Abstract
Periodontal disease is the most common osteolytic disease in humans and is significantly increased by diabetes mellitus. We tested the hypothesis that bacterial infection induces bone loss in diabetic animals through a mechanism that involves enhanced apoptosis. Type II diabetic rats were inoculated with Aggregatibacter actinomycetemcomitans and treated with a caspase-3 inhibitor, ZDEVD-FMK, or vehicle alone. Apoptotic cells were measured with TUNEL; osteoblasts and bone area were measured in H&E sections. New bone formation was assessed by labeling with fluorescent dyes and by osteocalcin mRNA levels. Osteoclast number, eroded bone surface, and new bone formation were measured by tartrate-resistant acid phosphatase staining. Immunohistochemistry was performed with an antibody against tumor necrosis factor-α. Bacterial infection doubled the number of tumor necrosis factor-α–expressing cells and increased apoptotic cells adjacent to bone 10-fold (P < 0.05). Treatment with caspase inhibitor blocked apoptosis, increased the number of osteoclasts, and eroded bone surface (P < 0.05); yet, inhibition of apoptosis resulted in significantly greater net bone area because of an increase in new bone formation, osteoblast numbers, and an increase in bone coupling. Thus, bacterial infection in diabetic rats stimulates periodontitis, in part through enhanced apoptosis of osteoblastic cells that reduces osseous coupling through a caspase-3–dependent mechanism.
Diabetes is a chronic inflammatory disease characterized by hyperglycemia that affects 26 million Americans.1 Diabetes has several complications, such as cardiovascular, renal, microvascular, and periodontal diseases. Periodontal disease is one of the most common forms of osteolytic bone disease and one of the most frequent complications of the diabetes.2 Recent research suggests that the relationship between periodontitis and diabetes is reciprocal.3, 4 People with diabetes are more susceptible to periodontitis, and periodontitis may affect serum glucose levels and contribute to progression of diabetes.5 Diabetes may contribute to periodontitis because of its effect on inflammation.6, 7 Despite being triggered by bacterial infection, periodontal bone loss is tied to the inflammatory host response, which leads to the generation of prostaglandins and cytokines that stimulate osteoclastogenesis and periodontal bone loss.8
Several of the detrimental aspects of periodontal disease have recently been shown to be mediated by elevated levels of tumor necrosis factor-α (TNF-α).9, 10 TNF-α is a proinflammatory cytokine produced by leukocytes and other cell types.11 Enhanced TNF-α levels have been directly linked to cellular changes in diabetic retinopathy, deficits in wound healing, and diabetes-enhanced periodontitis.12, 13, 14 Some of the detrimental effects of diabetes-enhanced TNF-α levels may be because of the induction of cell death by triggering caspase activity. Caspases are a family of cysteine proteases that can act as either initiators (caspases 2, 8, and 9) or executioners (caspases 3, 6, and 7) of apoptosis.15 Caspase-3 appears to play a central role in bacteria and lipopolysaccharide-mediated apoptosis.16, 17 In addition, it has been shown that TNF-α can stimulate the expression of several pro-apoptotic genes, many of which are regulated by the pro-apoptotic transcription factor, forkhead box-O1 (FOXO1).18
The functional role of apoptosis in pathological processes can be studied with caspase inhibitors, which are small peptides that block the activity of well-defined caspases.19 These inhibitors have been used in animal models to attenuate cell death and diminish tissue damage in ischemic conditions, sepsis, and other pathological processes.20, 21 Other studies using caspase inhibitors have shown that part of the detrimental effect of diabetes on healing after infection is the result of increased fibroblast or osteoblast apoptosis.16, 22 To understand how diabetes may affect periodontal bone loss through apoptosis, we used a caspase-3/7 inhibitor in a type 2 Goto-Kakizaki (GK) diabetic rat model of periodontal disease induced by bacterial infection. The aim of this study was to determine how apoptosis of osteoblasts contributed to periodontal bone loss by its effect on bone formation in diabetic animals.
Materials and Methods
Animals
GK male and female rats were purchased from Charles River Laboratories (Wilmington, MA). The GK rat naturally develops type 2 diabetes mellitus at the age of approximately 12 weeks. Rats were housed in separate cages and fed powdered food (Laboratory Rodent Meal Diet 5001; Purina Mills Feeds, St. Louis, MO). When glucose levels were >220 mg/dL, and glycated hemoglobin levels were >7.0%, they were classified as diabetic. All animal procedures were approved by the Institutional Animal Care and Use Committee.
Diabetic (GK) rats received antibiotics ad libitum in their drinking water for 4 days (20 mg kanamycin and 20 mg ampicillin) and were swabbed with a 0.12% chlorhexidine gluconate rinse during the past 2 days (Procter and Gamble, Cincinnati, OH) to facilitate colonization by Aggregatibacter actinomycetemcomitans. After 3 days without antibiotic treatment and fasting for 3 hours, 109 A. actinomycetemcomitans cells [adherent A. a strain; A. actinomycetemcomitans clinical isolate 1000 (CU1000NRif); Columbia University, New York, NY] were administered by oral gavage to diabetic (GK) rats, and 109 A. actinomycetemcomitans cells was also placed in their food once a day for 8 days, as previously described.23 Some groups received antibiotic or antibiotic plus caspase-3 inhibitor starting 4 weeks after the feeding regimen. Antibiotic treatment, as previously described, continued for 4 days to arrest A. actinomycetemcomitans infection.23 These animals were euthanized 1 and 2 weeks after the start of the antibacterial regimen (5 and 6 weeks after A. actinomycetemcomitans inoculation, respectively). Caspase-3 inhibitor, ZDEVD-FMK (SM Biochemicals, Anaheim, CA), was administered daily by 1.5 mg/kg i.p. injection starting at 4 weeks and rats were euthanized 1 or 2 weeks later. Animals not treated with caspase inhibitor were injected i.p. with the same volume of vehicle, 2% dimethyl sulfoxide (MP Biomedicals, Solon, OH).
