capable of transforming normal CD4+ T cells in vitro and of conferring on these cells morphological and immunophenotypic features characteristic of patient-derived ALK+ ALCL cells and tissues. Here, we have documented effective malignant transformation of normal human cells by a single potent oncogene, with the product cells faithfully recapitulating malignant cells encountered in ALK+ ALCL patients. Cells generated in this manner should prove invaluable in studying the early stages of oncogenesis and, probably, the mechanisms of progression. They should also be useful in evaluating anticancer agents, including ALK inhibitors, which have already shown substantial efficacy in ALK-driven malignancies.28, 29
Footnotes
Supported in part by NIH grants R01-CA89194 (M.A.W.), R01-CA96856 (M.A.W.), P01-AI080192 (J.L.R.), and R01-CA147795 (J.L.R.).
Q.Z. and F.W. contributed equally to this work.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2013.08.030.
Contributor Information
James L. Riley, Email: rileyj@exchange.upenn.edu.
Mariusz A. Wasik, Email: wasik@mail.med.upenn.edu.
Supplemental Data
Transfection efficiency of the NPM-ALK wild-type (WT) and KD constructs. Normal CD4+ T cells prestimulated with CD3/CD28 antibody-coated beads were transfected with the NPM-ALK and stained with an anti-ALK antibody (green) or IgG (red) 5 days later. Data are representative of three independent experiments in which expression of NPM-ALK or GFP (for the NPM-ALK–GFP constructs) was measured.
Growth time of CD4+ T lymphocytes transfected with NPM-ALK. Normal CD4+ T lymphocytes were transfected with NPM-ALK and remained in ongoing culture for 1 to 12 months. Nontransfected CD4+ T cells or CD4+ T cells transfected with the enzymatically inactive NPM-ALK-KD mutant served as controls.
Representative normal karyotypes of NA1 (top) and NA2 (bottom) cell lines derived from the NPM-ALK–transfected CD4+ T cells.
Effect of the ALK inhibitor CEP-28122 (ALK inh) on activation of ALK and its targets. The cells were exposed to the inhibitor at 100, 200, or 400 nmol/L and then were examined for expression of phosphorylated proteins. Total protein served as controls. L. Exp, long exposure; S. Exp, short exposure.
NPM-ALK–dependent survival of transduced CD4+ T cells. A: Effect of 100 nmol/L ALK inhibitor CEP-28122 on cell-surface annexin V expression and propidium iodide (PI) incorporation in cell lines NA1, NA2, SUDHL-1 (positive control), and MyLa3675 (negative control). B: Effect of NPM-ALK depletion using ALK-specific and nonspecific (NS) control siRNA on NA1 cell staining for annexin V and PI. Western blots show the degree of NPM-ALK depletion, with β-actin (ACTB) serving as control.
