Abstract
Telomere end-to-end fusions are an important source of chromosomal instability that arise in cells with critically shortened telomeres. We developed a nested real-time quantitative PCR method for telomere fusion detection in pancreatic ductal adenocarcinomas, intraductal papillary mucinous neoplasms (IPMNs), and IPMN cyst fluids. Ninety-one pancreatic cancer cell lines and xenograft samples, 93 IPMNs, and 93 surgically aspirated IPMN cyst fluid samples were analyzed. The association between telomere shortening, telomerase activity, and telomere fusion detection was evaluated. Telomere fusions were detected in 56 of 91 pancreatic cancers (61.5%). Telomere fusion-positive cell lines had significantly shorter telomere lengths than fusion-negative lines (P = 0.003). Telomere fusions were undetectable in normal pancreas or IPMNs with low-grade dysplasia (0.0%) and were detected in IPMN with high-grade dysplasia (HGD; 48.0%) (P < 0.001). In IPMN cyst fluids, telomere fusions were more frequent in IPMNs with HGD (26.9%) or associated invasive cancer (42.9%) than IPMN with intermediate-grade dysplasia (15.4%) or low-grade dysplasia (0%) (P = 0.025). Telomerase activity levels were higher in cyst fluids with fusions than in those without (P = 0.0414). Cyst fluid telomere fusion status was an independent predictor of HGD/invasive cancer by multivariate analysis (odds ratio, 6.23; 95% CI, 1.61–28.0). Telomere fusions are detected in later stages of IPMN progression and can serve as a marker for predicting the presence of HGD and/or invasive cancer.
CME Accreditation Statement: This activity (“JMD 2018 CME Program in Molecular Diagnostics”) has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of the American Society for Clinical Pathology (ASCP) and the American Society for Investigative Pathology (ASIP). ASCP is accredited by the ACCME to provide continuing medical education for physicians.
The ASCP designates this journal-based CME activity (“JMD 2018 CME Program in Molecular Diagnostics”) for a maximum of 18.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only credit commensurate with the extent of their participation in the activity.
CME Disclosures: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Telomeres are structures present at all chromosomal ends.1 Telomeric repeat sequences (TTAGGG) prevent the fusion of chromosomal ends; such end-to-end fusions can occur once telomeres lose most or all of their telomere repeat sequences.2 Telomere fusions that arise in critically short telomeres will either elicit DNA damage responses that lead to cell death or cause breakage-fusion-breakage cycles with ongoing chromosomal instability, which is a main mechanism that contributes to the progression of many precancerous neoplasms to invasive cancers.3, 4 Most cancer cells display significant telomere shortening,5, 6 and telomere shortening is observed in most pancreatic intraepithelial neoplasia lesions, including pancreatic intraepithelial neoplasia 1A.7 Similarly, intraductal papillary mucinous neoplasms (IPMNs) with low-grade dysplasia (LGD) typically have significant telomere shortening.8 Neoplastic cells with critically shortened telomeres can maintain their shortened telomere length and can overcome cell death by activating telomerase (the most common mechanism) or occasionally by using the alternate length telomere pathway.3, 9
Telomere fusions that arise from critical telomere shortening have been observed not only in cancers10, 11, 12 but also identified in 13% of colorectal adenomas in one study12; thus, they may be expected to occur in other precursor lesions that have critically shortened telomeres. If so, the detection of telomere fusions in diagnostic specimens may help identify precancerous neoplasms with critically short telomeres more likely to progress to invasive cancer.
IPMNs are one such precursor lesion, characterized by the papillary proliferation of mucin-producing epithelial cells and cystic dilatation of the main or branch pancreatic duct, and they are the most common type of neoplastic cyst. The main goal of the diagnostic evaluation of IPMNs is to determine their grade of dysplasia, that is, to distinguish IPMNs with LGD, having low malignant potential and a favorable prognosis and not requiring surgical intervention from those that have high-grade dysplasia (HGD) or an associated invasive carcinoma.13 Given the morbidity and risks related to pancreatic surgery, watchful observation is generally appropriate for patients with IPMNs with LGD and intermediate-grade dysplasia (IGD),14 unless patients have other risk factors for progression or extensive multifocal disease.15, 16 Clinicians rely on international consensus guidelines to help manage patients with IPMN.14
These guidelines are useful, but pancreatic imaging does not provide sufficient information about the neoplastic nature of a pancreatic cyst. Better characterization of pancreatic cysts may allow more patients with worrisome cysts to continue with surveillance.17, 18, 19, 20, 21 Cyst fluid biomarkers are being evaluated for their utility to better predict the neoplastic nature of IPMNs.22, 23, 24, 25, 26, 27, 28 For this study, we developed a telomere fusion assay and used it to detect telomere fusions in pancreatic cancer and IPMNs and to determine whether the detection of telomere fusions in pancreatic cyst fluid samples can predict the grade of dysplasia of IPMN.
Materials and Methods
Tissues and Cyst Fluid Samples
IPMN tissues and cyst fluids were obtained from patients undergoing pancreatic resection at the Johns Hopkins Hospital from 2004 to 2015. IPMNs were identified at the time of frozen-section analysis of pancreatic resection specimens by a pathologist specializing in pancreas pathology (R.H.H.). Frozen sections of primary resected IPMNs (n = 93), any adjacent adenocarcinoma, and adjacent normal pancreas tissue were obtained from fresh-frozen tissue blocks created in the surgical pathology suite shortly after the resection specimen was received and mounted onto membrane slides for subsequent laser capture microdissection. The collection and processing of surgical cyst fluid samples (n = 93) has been described previously.22 Frozen tissue samples of normal pancreas (n = 17) and duodenum (n = 22) were obtained from pancreatic resection specimens after diagnostic evaluation from cases with nonmalignant pancreatic disease, including small localized pancreatic neuroendocrine tumors and serous cyst neoplasm. Genomic DNA was also isolated from 60 pancreatic cancer xenografts established from primary pancreatic adenocarcinomas resected from our institution as previously described.29 All elements of this study were approved by the Johns Hopkins Institutional Review Board, and written informed consent was obtained from all patients.
Cell Lines
MIA PaCa-2, BxPC-3, Hs766T, PANC-1, AsPC-1, CFPAC-1, Capan-1, Capan-2, SU.86.86, HPAF-II, HPAC, and SW1990 were obtained from the ATCC (Rockville, MD). PK-8 and PK-9 cells are kind gifts from Dr. Akira Horii (Tohoku University, Sendai, Japan). Remaining cell lines were developed and maintained in our institution.29, 30, 31 An HPV-E6/E7 immortalized human pancreatic duct epithelial cell line, HPDE, was kindly provided by Dr. Ming-Sound Tsao (University of Toronto, Toronto, ON, Canada). The generation and culture of hTERT-immortalized human pancreatic nestin-expressing (HPNE) cells has previously been described.32 These cancer cell lines were recently authenticated with genetic markers by the Johns Hopkins Genetics Core facility. HPDE was authenticated by testing it for genetic markers of human papillomavirus (HPV), E6, and E7. Cell lines were grown under recommended conditions as described previously.22
Laser Capture Microdissection
Laser capture microdissection was performed as previously described.33
DNA Extraction
DNA extraction was performed as previously described.22 For cyst fluid samples with mucus, mucin was dissolved by increasing the length of proteinase K digestion. Whole-genome amplification was conducted for several cell line DNA samples with the REPLI-g Mini Kit (Qiagen, Valencia, CA) with the use of a 16-hour incubation. DNA was eluted with EB buffer (10 mmol/L Tris-HCl, pH 8.5) and quantified with Quantifiler (Applied Biosystems, Foster City, CA).