Analysis of TNF-α and Apoptosis in Histological Sections
Immunohistochemistry was performed with an antibody against TNF-α (IHCWORLD, Woodstock, MD). The number of immunopositive cells adjacent to bone was evaluated, as we have previously described,13 at ×600 magnification. Apoptotic cells were detected by TUNEL assay (DeadEnd Fluorometric TUNEL System kit; Promega, Madison, WI). Apoptosis was measured simultaneously with immunofluorescence with an anti-CD18 antibody (Novus Biological, Littleton, CO) to avoid counting apoptotic leukocytes. The number of nonleukocytic apoptotic cells (TUNEL+CD18−) was counted at ×200 magnification with an immunofluorescence microscope using NIS Elements software version 4.2 (Nikon, Melville, NY) in a region adjacent to bone and in bone-lining cells. Experiments were performed with six to seven animals per group.
Osteoblasts, Osteoclasts, Eroded Bone, New Bone Formation, and Bone Coupling
Osteoblasts were counted as cuboidal bone-lining cells in areas of bone remodeling in H&E-stained sections. Osteoclasts, eroded bone, and new bone formation were measured with tartrate-resistant acid phosphatase (TRAP)–stained sections, as previously described.13 Bone mineral apposition was also measured by using calcein blue (Sigma-Aldrich, St. Louis, MO) and alizarin red (Sigma-Aldrich) fluorescent staining. Calcein blue was given by i.p. injection at 4 weeks, and alizarin red was given 24 hours before euthanasia. Metabolic labeling of new bone formation was measured by fluorescence microscopy in methacrylate-embedded sections. The area between the two fluorescent labels was analyzed.24 Bone coupling was calculated as the newly formed bone area/the number of osteoclasts or the percentage of eroded bone. Bone area was measured as the percentage area occupied by bone. Experiments were performed with six to seven animals per group.
Real-Time PCR
Total RNA was extracted from the periodontium of the second and third molars, as previously described.13 mRNA levels of osteocalcin were assessed by real-time PCR using TaqMan primers and probe sets for osteocalcin (Applied Biosystems, Foster City, CA). Results were normalized to a housekeeping gene, ribosomal protein L32. The experiments were performed with six to seven animals per group, with triplicate samples, and performed twice, with similar results.
Statistical Analysis
Statistical analyses were performed using SPSS software version 20 (SPSS, Chicago, IL). One-way analysis of variance with contrast analysis was used to compare the differences between groups at a given time point and to compare differences from baseline values with the other time points. The significance level was set at P < 0.05.
Results
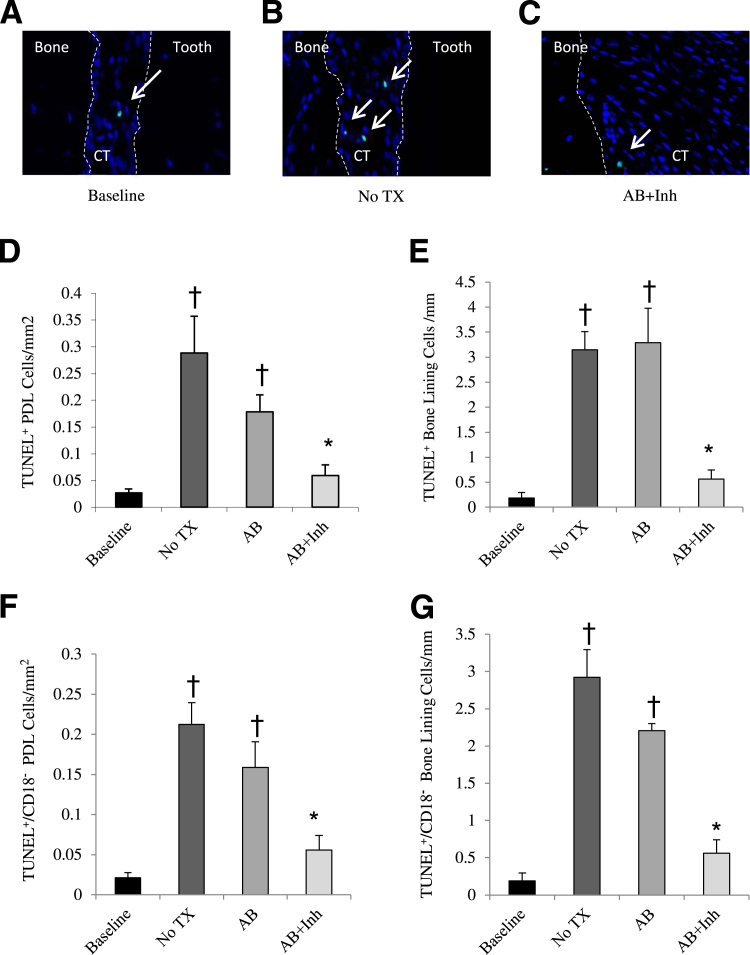
Apoptosis was measured as the total number of apoptotic cells (TUNEL+) and the number of nonleukocytic apoptotic cells (TUNEL+/CD18−) per mm2 in the periodontal ligament adjacent to bone and lining the bone surface. At baseline, the number of apoptotic cells was low. Five weeks after A. actinomycetemcomitans infection, the level of apoptosis increased by approximately 90% in cells adjacent to bone and lining the bone surface (P < 0.05). The increase in apoptosis with infection can be accounted for by a significant increase in expression of pro-apoptotic factors, such as TNF-α, in close proximity to bone. At baseline, the percentage TNF-α–positive cells was 11% and more than doubled to 25% at 5 weeks (P < 0.05). Treatment with antibiotic and caspase-3 inhibitor (AB + Inh) reduced apoptosis to near-baseline levels (Figure 1, A–E). The same pattern was shown with the number of TUNEL+/CD18− cells (Figure 1, F and G). In comparison, antibiotic treatment alone (AB) had only a small effect on these values.
Figure 1.
Diabetes increases apoptosis in bacteria-infected rats. Destructive periodontitis was initiated in GK diabetic rats by oral inoculation with the periodontal pathogen, A. actinomycetemcomitans, once daily for 4 consecutive days per week for 2 weeks. Four weeks after bacterial inoculation was completed, rats were treated with antibiotic to halt the infection. Caspase-3 was inhibited by injection with Z-DEVD-FMK daily 1 week before euthanasia. Control rats received vehicle alone. Rats were euthanized before the inoculation of the bacteria (baseline) and 5 or 6 weeks after inoculation was completed. A–C: TUNEL+ cells close to bone are indicated by arrows. The dotted lines demark the different tissue in the histologic section, bone, connective tissue (CT) and tooth. D and E: The number of TUNEL+ cells in the periodontal ligament close to bone and bone-lining cells was examined. F and G: The number of TUNEL+/CD18− cells in the periodontal ligament close to bone and bone-lining cells was examined. PDL, periodontal ligament; TX, treatment. ∗P < 0.05, a statistically significant difference between the AB and AB + Inh; †P < 0.05, a significant difference compared with baseline.
