A tailored tobacco cessation intervention for P/C smokers of fourth-grade children showed significant quit rates, which were confirmed by saliva cotinine over 4 years.
Abstract
OBJECTIVES:
There is no safe or risk-free level of tobacco use or tobacco smoke exposure. In this randomized controlled trial, we tested a tobacco control intervention in families and specifically evaluated a tailored cessation intervention for the parents and/or caregivers (Ps/Cs) who were smokers while their children were simultaneously enrolled in tobacco prevention.
METHODS:
Ps/Cs and children were recruited from 14 elementary schools across rural and urban settings. Approximately one-fourth (24.3%; n = 110) of the total Ps/Cs enrolled in the randomized controlled trial (n = 453) were smokers, predominantly women (80.9%), with a mean age of 37.7 years. (SD 12.2); 62.7% were African American, 44% had less than a high school education, and 58% earned <$20 000 annually. P/C smokers were offered a tailored cessation intervention in years 1 and 2. Self-report smoking status and saliva cotinine were obtained at baseline, the end of treatment (EOT) and/or year 2, and in the year 4 follow-up.
RESULTS:
Ps/Cs in the intervention group showed a larger increase in self-reported smoking abstinence over time (EOT: 6.5% [SE = 5.7%]; year 4: 40.6% [SE = 5.7%]) than the control group (EOT: 0.0% [SE = 6.5%]; year 4: 13.2% [SE = 6.4%]; F = 4.82; P = .0306). For cotinine, the intervention group showed a decrease from baseline (239.9 [SE = 1.3]) to EOT 99.3 [SE = 1.4]) and then maintenance through year 4 (109.6 [SE = 1.4]), whereas the control group showed increases from baseline (221.1 [SE = 1.4]) to EOT (239.0 [SE = 1.4]) to year 4 (325.8 [SE = 14]; F = 5.72; P = .0039).
CONCLUSIONS:
This study provides evidence that tailored cessation offered to Ps/Cs in their children’s schools during their children’s enrollment in tobacco prevention may contribute to more robust success in P/C cessation and a reduction of tobacco smoke exposure in children.
Tobacco smoke exposure (TSE), also referred to as secondhand smoke exposure, is exposure to smoke from a burning tobacco product or exhaled smoke.1–3 TSE results in increased morbidity from asthma and ear infections in children and increased mortality from sudden infant death syndrome.1,4,5 Parental smoking of at least half of 1 pack per day doubles the risk of being hospitalized for a respiratory sickness6 and results in 15 000 child hospitalizations per year. In the United States, from 2011 to 2012, 2 of every 5 children ages 3 to 11 years and 7 of every 10 African American (AA) children were exposed to tobacco smoke.7 According to the Centers for Disease Control and Prevention, there is no risk-free level of TSE, with even brief exposure being detrimental to health.1,3,4 Cotinine levels, which are the proximate metabolite of nicotine and are an accurate measure of smoking status or TSE, have continued to decline across all racial and ethnic groups; however, levels remain higher among AA (46.8% of nonsmokers) compared with white patients (21.8% of nonsmokers).7 TSE is also much higher among those living below the poverty level, with rates as high as 60.5%.1
Most children are exposed to tobacco smoke in the home from having parents and/or caregivers (Ps/Cs) who smoke. Forty percent of adolescents in the United States are exposed to at least 1 parent who smokes,8 and 50% to 67% of children <5 years old live in homes with at least 1 adult smoker.6 Parental smoking is also a risk factor for smoking initiation in adolescents, with up to 3 times the risk for adolescents whose parents smoke.9 Parents who are successful in quitting smoking may help prevent their children from initiating smoking by role-modeling a healthy behavior choice, decrease their children’s exposure to tobacco smoke, and improve both their children’s and their own personal health.
The US Public Health Service’s Clinical Practice Guideline for Treating Tobacco Use and Dependence combines cognitive behavioral therapy and pharmacotherapies for treating nicotine dependence.10 This guideline incorporates counseling and/or coaching, motivational interviewing (MI), smoking self-assessments, the establishment and selection of a quit plan, and integrates first- and second-line pharmacotherapies. The guideline’s interventions are applicable in many settings and modalities.10–12
The reduction of TSE in children, along with parental smoking cessation, has been the subject of numerous studies. In an early investigation13 using infant cotinine levels, 1 phone call from a physician, and a form letter presenting the results of the child’s cotinine resulted in no statistically significant reduction in TSE. Hovell et al14 used cotinine levels to monitor children’s TSE at baseline and 3, 6, 12, and 18 months after counseling sessions with mothers who smoked. Although there were sustained decreases in TSE among the children in the counseled group, children’s cotinine levels were not significantly different between the intervention and control groups. Liles et al15 reported that among mothers of lower socioeconomic status who smoked, those who received intensive, individualized counseling had more 24-hour quits (P = .02) and more 7-day quits (P = .03) than control group mothers. Three additional studies in P/C smokers with young children <6 years of age reported some success with P/C cessation and a decrease in children’s TSE; however, all studies used self-report and lacked biochemical verification of cessation outcomes.16–18 In a study with children <4 years old whose mothers were smokers, both mothers’ self-report and bioverified 7-day point prevalence quit rates19 were used. Also, a pilot study was conducted in which researchers educated nonsmoking adolescents in an effort to encourage their Ps/Cs to quit smoking.8 Both self-report and biochemical verification were used for parents’ cessation outcomes; however, results were not statistically significant. Schuck et al20 recruited P/C smokers from primary schools to compare intensive quitline counseling with standard self-help brochures and measured self-report 7-day point prevalence abstinence rates with a long-term assessment at 12 months. Twelve percent to 21% of quitline participants self-reported success with cessation compared with 3% to 5% of those in a control group, yet no biochemical verification was used.
