Abstract
Scaling up access to HIV viral load testing for individuals undergoing antiretroviral therapy in low-resource settings is a global health priority, as emphasised by research showing the benefits of suppressed viral load for the individual and the whole population. Historically, large-scale diagnostic test implementation has been slow and incomplete because of service delivery and other challenges. Building on lessons from the past, in this Personal View we propose a new framework to accelerate viral load scale-up and ensure equitable access to this essential test. The framework includes the following steps: (1) ensuring adequate financial investment in scaling up this test; (2) achieving pricing agreements and consolidating procurement to lower prices of the test; (3) strengthening functional tiered laboratory networks and systems to expand access to reliable, high-quality testing across countries; (4) strengthening national leadership, with prioritisation of laboratory services; and (5) demand creation and uptake of test results by clinicians, nurses, and patients, which will be vital in ensuring viral load tests are appropriately used to improve the quality of care. The use of dried blood spots to stabilise and ship samples from clinics to laboratories, and the use of point-of-care diagnostic tests, will also be important for ensuring access, especially in settings with reduced laboratory capacity. For countries that have just started to scale up viral load testing, lessons can be learnt from countries such as Botswana, Brazil, South Africa, and Thailand, which have already established viral load programmes. This framework might be useful for guiding the implementation of viral load with the aim of achieving the new global HIV 90-90-90 goals by 2020.
Introduction
In 2014 the Joint UN Programme on HIV/AIDS (UNAIDS) announced ambitious new global treatment targets for 2020: 90% of people with HIV will know their status, 90% of people living with HIV will have access to treatment, and 90% of people on treatment will have suppressed viral load.1 These targets are supported by WHO recommendations from 20162 for universal access to antiretroviral therapy (ART) and routine viral load testing to monitor treatment efficacy, and unequivocal evidence from the TEMPRANO ANRS 12136 study,3 the Strategic Timing of Antiretroviral Treatment (START) study,4 and the HIV Prevention Trials Network 0525 study showing that ART with sustained viral suppression should be started early in the clinical course of HIV infection.2
The UNAIDS 90-90-90 goals present great challenges to national governments, donors, and technical partners, and bring focus to the need to expand access to diagnostic tests. An urgent priority is the introduction of routine viral load monitoring within large-scale ART programmes. Although CD4 monitoring has been essential in clinical decisions around treatment initiation and monitoring of immune reconstitution immediately following treatment initiation, viral load monitoring enables the detection of early signs of adherence problems to treatment before immunological decline.6–8 This information is crucial for informing adherence interventions and timely switching to second-line drug regimens, before drug resistance develops. Approximately 17 million people living with HIV (PLHIV) were on ART by 2015, reflecting an increase from 7·5 million in 2010.9 As ART coverage expands, investments in the global response are shifting towards ensuring the long-term effectiveness of ART through sustained suppression of the virus to improve survival, reduce HIV transmission, and prevent large-scale HIV drug resistance.10
This goal cannot be met without a substantial effort towards ensuring minimal attrition in each step of the care continuum. Without optimal retention in care, sustained adherence to medication, and effective monitoring of therapeutic response to ART, the escalating treatment response could result in a rapid decaying of regimen efficacy and the spread of drug resistance.11 Achieving long-term viral suppression within the context of early ART initiation will require a shift in both patient management and governmental policies to offer routine viral load monitoring and improved access to viral load testing capabilities in low-income and middle-income countries (LMICs).
Access to viral load testing
In sub-Saharan Africa, where 70% of PLHIV reside, more than 6 million people who are on ART do not have access to viral load testing, and more than 10 million viral load tests are estimated to be needed in total every year.12,13 As national treatment programmes implement the recommendations in the 2016 WHO guidelines2—ie, immediate HIV treatment not just for sick patients, but for all HIV-infected individuals—the need for viral load testing globally will grow to as much as 30 million tests per year by 2020, with increases in test demand of up to 35% annually. The provision of viral load testing represents one of the most urgent and large-scale priorities facing countries and the global health community today. Cognisant of this urgency, UNAIDS launched a global Diagnostic Access Initiative in 201414 to strengthen partnerships and coordination of efforts to rapidly expand access to diagnostic tools for ART scale-up.