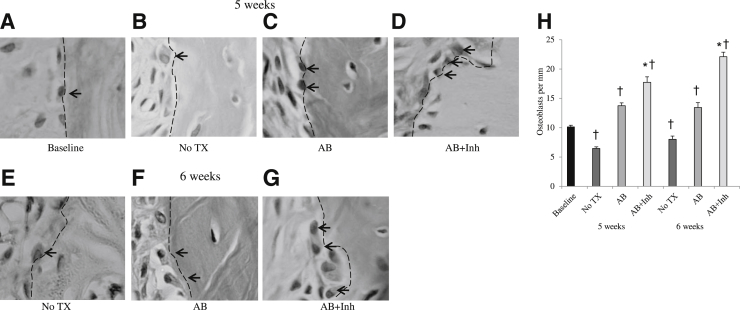
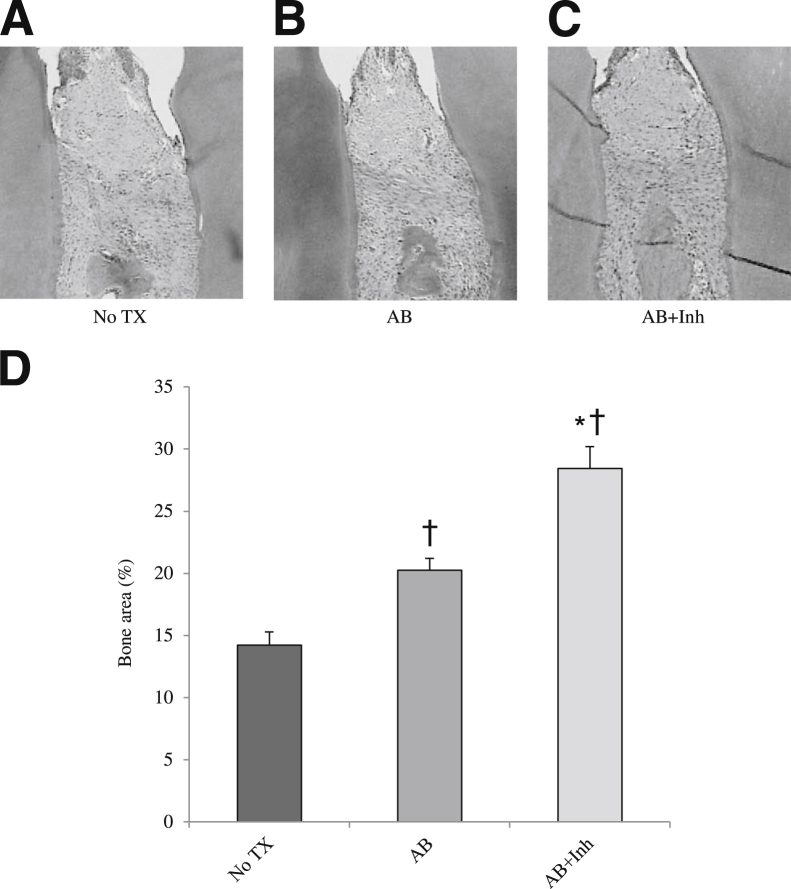
Bone resorption is followed by bone formation in a process referred to as coupling.25 In periodontal disease and other bone pathological conditions, bone coupling is decreased.10 We evaluated whether inhibition of apoptosis altered the number of osteoblasts present (Figure 2). Osteoblast numbers decreased after induction of periodontal disease (Figure 2H), consistent with an increase in apoptosis (P < 0.05). Halting periodontitis with the administration of antibiotics increased the number of osteoblasts by 50% (P < 0.05). However, treatment with caspase-3 inhibitor increased the number even further, by 68% at 5 weeks and 64% at 6 weeks, compared with the same time point without antibiotic or inhibitor (P < 0.05). Thus, much of the increase in osteoblast numbers could be directly tied to reduced apoptosis from treatment with the caspase-3 inhibitor. Furthermore, bone area increased 50% with caspase-3 inhibitor treatment (P < 0.05) (Figure 3).
Figure 2.
The loss of osteoblasts caused by periodontal disease in diabetic animals is reversed by inhibition of caspase-3. Periodontitis was induced in GK diabetic rats treated with antibiotic or antibiotic plus caspase-3 inhibitor, as described in Figure 1. A–G: H&E-stained histological images. Osteoblasts are indicated by arrows and dotted lines. H: The number of osteoblasts was measured per mm bone length at 5 and at 6 weeks after inoculation of bacteria. TX, treatment. ∗P < 0.05, a statistically significant difference between the AB and AB + Inh; †P < 0.05, a significant difference compared with baseline.
Figure 3.
Bacteria-induced periodontal bone loss is reduced by inhibition of apoptosis in diabetic rats. Periodontitis was induced in GK diabetic rats treated with antibiotic or antibiotic plus caspase-3 inhibitor, as described in Figure 1. A–C: H&E-stained histological sections. D: Bone area was calculated as the percentage area occupied by bone. TX, treatment. ∗P < 0.05, a statistically significant difference between the AB and AB + Inh; †P < 0.05, a significant difference compared with baseline.