The Russell Standard21 states that the standard of smoking cessation should be biochemically validated, continuous abstinence for 6 to 12 months to make meaningful comparisons among studies. Results across studies vary considerably, creating challenges in comparing cessation outcomes. This is due to the differences in measurement, a frequent lack of biochemical verification, and assessment time frames. The effectiveness of a tailored cessation intervention in P/C smokers delivered within the elementary school setting in which their children are concurrently enrolled in a smoking prevention program, and with longitudinal biochemical verification across 4 years, was not found through multiple literature searches. We addressed both these gaps in the literature.
The goals of the Surgeon General’s report1 are to prevent tobacco initiation in children, promote cessation in smokers, eliminate TSE, and eliminate health disparities related to tobacco use. This randomized controlled trial (RCT) targeted these 4 critical components of tobacco control in populations living in poverty through socioculturally appropriate family interventions. Thus, 1 of our primary aims in the RCT was to test the effectiveness of a tailored cessation intervention for P/C smokers while their children received tailored tobacco prevention. The objective of promoting the Ps/Cs cessation was threefold: (1) to improve the personal health of the Ps/Cs, (2) to reduce TSE in their children, and (3) to facilitate the P/C modeling healthy behavior by successfully quitting smoking. In this article, we report on the effectiveness of the P/C-tailored cessation intervention, including outcomes of self-reported smoking status (in percentages) and the biological measure of saliva cotinine (ng/mL), which were both obtained at baseline, the end of treatment (EOT) and/or year 2, and in the year-4 follow-up.
Methods
Design
This study was a 2-group RCT with repeated measures. Fourteen Title 1 elementary schools with high enrollment percentages of AA children (≥65%) in fourth grade and across 5 counties in a Southeastern state were randomly selected from a computer-generated list of 25 eligible schools. The selected schools were then randomly assigned to intervention or control arms on the basis of location (rural or urban; 7 schools to each arm). Any fourth-grade classes for accelerated students, students with learning disabilities, or students with behavioral disorders were excluded because the goal was to test the treatment arms with the typical, representative fourth-grade student population.
Sample
The RCT power analyses revealed that a total of 280 fourth-grade children (ages 8–11 years) and a minimum of 1 P/C per child (total N = 560; total families N = 280) were needed to detect clinically relevant effects between the intervention and control treatment arms for various child and P/C outcomes. Inclusion criteria stated that because this was a family study, voluntary participation from both the child and a minimum of 1 P/C with whom the child resided at least 50% of the time was needed for study enrollment. Exclusion criteria were families lacking access to a telephone or cellular phone. Smoking status was not an inclusion or exclusion criteria. Recruitment occurred through 2 methods: (1) family information sessions conducted by the research team about the research study were held within each school, and both Ps/Cs and their children were invited; and (2) for those children and Ps/Cs who did not attend the family session, low-literacy written materials describing the study were provided to the children through their classrooms to give to their parents. The goal was to make a concerted effort to offer study participation to all children and their Ps/Cs across the selected classrooms along with an emphasis on the voluntary nature of participation. Enrollment rates exceeded the needed sample size of 280 Ps/Cs because a total of 453 were recruited and enrolled. Further details on recruitment and enrollment are reported in Tingen et al.22
This article reports on 1 of the primary outcomes of the RCT, specifically, the effectiveness of the tailored cessation intervention in the Ps/Cs who were smokers enrolled in the trial. This study received approval regarding human research protection from the Institutional Review Board of Augusta University. Ps/Cs completed both written, informed consent and parental consent for their children, and children provided written assent for participation in the study.
Measures
Self-Report Measures
Ps/Cs completed 2 self-report surveys to measure their smoking status. The parent sociodemographic questionnaire, which was designed by the investigative team and used for the last 8 years in school-based studies, assesses sex, race and/or ethnicity, income, employment, tobacco status, frequency of usage, smoking in the home and its frequency, and other sociodemographic measures. The Assessment of Motivation: Readiness to Quit Ladder is a 1-item assessment with scores of 1 to 10, presented in a ladder format, that asks Ps/Cs to circle the number that most closely identifies their thoughts on quitting smoking.23 A 1 indicates they are not interested in quitting smoking in this lifetime. As the rungs of the ladder go up, the move toward quitting is greater, with a 10 indicating they have quit smoking. To participate in the intensive cessation component of the study, Ps/Cs had to score a minimum of 6 on the readiness ladder, which is an indication that they definitely were planning to quit smoking within the next 6 months.23 All Ps/Cs completed these 2 self-report measures on smoking status at baseline, EOT and/or 2 years, and 4 years.