Two essential considerations help define how scale-up of viral load testing should be approached. First, previous experiences with the introduction of new diagnostic tests in low-resource settings suggest that implementation challenges might slow scale-up. Viral load test access has increased modestly between 2013 and 2015, despite the target of 90% access by 2020.15–18 Previous large scale-up test implementation programmes provide perspective. For example, after nearly 10 years of CD4 test scale-up, an estimated 60% of patients in low-resource settings had access to WHO-recommended levels of CD4 testing in 2012. Second, viral load tests need to be used effectively by clinicians and by patients to improve clinical management and adherence to ensure the goal of 90% viral suppression. A recent review of the large-scale implementation of molecular tuberculosis diagnostic tests in South Africa showed minimal impact on patient mortality, probably as a result of inadequate patient access to the test and poor use of test results by clinicians and patients to improve management.19 Linkage to care and a strong laboratory–clinic interface are essential. The scale-up of viral load testing provides an opportunity to apply these lessons and take more effective approaches to test implementation.
Addressing barriers to access to viral load
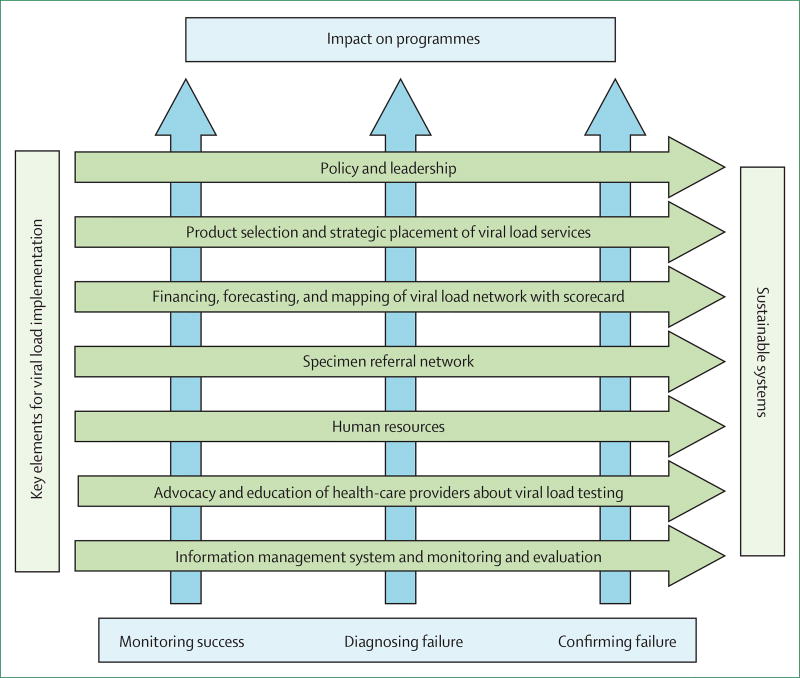
We propose the following framework (figure) for addressing barriers to access of viral load testing. First, substantial financial investments are needed at the outset to support the expansion of viral load monitoring, including laboratory infrastructure development, test commodities, operational costs, and downstream drug and clinical costs. In particular, increased switching to expensive second-line and third-line regimens could increase treatment budgets. Although viral load testing could lead to eventual cost savings on the basis of improved patient outcomes, reductions in unnecessary regimen switches, and reduced transmission, budget planning for increased investment in the short term will be necessary to ensure that these costs do not become barriers to testing scale-up. Additionally, increased efforts to improve access to viral load testing should be accompanied by efforts to improve access to effective and affordable second-line and third-line regimens. Governments should understand the full costs of integrating viral load testing into existing ART programmes to inform appropriate amounts of resource allocation towards not only testing supplies, but also strengthening of the supportive laboratory and clinical systems, and demand-generation initiatives, including patient and clinician education.
Figure.
Framework for implementing sustainable viral load networks
Guidance and strategies for the expansion of viral load testing programmes were released concurrently by the WHO, the Global Fund, the US President’s Emergency Plan for AIDS Relief (PEPFAR), and the African Society for Laboratory Medicine (ASLM).20 These strategies can help define cost-effective scale-up approaches. For example, although new laboratories would need to be established, existing capacity and systems for molecular HIV testing (eg, PCR-based testing for early infant diagnosis of HIV infection) should be exploited. In 2014, instrument capacity for 2 million viral load tests was estimated to exist in sub-Saharan Africa, on the basis of under-used instruments in laboratories doing viral load and PCR-based testing for early infant diagnosis of HIV infection. Using this spare capacity at the launch of the scale-up of viral load testing requires rational planning and could help reduce early financial hurdles to test scale-up.