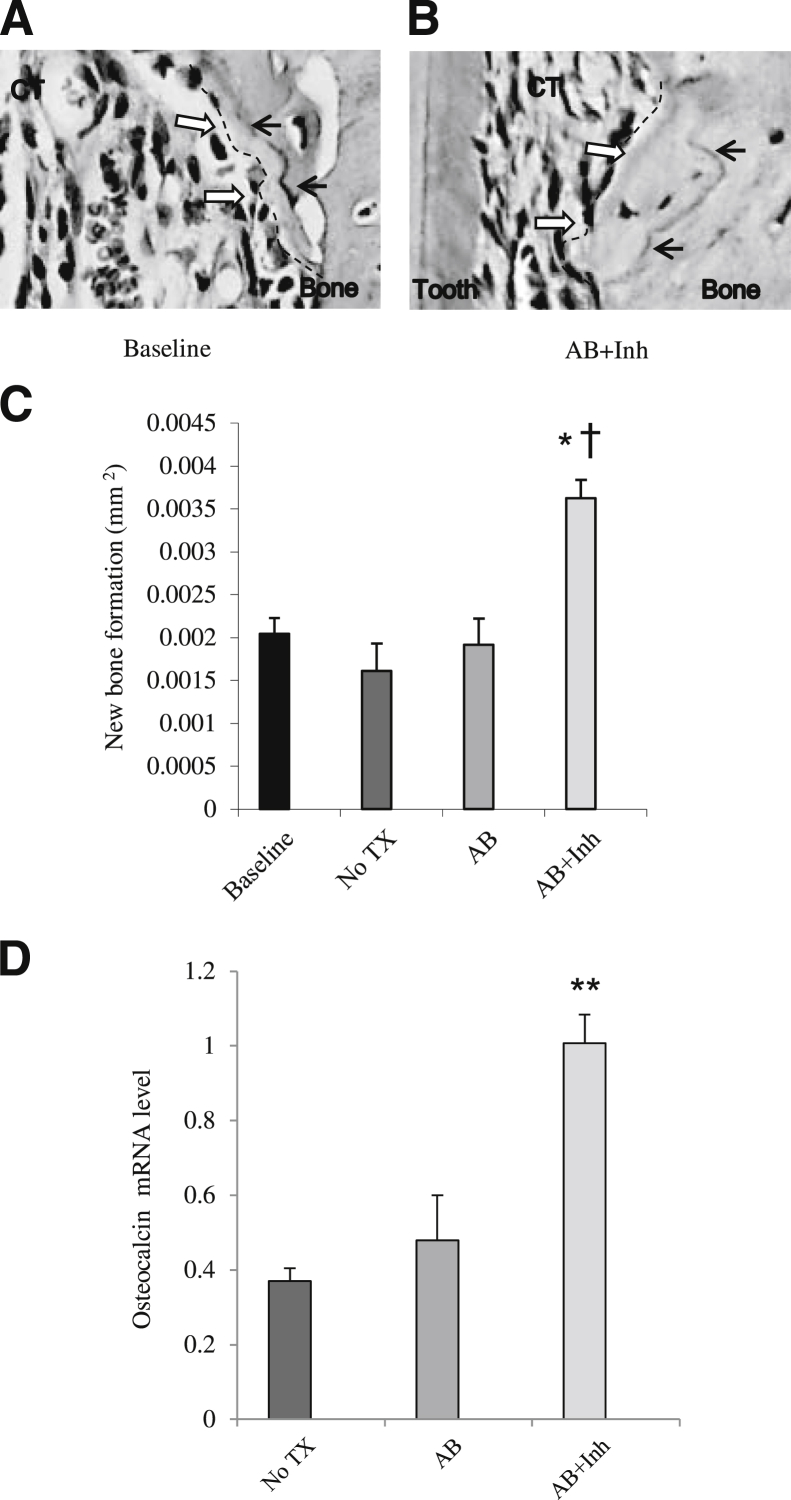
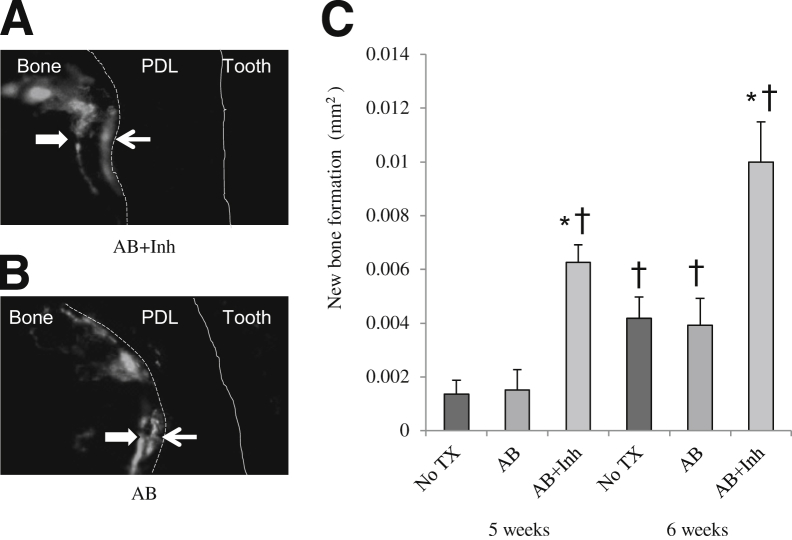
To measure the impact of caspase-3 inhibitor on bone, new bone formation was measured with different approaches. First, paraffin sections with TRAP staining, which used the reversal line as a guide (Figure 4, A and B), showed that the level of new bone formation increased 45% when diabetic rats were treated with caspase-3 inhibitor (P < 0.05) (Figure 4C). Treatment with the antibiotic alone had no discernible change in bone formation. Second, the amount of new bone formation was measured at the RNA level by expression of osteocalcin mRNA (Figure 4D). Diabetic rats had low osteocalcin mRNA levels at 5 weeks. When diabetic rats were treated with antibiotic + inhibitor, there was a 63% increase compared with the baseline (P < 0.05). Antibiotic alone did not show any effects. Third, new bone formation was assessed using fluorescent staining in methacrylate-embedded sections (Figure 5, A and B). The administration of caspase inhibitor increased the amount of new bone formation by 80% at 5 weeks and 60% at 6 weeks, compared with the antibiotic-treated group at the same time point (P < 0.05) (Figure 5C). Antibiotic alone had little effect on the amount of bone formed.
Figure 4.
Inhibition of apoptosis increases new bone formation and enhances osteocalcin mRNA levels. Periodontitis was induced in GK diabetic rats treated with antibiotic or antibiotic plus caspase-3 inhibitor, as described in Figure 1. A and B: TRAP-stained histological sections. Black arrows indicate the reversal line; white arrows and dotted lines, the extent of new bone formation. C: New bone formation was assessed per mm2 bone length. D: Osteocalcin mRNA levels were measured in total RNA obtained from the rat periodontium by real-time PCR. CT, connective tissue; TX, treatment. ∗P < 0.05, ∗∗P < 0.01, a statistically significant difference between the AB and AB + Inh; †P < 0.05, a significant difference compared with diabetic baseline.
Figure 5.