References
- 1.Li R., Morris S.W. Development of anaplastic lymphoma kinase (ALK) small-molecule inhibitors for cancer therapy. Med Res Rev. 2008;28:372–412. doi: 10.1002/med.20109. [DOI] [PubMed] [Google Scholar]
- 2.Wasik M.A., Zhang Q., Marzec M., Kasprzycka M., Wang H.Y., Liu X. Anaplastic lymphoma kinase (ALK)-induced malignancies: novel mechanisms of cell transformation and potential therapeutic approaches. Semin Oncol. 2009;36(2 Suppl 1):S27–S35. doi: 10.1053/j.seminoncol.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Tabbó F., Barreca A., Piva R., Inghirami G., European T-Cell Lymphoma Study Group ALK signaling and target therapy in anaplastic large cell lymphoma. Front Oncol. 2012;2:41. doi: 10.3389/fonc.2012.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris S.W., Kirstein M.N., Valentine M.B., Dittmer K.G., Shapiro D.N., Saltman D.L., Look A.T. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [Erratum appeared in Science 1995, 267:316–317] [DOI] [PubMed] [Google Scholar]
- 5.Shiota M., Fujimoto J., Semba T., Satoh H., Yamamoto T., Mori S. Hyperphosphorylation of a novel 80 kDa protein-tyrosine kinase similar to Ltk in a human Ki-1 lymphoma cell line, AMS3. Oncogene. 1994;9:1567–1574. [PubMed] [Google Scholar]
- 6.Fujimoto J., Shiota M., Iwahara T., Seki N., Satoh H., Mori S., Yamamoto T. Characterization of the transforming activity of p80, a hyperphosphorylated protein in a Ki-1 lymphoma cell line with chromosomal translocation t(2;5) Proc Natl Acad Sci USA. 1996;93:4181–4186. doi: 10.1073/pnas.93.9.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuefer M.U., Look A.T., Pulford K., Behm F.G., Pattengale P.K., Mason D.Y., Morris S.W. Retrovirus-mediated gene transfer of NPM-ALK causes lymphoid malignancy in mice. Blood. 1997;90:2901–2910. [PubMed] [Google Scholar]
- 8.Chiarle R., Gong J.Z., Guasparri I., Pesci A., Cai J., Liu J., Simmons W.J., Dhall G., Howes J., Piva R., Inghirami G. NPM-ALK transgenic mice spontaneously develop T-cell lymphomas and plasma cell tumors. Blood. 2003;101:1919–1927. doi: 10.1182/blood-2002-05-1343. [DOI] [PubMed] [Google Scholar]
- 9.Turner S.D., Merz H., Yeung D., Alexander D.R. CD2 promoter regulated nucleophosmin-anaplastic lymphoma kinase in transgenic mice causes B lymphoid malignancy. Anticancer Res. 2006;26:3275–3279. [PubMed] [Google Scholar]
- 10.Giuriato S., Foisseau M., Dejean E., Felsher D.W., Al Saati T., Demur C., Ragab A., Kruczynski A., Schiff C., Delsol G., Meggetto F. Conditional TPM3-ALK and NPM-ALK transgenic mice develop reversible ALK-positive early B-cell lymphoma/leukemia. Blood. 2010;115:4061–4070. doi: 10.1182/blood-2008-06-163386. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q., Raghunath P.N., Xue L., Majewski M., Carpentieri D.F., Odum N., Morris S., Skorski T., Wasik M.A. Multilevel dysregulation of STAT3 activation in anaplastic lymphoma kinase-positive T/null-cell lymphoma. J Immunol. 2002;168:466–474. doi: 10.4049/jimmunol.168.1.466. [DOI] [PubMed] [Google Scholar]
- 12.Zamo A., Chiarle R., Piva R., Howes J., Fan Y., Chilosi M., Levy D.E., Inghirami G. Anaplastic lymphoma kinase (ALK) activates Stat3 and protects hematopoietic cells from cell death. Oncogene. 2002;21:1038–1047. doi: 10.1038/sj.onc.1205152. [DOI] [PubMed] [Google Scholar]
- 13.Marzec M., Kasprzycka M., Liu X., El-Salem M., Halasa K., Raghunath P.N., Bucki R., Wlodarski P., Wasik M.A. Oncogenic tyrosine kinase NPM/ALK induces activation of the rapamycin-sensitive mTOR signaling pathway. Oncogene. 2007;26:5606–5614. doi: 10.1038/sj.onc.1210346. [DOI] [PubMed] [Google Scholar]