Second, novel approaches to financing viral load testing, such as consortium procurement and negotiated volume-based price reductions, instrument rental agreements, and public–private partnership initiatives with manufacturers, could be considered. For example, major test procurers, including PEPFAR, The Global Fund, and the South African Government recently partnered under the Diagnostics Access Initiative to negotiate an access pricing agreement with one viral load test supplier, who reduced the test price to less than US$10 per test, representing a nearly 40% reduction in price.21 This agreement exploited the higher purchasing power of these procurers to secure lower pricing for other countries and procurers, making the test accessible to most. Such agreements require accurate forecasts of test demand, realistic commodity costing, a competitive market for viral load goods and services, standardisation of test platforms, and predictable procurement patterns. Collaboration between governments and partners can help ensure that these conditions that facilitate sustainable low pricing are met.
Third, functional, tiered laboratory networks should be built to enable equitable test access from central levels to community levels of ART delivery. These networks will require both central, high-volume testing laboratories as well as point-of-care and mobile viral laboratories. Additionally, the networks will require effective specimen referral systems and results management and delivery systems to ensure decentralised access and rapid test turnaround times. These networks should include innovations in data capture and management, such as e-health and electronic medical records and geographical information systems, and the use of unmanned aerial vehicles for sample delivery from remote areas should be explored.22
Fourth, the scale-up of viral load testing requires the prioritisation of initiatives to ensure high-quality testing so that errors have a minimal impact on patients. The process of ensuring test quality has multiple dimensions, including product registration and regulatory requirements, post-market surveillance, supply chain management, proficiency testing, quality assurance, data management for test results (including remote wireless connectivity to monitor instrument and operator performance), instrument maintenance, and laboratory accreditation—eg, the WHO Regional Office for Africa Stepwise Laboratory Improvement Process Towards Accreditation (SLIPTA) programme.23 The value of quality is often underestimated and laboratory quality standards are not universally met. Developing an investment case framework for quality initiatives could help ensure consistent and sustainable resources are made available.
Finally, none of these efforts can occur without country ownership and leadership, as shown by South Africa, which as a result of its early and sustained prioritisation of viral load scale-up had the purchasing power to help negotiate reduced prices for viral load tests that benefited other countries.21 Governments should give greater priority to investment in laboratory services as a means of improving overall ART programme success and other health benefits. These HIV-related investments in laboratory services also benefit the management of other diseases, and help build higher capacity health systems that are better capable of responding to new disease threats.
Expansion of viral load testing
Simple and innovative technologies that minimise the complexities of the viral load testing process and extend the reach of the test to PLHIV in remote locations are also needed to ensure equity in access to viral load testing. These technologies include the use of dried blood spots instead of plasma samples,24 in line with recommendations in the 2014 WHO interim technical update for implementing viral load monitoring,20 and other sample collection and preservation devices to provide equitable access to the test to PLHIV in remote areas. Clear policy guidance for the use of viral load testing and the optimal frequency of routine monitoring for patient management also need to be made widely available.25 Validating the performance of point-of-care viral load tests, especially in low-volume or remote sites, should be prioritised in parallel with the expansion of existing viral load testing platforms and considered for targeted viral load testing as these technologies become readily available. Additionally, modelling is necessary to inform deployment of viral load testing technology that balances new diagnostics with existing test platforms. Long-term planning would ensure appropriate investment in technologies that enable new, improved platforms to be introduced in the future to further improve access.
In order to achieve the 90% viral suppression target, the global community should work collectively to increase equity by other means than price reduction. Demand creation, having the patients as stakeholders in the viral load scale-up efforts, training of health-care providers, and reviewing the lessons learned from past experiences in diagnostics will all help improve equity.