Telomere Fusion Assay
PCR primers designed to amplify telomere fusions in prior studies have placed primers well away from the telomere repeat region to take into account the potential for subtelomeric deletions.11, 34, 35 These PCR assays would not detect telomere fusions in highly fragmented DNA that are usually found in secondary fluids such as cyst fluids. Because most telomere fusions contain few telomere repeats, a telomere fusion PCR was designed to amplify short telomere fusion amplicons for detection in pancreatic cyst fluids. Primers were designed to anneal to the subtelomere region close to (approximately 100 bases from) telomeric repeat sequences. It is known that subtelomeric regions of many chromosomes have sequence homology.36 Thus, there is considerable homology between the (mostly) q arms 1q, 2q, 5q, 6q, 6p, 8p, 10q, 13q, 19p, 19q, 21q, 22q, and 2q13 and between the p arms 1p, 9p, 12p, 15q, and 16p such that with one subtelomere q-arm PCR primer and one subtelomere p-arm PCR primer pair one may potentially amplify many different chromosomal end-to-end fusions.35
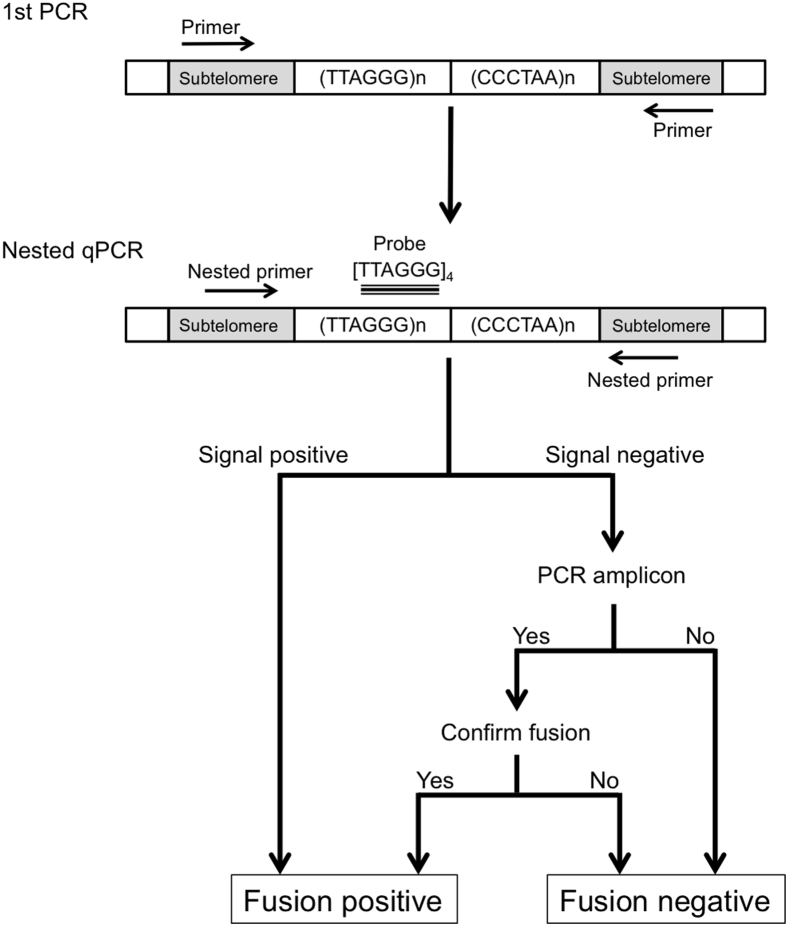
In the present study, we designed a nested real-time quantitative PCR (qPCR) assay, with a first-round PCR that used a subtelomere q-arm PCR (q-subtel) primer that targeted 1p, 1q, 2q, 4p, 4q, 5q, 6q, 9p, 10q, 13q, 16p, 16q, 19p, 21q, 22q, Xq, and Yq and a subtelomere p-arm PCR (p-subtel) primer that targeted the subtelomeric regions of 1p, 9p, 12p, 15q, 16p, Xq, and Yq, followed by a nested qPCR with the use of PCR primers located internal to the first-round PCR primers and detected with a [TTAGGG]4 probe. An overview of the assay protocol is provided in Figure 1. Telomere fusions were detected in pancreatic cancer cell line DNA with the use of 4 to 40 ng of DNA (Results). Therefore, the first PCR was performed in 10 μL with four replicates each containing 10 ng for pure genomic DNA, first primer set (500 nmol/L of p-subtel 1 and q-subtel 1), 100 nmol/L of 7-deaza-dGTP (New England Biolabs, Ipswich, MA), and Advantage GC genomic LA polymerase Mix (Clontech, Mountain View, CA). PCR conditions were 95°C for 3 minutes (1 cycle), followed by 30 cycles of 95°C for 15 seconds, 60°C for 30 seconds, and 72°C for 90 seconds. To account for the lower neoplastic cellularity of cyst fluids, 30 ng of cyst fluid DNA was used in the assay instead of 10 ng. The PCR products from the four replicates were collected, pooled, and diluted × 100-fold. The nested-qPCR was conducted with the use of a 7900HT thermocycler (Applied Biosystems) with 1 μL of first-round PCR diluted products, nested primers (500 nmol/L of p-subtel2 and q-subtel2), 100 nmol/L of 7-deaza-dGTP, Advantage GC genomic LA polymerase Mix, and 250 nmol/L of locked nucleic acid probe targeting the [TTAGGG]4 sequence with a 5′ reporter dye (FAM) and 3′ Iowa Black dark quencher (IABkFQ), synthesized by IDT (Integrated DNA Technologies, Inc., Coralville, IA). PCR conditions were 95°C for 3 minutes (1 cycle), followed by 50 cycles of 95°C for 15 seconds, 64°C for 30 seconds, 72°C for 45 seconds. Nontemplate controls from the first-round PCR were included in the second round PCR to rule out contamination. A Ct (the cycle number at which the fluorescence emission exceeds a fixed threshold) of 35 was considered the lowest level of reliable detection. Telomere fusion assays on IPMNs and cyst fluids were performed blinded (T.H.) for their histologic grade.
Figure 1.
Summary of the telomere fusion assay protocol. qPCR, real-time quantitative PCR.
Some telomere fusions do not have sufficient telomere repeat sequences to be detected with the telomere repeat probe. To detect these fusions, PCR products were run on 0.8% agarose gels. To confirm that qPCR-negative PCR amplicons generated by the fusion assay were telomere fusion amplicons, these amplicons were further characterized by applying another nested PCR to determine whether these amplicons were true telomere fusions. Amplicons generated from this PCR were also subcloned and sequenced. This third-round PCR was performed with × 300 dilution of nested-qPCR products and nested third-round primers (p-subtel 2 and q-subtel 3) with 71°C annealing and 30 cycles. To determine the sequence of telomere fusions, purified PCR products (using QIA quick Gel Extraction Kit; Qiagen) were cloned into pCR 2.1-TOPO TA vector with the use of the TOPO TA Cloning Kit (ThermoFisher Scientific, Waltham, MA) according to the manufacturer's instructions. White colonies on X-gal–containing agar plate were chosen randomly for colony PCR selection, plasmid DNA extraction (QIAprep Spin Mini kit), and Sanger sequenced with the use of M13 F and R primers at The Johns Hopkins Synthesis and Sequencing Facility. The PCR products of three to six colonies were sequenced, depending on whether multiple inserts were identified by colony PCR. The National Center for Biotechnology Information BLAST (https://blast.ncbi.nlm.nih.gov, last accessed September 10, 2016) of the telomere fusion sequences was used to identify the potential chromosomal arms involved.
The same nested qPCR approach was also used in an attempt to detect fusions that involved the subtelomeric region of the Xp and Yp chromosomal arms (with and without the p-subtel and q-subtel primers used above) by using primers that targeted these subtelomeric regions in the first-round PCR, followed by the nested qPCR assay with the [TTAGGG]4 probe. No telomere fusions were detected with the Xp or Yp primers (data not shown). Primer and probe sequences used in this study are provided in Table 1.
Table 1.
Primer and Probe Sequences Used in This Study
| Primer/probe | Purpose | Sequence | Covered chromosomal arms |
|---|---|---|---|
| p-subtel 1 primer | First PCR | 5′-GACGCGCTAGCATGTGTCTCTG-3′ | 1p, 9p, 12p, 15q, 16p, Xq, Yq |
| p-subtel 2 primer | Nested-qPCR, third PCR | 5′-CTAGCATGTGTCTCTGCGCCTG-3′ | |
| q-subtel 1 primer | First PCR | 5′-GAATCCTGCGCACCGAGATTCTC-3′ | 1p, 1q, 2q, 4p, 4q, 5q, 6q, 9p, 10q, 13q, 16p, 16q, 19p, 21q, 22q, Xq, Yq |
| q-subtel 2 primer | Nested-qPCR | 5′-CACCGAGATTCTCCCAAGGCAAG-3′ | |
| q-subtel 3 primer | Third PCR | 5′-CAAGGCAAGGSGAGGGGCTG-3′ | |
| XpYp primer | First PCR | 5′-GGCTCAGGCAGTCTGCTTTTATTC-3′ | Xp, Yp |
| XpYp primer | Nested-qPCR | 5′-CTCTAATCTGCTCCCACCCACATC-3′ | |
| [TTAGGG]4 probe | Nested-qPCR | /56-FAM/TTA+GGGTTA+GGGTTA+GGGTTA+GGG/3IABkFQ/ | |
p-subtel, subtelomere p-arm PCR; qPCR, real-time quantitative PCR; q-subtel, subtelomere q-arm PCR.
Telomere Length Assay
Telomere length was determined as the relative ratio of telomere repeat copy number to a single copy gene copy number (T/S ratio) with the use of real-time qPCR with minor modifications.37 Quantitative PCR was performed with QuantiTect SYBR Green PCR Master Mix (Qiagen) by using a 7900HT thermocycler (Applied Biosystems) with an annealing temperature of 49°C. The reference housekeeping gene 36B4 (known as ribosomal protein lateral stalk subunit P0) was amplified with the following conditions: 95°C for 10 minutes, followed by 40 cycles of denaturation at 95°C for 15 seconds, and annealing and extension at 60°C for 60 seconds. To calculate the T/S ratios, each DNA standard curve was generated with the use of commercialized human whole blood genomic DNA from multiple healthy anonymous donors (Promega, Madison, WI). Primer sequences were as described previously.37 Relative telomere erosion, the change in telomere length in tumor DNA compared with the matched adjacent normal tissue DNA, was also calculated.38
Statistical Analysis
Nonparametric U-test was used to compare unpaired continuous variables. Wilcoxon matched-pairs signed rank test was used to compare paired continuous variables. Fisher exact test was used to compare categorical variables. Survival time was examined with the Kaplan-Meier method and compared with the log-rank test. A multivariate analysis was performed with the logistic regression model. All statistical analysis was performed with the JMP Pro statistical software version 12.2.0 (SAS Institute Inc., Cary, NC) and GraphPad Prism version 7.0 (GraphPad Software, San Diego, CA). P < 0.05 was considered statistically significant.