Biochemical Measures
Smoking status and cessation outcomes were assessed with saliva cotinine at baseline, EOT and/or 2 years, and 4 years follow-up. Exhaled carbon monoxide (CO) was assessed in the intervention group at baseline and 3 months because the pharmacotherapy for cessation was the nicotine replacement patch. Exhaled CO is an immediate response measure of smoking status, and >5.5 ppm is established as an optimal discrimination cutoff point.24 Saliva cotinine is the gold standard for measuring smoking status and the severity of it.25,26 Previous studies have revealed that self-report of smoking status among Ps/Cs is often not supported with cotinine levels.27,28 Thus, to have a precise measure of the effectiveness of the tailored intervention and test the primary aim of Ps/Cs success with smoking cessation, saliva cotinine was used to biochemically validate self-report measures. Established cotinine cutoff points by Benowitz et al26 that delineate smoking status for adults by race and/or ethnicity were used as follows: 5.92 ng/mL and 4.85 ng/mL for non-Hispanic AA and white participants, respectively. Cotinine analysis was performed by Salimetrics LLC by using an enzyme immunoassay that has exceptional sensitivity and detects levels as low as 0.05 ng/mL.29
Treatment Groups: Intervention and Control
Intervention
P/C smokers in the intervention group received 3 components of intensive and tailored cessation that included 8 individual face-to-face or telephone MI sessions with a matched sex and/or racial and/or ethnically similar counselor, self-help tailored written materials, and 8 weeks of nicotine replacement therapy. P/C smokers were offered the cessation intervention in years 1 and 2 (if relapsed/refused in year 1) at their children’s respective schools or a local community setting of their choice in close proximity to the children’s schools. The MI sessions were based on procedures by Miller and Rollnick30,31 and allowed the counselor and P/C to work collaboratively to identify and overcome barriers to achieve and maintain abstinence and appropriate goal setting. This approach is P/C-centered and uses a directive method for increasing intrinsic motivation while avoiding attempts to impose change externally. Communication strategies used reflective listening, affirmation, elicitation, and reinforcement of self-motivational statements rather than direct advice giving. The P/C, rather than the counselor, made the argument for change and described his or her course of action. The MI-trained study counselors conducted the initial face-to-face MI sessions at each annual assessment (at baseline [year 1] and year 2). The counselors had extensive experience with patient counseling and used a scripted protocol to provide the face-to-face and/or telephone MI sessions, which were derived from MI literature and previous MI smoking cessation interventions.31–33 For Ps/Cs who preferred it or had challenges with transportation, telephone MI counseling sessions (5–15 minutes each) were conducted over the 12 weeks after the initial face-to-face MI sessions. The frequencies of the phone calls and the face-to-face MI sessions were based on a relapse-sensitive schedule34,35 and tailored to the individual needs and readiness to change of each P/C. The probability of relapse is much greater with initial attempts of cessation, especially during the first few days and weeks after cessation attempts. Therefore, to optimize the effect of counseling, the scheduled face-to-face sessions or calls occurred in a logarithmic manner rather than at equal intervals.
Self-help written materials were tailored brochures distributed at the time of the first MI session on the basis of the readiness of the Ps/Cs to quit smoking. 8 brochures were used for participants who indicated they had no plans to quit smoking (ie, ladder score <5) over the 2 years of the intervention (ie, 4 in years 1 and 2; the number of brochures equaled the number of weeks of the child intervention/year). The self-help brochures focused on the risks of smoking and TSE, the prevalence of tobacco use in children and adults, strategies that Ps/Cs could use in keeping their children tobacco free, and the positive and beneficial effects of smoking cessation. In addition to the above self-help materials, Ps/Cs who indicated a plan to quit smoking in the next 6 months or sooner (ladder score >6) received Pathways to Freedom,23 a culturally sensitive self-help guide that was codeveloped by the Centers for Disease Control and Prevention and the US Department of Health and Human Services36 because of concern for the high rates of smoking among the AA population. For the Ps/Cs who were not AA, the self-help booklet “Freedom from Smoking” by the American Lung Association was provided.36
An 8-week supply of over-the-counter nicotine replacement therapy, the transdermal nicotine patch, was offered to Ps/Cs with a score of >6 on the readiness ladder, an established quit date, and no medical contraindications. The patch was provided for 2-week increments, and the dose was based on the nicotine dependence score and number of cigarettes smoked per day. The patch was not required for participation in the study. Ps/Cs with ladder scores <5 (no cessation contract initiated) at the end of the first face-to-face MI counseling session received follow-up MI counseling by telephone every 4 weeks for 12 weeks (ie, 3 telephone calls). If during this time frame the Ps/Cs progressed to readiness and motivation to quit smoking (ladder score >6), they received the same proactive telephone MI counseling as the other smokers who were trying to quit and were offered nicotine replacement therapy as well.
Control
Ps/Cs in the control group received time- and attention-matched health education materials on the updated American Diabetes Association food recommendations, the importance of physical activity, and lifestyle choices that decrease risk factors for cancer (American Cancer Society) and cardiovascular disease (American Heart Association). Ps/Cs self-identified as smokers at baseline received self-help cessation materials by mail on the same time schedule that the Ps/Cs in the intervention group received them. These materials included information for contacting the Georgia Tobacco Quit Line (toll free) and the same self-help brochures as the Ps/Cs in intervention schools.
Statistical Analysis
Descriptive statistics by intervention and control group at each measurement time (baseline, EOT and/or year 2, and 4 years) were determined for all variables in the analysis among P/C smokers only (n = 110). We examined whether parents in the intervention group had lower rates of cessation, as measured by self-report and cotinine, than parents in the control group at EOT and/or year 2 and year 4. Repeated measures mixed models for the cotinine outcome measure and generalized estimating equation (GEE) models for the self-report quit smoking and Benowitz-based smoking category outcome measures were used. Different correlation structures among measurement times were examined, including unstructured, compound symmetric, and autoregressive order 1. The compound symmetric correlation structure gave the best model fit by using Akaike information criteria. Fixed effects in the model included group, measurement time, and the group-by-measurement time interaction. Subject nested within group was considered a random effect. A Bonferroni adjustment to the overall α level was used to examine pairwise differences between intervention and control groups within a time point and among baseline and all other time points within the group. Additionally, to examine whether CO levels changed among those in the intervention group, a paired t test was performed. Benowitz et al26 categorizations of cotinine were used to determine smoking cessation outcomes.