Medical professionals and patients as stakeholders
For viral load monitoring to be successful, both medical professionals and patients should work together to request this test when needed. Demand for the test remains low, and although annual viral load testing is recommended for PLHIV on ART,25 many physicians, nurses, and clinical officers might not order viral load tests because they are unaware of the advantages of virological monitoring to guide patient management or they do not know that the test is readily available. Alternatively, they might not have been trained on the viral load testing algorithm and might have difficulty interpreting viral load test results accurately or not know how to use the results to make appropriate decisions about when to switch therapies. Patients might not be aware of viral load testing, which can also contribute to low uptake; therefore, the opportunity to empower patients to self-manage their disease might be diminished. The need to educate both medical pro fessionals and patients about the reasons for viral load testing is clear. As these groups become familiar with the benefits of viral suppression and are trained on the testing process, the demand for viral load testing might rise while patient attrition in the clinical cascade, adherence to ART, and inappropriate switches to second-line drugs might diminish, improving the management of HIV disease and overall health.
Lessons from the past
Many of the existing barriers to viral load adoption are not new to LMICs and can be mitigated through renewed focus, concerted action, and novel approaches in expanding access to viral load testing to millions of patients. Using lessons learned from global initiatives in the 1990s that helped to improve access and equity of ART in developing countries, a similar approach in coordinated partnerships between country governments, implementing partners, donors, and industry will be needed to improve access and equity to viral load testing.26 Much can be learned from existing national viral load testing programmes such as those in South Africa, Brazil, Botswana, Thailand, and Kenya on how to scale up viral load testing in an effective and efficient manner. Partnerships, political commitment, and substantial financial, operational, and technical investments were necessary.
Conclusion
HIV viral load monitoring is now the recommended standard of care for monitoring the impact of treatment and is crucial to sustain quality of care at both the patient and programmatic level. Expanding equitable access to viral load testing provides a clear path for preventing future infections and saving lives, and can be achieved by a global partnership. The suggestions we provide offer a framework for a concerted and harmonised implementation strategy that enables a rapid and cost-effective scale-up of viral load testing for HIV treatment monitoring. Regional bodies such as ASLM can help to galvanise countries to develop strategic plans for implementing viral load testing. These plans offer a platform for countries and partners to scale up viral load testing in a coordinated manner as access to ART expands and overcomes anticipated barriers at the outset.
Acknowledgments
The findings and conclusions in this Personal View are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention
Footnotes
Contributors
TP and JNN were responsible for the scope of the report. TP and DE wrote and made amendments to the report. All authors commented on the report and contributed amendments.
Declaration of interests
We declare no competing interests.
References
- 1.Joint UN on HIV/AIDS. 90-90-90: a transformative agenda to leave no one behind. [accessed Sept 15, 2015];2014 Oct 25; http://www.unaids.org/en/resources/presscentre/unaidsspeeches/2014/20141025_SP_EXD_Vietnam_launch_of_909090_en.pdf.
- 2.WHO. Recommendations for a public health approach. second. Geneva: World Health Organization; 2016. [accessed Oct 4, 2016]. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf?ua=1. [PubMed] [Google Scholar]
- 3.TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808–22. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 4.The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen M, Chen Y, McCauley M, et al. Final results of the HPTN 052 randomized controlled trial: antiretroviral therapy prevents HIV transmission. J Int AIDS Soc. 2015;18(suppl 4):20479. [Google Scholar]
- 6.Boyer S, March L, Kouanfack C, et al. Monitoring of HIV viral load, CD4 cell count, and clinical assessment versus clinical monitoring alone for antiretroviral therapy in low-resource settings (Stratall ANRS 12110/ESTHER): a cost-effectiveness analysis. Lancet Infect Dis. 2013;13:577–86. doi: 10.1016/S1473-3099(13)70073-2. [DOI] [PubMed] [Google Scholar]
- 7.Estill J, Egger M, Blaser N, et al. IeDEA Southern Africa. Cost-effectiveness of point-of-care viral load monitoring of antiretroviral therapy in resource-limited settings: mathematical modelling study. AIDS. 2013;27:1483–92. doi: 10.1097/QAD.0b013e328360a4e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahn JG, Marseille E, Moore D, et al. CD4 cell count and viral load monitoring in patients undergoing antiretroviral therapy in Uganda: cost effectiveness study. BMJ. 2011;343:d6884. doi: 10.1136/bmj.d6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UNAIDS. Geneva: Joint United Nations Programme on HIV/AIDS; 2016. [accessed Oct 4, 2016]. Fact sheet 2016. http://www.un.am/up/library/Fact%20Sheet%20UNAIDS_eng.pdf. [Google Scholar]
- 10.Piot P, Quinn TC. Response to the AIDS pandemic—a global health model. N Engl J Med. 2013;368:2210–18. doi: 10.1056/NEJMra1201533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta RK, Jordan MR, Sultan BJ, et al. Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: a global collaborative study and meta-regression analysis. Lancet. 2012;380:1250–58. doi: 10.1016/S0140-6736(12)61038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford N, Roberts T, Calmy A. Viral load monitoring in resource-limited settings: a medical and public health priority. AIDS. 2012;26:1719–20. doi: 10.1097/QAD.0b013e3283543e2c. [DOI] [PubMed] [Google Scholar]
- 13.WHO. HIV diagnostic tests in low- and middle-income countries: forecasts of global demand for 2014–2018. [accessed Sept 14, 2015];2015 Jul; http://www.who.int/hiv/pub/amds/diagnostic-forecast2014-2018/en/
- 14.Joint UN on HIV/AIDS. UNAIDS and partners launch initiative to improve HIV diagnostics. [accessed Sept 15, 2015];2014 Jul 23; http://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2014/july/20140723dai.