Results
Telomere Fusion Detection in Pancreatic Cancer Samples
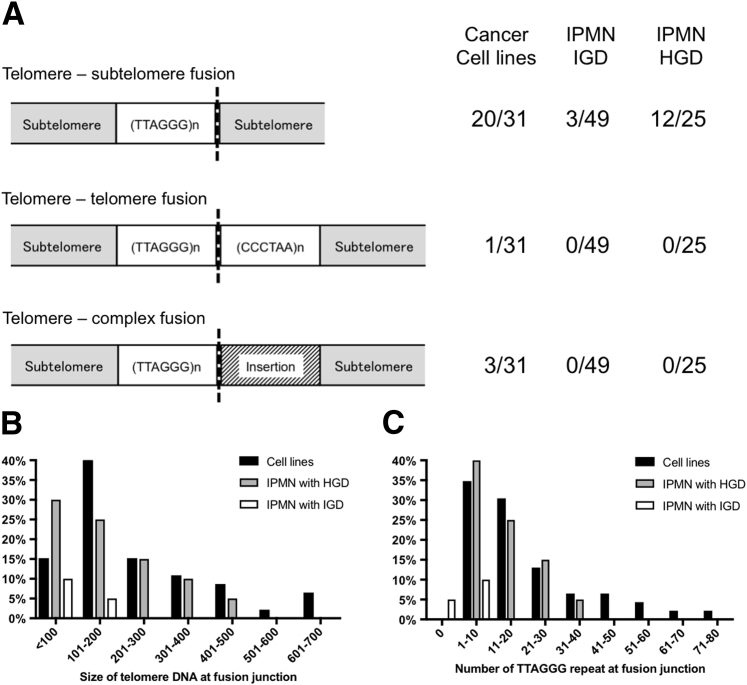
Telomere fusions were detected in 22 of 31 pancreatic cancer cell lines with the use of the telomere fusion assay (Supplemental Figure S1, A and B). Twenty-one of these fusions were detected with the telomere repeat probe by qPCR. One cell line (PL4) had a telomere fusion with variant telomere repeat sequence, identified first by electrophoresis of the nested-qPCR products and confirmed by an additional nested PCR (lane 30) (Supplemental Figure S1C). A telomere fusion was also detected in the HPDE cell line that is known to harbor chromosomal abnormalities,39 but not in the HPNE line or in normal tissue samples. The presence of telomere fusions in the cancer cell lines was confirmed in all qPCR-positive samples by subcloning and sequencing the nested-qPCR products (Supplemental Table S1). As expected, all qPCR-positive cell lines had subtelomeric sequences with TTAGGG repeats. Supplemental Figure S1D shows a representative example of a fusion junction sequence from AsPC-1 cells. Supplemental Figure S1E shows that most of the PL4 fusion contained variant repeat sequences (TGAGGG, TGGGGG, TTGGGG, and TTCGGG). Among the 23 fusion-positive cell lines, 20 cell lines had a telomere–subtelomere fusion, that is, the fusion of a short telomeric repeat from one chromosomal end to a subtelomere region without any telomeric repeats from the other chromosomal end (Supplemental Table S1 and Figure 2). Three cell lines (SU.86.86, Panc 3.014, and PANC 486) had fusions that contained small insertions between the telomeric repeats of one chromosome and the subtelomere of the other (complex telomere–fusions) (Supplemental Table S1 and Figure 2A). Supplemental Figure S2 shows an example of a complex fusion junction found in PANC 486 cells. Some cell lines had multiple different fusions (47 unique clones were identified) (Supplemental Table S1) as has been shown previously in other tumor types.35
Figure 2.
Characteristics of telomere fusions. A: Classification of telomere fusions in pancreatic cancer cell lines and intraductal papillary mucinous neoplasm (IPMN) tissues. Telomere–telomere fusions contain telomere repeat sequences from both chromosomal ends (TTAGGG and its complement CCCTAA). Telomere–subtelomere fusions contain telomeric repeats from one chromosome fused with the subtelomeric region of another chromosome. Telomere–complex fusions contain short insertion between the subtelomere-telomere fusion junction. B: The length of telomeric DNA within fusion junctions. C: The number of TTAGGG repeats within fusions. HGD, high-grade dysplasia; IGD, intermediate-grade dysplasia.
The fusion assay was also performed on DNA from 60 pancreatic cancer xenograft samples. A summary of these cases, including the American Joint Committee on Cancer eighth edition40 staging of the primary cancers from which they were derived, is provided in Supplemental Table S2. Overall, 34 of the 60 pancreatic cancer xenografts (56.7%) were fusion positive (Supplemental Figure S3). No fusions were detected in mouse DNA (Supplemental Figure S3C). No significant association was found between tumor pathology (ie, tumor size, lymph node metastasis, and histologic differentiation) and telomere fusion status (Supplemental Table S3). Patient survival was similar irrespective of the fusion status of their pancreatic cancer (P = 0.54) (Supplemental Figure S4).
To better estimate the amount of cancer DNA required to identify a fusion with the designed assay, fusion assay was performed with lower amounts of input DNA from several fusion-positive and -negative cells lines. Fusions were detected in four fusion-positive cell lines with the use of 10 ng of input DNA, but fusions were not detected reliably with the use of amounts of DNA below this amount (Supplemental Table S4). To exclude PCR efficiency as an explanation for these results, telomere fusion PCR products of two different fusion amplicons were quantified with the use of a Bioanalyzer, and these PCR products were spiked into wild-type DNA, and the number of amplicons required to get amplification was determined. After obtaining fusion products generated from performing the first- and second-round PCR on cancer cell lines that consistently generated fusions, the spiked fusion amplicons could be amplified with the use of the third-round PCR at concentrations as low as 1 amplicon. Similar results were obtained when fusion amplicons were generated with two rounds of the first-round PCR (30 cycles each); fusion amplicons could be detected with the use of the second-round PCR at amplicon concentrations of approximately 1 amplicon per sample (data not shown). These results indicated that PCR efficiency was not the primary reason for the inability to amplify telomere fusions with low amounts of input DNA.
Telomere fusion sequences had a variable number of telomeric repeats, including in some cases the presence of telomere variant sequences (Supplemental Table S1). Most fusions had variant repeat sequences; the presence of variant repeats was thought to contribute to telomere dysfunction and fusion formation.41 Most telomere fusion variant sequences involved substitutions consistent with nucleotide misincorporation that arise when telomerase performs telomere extensions.42 It was examined how quantification by the qPCR assay was affected by the number of telomere repeats in the telomere fusions. Cloned plasmid DNA from PANC 215, Panc 08.13, and AsPC-1 cells was used. Clones with more TTAGGG repeats generated lower Ct values than clones with fewer repeats (Supplemental Table S5 and Supplemental Figure S5A). Amplification of PANC 215 (clone 8), which generated a higher Ct value had only six TTAGGG repeats in the fusion, and they were not present as a continuous [TTAGGG]4 repeat. Instead, one or more TTAGGG repeats were dispersed throughout the fusion junction with intervening variant sequences such as TTCGGG, TCGGGG, and TTGGGG, which resulted in atypical amplification curve (Supplemental Figure S5, B and C), unlike the linear amplification observed for PANC 215 (clone 1) fusion (Supplemental Figure S5, D and E). Other telomere-fusion–positive DNA samples with atypical variant repeat sequences were also identified with the use of the [TTAGGG]4 probe; many of these samples also had the [TTAGGG]4 target sequence, and others may have been identified because there was sufficient annealing of the [TTAGGG]4 for detection. These results indicated that Ct values are affected by both the number of fusion templates in samples and by the number and sequence of the telomeric repeats within the fusion junction.
Telomere Length and Telomere Fusions in Pancreatic Cancer Cell Lines
Because telomere fusions are thought to be more likely to occur in cells with critically short telomeres, the association between telomere length and telomere fusion status was examined. The telomere length assay was evaluated for its specificity, linearity, and reproducibility (Supplemental Figure S6). Fusion-positive pancreatic cancer cell lines had significant shorter mean telomere length than fusion-negative cell lines (P = 0.0033) (Supplemental Figure S7).
Telomere Fusion Detection and Telomere Length in IPMN
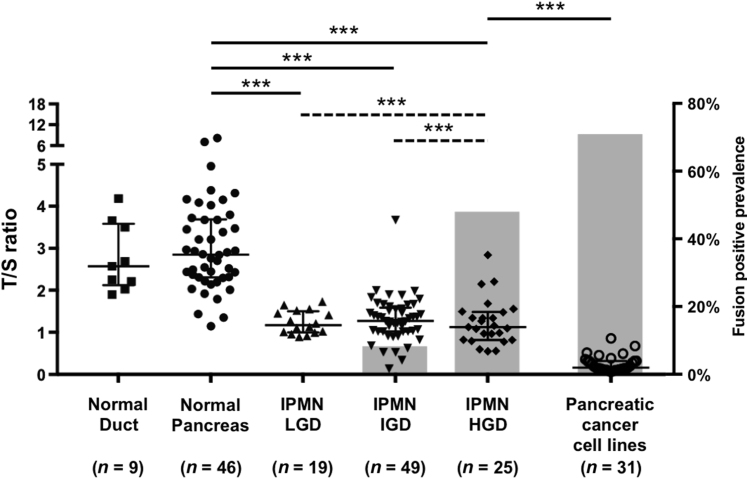
The fusion assay was next performed on DNA isolated from IPMN, normal pancreas, and normal duodenum. No fusions were detected in 39 normal pancreas and duodenal tissue samples. Ninety-three laser capture microdissected IPMNs of different histologic grades were then analyzed along with adjacent normal pancreas tissue obtained from the same tissue sections. A summary of the description of the IPMN cases is provided in Supplemental Table S6 and representative examples of IPMN tissue before and after laser capture microdissection is provided in Supplemental Figure S8. Notably, telomere fusions were predominantly detected in IPMNs of higher histologic grades; 12 of 25 IPMN with HGD (48.0%) and four of 49 IPMN with IGD (8.2%) (Figure 3). No telomere fusions were detected in 19 IPMN with LGD. The prevalence of telomere fusions in the IPMN cases with HGD was significantly higher than in IPMN with IGD and LGD (both P < 0.001). No fusions were detected in DNA from available adjacent normal pancreas tissue microdissected from 46 of these cases (Figure 3). Some cases had multiple fusions detected (only those with HGD). Representative examples of fusions detected in IPMNs are shown in Supplemental Figure S9.