Results
The total P/C sample (N = 453) in the RCT was predominantly women (89%), AA (73%), and had a single head of household (51%); nearly half of the sample (47%) earned <$20 000 annually, 40% had less than a high school education, and 54% were insured by Medicaid and/or Medicare. Control subjects were more likely to be younger than intervention subjects (P = .017). Table 1 provides the demographic information on all Ps/Cs who participated in the RCT by intervention and control group.
TABLE 1.
Demographic Characteristics for All Study Ps/Cs at Baseline (N = 453)
| Variable | Control (N = 191 [42%]) | Intervention (N = 262 [58%]) | P |
|---|---|---|---|
| Sex, n (%) | .70 | ||
| Male | 19 (10) | 29 (11) | |
| Female | 172 (90) | 233 (89) | |
| Race, n (%) | .65 | ||
| White, Hispanic, other | 54 (28) | 69 (26) | |
| AA | 137 (72) | 193 (74) | |
| Setting, n (%) | .68 | ||
| Urban | 75 (39) | 108 (41) | |
| Rural | 116 (61) | 154 (59) | |
| Age, y, mean (SD) | 36.6 (9) | 39.4 (16) | .02 |
| Marital status,a n (%) | .58 | ||
| Married | 75 (39) | 89 (36) | |
| Single | 83 (44) | 112 (45) | |
| Other | 31 (16) | 49 (20) | |
| Education,a n (%) | .11 | ||
| Less than high school | 81 (43) | 94 (37) | |
| High school | 54 (28) | 96 (38) | |
| Some college | 39 (20) | 49 (19) | |
| College or more | 16 (8) | 12 (5) | |
| Income,a n (%) | .33 | ||
| $0–$9999 | 50 (27) | 83 (34) | |
| $10 000–$19 999 | 26 (14) | 41 (17) | |
| $20 000–$29 999 | 34 (19) | 40 (16) | |
| $30 000–$39 999 | 26 (14) | 36 (15) | |
| $40 000–$49 999 | 11 (6) | 12 (5) | |
| ≥$50 000 | 36 (20) | 31 (13) | |
| Health care payment source,a n (%) | .51 | ||
| Medicare or Medicaid | 96 (52) | 136 (55) | |
| Other | 14 (8) | 25 (10) | |
| Insurance | 73 (40) | 88 (35) | |
| Household parent status,a n (%) | .31 | ||
| Single parent | 91 (49) | 134 (54) | |
| Two parents | 95 (51) | 115 (46) |
Missing data will result in frequencies that do not add to 453.
Table 2 provides sociodemographic information about the P/C smokers by intervention and control group at baseline. The P/C smokers (n = 110) comprised 24% of the total P/C enrolled sample (n = 453). The P/C smokers were also predominantly women (81%) with a mean age of 37.7 years (SD 12.2) and a similar distribution of AA participants (63%) as the total sample. Those with less than a high school education represented a slightly higher percentage (44%), as well as those earning <$20 000 annually (58%) and those covered by Medicaid and/or Medicare (64%), than the total RCT sample. Although there were low numbers of male Ps/Cs in the RCT, 44% (21 of 48) were smokers, whereas 22% (89 of 405) of the female Ps/Cs were smokers. There were no statistically significant differences in demographic variables at baseline between the intervention and control groups.
TABLE 2.
Demographic Characteristics for P/C Smokers at Baseline (n = 110)
| Variable | Control (N = 42 [38%]) | Intervention (N = 68 [62%]) | P |
|---|---|---|---|
| Sex, n (%) | .99 | ||
| Male | 8 (19) | 13 (19) | |
| Female | 34 (81) | 55 (81) | |
| Race, n (%) | .17 | ||
| White, Hispanic, other | 19 (45) | 22 (32) | |
| AA | 23 (55) | 46 (68) | |
| Setting, n (%) | .59 | ||
| Urban | 20 (48) | 36 (53) | |
| Rural | 22 (52) | 32 (47) | |
| Age, y, mean (SD) | 35.3 (8.4) | 39.2 (13.8) | .08 |
| Marital status,a n (%) | .64 | ||
| Married | 15 (36) | 17 (27) | |
| Single | 17 (40) | 29 (46) | |
| Other | 10 (24) | 17 (27) | |
| Education,a n (%) | .07 | ||
| Less than high school | 24 (57) | 24 (36) | |
| High school | 9 (21) | 27 (40) | |
| Some college | 6 (14) | 14 (21) | |
| College or more | 3 (7) | 2 (3) | |
| Income,a n (%) | .84 | ||
| $0–$9999 | 14 (35) | 22 (34) | |
| $10 000–$19 999 | 8 (20) | 16 (25) | |
| $20 000–$29 999 | 5 (12) | 10 (15) | |
| $30 000–$39 999 | 5 (12) | 10 (15) | |
| $40 000–$49 999 | 3 (8) | 3 (5) | |
| ≥$50 000 | 5 (13) | 4 (6) | |
| Health care payment source,a n (%) | .86 | ||
| Medicare or Medicaid | 26 (65) | 42 (64) | |
| Other | 3 (7) | 7 (11) | |
| Insurance | 11 (28) | 17 (26) | |
| Household parent status,a n (%) | .81 | ||
| Single parent | 22 (52) | 33 (50) | |
| Two parents | 20 (48) | 33 (50) |
Missing data will result in frequencies that don’t add to 110.
There were 17 subjects (15% attrition rate) who were lost to follow-up in year 2 or year 4 of the study. Of these, 5 of 42 (12%) were in the control group, and 12 of 68 (18%) were in the intervention group. There were no differences in attrition between the intervention and control groups (χ2 test statistic = 0.66; P = .4183). Of the 5 subjects who were lost to follow-up in the control group, 4 were lost in year 2, and 1 was lost in year 4. Of the 12 subjects who were lost to follow-up in the intervention group, 10 were lost in year 2, and 2 were lost in year 4 (Supplemental Fig 4).