Periodontal bone formation is increased by caspase 3-inhibitor. Periodontitis was induced in GK diabetic rats, which were treated with antibiotic or antibiotic plus caspase-3 inhibitor, as described in Figure 1. A and B: New bone formation was measured using fluorescent staining in methacrylate-embedded sections. The distance between fluorescent labels (white arrows) was used to assess new bone formation. The dotted line represents the boundary between bone and periodontal ligament; solid line, boundary between periodontal ligament and tooth. C: New bone formation was assessed at 5- and 6-week time points. ∗P < 0.05, a statistically significant difference between the AB and AB + Inh; †P < 0.05, a significant difference compared with the 5 weeks no treatment (TX) group.
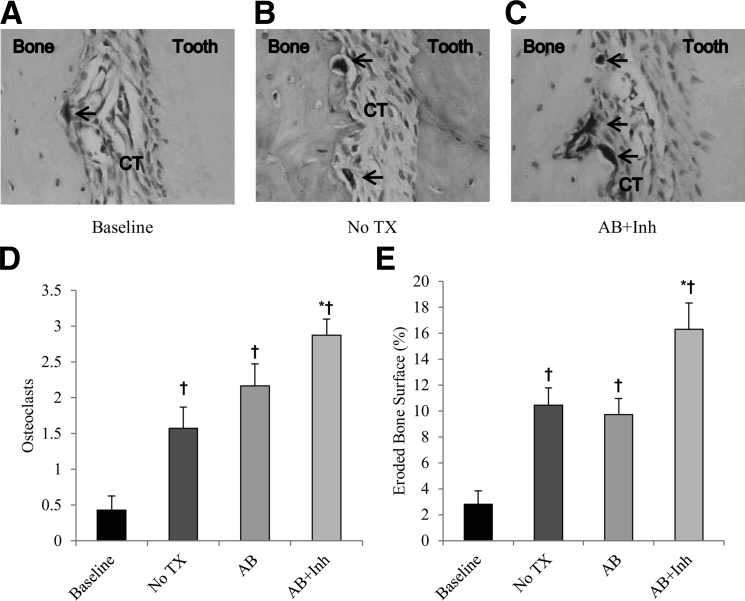
To analyze the effect of caspase-3 inhibitor treatment on bone resorption, the number of osteoclasts (Figure 6, A–C) and the percentage eroded bone surface that measures osteoclast activity were assessed. Osteoclast numbers and osteoclast activity increased by approximately 70% after the infection (Figure 6, D and E). Antibiotic alone did not show any significant increase compared with the 5-week time point with no treatment, but the treatment with caspase inhibitor increased the number of osteoclasts by 45% and eroded bone surface by 80% (P < 0.05).
Figure 6.
Osteoclast numbers and activity are increased by caspase-3 inhibitor. Periodontitis was induced in GK diabetic rats, which were treated with antibiotic or antibiotic plus caspase-3 inhibitor, as described in Figure 1. A–C: TRAP-stained histological images. The arrows indicate osteoclasts. D and E: Quantitative assessment of osteoclast numbers and the eroded bone surface per total bone surface. CT, connective tissue; TX, treatment. ∗P < 0.05, a statistically significant difference between the AB and AB + Inh; †P < 0.05, a significant difference compared with baseline.
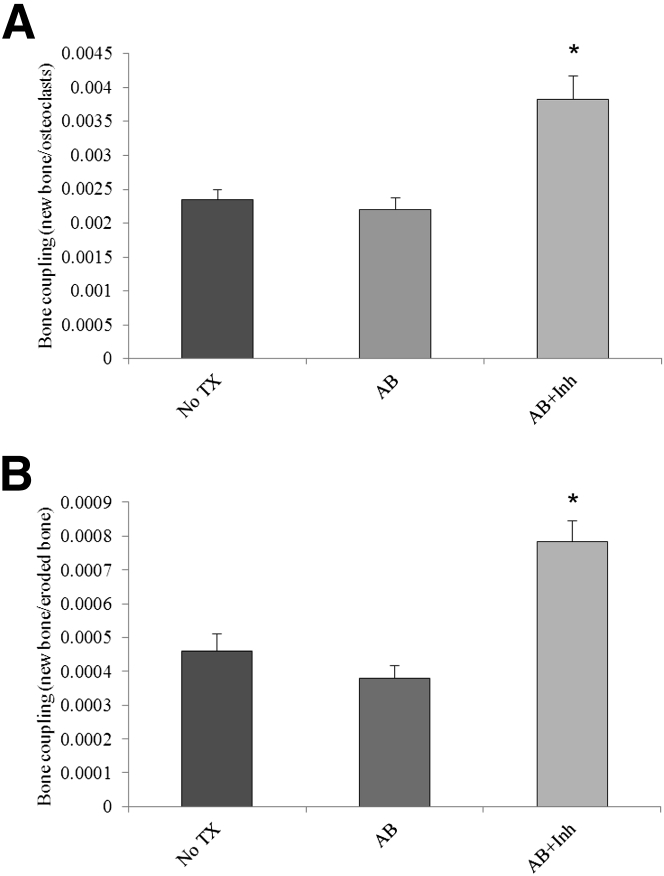
To assess the impact of diabetes on bone coupling, the amount of new bone formation determined by metabolic labeling was divided by an assessment of osteoclast numbers or eroded bone surface (Figure 7). Treatment with antibiotics alone did not increase in bone coupling. Treatment with caspase-3 inhibitor significantly increased bone coupling by approximately 40% when measured relative to osteoclast numbers (Figure 7A) or by 41% when examined per eroded bone surface (Figure 7B) (P < 0.05).
Figure 7.
Bone coupling is increased by the administration of the caspase-3 inhibitor. Periodontitis was induced in GK diabetic rats, which were treated with antibiotic or antibiotic plus caspase-3 inhibitor, as described in Figure 1. A: Bone coupling assessed by new bone formation (see Figure 5)/osteoclast numbers. B: Bone coupling assessed by new bone formation/eroded bone surface. ∗P < 0.05, a statistically significant difference compared with AB and compared with diabetic no treatment (TX).