Figure 3.
Telomere fusion and telomere length in normal and intraductal papillary mucinous neoplasm (IPMN) tissues. Scatter plot graph with left y axis indicates telomere length. The longer horizontal bar represents the median value and shorter ones represent values of the 75th and 25th percentiles, respectively. Gray bar graph with right y axis indicates telomere fusion prevalence. There was insufficient DNA available from three low-grade dysplasia (LGD) cases for telomere length analysis. ∗∗∗P < 0.001, solid comparison bars represent the comparison of telomere lengths, and dashed comparison bars represent the comparison of telomere fusion prevalence. HGD, high-grade dysplasia; IGD, intermediate-grade dysplasia; T/S ratio, ratio of telomere repeat copy number to a single copy gene copy number.
The intra and interassay variability of the fusion assay was estimated with the use of representative fusion-positive tissue and cell line samples, which revealed excellent reproducibility (Supplemental Figure S10).
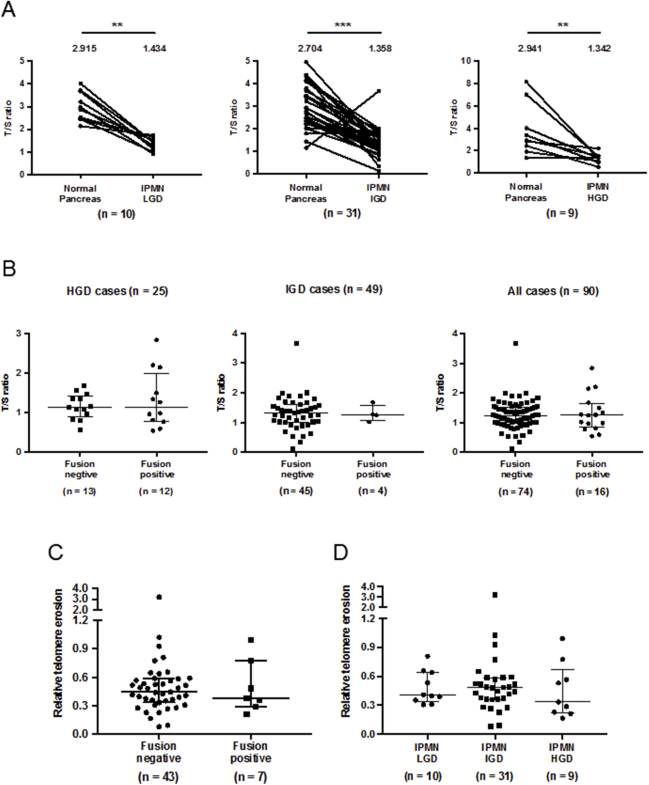
The telomere lengths of DNA from IPMNs and adjacent normal pancreas samples were also measured. As expected, IPMN cases of all histologic grades had significantly shorter mean telomere length than normal pancreas (Figure 3). The telomere length of microdissected normal pancreas tissue DNA (n = 46) and that of normal pancreatic ductal cells (n = 9) were similar (Figure 3).43 The mean telomere length of IPMN samples and samples of adjacent normal pancreas from the same individuals was compared. Mean telomere length was significantly shorter in IPMNs of all grades compared with adjacent normal pancreas (Supplemental Figure S11A), but there was no difference in the mean telomere length of fusion-positive compared with fusion-negative IPMNs (Supplemental Figure S11, B and C) or within the three histologic grades of IPMN (Supplemental Figure S11D). The average telomere length of IPMN DNA was significantly higher than that of pancreatic cancer cell lines (data not shown).
Fusion-positive PCR products from 16 fusion-positive IPMN samples were cloned and sequenced to isolate specific fusion junctions (Supplemental Table S7). Fifteen of the 16 fusions were subtelomere–telomere fusions, that is, telomeric repeats from one chromosomal end were fused to the subtelomere sequence of another chromosome (Figure 2A and Supplemental Table S7). One IPMN (a case with IGD) had an interstitial fusion; that is, shortened telomeric repeats from one chromosomal end fused to a broken 2q arm that had ancestral interstitial telomeric repeats integrated within chromosome 2q13-q14.144 (Supplemental Table S7). Telomere fusions from IPMN cases contained a variety of telomeric repeat lengths (from 48 to 430 bp) and TTAGGG repeats (from 0 to 38 repeats) (Figure 2, B and C).
Telomere Fusion Detection in Surgically Aspirated Cyst Fluid Samples
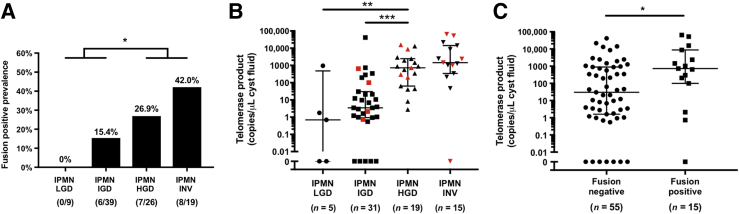
To investigate the utility of telomere fusion detection as a biomarker for predicting the grade of dysplasia of IPMNs, the telomere fusion assay was performed with the use of 93 surgically aspirated cyst fluid samples derived from resected IPMNs. The characteristics of these patients and their IPMNs are summarized in Supplemental Table S8. No fusions were detected in the cyst fluid samples from IPMNs with LGD (0 of nine, 0%) and the prevalence of detected telomere fusions increased with histologic grade: IGD (6 of 39, 15.4%) to HGD (7 of 26, 26.9%) and an associated invasive cancer (8 of 19, 42.9%) (Figure 4A). The difference in the prevalence of telomere fusions in higher grade lesions (IPMNs with HGD and/or an associated invasive cancer) versus lower grades (IPMN with IGD or LGD) was statistically significant (P = 0.025). The use of lower amounts of cyst fluid DNA to detect telomere fusions resulted in a lower rate of fusion detection (Supplemental Table S9). The concentration of KRAS and GNAS mutant allele frequencies in these cyst fluid samples was provided for comparison. Several cases in which fusions were detected in the IPMN but not in the patients corresponding cyst fluid were identified. This suggested that for some cases more cyst fluid DNA might need to be sampled to detect fusions or that the DNA from cells with fusions was not shed into cyst fluid. Table 2 shows that the presence of telomere fusions in cyst fluid samples was independent of other predictive factors of malignancy (HGD/invasive cancer) by multivariate analysis (odds ratio, 6.229; 95% CI, 1.61–28.0). The concordance of telomere fusion detection in 19 paired cases of IPMN tissue and surgically aspirated cyst fluid samples was compared. Fourteen of the 19 cases (73.7%, 2 of positive and 12 of negative) had concordant results between the tissue and cyst fluid samples; only one case was fusion positive in the cyst fluid but negative in the IPMN tissue sample (Supplemental Table S10).
Figure 4.
Telomere fusions detected in intraductal papillary mucinous neoplasm (IPMN) cyst fluid samples. A: The prevalence of telomere fusions in IPMN cyst fluid samples according to their grade of dysplasia. B: Cyst fluid telomerase activity. The longer horizontal represents the median value and shorter ones represent values of the 75th and 25th percentiles, respectively. Red markers indicate fusion-positive samples. C: Telomerase activities in cyst fluid samples with or without telomere fusions. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. HGD, high-grade dysplasia; IGD, intermediate-grade dysplasia; INV, invasive cancer; LGD, low-grade dysplasia.
Table 2.
Univariate and Multivariate Analyses of Factors Predictive of Invasive Cancer or High-Grade Dysplasia in Pancreatic Cysts
| Variables | LGD IGD | HGD INV | Univariate P | Multivariate |
||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | ||||
| Age | ||||||
| <65 years | 16 | 9 | 0.167 | |||
| ≥65 years | 32 | 36 | ||||
| Sex | ||||||
| Female | 27 | 18 | 0.148 | |||
| Male | 21 | 27 | ||||
| Fluid appearance | ||||||
| Serous | 37 | 20 | 0.002∗ | 1.000 | ||
| Mucinous | 11 | 25 | 2.671 | 0.871–8.473 | 0.086 | |
| Cyst size | ||||||
| <30 mm | 29 | 21 | 0.215 | |||
| ≥30 mm | 19 | 24 | ||||
| MPD dilatation | ||||||
| <5 mm | 40 | 13 | <0.001∗ | 1.000 | ||
| ≥5 mm | 8 | 32 | 12.924 | 4.207–46.000 | <0.001∗ | |
| Mural nodule/solid component | ||||||
| Absent | 36 | 27 | 0.183∗ | |||
| Present | 12 | 18 | ||||
| Clinical symptoms | ||||||
| Absent | 39 | 20 | <0.001 | 1.000 | ||
| Present | 9 | 25 | 4.019 | 1.272–13.878 | 0.018∗ | |
| Telomere fusion | ||||||
| Negative | 42 | 30 | 0.025∗ | 1.000 | ||
| Positive | 6 | 15 | 6.229 | 1.605–27.988 | 0.008∗ | |
HGD, high-grade dysplasia; IGD, intermediate-grade dysplasia; INV, invasive cancer; LGD, low-grade dysplasia; MPD, main pancreatic duct; OR, odds ratio.