Table 3 gives the descriptive statistics for all P/C smoking outcome variables by group and measurement time.
TABLE 3.
Descriptive Statistics for P/C Smokers by Group and Measurement Time
| Variable | Control | Intervention | ||||
|---|---|---|---|---|---|---|
| Baseline | EOT and/or Year 2 | Year 4 | Baseline | EOT and/or Year 2 | Year 4 | |
| Self-reported smoking abstinence, n (%) | ||||||
| Yes | 0 (0) | 0 (0) | 5 (14) | 0 (0) | 6 (11) | 24 (44) |
| No | 42 (100) | 36 (100) | 31 (86) | 68 (100) | 50 (89) | 31 (56) |
| Cotinine, geometric mean (SD) | 172.9 (8.4) | 185.9 (9.4) | 241.7 (5.4) | 261.2 (2.7) | 76.8 (12.4) | 79.3 (13.4) |
| Benowitz cotinine category, n (%) | ||||||
| Exposed | 4 (10) | 3 (8) | 1 (3) | 0 (0) | 9 (17) | 10 (19) |
| Smoker | 36 (90) | 33 (92) | 33 (97) | 63 (100) | 45 (83) | 43 (81) |
| CO, mean (SD) | — | — | — | 10.7 (7.1) | — | 6.4 (6.2) |
| CO-based quit status at last MI visit, n (%) | ||||||
| No | — | — | — | 13 (38) | 11 (35) | 14 (40) |
| Yes | — | — | — | 21 (62) | 20 (65) | 21 (60) |
—, not applicable.
Table 4 provides the results of the mixed or GEE models. It should be noted that the GEE models for quitting smoking and Benowitz-based cotinine categories did not converge. Thus, a mixed model on the binary outcome variable was used to estimate the percentage reported. Statistically significant interactions between group and measurement time were seen for all variables (self-reported smoking abstinence, geometric mean cotinine levels, and Benowitz-based cotinine categories), indicating that the change in the means or percentages over time were different in the intervention and control groups.
TABLE 4.
Mixed Model or GEE Analysis Results of P/C Outcomes for Differences by Group Over Time
| Outcome | Variable | Group Level | Time Level | LS Mean | SE | F | P |
|---|---|---|---|---|---|---|---|
| Self-reported smoking abstinence, % | Race | — | — | — | — | 2.64 | .11 |
| Sex | — | — | — | — | 0.05 | .82 | |
| Group | Intervention | — | 23.6 | 4.9 | 8.32 | .005 | |
| Control | — | 6.2 | 5.4 | ||||
| Time | — | EOT, year 2 | 2.9 | 4.8 | 27.76 | <.001 | |
| Year 4 | 2.7 | 4.7 | |||||
| Group × time | Intervention | EOT, year 2 | 6.5 | 5.7 | 4.82 | .03 | |
| Year 4 | 40.6 | 5.7 | |||||
| Control | EOT, year 2 | 0.0 | 6.5 | ||||
| Year 4 | 13.2 | 6.4 | |||||
| Cotinine, geometric mean | Race | — | — | — | — | 0.00 | .95 |
| Sex | — | — | — | — | 1.60 | .21 | |
| Group | Intervention | — | 137.7 | 1.3 | 3.03 | .08 | |
| Control | — | 258.2 | 1.4 | ||||
| Time | Baseline | Baseline | 230.3 | 1.3 | 2.43 | .09 | |
| EOT, year 2 | EOT, year 2 | 154.1 | 1.3 | ||||
| Year 4 | Year 4 | 189.0 | 1.3 | ||||
| Group × time | Intervention | Baseline | 239.9 | 1.3 | 5.72 | .004 | |
| EOT, year 2 | 99.3 | 1.4 | |||||
| Year 4 | 109.6 | 1.4 | |||||
| Control | Baseline | 221.1 | 1.4 | ||||
| EOT, year 2 | 239.0 | 1.4 | |||||
| Year 4 | 325.8 | 1.4 | |||||
| Benowitz cotinine-based tobacco exposure (exposed or smoker), % | Race | — | — | — | — | 0.01 | .90 |
| Sex | — | — | — | — | 3.35 | .07 | |
| Group | Intervention | — | 91.1 | 3.4 | 1.69 | .20 | |
| Control | — | 96.9 | 3.9 | ||||
| Time | Baseline | Baseline | 96.9 | 3.4 | 1.23 | .29 | |
| EOT, year 2 | EOT, year 2 | 91.7 | 3.6 | ||||
| Year 4 | Year 4 | 93.3 | 3.7 | ||||
| Group × time | Intervention | Baseline | 100.0 | 4.0 | 5.95 | .003 | |
| EOT, year 2 | 87.6 | 4.4 | |||||
| Year 4 | 85.2 | 4.4 | |||||
| Control | Baseline | 93.4 | 4.8 | ||||
| EOT, year 2 | 95.8 | 5.0 | |||||
| Year 4 | 100.0 | 5.1 |
LS, Least Squares; —, not applicable.