- 15.Haas AD, Keiser O, Balestre E, et al. Monitoring and switching of first-line antiretroviral therapy in sub-Saharan Africa: collaborative analysis of adult treatment cohorts. Lancet HIV. 2015;2:e271–78. doi: 10.1016/S2352-3018(15)00087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.President’s Emergency Plan for AIDS Relief. PEPFAR blueprint: creating an AIDS-free generation. [accessed Sept 12, 2015];2012 Nov; http://www.pepfar.gov/documents/organization/201386.pdf.
- 17.Joint UN on HIV/AIDS. Treatment 2015. [accessed Sept 12, 2015];2013 Jul 13; http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2013/JC2484_treatment-2015_en.pdf.
- 18.Joint UN on HIV/AIDS. Ambitious treatment targets: writing the final chapter of the AIDS epidemic. [accessed Sept 12, 2015]; http://www.unaids.org/sites/default/files/media_asset/JC2670_UNAIDS_Treatment_Targets_en.pdf.
- 19.Churchyard GJ, Stevens WS, Mametja LD, et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Glob Health. 2015;3:e450–57. doi: 10.1016/S2214-109X(15)00100-X. [DOI] [PubMed] [Google Scholar]
- 20.WHO. Technical and operational considerations for implementing HIV viral load testing. [accessed Sept 10, 2015];Interim technical update. 2014 Jul; http://www.who.int/hiv/pub/arv/viral-load-testing-technical-update/en/
- 21.Roche. Roche launches global access program for HIV viral load testing. [accessed Jan 5, 2015];2014 Sep 26; http://www.roche.com/media/media_releases/med-cor-2014-09-26.htm.
- 22.Amukele TK, Sokoll LJ, Pepper D, Howard DP, Street J. Can unmanned aerial systems (drones) be used for the routine transport of chemistry, hematology, and coagulation laboratory specimens? PLoS One. 2015;10:e0134020. doi: 10.1371/journal.pone.0134020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO Regional Office for Africa. WHO guide for the stepwise laboratory improvement process towards accreditation in the African region (SLIPTA) [accessed Sept 11, 2015];2015 http://www.afro.who.int/index.php?option=com_docman&task=doc_download&gid=8642&Itemid=2593.
- 24.Rottinghaus EK, Ugbena R, Diallo K, et al. Dried blood spot specimens are a suitable alternative sample type for HIV-1 viral load measurement and drug resistance genotyping in patients receiving first-line antiretroviral therapy. Clin Infect Dis. 2012;54:1187–95. doi: 10.1093/cid/cis015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. [accessed Sept 14, 2015];Recommendations for a public health approach. 2013 Jun; http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf. [PubMed]
- 26.Joint UN on HIV/AIDS. UNAIDS HIV drug access initiative: providing wider access to HIV-related drugs in developing countries, pilot phase. [accessed Sept 14, 2015];1999 Aug; http://quod.lib.umich.edu/c/cohenaids/5571095.0368.003?rgn=main;view=fulltext.