- 14.Kasprzycka M., Marzec M., Liu Z., Zhang Q., Wasik M.A. Nucleophosmin/anaplastic lymphoma kinase (NPM/ALK) oncoprotein induces the T regulatory cell phenotype by activating STAT3. Proc Natl Acad Sci USA. 2006;103:9964–9969. doi: 10.1073/pnas.0603507103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marzec M., Zhang Q., Goradia A., Raghunath P.N., Liu X., Paessler M., Wang H.Y., Wysocka M., Cheng M., Ruggeri B.A., Wasik M.A. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1) Proc Natl Acad Sci USA. 2008;105:20852–20857. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng M., Quail M.R., Gingrich D.E., Ott G.R., Lu L., Wan W., Albom M.S., Angeles T.S., Aimone L.D., Cristofani F., Machiorlatti R., Abele C., Ator M.A., Dorsey B.D., Inghirami G., Ruggeri B.A. CEP-28122, a highly potent and selective orally active inhibitor of anaplastic lymphoma kinase with antitumor activity in experimental models of human cancers. Mol Cancer Ther. 2012;11:670–679. doi: 10.1158/1535-7163.MCT-11-0776. [DOI] [PubMed] [Google Scholar]
- 17.Hsu F.Y., Johnston P.B., Burke K.A., Zhao Y. The expression of CD30 in anaplastic large cell lymphoma is regulated by nucleophosmin-anaplastic lymphoma kinase-mediated JunB level in a cell type-specific manner. Cancer Res. 2006;15:9002–9008. doi: 10.1158/0008-5472.CAN-05-4101. [DOI] [PubMed] [Google Scholar]
- 18.Klein G., Klein E., Kashuba E. Interaction of Epstein-Barr virus (EBV) with human B-lymphocytes. Biochem Biophys Res Commun. 2010;396:67–73. doi: 10.1016/j.bbrc.2010.02.146. [DOI] [PubMed] [Google Scholar]
- 19.Hahn W.C., Counter C.M., Lundberg A.S., Beijersbergen R.L., Brooks M.W., Weinberg R.A. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 20.Elenbaas B., Spirio L., Koerner F., Fleming M.D., Zimonjic D.B., Donaher J.L., Popescu N.C., Hahn W.C., Weinberg R.A. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 2001;15:50–65. doi: 10.1101/gad.828901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ince T.A., Richardson A.L., Bell G.W., Saitoh M., Godar S., Karnoub A.E., Iglehart J.D., Weinberg R.A. Transformation of different human breast epithelial cell types leads to distinct tumor phenotypes. Cancer Cell. 2007;12:160–170. doi: 10.1016/j.ccr.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Shah N.P., Shannon K. Advancing the STATus of MPN pathogenesis. Blood. 2012;119:3374–3376. doi: 10.1182/blood-2012-02-406611. [DOI] [PubMed] [Google Scholar]
- 23.Lee R.S., Stewart C., Carter S.L., Ambrogio L., Cibulskis K., Sougnez C., Lawrence M.S., Auclair D., Mora J., Golub T.R., Biegel J.A., Getz G., Roberts C.W. A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. J Clin Invest. 2012;122:2983–2988. doi: 10.1172/JCI64400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young L.C., Bone K.M., Wang P., Wu F., Adam B.A., Hegazy S., Gelebart P., Holovati J., Li L., Andrew S.E., Lai R. Fusion tyrosine kinase NPM-ALK deregulates MSH2 and suppresses DNA mismatch repair function. Novel insights into a potent oncoprotein. Am J Pathol. 2011;179:411–421. doi: 10.1016/j.ajpath.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuyama H., Suzuki H.I., Nishimori H., Noguchi M., Yao T., Komatsu N., Mano H., Sugimoto K., Miyazono K. miR-135b mediates NPM-ALK–driven oncogenicity and renders IL-17-producing immunophenotype to anaplastic large cell lymphoma. Blood. 2011;118:6881–6892. doi: 10.1182/blood-2011-05-354654. [DOI] [PubMed] [Google Scholar]