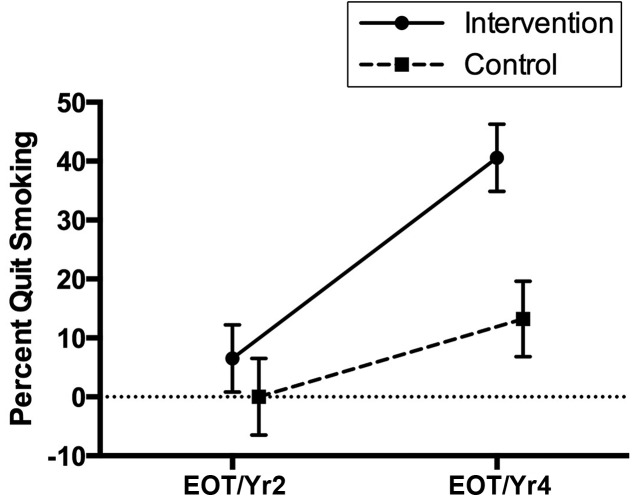
Figures 1–3 illustrate the different changes over time for each variable. For self-reported smoking abstinence (Fig 1), the intervention group showed a larger increase in quitting smoking over time than the control group.
FIGURE 1.
Self-reported smoking status.
FIGURE 3.
Benowitz cotinine smoking categories.
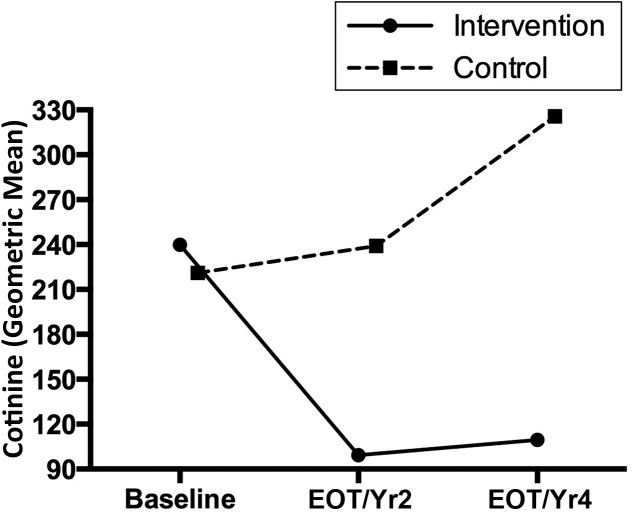
For cotinine (Fig 2), the intervention group showed a decrease from baseline to EOT and/or year 2 and then maintenance through year 4, whereas the control group showed increases from baseline to EOT and/or year 2 to year 4.
FIGURE 2.
Cotinine (geometric mean).
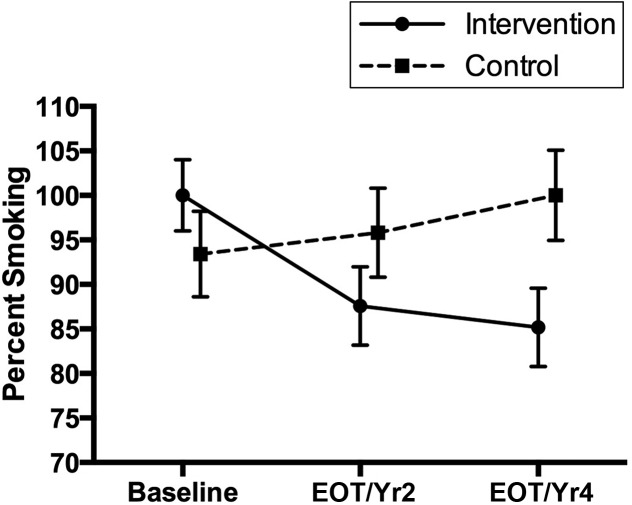
The Benowitz-based (Fig 3) cotinine smoking category percentages decreased over time from baseline to EOT and/or year 2 and year 4 in the intervention group, whereas they increased over time in the control group.
Specific post hoc pairwise differences between the intervention and control groups within each time point and between time points within a group were examined. For self-reported smoking abstinence, percentages were significantly lower at EOT and/or year 2 than at year 4 (P < .001) in the intervention group (6% vs 41%), whereas there were no differences in the control group (0% vs 13%). At year 4, the intervention group (41%) had a significantly higher smoking abstinence rate than the control group (13%; P < .001). For cotinine levels, in the intervention group, baseline (mean = 239.9) was significantly higher than EOT and/or year 2 (mean = 99.3; P <.001) and year 4 (mean = 109.6; P = .001). There were no differences in cotinine in the control group between time points (baseline mean = 221.1; EOT and/or year 2 mean = 239.0; year 4 mean = 325.8). There were no differences between intervention and control groups within a time point. For the Benowitz-based smoking category, in the intervention group, baseline (100%) was significantly higher than EOT and/or year 2 (88%; P = .003) and year 4 (85%; P < .001). There were no differences in cotinine in the control group between time points (baseline 93%; EOT and/or year 2 96%; year 4 100%). There were no differences in Benowitz-based smoking categories between the intervention and control groups within a time point (baseline 100% vs 93%, EOT and/or year 2, 88% vs 96%, year 4, 85% vs 100%). Examining the intervention group only, CO levels were significantly higher (mean difference = 4.2; SD = 9.3; paired t test: t = 2.50; P = .02) at baseline (mean = 10.7) compared with the last CO level obtained (mean = 6.4). Ps/Cs in the intervention group had smoking abstinence rates of 37% at 3 months; Ps/Cs in the control group had rates of 17% (Fisher’s exact test; P = .07).
Discussion
In this study, we demonstrated that recruiting Ps/Cs through their children’s elementary schools for participation in a research study on tobacco control is a successful strategy. Furthermore, P/C smokers may be more likely to be encouraged to participate and stay engaged in cessation treatment when their children are simultaneously enrolled in a tobacco prevention program. This study is particularly novel in the measurement of quit rates at 2 and 4 years after baseline, the statistically significant high quit rates maintained through year 4, the inclusion of both self-report and biochemical verification measures, and the predominantly AA sample.