Discussion
We previously reported that diabetes significantly enhanced inflammation in the periodontium and that the diabetes-enhanced inflammation was tied to high levels of apoptosis in the epithelium and connective tissue of diabetic animals.13, 26 In the present study, we functionally examined the role of high levels of apoptosis in diabetic animals to determine its contribution to periodontal bone loss, examining parameters of bone formation and bone resorption to assess bone coupling. We examined a 5- to 6-week time period, which is 7 to 8 weeks after periodontal infection was initiated, to allow sufficient time for bone resorption to occur and subsequent bone coupling after infection was treated.27, 28 The results indicate that blocking apoptosis significantly increases the number of bone-lining and periodontal ligament cells capable of forming osteoblasts. This ultimately affected osteoblast numbers, which were significantly increased when caspase-3–mediated apoptosis was inhibited. The caspase-3–specific inhibitor also increased significantly the amount of new bone formed by assessing paraffin sections, methacrylate sections from metabolically labeled bone specimens, and mRNA levels of the bone matrix protein, osteocalcin. Furthermore, the number of osteoclasts and their activity were increased by the caspase-3 inhibitor. However, the net effect was an increase in bone area because of enhanced bone coupling, indicating that bacterial infection in diabetic animals during the period of bone formation has a significant impact on periodontal disease through enhanced apoptosis of osteoblasts that form bone or their precursors. This is the first demonstration, to our knowledge, that shows that apoptosis significantly contributes to periodontal bone loss in bacteria-induced periodontitis.
Other diabetic complications have been associated with enhanced levels of apoptosis. For instance, diabetic neuropathy and diabetes-enhanced cardiovascular disease are significantly affected by apoptosis of neuronal cells and myocardial cells, respectively.29, 30 The inhibition of apoptosis through caspase-3 has been linked to reduce pathological characteristics. For example, a recent in vitro study showed blocking caspase-3 partly blocked tight-junction protein degradation that leads to stroke.31 Another in vivo study, in mice with a reovirus central nervous system infection, showed that mice lacking the caspase-3 gene were associated with a reduction in severity of central nervous system tissue injury.32
Diabetes leads to the up-regulation of pro-apoptotic factors, including the formation of reactive oxidative species, TNF-α, and advanced glycation end products.3, 18 Each may contribute to apoptosis. In addition, expression of several genes affecting propagation of intracellular pro-apoptotic signals or decreased expression of anti-apoptotic intracellular signals and effectors is enhanced by diabetes.14, 16 The balance between pro-apoptotic and anti-apoptotic extracellular and intracellular signals of these factors may be influenced by members of the forkhead transcriptions factors, such as FOXO. FOXO plays a pivotal role in cell cycle differentiation and apoptosis and may be a factor to overcome NF-κB–associated anti-apoptosis.18, 33 In periodontal disease, much of the pro-apoptotic effect of infection has been linked to the host response.10, 34 In vivo studies have demonstrated that the inhibition of TNF-α reduces apoptosis of connective tissue and bone-lining cells.13, 35 Bacteria or their components, such as lipopolysaccharide, stimulate apoptosis through a TNF-dependent mechanism.17, 35 We demonstrated herein that TNF-α expression was up-regulated in close proximity to bone after bacterial infection. However, bacterial toxins from A. actinomycetemcomitans or other bacteria may also enhance apoptosis, although this has not been demonstrated in in vivo studies of periodontal disease.36, 37
We have previously shown that inflammation stimulated by periodontal pathogens induces apoptosis of fibroblasts or osteoblasts through enhanced TNF-α levels.35, 38 The host response to periodontal pathogens in vivo has previously been shown to stimulate apoptosis of bone cells through activation of caspase-3.39 Inhibition of caspase-3 protects bone-lining cells from inflammation- and oxidative stress–induced apoptosis in vivo.40 Our results demonstrate that by inhibiting caspase-3, apoptosis of bone-lining cells and their precursors is diminished and that the diminished cell death plays a significant role in preventing periodontal bone loss in diabetic animals. Thus, apoptosis of osteoblasts or their precursors is a limiting factor in bone coupling.
In contrast to osteoblasts, inflammatory mediators enhance osteoclast lifespan by decreasing caspase-3 activation. For example, IL-1α reduces osteoclast apoptosis by inhibiting caspase-3 activity in vitro.41 Caspase-3 inhibitors suppress osteoclast apoptosis in vitro.41, 42 These findings agree with our results, in which the number of osteoclasts and their activity were increased when caspase-3 is inhibited. However, there is a greater increase in bone formation than resorption so that the net effect of inhibiting apoptosis in diabetic animals is to enhance bone formation. This is consistent with other pathological conditions in which inflammation and osteoblast apoptosis, through the caspase-3 pathway, have been shown to contribute to reduced bone formation and decreased bone mass in rheumatoid arthritis, ankylosing spondylitis, and inflammatory bowel disease.43, 44
We administered antibiotics to prevent persistent infection based on studies in which administration of antibiotics causes a cessation of periodontitis.45 In our study, antibiotic treatment helped improve some parameters, such as increasing the number of osteoblasts; however, in general, the effect of antibiotic alone was relatively small compared with the addition of the caspase inhibitor.
In summary, diabetes has been shown to have a negative effect on bone in periodontitis and on dental implants.42 We demonstrate herein that diabetes has an important effect on bone through apoptosis of bone-lining cells. These data, combined with previous experimental results, indicate that diabetes-enhanced inflammation causes accelerated loss of osteoblasts and, thereby, reduces bone coupling. In the absence of robust osseous coupling, there is loss of periodontal bone, which is one of the characteristic manifestations of periodontitis. This provides new insight as to how diabetes can affect bone and may contribute to diabetes-enhanced periodontitis and other pathological conditions in which diabetes has a negative impact on bone, including osteoporosis and impaired fracture healing.46 We have recently shown that diabetes-enhanced TNF-α plays a significant role in the resolution of periodontal inflammation and deficient bone formation.13, 47 These studies, along with results presented herein, indicate that diabetes-enhanced periodontitis is significantly affected by elevated levels of inflammation and apoptosis. Future studies may investigate mechanisms by which diabetes leads to enhanced TNF-α levels, which may be helpful in improving diabetes-impaired bone coupling.
Acknowledgments
We thank Sunitha Batchu for help in preparing the manuscript and Helly Shah for help in preparing the histological images.
Footnotes
Supported by NIH grant DE017732 (D.T.G.). B.B.B. was the recipient of a scholarship from the Coordination for the Improvement of Higher Education Personnel (CAPES) Foundation in Brazil. Dynamic bone labeling analysis was supported in part by the Penn Center for Musculoskeletal Disorders P30AR050950 from NIAMS.