- 26.Vicente-Dueñas C., Fontán L., Gonzalez-Herrero I., Romero-Camarero I., Segura V., Aznar M.A., Alonso-Escudero E., Campos-Sanchez E., Ruiz-Roca L., Barajas-Diego M., Sagardoy A., Martinez-Ferrandis J.I., Abollo-Jimenez F., Bertolo C., Peñuelas I., Garcia-Criado F.J., García-Cenador M.B., Tousseyn T., Agirre X., Prosper F., Garcia-Bragado F., McPhail E.D., Lossos I.S., Du M.Q., Flores T., Hernandez-Rivas J.M., Gonzalez M., Salar A., Bellosillo B., Conde E., Siebert R., Sagaert X., Cobaleda C., Sanchez-Garcia I., Martinez-Climent J.A. Expression of MALT1 oncogene in hematopoietic stem/progenitor cells recapitulates the pathogenesis of human lymphoma in mice. Proc Natl Acad Sci USA. 2012;109:10534–10539. doi: 10.1073/pnas.1204127109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newrzela S., Al-Ghaili N., Heinrich T., Petkova M., Hartmann S., Rengstl B., Kumar A., Jäck H.M., Gerdes S., Roeder I., Hansmann M.L., von Laer D. T-cell receptor diversity prevents T-cell lymphoma development. Leukemia. 2012;12:2499–2507. doi: 10.1038/leu.2012.142. [DOI] [PubMed] [Google Scholar]
- 28.Kwak E.L., Bang Y.J., Camidge D.R., Shaw A.T., Solomon B., Maki R.G., Ou S.H., Dezube B.J., Jänne P.A., Costa D.B., Varella-Garcia M., Kim W.H., Lynch T.J., Fidias P., Stubbs H., Engelman J.A., Sequist L.V., Tan W., Gandhi L., Mino-Kenudson M., Wei G.C., Shreeve S.M., Ratain M.J., Settleman J., Christensen J.G., Haber D.A., Wilner K., Salgia R., Shapiro G.I., Clark J.W., Iafrate A.J. Crizotinib for EML4-ALK positive lung adenocarcinoma: a hope for the advanced disease? N Engl J Med. 2010;363:1693–1703. [Erratum appeared in N Engl J Med 2011, 364:588] [Google Scholar]
- 29.Gambacorti-Passerini C., Messa C., Pogliani E.M. Crizotinib in anaplastic large-cell lymphoma. N Engl J Med. 2011;364:775–776. doi: 10.1056/NEJMc1013224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transfection efficiency of the NPM-ALK wild-type (WT) and KD constructs. Normal CD4+ T cells prestimulated with CD3/CD28 antibody-coated beads were transfected with the NPM-ALK and stained with an anti-ALK antibody (green) or IgG (red) 5 days later. Data are representative of three independent experiments in which expression of NPM-ALK or GFP (for the NPM-ALK–GFP constructs) was measured.
Growth time of CD4+ T lymphocytes transfected with NPM-ALK. Normal CD4+ T lymphocytes were transfected with NPM-ALK and remained in ongoing culture for 1 to 12 months. Nontransfected CD4+ T cells or CD4+ T cells transfected with the enzymatically inactive NPM-ALK-KD mutant served as controls.
Representative normal karyotypes of NA1 (top) and NA2 (bottom) cell lines derived from the NPM-ALK–transfected CD4+ T cells.
Effect of the ALK inhibitor CEP-28122 (ALK inh) on activation of ALK and its targets. The cells were exposed to the inhibitor at 100, 200, or 400 nmol/L and then were examined for expression of phosphorylated proteins. Total protein served as controls. L. Exp, long exposure; S. Exp, short exposure.
NPM-ALK–dependent survival of transduced CD4+ T cells. A: Effect of 100 nmol/L ALK inhibitor CEP-28122 on cell-surface annexin V expression and propidium iodide (PI) incorporation in cell lines NA1, NA2, SUDHL-1 (positive control), and MyLa3675 (negative control). B: Effect of NPM-ALK depletion using ALK-specific and nonspecific (NS) control siRNA on NA1 cell staining for annexin V and PI. Western blots show the degree of NPM-ALK depletion, with β-actin (ACTB) serving as control.