Although researchers in previous studies have recruited Ps/Cs from pediatric practices; Supplemental Nutrition Program for Women, Infants, and Children’s offices; preschool and/or day care programs; and middle and high schools;8,14,16–19 this study is the first in which researchers recruited both Ps/Cs and children from elementary schools in both rural and urban settings and enrolled them concurrently in a tobacco control program. A unique feature of this study is the high smoking rate among Ps/Cs. In Georgia, 21.2% of the adult population (≥18 years old) are current smokers. Across all states, the prevalence of cigarette smoking among adults ranges from 11.8% to 29.0%.37 In this study population, 24.3% of the total P/C sample enrolled in the RCT were smokers, substantially exceeding the state’s smoking prevalence rate. Also, although the national smoking averages are 22.2% and 19.9% for men and women, respectively,38 44% of male and 22% of the female Ps/Cs were smokers in the RCT, with both sexes exceeding the national smoking rates. Important and also novel is the high percentage of AA Ps/Cs in this study. With 70% of AA children being exposed to tobacco smoke, efforts for successful cessation, as presented in this study, must be disseminated on a large scale because children are a vulnerable population for TSE from their Ps/Cs. The high rates of P/C smokers place the children of Georgia at a substantially greater risk for TSE; thus, a call to action is needed for robust and effective cessation interventions for Ps/Cs, which will result in a reduction of TSE in children. Furthermore, by improving the success in and maintenance of tobacco cessation by Ps/Cs, an approach including both parents and children may also reinforce the children’s resistance to the lure of tobacco products, and thus prevent their initiation of personal smoking.
The generalizability of these findings to other cessation studies with Ps/Cs may be limited because of the family approach with both child and P/C simultaneously enrolled in tobacco prevention and cessation and the long-term biochemical validation at 2 years and 4 years. No other studies were found in the literature with these 2 characteristics for comparison. Although we are unable to confirm these presumptions, the investigative team anticipates that the high abstinence rates sustained over time in the P/C intervention group may be partially attributed to their children being enrolled in a tobacco prevention intervention during the same time period.
Acknowledgments
We especially appreciate the research team members for their many contributions to a successful study implementation: Maudesta Caleb, MEd; Sheree Cartee, BS; Kelora Cofer, MPH; Sandra Inglett, PhD; Matthew Humphries, MS; Zubin Mehta, MD; Kendra Piper, MPH; Ashley Stevenson, MS; Joseph Tingen, MD; Ashley Turnmire, MS; and Lydia Veihman, DPT. We also sincerely thank the school systems in the rural and urban settings and the parents and children who participated in the study.
Glossary
- AA
African American
- CO
carbon monoxide
- EOT
end of treatment
- GEE
generalized estimating equation
- MI
motivational interviewing
- P/C
parent and/or caregiver
- RCT
randomized controlled trial
- TSE
tobacco smoke exposure
Footnotes
Dr Caldwell drafted the initial manuscript, assisted with data analyses, and reviewed the manuscript; Dr Tingen conceptualized, designed, and provided oversight for the study implementation and reviewed and revised the manuscript; Mr Nguyen assisted with the initial draft and review of the manuscript; Drs Andrews, Heath, and Treiber assisted with conceptualizing and designing the study and reviewed the manuscript; Dr Waller conducted the data analyses, provided the Statistical Analyses and Results sections of the manuscript, and reviewed the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
This trial has been registered at www.clinicaltrials.gov (identifier NCT01164306).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the National Cancer Institute of the National Institutes of Health (award R01CA118066; principal investigator: Dr Tingen). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.US Department of Health and Human Services The Health Consequences of Smoking—50 years of Progress: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014 [Google Scholar]
- 2.Institute of Medicine Secondhand Smoke Exposure and Cardiovascular Effects: Making Sense of the Evidence. Washington, DC: National Academy of Sciences, Institute of Medicine; 2009 [Google Scholar]
- 3.National Toxicology Program Report on Carcinogens. 13th ed. Washington, DC: US Department of Health and Human Services, Public Health Service; 2014 [Google Scholar]
- 4.US Department of Health and Human Services A Report of the Surgeon General: How Tobacco Smoke Causes Disease: What It Means to You. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010 [Google Scholar]
- 5.US Department of Health and Human Services The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006 [Google Scholar]
- 6.American Academy of Otolaryngology-Head and Neck Surgery Secondhand smoke and children. 2016. Available at: www.entnet.org/content/secondhand-smoke-and-children. Accessed February 21, 2017
- 7.Homa DM, Neff LJ, King BA, et al. ; Centers for Disease Control and Prevention . Vital signs: disparities in nonsmokers’ exposure to secondhand smoke–United States, 1999-2012. MMWR Morb Mortal Wkly Rep. 2015;64(4):103–108 [PMC free article] [PubMed] [Google Scholar]
- 8.Patten CA, Hughes CA, Lopez KN, et al. Web-based intervention for adolescent nonsmokers to help parents stop smoking: a pilot feasibility study. Addict Behav. 2012;37(1):85–91 [DOI] [PubMed] [Google Scholar]
- 9.Schuck K, Otten R, Kleinjan M, Bricker JB, Engels RC. School-based promotion of cessation support: reach of proactive mailings and acceptability of treatment in smoking parents recruited into cessation support through primary schools. BMC Public Health. 2013;13:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiore M, Jae’n C, Baker T, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: US Department of Health and Human Services; 2008 [Google Scholar]