Statistically significant.
The relationship between telomere fusion status and telomerase activity in IPMN cyst fluids was examined. Telomerase activity data were available from most cyst fluid samples and, as we previously reported, was associated with HGD (Figure 4B).22 Most but not all IPMNs with telomere fusions had high telomerase activity and vice versa. Telomere fusion-positive cases had higher telomerase activity on average than fusion-negative cases (Figure 4C).
Discussion
During IPMN development telomere fusions are only commonly detected in IPMNs with HGD, a stage of tumor development in which chromosomal abnormalities become prevalent.45 The telomere fusion assay was able to identify telomere fusions in 61.5% of pancreatic cancer cell lines and xenografts and in 48% of IPMNs with an associated invasive adenocarcinoma. Consistent with prior studies, telomere fusions in normal tissue were absent.11, 12 Pancreatic cancer cell lines with telomere fusions had even shorter telomeres than those without fusions, consistent with evidence that telomere fusions occur once telomeres become critically short. Cyst fluids that contain telomere fusions were more likely to have elevated levels of telomerase, consistent with our understanding that telomerase is induced in cells with critically shortened telomeres to overcome crisis.
Few studies have described the detection of telomere fusions in solid tumors,12 in part because laborious methods such as Southern blot analysis have been used to identify them. The assay uses qPCR methods that include a telomere repeat probe for assay specificity. Because the assay does not require laborious experimental procedures, it can be readily applied to diagnostic laboratories to detect telomere fusions in clinical samples.
The use of telomere fusions as a biomarker can provide additional information beyond that provided by existing biomarkers. Gene mutations are useful for classifying the type of pancreatic cyst and can be helpful at predicting the grade of dysplasia, particularly when mutations associated with HGD are detected (such as mutations in TP53 or SMAD4), but these are present in <50% of such cases.46, 47, 48 The detection of chromosomal copy number alterations can help predict the neoplastic grade of a pancreatic cyst, but sophisticated assays are required to detect these alterations in secondary fluids. Telomerase activity is a promising biomarker for predicting the grade of dysplasia of an IPMN; although telomerase activity and telomere fusions emerge at a similar stage in IPMN development, these two biomarkers have complementary diagnostic utility.22
Telomere fusions were not identified as clonal events in the pancreatic cancer cell line/xenograft and IPMN samples and were detected in only a small fraction of genome equivalents (<1/1000), consistent with prior observations.10, 12, 35 Although telomere fusions that arise from critically short telomeres can cause chromosomal breakage-fusion-breakage cycles,49 most telomere fusions are not tolerated by the cell,50 and they represent transient events that arise in neoplastic cells before they die. Therefore, it is likely that telomere fusions were not found to be prognostic. By the time a neoplastic cell has evolved into a pancreatic cancer, it has acquired several oncogenic driver mutations that drive prognosis and often has other mechanisms besides critical telomere shortening that contribute to chromosomal instability.
The telomere fusion assay was designed to detect relatively small fusion amplicons which is useful for certain biomarker applications. Because most telomere fusions arise at critically shortened telomeres, most fusions have few telomere repeats. Because the assay also amplifies subtelomeric DNA, most telomere fusion amplicons are >150 bp; therefore, they may not reliably detect fusions in formalin-fixed DNA. The telomere fusion assay cannot detect fusions that arise where the target subtelomeric region has been deleted. The assay cannot detect telomere fusions that involve the many telomere ends not amplified by the telomere fusion primers. It should also be noted that, although the assay uses qPCR, it is not a quantitative assay; the qPCR probe improves the specificity of the assay by targeting telomere repeats. Although most telomere fusions can be detected with the telomere repeat qPCR probe, telomere fusions that lack telomere repeats can also be identified.10, 12, 34 The detection of telomere fusions with the assay was also a function of the amount of input DNA. To increase the efficiency of each assay, the first-round products from four 10-ng PCR reactions were pooled into one second-round PCR. Performing the second-round PCR on the products of each first-round PCR would better quantify the number of telomere fusions, although most samples had only fusion. It may be the case that some neoplasms with rare telomere fusion events may be identified by sampling more tumor DNA.
Data indicate that telomere fusions may serve as a novel biomarker for predicting the presence of HGD lesion within a cyst. Although patients in this study underwent surgical resection and therefore had defined histologic findings, the results of analyses cannot be directly applied to patients who undergo surveillance without surgical resection. Therefore, prospective studies are needed to evaluate the diagnostic utility of using telomere fusion detection for patients undergoing endoscopic ultrasound evaluation and pancreatic cyst fluid sampling.
Conclusions
We observe telomere fusion events in most pancreatic ductal adenocarcinomas and in IPMNs with HGD where they are related to high telomerase activity and critically short telomeres. These telomere fusion events can be readily detected in pancreatic cyst fluid and are helpful for predicting the grade of dysplasia of IPMNs.
Acknowledgments
We thank Drs. Akira Horii (Tohoku University, Sendai, Japan) and Ming-Sound Tsao (University of Toronto, ON, Canada) for PK-8 and PK-9 cells, and HPV-E6/E7 immortalized human pancreatic duct epithelial cell lines, respectively.
Footnotes
Supported by NIH grants U01CA210170, CA62924, and R01CA176828 (M.G.), Susan Wojcicki and Dennis Troper, and the Rolfe Pancreatic Cancer Foundation (M.G.).
Disclosures: M.G. and T.H. have submitted a patent describing telomere fusions and their detection in pancreatic cyst fluids.
See related Commentary on page 31
Supplemental material for this article can be found at https://doi.org/10.1016/j.jmoldx.2017.09.006.
Supplemental Data
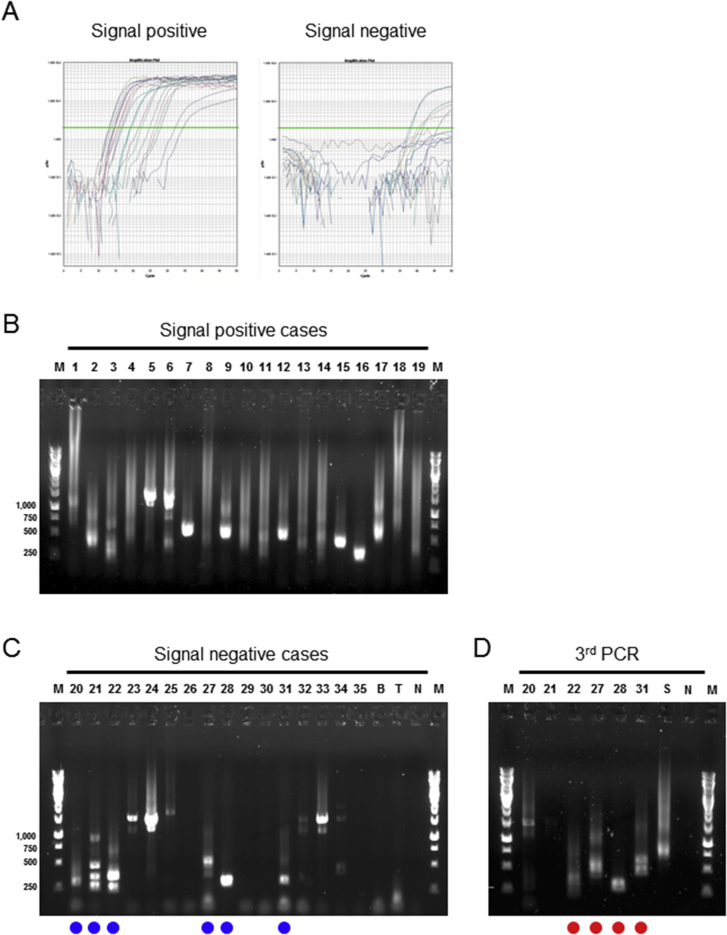
Supplemental Figure S1.
Examples of telomere fusion assay results. A: Amplification curves after nested real-time quantitative PCR (qPCR) classified by signal positive and negative. Green lines indicate the Ct value. B: Gel imaging after the electrophoresis of nested-qPCR products. Samples marked with blue circles are applied to third PCR. C: Gel imaging after the electrophoresis of third PCR. Sample in lane 30 (PL4 cell line) marked with red circles highlights the amplicon of similar size to that obtained after the nested-qPCR. D and E: DNA sequence in one of the subcloned AsPC-1 (D) and PL4 (E) fusion junctions. Rectangles indicate primer sequences, and telomeric repeats are underlined. M, DNA marker; N, nontemplate control.
The DNA sequence of a telomere fusion from PANC 486 (clone 4) cells. Rectangles indicate primer sequences, and telomeric repeats are underlined.
Supplemental Figure S3.