References
- 1.Gong Z., Muzumdar R.H. Pancreatic function, type 2 diabetes, and metabolism in aging. Int J Endocrinol. 2012;2012:320482. doi: 10.1155/2012/320482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamster I.B., Lalla E., Borgnakke W.S., Taylor G.W. The relationship between oral health and diabetes mellitus. J Am Dent Assoc. 2008;139(Suppl):19S–24S. doi: 10.14219/jada.archive.2008.0363. [DOI] [PubMed] [Google Scholar]
- 3.Lalla E., Papapanou P.N. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. 2011;7:738–748. doi: 10.1038/nrendo.2011.106. [DOI] [PubMed] [Google Scholar]
- 4.Bascones-Martinez A., Arias-Herrera S., Criado-Camara E., Bascones-Ilundain J., Bascones-Ilundain C. Periodontal disease and diabetes. Adv Exp Med Biol. 2012;771:76–87. doi: 10.1007/978-1-4614-5441-0_9. [DOI] [PubMed] [Google Scholar]
- 5.Gurav A.N. Periodontitis and insulin resistance: casual or causal relationship? Diabetes Metab J. 2012;36:404–411. doi: 10.4093/dmj.2012.36.6.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otomo-Corgel J., Pucher J.J., Rethman M.P., Reynolds M.A. State of the science: chronic periodontitis and systemic health. J Evid Based Dent Pract. 2012;12(Suppl):20–28. doi: 10.1016/S1532-3382(12)70006-4. [DOI] [PubMed] [Google Scholar]
- 7.Nassar H., Kantarci A., van Dyke T.E. Diabetic periodontitis: a model for activated innate immunity and impaired resolution of inflammation. Periodontol 2000. 2007;43:233–244. doi: 10.1111/j.1600-0757.2006.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q., Valerio M.S., Kirkwood K.L. MAPK usage in periodontal disease progression. J Signal Transduct. 2012;2012:308943. doi: 10.1155/2012/308943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garlet G.P. Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dent Res. 2010;89:1349–1363. doi: 10.1177/0022034510376402. [DOI] [PubMed] [Google Scholar]
- 10.Graves D.T., Li J., Cochran D.L. Inflammation and uncoupling as mechanisms of periodontal bone loss. J Dent Res. 2011;90:143–153. doi: 10.1177/0022034510385236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croft M., Benedict C.A., Ware C.F. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov. 2013;12:147–168. doi: 10.1038/nrd3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behl Y., Krothapalli P., Desta T., Dipiazza A., Roy S., Graves D.T. Diabetes-enhanced tumor necrosis factor-{alpha} production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am J Pathol. 2008;172:1411–1418. doi: 10.2353/ajpath.2008.071070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pacios S., Kang J., Galicia J., Gluck K., Patel H., Ovaydi-Mandel A., Petrov S., Alawi F., Graves D.T. Diabetes aggravates periodontitis by limiting repair through enhanced inflammation. FASEB J. 2012;26:1423–1430. doi: 10.1096/fj.11-196279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kayal R.A., Siqueira M., Alblowi J., McLean J., Krothapalli N., Faibish D., Einhorn T.A., Gerstenfeld L.C., Graves D.T. TNF-alpha mediates diabetes-enhanced chondrocyte apoptosis during fracture healing and stimulates chondrocyte apoptosis through FOXO1. J Bone Miner Res. 2010;25:1604–1615. doi: 10.1002/jbmr.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chowdhury I., Tharakan B., Bhat G.K. Caspases: an update. Comp Biochem Physiol B Biochem Mol Biol. 2008;151:10–27. doi: 10.1016/j.cbpb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Al-Mashat H.A., Kandru S., Liu R., Behl Y., Desta T., Graves D.T. Diabetes enhances mRNA levels of proapoptotic genes and caspase activity, which contribute to impaired healing. Diabetes. 2006;55:487–495. doi: 10.2337/diabetes.55.02.06.db05-1201. [DOI] [PubMed] [Google Scholar]
- 17.Alikhani M., Alikhani Z., He H., Liu R., Popek B.I., Graves D.T. Lipopolysaccharides indirectly stimulate apoptosis and global induction of apoptotic genes in fibroblasts. J Biol Chem. 2003;278:52901–52908. doi: 10.1074/jbc.M307638200. [DOI] [PubMed] [Google Scholar]
- 18.Ponugoti B., Dong G., Graves D.T. Role of forkhead transcription factors in diabetes-induced oxidative stress. Exp Diabetes Res. 2012;2012:939751. doi: 10.1155/2012/939751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callus B.A., Vaux D.L. Caspase inhibitors: viral, cellular and chemical. Cell Death Differ. 2007;14:73–78. doi: 10.1038/sj.cdd.4402034. [DOI] [PubMed] [Google Scholar]
- 20.Concha N.O., Abdel-Meguid S.S. Controlling apoptosis by inhibition of caspases. Curr Med Chem. 2002;9:713–726. doi: 10.2174/0929867023370761. [DOI] [PubMed] [Google Scholar]
- 21.Wilson S.E., Mohan R.R., Hong J., Lee J., Choi R., Liu J.J. Apoptosis in the cornea in response to epithelial injury: significance to wound healing and dry eye. Adv Exp Med Biol. 2002;506:821–826. doi: 10.1007/978-1-4615-0717-8_116. [DOI] [PubMed] [Google Scholar]
- 22.Siqueira M.F., Li J., Chehab L., Desta T., Chino T., Krothpali N., Behl Y., Alikhani M., Yang J., Braasch C., Graves D.T. Impaired wound healing in mouse models of diabetes is mediated by TNF-alpha dysregulation and associated with enhanced activation of forkhead box O1 (FOXO1) Diabetologia. 2010;53:378–388. doi: 10.1007/s00125-009-1529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schreiner H.C., Sinatra K., Kaplan J.B., Furgang D., Kachlany S.C., Planet P.J., Perez B.A., Figurski D.H., Fine D.H. Tight-adherence genes of Actinobacillus actinomycetemcomitans are required for virulence in a rat model. Proc Natl Acad Sci U S A. 2003;100:7295–7300. doi: 10.1073/pnas.1237223100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu D.L., Jiang Q.H., He F.M., Yang G.L., Liu L. Fluorescence microscopic analysis of bone osseointegration of strontium-substituted hydroxyapatite implants. J Zhejiang Univ Sci B. 2012;13:364–371. doi: 10.1631/jzus.B1100381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sims N.A., Gooi J.H. Bone remodeling: multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol. 2008;19:444–451. doi: 10.1016/j.semcdb.