- 11.Zeng EY, Vilardaga R, Heffner JL, Mull KE, Bricker JB. Predictors of utilization of a novel smoking cessation smartphone app. Telemed J E Health. 2015;21(12):998–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bricker JB, Mull KE, Kientz JA, et al. Randomized, controlled pilot trial of a smartphone app for smoking cessation using acceptance and commitment therapy. Drug Alcohol Depend. 2014;143:87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chilmonczyk BA, Palomaki GE, Knight GJ, Williams J, Haddow JE. An unsuccessful cotinine-assisted intervention strategy to reduce environmental tobacco smoke exposure during infancy. Am J Dis Child. 1992;146(3):357–360 [DOI] [PubMed] [Google Scholar]
- 14.Hovell MF, Zakarian JM, Matt GE, et al. Counseling to reduce children’s secondhand smoke exposure and help parents quit smoking: a controlled trial. Nicotine Tob Res. 2009;11(12):1383–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liles S, Hovell MF, Matt GE, Zakarian JM, Jones JA. Parent quit attempts after counseling to reduce children’s secondhand smoke exposure and promote cessation: main and moderating relationships. Nicotine Tob Res. 2009;11(12):1395–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joseph A, Murphy S, Thomas J, et al. A pilot study of concurrent lead and cotinine screening for childhood tobacco smoke exposure: effect on parental smoking. Am J Health Promot. 2014;28(5):316–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdullah AS, Hua F, Khan H, et al. Secondhand smoke exposure reduction intervention in Chinese households of young children: a randomized controlled trial. Acad Pediatr. 2015;15(6):588–598 [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Huang Z, Yang M, Wang F, Xiao S. Reducing environmental tobacco smoke exposure of preschool children: a randomized controlled trial of class-based health education and smoking cessation counseling for caregivers. Int J Environ Res Public Health. 2015;12(1):692–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins BN, Nair US, Hovell MF, et al. Reducing underserved children’s exposure to tobacco smoke: a randomized counseling trial with maternal smokers. Am J Prev Med. 2015;49(4):534–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuck K, Bricker JB, Otten R, Kleinjan M, Brandon TH, Engels RC. Effectiveness of proactive quitline counselling for smoking parents recruited through primary schools: results of a randomized controlled trial. Addiction. 2014;109(5):830–841 [DOI] [PubMed] [Google Scholar]
- 21.Schuck K, Otten R, Kleinjan M, Bricker JB, Engels RC. Predictors of cessation treatment outcome and treatment moderators among smoking parents receiving quitline counselling or self-help material. Prev Med. 2014;69:126–131 [DOI] [PubMed] [Google Scholar]
- 22.Tingen MS, Andrews JO, Heath J, Turnmire AE, Waller JL, Treiber FA. Comparison of enrollment rates of African-American families into a school-based tobacco prevention trial using two recruitment strategies in urban and rural settings. Am J Health Promot. 2013;27(4):e91–e100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biener L, Abrams DB. The contemplation ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991;10(5):360–365 [DOI] [PubMed] [Google Scholar]
- 24.Kapusta ND, Pietschnig J, Plener PL, Blüml V, Lesch OM, Walter H. Does breath carbon monoxide measure nicotine dependence? J Addict Dis. 2010;29(4):493–499 [DOI] [PubMed] [Google Scholar]
- 25.Society for Research on Nicotine and Tobacco Subcommittee on Biochemical Verification Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159 [DOI] [PubMed] [Google Scholar]
- 26.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236–248 [DOI] [PubMed] [Google Scholar]
- 27.Klesges RC, Krukowski RA, Klosky JL, et al. Efficacy of a tobacco quitline among adult survivors of childhood cancer. Nicotine Tob Res. 2015;17(6):710–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis CL, Tingen MS, Jia J, et al. Passive smoke exposure and its effects on cognition, sleep, and health outcomes in overweight and obese children. Child Obes. 2016;12(2):119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salimetrics LLC Available at: www.salimetrics.com. Accessed February 1, 2016
- 30.Miller W, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behavior. New York, NY: Guilford Press; 1991 [Google Scholar]
- 31.Miller W, Rollnick S. Motivational Interviewing: Preparing People for Change. New York, NY: Guilford Press; 2002 [Google Scholar]
- 32.Burke BL, Dunn CW, Atkins DC, Phelps JS. The emerging evidence base for motivational interviewing: a meta-analytic and qualitative inquiry. J Cogn Psychother. 2004;18(4):309–322 [Google Scholar]
- 33.Resnicow K, DiIorio C, Soet JE, Ernst D, Borrelli B, Hecht J. Motivational interviewing in health promotion: it sounds like something is changing. Health Psychol. 2002;21(5):444–451 [PubMed] [Google Scholar]
- 34.Zhu SH, Stretch V, Balabanis M, Rosbrook B, Sadler G, Pierce JP. Telephone counseling for smoking cessation: effects of single-session and multiple-session interventions. J Consult Clin Psychol. 1996;64(1):202–211 [DOI] [PubMed] [Google Scholar]
- 35.Zhu SH, Rosbrook B, Anderson C, Gilpin E, Sadler G, Pierce JP. The demographics of help-seeking for smoking cessation in California and the role of the California Smokers’ Helpline. Tob Control. 1995;4(suppl 1):S9–S15 [Google Scholar]
- 36.Robinson RG, Sutton CG, James DA, Orleans CT. Pathways to Freedom: Winning the Fight Against Tobacco. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2002 [Google Scholar]
- 37.Centers for Disease Control and Prevention Georgia smoking and tobacco use state highlights. 2011. Available at: https://www.cdc.gov/tobacco/data_statistics/state_data/state_highlights/2012/states/georgia/index.htm. Accessed January 25, 2016
- 38.Institute for Health Metrics and Evaluation US County Profile: Richmond County, Georgia. Seattle, WA: Institute for Health Metrics and Evaluation; 2015 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.