Representative results of telomere fusion assays in pancreatic cancer xenograft samples. A: Amplification curves after nested real-time quantitative PCR (qPCR) classified by signal positive and negative. Green lines indicate the Ct value. B: Gel imaging after the electrophoresis of signal-positive nested-qPCR products. C: Gel imaging after the electrophoresis of signal-negative nested-qPCR products. Samples marked with blue circles are applied to third PCR. D: Gel imaging after the electrophoresis of third PCR. Samples marked with red circles highlight the amplicons of similar size to that obtained after the second-round nested-qPCR; these amplicons were subcloned and sequenced for validation. B, mouse whole blood DNA; M, DNA marker; N, nontemplate control; S, SU.86.86 cells; T, mouse tail DNA.
Supplemental Figure S4.

Kaplan-Meier curve of overall survival characterized by telomere fusion status in pancreatic cancer xenograft samples. Patients lost to follow-up were censored at their last visit.
Supplemental Figure S5.
Nested real-time quantitative PCR (qPCR) performance using various telomere fusion junction sequences as templates. A: Standard curve of serially diluted DNA for all tested clones. B and C: Amplification curve of serially diluted DNA (B) and fusion junction sequence (C) of PANC 215 clone 8. D and E: Amplification curve of serially diluted DNA (D) and fusion junction sequence (E) of PANC 215 clone 1. Rectangles indicate primer sequence, and telomeric repeats are underlined. The green lines indicate the Ct value (B and D).
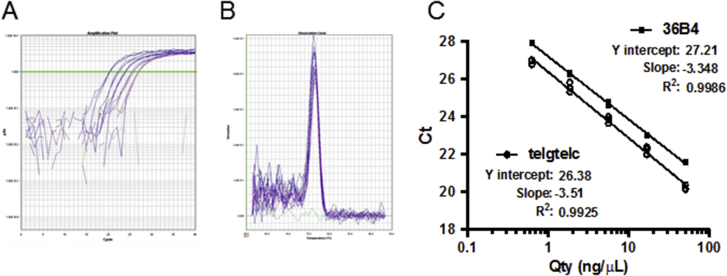
Supplemental Figure S6.
Methodologic validation of telomere length measurement assay with the use of real-time quantitative PCR (qPCR) technique. A: Amplification curves using telomere telg/telc primers and serially diluted human genomic DNA. Green line indicates the Ct value. B: Dissociation curves of single product amplifications using telg/telc primers. C: Standard curves for telomeric repeat length and single copy reference (36B4).
Supplemental Figure S7.

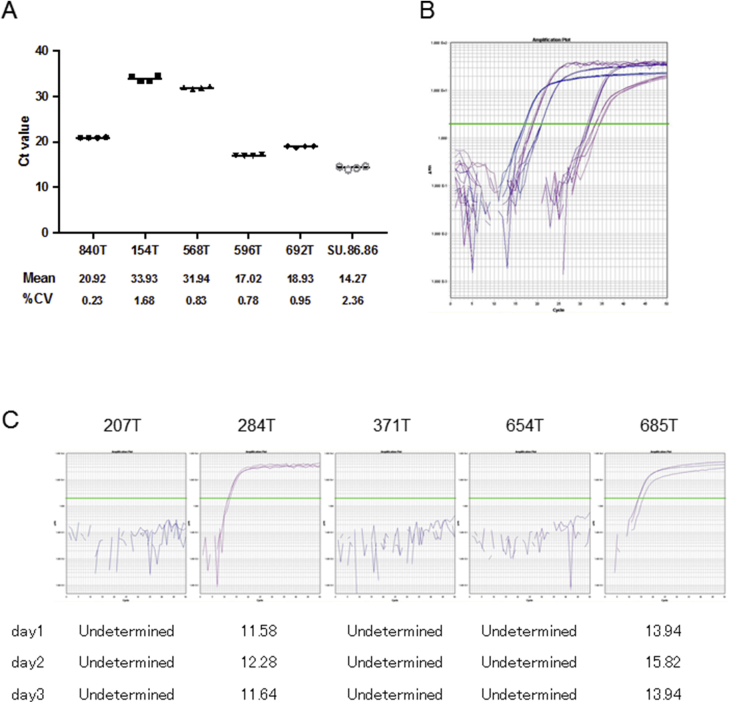
Telomere lengths measured in 31 pancreatic cancer cell lines and HPDE and HPNE cells classified by their telomere fusion status. The longer horizontal bar represents the median value and shorter ones represent values of the 75th and 25th percentiles, respectively.
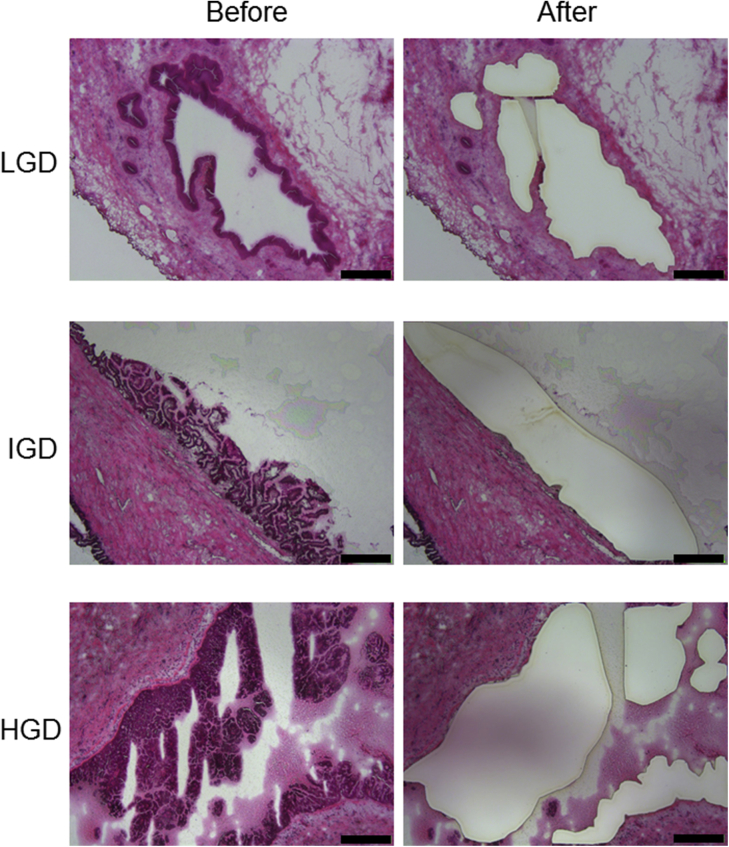
Supplemental Figure S8.
Representative hematoxylin and eosin images of intraductal papillary mucinous neoplasm frozen sections before and after laser capture microdissection. Scale bars: 400 μm. HGD, high-grade dysplasia; IGD, intermediate-grade dysplasia; LGD, low-grade dysplasia.
Supplemental Figure S9.
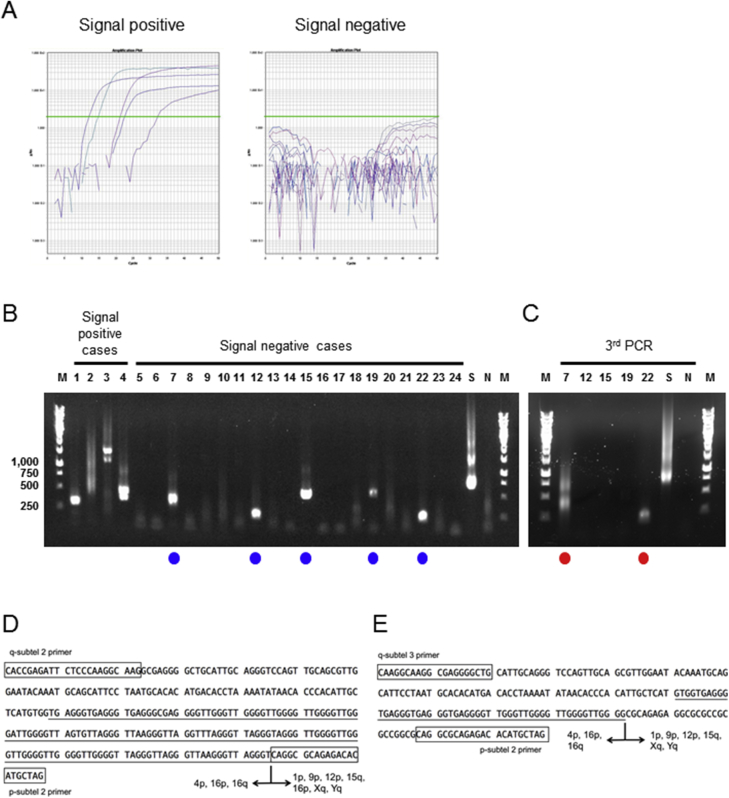
Representative examples of telomere fusion assay results. A: Nested real-time quantitative PCR (qPCR) amplification from fusion-positive and fusion-negative cases. Green lines indicate the Ct value. B: Gel electrophoresis images of nested-qPCR products. Samples marked with blue circles represent PCR-positive but qPCR negative telomere fusion amplicons. C–E: Gel electrophoresis images of third-round PCR to further evaluate telomere fusion amplicons. Examples of telomere fusion sequences from intraductal papillary mucinous neoplasms, including qPCR-positive (lane 3, D) and qPCR-negative (lane 22, E) fusions. Rectangles indicate primer sequences and underlined sequences are telomeric repeats. Lanes marked with red circles highlight the amplicons generated with the third-round PCR that are of similar size to that obtained after the second-round nested-qPCR. M, DNA marker; N, nontemplate control; S, SU.86.86 cell line as positive control.