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Kang J., de Brito Bezerra B., Pacios S., Andriankaja O., Li Y., Tsiagbe V., Schreiner H., Fine D.H., Graves D.T. Aggregatibacter actinomycetemcomitans infection enhances apoptosis in vivo through a caspase-3-dependent mechanism in experimental periodontitis. Infect Immun. 2012;80:2247–2256. doi: 10.1128/IAI.06371-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graves D.T., Fine D., Teng Y.T., Van Dyke T.E., Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008;35:89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu R., Bal H.S., Desta T., Krothapalli N., Alyassi M., Luan Q., Graves D.T. Diabetes enhances periodontal bone loss through enhanced resorption and diminished bone formation. J Dent Res. 2006;85:510–514. doi: 10.1177/154405910608500606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vincent A.M., Brownlee M., Russell J.W. Oxidative stress and programmed cell death in diabetic neuropathy. Ann N Y Acad Sci. 2002;959:368–383. doi: 10.1111/j.1749-6632.2002.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 30.Cai L., Li W., Wang G., Guo L., Jiang Y., Kang Y.J. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes. 2002;51:1938–1948. doi: 10.2337/diabetes.51.6.1938. [DOI] [PubMed] [Google Scholar]
- 31.Zehendner C.M., Librizzi L., de Curtis M., Kuhlmann C.R., Luhmann H.J. Caspase-3 contributes to ZO-1 and Cl-5 tight-junction disruption in rapid anoxic neurovascular unit damage. PLoS One. 2011;6:e16760. doi: 10.1371/journal.pone.0016760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beckham J.D., Tuttle K.D., Tyler K.L. Caspase-3 activation is required for reovirus-induced encephalitis in vivo. J Neurovirol. 2010;16:306–317. doi: 10.3109/13550284.2010.499890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greer E.L., Brunet A. FOXO transcription factors in ageing and cancer. Acta Physiol. 2008;192:19–28. doi: 10.1111/j.1748-1716.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- 34.Scott D.A., Krauss J. Neutrophils in periodontal inflammation. Front Oral Biol. 2012;15:56–83. doi: 10.1159/000329672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu R., Bal H.S., Desta T., Behl Y., Graves D.T. Tumor necrosis factor-alpha mediates diabetes-enhanced apoptosis of matrix-producing cells and impairs diabetic healing. Am J Pathol. 2006;168:757–764. doi: 10.2353/ajpath.2006.050907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kachlany S.C. Aggregatibacter actinomycetemcomitans leukotoxin: from threat to therapy. J Dent Res. 2010;89:561–570. doi: 10.1177/0022034510363682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dickinson B.C., Moffatt C.E., Hagerty D., Whitmore S.E., Brown T.A., Graves D.T., Lamont R.J. Interaction of oral bacteria with gingival epithelial cell multilayers. Mol Oral Microbiol. 2011;26:210–220. doi: 10.1111/j.2041-1014.2011.00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graves D., Oskoui M., Volejnikova S., Naguib G., Cai S., Desta T., Kakouras A., Jiang Y. Tumor necrosis factor modulates fibroblast apoptosis, PMN recruitment, and osteoclast formation in response to P gingivalis infection. J Dent Res. 2001;80:1875–1879. doi: 10.1177/00220345010800100301. [DOI] [PubMed] [Google Scholar]
- 39.Alikhani M., Maclellan C.M., Raptis M., Vora S., Trackman P.C., Graves D.T. Advanced glycation end products induce apoptosis in fibroblasts through activation of ROS, MAP kinases, and the FOXO1 transcription factor. Am J Physiol Cell Physiol. 2007;292:C850–C856. doi: 10.1152/ajpcell.00356.2006. [DOI] [PubMed] [Google Scholar]
- 40.Chen R.M., Wu G.J., Chang H.C., Chen J.T., Chen T.F., Lin Y.L., Chen T.L. 2,6-Diisopropylphenol protects osteoblasts from oxidative stress-induced apoptosis through suppression of caspase-3 activation. Ann N Y Acad Sci. 2005;1042:448–459. doi: 10.1196/annals.1338.038. [DOI] [PubMed] [Google Scholar]
- 41.Okahashi N., Koide M., Jimi E., Suda T., Nishihara T. Caspases (interleukin-1beta-converting enzyme family proteases) are involved in the regulation of the survival of osteoclasts. Bone. 1998;23:33–41. doi: 10.1016/s8756-3282(98)00069-6. [DOI] [PubMed] [Google Scholar]
- 42.Benford H.L., McGowan N.W., Helfrich M.H., Nuttall M.E., Rogers M.J. Visualization of bisphosphonate-induced caspase-3 activity in apoptotic osteoclasts in vitro. Bone. 2001;28:465–473. doi: 10.1016/s8756-3282(01)00412-4. [DOI] [PubMed] [Google Scholar]
- 43.D’Lima D., Hermida J., Hashimoto S., Colwell C., Lotz M. Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum. 2006;54:1814–1821. doi: 10.1002/art.21874. [DOI] [PubMed] [Google Scholar]
- 44.Bradford P.G., Gerace K.V., Roland R.L., Chrzan B.G. Estrogen regulation of apoptosis in osteoblasts. Physiol Behav. 2010;99:181–185. doi: 10.1016/j.physbeh.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bartold P.M., du Bois A.H., Gannon S., Haynes D.R., Hirsch R.S. Antibacterial and immunomodulatory properties of azithromycin treatment implications for periodontitis. Inflammopharmacology. 2013;21:321–338. doi: 10.1007/s10787-012-0165-1. [DOI] [PubMed] [Google Scholar]
- 46.Mi M., Jin H., Wang B., Yukata K., Sheu T.J., Ke Q.H., Tong P., Im H.J., Xiao G., Chen D. Chondrocyte BMP2 signaling plays an essential role in bone fracture healing. Gene. 2013;512:211–218. doi: 10.1016/j.gene.2012.09.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andriankaja O.M., Galicia J., Dong G., Xiao W., Alawi F., Graves D.T. Gene expression dynamics during diabetic periodontitis. J Dent Res. 2012;91:1160–1165. doi: 10.1177/0022034512465292. [DOI] [PMC free article] [PubMed] [Google Scholar]