Supplemental Figure S10.
Intra and interassay variation of telomere fusion assay. A: Ct values of four technical replicates measured by nested real-time quantitative PCR (qPCR) in the same assay, using five representative intraductal papillary mucinous neoplasm (IPMN) DNA samples and the SU.86.86 cell line. B: Amplification curves of five DNA samples corresponding to A. C: Interassay variation using five representative fusion-positive or fusion-negative DNA samples. Green lines indicate the Ct value (B and C). Green bars indicate mean values. CV; coefficient of variation.
Supplemental Figure S11.
Telomere lengths of intraductal papillary mucinous neoplasm (IPMN) stratified by histologic grade and telomere fusion status. A: Telomere length measurement and comparison by using paired of normal pancreas and IPMN. Median ratio of telomere repeat copy number to a single copy gene copy number is shown above the aligned dots. B: Mean Telomere lengths of fusion-positive and -negative IPMNs. The longer horizontal bar represents the median value and shorter ones represent values of the 75th and 25th percentiles, respectively. C: Relative telomere erosion between fusion-positive and -negative IPMN tissues. D: Relative telomere erosion across the three histologic grades of IPMN. ∗∗P < 0.01, ∗∗∗P < 0.001. HGD, high-grade dysplasia; IGD, intermediate-grade dysplasia; LGD, low-grade dysplasia.
References
- 1.de Lange T. How telomeres solve the end-protection problem. Science. 2009;326:948–952. doi: 10.1126/science.1170633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Sullivan R.J., Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol. 2010;11:171–181. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maser R.S., DePinho R.A. Connecting chromosomes, crisis, and cancer. Science. 2002;297:565–569. doi: 10.1126/science.297.5581.565. [DOI] [PubMed] [Google Scholar]
- 4.Feldser D.M., Hackett J.A., Greider C.W. Telomere dysfunction and the initiation of genome instability. Nat Rev Cancer. 2003;3:623–627. doi: 10.1038/nrc1142. [DOI] [PubMed] [Google Scholar]
- 5.Meeker A.K., Hicks J.L., Gabrielson E., Strauss W.M., De Marzo A.M., Argani P. Telomere shortening occurs in subsets of normal breast epithelium as well as in situ and invasive carcinoma. Am J Pathol. 2004;164:925–935. doi: 10.1016/S0002-9440(10)63180-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meeker A.K., Hicks J.L., Iacobuzio-Donahue C.A., Montgomery E.A., Westra W.H., Chan T.Y., Ronnett B.M., De Marzo A.M. Telomere length abnormalities occur early in the initiation of epithelial carcinogenesis. Clin Cancer Res. 2004;10:3317–3326. doi: 10.1158/1078-0432.CCR-0984-03. [DOI] [PubMed] [Google Scholar]
- 7.van Heek N.T., Meeker A.K., Kern S.E., Yeo C.J., Lillemoe K.D., Cameron J.L., Offerhaus G.J., Hicks J.L., Wilentz R.E., Goggins M.G., De Marzo A.M., Hruban R.H., Maitra A. Telomere shortening is nearly universal in pancreatic intraepithelial neoplasia. Am J Pathol. 2002;161:1541–1547. doi: 10.1016/S0002-9440(10)64432-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto Y., Murakami Y., Uemura K., Hayashidani Y., Sudo T., Ohge H., Fukuda E., Shimamoto F., Sueda T., Hiyama E. Telomere shortening and telomerase expression during multistage carcinogenesis of intraductal papillary mucinous neoplasms of the pancreas. J Gastrointest Surg. 2008;12:17–28. doi: 10.1007/s11605-007-0383-9. discussion 28–29. [DOI] [PubMed] [Google Scholar]
- 9.Heaphy C.M., Subhawong A.P., Hong S.M., Goggins M.G., Montgomery E.A., Gabrielson E., Netto G.J., Epstein J.I., Lotan T.L., Westra W.H., Shih Ie M., Iacobuzio-Donahue C.A., Maitra A., Li Q.K., Eberhart C.G., Taube J.M., Rakheja D., Kurman R.J., Wu T.C., Roden R.B., Argani P., De Marzo A.M., Terracciano L., Torbenson M., Meeker A.K. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am J Pathol. 2011;179:1608–1615. doi: 10.1016/j.ajpath.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin T.T., Letsolo B.T., Jones R.E., Rowson J., Pratt G., Hewamana S., Fegan C., Pepper C., Baird D.M. Telomere dysfunction and fusion during the progression of chronic lymphocytic leukemia: evidence for a telomere crisis. Blood. 2010;116:1899–1907. doi: 10.1182/blood-2010-02-272104. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka H., Abe S., Huda N., Tu L., Beam M.J., Grimes B., Gilley D. Telomere fusions in early human breast carcinoma. Proc Natl Acad Sci U S A. 2012;109:14098–14103. doi: 10.1073/pnas.1120062109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roger L., Jones R.E., Heppel N.H., Williams G.T., Sampson J.R., Baird D.M. Extensive telomere erosion in the initiation of colorectal adenomas and its association with chromosomal instability. J Natl Cancer Inst. 2013;105:1202–1211. doi: 10.1093/jnci/djt191. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt C.M., White P.B., Waters J.A., Yiannoutsos C.T., Cummings O.W., Baker M., Howard T.J., Zyromski N.J., Nakeeb A., DeWitt J.M., Akisik F.M., Sherman S., Pitt H.A., Lillemoe K.D. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg. 2007;246:644–651. doi: 10.1097/SLA.0b013e318155a9e5. discussion 651–654. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka M., Fernandez-del Castillo C., Adsay V., Chari S., Falconi M., Jang J.Y., Kimura W., Levy P., Pitman M.B., Schmidt C.M., Shimizu M., Wolfgang C.L., Yamaguchi K., Yamao K., International Association of Pancreatology International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Canto M.I., Harinck F., Hruban R.H., Offerhaus G.J., Poley J.W., Kamel I., Nio Y., Schulick R.S., Bassi C., Kluijt I., Levy M.J., Chak A., Fockens P., Goggins M., Bruno M., International Cancer of Pancreas Screening (CAPS) Consortium International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013;62:339–347. doi: 10.1136/gutjnl-2012-303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasen H., Ibrahim I., Ponce C.G., Slater E.P., Matthai E., Carrato A., Earl J., Robbers K., van Mil A.M., Potjer T., Bonsing B.A., de Vos Tot Nederveen Cappel W.H., Bergman W., Wasser M., Morreau H., Kloppel G., Schicker C., Steinkamp M., Figiel J., Esposito I., Mocci E., Vazquez-Sequeiros E., Sanjuanbenito A., Munoz-Beltran M., Montans J., Langer P., Fendrich V., Bartsch D.K. Benefit of surveillance for pancreatic cancer in high-risk individuals: outcome of long-term prospective follow-up studies from three European expert centers. J Clin Oncol. 2016;34:2010–2019. doi: 10.1200/JCO.2015.64.0730. [DOI] [PubMed] [Google Scholar]
- 17.Crippa S., Bassi C., Salvia R., Malleo G., Marchegiani G., Rebours V., Levy P., Partelli S., Suleiman S.L., Banks P.A., Ahmed N., Chari S.T., Fernandez-Del Castillo C., Falconi M. Low progression of intraductal papillary mucinous neoplasms with worrisome features and high-risk stigmata undergoing non-operative management: a mid-term follow-up analysis. Gut. 2017;66:495–506. doi: 10.1136/gutjnl-2015-310162. [DOI] [PubMed] [Google Scholar]
- 18.Sahora K., Mino-Kenudson M., Brugge W., Thayer S.P., Ferrone C.R., Sahani D., Pitman M.B., Warshaw A.L., Lillemoe K.D., Fernandez-del Castillo C.F. Branch duct intraductal papillary mucinous neoplasms: does cyst size change the tip of the scale? A critical analysis of the revised international consensus guidelines in a large single-institutional series. Ann Surg. 2013;258:466–475. doi: 10.1097/SLA.0b013e3182a18f48. [DOI] [PubMed] [Google Scholar]
- 19.Ridtitid W., DeWitt J.M., Schmidt C.M., Roch A., Stuart J.S., Sherman S., Al-Haddad M.A. Management of branch-duct intraductal papillary mucinous neoplasms: a large single-center study to assess predictors of malignancy and long-term outcomes. Gastrointest Endosc. 2016;84:436–445. doi: 10.1016/j.gie.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Yamada S., Fujii T., Murotani K., Kanda M., Sugimoto H., Nakayama G., Koike M., Fujiwara M., Nakao A., Kodera Y. Comparison of the international consensus guidelines for predicting malignancy in intraductal papillary mucinous neoplasms. Surgery. 2016;159:878–884. doi: 10.1016/j.surg.2015.08.042. [DOI] [PubMed] [Google Scholar]
- 21.Mukewar S., de Pretis N., Aryal-Khanal A., Ahmed N., Sah R., Enders F., Larson J.J., Levy M.J., Takahashi N., Topazian M., Pearson R., Vege S.S., Chari S.T. Fukuoka criteria accurately predict risk for adverse outcomes during follow-up of pancreatic cysts presumed to be intraductal papillary mucinous neoplasms. Gut. 2017;66:1811–1817. doi: 10.1136/gutjnl-2016-311615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hata T., Dal Molin M., Suenaga M., Yu J., Pittman M., Weiss M., Canto M.I., Wolfgang C., Lennon A.M., Hruban R.H., Goggins M. Cyst fluid telomerase activity predicts the histologic grade of cystic neoplasms of the pancreas. Clin Cancer Res. 2016;22:5141–5151. doi: 10.1158/1078-0432.CCR-16-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maker A.V., Katabi N., Qin L.X., Klimstra D.S., Schattner M., Brennan M.F., Jarnagin W.R., Allen P.J. Cyst fluid interleukin-1beta (IL1beta) levels predict the risk of carcinoma in intraductal papillary mucinous neoplasms of the pancreas. Clin Cancer Res. 2011;17:1502–1508. doi: 10.1158/1078-0432.CCR-10-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthaei H., Wylie D., Lloyd M.B., Dal Molin M., Kemppainen J., Mayo S.C., Wolfgang C.L., Schulick R.D., Langfield L., Andruss B.F., Adai A.T., Hruban R.H., Szafranska-Schwarzbach A.E., Maitra A. miRNA biomarkers in cyst fluid augment the diagnosis and management of pancreatic cysts. Clin Cancer Res. 2012;18:4713–4724. doi: 10.1158/1078-0432.CCR-12-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt C.M., Yip-Schneider M.T., Ralstin M.C., Wentz S., DeWitt J., Sherman S., Howard T.J., McHenry L., Dutkevitch S., Goggins M., Nakeeb A., Lillemoe K.D. PGE(2) in pancreatic cyst fluid helps differentiate IPMN from MCN and predict IPMN dysplasia. J Gastrointest Surg. 2008;12:243–249. doi: 10.1007/s11605-007-0404-8. [DOI] [PubMed] [Google Scholar]
- 26.Brugge W.R., Lewandrowski K., Lee-Lewandrowski E., Centeno B.A., Szydlo T., Regan S., del Castillo C.F., Warshaw A.L. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Springer S., Wang Y., Molin M.D., Masica D.L., Jiao Y., Kinde I. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology. 2015;149:1501–1510. doi: 10.1053/j.gastro.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masica D.L., Dal Molin M., Wolfgang C.L., Tomita T., Ostovaneh M.R., Blackford A., Moran R.A., Law J.K., Barkley T., Goggins M., Irene Canto M., Pittman M., Eshleman J.R., Ali S.Z., Fishman E.K., Kamel I.R., Raman S.P., Zaheer A., Ahuja N., Makary M.A., Weiss M.J., Hirose K., Cameron J.L., Rezaee N., He J., Joon Ahn Y., Wu W., Wang Y., Springer S., Diaz L.L., Jr., Papadopoulos N., Hruban R.H., Kinzler K.W., Vogelstein B., Karchin R., Lennon A.M. A novel approach for selecting combination clinical markers of pathology applied to a large retrospective cohort of surgically resected pancreatic cysts. J Am Med Inform Assoc. 2017;24:145–152. doi: 10.1093/jamia/ocw069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones S., Zhang X., Parsons D.W., Lin J.C., Leary R.J., Angenendt P. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffin C.A., Morsberger L., Hawkins A.L., Haddadin M., Patel A., Ried T., Schrock E., Perlman E.J., Jaffee E. Molecular cytogenetic characterization of pancreas cancer cell lines reveals high complexity chromosomal alterations. Cytogenet Genome Res. 2007;118:148–156. doi: 10.1159/000108295. [DOI] [PubMed] [Google Scholar]
- 31.Cui Y., Brosnan J.A., Blackford A.L., Sur S., Hruban R.H., Kinzler K.W., Vogelstein B., Maitra A., Diaz L.A., Jr., Iacobuzio-Donahue C.A., Eshleman J.R. Genetically defined subsets of human pancreatic cancer show unique in vitro chemosensitivity. Clin Cancer Res. 2012;18:6519–6530. doi: 10.1158/1078-0432.CCR-12-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee K.M., Yasuda H., Hollingsworth M.A., Ouellette M.M. Notch 2-positive progenitors with the intrinsic ability to give rise to pancreatic ductal cells. Lab Invest. 2005;85:1003–1012. doi: 10.1038/labinvest.3700298. [DOI] [PubMed] [Google Scholar]
- 33.Kanda M., Matthaei H., Wu J., Hong S.M., Yu J., Borges M., Hruban R.H., Maitra A., Kinzler K., Vogelstein B., Goggins M. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology. 2012;142:730–733.e9. doi: 10.1053/j.gastro.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capper R., Britt-Compton B., Tankimanova M., Rowson J., Letsolo B., Man S., Haughton M., Baird D.M. The nature of telomere fusion and a definition of the critical telomere length in human cells. Genes Dev. 2007;21:2495–2508. doi: 10.1101/gad.439107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Letsolo B.T., Rowson J., Baird D.M. Fusion of short telomeres in human cells is characterized by extensive deletion and microhomology, and can result in complex rearrangements. Nucleic Acids Res. 2010;38:1841–1852. doi: 10.1093/nar/gkp1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown W.R., MacKinnon P.J., Villasante A., Spurr N., Buckle V.J., Dobson M.J. Structure and polymorphism of human telomere-associated DNA. Cell. 1990;63:119–132. doi: 10.1016/0092-8674(90)90293-n. [DOI] [PubMed] [Google Scholar]
- 37.Cawthon R.M. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37:e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka H., Beam M.J., Caruana K. The presence of telomere fusion in sporadic colon cancer independently of disease stage, TP53/KRAS mutation status, mean telomere length, and telomerase activity. Neoplasia. 2014;16:814–823. doi: 10.1016/j.neo.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li A., Omura N., Hong S.M., Goggins M. Pancreatic cancer DNMT1 expression and sensitivity to DNMT1 inhibitors. Cancer Biol Ther. 2010;9:321–329. doi: 10.4161/cbt.9.4.10750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amin M.B., Edge S., Greene F., Byrd D.R., Brookland R.K., Washington M.K., Gershenwald J.E., Compton C.C., Hess K.R., Sullivan D.C., Jessup J.M., Brierley J.D., Gaspar L.E., Schilsky R.L., Balch C.M., Winchester D.P., Asare E.A., Madera M., Gress D.M., Meyer L.R., editors. AJCC Cancer Staging Manual. ed 8. Springer International Publishing; Chicago, IL: 2017. [Google Scholar]
- 41.Stohr B.A., Xu L., Blackburn E.H. The terminal telomeric DNA sequence determines the mechanism of dysfunctional telomere fusion. Mol Cell. 2010;39:307–314. doi: 10.1016/j.molcel.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee M., Hills M., Conomos D., Stutz M.D., Dagg R.A., Lau L.M., Reddel R.R., Pickett H.A. Telomere extension by telomerase and ALT generates variant repeats by mechanistically distinct processes. Nucleic Acids Res. 2014;42:1733–1746. doi: 10.1093/nar/gkt1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong S.M., Heaphy C.M., Shi C., Eo S.H., Cho H., Meeker A.K., Eshleman J.R., Hruban R.H., Goggins M. Telomeres are shortened in acinar-to-ductal metaplasia lesions associated with pancreatic intraepithelial neoplasia but not in isolated acinar-to-ductal metaplasias. Mod Pathol. 2011;24:256–266. doi: 10.1038/modpathol.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.IJdo J.W., Baldini A., Ward D.C., Reeders S.T., Wells R.A. Origin of human chromosome 2: an ancestral telomere-telomere fusion. Proc Natl Acad Sci U S A. 1991;88:9051–9055. doi: 10.1073/pnas.88.20.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maciejowski J., de Lange T. Telomeres in cancer: tumour suppression and genome instability. Nat Rev Mol Cell Biol. 2017;18:175–186. doi: 10.1038/nrm.2016.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hruban R.H., Takaori K., Klimstra D.S., Adsay N.V., Albores-Saavedra J., Biankin A.V., Biankin S.A., Compton C., Fukushima N., Furukawa T., Goggins M., Kato Y., Kloppel G., Longnecker D.S., Luttges J., Maitra A., Offerhaus G.J., Shimizu M., Yonezawa S. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–987. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 47.Amato E., Molin M.D., Mafficini A., Yu J., Malleo G., Rusev B., Fassan M., Antonello D., Sadakari Y., Castelli P., Zamboni G., Maitra A., Salvia R., Hruban R.H., Bassi C., Capelli P., Lawlor R.T., Goggins M., Scarpa A. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol. 2014;233:217–227. doi: 10.1002/path.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan M.C., Basturk O., Brannon A.R., Bhanot U., Scott S.N., Bouvier N., LaFemina J., Jarnagin W.R., Berger M.F., Klimstra D., Allen P.J. GNAS and KRAS mutations define separate progression pathways in intraductal papillary mucinous neoplasm-associated carcinoma. J Am Coll Surg. 2015;220:845–854.e1. doi: 10.1016/j.jamcollsurg.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gisselsson D., Jonson T., Petersen A., Strombeck B., Dal Cin P., Hoglund M., Mitelman F., Mertens F., Mandahl N. Telomere dysfunction triggers extensive DNA fragmentation and evolution of complex chromosome abnormalities in human malignant tumors. Proc Natl Acad Sci U S A. 2001;98:12683–12688. doi: 10.1073/pnas.211357798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhivotovsky B., Kroemer G. Apoptosis and genomic instability. Nat Rev Mol Cell Biol. 2004;5:752–762. doi: 10.1038/nrm1443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The DNA sequence of a telomere fusion from PANC 486 (clone 4) cells. Rectangles indicate primer sequences, and telomeric repeats are underlined.